Exploring Oceans for Curative Compounds: Potential New Antimicrobial and Anti-Virulence Molecules against Pseudomonas aeruginosa
Abstract
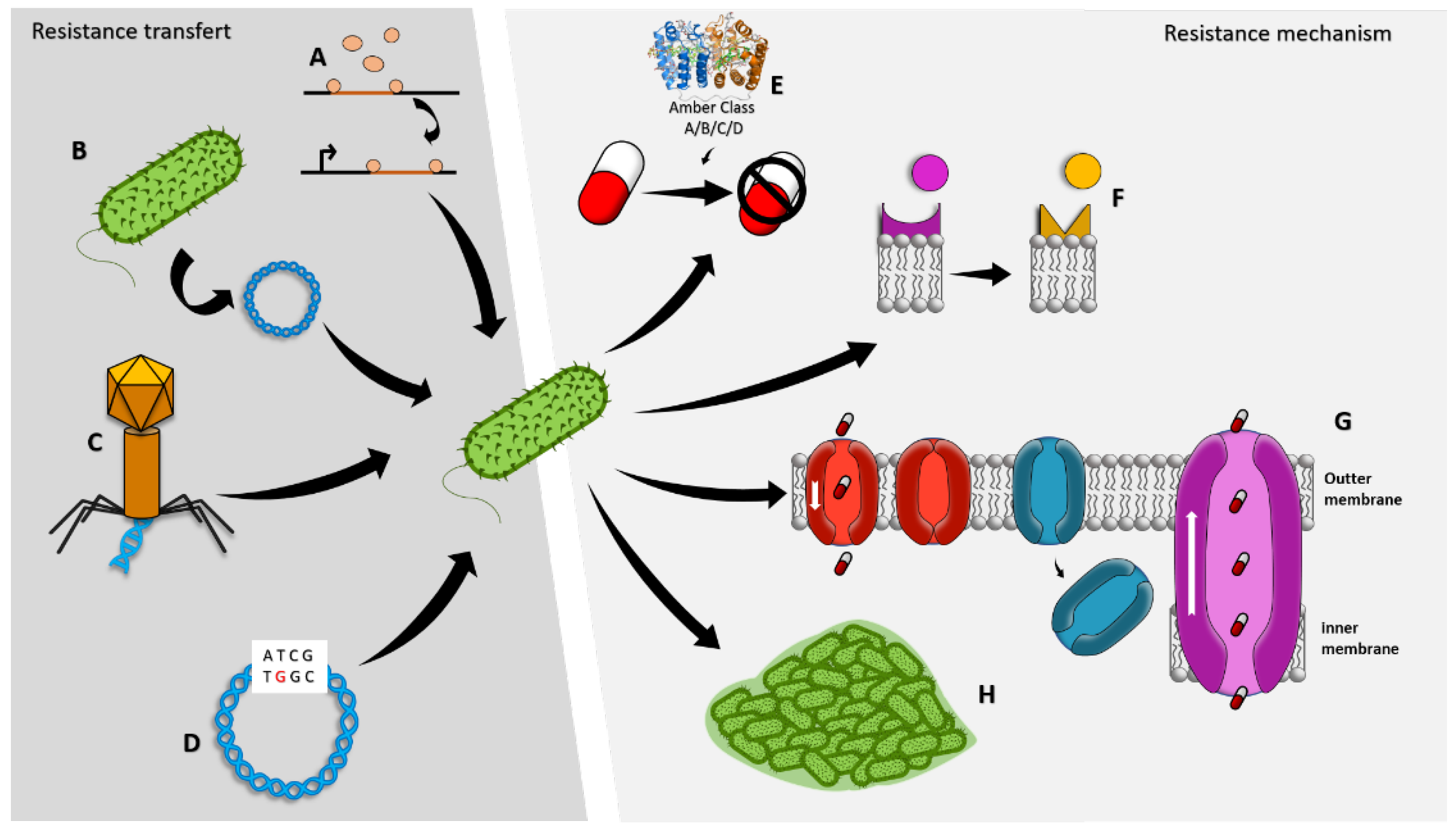
:1. Introduction
2. Pseudomonas aeruginosa
3. Antimicrobial Resistance Mechanisms of Pseudomonas aeruginosa
3.1. Biofilm Production
3.2. Drug Inactivation
3.3. Modification of Drug Binding Sites/Targets
3.4. Changes in Cell Permeability
4. Marine Natural Products against P. aeruginosa
4.1. Antimicrobials
4.1.1. Peptides and Proteins
4.1.2. Terpenes and Terpenoids
4.1.3. Polyketides
4.1.4. Alkaloids
4.1.5. Miscellaneous
4.2. Anti-Virulence Compounds
4.2.1. Proteins and Peptides
4.2.2. Polyketides
4.2.3. Alkaloids
4.2.4. Miscellaneous
4.2.5. β-Lactamases and Efflux Pump Inhibitors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kupferschmidt, K. Resistance Fighters; American Association for the Advancement of Science: Washington, DC, USA, 2016. [Google Scholar]
- Fernández, J.; Bert, F.; Nicolas-Chanoine, M.-H. The challenges of multi-drug-resistance in hepatology. J. Hepatol. 2016, 65, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control, and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Tacconelli, E. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- De Bentzmann, S.; Plésiat, P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol. 2011, 13, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.V.; Galdino, A.C.M.; Nunes, A.P.F.; dos Santos, K.R.; Moreira, B.M.; Cacci, L.C.; Sodré, C.L.; Ziccardi, M.; Branquinha, M.H.; Santos, A.L. Virulence attributes in Brazilian clinical isolates of Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2014, 304, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313. [Google Scholar] [CrossRef]
- Vasil, M.L. Pseudomonas aeruginosa: Biology, mechanisms of virulence, epidemiology. J. Pediatr. 1986, 108, 800–805. [Google Scholar] [CrossRef]
- Jayaseelan, S.; Ramaswamy, D.; Dharmaraj, S. Pyocyanin: Production, applications, challenges and new insights. World J. Microbiol. Biotechnol. 2014, 30, 1159–1168. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]
- Bendiak, G.N.; Ratjen, F. The Approach to Pseudomonas Aeruginosa in Cystic Fibrosis; Seminars in respiratory and critical care medicine, 2009; Thieme Medical Publishers: New York, NY, USA, 2009; pp. 587–595. [Google Scholar]
- Sousa, A.M.; Pereira, M.O. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs—A review. Pathogens 2014, 3, 680–703. [Google Scholar] [CrossRef]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updates 2015, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, M.M.; Murad, H.A.S. Cystic fibrosis: Current therapeutic targets and future approaches. J. Transl. Med. 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Breidenstein, E.B.; de la Fuente-Núñez, C.; Hancock, R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Speert, D.P. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and impact on treatment. Drug Resist. Updates 2000, 3, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghodhbane, H.; Elaidi, S.; Sabatier, J.-M.; Achour, S.; Benhmida, J.; Regaya, I. Bacteriocins active against multi-resistant gram negative bacteria implicated in nosocomial infections. Infect. Disord. Drug Targets (Former. Curr. Drug Targets-Infect. Disord.) 2015, 15, 2–12. [Google Scholar] [CrossRef] [PubMed]
- El Zowalaty, M.E.; Al Thani, A.A.; Webster, T.J.; El Zowalaty, A.E.; Schweizer, H.P.; Nasrallah, G.K.; Marei, H.E.; Ashour, H.M. Pseudomonas aeruginosa: Arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol. 2015, 10, 1683–1706. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, 1–20. [Google Scholar] [CrossRef]
- Kung, V.L.; Ozer, E.A.; Hauser, A.R. The accessory genome of Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2010, 74, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Gill, E.E.; Franco, O.L.; Hancock, R.E. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem. Biol. Drug Des. 2015, 85, 56–78. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Rao, S.; Bansal, A.; Dang, S.; Gupta, S.; Gabrani, R. Pseudomonas aeruginosa biofilm: Potential therapeutic targets. Biologicals 2014, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Mandsberg, L.F.; Wang, H.; Høiby, N. Phenotypes selected during chronic lung infection in cystic fibrosis patients: Implications for the treatment of Pseudomonas aeruginosa biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macia, M.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob. Agents Chemother. 2012, 56, 2683–2690. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lu, J.; Ying, M.; Mu, J.; Li, P.; Liu, Y. Docking and molecular dynamics studies on triclosan derivatives binding to FabI. J. Mol. Model. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Maiden, M.M.; Hunt, A.M.A.; Zachos, M.P.; Gibson, J.A.; Hurwitz, M.E.; Mulks, M.H.; Waters, C.M. Triclosan is an aminoglycoside adjuvant for eradication of Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2018, 62, e00146-18. [Google Scholar] [CrossRef] [Green Version]
- Jackson, K.D.; Starkey, M.; Kremer, S.; Parsek, M.R.; Wozniak, D.J. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. Am. Soc. Microbiol. 2004, 186, 4466–4475. [Google Scholar] [CrossRef]
- Matsukawa, M.; Greenberg, E. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. Am. Soc. Microbiol. 2004, 186, 4449–4456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozniak, D.J.; Wyckoff, T.J.; Starkey, M.; Keyser, R.; Azadi, P.; O’Toole, G.A.; Parsek, M.R. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 2003, 100, 7907–7912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.; Parsek, M.R. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Vogeleer, P.; Tremblay, Y.D.; Mafu, A.A.; Jacques, M.; Harel, J. Life on the outside: Role of biofilms in environmental persistence of Shiga-toxin producing Escherichia coli. Front. Microbiol. 2014, 5, 317. [Google Scholar] [CrossRef] [Green Version]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [Green Version]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef]
- Pamp, S.J.; Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef] [Green Version]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Van Gennip, M.; Christensen, L.D.; Alhede, M.; Phipps, R.; Jensen, P.Ø.; Christophersen, L.; Pamp, S.J.; Moser, C.; Mikkelsen, P.J.; Koh, A.Y. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. Apmis 2009, 117, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015, 21 (Suppl. S1), S1–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. Apmis 2017, 125, 304–319. [Google Scholar] [CrossRef] [Green Version]
- Stewart, P.S.; Franklin, M.J.; Williamson, K.S.; Folsom, J.P.; Boegli, L.; James, G.A. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2015, 59, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef]
- Poole, K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2005, 49, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Wolter, D.J.; Lister, P.D. Mechanisms of β-lactam resistance among Pseudomonas aeruginosa. Curr. Pharm. Des. 2013, 19, 209–222. [Google Scholar] [CrossRef]
