A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors
Abstract
1. Introduction
2. Results
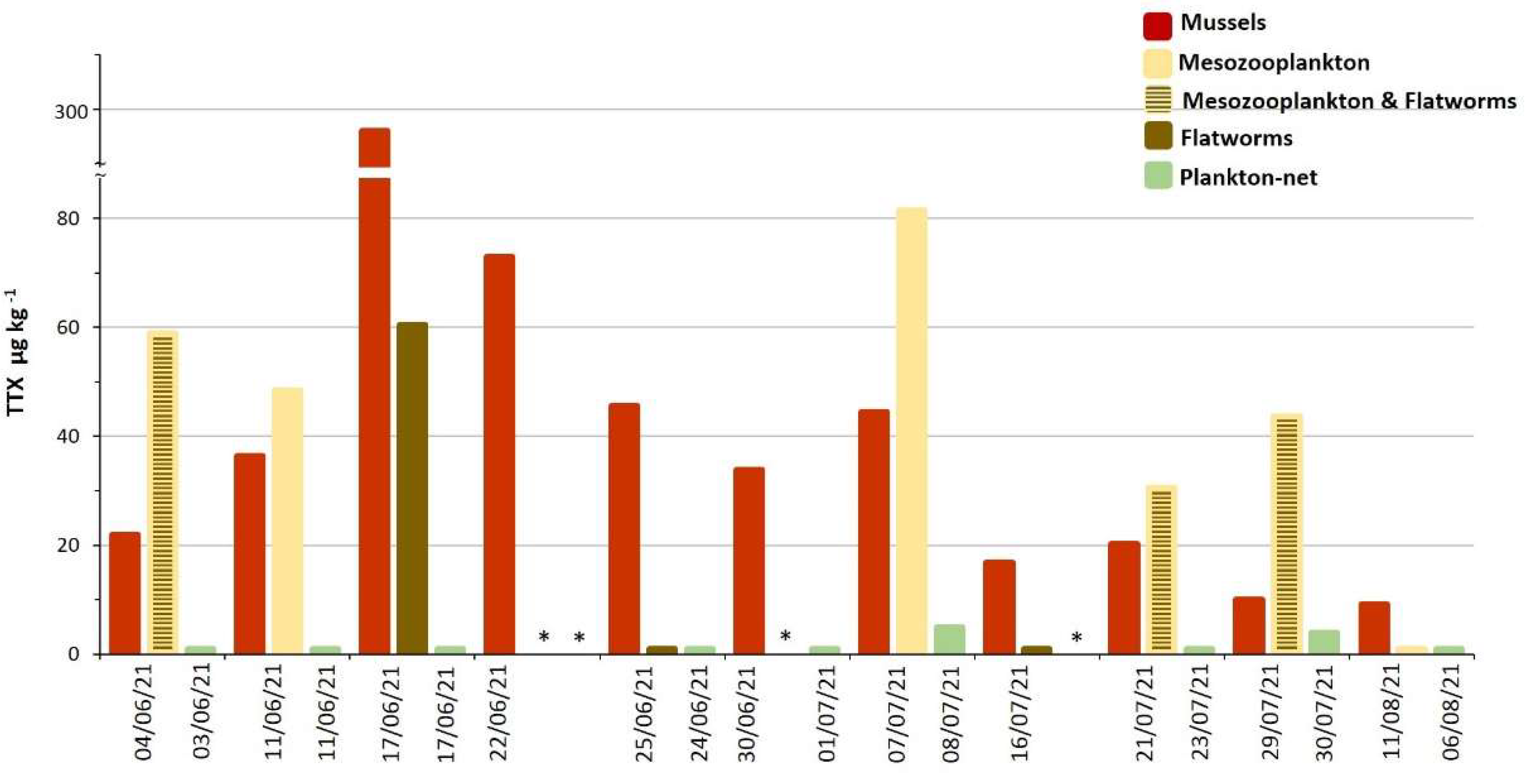
2.1. TTX in Mussels Natural Beds
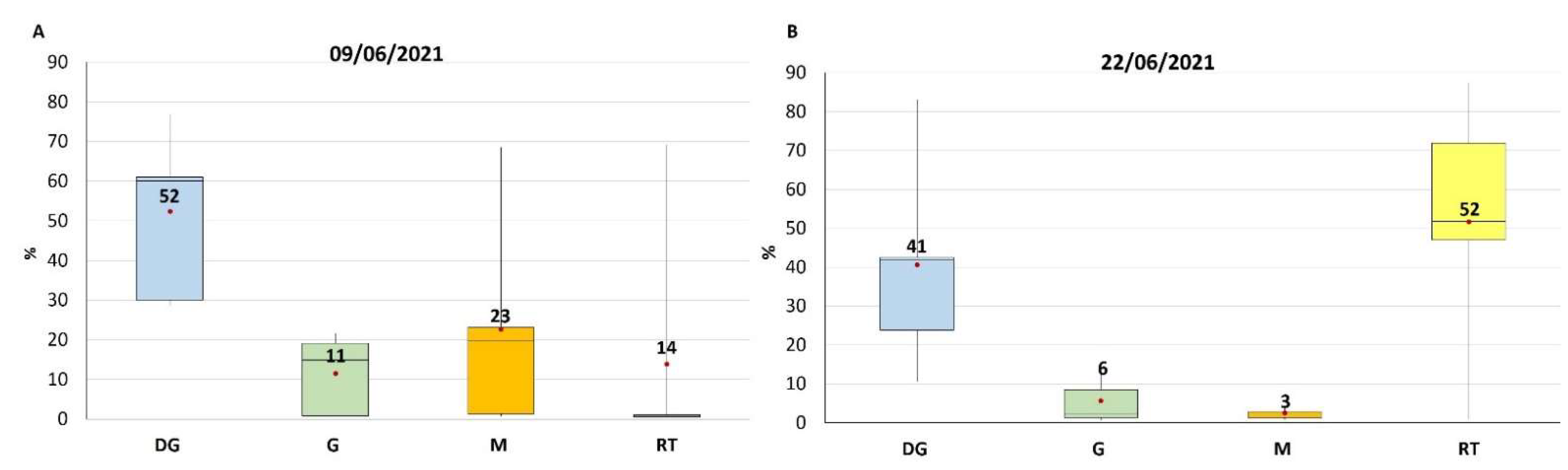
2.2. TTX Distribution in Mussel Tissues
2.3. TTX in Biota from Molo Portonovo
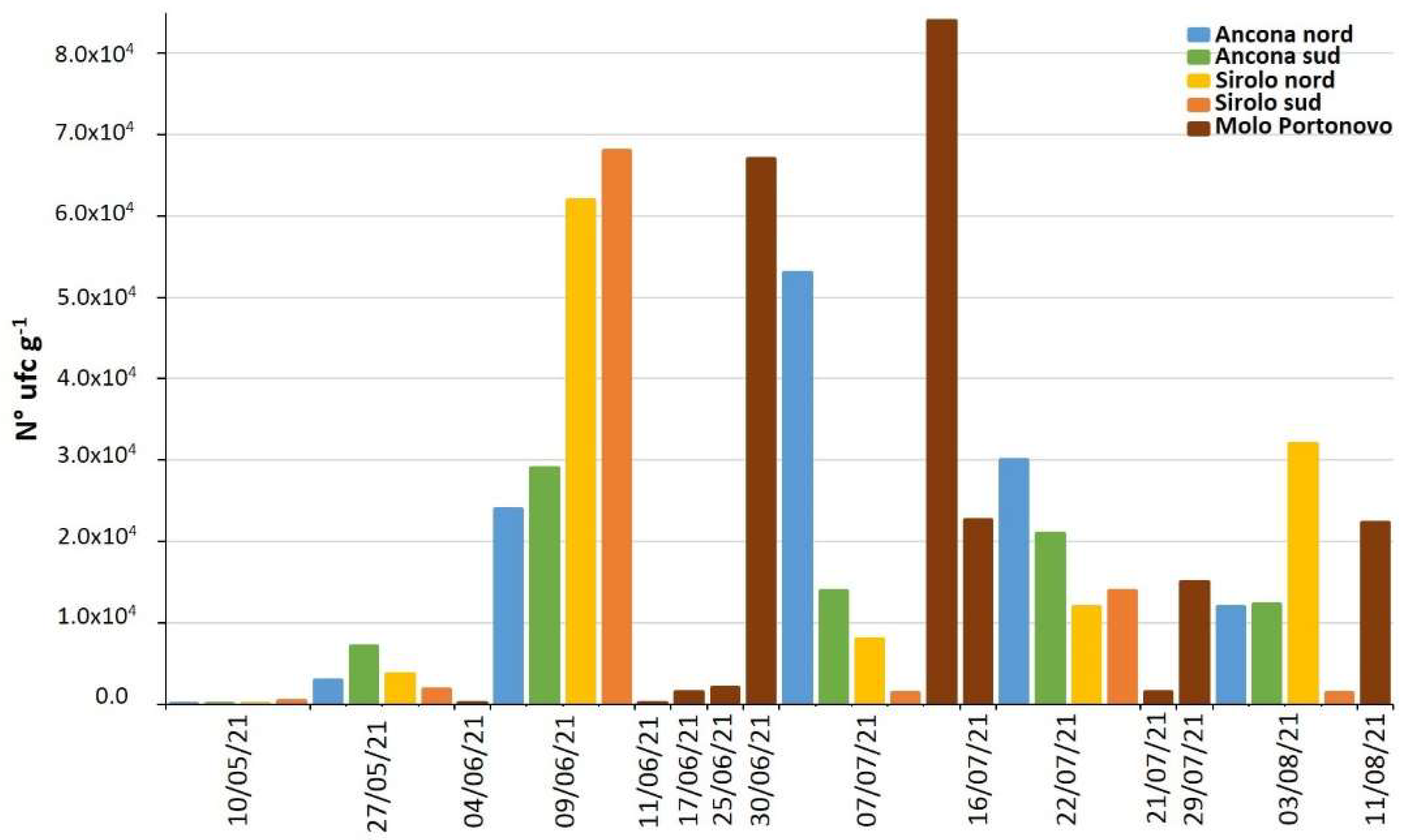
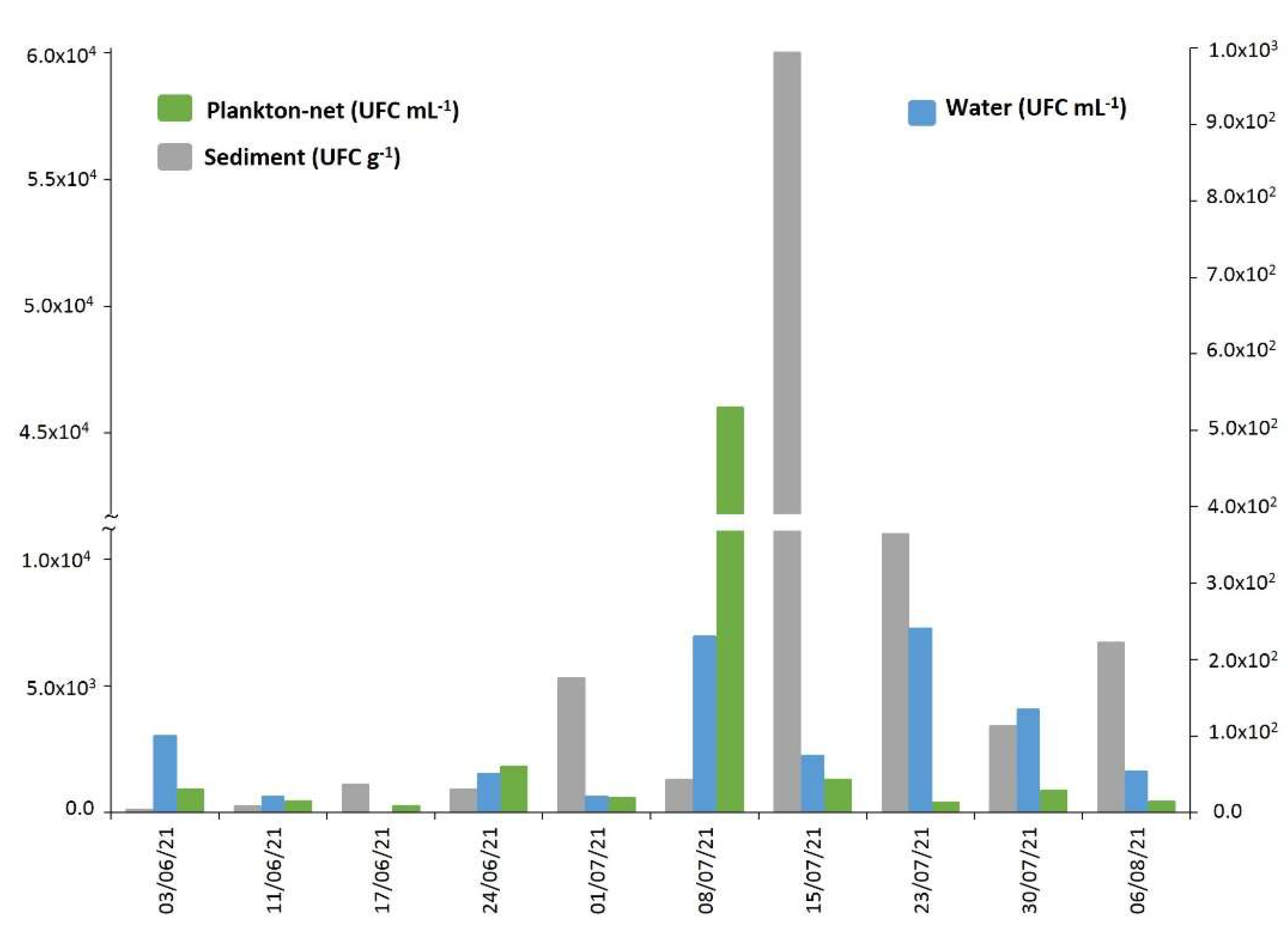
2.4. Vibrio alginolyticus in Biotic and Abiotic Samples
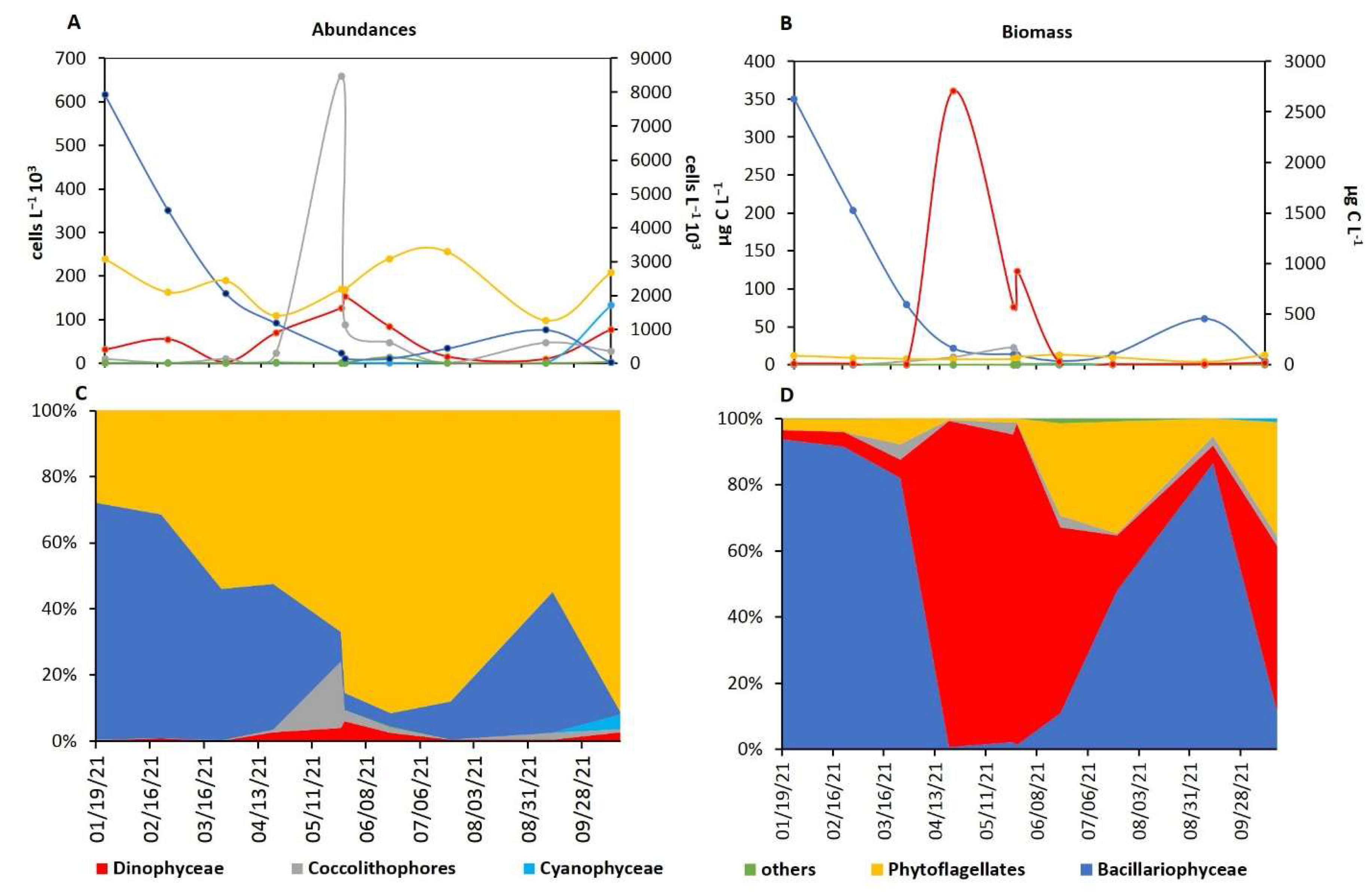
2.5. Phytoplankton Characterization
3. Discussion
4. Materials and Methods
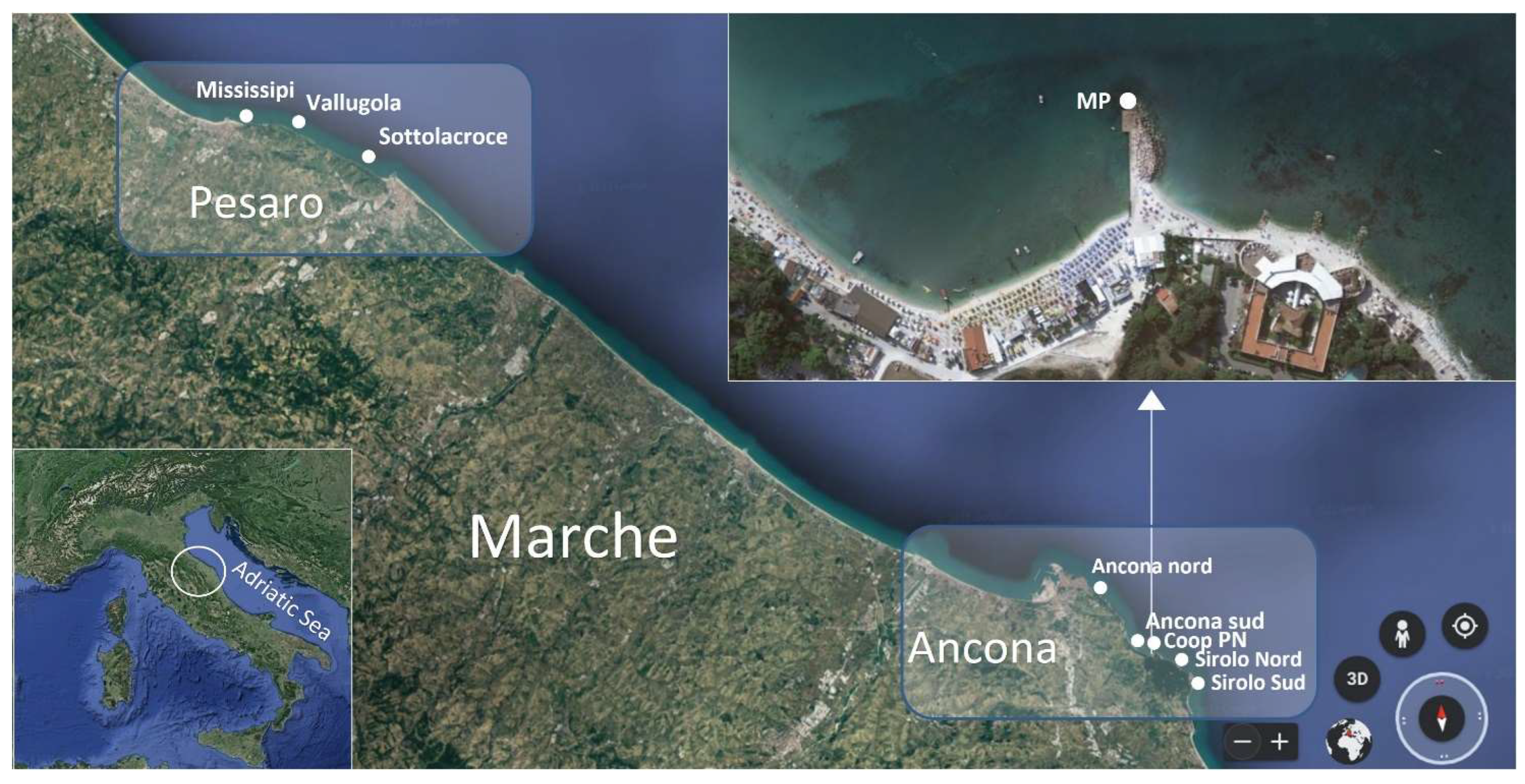
4.1. Sampling
4.2. Sample Treatment
4.3. Phytoplankton Identification and Counting
4.4. Chemical Analysis
4.4.1. Chemicals and Standards
4.4.2. TTXs Extraction
Mussels, Flatworms and Mesozooplankton
Net-Phytoplankton
4.4.3. HILIC-MS/MS Analysis
4.4.4. Quality Control
4.5. Microbiological Analysis
4.5.1. Vibrio Alginolyticus Isolation and Enumeration
4.5.2. Vibrio Alginolyticus Molecular Identification and Characterization
DNA Extraction—Operative Method
PCR Analysis
- gyrB species-specific gene
- NRPS and PKS biosynthesis genes
4.6. Nutrient Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, K.; Barnes, P.; Haughey, S.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C. Development and Single Laboratory Validation of an Optical Biosensor Assay for Tetrodotoxin Detection as a Tool to Combat Emerging Risks in European Seafood Rapid Detection in Food and Feed. