Fish Collagen Peptides Enhance Thymopoietic Gene Expression, Cell Proliferation, Thymocyte Adherence, and Cytoprotection in Thymic Epithelial Cells via Activation of the Nuclear Factor-κB Pathway, Leading to Thymus Regeneration after Cyclophosphamide-Induced Injury
Abstract
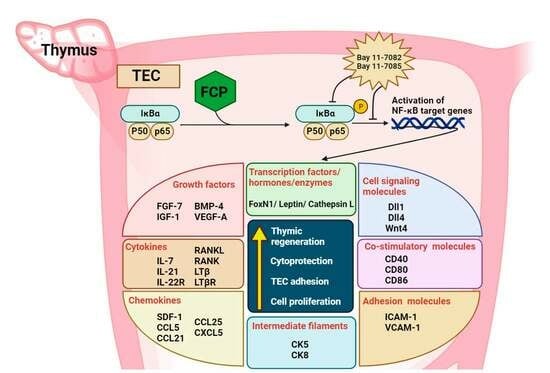
:1. Introduction
2. Results
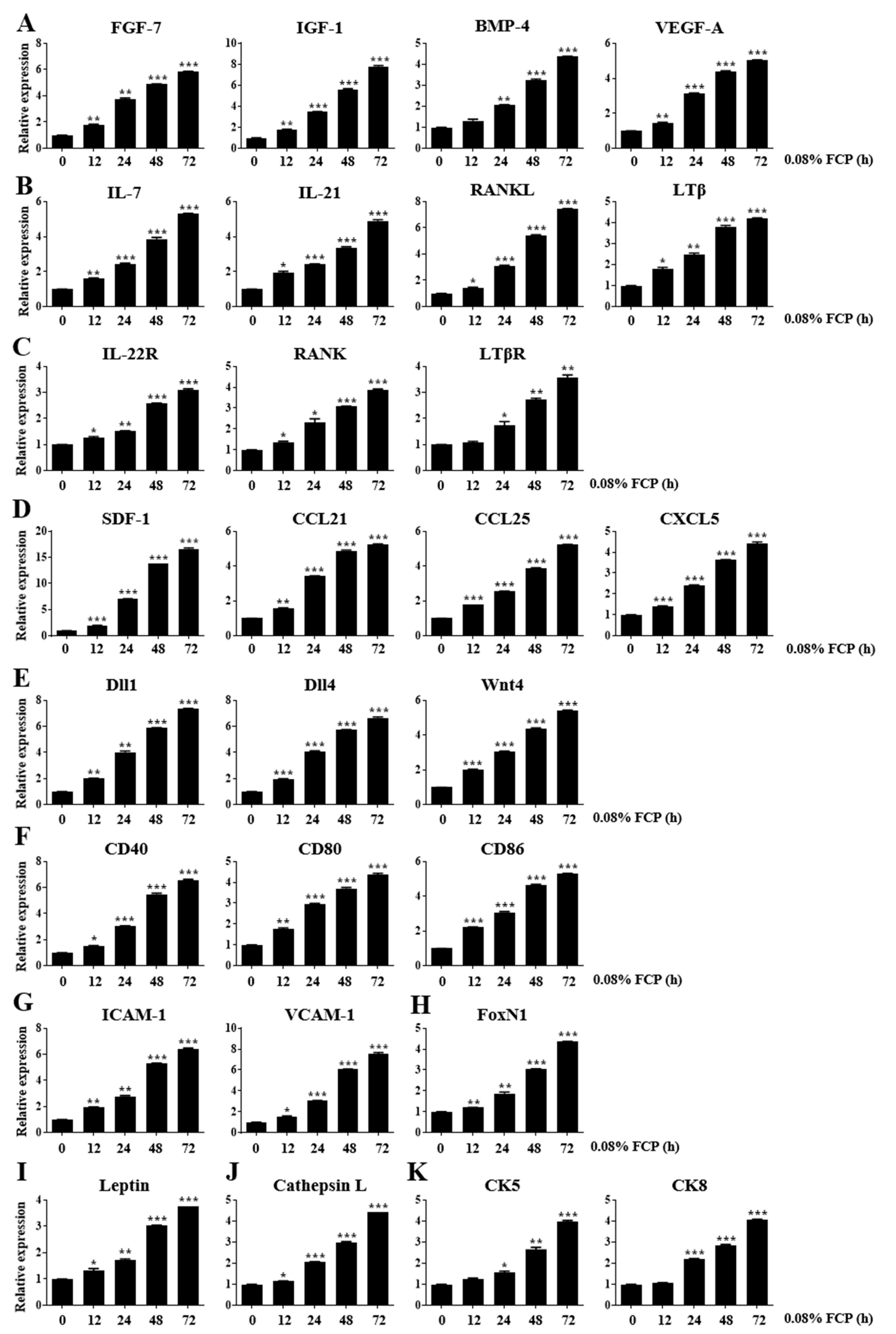
2.1. FCPs Stimulate Thymopoietic Gene Expression in TECs
2.2. FCPs Promote TEC Proliferation through the NF-κB Signal Transduction Pathway
2.3. FCPs Enhance Thymocyte Adhesion to TECs through the NF-κB Signal Transduction Pathway
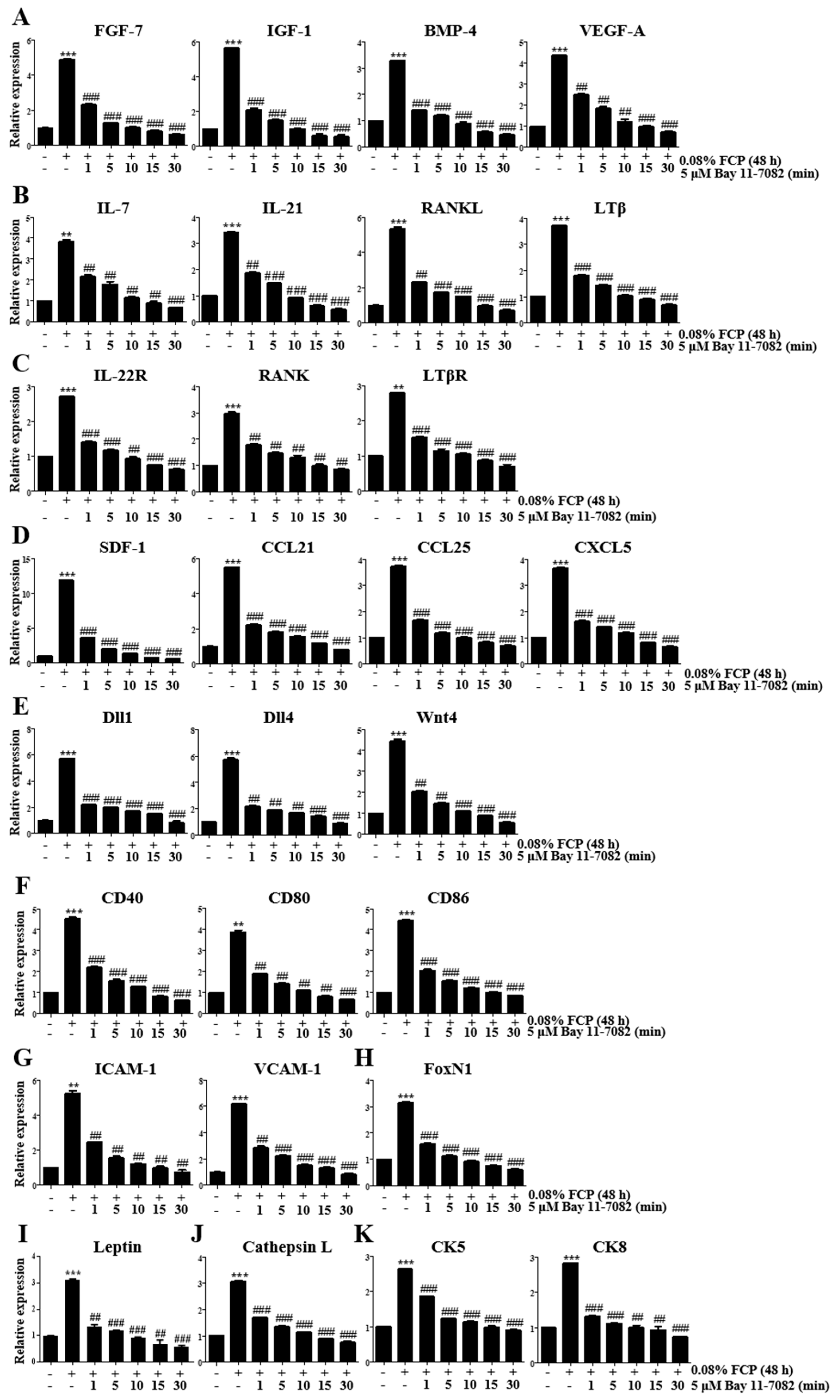
2.4. FCPs Augment Thymopoietic Gene Expression in TECs through the NF-κB Signaling Pathway
2.5. FCPs Protect TECs against CP-Induced Cellular Injury through the NF-κB Signaling Pathway
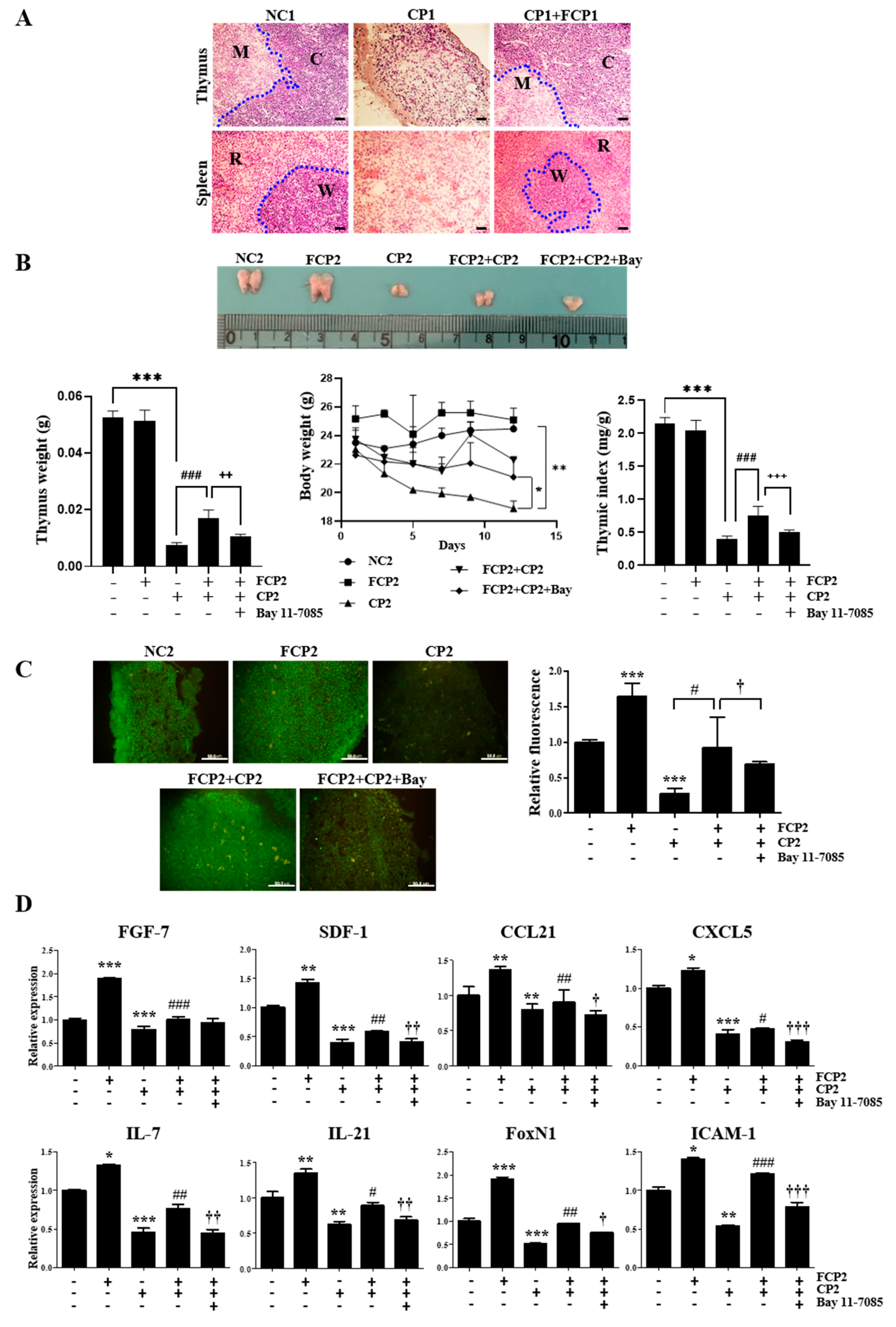
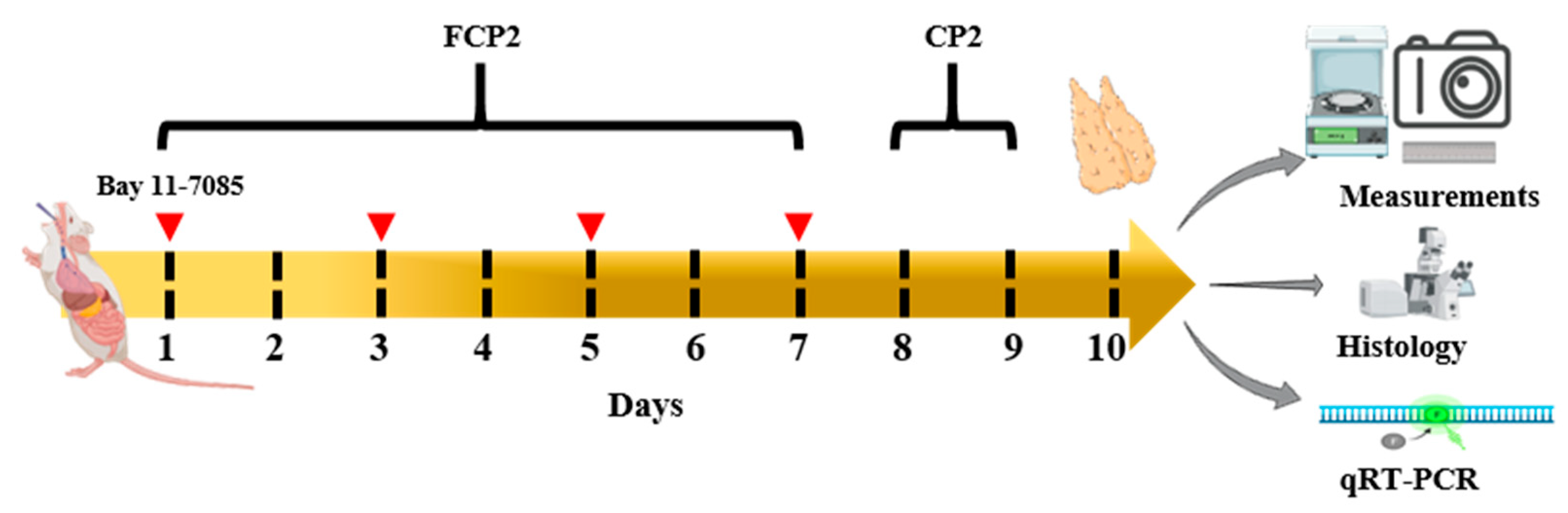
2.6. FCPs Promote Regeneration In Vivo from CP-Induced Thymus Injury through the NF-κB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Proliferation Assay
4.3. Evaluation of Immunofluorescence Using Confocal Microscopy
4.4. RNA Isolation and cDNA Synthesis
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Quantitation of Thymocyte Adhesion to TECs
4.7. Flow Cytometry
4.8. In Vitro TEC Treatment of CP
4.9. Animals and Experimental Design
4.10. HE Staining
4.11. Effect of FCPs on Mouse Thymus Index
4.12. Immunofluorescence Analysis of CD4 Expressions in the Thymus
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, G.P.; Allen, P.M. How the TCR Balances Sensitivity and Specificity for the Recognition of Self and Pathogens. Nat. Immunol. 2012, 13, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Anderson, G. Thymic Epithelial Cells. Annu. Rev. Immunol. 2017, 35, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Mo, X.; Liu, J.; Ye, S.; Zeng, X.; Chen, D. Thymic Function in the Regulation of T Cells, and Molecular Mechanisms Underlying the Modulation of Cytokines and Stress Signaling (Review). Mol. Med. Rep. 2017, 16, 7175–7184. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Pan, W.; Zheng, L.; Zhong, X.P.; Tan, L.; Liang, Z.; He, J.; Feng, P.; Zhao, Y.; Qiu, Y.-R. Corrigendum: Thymic Epithelial Cells Contribute to Thymopoiesis and T Cell Development. Front. Immunol. 2020, 11, 628464. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.S.; Velardi, E.; Dudakov, J.A.; van den Brink, M.R.M. Thymus: The next (Re)Generation. Immunol. Rev. 2016, 271, 56–71. [Google Scholar] [CrossRef]
- Gray, D.H.D.; Seach, N.; Ueno, T.; Milton, M.K.; Liston, A.; Lew, A.M.; Goodnow, C.C.; Boyd, R.L. Developmental Kinetics, Turnover, and Stimulatory Capacity of Thymic Epithelial Cells. Blood 2006, 108, 3777–3785. [Google Scholar] [CrossRef]
- Liang, Z.; Dong, X.; Zhang, Z.; Zhang, Q.; Zhao, Y. Age-Related Thymic Involution: Mechanisms and Functional Impact. Aging Cell 2022, 21, e13671. [Google Scholar] [CrossRef]
- Kilpatrick, R.D.; Rickabaugh, T.; Hultin, L.E.; Hultin, P.; Hausner, M.A.; Detels, R.; Phair, J.; Jamieson, B.D. Homeostasis of the Naive CD4+ T Cell Compartment during Aging. J. Immunol. 2008, 180, 1499–1507. [Google Scholar] [CrossRef]
- Zediak, V.P.; Maillard, I.; Bhandoola, A. Multiple Prethymic Defects Underlie Age-Related Loss of T Progenitor Competence. Blood 2007, 110, 1161–1167. [Google Scholar] [CrossRef]