- Ratjen, F.; Brockhaus, F.; Angyalosi, G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: A review. J. Cyst. Fibros. 2009, 8, 361–369. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Hainrichson, M.; Yaniv, O.; Cherniavsky, M.; Nudelman, I.; Shallom-Shezifi, D.; Yaron, S.; Baasov, T. Overexpression and initial characterization of the chromosomal aminoglycoside 3′-O-phosphotransferase APH (3′)-IIb from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2007, 51, 774–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hächler, H.; Santanam, P.; Kayser, F.H. Sequence and characterization of a novel chromosomal aminoglycoside phosphotransferase gene, aph (3′)-IIb, in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1996, 40, 1254–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, D.; Vijay, A.K.; Willcox, M. Overview of mechanisms of antibiotic resistance in Pseudomonas aeruginosa: An ocular perspective. Clin. Exp. Optom. 2018, 101, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, G.A.; Blaser, M.; Santanam, P.; Hächler, H.; Kayser, F.; Hare, R.; Miller, G. Appearance of amikacin and tobramycin resistance due to 4′-aminoglycoside nucleotidyltransferase [ANT (4′)-II] in gram-negative pathogens. Antimicrob. Agents Chemother. 1990, 34, 2381–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrazeg, M.; Jeannot, K.; Ntsogo Enguéné, V.Y.; Broutin, I.; Loeffert, S.; Fournier, D.; Plésiat, P. Mutations in β-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob. Agents Chemother. 2015, 59, 6248–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, D.; Nair, D. Extended-spectrum β-lactamases in Gram Negative Bacteria. J. Glob. Infect. Dis. 2010, 2, 263. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Juan, C.; Maciá, M.D.; Gutiérrez, O.; Vidal, C.; Pérez, J.L.; Oliver, A. Molecular mechanisms of β-lactam resistance mediated by AmpC hyperproduction in Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 2005, 49, 4733–4738. [Google Scholar] [CrossRef] [Green Version]
- Bruchmann, S.; Dötsch, A.; Nouri, B.; Chaberny, I.F.; Häussler, S. Quantitative contributions of target alteration and decreased drug accumulation to Pseudomonas aeruginosa fluoroquinolone resistance. Antimicrob Agents Chemother 2013, 57, 1361–1368. [Google Scholar] [CrossRef]
- El’Garch, F.; Jeannot, K.; Hocquet, D.; Llanes-Barakat, C.; Plésiat, P. Cumulative effects of several nonenzymatic mechanisms on the resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 2007, 51, 1016–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyá, B.; Beceiro, A.; Cabot, G.; Juan, C.; Zamorano, L.; Alberti, S.; Oliver, A. Pan-β-lactam resistance development in Pseudomonas aeruginosa clinical strains: Molecular mechanisms, penicillin-binding protein profiles, and binding affinities. Antimicrob. Agents Chemother. 2012, 56, 4771–4778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boll, M.; Radziejewska-Lebrecht, J.; Warth, C.; Krajewska-Pietrasik, D.; Mayer, H. 4-Amino-4-deoxy-L-arabinose in LPS of enterobacterial R-mutants and its possible role for their polymyxin reactivity. FEMS Immunol. Med. Microbiol. 1994, 8, 329–341. [Google Scholar] [PubMed] [Green Version]
- Miller, A.K.; Brannon, M.K.; Stevens, L.; Johansen, H.K.; Selgrade, S.E.; Miller, S.I.; Høiby, N.; Moskowitz, S.M. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob. Agents Chemother. 2011, 55, 5761–5769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owusu-Anim, D.; Kwon, D.H. Differential role of two-component regulatory systems (phoPQ and pmrAB) in polymyxin B susceptibility of Pseudomonas aeruginosa. Adv. Microbiol. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef] [Green Version]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Poole, K. Resistance to β-lactam antibiotics. Cell. Mol. Life Sci. CMLS 2004, 61, 2200–2223. [Google Scholar] [CrossRef]
- Bellido, F.; Martin, N.L.; Siehnel, R.J.; Hancock, R. Reevaluation, using intact cells, of the exclusion limit and role of porin OprF in Pseudomonas aeruginosa outer membrane permeability. J. Bacteriol. 1992, 174, 5196–5203. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Sugawara, E.; Nagano, K.; Nikaido, H. Alternative folding pathways of the major porin OprF of Pseudomonas aeruginosa. FEBS J. 2012, 279, 910–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestorovich, E.M.; Sugawara, E.; Nikaido, H.; Bezrukov, S.M. Pseudomonas aeruginosa porin OprF: Properties of the channel. J. Biol. Chem. 2006, 281, 16230–16237. [Google Scholar] [CrossRef] [Green Version]
- Piddock, L.; Hall, M.; Bellido, F.; Bains, M.; Hancock, R. A pleiotropic, posttherapy, enoxacin-resistant mutant of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1992, 36, 1057–1061. [Google Scholar] [CrossRef] [Green Version]
- Trias, J.; Nikaido, H. Protein D2 channel of the Pseudomonas aeruginosa outer membrane has a binding site for basic amino acids and peptides. J. Biol. Chem. 1990, 265, 15680–15684. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Yamano, Y.; Nakae, T. Role of porins in the antibiotic susceptibility of Pseudomonas aeruginosa: Construction of mutants with deletions in the multiple porin genes. Biochem. Biophys. Res. Commun. 1995, 213, 88–95. [Google Scholar] [CrossRef]
- Richardot, C.; Plésiat, P.; Fournier, D.; Monlezun, L.; Broutin, I.; Llanes, C. Carbapenem resistance in cystic fibrosis strains of Pseudomonas aeruginosa as a result of amino acid substitutions in porin OprD. Int. J. Antimicrob. Agents 2015, 45, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef] [Green Version]
- Dreier, J.; Ruggerone, P. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 660. [Google Scholar] [CrossRef] [Green Version]
- Masuda, N.; Sakagawa, E.; Ohya, S.; Gotoh, N.; Tsujimoto, H.; Nishino, T. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000, 44, 3322–3327. [Google Scholar] [CrossRef]
- Okamoto, K.; Gotoh, N.; Nishino, T. Extrusion of penem antibiotics by multicomponent efflux systems MexAB-OprM, MexCD-OprJ, and MexXY-OprM of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2002, 46, 2696–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llanes, C.; Köhler, T.; Patry, I.; Dehecq, B.; Van Delden, C.; Plésiat, P. Role of the MexEF-OprN efflux system in low-level resistance of Pseudomonas aeruginosa to ciprofloxacin. Antimicrob. Agents Chemother. 2011, 55, 5676–5684. [Google Scholar] [CrossRef] [Green Version]
- Hocquet, D.; Vogne, C.; El Garch, F.; Vejux, A.; Gotoh, N.; Lee, A.; Lomovskaya, O.; Plésiat, P. MexXY-OprM efflux pump is necessary for adaptive resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 2003, 47, 1371–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llanes, C.; Hocquet, D.; Vogne, C.; Benali-Baitich, D.; Neuwirth, C.; Plésiat, P. Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously. Antimicrob. Agents Chemother. 2004, 48, 1797–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigemura, K.; Osawa, K.; Kato, A.; Tokimatsu, I.; Arakawa, S.; Shirakawa, T.; Fujisawa, M. Association of overexpression of efflux pump genes with antibiotic resistance in Pseudomonas aeruginosa strains clinically isolated from urinary tract infection patients. J. Antibiot. 2015, 68, 568–572. [Google Scholar] [CrossRef]
- Llanes, C.; Pourcel, C.; Richardot, C.; Plésiat, P.; Fichant, G.; Cavallo, J.-D.; Mérens, A.; Group, G.S.; Vu-Thien, H.; Leclercq, R. Diversity of β-lactam resistance mechanisms in cystic fibrosis isolates of Pseudomonas aeruginosa: A French multicentre study. J. Antimicrob. Chemother. 2013, 68, 1763–1771. [Google Scholar] [CrossRef] [Green Version]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa–Mechanisms, epidemiology and evolution. Drug Resist. Updates 2019, 44, 100640. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Starkey, M.; Lepine, F.; Maura, D.; Bandyopadhaya, A.; Lesic, B.; He, J.; Kitao, T.; Righi, V.; Milot, S.; Tzika, A. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog. 2014, 10, e1004321. [Google Scholar] [CrossRef]
- Kang, D.; Zhang, L.; Kirienko, N.V. High-throughput approaches for the identification of Pseudomonas aeruginosa antivirulents. mBio 2021, 12, e02240-20. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Antibiotic Resistance: Biofilm Dispersing Agent Rejuvenates Older Antibiotics; National Institute of Environmental Health Sciences: Durham, NC, USA, 2010.
