Antidiabetic Effect of Collagen Peptides from Harpadon nehereus Bones in Streptozotocin-Induced Diabetes Mice by Regulating Oxidative Stress and Glucose Metabolism
Abstract
:1. Introduction
2. Results
2.1. Preparation of HNCP and Its Antioxidative Activity
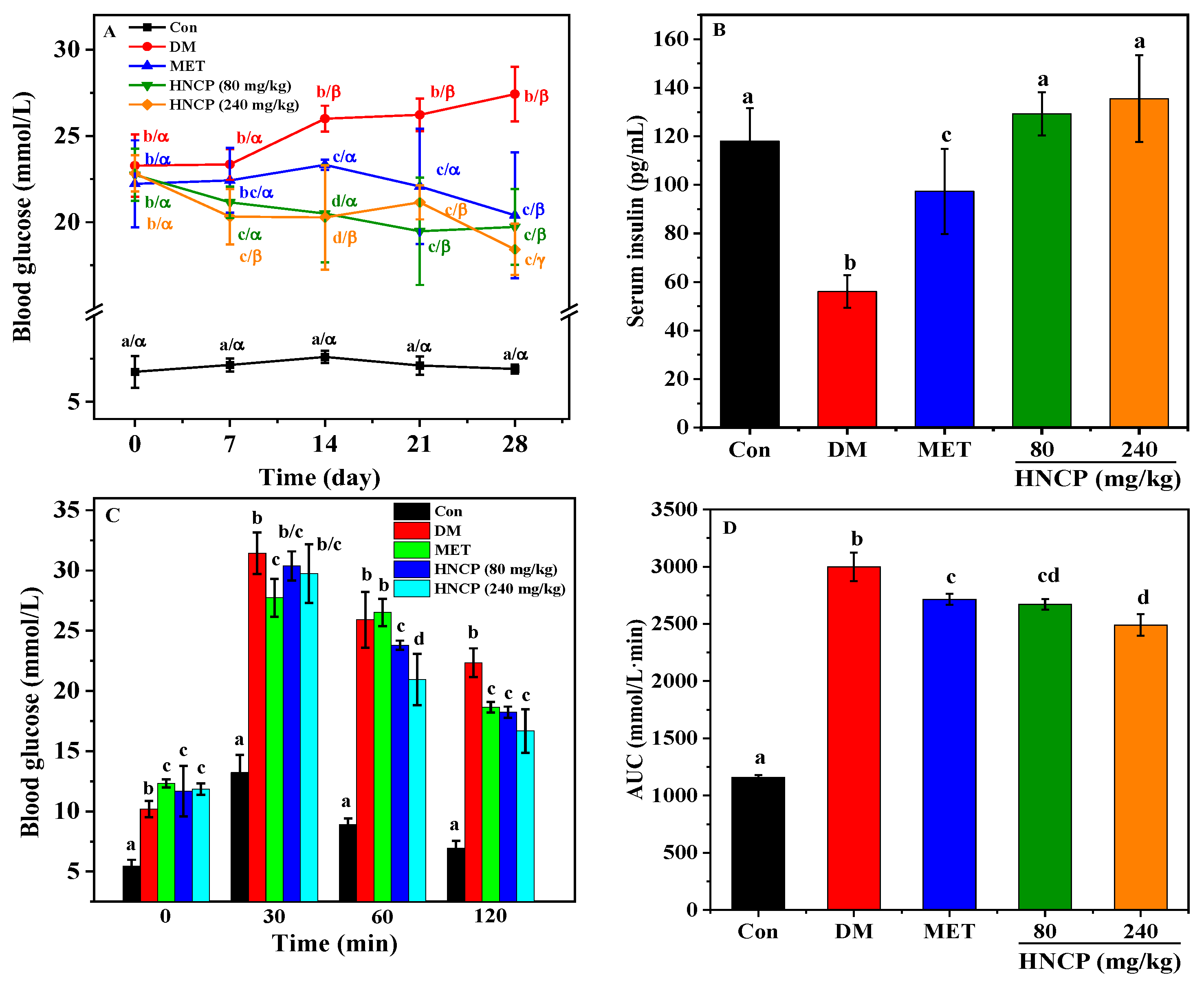
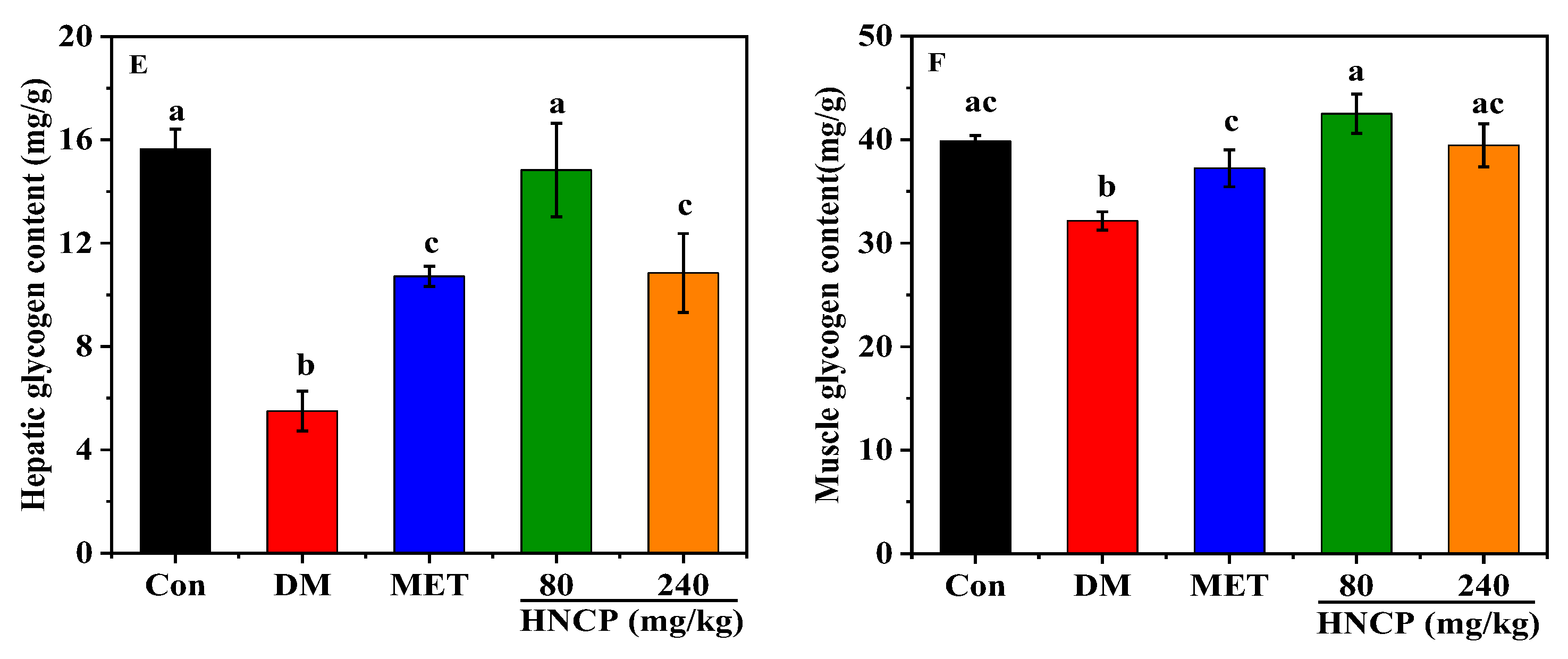
2.2. Effects of HNCP on Glucose Metabolism in Diabetic Mice
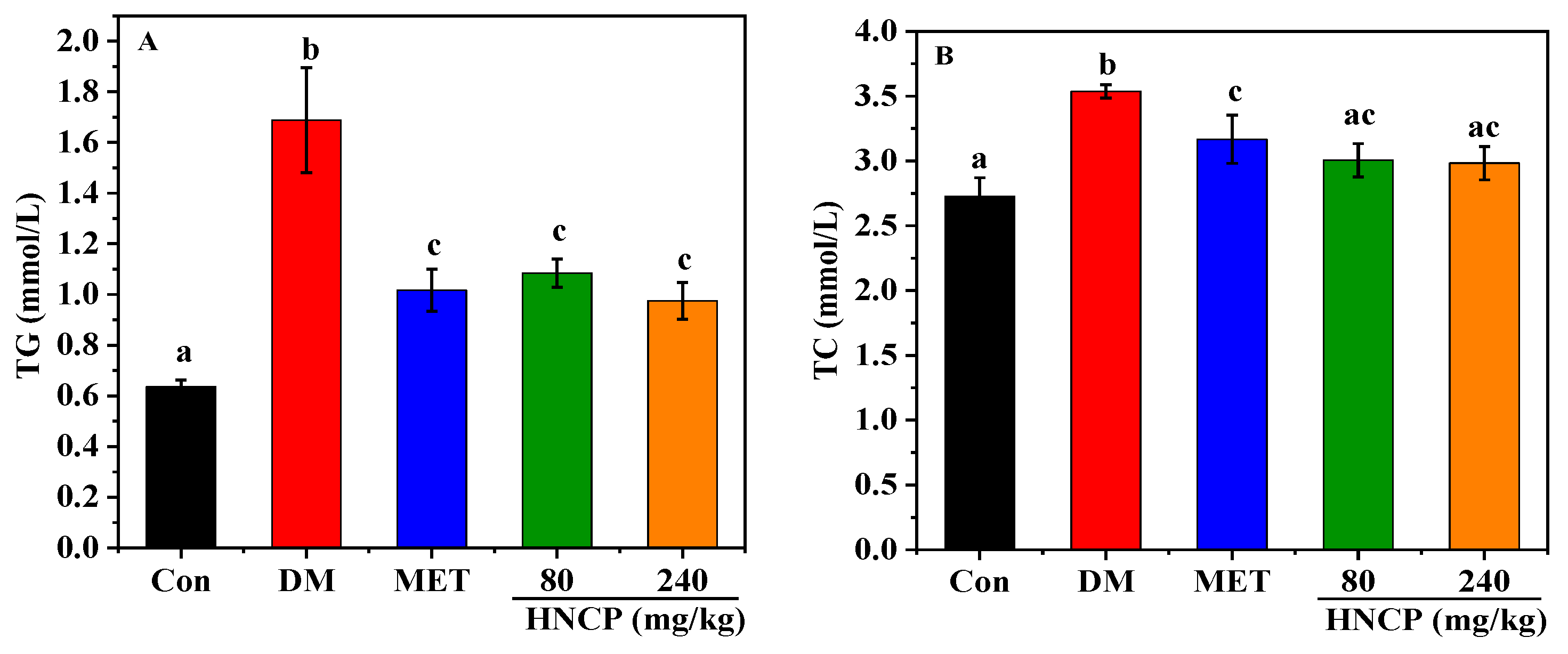
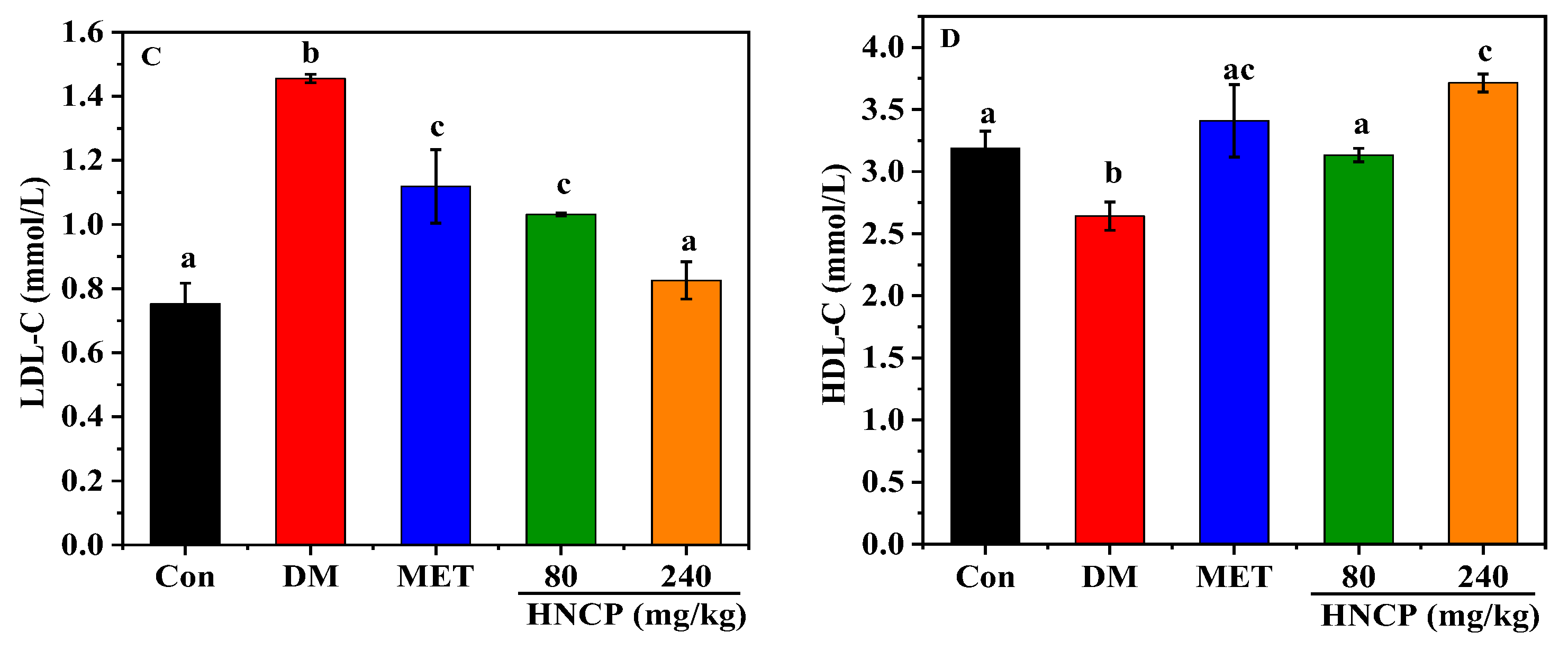
2.3. Effects of HNCP on Blood Lipids in Diabetic Mice
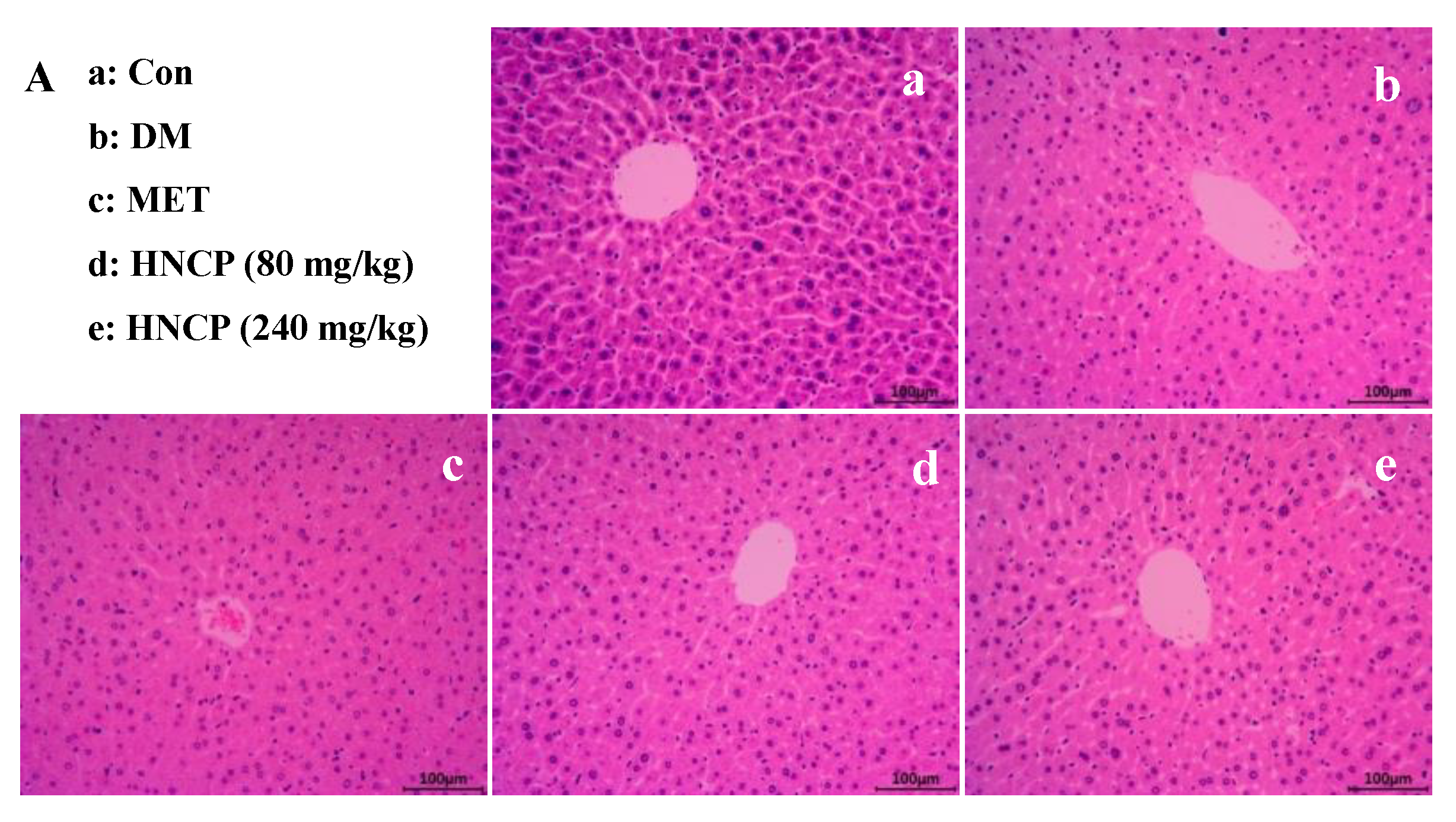
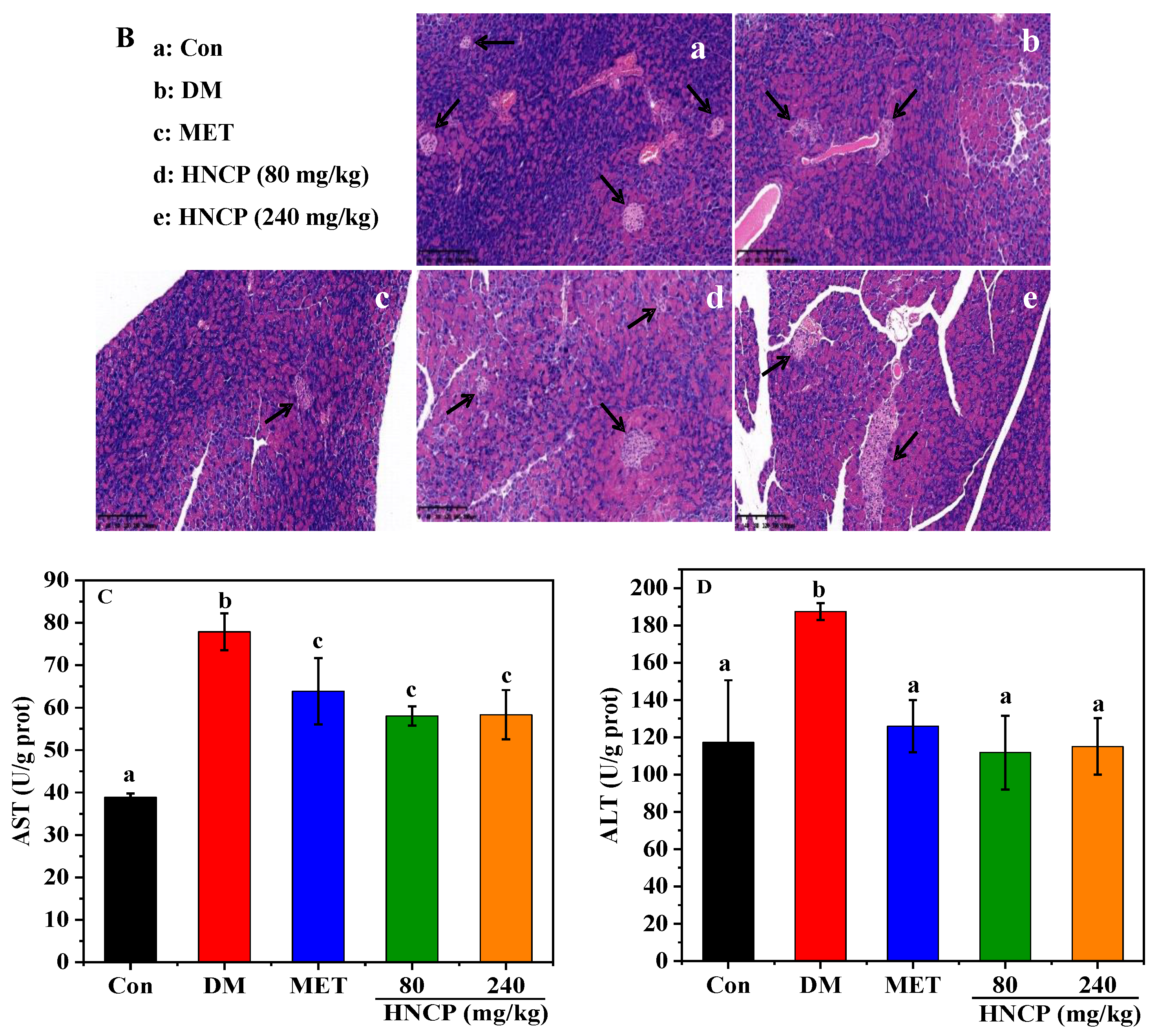
2.4. Improvement of HNCP on Liver and Pancreas Injury in Diabetes Mice
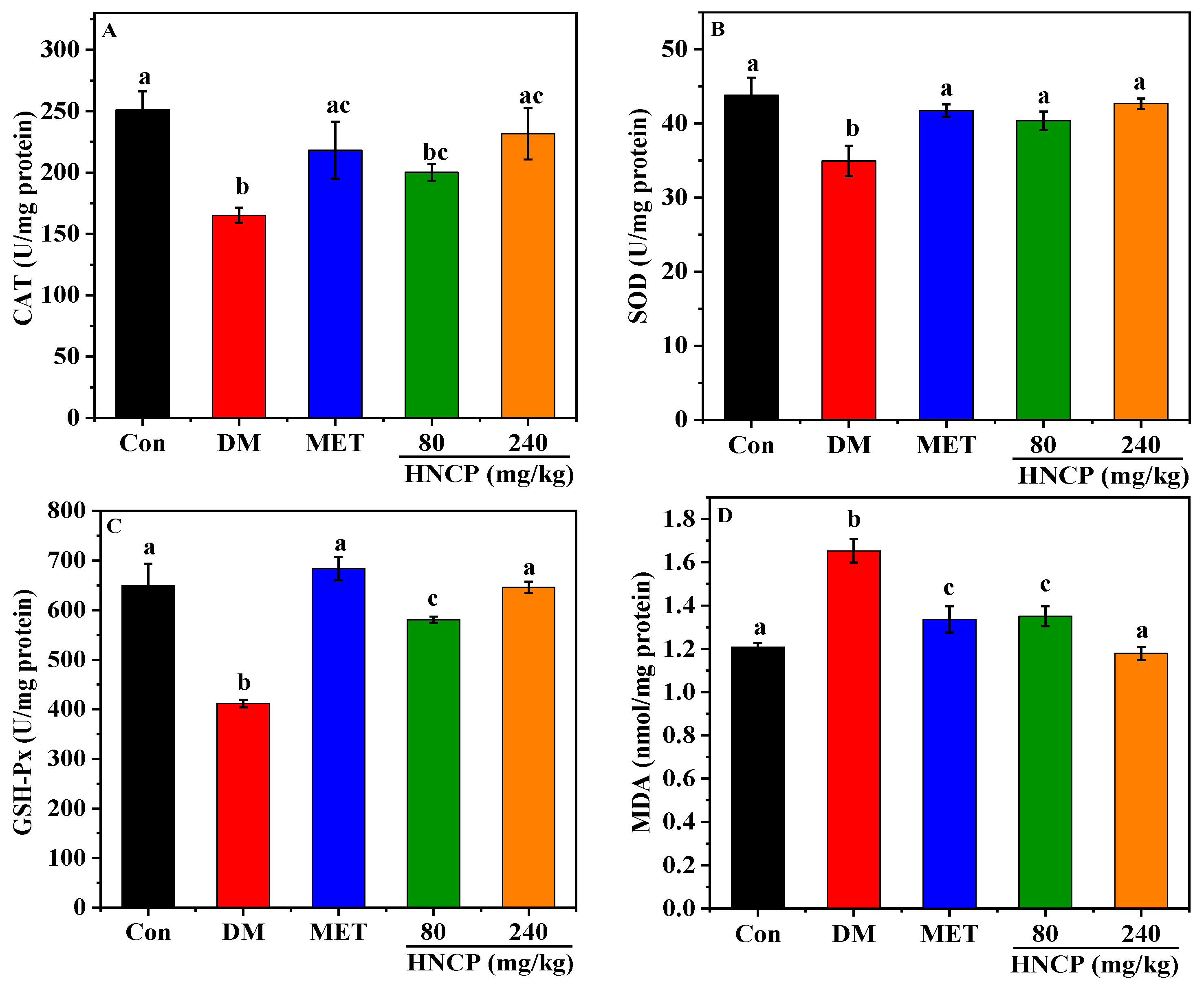
2.5. Effects of HNCP on Hepatic Oxidative Damage in Diabetic Mice
2.6. Effects of HNCP on Nrf2 Signaling Pathway
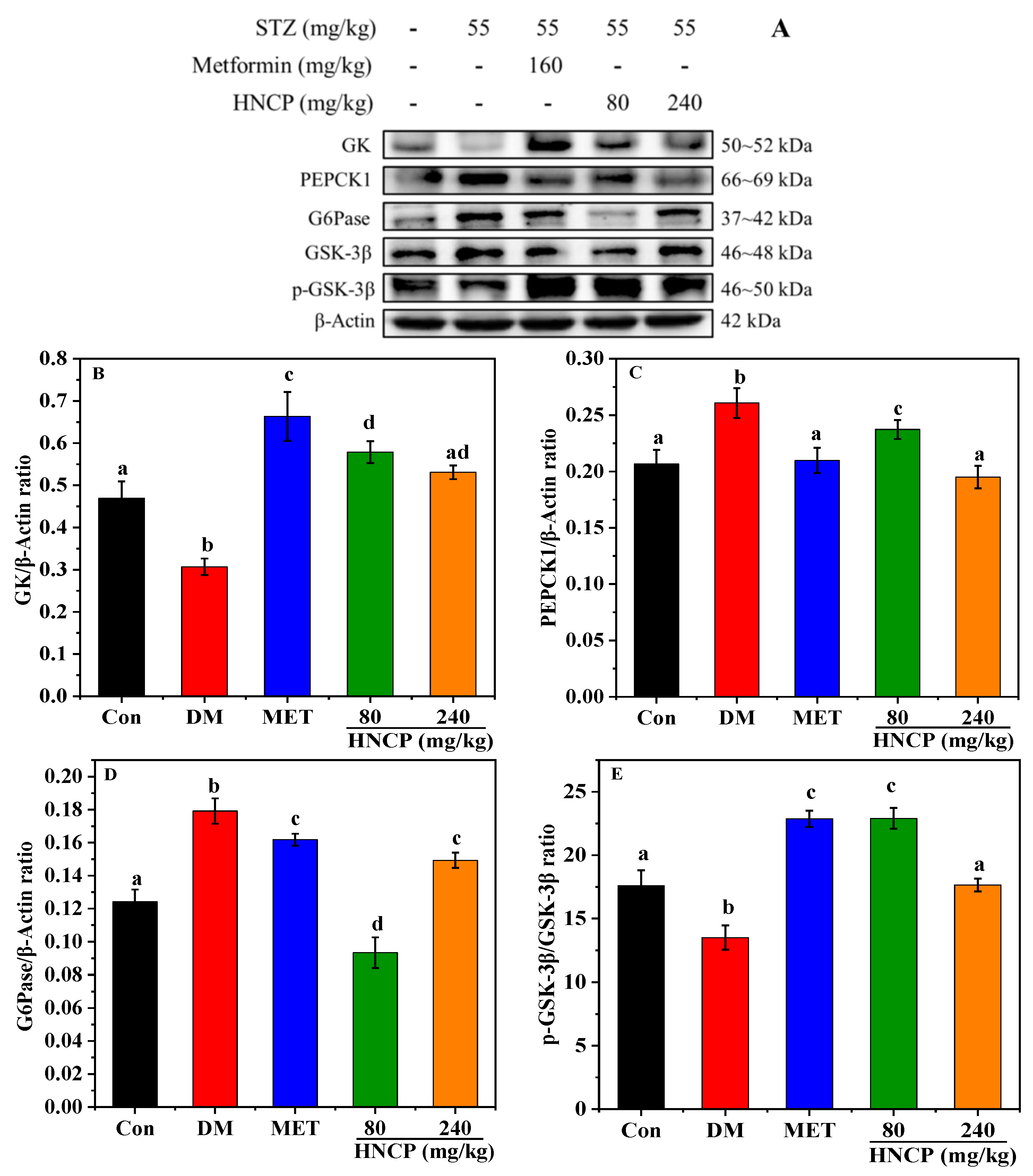
2.7. Effects of HNCP on the Expression of Glucometabolic-Related Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Collagen Peptides from Harpadon nehereus Bone
4.3. Laboratory Animals
4.4. Measurement of Blood Glucose, Insulin, and Glycogen
4.5. Oral Glucose Tolerance Test (OGTT)
4.6. Determination of Lipid-Related Indexes in Mice
4.7. Histological Evaluation of Liver and Pancreas
4.8. Detection of ALT, AST, MDA, CAT, SOD, and GSH-Px Levels in Liver
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, G.; Mo, F.; Ling, C.; Peng, H.; Gu, W.; Li, M.; Chen, Z. Portulaca oleracea L. alleviates liver injury in streptozotocin-induced diabetic mice. Drug Des. Devel Ther. 2018, 12, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M.; Alsalmi, F.A. Influence of olive leaves extract on hepatorenal injury in streptozotocin diabetic rats. Saudi J. Biol. Sci. 2019, 26, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.; Sypniewska, G. Diabetes as a complication of adipose tissue dysfunction. Is there a role for potential new biomarkers? Clin. Chem. Lab. Med. 2013, 51, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, H.; Chen, L.; Duan, Y.; Chen, Q.; Xi, S. Effects of a Novel Glucokinase Activator, HMS5552, on Glucose Metabolism in a Rat Model of Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 5812607. