Abstract
The Arctic-derived fungus Eutypella sp. D-1 can produce numerous secondary metabolites, and some compounds exhibit excellent biological activity. Seven pimarane-type diterpenes, including three new compounds eutypellenone F (1), libertellenone Y (2), and libertellenone Z (3), and four known compounds (4–7), were isolated from fermentation broth of Eutypella sp. D-1 by the OSMAC strategy of adding ethanol as a promoter in the culture medium. Compound 2 has a rare tetrahydrofuran-fused pimarane diterpene skeleton. The anti-inflammatory activity of all compounds was evaluated. Compounds 3–6 showed a significant inhibitory effect on cell NO release at 10 μmol/L by in vitro experiments, of which 3–5 had inhibitory rates over 60% on nitric oxide (NO) release. Subsequently, the anti-inflammatory activity of 3–5 was evaluated based on a zebrafish model, and the results showed that 3 had a significant inhibitory effect on inflammatory cells migration at 40 μmol/L, while 4 and 5 had a significant inhibitory effect at 20 μmol/L. Moreover, compounds 3–5 have the same conjugated double bond structure, which may be an important group for these compounds to exert anti-inflammatory activity.
1. Introduction
Inflammation is a reaction caused by organism damage, which is a way for higher animals to combat infection and trauma. The inflammation can remove harmful factors and damaged tissues and restore the organism to normal [1,2,3]. However, chronic and intense inflammation can participate in the pathological processes of many diseases, such as arthritis, tumors, and metabolic syndrome [4]. Therefore, the development of anti-inflammatory drugs possesses great significance. Currently, the commonly used anti-inflammatory drugs in the clinic are still insufficient, and both of them have obvious adverse reactions [5]. Consequently, there is an urgent need to search for some anti-inflammatory leading drugs with efficient and low toxicity.
Microorganisms can produce a wide variety of secondary metabolites (e.g., polyketides, terpenes, and non-ribosomal peptides), some of which show excellent biological activity, such as antibacterial, anti-tumor, and immunoregulation activities [6,7,8]. Due to the extreme living environment (e.g., bitter cold and hypoxia), polar fungi could induce the production of secondary metabolites with novel skeletons and rich biological activity [9,10]. Eutypella sp. D-1, a fungus isolated from high-latitude areas in the Arctic, can produce structurally diverse secondary metabolites; for instance, terpenes, cytochalasins, steroids, lactones, etc. [11,12,13].
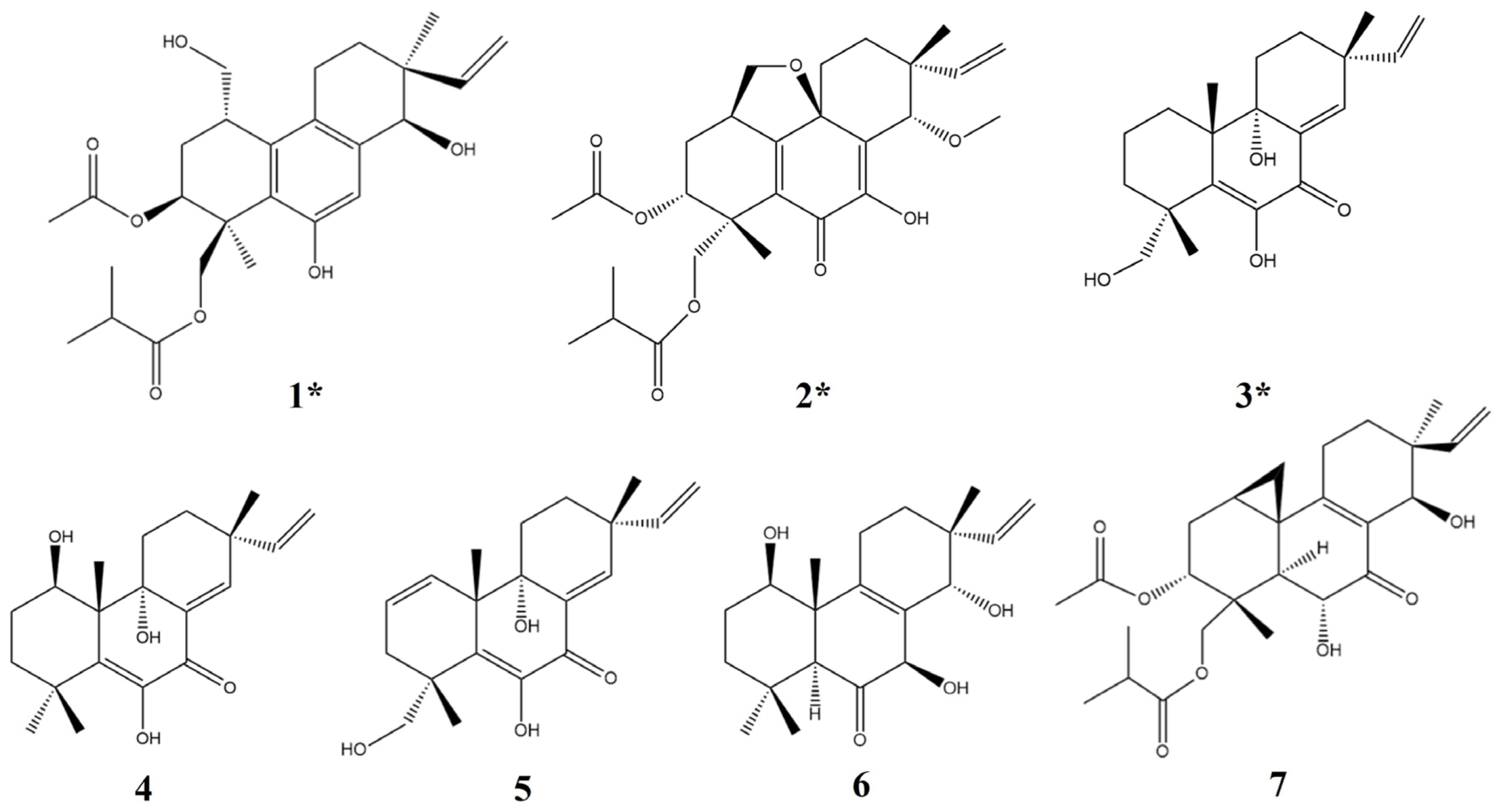
The pimarane-type diterpenes are the main secondary metabolites of Eutypella sp. D-1, some of which showed significant anti-tumor activity, such as libertellenone H [14,15,16]. Through further research, it was demonstrated that the addition of ethanol can prominently increase the yield of libertellenone H. Transcriptome analysis showed that the addition of ethanol could increase the expression of genes related to terpene biosynthesis and enhance the activity of rate-limiting enzymes related to the mevalonate pathway, which may promote an increment in the types and yield of terpenes in Eutypella sp. D-1 [17]. Consequently, in this study, seven pimarane-type diterpenes were obtained from Eutypella sp. D-1 fermentation broth by adding ethanol (Figure 1), including the three new compounds eutypellenone F (1), libertellenone Y (2), and libertellenone Z (3), with four known compounds (4–7). Meanwhile, based on the extensive biological activities of terpene, the anti-inflammatory activity is also evaluated in vivo and in vitro.