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geske, G.D.; Wezeman, R.J.; Siegel, A.P.; Blackwell, H.E. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J. Am. Chem. Soc. 2005, 127, 12762–12763. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [Green Version]
- Marx, V. Stop the microbial chatter. Nature 2014, 511, 493–497. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Leoni, G.; De Poli, A.; Mardirossian, M.; Gambato, S.; Florian, F.; Venier, P.; Wilson, D.N.; Tossi, A.; Pallavicini, A.; Gerdol, M. Myticalins: A Novel Multigenic Family of Linear, Cationic Antimicrobial Peptides from Marine Mussels (Mytilus spp.). Mar. Drugs 2017, 15, 261. [Google Scholar] [CrossRef] [Green Version]
- Oh, R.; Lee, M.J.; Kim, Y.-O.; Nam, B.-H.; Kong, H.J.; Kim, J.-W.; Park, J.-Y.; Seo, J.-K.; Kim, D.-G. Myticusin-beta, antimicrobial peptide from the marine bivalve, Mytilus coruscus. Fish Shellfish. Immunol. 2020, 99, 342–352. [Google Scholar] [CrossRef]
- Seo, J.-K.; Lee, M.J.; Go, H.-J.; Kim, G.D.; Jeong, H.D.; Nam, B.-H.; Park, N.G. Purification and antimicrobial function of ubiquitin isolated from the gill of Pacific oyster, Crassostrea gigas. Mol. Immunol. 2013, 53, 88–98. [Google Scholar] [CrossRef]
- Solstad, R.G.; Li, C.; Isaksson, J.; Johansen, J.; Svenson, J.; Stensvåg, K.; Haug, T. Novel Antimicrobial Peptides EeCentrocins 1, 2 and EeStrongylocin 2 from the Edible Sea Urchin Echinus esculentus Have 6-Br-Trp Post-Translational Modifications. PLoS ONE 2016, 11, e0151820. [Google Scholar] [CrossRef]
- Rekha, R.; Vaseeharan, B.; Ishwarya, R.; Anjugam, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Govindarajan, M. Searching for crab-borne antimicrobial peptides: Crustin from Portunus pelagicus triggers biofilm inhibition and immune responses of Artemia salina against GFP tagged Vibrio parahaemolyticus Dahv2. Mol. Immunol. 2018, 101, 396–408. [Google Scholar] [CrossRef]
- Moe, M.K.; Haug, T.; Sydnes, M.O.; Sperstad, S.V.; Li, C.; Vaagsfjord, L.C.; de la Vega, E.; Stensvåg, K. Paralithocins, Antimicrobial Peptides with Unusual Disulfide Connectivity from the Red King Crab, Paralithodes camtschaticus. J. Nat. Prod. 2018, 81, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, F.; Wang, X.; Peng, H.; Zhang, H.; Wang, K.-J. The Synergistic Effect of Mud Crab Antimicrobial Peptides Sphistin and Sph12−38 With Antibiotics Azithromycin and Rifampicin Enhances Bactericidal Activity Against Pseudomonas Aeruginosa. Front. Cell. Infect. Microbiol. 2020, 10, 572849. [Google Scholar] [CrossRef] [PubMed]
- Kawano, K.; Yoneya, T.; Miyata, T.; Yoshikawa, K.; Tokunaga, F.; Terada, Y.; Iwanaga, S. Antimicrobial peptide, tachyplesin I, isolated from hemocytes of the horseshoe crab (Tachypleus tridentatus). NMR determination of the beta-sheet structure. J. Biol. Chem. 1990, 265, 15365–15367. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Lee, M.J.; Jung, H.G.; Go, H.J.; Kim, Y.J.; Park, N.G. Antimicrobial function of SHbetaAP, a novel hemoglobin beta chain-related antimicrobial peptide, isolated from the liver of skipjack tuna, Katsuwonus pelamis. Fish Shellfish. Immunol. 2014, 37, 173–183. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Chen, J.-C.; Sheen, J.-F.; Lin, T.-L.; Chen, J.-Y. Epinecidin-1 has immunomodulatory effects, facilitating its therapeutic use in a mouse model of Pseudomonas aeruginosa sepsis. Antimicrob. Agents Chemother. 2014, 58, 4264–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.-C.; Lee, S.-H.; Hour, A.-L.; Pan, C.-Y.; Lee, L.-H.; Chen, J.-Y. Five Different Piscidins from Nile Tilapia, Oreochromis niloticus: Analysis of Their Expressions and Biological Functions. PLoS ONE 2012, 7, e50263. [Google Scholar] [CrossRef]
- Acosta, J.; Montero, V.; Carpio, Y.; Velázquez, J.; Garay, H.E.; Reyes, O.; Cabrales, A.; Masforrol, Y.; Morales, A.; Estrada, M.P. Cloning and functional characterization of three novel antimicrobial peptides from tilapia (Oreochromis niloticus). Aquaculture 2013, 372–375, 9–18. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.; Zhang, S.; Wu, S.; Xie, Z.; Ling, G.; Kuang, Y.-Q.; Yang, Y.; Yu, H.; Wang, Y. Identification and Characterization of the First Cathelicidin from Sea Snakes with Potent Antimicrobial and Anti-inflammatory Activity and Special Mechanism *. J. Biol. Chem. 2015, 290, 16633–16652. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Ross, N.W.; MacKinnon, S.L. Myxinidin, A Novel Antimicrobial Peptide from the Epidermal Mucus of Hagfish, Myxine glutinosa L. Mar. Biotechnol. 2009, 11, 748. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Sun, S.; Chang, A.; Dai, X.; Li, H.; Wang, Y.; Zhu, H. A cyclic dipeptide from marine fungus Penicillium chrysogenum DXY-1 exhibits anti-quorum sensing activity. ACS Omega 2021, 6, 7693–7700. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, X.-M.; Hu, X.-Y.; Li, X.; Chi, L.-P.; Li, H.-L.; Wang, B.-G. Antibacterial metabolites from Ascidian-derived fungus Aspergillus clavatus AS-107. Phytochem. Lett. 2019, 34, 30–34. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, J.-S.; Lee, Y.-J.; Shin, H.J. Gageostatins A–C, Antimicrobial Linear Lipopeptides from a Marine Bacillus subtilis. Mar. Drugs 2014, 12, 871–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahiya, R.; Pathak, D. First total synthesis and biological evaluation of halolitoralin A. J. Serb. Chem. Soc. 2007, 72. [Google Scholar] [CrossRef]
- Dahiya, R.; Pathak, D. Synthesis, Characterization and Biological Evaluation of Halolitoralin B—A Natural Cyclic Peptide. Asian J. Chem. 2007, 19, 1499–1505. [Google Scholar]
- Yang, H.; Johnson, P.M.; Ko, K.C.; Kamio, M.; Germann, M.W.; Derby, C.D.; Tai, P.C. Cloning, characterization and expression of escapin, a broadly antimicrobial FAD-containing L-amino acid oxidase from ink of the sea hare Aplysia californica. J. Exp. Biol. 2005, 208 Pt 18, 3609–3622. [Google Scholar] [CrossRef] [Green Version]
- Dusane, D.H.; Damare, S.R.; Nancharaiah, Y.V.; Ramaiah, N.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Disruption of Microbial Biofilms by an Extracellular Protein Isolated from Epibiotic Tropical Marine Strain of Bacillus licheniformis. PLoS ONE 2013, 8, e64501. [Google Scholar] [CrossRef] [Green Version]