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.R.; Ye, Y.L.; Feng, Y.; Xu, T.F.; Shen, Y.; Liu, J.; Huang, S.L.; Shen, J.H.; Leng, Y. SL010110, a lead compound, inhibits gluconeogenesis via SIRT2-p300-mediated PEPCK1 degradation and improves glucose homeostasis in diabetic mice. Acta Pharmacol. Sin. 2021, 42, 1834–1846. [Google Scholar] [CrossRef]
- Gurumayum, S.; Bharadwaj, S.; Sheikh, Y.; Barge, S.R.; Saikia, K.; Swargiary, D.; Ahmed, S.A.; Thakur, D.; Borah, J.C. Taxifolin-3-O-glucoside from Osbeckia nepalensis mediates antihyperglycemic activity in CC1 hepatocytes and in diabetic Wistar rats via regulating AMPK/G6Pase/PEPCK signaling axis. J. Ethnopharmacol. 2023, 303, 115936. [Google Scholar] [CrossRef]
- Desai, S.M.; Sanap, A.P.; Bhonde, R.R. Treat liver to beat diabetes. Med. Hypotheses 2020, 144, 110034. [Google Scholar] [CrossRef]
- Loguercio, C.; Federico, A. Oxidative stress in viral and alcoholic hepatitis. Free Radic. Biol. Med. 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Bedi, O.; Aggarwal, S.; Trehanpati, N.; Ramakrishna, G.; Krishan, P. Molecular and Pathological Events Involved in the Pathogenesis of Diabetes-Associated Nonalcoholic Fatty Liver Disease. J. Clin. Exp. Hepatol. 2019, 9, 607–618. [Google Scholar] [CrossRef]
- Leo, E.E.M.; Fernandez, J.J.A.; Campos, M.R.S. Biopeptides with antioxidant and anti-inflammatory potential in the prevention and treatment of diabesity disease. Biomed. Pharmacother. 2016, 83, 816–826. [Google Scholar]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Gargiulo, S.; Russo, I.; Barale, C.; Poli, G.; Cavalot, F. Postprandial Dysmetabolism and Oxidative Stress in Type 2 Diabetes: Pathogenetic Mechanisms and Therapeutic Strategies. Med. Res. Rev. 2015, 35, 968–1031. [Google Scholar] [CrossRef]