- Yoon, S.; Yoo, Y.H.; Kim, B.S.; Kim, J.J. Ultrastructural Alterations of the Cortical Epithelial Cells of the Rat Thymus after Cyclophosphamide Treatment. Histol. Histopathol. 1997, 12, 401–413. [Google Scholar]
- Kinsella, S.; Dudakov, J.A. When the Damage Is Done: Injury and Repair in Thymus Function. Front. Immunol. 2020, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Velardi, E.; Tsai, J.J.; van den Brink, M.R.M. T Cell Regeneration after Immunological Injury. Nat. Rev. Immunol. 2021, 21, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.; Park, D.; Selden, H.J.; Seita, J.; Chung, H.; Kim, J.; Iyer, V.R.; Ehrlich, L.I.R. Global Transcriptional Profiling Reveals Distinct Functions of Thymic Stromal Subsets and Age-Related Changes during Thymic Involution. Cell Rep. 2014, 9, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Lepletier, A.; Chidgey, A.P.; Savino, W. Perspectives for Improvement of the Thymic Microenvironment through Manipulation of Thymic Epithelial Cells: A Mini-Review. Gerontology 2015, 61, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Ludeman, S.M. The Chemistry of the Metabolites of Cyclophosphamide. Curr. Pharm. Des. 1999, 5, 627–643. [Google Scholar] [CrossRef]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Subramanian, R.; Pathak, H.; Ravindran, V. Safety of Cyclophosphamide Therapy in Autoimmune Rheumatic Diseases. Indian J. Rheumatol. 2019, 14, 127. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Song, W.; Kim, H.Y.; Lim, Y.S.; Hwang, S.Y.; Lee, C.; Lee, D.Y.; Moon, Y.; Song, Y.J.; Yoon, S. Fish Collagen Peptides Protect against Cisplatin-Induced Cytotoxicity and Oxidative Injury by Inhibiting MAPK Signaling Pathways in Mouse Thymic Epithelial Cells. Mar. Drugs 2022, 20, 232. [Google Scholar] [CrossRef]
- Fiorini, E.; Marchisio, P.C.; Scupoli, M.T.; Poffe, O.; Tagliabue, E.; Brentegani, M.; Colombatti, M.; Santini, F.; Tridente, G.; Ramarli, D. Adhesion of Immature and Mature T Cells Induces in Human Thymic Epithelial Cells (TEC) Activation of IL-6 Gene Trascription Factors (NF-κB and NF-IL6) and IL-6 Gene Expression: Role of α3β1 and α6β4 Integrins. Dev. Immunol. 2000, 7, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Scupoli, M.T.; Fiorini, E.; Marchisio, P.C.; Poffe, O.; Tagliabue, E.; Brentegani, M.; Tridente, G.; Ramarli, D. Lymphoid Adhesion Promotes Human Thymic Epithelial Cell Survival via NF-κB Activation. J. Cell Sci. 2000, 113 Pt 1, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Nagib, P.; Verinaud, L. The Thymus Microenvironment in Regulating Thymocyte Differentiation. Cell Adh. Migr. 2010, 4, 382–390. [Google Scholar] [CrossRef]
- Finch, P.W.; Rubin, J.S. Keratinocyte Growth Factor/Fibroblast Growth Factor 7, a Homeostatic Factor with Therapeutic Potential for Epithelial Protection and Repair. Adv. Cancer Res. 2004, 91, 69–136. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Degenstein, L.; Fuchs, E. Keratinocyte Growth Factor Is Required for Hair Development but Not for Wound Healing. Genes Dev. 1996, 10, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.W.; Jeker, L.T.; Ueno, T.; Kuse, S.; Keller, M.P.; Zuklys, S.; Gudkov, A.V.; Takahama, Y.; Krenger, W.; Blazar, B.R.; et al. Keratinocyte Growth Factor (KGF) Enhances Postnatal T-Cell Development via Enhancements in Proliferation and Function of Thymic Epithelial Cells. Blood 2007, 109, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Chung, B.; Danilenko, D.M.; Farrell, C.; Lacey, D.L.; Blazar, B.R.; Weinberg, K.I. Protection from Thymic Epithelial Cell Injury by Keratinocyte Growth Factor: A New Approach to Improve Thymic and Peripheral T-Cell Reconstitution after Bone Marrow Transplantation. Blood 2002, 99, 4592–4600. [Google Scholar] [CrossRef]