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Gageotetrins A–C, noncytotoxic antimicrobial linear lipopeptides from a marine bacterium Bacillus subtilis. Org. Lett. 2014, 16, 928–931. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Zhang, Y.; Li, C.S.; Wang, B.G. Conidiogenones H and I, two new diterpenes of Cyclopiane class from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Chem. Biodivers. 2011, 8, 1748–1753. [Google Scholar] [CrossRef]
- Chi, L.-P.; Li, X.-M.; Wan, Y.-P.; Li, X.; Wang, B.-G. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J. Nat. Prod. 2020, 83, 3652–3660. [Google Scholar] [CrossRef]
- Jebakumar, S.; Velayudhan, S.S. Purification of bioactive natural product against human microbial pathogens from marine sea weed Dictyota acutiloba J. Ag. World J. Microbiol. Biotechnol. 2008, 24, 1747–1752. [Google Scholar] [CrossRef]
- Gomaa, M.N.; Soliman, K.; Ayesh, A.; Abd El-Wahed, A.; Hamza, Z.; Mansour, H.M.; Khalifa, S.A.; Mohd Ali, H.B.; El-Seedi, H.R. Antibacterial effect of the red sea soft coral Sarcophyton trocheliophorum. Nat. Prod. Res. 2016, 30, 729–734. [Google Scholar] [CrossRef]
- Lutta, K.; Bii, C.; Akenga, T.; Cornelius, W. Antimicrobial Marine Natural Products from the Sponge, Axinella infundibuliformis. Rec. Nat. Prod. 2008, 2. [Google Scholar]
- Akhter, N.; Liu, Y.; Auckloo, B.N.; Shi, Y.; Wang, K.; Chen, J.; Wu, X.; Wu, B. Stress-Driven Discovery of New Angucycline-Type Antibiotics from a Marine Streptomyces pratensis NA-ZhouS1. Mar. Drugs 2018, 16, 331. [Google Scholar] [CrossRef] [Green Version]
- Orfali, R.; Perveen, S.; Al-Taweel, A.; Ahmed, A.F.; Majrashi, N.; Alluhay, K.; Khan, A.; Luciano, P.; Taglialatela-Scafati, O. Penipyranicins A–C: Antibacterial Methylpyran Polyketides from a Hydrothermal Spring Sediment Penicillium sp. J. Nat. Prod. 2020, 83, 3591–3597. [Google Scholar] [CrossRef]
- Raju, R.; Khalil, Z.G.; Piggott, A.M.; Blumenthal, A.; Gardiner, D.L.; Skinner-Adams, T.S.; Capon, R.J. Mollemycin A: An antimalarial and antibacterial glyco-hexadepsipeptide-polyketide from an Australian marine-derived Streptomyces sp. (CMB-M0244). Org. Lett. 2014, 16, 1716–1719. [Google Scholar] [CrossRef]
- Auckloo, B.N.; Pan, C.; Akhter, N.; Wu, B.; Wu, X.; He, S. Stress-driven discovery of novel cryptic antibiotics from a marine fungus Penicillium sp. BB1122. Front. Microbiol. 2017, 8, 1450. [Google Scholar] [CrossRef] [Green Version]
- Schneemann, I.; Kajahn, I.; Ohlendorf, B.; Zinecker, H.; Erhard, A.; Nagel, K.; Wiese, J.; Imhoff, J.F. Mayamycin, a cytotoxic polyketide from a Streptomyces strain isolated from the marine sponge Halichondria panicea. J. Nat. Prod. 2010, 73, 1309–1312. [Google Scholar] [CrossRef]
- Shah, M.; Sun, C.; Sun, Z.; Zhang, G.; Che, Q.; Gu, Q.; Zhu, T.; Li, D. Antibacterial polyketides from antarctica sponge-derived fungus Penicillium sp. HDN151272. Mar. Drugs 2020, 18, 71. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Macrocyclic polyketides with siderophore mode of action from marine heterotrophic Shewanella algae: Prospective anti-infective leads attenuate drug-resistant pathogens. J. Appl. Microbiol. 2021, 130, 1552–1570. [Google Scholar] [CrossRef]
- Kizhakkekalam, V.K.; Chakraborty, K.; Joy, M. Oxygenated elansolid-type of polyketide spanned macrolides from a marine heterotrophic Bacillus as prospective antimicrobial agents against multidrug-resistant pathogens. Int. J. Antimicrob. Agents 2020, 55, 105892. [Google Scholar] [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Polyketide-derived macrobrevins from marine macroalga-associated Bacillus amyloliquefaciens as promising antibacterial agents against pathogens causing nosocomial infections. Phytochemistry 2022, 193, 112983. [Google Scholar] [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Dhara, S. Difficidin class of polyketide antibiotics from marine macroalga-associated Bacillus as promising antibacterial agents. Appl. Microbiol. Biotechnol. 2021, 105, 6395–6408. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Abdel-Razek, A.S.; Hamed, A.; Soliman, H.S.M.; Ponomareva, L.V.; Thorson, J.S.; Shaaban, K.A.; Shaaban, M. RF-3192C and other polyketides from the marine endophytic Aspergillus niger ASSB4: Structure assignment and bioactivity investigation. Med. Chem. Res. 2021, 30, 647–654. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Zhang, Y.-H.; Shao, C.-L.; Cao, F.; Wang, C.-Y. Microketides A and B, polyketides from a gorgonian-derived Microsphaeropsis sp. fungus. J. Nat. Prod. 2020, 83, 1300–1304. [Google Scholar] [CrossRef]
- Fu, P.; Wang, S.; Hong, K.; Li, X.; Liu, P.; Wang, Y.; Zhu, W. Cytotoxic Bipyridines from the Marine-Derived Actinomycete Actinoalloteichus cyanogriseus WH1-2216-6. J. Nat. Prod. 2011, 74, 1751–1756. [Google Scholar] [CrossRef]
- Sun, X.; Sun, S.; Ference, C.; Zhu, W.; Zhou, N.; Zhang, Y.; Zhou, K. A potent antimicrobial compound isolated from Clathria cervicornis. Bioorganic Med. Chem. Lett. 2015, 25, 67–69. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Pérez-Povedano, M.; Martinez-Guitian, M.; Lasarte-Monterrubio, C.; Vázquez-Ucha, J.C.; Bou, G.; Rodríguez, J.; Beceiro, A.; Jimenez, C. In vitro and in vivo assessment of the efficacy of bromoageliferin, an alkaloid isolated from the sponge Agelas dilatata, against Pseudomonas aeruginosa. Mar. Drugs 2020, 18, 326. [Google Scholar] [CrossRef]
- De Oliveira, J.H.H.L.; Seleghim, M.H.R.; Timm, C.; Grube, A.; Köck, M.; Nascimento, G.G.F.; Martins, A.C.T.; Silva, E.G.O.; De Souza, A.O.; Minarini, P.R.R.; et al. Antimicrobial and Antimycobacterial Activity of Cyclostellettamine Alkaloids from Sponge Pachychalina sp. Mar. Drugs 2006, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A. Bioactive brominated oxindole alkaloids from the Red Sea sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef] [Green Version]
- Murali Krishna Kumar, M.; Devilal Naik, J.; Satyavathi, K.; Ramana, H.; Raghuveer Varma, P.; Purna Nagasree, K.; Smitha, D.; Venkata Rao, D. Denigrins A–C: New antitubercular 3, 4-diarylpyrrole alkaloids from Dendrilla nigra. Nat. Prod. Res. 2014, 28, 888–894. [Google Scholar] [CrossRef]