- Jung, E.Y.; Lee, H.-S.; Choi, J.W.; Ra, K.S.; Kim, M.-R.; Suh, H.J. Glucose tolerance and antioxidant activity of spent brewer’s yeast hydrolysate with a high content of cyclo-His-Pro (CHP). J. Food Sci. Technol. 2011, 76, C272–C278. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Fitzgerald, R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, A.; Pokora, M.; Setner, B.; Dabrowska, A.; Szoltysik, M.; Babij, K.; Szewczuk, Z.; Trziszka, T.; Lubec, G.; Chrzanowska, J. Multifunctional peptides derived from an egg yolk protein hydrolysate: Isolation and characterization. Amino Acids 2015, 47, 369–380. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Kizhakkethil, B.P.; George, J.C.; Abubacker, Z.A.; Ninan, G.; Nagarajarao, R.C. Antioxidant Peptides from Dark Meat of Yellowfin Tuna (Thunnus albacares): Process Optimization and Characterization. Waste Biomass Valorization 2021, 12, 1845–1860. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.T.; Wang, Y.M.; Chi, C.F.; Wang, B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenser baerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2-Damaged Human Umbilical Vein Endothelial Cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef]
- Wang, Y.M.; Li, X.Y.; Wang, J.; He, Y.; Chi, C.F.; Wang, B. Antioxidant peptides from protein hydrolysate of skipjack tuna milt: Purification, identification, and cytoprotection on H2O2 damaged human umbilical vein endothelial cells. Process Biochem. 2022, 113, 258–269. [Google Scholar] [CrossRef]
- Gaikwad, S.B.; More, P.R.; Sonawane, S.K. Antioxidant and Anti-hypertensive Bioactive Peptides from Indian Mackerel Fish Waste. J. Int. J. Pept. Res. Ther. 2021, 27, 2671–2684. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.J.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef]
- Abachi, S.; Offret, C.; Fliss, I.; Marette, A.; Bazinet, L.; Beaulieu, L. Isolation of Immunomodulatory Biopeptides from Atlantic Mackerel (Scomber scombrus) Protein Hydrolysate based on Molecular Weight, Charge, and Hydrophobicity. Food Bioprocess. Technol. 2022, 15, 852–874. [Google Scholar] [CrossRef]