- Dooley, J.; Erickson, M.; Larochelle, W.J.; Gillard, G.O.; Farr, A.G. FGFR2IIIb Signaling Regulates Thymic Epithelial Differentiation. Dev. Dyn. 2007, 236, 3459–3471. [Google Scholar] [CrossRef]
- Erickson, M.; Morkowski, S.; Lehar, S.; Gillard, G.; Beers, C.; Dooley, J.; Rubin, J.S.; Rudensky, A.; Farr, A.G. Regulation of Thymic Epithelium by Keratinocyte Growth Factor. Blood 2002, 100, 3269–3278. [Google Scholar] [CrossRef]
- Alpdogan, O.; Hubbard, V.M.; Smith, O.M.; Patel, N.; Lu, S.; Goldberg, G.L.; Gray, D.H.; Feinman, J.; Kochman, A.A.; Eng, J.M.; et al. Keratinocyte Growth Factor (KGF) Is Required for Postnatal Thymic Regeneration. Blood 2006, 107, 2453–2460. [Google Scholar] [CrossRef]
- Min, D.; Panoskaltsis-Mortari, A.; Kuro-O, M.; Holländer, G.A.; Blazar, B.R.; Weinberg, K.I. Sustained Thymopoiesis and Improvement in Functional Immunity Induced by Exogenous KGF Administration in Murine Models of Aging. Blood 2007, 109, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Yakar, S. Mechanisms of Disease: Metabolic Effects of Growth Hormone and Insulin-like Growth Factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Strasser, J.; McCabe, S.; Robbins, K.; Jardieu, P. Insulin-like Growth Factor-1 Stimulation of Lymphopoiesis. J. Clin. Investig. 1993, 92, 540–548. [Google Scholar] [CrossRef]
- Walsh, P.T.; Smith, L.M.; O’Connor, R. Insulin-like Growth Factor-1 Activates Akt and Jun N-Terminal Kinases (JNKs) in Promoting the Survival of T Lymphocytes. Immunology 2002, 107, 461–471. [Google Scholar] [CrossRef]
- Bernabei, P.; Bosticardo, M.; Losana, G.; Regis, G.; Di Paola, F.; De Angelis, S.; Giovarelli, M.; Novelli, F. IGF-1 down-Regulates IFN-γR2 Chain Surface Expression and Desensitizes IFN-γ/STAT-1 Signaling in Human T Lymphocytes. Blood 2003, 102, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-W.; Schmitz, S.; Choudhury, B.; Telford, W.; Kapoor, V.; Garfield, S.; Howe, D.; Gress, R.E. Exogenous Insulin-like Growth Factor 1 Enhances Thymopoiesis Predominantly through Thymic Epithelial Cell Expansion. Blood 2008, 112, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.C.; Dean, G.A.; Lavoy, A.; Clark, R.; Moore, P.F. Investigation of Recombinant Human Insulin-like Growth Factor Type I in Thymus Regeneration in the Acute Stage of Experimental FIV Infection in Juvenile Cats. AIDS Res. Hum. Retroviruses 1999, 15, 1377–1388. [Google Scholar] [CrossRef]
- Bleul, C.C.; Boehm, T. BMP Signaling Is Required for Normal Thymus Development. J. Immunol. 2005, 175, 5213–5221. [Google Scholar] [CrossRef]
- Gordon, J.; Patel, S.R.; Mishina, Y.; Manley, N.R. Evidence for an Early Role for BMP4 Signaling in Thymus and Parathyroid Morphogenesis. Dev. Biol. 2010, 339, 141–154. [Google Scholar] [CrossRef]
- Parent, A.V.; Russ, H.A.; Khan, I.S.; LaFlam, T.N.; Metzger, T.C.; Anderson, M.S.; Hebrok, M. Generation of Functional Thymic Epithelium from Human Embryonic Stem Cells That Supports Host T Cell Development. Cell Stem Cell 2013, 13, 219–229. [Google Scholar] [CrossRef]