- Tadesse, M.; Tabudravu, J.N.; Jaspars, M.; Strøm, M.B.; Hansen, E.; Andersen, J.H.; Kristiansen, P.E.; Haug, T. The antibacterial ent-eusynstyelamide B and eusynstyelamides D, E, and F from the Arctic bryozoan Tegella cf. spitzbergensis. J. Nat. Prod. 2011, 74, 837–841. [Google Scholar] [CrossRef]
- Hassan, S.; Hamed, S.; Almuhayawi, M.; Hozzein, W.; Selim, S.; AbdElgawad, H. Bioactivity of Ellagic Acid and Velutin: Two Phenolic Compounds Isolated from Marine Algae. Egypt. J. Bot. 2021, 61, 219–231. [Google Scholar] [CrossRef]
- Alam, P.; Alqahtani, A.S.; Mabood Husain, F.; Tabish Rehman, M.; Alajmi, M.F.; Noman, O.M.; El Gamal, A.A.; Al-Massarani, S.M.; Shavez Khan, M. Siphonocholin isolated from red sea sponge Siphonochalina siphonella attenuates quorum sensing controlled virulence and biofilm formation. Saudi. Pharm. J. 2020, 28, 1383–1391. [Google Scholar] [CrossRef]
- Sun, S.; Canning, C.B.; Bhargava, K.; Sun, X.; Zhu, W.; Zhou, N.; Zhang, Y.; Zhou, K. Polybrominated diphenyl ethers with potent and broad spectrum antimicrobial activity from the marine sponge Dysidea. Bioorganic Med. Chem. Lett. 2015, 25, 2181–2183. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Wang, B.; Xu, Y.; Zhu, G.; Lan, M.; Zhu, W.; Sun, K. Diketopiperazine and Diphenylether Derivatives from Marine Algae-Derived Aspergillus versicolor OUCMDZ-2738 by Epigenetic Activation. Mar. Drugs 2019, 17, 6. [Google Scholar] [CrossRef] [Green Version]
- Wyche, T.P.; Alvarenga, R.F.R.; Piotrowski, J.S.; Duster, M.N.; Warrack, S.R.; Cornilescu, G.; De Wolfe, T.J.; Hou, Y.; Braun, D.R.; Ellis, G.A. Chemical genomics, structure elucidation, and in vivo studies of the marine-derived anticlostridial ecteinamycin. ACS Chem. Biol. 2017, 12, 2287–2295. [Google Scholar] [CrossRef] [Green Version]
- Lotfy, W.A.; Mostafa, S.W.; Adel, A.A.; Ghanem, K.M. Production of di-(2-ethylhexyl) phthalate by Bacillus subtilis AD35: Isolation, purification, characterization and biological activities. Microb. Pathog. 2018, 124, 89–100. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Shin, H.J. Ieodoglucomide C and Ieodoglycolipid, New Glycolipids from a Marine-Derived Bacterium Bacillus licheniformis 09IDYM23. Lipids 2015, 50, 513–519. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Pournejati, R.; Karbalaei-Heidari, H.R. Pseudomonas aeruginosa Growth Inhibitor, PAGI264: A Natural Product from a Newly Isolated Marine Bacterium, Bacillus sp. Strain REB264. Iran. J. Sci. Technol. Trans. A: Sci. 2021, 45, 1165–1175. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Shin, H.J. Antibacterial and antiyeast compounds from marine-derived bacteria. Mar. Drugs 2014, 12, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, J.-S.; Lee, Y.-J.; Shin, H.J. Antimicrobial gageomacrolactins characterized from the fermentation of the marine-derived bacterium Bacillus subtilis under optimum growth conditions. J. Agric. Food Chem. 2013, 61, 3428–3434. [Google Scholar] [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. Moving away from traditional antibiotic treatment: Can macrocyclic lactones from marine macroalga-associated heterotroph be the alternatives? Appl. Microbiol. Biotechnol. 2020, 104, 7117–7130. [Google Scholar] [CrossRef]
- Chakraborty, K.; Thilakan, B.; Kizhakkekalam, V. Antibacterial aryl-crowned polyketide from Bacillus subtilis associated with seaweed Anthophycus longifolius. J. Appl. Microbiol. 2018, 124, 108–125. [Google Scholar] [CrossRef]
- Daboor, S.M.; Rohde, J.R.; Cheng, Z. Disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate lyase enhances pathogen eradication by antibiotics. J. Cyst. Fibros. 2021, 20, 264–270. [Google Scholar] [CrossRef]
- Daboor, S.M.; Raudonis, R.; Cohen, A.; Rohde, J.R.; Cheng, Z. Marine bacteria, a source for alginolytic enzyme to disrupt Pseudomonas aeruginosa biofilms. Mar. Drugs 2019, 17, 307. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, G.; Zhang, D.; Li, C.; Sun, C. Purification and biochemical characterization of an alkaline protease from marine bacteria Pseudoalteromonas sp. 129-1. J. Basic Microbiol. 2015, 55, 1427–1434. [Google Scholar] [CrossRef]
- Jouault, A.; Gobet, A.; Simon, M.; Portier, E.; Perennou, M.; Corre, E.; Gaillard, F.; Vallenet, D.; Michel, G.; Fleury, Y. Alterocin, an antibiofilm protein secreted by Pseudoalteromonas sp. strain 3J6. Appl. Environ. Microbiol. 2020, 86, e00893-20. [Google Scholar] [CrossRef]
- Sun, X.; Hill, P.; Liu, J.; Qian, J.; Ma, Y.; Zhou, S. Marine-Source Quorum Quenching Enzyme YtnP to Improve Hygiene Quality in Dental Units. Mar. Drugs 2021, 19, 225. [Google Scholar] [CrossRef]
- Reina, J.C.; Pérez-Victoria, I.; Martín, J.; Llamas, I. A quorum-sensing inhibitor strain of Vibrio alginolyticus blocks Qs-controlled phenotypes in Chromobacterium violaceum and Pseudomonas aeruginosa. Mar. Drugs 2019, 17, 494. [Google Scholar] [CrossRef] [Green Version]
- Kiran, G.S.; Sajayan, A.; Gopal, P.; Balakrishnan, A.; Prathiviraj, R.; Sabu, A.; Selvin, J. A novel anti-infective molecule nesfactin identified from sponge associated bacteria Nesterenkonia sp. MSA31 against multidrug resistant Pseudomonas aeruginosa. Microb. Pathog. 2021, 157, 104923. [Google Scholar] [CrossRef]
- Chang, A.; Sun, S.; Li, L.; Dai, X.; Li, H.; He, Q.; Zhu, H. Tyrosol from marine Fungi, a novel Quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. Bioorganic Chem. 2019, 91, 103140. [Google Scholar] [CrossRef]
- Sun, S.; Dai, X.; Sun, J.; Bu, X.; Weng, C.; Li, H.; Zhu, H. A diketopiperazine factor from Rheinheimera aquimaris QSI02 exhibits anti-quorum sensing activity. Sci. Rep. 2016, 6, 1–10. [Google Scholar]
- Parasuraman, P.; Devadatha, B.; Sarma, V.V.; Ranganathan, S.; Ampasala, D.R.; Reddy, D.; Kumavath, R.; Kim, I.-W.; Patel, S.K.; Kalia, V.C. Inhibition of microbial quorum sensing mediated virulence factors by Pestalotiopsis sydowiana. J. Microbiol. Biotechnol. 2020, 30, 571–582. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, L.; Wu, H.; Zhao, C.; Gong, Q.; Yu, W. Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa. Mar. Drugs 2020, 18, 205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, M.; Zhu, X.; Yu, W.; Gong, Q. Equisetin as potential quorum sensing inhibitor of Pseudomonas aeruginosa. Biotechnol. Lett. 2018, 40, 865–870. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Yan, Y.; Chen, S.; Xu, X.; Zhang, H.; Wang, H. Quorum sensing inhibitors from marine bacteria Oceanobacillus sp. XC22919. Nat. Prod. Res. 2019, 33, 1819–1823. [Google Scholar] [CrossRef]