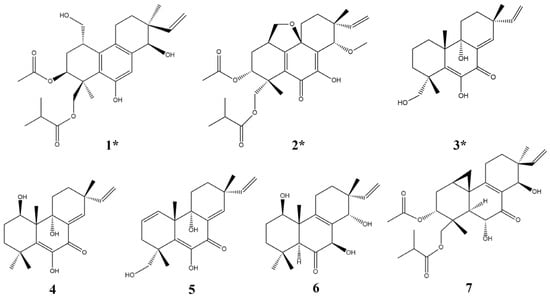
Figure 1.
Structures of compounds 1–7 (* new compounds).
2. Results and Discussion
2.1. Structural Analysis of Compounds
Eutypellenone F (1) was isolated as a white powder. The molecular formula was determined to be C26H36O7 by high-resolution electrospray ionization mass spectroscopy (HRESIMS), implying nine degrees of unsaturation. The ultraviolet (UV) spectrum exhibited absorption at λmax 206 nm. The 1H-NMR and 13C-NMR data are shown in Table 1 and Supplementary Materials Figures S1 and S2. The 1H-NMR data display three characteristic methyl proton signals at δH 1.18 (3H, s), 1.54 (3H, s), and 2.17 (3H, s); one olefinic proton at δH 6.86 (1H, s) and a terminal vinyl group at δH 5.83 (1H, q, J = 7.24 Hz) and 5.21 (2H, m). Analysis of the 13C-NMR data and DEPT spectrum revealed a total of 26 carbons including five methyls, six methylenes, six hypomethylenes, and nine quaternary carbon signals. Four carbons connecting oxygen atom at δC 63.96, 65.42, 74.90, and 75.42, and two ketone carbonyl signals at δC 171.50 and 176.80 could be observed from the 13C-NMR.

Table 1.
1H and 13C-NMR data of compounds 1–3 in CDCl3.
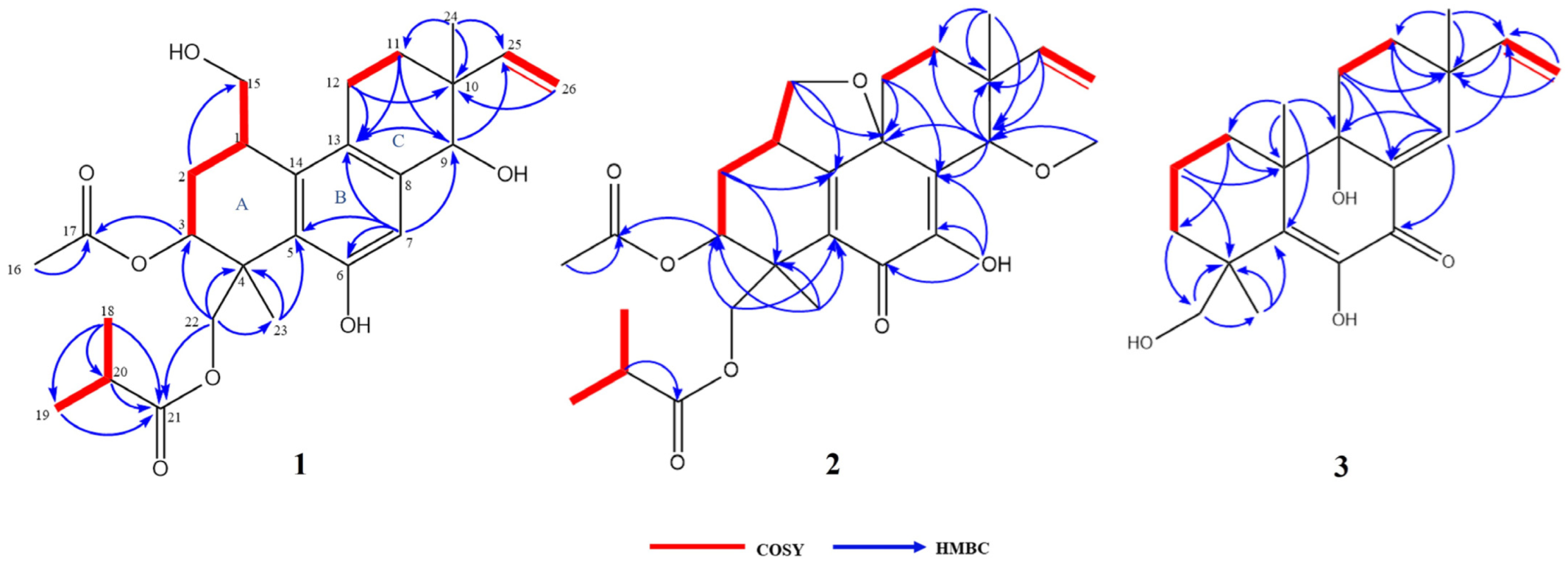
The COSY correlations of H-1/H-2b, H-1/H-15, H-2b/H-3, H-11a/H-12, H-18/H-20, and H-19/H-20 revealed the presence of four isolated spin systems: C-15/C-1/C-2/C-3, C-11/C-12, C-25/C-26, and C-18/C-20/C-19 (Figure 2). The diagnostic HMBC correlations from H-9 to C-13/C-25, H-11a to C-9/C-12/C-13, and H-12 to C-10/C-11/C-13 indicated the existence of a ring C. In addition, the correlations from H-18 to C-19/C-20/C-21, H-23 to C-3/C-4/C-5/C-22, and H-24 to C-9/C-10/C-11/C-25 indicated the presence of a pimarane-type diterpene. Due to the presence of four oxygenated carbon signals, the oxygenated carbon at C-9/C-15 had to be substituted with a hydroxy group to satisfy the molecular formula (Figure 2). All of these spectroscopic characteristics revealed that 1 is a member of the libertellenone class [14].