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Noguchi, T.; Maruyama, J.; Ogata, T.; Hashimoto, K. Release of Tetrodotoxin and Paralytic Shellfish Poison from Puffer Liver by RNase. J. Biochem. 1983, 93, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX Accumulation in Pufferfish. Part D: Genomics and Proteomics. Comp. Biochem. Physiol. C Toxicol. Pharmacol 2006, 1, 145–152. [Google Scholar]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the Origins and Biosynthesis of Tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef]
- Denac, H.; Mevissen, M.; Scholtysik, G. Structure, Function and Pharmacology of Voltage-Gated Sodium Channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Scheib, H.; McLay, I.; Guex, N.; Clare, J.J.; Blaney, F.E.; Dale, T.J.; Tate, S.N.; Robertson, G.M. Modeling the Pore Structure of Voltage-Gated Sodium Channels in Closed, Open, and Fast-Inactivated Conformation Reveals Details of Site 1.Toxin and Local Anesthetic Binding. J. Mol. Model. 2006, 12, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins 2014, 6, 693. [Google Scholar] [CrossRef]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the Pufferfish Toxin. Tetrodotoxin in European Bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009. [Google Scholar] [CrossRef]
- Leão, J.M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, Ó.; Gago-Martínez, A. Preliminary Results on the Evaluation of the Occurrence of Tetrodotoxin Associated to Marine Vibrio Spp. in Bivalves from the Galician Rias (North-west of Spain). Mar. Drugs 2018, 16, 81. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First Detection of Tetrodotoxin in Greek Shellfish by UPLC-MS/MS Potentially Linked to the Presence of the Dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef]
- Gerssen, A.; Bovee, T.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R. First Report on the Occurrence of Tetrodotoxins in Bivalve Mollusks in The Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First Toxicity Report of Tetrodotoxin and 5, 6,11-TrideoxyTTX in the Trumpet Shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of Tetrodotoxin in Alexandrium tamarense, a Causative Dinoflagellate of Paralytic Shellfish Poisoning. Toxicon 1996, 34, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First Detection of Tetrodotoxin in the Bivalve Paphies australis by Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry With and Without Precolumn Reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, L.A.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; et al. Detection of Tetrodotoxin from the Grey Side-Gilled Sea Slug—Pleurobranchaea maculata, and Associated Dog Neurotoxicosis on Beaches Adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473. [Google Scholar] [CrossRef]
- Wood, S.A.; Casas, M.; Taylor, D.I.; McNabb, P.; Salvitti, L.; Ogilvie, S.; Cary, S.C. Depuration of Tetrodotoxin and Changes in Bacterial Communities in Pleurobranchea maculata Adults and Egg Masses Maintained in Captivity. J. Chem. Ecol. 2012, 38, 1342–1350. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New Gastropod Vectors and Tetrodotoxin Potential Expansion in Temperate Waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and Related Substances in a Ribbon Worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic Toxins in a Ribbon Worm Cephalothrix species (Nemertean) Adherent to Cultured Oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 2000, 38, 763–773. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First Detection of Tetrodotoxin and High Levels of Paralytic Shellfish Poisoning Toxins in Shellfish from Sicily (Italy) by Three Different Analytical Methods. Chemosphere 2019, 215, 881–892. [Google Scholar] [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First Occurrence of Tetrodotoxins in Bivalve Mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Leoni, F.; Tavoloni, T.; Accoroni, S.; Gorbi, S.; Giuliani, M.E.; Stramenga, A.; et al. Tetrodotoxins (Ttxs) and Vibrio alginolyticus in Mussels from Central Adriatic Sea (Italy): Are They Closely Related? Marine Drugs 2021, 19, 304. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. A Risks for Public Health Related to the Presence of Tetrodotoxin (TTX) and TTX Analogues in Marine Bivalves and Gastropods. EFSA J. 2017, 15, 47–52. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-Producing Bacteria: Detection, Distribution and Migration of the Toxin in Aquatic Systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Jeong, D.