- Ye, J.; Shen, C.; Huang, Y.; Zhang, X.; Xiao, M. Anti-fatigue activity of sea cucumber peptides prepared from Stichopus japonicus in an endurance swimming rat model. J. Sci. Food Agric. 2017, 97, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Sahoo, S.; Bhattacharyya, D.K.; Ghosh, M. Marine lizardfish (Harpadon nehereus) meal concentrate in preparation of ready-to-eat protein and calcium rich extruded snacks. J. Food Sci. Technol. 2020, 57, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; Panisello-Rosello, A.; Sanchez-Nuno, S.; Alva, N.; Rosello-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. Febs. J. 2022, 289, 5463–5479. [Google Scholar] [CrossRef]
- Paul, N.; Truyen, N.; Sherratt Philip, J.; Pickett Cecil, B. The Carboxy-Terminal Neh3 Domain of Nrf2 Is Required for Transcriptional Activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar]
- He, S.; Zhao, W.; Chen, X.; Li, J.; Zhang, L.; Jin, H. Ameliorative Effects of Peptide Phe-Leu-Ala-Pro on Acute Liver and Kidney Injury Caused by CCl4 via Attenuation of Oxidative Stress and Inflammation. ACS Omega 2022, 7, 44796–44803. [Google Scholar] [CrossRef]
- Wu, H.; Kong, L.; Tan, Y.; Epstein, P.N.; Zeng, J.; Gu, J.; Liang, G.; Kong, M.; Chen, X.; Miao, L. C66 ameliorates diabetic nephropathy in mice by both upregulating NRF2 function via increase in miR-200a and inhibiting miR-21. Diabetologia 2016, 59, 1558–1568. [Google Scholar] [CrossRef]
- Wang, J.B.; Xie, Y.; Pei, X.R.; Yang, R.Y.; Zhang, Z.F.; Li, Y. The lipid-lowering and antioxidative effects of marine collagen peptides. Zhonghua Yu Fang Yi Xue Za Zhi 2008, 42, 226–230. [Google Scholar]
- Zhu, C.F.; Li, G.Z.; Peng, H.B.; Zhang, F.; Chen, Y.; Li, Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metab. 2010, 35, 797–804. [Google Scholar] [CrossRef]
- Castellano, P.; Mora, L.; Escudero, E.; Vignolo, G.; Aznar, R.; Toldra, F. Antilisterial peptides from Spanish dry-cured hams: Purification and identification. Food Microbiol. 2016, 59, 133–141. [Google Scholar] [CrossRef]