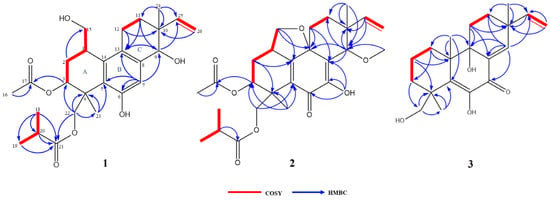
Figure 2.
COSY and key HMBC correlations of compounds 1–3.
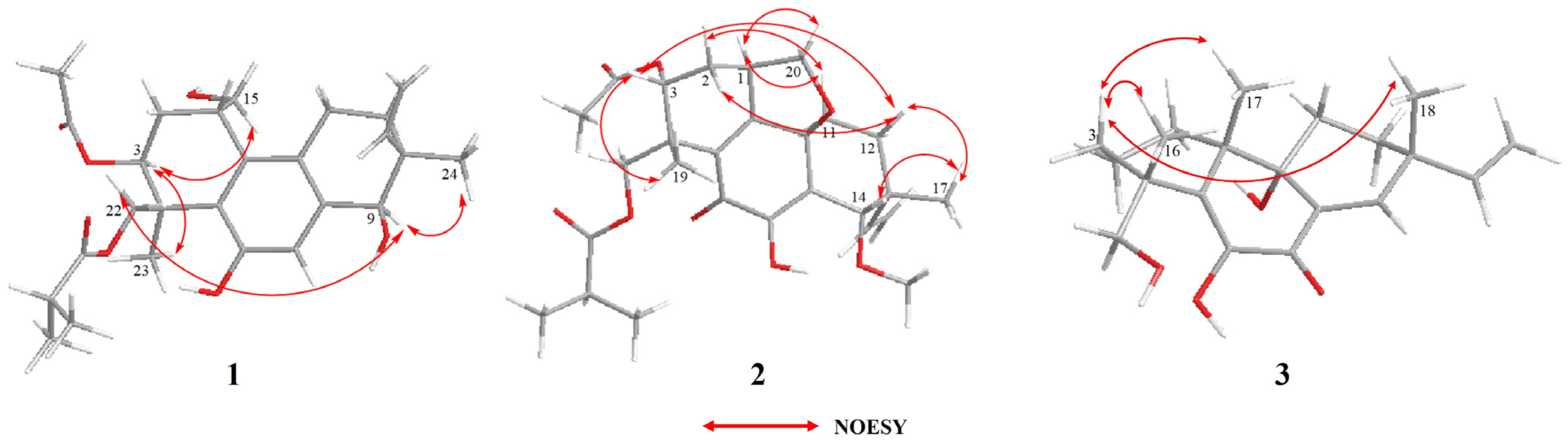
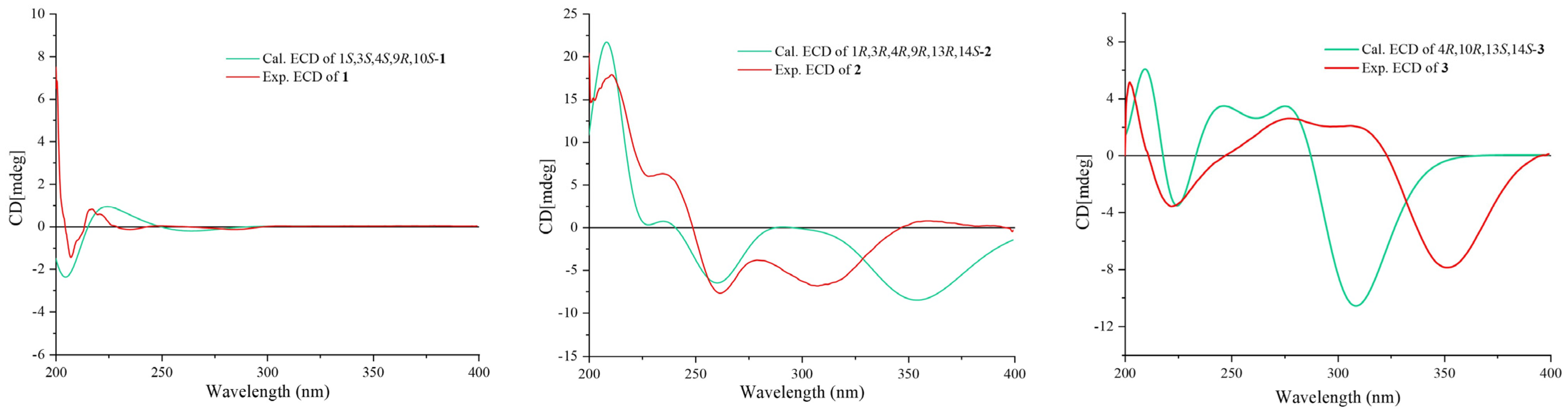
Once the planar structure was established, its relative configuration was addressed by NOESY experiments (Figure 3). The NOESY cross-peaks of H-3/H-15 and H-3/H-23 indicated the same orientation of these protons. Correspondingly, H-9/H-22a/H-24 oriented in the other direction could be obtained. Therefore, the relative structure was determined. In order to determine the whole absolute configurations of 1, the quantum chemical electronic circular dichroism (ECD) calculation method was employed. The negative Cotton effect (CE) at 205 nm in the calculated spectrum of the 1S, 3S, 4S, 9R, 10S enantiomer approximately matched the CE observed in the experimental ECD spectrum of 1 (Figure 4), enabling assignment of the absolute configurations of 1 as shown.