-Y.; Kim, W.-S.; Kim, H.-D.; Kim, C.-H.; Park, W.-W.; Park, Y.-H.; Kim, K.-S.; Kim, H.-M.; Kim, D.-S. A Tetrodotoxin-Producing Vibrio Strain, LM-1, from the Puffer Fish Fugu Vermicularis radiatus. Appl. Environ. Microbiol. 2000, 66, 1698–1701. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Y.; Xie, L.; Xia, G.; Hu, J.; Wang, S.; Zhang, R. Toxicity and Distribution of Tetrodotoxin-Producing Bacteria in Puffer Fish Fugu rubripes Collected from the Bohai Sea of China. Toxicon 2005, 46, 471–476. [Google Scholar] [CrossRef]
- Do, H.K.; Kogure, K.; Simidu, U. Identification of deep-sea-sediment bacteria which produce tetrodotoxin. Appl. Environ. Microbiol. 1993, 56, 1162–1163. [Google Scholar] [CrossRef]
- Rodríguez, I.; Alfonso, A.; Alonso, E.; Rubiolo, J.A.; Roel, M.; Vlamis, A.; Katikou, P.; Jackson, S.A.; Menon, M.L.; Dobson, A.; et al. The association of bacterial C 9 -based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Kotaki, Y.; Shimizu, Y. 1-Hydroxy-5,11-Dideoxytetrodotoxin, the First N-Hydroxy and Ring-Deoxy derivative of Tetrodotoxin Found in the Newt Taricha granulosa. J. Am. Chem. Soc. 1993, 115, 827–830. [Google Scholar] [CrossRef]
- Wang, X.J.; Yu, R.C.; Luo, X.; Zhou, M.J.; Lin, X.T. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61. [Google Scholar] [CrossRef]
- Biessy, L.; Pearman, J.K.; Smith, K.F.; Hawes, I.; Wood, S.A. Seasonal and spatial variations in bacterial communities from tetrodotoxin-bearing and non-tetrodotoxin-bearing clams. Front. Microb. 2020. [Google Scholar] [CrossRef]