- Kuerban, A.; Al-Malki, A.L.; Kumosani, T.A.; Sheikh, R.A.; Al-Abbasi, F.A.M.; Alshubaily, F.A.; Abulnaja, K.O.; Moselhy, S.S. Identification, protein antiglycation, antioxidant, antiproliferative, and molecular docking of novel bioactive peptides produced from hydrolysis ofLens culinaris. J. Food Biochem. 2020, 44, e13494. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Cao, Y.; Li, H. Antioxidant activity of peptide fractions derived from cottonseed protein hydrolysate. J. Sci. Food Agric. 2010, 90, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Compean, D.; Jaquez-Quintana, J.O.; Gonzalez-Gonzalez, J.A.; Maldonado-Garza, H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J. Gastroenterol. 2009, 15, 280–288. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, S.J.; Zhu, J.; Shang, J.; Gao, X.D. Hypoglycemic, Hypolipidemic and Antioxidant Effects of Peptides from Red Deer Antlers in Streptozotocin-Induced Diabetic Mice. Tohoku J. Exp. Med. 2015, 236, 71–79. [Google Scholar] [CrossRef]
- Woo, M.; Seol, B.G.; Kang, K.H.; Choi, Y.H.; Cho, E.J.; Noh, J.S. Effects of collagen peptides from skate (Raja kenojei) skin on improvements of the insulin signaling pathway via attenuation of oxidative stress and inflammation. Food Funct. 2020, 11, 2017–2025. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, H.-H.; Liu, Z.-Z.; Huang, M.-Q.; Su, M.; Zhang, C.-W.; Gao, B.-Y.; Niu, J. A newly isolated strain of Haematococcus pluvialis JNU35 improves the growth, antioxidation, immunity and liver function of golden pompano (Trachinotus ovatus). Aquac. Nutr. 2021, 27, 342–354. [Google Scholar] [CrossRef]
- Najmeh, S.; Hossein, T.-N.; Ali, K.O.; Hamed, N.E.M. Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2011, 38, 413–419. [Google Scholar]