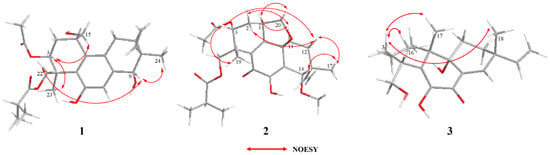
Figure 3.
Key NOESY correlations of compounds 1–3.
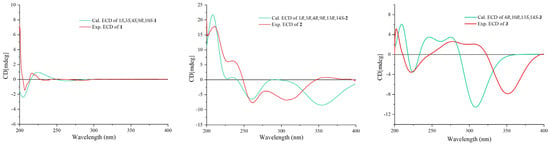
Figure 4.
Calculated and experimental ECD spectra of compounds 1–3.
Libertellenone Y (2) was isolated as a yellow oil. The molecular formula was determined to be C27H36O8 by HRESIMS, implying 10 degrees of unsaturation. The UV spectrum exhibited absorption at λmax 205 and 250 nm. The 1H-NMR and 13C-NMR data are shown in Table 1 and Supplementary Materials Figures S11 and S12. The 1H-NMR data display three characteristic methyl proton signals at δH 1.20 (3H, s), 1.36 (3H, s), and 2.00 (3H, s). The detailed analysis of the 13C-NMR data and DEPT spectrum revealed a total of 27 carbons, including five methyls, six methylenes, five hypomethylenes, and ten quaternary carbons.
The COSY and HMBC correlations revealed that compound 2 is similar to eutypellenoid B [15], except for the replacement of a hydroxy with a methoxy group at the C-14 position (Figure 2). Once the planar structure was established, its relative configuration was addressed by NOESY experiments (Figure 3). The NOESY cross-peaks of H-1/H-11a, H-1/H-20a, and H-11a/H-2a indicated the same orientation of these protons. Correspondingly, H-2b/H-3/H-12b/H-14/H-17/H-19 oriented in the other direction could be obtained. Therefore, the relative structure was determined. In order to determine the whole absolute configurations of 2, the ECD calculation method was employed. Likewise, the experimental ECD spectrum of 2 was in good agreement with the calculated spectrum for 1R, 3R, 4R, 9R, 13R, 14S (Figure 4).
Libertellenone Z (3) was isolated as a yellow oil. The molecular formula was determined to be C20H28O4 by HRESIMS, implying seven degrees of unsaturation. The UV spectrum of 3 exhibited absorption at λmax 266 nm and 324 nm. The 1H-NMR and 13C-NMR data of 3 are shown in Table 1 and Supplementary Materials Figures S19 and S20. The 1H-NMR data of 3 display three characteristic methyl proton signals at δH 1.12 (3H, s), 1.17 (3H, s), and 1.20 (3H, s); one olefinic proton at δH 7.04 (1H, d, J = 1.86 Hz), and a terminal vinyl group at δH 5.88 (1H, q, J = 6.41 Hz) and 5.08 (2H, m). Detailed analysis of the 13C-NMR and DEPT spectrum revealed a total of 20 carbons, including three methyls, seven methylenes, two hypomethylenes, and eight quaternary carbons. Two carbons connecting oxygen atoms at δC 70.49 and 74.86, and one ketone carbonyl signal at δC 181.80 could be observed from the 13C-NMR.
The COSY and HMBC correlations revealed that the structure of 3 is similar to libertellenone A [18], except for the replacement of a hydroxy with a hydrogen atom at the C-1 position, and the replacement of a hydrogen atom with a hydroxy at C-15 position (Figure 2). Once the planar structure of 3 was established, its relative configuration was addressed by NOESY experiments (Figure 3). The NOESY cross-peaks of H-3b/H-16, H-3b/H-17, and H-3b/H-18 indicated the same orientation of these protons. Therefore, the relative structure of 3 was determined. In order to determine the whole absolute configurations of 3, the ECD calculation method was employed. The positive CE at 204 nm, and the negative CE at 220 nm and 352 nm in the calculated spectrum of the 4R, 10R, 13S, 14S enantiomer approximately matched the CE observed in the experimental ECD spectrum of 3 (Figure 4), which enabled the assignment of the absolute configurations of 3 as shown.
In addition, four known compounds (4–7) were also isolated as metabolites of Eutypella sp. D-1 (Figure 1). These compounds were identified by comparing their spectral data with spectroscopic data reported in the corresponding literature, and all of these belong to diterpenes of the pimarane type [13,14,18,19].