- Lepletier, A.; Hun, M.L.; Hammett, M.V.; Wong, K.; Naeem, H.; Hedger, M.; Loveland, K.; Chidgey, A.P. Interplay between Follistatin, Activin A, and BMP4 Signaling Regulates Postnatal Thymic Epithelial Progenitor Cell Differentiation during Aging. Cell Rep. 2019, 27, 3887–3901. [Google Scholar] [CrossRef]
- Wertheimer, T.; Velardi, E.; Tsai, J.; Cooper, K.; Xiao, S.; Kloss, C.C.; Ottmüller, K.J.; Mokhtari, Z.; Brede, C.; deRoos, P.; et al. Production of BMP4 by Endothelial Cells Is Crucial for Endogenous Thymic Regeneration. Sci. Immunol. 2018, 3, eaal2736. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, M.; Lim, J.M.C.; Hun, M.L.; Lister, N.; Wong, K.; Hammett, M.V.; Lepletier, A.; Boyd, R.L.; Giudice, A.; Chidgey, A.P. A Novel Foxn1eGFP/+ Mouse Model Identifies Bmp4-Induced Maintenance of Foxn1 Expression and Thymic Epithelial Progenitor Populations. Eur. J. Immunol. 2017, 47, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, M.N.; Kim, J.G.; Bae, Y.H.; Bae, M.K.; Wee, H.J.; Kim, T.W.; Kim, B.S.; Kim, J.B.; Bae, S.K.; et al. Up-Regulation of VEGF Expression by NGF That Enhances Reparative Angiogenesis during Thymic Regeneration in Adult Rat. Biochim. Biophys. Acta 2007, 1773, 1462–1472. [Google Scholar] [CrossRef]
- Irla, M. RANK Signaling in the Differentiation and Regeneration of Thymic Epithelial Cells. Front. Immunol. 2021, 11, 623265. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, R.; Henson, S.; Pido-Lopez, J.; Ngom, P.A.T. Interleukin-7: An Interleukin for Rejuvenating the Immune System. Ann. N. Y. Acad. Sci. 2004, 1019, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Adoro, S.; Guinter, T.; Erman, B.; Alag, A.S.; Catalfamo, M.; Kimura, M.Y.; Cui, Y.; Lucas, P.J.; Gress, R.E.; et al. Signaling by Intrathymic Cytokines, Not T Cell Antigen Receptors, Specifies CD8 Lineage Choice and Promotes the Differentiation of Cytotoxic-Lineage T Cells. Nat. Immunol. 2010, 11, 257–264. [Google Scholar] [CrossRef]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef]
- Surh, C.D.; Sprent, J. Homeostasis of Naive and Memory T Cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Rodrigues, P.M.; Meireles, C.; Di Santo, J.P.; Alves, N.L. Thymocyte Selection Regulates the Homeostasis of IL-7-Expressing Thymic Cortical Epithelial Cells in vivo. J. Immunol. 2013, 191, 1200–1209. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A Double-Edged Sword with Therapeutic Potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Lacraz, S.; Chicheportiche, R.; Schneiter, G.; Molnarfi, N.; Villard, J.; Dayer, J.M. IL-21 Promotes Survival and Maintains a Naive Phenotype in Human CD4+ T Lymphocytes. Int. Immunol. 2008, 20, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Rafei, M.; Rouette, A.; Brochu, S.; Vanegas, J.-R.; Perreault, C. Differential Effects of γc Cytokines on Postselection Differentiation of CD8 Thymocytes. Blood 2013, 121, 107–117. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and Pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; Jenq, R.R.; Young, L.F.; Ghosh, A.; Singer, N.V.; West, M.L.; Smith, O.M.; Holland, A.M.; Tsai, J.J.; et al. Interleukin-22 Drives Endogenous Thymic Regeneration in Mice. Science 2012, 336, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL Is a Key Regulator of Osteoclastogenesis, Lymphocyte Development and Lymph-Node Organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef]