- Turner, A.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; Oneill, A.; Faulkner, D.; Mceneny, H.; Baker-Austin, C.; Lees, D.; et al. Detection of Tetrodotoxin Shellfish Poisoning (TSP) Toxins and Causative Factors in Bivalve Molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef]
- Totti, C.; Romagnoli, T.; Accoroni, S.; Coluccelli, A.; Pellegrini, M.; Campanelli, A.; Grilli, F.; Marini, M. Phytoplankton communities in the North Western Adriatic Sea: Interdecadal variability over a 30-years period (1988–2016) and relationships with meteoclimatic drivers. J. Mar. Syst. 2019, 193, 137–153. [Google Scholar] [CrossRef]
- Pratheepa, V.; Alex, A.; Silva, M.; Vasconcelos, V. Bacterial Diversity and Tetrodotoxin Analysis in the Viscera of the Gastropods from Portuguese Coast. Toxicon 2016, 119, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Savar, V.; Schaefer, E.; Chevé, J.; Halm-Lemeille, M.-P.; Hervio-Heath, D.; Travers, M.-A.; Abadie, E.; Rolland, J.-L.; Hess, P. Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections. Toxins 2021, 13, 740. [Google Scholar] [CrossRef]
- Itoi, S.; Ueda, H.; Yamada, R.; Takei, M.; Sato, T.; Oshikiri, S.; Wajima, Y.; Ogata, R.; Oyama, H.; Shitto, T.; et al. Including Planocerid Flatworms in the Diet Effectively Toxifies the Pufferfish, Takifugu Niphobles. Sci. Rep. 2018, 8, 12302. [Google Scholar] [CrossRef]