- Zhu, C.F.; Peng, H.B.; Liu, G.Q.; Zhang, F.; Li, Y. Beneficial effects of oligopeptides from marine salmon skin in a rat model of type 2 diabetes. Nutrition 2010, 26, 1014–1020. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, W.; Mu, B.; Zhang, F.; Lai, N.; Zhou, J.; Xu, A.; Liu, J.; Li, Y. Effects of marine collagen peptides on glucose metabolism and insulin resistance in type 2 diabetic rats. J. Food Sci. Technol. 2017, 54, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, X.; Li, D.; Sun, N.; Lin, S. Sea Cucumber Peptides Attenuated the Scopolamine-Induced Memory Impairment in Mice and Rats and the Underlying Mechanism. J. Agric. Food Chem. 2022, 70, 157–170. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xu, Z.; Li, J.; Guo, Y.; Lin, Q.; Jin, H. Peptides from Harpadon nehereus protect against hyperglycemia-induced HepG2 via oxidative stress and glycolipid metabolism regulation. J. Funct. Foods 2023, 108, 105723. [Google Scholar] [CrossRef]
- Kumar, A.; Mittal, R. Nrf2: A potential therapeutic target for diabetic neuropathy. Inflammopharmacology 2017, 25, 393–402. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Jiang, Z.; Jiang, J.; Xu, L.; Tian, A.; Sun, X.; Meng, H.; Li, Y.; Huang, W.; et al. Protective role of NRF2 in macrovascular complications of diabetes. J. Cell Mol. Med. 2020, 24, 8903–8917. [Google Scholar] [CrossRef] [PubMed]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Sanchez-Chavez, G.; Salceda, R. Retinal Nrf2 expression in normal and early streptozotocin-diabetic rats. Neurochem. Int. 2021, 145, 105007. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Zheng, A.; Zhang, H.; Cao, H. Down-regulation of betatrophin enhances insulin sensitivity in type 2 diabetes mellitus through activation of the GSK-3beta/PGC-1alpha signaling pathway. J. Endocrinol. Investig. 2021, 44, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wan, J.; Shan, Y.; Song, X.; Jin, J.; Su, Q.; Chen, S.; Lu, X.; Yang, J.; Li, Q.; et al. MicroRNA-185-5p inhibits hepatic gluconeogenesis and reduces fasting blood glucose levels by suppressing G6Pase. Theranostics 2021, 11, 7829–7843. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Jiang, X.; Yin, L.; Zhang, X. Antioxidant and hypoglycaemic effects of tilapia skin collagen peptide in mice. Int. J. Food Sci. Technol. 2016, 51, 2157–2163. [Google Scholar] [CrossRef]