- Akiyama, N.; Takizawa, N.; Miyauchi, M.; Yanai, H.; Tateishi, R.; Shinzawa, M.; Yoshinaga, R.; Kurihara, M.; Demizu, Y.; Yasuda, H.; et al. Identification of Embryonic Precursor Cells That Differentiate into Thymic Epithelial Cells Expressing Autoimmune Regulator. J. Exp. Med. 2016, 213, 1441–1458. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Shimo, Y.; Yanai, H.; Qin, J.; Ohshima, D.; Maruyama, Y.; Asaumi, Y.; Kitazawa, J.; Takayanagi, H.; Penninger, J.M.; et al. The Tumor Necrosis Factor Family Receptors RANK and CD40 Cooperatively Establish the Thymic Medullary Microenvironment and Self-Tolerance. Immunity 2008, 29, 423–437. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, B.S.; Kim, H.J.; Lee, C.W.; Yoo, H.J.; Kim, J.B.; Yoon, S. Upregulation of Receptor Activator of Nuclear Factor-κB Ligand Expression in the Thymic Subcapsular, Paraseptal, Perivascular, and Medullary Epithelial Cells during Thymus Regeneration. Histochem. Cell Biol. 2005, 123, 491–500. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, H.K.; Na, Y.J.; Kim, C.D.; Lee, J.H.; Kim, B.S.; Kim, J.B.; Lee, C.W.; Moon, J.O.; Yoon, S. RANKL Stimulates Proliferation, Adhesion and IL-7 Expression of Thymic Epithelial Cells. Exp. Mol. Med. 2008, 40, 59–70. [Google Scholar] [CrossRef]
- Borelli, A.; Irla, M. Lymphotoxin: From the Physiology to the Regeneration of the Thymic Function. Cell Death Differ. 2021, 28, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.; James, K.D.; Cosway, E.J.; Parnell, S.M.; Tumanov, A.V.; Ware, C.F.; Jenkinson, W.E.; Anderson, G. Lymphotoxin β Receptor Controls T Cell Progenitor Entry to the Thymus. J. Immunol. 2016, 197, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, T.; Tai, X.; Kruhlak, M.; Wisniewski, J.; Hwang, I.Y.; Roy, S.; Guinter, T.I.; Alag, A.; Kehrl, J.H.; Zhuang, Y.; et al. E-Protein-Regulated Expression of CXCR4 Adheres Preselection Thymocytes to the Thymic Cortex. J. Exp. Med. 2019, 216, 1749–1761. [Google Scholar] [CrossRef]
- Hernández-López, C.; Varas, A.; Sacedón, R.; Jiménez, E.; Muñoz, J.J.; Zapata, A.G.; Vicente, A. Stromal Cell–Derived Factor 1/CXCR4 Signaling Is Critical for Early Human T-Cell Development. Blood 2002, 99, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, L. CCL21/CCR7 Axis as a Therapeutic Target for Autoimmune Diseases. Int. Immunopharmacol. 2023, 121, 110431. [Google Scholar] [CrossRef]
- Kozai, M.; Kubo, Y.; Katakai, T.; Kondo, H.; Kiyonari, H.; Schaeuble, K.; Luther, S.A.; Ishimaru, N.; Ohigashi, I.; Takahama, Y. Essential Role of CCL21 in Establishment of Central Self-Tolerance in T Cells. J. Exp. Med. 2017, 214, 1925–1935. [Google Scholar] [CrossRef]
- James, K.D.; Legler, D.F.; Purvanov, V.; Ohigashi, I.; Takahama, Y.; Parnell, S.M.; White, A.J.; Jenkinson, W.E.; Anderson, G. Medullary Stromal Cells Synergize Their Production and Capture of CCL21 for T-Cell Emigration from Neonatal Mouse Thymus. Blood Adv. 2021, 5, 99–112. [Google Scholar] [CrossRef]
- Williams, K.M.; Lucas, P.J.; Bare, C.V.; Wang, J.; Chu, Y.-W.; Tayler, E.; Kapoor, V.; Gress, R.E. CCL25 Increases Thymopoiesis after Androgen Withdrawal. Blood 2008, 112, 3255–3263. [Google Scholar] [CrossRef]