- Itoi, S.; Sato, T.; Takei, M.; Yamada, R.; Ogata, R.; Oyama, H.; Teranishi, S.; Kishiki, A.; Wada, T.; Noguchi, K.; et al. The Planocerid Flatworm Is a Main Supplier of Toxin to Tetrodotoxin-Bearing Fish Juveniles. Chemosphere 2020, 249, 126217. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Oyama, H.; Kashitani, M.; Ishimaru, Y.; Suo, R.; Sugita, H.; Itoi, S. Toxic Flatworm Egg Plates Serve as a Possible Source of Tetrodotoxin for Pufferfish. Toxins 2019, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Saito, R.; Yamamoto, K.; Watanabe, R.; Kaneko, Y.; Yanaoka, M.; Furukoshi, S.; Yasukawa, S.; Ito, M.; Oyama, H.; et al. The Role of Toxic Planocerid Flatworm Larvae on Tetrodotoxin Accumulation in Marine Bivalves. Aquat Toxicol 2021, 237, 105908. [Google Scholar] [CrossRef] [PubMed]
- Kashitani, M.; Okabe, T.; Oyama, H.; Noguchi, K.; Yamazaki, H.; Suo, R.; Mori, T.; Sugita, H.; Itoi, S. Taxonomic Distribution of Tetrodotoxin in Acotylean Flatworms (Polycladida: Platyhelminthes). Mar. Biotechnol. (NY) 2020, 22, 805–811. [Google Scholar] [CrossRef]
- Galleni, L.; Tongiorgi, P.; Ferrero, E.; Salghetti, U. Stylochus Mediterraneus (Turbellaria: Polycladida), Predator on the Mussel Mytilus Galloprovincialis. Mar. Biol. 1980, 55, 317–326. [Google Scholar] [CrossRef]
- Cozzi & Giani. River Water and Nutrient Discharges in the Northern Adriatic Sea: Current Importance and Long Term Changes. 2011. Available online: https://www.lescienze.it/pubblicazioni/2011/09/03/.4436300/ (accessed on 13 November 2022).