- Abdelmawgood, I.A.; Mahana, N.A.; Badr, A.M.; Mohamed, A.S.; Al Shawoush, A.M.; Atia, T.; Abdelrazak, A.E.; Sakr, H.I. Echinochrome Ameliorates Physiological, Immunological, and Histopathological Alterations Induced by Ovalbumin in Asthmatic Mice by Modulating the Keap1/Nrf2 Signaling Pathway. Mar. Drugs 2023, 21, 455. [Google Scholar] [CrossRef]
- Okan, A.; Doganyigit, Z.; Eroglu, E.; Akyuz, E.; Demir, N. Immunoreactive definition of TNF- alpha, HIF-1 alpha, Kir6.2, Kir3.1 and M2 muscarinic receptor for cardiac and pancreatic tissues in a mouse model for type 1 diabetes. Life Sci. 2021, 284, 119886. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Lu, J.; Chen, H.H.; Chen, W.S.; Xu, H.G. Individualized correction of insulin measurement in hemolyzed serum samples. Immunol. Res. 2017, 65, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Li, Q.M.; Yu, N.J.; Chen, W.D.; Zha, X.Q.; Wu, D.L.; Pan, L.H.; Duan, J.; Luo, J.P. Dendrobium huoshanense polysaccharide regulates hepatic glucose homeostasis and pancreatic ss-cell function in type 2 diabetic mice. Carbohyd Polym. 2019, 211, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Gao, Z.Z.; Guo, Q.T.; Wang, T.; Lu, C.E.; Chen, Y.; Sheng, Q.; Chen, J.; Nie, Z.M.; Zhang, Y.Z.; et al. Anti-Diabetic Effects of CTB-APSL Fusion Protein in Type 2 Diabetic Mice. Mar. Drugs 2014, 12, 1512–1529. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Zhao, R.; Pu, Q.Y.; Jiang, S.; Yu, F.M.; Yang, Z.S.; Han, T. Investigation of nephrotoxicity on mice exposed to polystyrene nanoplastics and the potential amelioration effects of DHA-enriched phosphatidylserine. Sci. Total Environ. 2023, 892, 164808. [Google Scholar] [CrossRef]
- Park, S.Y.; Fernando, I.P.S.; Han, E.J.; Kim, M.J.; Jung, K.; Kang, D.S.; Ahn, C.B.; Ahn, G. In Vivo Hepatoprotective Effects of a Peptide Fraction from Krill Protein Hydrolysates against Alcohol-Induced Oxidative Damage. Mar. Drugs 2019, 17, 690. [Google Scholar] [CrossRef]