2.2. Evaluation of the Inhibitory Effect on the NO Release
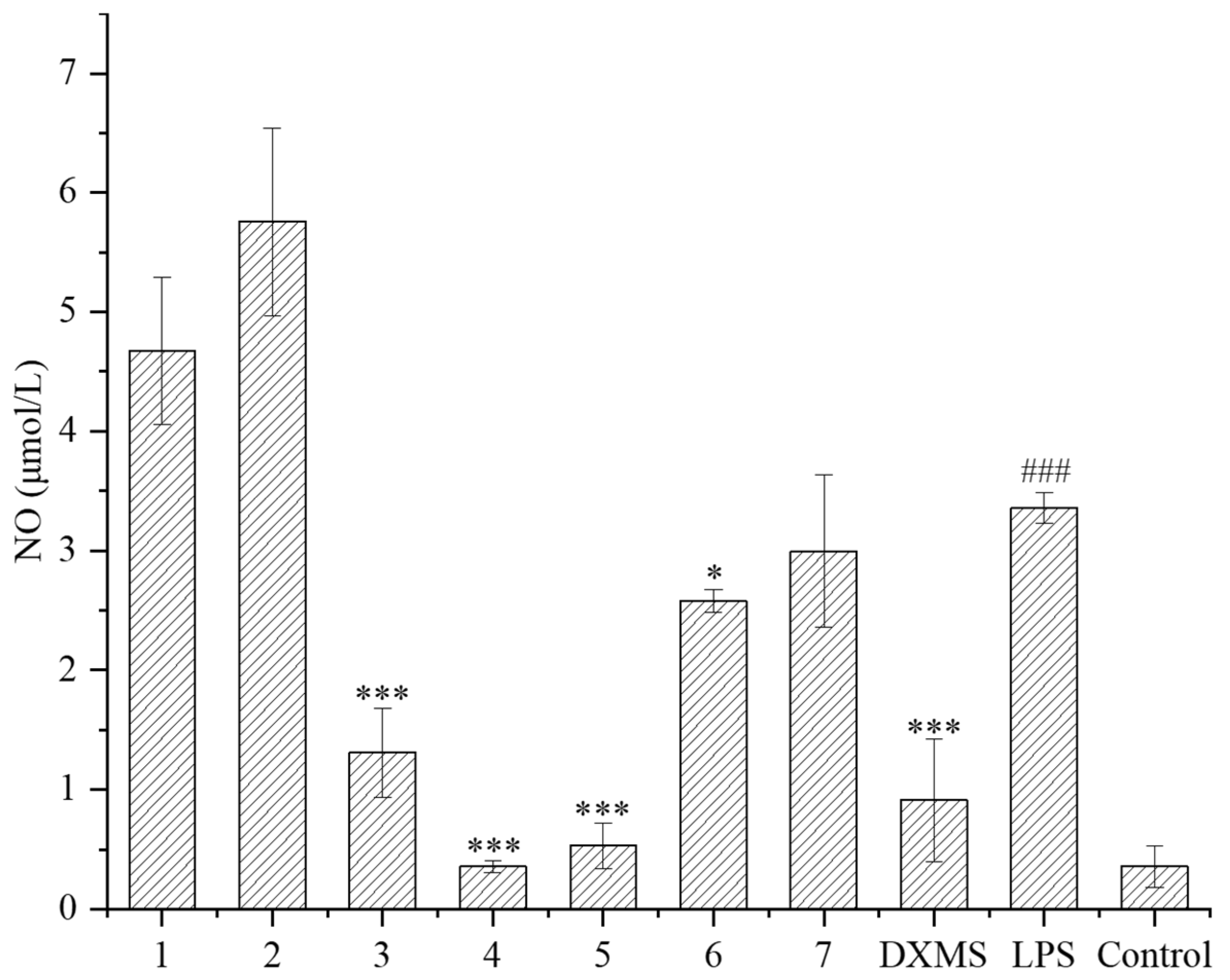
The LPS-induced RAW264.7 cell was used as an inflammatory model. RAW264.7 macrophages can stimulate inflammatory reactions under the induction of LPS, and release various inflammatory mediators such as NO, TNF-α, and interleukin-6, which can be used to evaluate the anti-inflammatory activity of secondary metabolites. In this study, the inhibitory effects of compounds on the NO released from cells were evaluated. Firstly, the safe concentration of compounds was determined. Except for compound 6, the safe concentration of all the other compounds was greater than 40 μmol/L, indicating that these compounds have little effect on cell proliferation at high concentration. Subsequently, the NO releasing was measured at 10 μmol/L of these compounds. It is shown that compounds 3–6 had a significant inhibitory effect on the NO releasing compared to the LPS treatment group (Figure 5), of which compounds 3–5 had a very noticeable inhibitory effect, close to or exceeding the effect of the positive drug dexamethasone (DXMS). Compounds 3–5 exhibited that strong inhibition rates of NO releasing exceeded 60%, and compound 4 had a maximum inhibition rate of 89%, which was already superior to the effect of the positive drug dexamethasone (72%) (Table 2).
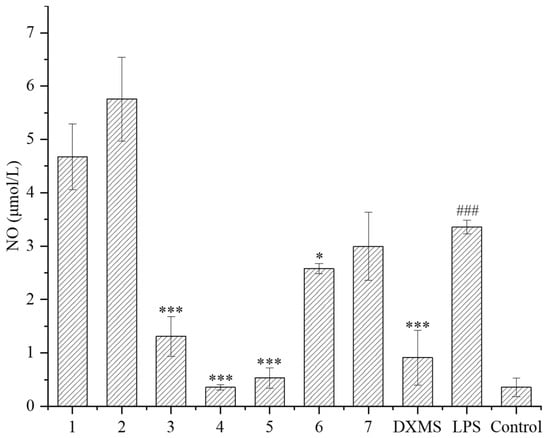
Figure 5.
NO release of LPS-induced RAW264.7 inflammatory cells under compounds treatment (DXMS: dexamethasone group. The compounds group was compared with the LPS group, * p < 0.05, and *** p < 0.001. ### p < 0.001 versus the control group).

Table 2.
The NO release inhibition rate of compounds.