- Danaraj, J.; Mariasingarayan, Y.; Ayyappan, S.; Karuppiah, V. Seagrass Halodule pinifolia active constituent 4-methoxybenzioic acid (4-MBA) inhibits quorum sensing mediated virulence production of Pseudomonas aeruginosa. Microb. Pathog. 2020, 147, 104392. [Google Scholar] [CrossRef]
- Yin, Q.; Liang, J.; Zhang, W.; Zhang, L.; Hu, Z.-L.; Zhang, Y.; Xu, Y. Butenolide, a marine-derived broad-spectrum antibiofilm agent against both Gram-positive and Gram-negative pathogenic bacteria. Mar. Biotechnol. 2019, 21, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.-P.; Song, Y.; Cai, Z.-H.; Lin, Z.-J.; Lin, G.-H.; Wang, Y.; Zhou, J. Anti-quorum sensing activities of selected coral symbiotic bacterial extracts from the South China Sea. Front. Cell. Infect. Microbiol. 2018, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Balan, S.S.; Mani, P.; Kumar, C.G.; Jayalakshmi, S. Structural characterization and biological evaluation of Staphylosan (dimannooleate), a new glycolipid surfactant produced by a marine Staphylococcus saprophyticus SBPS-15. Enzyme Microb. Technol. 2019, 120, 1–7. [Google Scholar] [CrossRef]
- Dusane, D.H.; Pawar, V.S.; Nancharaiah, Y.V.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 2011, 27, 645–654. [Google Scholar] [CrossRef]
- Kwan, J.C.; Meickle, T.; Ladwa, D.; Teplitski, M.; Paul, V.; Luesch, H. Lyngbyoic acid, a “tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol. Biosyst. 2011, 7, 1205–1216. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Gulbake, A.; Shilpi, S.; Hurkat, P.; Jain, S.K. Peptide and protein delivery using new drug delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 293–329. [Google Scholar] [CrossRef]
- Semreen, M.H.; El-Gamal, M.I.; Abdin, S.; Alkhazraji, H.; Kamal, L.; Hammad, S.; El-Awady, F.; Waleed, D.; Kourbaj, L. Recent updates of marine antimicrobial peptides. Saudi Pharm. J. 2018, 26, 396–409. [Google Scholar] [CrossRef]
- Ma, X.-W.; Hou, L.; Chen, B.; Fan, D.-Q.; Chen, Y.-C.; Yang, Y.; Wang, K.-J. A truncated Sph12-38 with potent antimicrobial activity showing resistance against bacterial challenge in Oryzias melastigma. Fish Shellfish. Immunol. 2017, 67, 561–570. [Google Scholar] [CrossRef]
- Chen, B.; Fan, D.-Q.; Zhu, K.-X.; Shan, Z.-G.; Chen, F.-Y.; Hou, L.; Cai, L.; Wang, K.-J. Mechanism study on a new antimicrobial peptide Sphistin derived from the N-terminus of crab histone H2A identified in haemolymphs of Scylla paramamosain. Fish Shellfish. Immunol. 2015, 47, 833–846. [Google Scholar] [CrossRef]
- Yu, R.; Wang, J.; So, L.-Y.; Harvey, P.J.; Shi, J.; Liang, J.; Dou, Q.; Li, X.; Yan, X.; Huang, Y.-H. Enhanced activity against multidrug-resistant bacteria through coapplication of an analogue of Tachyplesin I and an inhibitor of the QseC/B signaling pathway. J. Med. Chem. 2020, 63, 3475–3484. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Chen, J.-Y.; Cheng, Y.-S.E.; Chen, C.-Y.; Ni, I.-H.; Sheen, J.-F.; Pan, Y.-L.; Kuo, C.-M. Gene expression and localization of the epinecidin-1 antimicrobial peptide in the grouper (Epinephelus coioides), and its role in protecting fish against pathogenic infection. DNA Cell Biol. 2007, 26, 403–413. [Google Scholar] [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids; IntechOpen: London, UK, 2018. [Google Scholar]
- Yang, S.-T.; Liu, X.; Zhang, Y. Chapter 4–Metabolic Engineering—Applications, Methods, and Challenges. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 73–118. [Google Scholar]
- Verpoorte, R. ALKALOIDS. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 56–61. [Google Scholar]
- Bharate, S.B.; Manda, S.; Mupparapu, N.; Battini, N.; Vishwakarma, R.A. Chemistry and biology of fascaplysin, a potent marine-derived CDK-4 inhibitor. Mini Rev. Med. Chem. 2012, 12, 650–664. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Smirnova, P.A.; Tryapkin, O.A.; Kantemirov, A.V.; Khudyakova, Y.V.; Malyarenko, O.S.; Ermakova, S.P.; Grigorchuk, V.P.; Kaune, M.; von Amsberg, G. Total syntheses and preliminary biological evaluation of brominated fascaplysin and reticulatine alkaloids and their analogues. Mar. Drugs 2019, 17, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho, M.P.; Abraham, W.-R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Zhang, H.; He, L.; Liu, C.; Xu, Y.; Qian, P.-Y. Butenolide inhibits marine fouling by altering the primary metabolism of three target organisms. ACS Chem. Biol. 2012, 7, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Docquier, J.-D.; Mangani, S. An update on β-lactamase inhibitor discovery and development. Drug Resist. Updates 2018, 36, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Durães, F.; Palmeira, A.; Cruz, B.; Freitas-Silva, J.; Szemerédi, N.; Gales, L.; da Costa, P.M.; Remião, F.; Silva, R.; Pinto, M. Antimicrobial activity of a library of thioxanthones and their potential as efflux pump inhibitors. Pharmaceuticals 2021, 14, 572. [Google Scholar] [CrossRef]
- Dolgin, E. Sequencing of superbugs seen as key to combating their spread. Nat. Med. 2010, 16, 1054. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
| Producer Organism | Type of Organism | Compound | Class a | Activity | Assay b | P. aeruginosa Strain | Ref. |
|---|---|---|---|---|---|---|---|
| Mytilus spp. | Mussel | Myticalin A5 Myticalin A8 Myticalin C9 Myticalin D2 | AMP | <8 µM 8 µM 8 µM 4 µM | MDA | ATCC27853 | [102] |
| Mytilus coruscus | Mussel | Myticusin-beta | AMP | 9.2 mm | URDA | KCTC1636 | [103] |
| Crassostrea gigas | Pacific oyster | cgUbiquitin | AMP | 0.6 µM | URDA | KCTC2004 | [104] |
| Echinus esculentus | Sea urchin | EeCentrocin 1 EeCentrocin 2 EeStrongylocin 2 | AMP | 0.78 µM 0.78 µM 1.56 µM | MDA | ATCC27853 | [105] |
| Portunus pelagicus | Crab | Crustin | AMP | 50 µg/mL | Resazurin | HQ4006631 | [106] |
| Paralithodes camtschaticus | King crab | Paralithocin 1-3 | AMP | >100 µM | MDA | ATCC25853 | [107] |