- Jenkinson, W.E.; Rossi, S.W.; Parnell, S.M.; Agace, W.W.; Takahama, Y.; Jenkinson, E.J.; Anderson, G. Chemokine Receptor Expression Defines Heterogeneity in the Earliest Thymic Migrants. Eur. J. Immunol. 2007, 37, 2090–2096. [Google Scholar] [CrossRef]
- Gossens, K.; Naus, S.; Corbel, S.Y.; Lin, S.; Rossi, F.M.V.; Kast, J.; Ziltener, H.J. Thymic Progenitor Homing and Lymphocyte Homeostasis Are Linked via S1P-Controlled Expression of Thymic P-Selectin/CCL25. J. Exp. Med. 2009, 206, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Balamayooran, G.; Batra, S.; Cai, S.; Mei, J.; Worthen, G.S.; Penn, A.L.; Jeyaseelan, S. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am. J. Respir. Cell Mol. Biol. 2012, 47, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zubkova, I.; Mostowski, H.; Zaitseva, M. Up-Regulation of IL-7, Stromal-Derived Factor-1 Alpha, Thymus-Expressed Chemokine, and Secondary Lymphoid Tissue Chemokine Gene Expression in the Stromal Cells in Response to Thymocyte Depletion: Implication for Thymus Reconstitution. J. Immunol. 2005, 175, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Na, Y.J.; Jung, P.K.; Kim, M.N.; Kim, S.M.; Chung, J.S.; Kim, B.S.; Kim, J.B.; Moon, J.O.; Yoon, S. Nerve Growth Factor Stimulates Proliferation, Adhesion and Thymopoietic Cytokine Expression in Mouse Thymic Epithelial Cells in Vitro. Regul. Pept. 2008, 147, 72–81. [Google Scholar] [CrossRef]
- Hozumi, K.; Mailhos, C.; Negishi, N.; Hirano, K.-I.; Yahata, T.; Ando, K.; Zuklys, S.; Holländer, G.A.; Shima, D.T.; Habu, S. Delta-like 4 Is Indispensable in Thymic Environment Specific for T Cell Development. J. Exp. Med. 2008, 205, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Zarin, P.; In, T.S.; Chen, E.L.; Singh, J.; Wong, G.W.; Mohtashami, M.; Wiest, D.L.; Anderson, M.K.; Zúñiga-Pflücker, J.C. Integration of T-Cell Receptor, Notch and Cytokine Signals Programs Mouse γδ T-Cell Effector Differentiation. Immunol. Cell Biol. 2018, 96, 994–1007. [Google Scholar] [CrossRef]
- Wilson, A.; MacDonald, H.R.; Radtke, F. Notch 1-Deficient Common Lymphoid Precursors Adopt a B Cell Fate in the Thymus. J. Exp. Med. 2001, 194, 1003–1012. [Google Scholar] [CrossRef]
- Heinonen, K.M.; Vanegas, J.R.; Brochu, S.; Shan, J.; Vainio, S.J.; Perreault, C. Wnt4 regulates thymic cellularity through the expansion of thymic epithelial cells and early thymic progenitors. Blood 2011, 118, 5163–5173. [Google Scholar] [CrossRef]
- Williams, J.A.; Hathcock, K.S.; Klug, D.; Harada, Y.; Choudhury, B.; Allison, J.P.; Abe, R.; Hodes, R.J. Regulated Costimulation in the Thymus Is Critical for T Cell Development: Dysregulated CD28 Costimulation Can Bypass the Pre-TCR Checkpoint. J. Immunol. 2005, 175, 4199–4207. [Google Scholar] [CrossRef]
- Williams, J.A.; Zhang, J.; Jeon, H.; Nitta, T.; Ohigashi, I.; Klug, D.; Kruhlak, M.J.; Choudhury, B.; Sharrow, S.O.; Granger, L.; et al. Thymic Medullary Epithelium and Thymocyte Self-Tolerance Require Cooperation between CD28–CD80/86 and CD40–CD40L Costimulatory Pathways. J. Immunol. 2014, 192, 630–640. [Google Scholar] [CrossRef]
- Singer, K.H.; Le, P.T.; Denning, S.M.; Whichard, L.P.; Haynes, B.F. The Role of Adhesion Molecules in Epithelial–T-Cell Interactions in Thymus and Skin. J. Investig. Dermatol. 1990, 94, s85–s90. [Google Scholar] [CrossRef] [PubMed]