2.3. Evaluation of Anti-Inflammatory Activity Based on Zebrafish Model
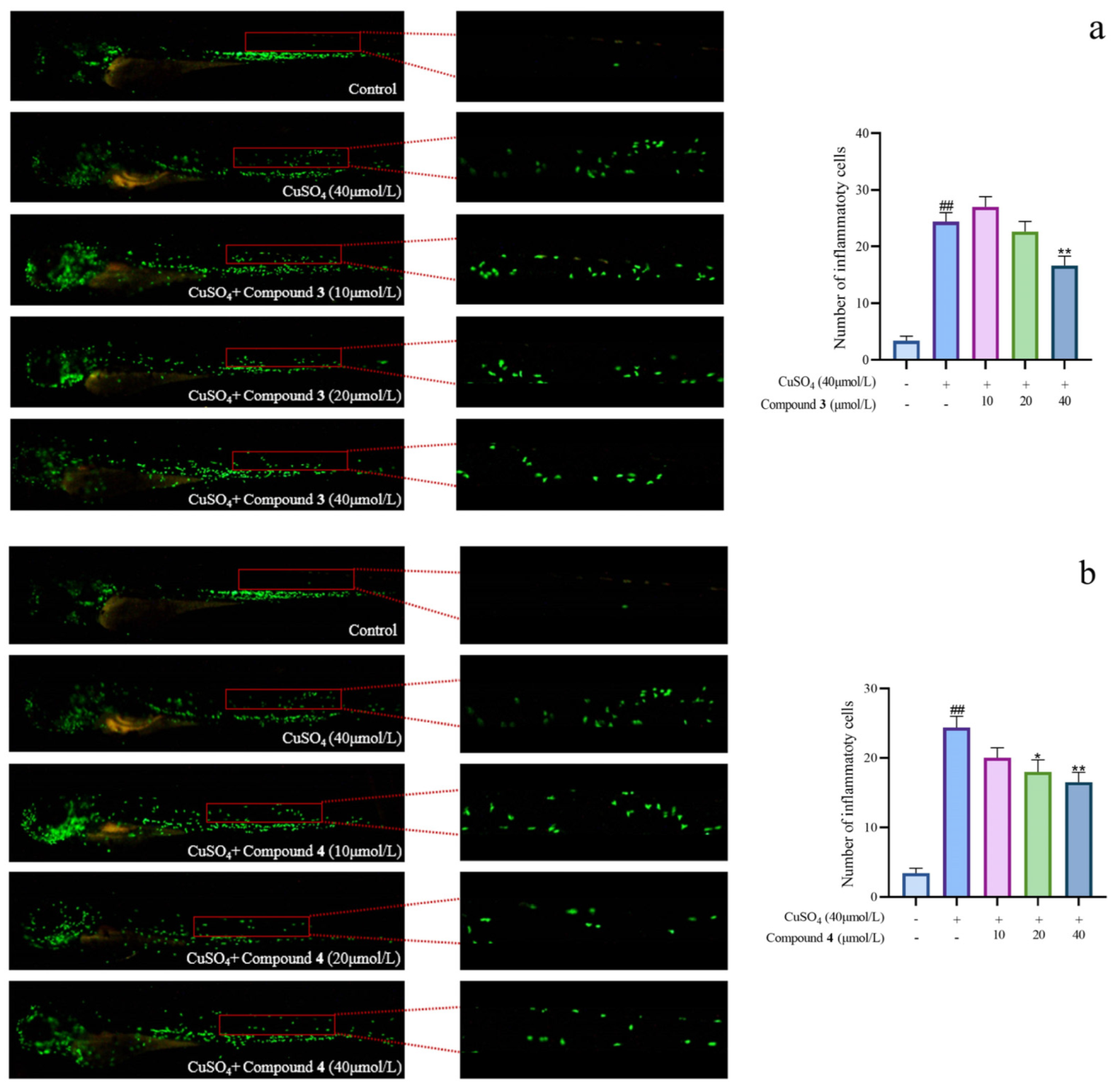
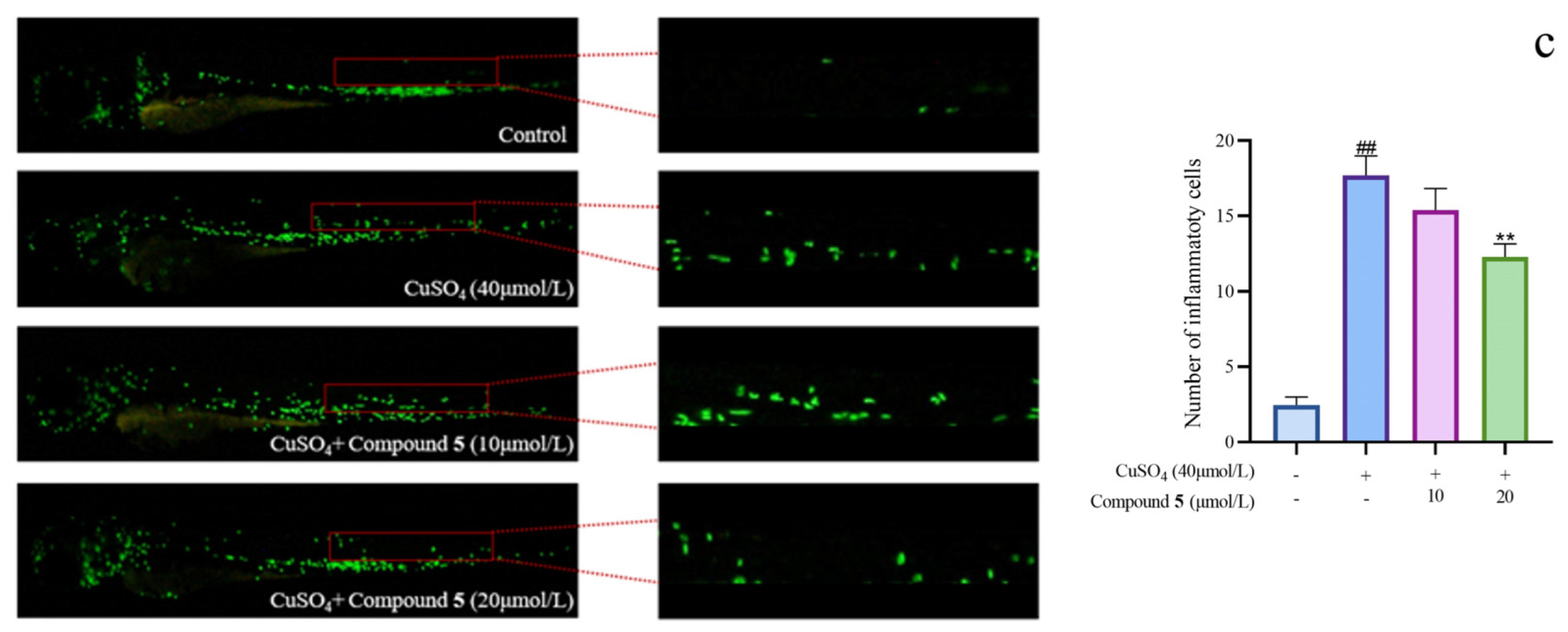
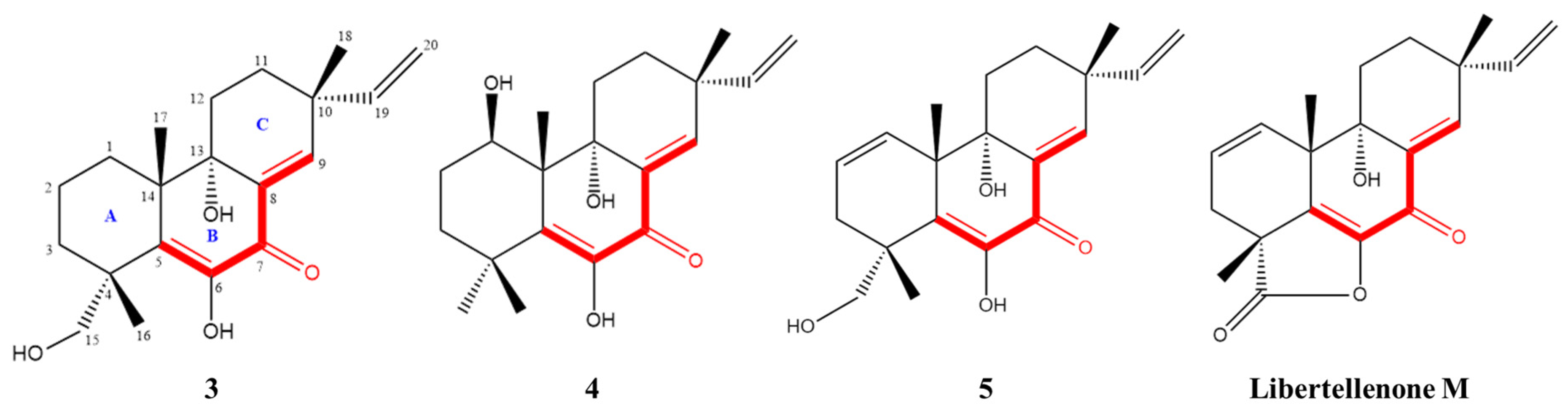
Given the significant anti-inflammatory activity of 3–5, further in-depth research was conducted based on the zebrafish model. The effect of 3–5 on the migration of zebrafish inflammatory cells induced by CuSO4 was investigated. Compared with the control group, the number of inflammatory cells migrating to the lateral line of the zebrafish of the CuSO4 model group apparently increased, indicating the success of the acute inflammation model (Figure 6). Compared with the CuSO4 group, the compound 3 treatment group clearly reduced the number of inflammatory cells migrating to the lateral line of zebrafish at 40 μmol/L (Figure 6a), while compounds 4–5 at 20 μmol/L performed likewise (Figure 6b,c). According to the in vivo and in vitro activity experiment, it was observed that compounds 3–5 all have the same conjugated double bond structure at C-5/C-6 and C-8/C-9 positions (Figure 7), with the hydroxyl group at C-6 and