- Jiang, Q.J.; Chen, Q.; Li, C.P.; Gong, Z.G.; Li, Z.G.; Ding, S.F. ox-LDL-Induced Endothelial Progenitor Cell Oxidative Stress via p38/Keap1/Nrf2 Pathway. Stem Cells Int. 2022, 2022, 5897194. [Google Scholar] [CrossRef]


| Amino Acid | Residues/1000 Residues |
|---|---|
| Aspartic acid (Asp) | 47.4 |
| Threonine (Thr) | 29.2 |
| Serine (Ser) | 34.8 |
| Glutamic acid (Glu) | 71.4 |
| Glycine (Gly) | 336.2 |
| Alanine (Ala) | 117.3 |
| Valine (Val) | 26.1 |
| Methionine (Met) | 11.6 |
| Isoleucine (Ile) | 12.3 |
| Leucine (Leu) | 27.8 |
| Tyrosine (Tyr) | 4.2 |
| Phenylalanine (Phe) | 12.3 |
| Lysine (Lys) | 22.0 |
| Histidine (His) | 6.0 |
| Arginine (Arg) | 37.3 |
| Proline (Pro) | 116.0 |
| Hydroxyproline (Hyp) | 76.5 |
| Hydroxylysine (Hyl) | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Q.; Guo, Y.; Li, J.; He, S.; Chen, Y.; Jin, H. Antidiabetic Effect of Collagen Peptides from Harpadon nehereus Bones in Streptozotocin-Induced Diabetes Mice by Regulating Oxidative Stress and Glucose Metabolism. Mar. Drugs 2023, 21, 518. https://doi.org/10.3390/md21100518
Lin Q, Guo Y, Li J, He S, Chen Y, Jin H. Antidiabetic Effect of Collagen Peptides from Harpadon nehereus Bones in Streptozotocin-Induced Diabetes Mice by Regulating Oxidative Stress and Glucose Metabolism. Marine Drugs. 2023; 21(10):518. https://doi.org/10.3390/md21100518
Chicago/Turabian StyleLin, Qianxia, Yueping Guo, Jie Li, Shuqi He, Yan Chen, and Huoxi Jin. 2023. "Antidiabetic Effect of Collagen Peptides from Harpadon nehereus Bones in Streptozotocin-Induced Diabetes Mice by Regulating Oxidative Stress and Glucose Metabolism" Marine Drugs 21, no. 10: 518. https://doi.org/10.3390/md21100518
APA StyleLin, Q., Guo, Y., Li, J., He, S., Chen, Y., & Jin, H. (2023). Antidiabetic Effect of Collagen Peptides from Harpadon nehereus Bones in Streptozotocin-Induced Diabetes Mice by Regulating Oxidative Stress and Glucose Metabolism. Marine Drugs, 21(10), 518. https://doi.org/10.3390/md21100518