- Žuklys, S.; Handel, A.; Zhanybekova, S.; Govani, F.; Keller, M.; Maio, S.; Mayer, C.E.; Teh, H.Y.; Hafen, K.; Gallone, G.; et al. Foxn1 Regulates Key Target Genes Essential for T Cell Development in Postnatal Thymic Epithelial Cells. Nat. Immunol. 2016, 17, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Corbeaux, T.; Hess, I.; Swann, J.B.; Kanzler, B.; Haas-Assenbaum, A.; Boehm, T. Thymopoiesis in Mice Depends on a Foxn1-Positive Thymic Epithelial Cell Lineage. Proc. Natl. Acad. Sci. USA 2010, 107, 16613–16618. [Google Scholar] [CrossRef] [PubMed]
- Rode, I.; Martins, V.C.; Küblbeck, G.; Maltry, N.; Tessmer, C.; Rodewald, H.-R. Foxn1 Protein Expression in the Developing, Aging, and Regenerating Thymus. J. Immunol. 2015, 195, 5678–5687. [Google Scholar] [CrossRef]
- Bredenkamp, N.; Nowell, C.S.; Blackburn, C.C. Regeneration of the Aged Thymus by a Single Transcription Factor. Development 2014, 141, 1627–1637. [Google Scholar] [CrossRef]
- Calderón, L.; Boehm, T. Synergistic, Context-Dependent, and Hierarchical Functions of Epithelial Components in Thymic Microenvironments. Cell 2012, 149, 159–172. [Google Scholar] [CrossRef]
- Sreenivasan, J.; Schlenner, S.; Franckaert, D.; Dooley, J.; Liston, A. The Thymoprotective Function of Leptin Is Indirectly Mediated via Suppression of Obesity. Immunology 2015, 146, 122–129. [Google Scholar] [CrossRef]
- Howard, J.K.; Lord, G.M.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin Protects Mice from Starvation-Induced Lymphoid Atrophy and Increases Thymic Cellularity in Ob/Ob Mice. J. Clin. Investig. 1999, 104, 1051–1059. [Google Scholar] [CrossRef]
- Dooley, J.; Liston, A. Molecular Control over Thymic Involution: From Cytokines and MicroRNA to Aging and Adipose Tissue. Eur. J. Immunol. 2012, 42, 1073–1079. [Google Scholar] [CrossRef]
- Nakagawa, T.; Roth, W.; Wong, P.; Nelson, A.; Farr, A.; Deussing, J.; Villadangos, J.A.; Ploegh, H.; Peters, C.; Rudensky, A.Y. Cathepsin L: Critical Role in Ii Degradation and CD4 T Cell Selection in the Thymus. Science 1998, 280, 450–453. [Google Scholar] [CrossRef]
- Gommeaux, J.; Grégoire, C.; Nguessan, P.; Richelme, M.; Malissen, M.; Guerder, S.; Malissen, B.; Carrier, A. Thymus-Specific Serine Protease Regulates Positive Selection of a Subset of CD4+ Thymocytes. Eur. J. Immunol. 2009, 39, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.N.; Park, J.K.; Lee, J.R.; Oh, S.O.; Baek, S.Y.; Kim, B.S.; Yoon, S. Characterization of the Expression of Cytokeratins 5, 8, and 14 in Mouse Thymic Epithelial Cells during Thymus Regeneration Following Acute Thymic Involution. Anat. Cell Biol. 2011, 44, 14. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB Signaling Is Key in the Wound Healing Processes of Silk Fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Shi, T.; Lu, L. Epidermal Growth Factor (EGF)-induced Corneal Epithelial Wound Healing through Nuclear Factor κB Subtype-regulated CCCTC Binding Factor (CTCF) Activation. J. Biol. Chem. 2013, 288, 24363–24371. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.S.; Kruisbeek, A.M. The Role of LFA-1/ICAM-1 Interactions during Murine T Lymphocyte Development. J. Immunol. 1991, 147, 2852–2859. [Google Scholar] [CrossRef]