| Scylla paramamosain | Mud crab | Sphistin | AMP | 24 µM | MDA | ATCC 9027 | [108] |
| Tachypleus tridentatus | Horseshoe crab | TPAD | AMP | 8 μg/mL 8–16 μg/mL | MDA | BAA 2108 ATCC 27853 | [109] |
| Katsuwonus pelamis | Tuna | SHbAP | AMP | 19 µg/mL | MDA | KCTC2004 | [110] |
| Epinephelus coioides | Teleost fish | Epinecidin-1 | AMP | 50 µg/mL 3.12 µg/mL | MDA | ATCC 19660 R | [111] |
| Oreochromis niloticus | Teleost fish | TP-4 | AMP | 0.52 µg/mL | MDA | ATCC19660 | [112] |
| Oreochromis niloticus | Teleost fish | Oreoch-1 Oreoch-2 | AMP | 35 µM 6.67 µM | MDA | NS* | [113] |
| Hydrophis cyanocinctus | Sea snake | Hc-CATH | AMP | 5.17 µM | MDA | ATCC27853 | [114] |
| Myxine glutinosa L. | Hagfish | Myxinidin | AMP | <10 µg/mL | MDA | Z61; K799 | [115] |
| Penicillium chrysogenum DXY-1 | Fungus | cyclo(L-Tyr-L-Pro) | AMP | 6.2 mg/mL | MDA | PAO1 | [116] |
| Aspergillus clavatus AS-107 | Fungus | Cyclodepsipeptide | AMP | 8.8 µM | MDA | NS* | [117] |
| Bacillus subtilis | Bacterium | Gageostatin A, B Gageostatin A+B | AMP | 16 µg/mL 8 µg/mL | MDA | NS* | [118] |
| Halobacillus litoralis YS3016 | Bacterium | Hololitaralin A Hololitaralin B | AMP | 24 mm 17 mm | DDA | NS* | [119,120] |
| Aplysia californica | Sea hare | Escapin | Protein | 0.31 µg/mL | MDA | PAO1 | [121] |
| Bacillus licheniformis D1 | Bacterium | BL-DZ1 | Protein | 3.12 µg/mL | MDA | PAO1 | [122] |
| Bacillus subtilis | Bacterium | Gageotetrins A Gageotetrins B Gageotetrins C | Lipopeptides | 0.06 µM 0.04 µM 0.02 µM | MDA | NS* | [123] |
| Penicillium chrysogenum QEN-24S | Fungus | Conidiogenone B | Diterpene | 8 µg/mL | MDA | NS* | [124] |
| Aspergillus insuetus SD-512 | Fungus | (5S,6S)-16,17-dihydroophiobolin H (6α)-21,21-O-dihydroophiobolin G Farnesylemefuranone D Farnesylemefuranone E Farnesylemefuranone F | Terpenoid | 8 µg/mL 8 µg/mL 16 µg/mL 16 µg/mL 8 µg/mL | MDA | a QDIO-2 | [125] |
| Dictyoata acutiloba | Seaweed | A1 C1 | Terpenoid | 0.9 µg/mL 0.89 µg/mL | MDA | MTCC741 | [126] |
| Sarcophyton trocheliophorum | Soft coral | (5S)-3-[(3E,5S)-5-hydroxy-3-hepten-6-yn-1-yl]-5-methyl-2(5H)-furanone | Terpenoid | 8 mm | DDA | NS* | [127] |
| Axinella infundibuliformis | Sponge | 3β-Hydroxylup-20(29)-ene 3β-Hydroxylup-20(29)-en28-oic acid 3-oxo-lup-20(29)-en-28-oic acid | Terpenoid | 24 mm 7 mm 10 mm | DDA | ATCC27853 | [128] |
| Streptomyces pratensis NA-ZhouS1 | Bacterium | Stremycin A-B | PK | 16 µg/mL | MDA | NS* | [129] |
| Penicillium sp. RO-11 | Fungus | Penipyranicins A Penipyranicins B Penipyranicins C Isopyrenulin | PK | 18.4 µg/mL 5.2 µg/mL 1.4 µg/mL 4.7 µg/mL | MDA | NR-117678.1 | [130] |
| Streptomyces sp. CMB-M0244 | Bacterium | Mollemycin A | PK | IC50 50 nM | MDA | ATCC 27853 | [131] |
| Penicillium sp. BB1122 | Fungus | Neocitreoviridin Penicillstressol Isopenicillstressol 10Z-isocitreoviridinol | PK | 4 µg/mL 4 µg/mL 4 µg/mL 8 µg/mL | MDA | CMCC(B)10104 | [132] |
| Streptomyces sp. HB202 | Bacterium | Mayamycin | PK | IC50 2.5 µg/mL | MDA | DSM 50071 | [133] |
| Penicillium sp. HDN151272 | Fungus | Ketidocillinone B Ketidocillinone C | PK | 1.56 mg/mL 6.25 mg/mL | MDA | NS* | [134] |
| Shewanella algae MTCC 12715 | Bacterium | 14-(14b,14c-dimethylbutyl)-12-methoxy-18-oxo-11,15-dioxacyclododecan-8-yl 1-((50-hydroxyfuran-10-yl)oxy)benzoate; | PK | 21 mm–1.5 µg/mL | DDA; MDA | ATCC27853 | [135] |
| 14-(sec-butyl)-12-methoxy-12-methyl-18-oxo-11,15-dioxacyclododecan-8-yl1-((50-hydroxyfuran-10-yl)oxy)benzoate | 24 mm–3 µg/mL | ||||||
| Bacillus Amyloliquefacien MTCC 12716 | Bacterium | Methyl 1′-((2E,4E,14E)-9,12-dihydroxy-15-isopropyl-1,6- dioxohexadecahydro [1]oxacyclononadecino[3,4-f]isobenzofuranyl) benzoate; | PK | 3.12 µg/mL | MDA | ATCC27853 | [136] |
| E)-Ethyl 15-ethyl-9,12-dihydroxy-25-(2-hydroxy-3-(methoxycarbonyl)phenyl)-1-oxo-octadecahydro-1 H -furopyrano[2,3- c]oxacyclononadecine-6-carboxylate; | 0.75 µg/mL | ||||||
| ((E)-Ethyl 15-ethyl-12-hydroxy-25-(2 -hydroxy-3-(methoxycarbonyl)phenyl)-24-methyl-1-oxo-icosahydro-1 H-furopyrano[2,3-c]oxacyclononadecine-6-carboxylate | 1.50 µg/mL | ||||||
| Bacillus amyloliquefacien MTCC 12713 | Bacterium | 4,27,39-Trihydroxy-7,8,10,11,16,17,25,26,27,28-decahydro-37-methyl-macrobrevin; | PK | 3.12 µg/mL–19 mm | MDA, DDA | ATCC27853 | [137] |
| 7,8,16,17,25,26-Hexahydro-macrobrevin; | 6.25 µg/mL–13 mm | ||||||
| 7,8,16,17,25,26-Hexahydro-41-hydroxy-macrobrevin-31-acetate; | 1.56 µg/mL–23 mm | ||||||
| 7,8,16,17,25,26-Hexahydro-28-nor-methyl-5-methoxy-macrobrevin | 3.12 µg/mL–22mm | ||||||
| Bacillus amyloliquefacien MTCC 12713 | Bacterium | 18,19-Dihydro-6-hydroxy-8-propyl carboxylate difficidin | PK | 0.006 µM–17 mm | MDA, DDA | ATCC27853 | [138] |
| 5-Ethoxy-28-methyl-(9-methyl-19-propyl dicarboxylate) difficidin | 0.004 µM–26 mm | ||||||
| (6-Methyl-9-propyl dicarboxylate)-19-propanone difficidin | 0.002 µM–23 mm | ||||||
| 20-Acetyl-(6-methyl-9-isopentyl dicarboxylate) difficidin | 0.002 µM-25 mm | ||||||
| Aspergillus niger ASSB4 | Fungus | RF-3192C | PK | 15 mm | DDA | ATCC27853 | [139] |
| Microsphaeropsis sp. RA10-14 | Fungus | Microketides A Microketides B | PK | 0.19 µg/mL 1.56 µg/mL | MDA | NS* | [140] |
| Actinoalloteichus cyanogriseus WH1-2216 | Bacterium | Caerulomycin A Caerulomycin C | Alkaloid | 21.8 µg/mL 38.6 µg/mL | ADA | NS* | [141] |
| Clathria cervicornis | Sponge | Crambescidin 800 | Alkaloid | 1 µg/mL | MDA | ATCC 10145 | [142] |
| Agelas dilatata | Sponge | Bromoageliferin | Alkaloid | 32 µg/mL 8 µg/mL | MDA | PAO1 ATCC 27853 | [143] |
| Pachychalina sp. | Sponge | Cyclostellettamine C | Alkaloid | 8.6 µg/mL 18.8 µg/mL | MDA | ATCC27853 Pa13, PaP1 | [144] |
| Cyclostellettamine E | 18.8 µg/mL 18.8 µg/mL 9.4 µg/mL | ATCC27853, PaP1 Pa13 | |||||
| Cyclostellettamine F | 4.7 µg/mL 9.4 µg/mL | PaP1 Pa13 | |||||
| Callyspongia siphonella | Sponge | 5-bromo trisindoline 6-bromo trisindoline | Alkaloid | 256 µg/mL 256 µg/mL | MDA | PAO1 | [145] |
| Dendrilla nigra | Sponge | Denigrins A Denigrins B Denigrins C | Alkaloid | 100 µg/mL 25 µg/Ml 12.5 µg/mL | MDA | ATCC 27853 | [146] |
| Tegella cf. spitzbergensis | Bryozoan | Ent-eusynstyelamide B Eusynstyelamide D Eusynstyelamide E Eusynstyelamide F | Alkaloid | 25 µg/mL 25 µg/mL 25 µg/mL 12.5 µg/mL | MDA | ATCC 27853 | [147] |