carbonyl group at the C-7 position. It is speculated that the conjugated structure with the carbonyl and hydroxyl groups in the B and C ring is an important pharmacophore group for their anti-inflammatory activity. Meanwhile, a study reported that the compound Libertellenone M, with the same conjugated double bond structure, also has good anti-inflammatory activity, and researchers conducted in-depth research on its anti-inflammatory mechanism [20] (Figure 7). On the other hand, similar compounds (1–2 and 6–7) without this structure fragment do not have good anti-inflammatory activity (Figure 5).
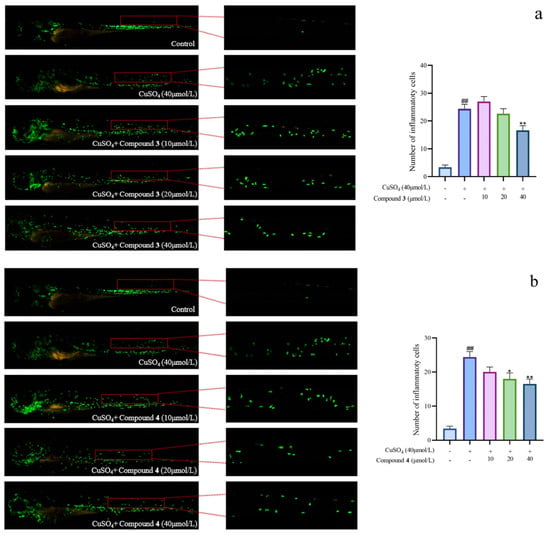
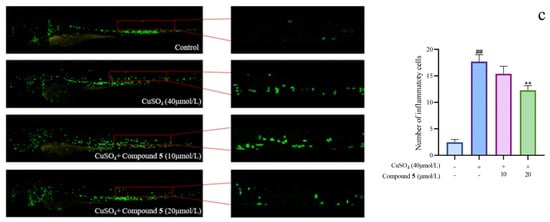
Figure 6.
The migration of zebrafish inflammatory cells induced by CuSO4 ((a–c) represent the number of inflammatory cells that migrated to the lateral line in zebrafish treated with compound 3–5, respectively. The compounds group was compared with the CuSO4 group, * p < 0.05, and ** p < 0.01. ## p < 0.01 versus the control group).
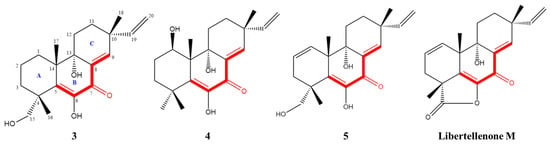
Figure 7.
Compounds with conjugated double bond structure.