- Salomon, D.R.; Crisa, L.; Mojcik, C.F.; Ishii, J.K.; Klier, G.; Shevach, E.M. Vascular Cell Adhesion Molecule-1 Is Expressed by Cortical Thymic Epithelial Cells and Mediates Thymocyte Adhesion. Implications for the Function of α4β1 (VLA4) Integrin in T-Cell Development. Blood 1997, 89, 2461–2471. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, N.; Guo, Z.; Chi, F.; Song, Y.; Zhu, X. Wnt4 Signaling is Associated with the Decrease of Proliferation and Increase of Apoptosis During Age-Related Thymic Involution. Mol. Med. Rep. 2015, 5, 7568–7576. [Google Scholar] [CrossRef]
- Zhu, F.; Hu, Y. Integrity of IKK/NF-κB Shields Thymic Stroma That Suppresses Susceptibility to Autoimmunity, Fungal Infection, and Carcinogenesis. Bioessays 2018, 4, e1700131. [Google Scholar] [CrossRef]
- Sikora, M.J.; Jacobsen, B.M.; Levine, K.; Chen, J.; Davidson, N.E.; Lee, A.V.; Alexander, C.M.; Oesterreich, S. WNT4 Mediates Estrogen Receptor Signaling and Endocrine Resistance in Invasive Lobular Carcinoma Cell Lines. Breast Cancer Res. 2016, 1, 92. [Google Scholar] [CrossRef]
- Subhan, F.; Kang, H.Y.; Lim, Y.; Ikram, M.; Baek, S.-Y.; Jin, S.; Jeong, Y.H.; Kwak, J.Y.; Yoon, S. Fish Scale Collagen Peptides Protect against CoCl2/TNF-α-Induced Cytotoxicity and Inflammation via Inhibition of ROS, MAPK, and NF-κB Pathways in HaCaT Cells. Oxid. Med. Cell. Longev. 2017, 2017, 9703609. [Google Scholar] [CrossRef]
- Barda-Saad, M.; Rozenszajn, L.A.; Globerson, A.; Zhang, A.S.; Zipori, D. Selective Adhesion of Immature Thymocytes to Bone Marrow Stromal Cells: Relevance to T Cell Lymphopoiesis. Exp. Hematol. 1996, 24, 386–391. [Google Scholar] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.Y.; Song, W.H.; Lim, Y.S.; Lee, C.; Rajbongshi, L.; Hwang, S.Y.; Kim, B.S.; Lee, D.; Song, Y.J.; Kim, H.-G.; et al. Fish Collagen Peptides Enhance Thymopoietic Gene Expression, Cell Proliferation, Thymocyte Adherence, and Cytoprotection in Thymic Epithelial Cells via Activation of the Nuclear Factor-κB Pathway, Leading to Thymus Regeneration after Cyclophosphamide-Induced Injury. Mar. Drugs 2023, 21, 531. https://doi.org/10.3390/md21100531
Lee DY, Song WH, Lim YS, Lee C, Rajbongshi L, Hwang SY, Kim BS, Lee D, Song YJ, Kim H-G, et al. Fish Collagen Peptides Enhance Thymopoietic Gene Expression, Cell Proliferation, Thymocyte Adherence, and Cytoprotection in Thymic Epithelial Cells via Activation of the Nuclear Factor-κB Pathway, Leading to Thymus Regeneration after Cyclophosphamide-Induced Injury. Marine Drugs. 2023; 21(10):531. https://doi.org/10.3390/md21100531
Chicago/Turabian StyleLee, Do Young, Won Hoon Song, Ye Seon Lim, Changyong Lee, Lata Rajbongshi, Seon Yeong Hwang, Byoung Soo Kim, Dongjun Lee, Yong Jung Song, Hwi-Gon Kim, and et al. 2023. "Fish Collagen Peptides Enhance Thymopoietic Gene Expression, Cell Proliferation, Thymocyte Adherence, and Cytoprotection in Thymic Epithelial Cells via Activation of the Nuclear Factor-κB Pathway, Leading to Thymus Regeneration after Cyclophosphamide-Induced Injury" Marine Drugs 21, no. 10: 531. https://doi.org/10.3390/md21100531
APA StyleLee, D. Y., Song, W. H., Lim, Y. S., Lee, C., Rajbongshi, L., Hwang, S. Y., Kim, B. S., Lee, D., Song, Y. J., Kim, H.-G., & Yoon, S. (2023). Fish Collagen Peptides Enhance Thymopoietic Gene Expression, Cell Proliferation, Thymocyte Adherence, and Cytoprotection in Thymic Epithelial Cells via Activation of the Nuclear Factor-κB Pathway, Leading to Thymus Regeneration after Cyclophosphamide-Induced Injury. Marine Drugs, 21(10), 531. https://doi.org/10.3390/md21100531