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent Changes in the Marine Ecosystems of the Northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of Vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Falzano, L.; Fiorentini, C.; Pianetti, A.; Baffone, W.; Fabbri, A.; Matarrese, P.; Casiere, A.; Katouli, M.; Kühn, I.; et al. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. And non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl. Environ.Microbiol. 1999, 65, 2748–2753. [Google Scholar] [CrossRef] [PubMed]
- Throndsen, J. Phytoplankton Manual—Monographs on Oceanographic Methodology, 6A. Sournia (Editor) UNESCO, Paris, 1978, 337. Book Rev. 1981, 6, 247–248. [Google Scholar] [CrossRef]
- Edler, L.; Elbrächter, M. The Utermöhl Method for Quantitative Phytoplankton Analysis. 2010, 13. Available online: https://www.researchgate.net/publication/284040936 (accessed on 13 November 2022).
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.; Menden-Deuer, S.; Lessard, E.J. Carbon to Volume Relationships for Dinoflagellates, Diatoms, and Other Protest Plankton. Limnol Oceanogr 45: 569-579. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- EURLMB SOP-Determination of Tetrodotoxin by HILIC-MS/MS-, June 2017. Available online: https://www.aesan.gob.es/en/CRLMB/web/public_documents/seccion/crlmb_standard_operating_procedures.htmI (accessed on 13 March 2021).
- Chen, M.-X.; Li, H.-Y.; Li, G.; Zheng, T.-L. Distribution of Vibrio Alginolyticus-like Species in Shenzhen Coastal Waters, China. Braz. J. Microbiol. 2011, 42, 884–896. [Google Scholar] [CrossRef]
- Froelich, B.A.; Ayrapetyan, M.; Fowler, P.; Oliver, J.D.; Noble, R.T. Development of a Matrix Tool for the Prediction of Vibrio Species in Oysters Harvested from North Carolina. Appl Environ Microbiol 2015, 81, 1111–1119. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.; Palmer, J.D. Investigating Deep Phylogenetic Relationships among Cyanobacteria and Plastids by Small Subunit RRNA Sequence Analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Luo, P.; Hu, C. Vibrio alginolyticus gyrB Sequence Analysis and gyrB-Targeted PCR Identification in Environmental Isolates. Dis. Aquat. Organ. 2008, 82, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tambadou, F.; Lanneluc, I.; Sablé, S.; Klein, G.L.; Doghri, I.; Sopéna, V.; Didelot, S.; Barthélémy, C.; Thiéry, V.; Chevrot, R. Novel Nonribosomal Peptide Synthetase (NRPS) Genes Sequenced from Intertidal Mudflat Bacteria. FEMS Microbiol. Lett. 2014, 357, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, M.C.; Neilan, B.A. On the Presence of Peptide Synthetase and Polyketide Synthase Genes in the Cyanobacterial Genus Nodularia. FEMS Microbiol. Lett. 2001, 196, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Wiley Online Library: Ottawa, ON, Canada, 1972; p. 310. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Accoroni, S.; Romagnoli, T.; Campanelli, A.; Griffoni, F.; Tavoloni, T.; Gorbi, S.; et al. A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors. Mar. Drugs 2023, 21, 8. https://doi.org/10.3390/md21010008
Bacchiocchi S, Campacci D, Siracusa M, Dubbini A, Accoroni S, Romagnoli T, Campanelli A, Griffoni F, Tavoloni T, Gorbi S, et al. A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors. Marine Drugs. 2023; 21(1):8. https://doi.org/10.3390/md21010008
Chicago/Turabian StyleBacchiocchi, Simone, Debora Campacci, Melania Siracusa, Alessandra Dubbini, Stefano Accoroni, Tiziana Romagnoli, Alessandra Campanelli, Francesco Griffoni, Tamara Tavoloni, Stefania Gorbi, and et al. 2023. "A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors" Marine Drugs 21, no. 1: 8. https://doi.org/10.3390/md21010008
APA StyleBacchiocchi, S., Campacci, D., Siracusa, M., Dubbini, A., Accoroni, S., Romagnoli, T., Campanelli, A., Griffoni, F., Tavoloni, T., Gorbi, S., Totti, C., & Piersanti, A. (2023). A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors. Marine Drugs, 21(1), 8. https://doi.org/10.3390/md21010008