| Acanthophora spicifera | Sponge | Velutin | Flavone | 26.8 mm | DDA | ATCC9027 | [148] |
| Siphonochalina siphonella | Sponge | Siphonocholin | Steroid | 64 µg/mL | MDA | PAO1 | [149] |
| Dysidea granulosa | Sponge | 2-(20,40-dibromophenoxy)-3,5-dibromophenol | Diphenylether derivative | 4 µg/mL | MDA | NS* | [150] |
| Aspergillus versicolor OUCMDZ-2738 | Fungus | brevianamide K Diorcinol C Diorcinol E Diorcinol J Diorcinol Methyl diorcinol-4-carboxylate | Diphenylether derivative | IC50 92.2 µM IC50 46.2 µM IC50 101.9 µM IC50 50.9 µM IC50 17.4 µM IC50 13.9 µM | MDA | ATCC10145 | [151] |
| Ecteinascidia turbinate | Ascidian | Ecteinamycin | Polyether | 8.0 μg/mL | MDA | ATCC 27853 | [152] |
| Bacillus subtilis | Bacterium | DEHP | Phthalate derivative | 8 µg/mL | MDA | ATCC 9027 | [153] |
| Bacillus licheniformis 09IDYM23 | Bacterium | Ieodoglucomide C Ieodoglycolipid | Glycolipid | 0.01 µM 0.03 µM | MDA | NS* | [154] |
| Bacillus sp. REB264 | Bacterium | PAGI264 | NS | 15 µg/mL | MDA | NS* | [155] |
| Bacillus sp. | Bacterium | Macrolactins A1 Macrolactins B1 | Macrolide | 0.055 µM 0.055 µM | MDA | NS* | [156] |
| Bacillus subtilis | Bacterium | Gageomacrolactins 1 Gageomacrolactins 2 Gageomacrolactins 3 | Macrolide | 0.03 µM 0.05 µM 0.05 µM | MDA | NS* | [157] |
| B. amyloliquefaciens MTCC 12716 | Bacterium | Bacvalactones 1 Bacvalactones 2 Bacvalactones 3 | Macrolide | 3.12 µg/mL 3.00 µg/mL 1.5 µg/mL | MDA | ATCC 27853 | [158] |
| Bacillus subtilis MTCC 10403 | Bacterium | 7-O-6′-(2″-acetylphenyl)-5′-hydroxyhexanoate-macrolactin | Macrolide | 12.5 µg/mL | MDA | MTCC 429 | [159] |
| Producer Organism | Type of Organism | Compound | Class | Activity | Assay | P. aeruginosa Strain | Ref. |
|---|---|---|---|---|---|---|---|
| Pseudoalteromonas sp. 1400 | Bacterium | AlyP1400 | Protein | Antibiofilm | Biofilm disruption | CF27 PA14 | [160,161] |
| Pseudoalteromonas sp. 129-1 | Bacterium | Protease | Protein | Antibiofilm | Biofilm inhibition | PAO1 | [162] |
| Pseudoalteromonas sp. 3j6 | Bacterium | Alterocin | Protein | Antibiofilm | Biofilm inhibition | PAO1 | [163] |
| Bacillus velezensis DH82 | Bacterium | YtnP | Protein | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [164] |
| Psychrobacter sp. M9-54-1 | Bacterium | AHL-Acylase | Protein | Anti-QS | QS genes downregulation | PAO1 | [165] |
| Nesterenkonia sp. MSA31 | Bacterium | Nesfactin | Lipopeptide | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm disruption | PAO1 FSPA02 | [166] |
| Penicillium chrysogenum DXY-1 | Fungus | cyclo(L-Tyr-L-Pro) | Dipeptide | Annt-QS Antibiofilm | las and rhl reduced expression Biofilm inhibition | PAO1 | [116] |
| Penicillium chrysogenum DXY-1 | Fungus | Tyrosol | Dipeptide | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [167] |
| Rheinheimera aquimaris QSI02 | Bacterium | Cyclo(Trp-Ser) | Dipeptide | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [168] |
| Pestalotiopsis sydowiana PPR | Fungus | Cyclo(Leu-Pro) | Dipeptide | Anti-QS | Inhibition of virulence factors | PAO1 | [169] |
| Cladosporium sp. Z148 | Fungus | Cladodionen | PK | Anti-QS | Inhibition of virulence factors | PAO1 | [170] |
| Fusarium sp. Z10 | Fungus | Equisetin | PK | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [171] |
| Agelas dilatata | Sponge | Bromoageliferin | Alkaloid | Antibiofilm Anti-virulence | Biofilm inhibition G. mellonella survival assay | PAO1 ATCC 27853 | [143] |
| Callyspongia siphonella | Sponge | 5-bromo trisindoline 6-bromo trisindoline | Alkaloid | Antibiofilm | Biofilm inhibition | PAO1 | [145] |
| Oceanobacillus sp. XC22919 | Bacterium | 2-methyl-N-(2′-phenylethyl) butyramide; 3-methyl-N-(2′-phenylethyl)-butyramide | Alkaloid | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [172] |
| Halodule pinifolia | Seagrass | 4-methoxybeanzoic acid (4-MBA) | Benzoic acid derivative | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [173] |
| Pestalotiopsis sydowiana PPR | Fungus | 4-Hydroxyphenylacetamide | phenylacetic acid derivative | Anti-QS | Inhibition of virulence factors | PAO1 | [169] |
| Vibrio alginolyticus | Bacterium | Tyramine N-acetyltyramine | Amine | Anti-QS | Inhibition of virulence factors | PAO1 | [165] |
| Siphonochalina siphonella | Sponge | Syph-1 | Steroid | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [149] |
| Oceanobacillus sp. XC22919 | Bacterium | Benzyl benzoate | Benzoic acid derivative | Anti-QS Antibiofilm | Inhibition of virulence factors Biofilm inhibition | PAO1 | [172] |
| Streptomyces sp. | Bacterium | 5-octylfuran-2(5H)-one | Lactone butenolide | Antibiofilm | Biofilm degradation Biofilm disruption | PAO1 | [174] |
| Staphylococcus hominis | Bacterium | DL-homocysteine thiolacton | Lactone | Anti-QS Antibiofilm | QS genes downregulation Biofilm inhibition | PAO1 | [175] |
| Staphylococcus saprophyticus SBPS-15 | Bacterium | Staphylosan | Glycolipid | Antibiofilm | Biofilm degradation Biofilm disruption | BHKH | [176]. |
| Serratia marcescens | Bacterium | - | Glycolipid | Antibiofilm | Attachment inhibition Biofilm degradation Biofilm disruption | PAO1 | [177] |
| Lyngbya majuscola | Cyanobacterium | Lyngbyoic acid | Fatty Acid | Anti-QS | Inhibition of virulence factors | PAO1 | [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, D.; Buonocore, C.; Palisse, M.; Tedesco, P.; de Pascale, D. Exploring Oceans for Curative Compounds: Potential New Antimicrobial and Anti-Virulence Molecules against Pseudomonas aeruginosa. Mar. Drugs 2023, 21, 9. https://doi.org/10.3390/md21010009
Coppola D, Buonocore C, Palisse M, Tedesco P, de Pascale D. Exploring Oceans for Curative Compounds: Potential New Antimicrobial and Anti-Virulence Molecules against Pseudomonas aeruginosa. Marine Drugs. 2023; 21(1):9. https://doi.org/10.3390/md21010009
Chicago/Turabian StyleCoppola, Daniela, Carmine Buonocore, Morgan Palisse, Pietro Tedesco, and Donatella de Pascale. 2023. "Exploring Oceans for Curative Compounds: Potential New Antimicrobial and Anti-Virulence Molecules against Pseudomonas aeruginosa" Marine Drugs 21, no. 1: 9. https://doi.org/10.3390/md21010009