3. Materials and Methods
3.1. General Experimrntal Procedures
Nuclear magnetic resonance (NMR) spectra were measured on a Bruker AMX-500 instrument (Bruker Biospin Corp., Billerica, MA, USA) at 500 MHz for 1H-NMR and 125 MHz for 13C-NMR. High-resolution electrospray ionization mass spectroscopy (HRESIMS) data were obtained on an Agilent 6210 LC/MSD TOF mass spectrometer (Agilent Technologies Inc. Lake Forest, CA, USA), and ultraviolet (UV) spectra were obtained on a UV-8000 spectrophotometer (Shanghai Metash instruments Co., Shanghai, China). Optical rotations were measured on an Anton Paar MCP 5500 (Anton Paar Co, Graz, Austria). Semi-preparative high-performance liquid chromatography (HPLC) was performed on a Waters 1525 separation module (Waters Corp., Milford, MA, USA) equipped with a Waters 2996 photodiode array detector (Waters Corp., Milford, MA, USA) by using YMC-Pack Pro C18 RS (5 µm) columns (YMC Co. Ltd., Kyoto, Japan). Purifications were conducted by Agilent LC1263 Infinity Ⅱ (Agilent Technologies Inc., CA, USA). Thin-layer chromatography analysis was run on HSGF254-precoated silica gel plates (10–40 mm, Yantai Chemical Plant, Yantai, China).
3.2. Fungal Strain
The fungus Eutypella sp. D-1 (CCTCC NO: M2013144) was isolated from the soil of London Island of Kongsfjorden of Ny-Ålesund District (altitude of 100 m) of Arctic (78°55′ N). Due to its chemical and morphological features as well as the 18S rDNA (GenBank accession number FJ430580, 100% similarity), the strain can be assigned to the genus Eutypella sp. The fungus was deposited in the PDA medium at the China Center for Type Culture Collection, Wuhan, China. The mycelium of the strain was picked and inoculated in a 50 mL flask filled with PDB (24 g/L of potato dextrose broth) medium, and cultured at 28 °C with 180 rpm for 3 days to obtain the seed culture.
3.3. Fermentation
The seed was added to 500 mL of PDB medium in a ratio of 5% (V/V), and the medium was placed in a shaker at 180 rpm under condition of 20 °C for 10 days. The ethanol was added at 72, 96, and 120 h of cultivation, respectively, with a concentration of 4%.
3.4. Extraction and Isolation
The fermentation broth was filtered using eight layers of gauze, and the filtrate was thoroughly mixed with an equal volume of ethyl acetate. The ethyl acetate layer was collected after layering, and this process was repeated three times. The ethyl acetate was evaporated using a rotary evaporator to obtain the fermentation products.
The crude extracts (58.8 g) were separated into 14 fractions (A–N) using middle-pressure liquid chromatography with MeOH/H2O (0–70 min, 10–50%; 71–310 min, and 50–100%). The fraction H was separated on an ODS (50 µm) column followed by stepwise gradient elution with MeOH/H2O (50–100%) to obtain five subfractions (H1–H9). The fraction H6 was subjected to vacuum liquid chromatography on silica gel eluting with a step gradient of a mixture of petroleum ether and EtOAc (from 50:1 to 0:1) to afford seven fractions. Fractions were purified by HPLC (MeOH/H2O, 2.0 mL/min) detected at the wavelength of 210 nm to afford compound 1 (1.7 mg), compound 2 (6.2 mg), and compound 3 (2.1 mg). The separation process of known compounds is not elaborated.
Eutypellenone F (1): white powder; [α-90.35 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 206 (4.24) nm; CD (MeOH) (∆ε) 205 (−1.99), 235 (−0.89), 285 (−0.78); 1H-NMR and 13C-NMR data, see Table 1; and HRESIMS m/z 483.2348 [M+Na]+ (calcd for C26H36O7Na, 483.2353).
Libertellenone Y (2): yellow oil; [α-15.8 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 205 (3.94), 250 (3.73), 311 (3.26) nm; CD (MeOH) (Δε) 210 (+8.28), 237 (+2.99), 278 (−1.67), 351 (+1.34) nm; 1H-NMR and 13C-NMR data, see Table 1; and HRESIMS m/z 511.2310 [M+Na]+ (calcd for C27H36O8Na, 511.2302).
Libertellenone Z (3): yellow oil; [α-78.63 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 266 (4.14) nm, 324 (4.34) nm; CD (MeOH) (∆ε) 204 (+1.93), 220 (−1.44), 275 (+0.82), 312 (+0.64), 352 (−2.56); 1H-NMR and 13C-NMR data, see Table 1; and HRESIMS m/z 355.1880 [M+Na]+ (calcd for C20H28O4Na, 355.1879).
3.5. ECD Calculations
The conformational analyses for compounds 1–3 were initially performed using Spartan’10 software (Wave-function, Inc., Irvine, CA, USA) in the MMFF94 force field. Subsequently, the conformers with a Boltzmann population of over 5% were optimized at the B3LYP/6-31+G (d, p) level by employing the conductor-like polarizable continuum model (CPCM) in MeOH. The theoretical calculation of ECD for 1–3 was calculated using the time-dependent density functional theory (TDDFT) methodology at the B3LYP/6-311++G (2d, 2p) level in MeOH, respectively. The ECD spectra were generated by the program SpecDis 1.6 (University of Würzburg, Würzburg, Germany) using a Gaussian band shape with 0.3 eV exponential half-width from dipole-length dipolar and rotational strengths.
3.6. Assay of Anti-Inflammatory Activity Based on LPS-Induced RAW264.7 Model
Mouse macrophage RAW264.7 was diluted with DMEM medium containing 10% serum to 2 × 105 cells/mL into 96 well plate, and cultured in 37 °C for 24 h. The compounds 1–7 were dissolved in DMSO and prepared in different concentrations using DMEM medium to add to the 96-well plate. CCK-8 reagent was added to the 96-well plate, and the cells were cultured for three hours. The absorbance of medium was measured at 450 nm to obtain the cell survival rate. LPS (TLR4 activator) was added to the 96-well plate with a concentration of 1 μg/mL, and the cells were cultured in 37 °C for 24 h. The absorbance of supernatant was measured at 540 nm to detect the content of NO. The formula for calculating the inhibition rate of NO release is: inhibition rate = (LPS group NO content—compound group NO content)/LPS group NO content × 100%.
3.7. Assay of Anti-Inflammatory Activity Based on Zebrafish Model
The transgenic zebrafish Tg (zlyz:EGFP) provided by Engineering Research Center of Zebrafish Models for Human Diseases and Drug Screening of Shandong Province was selected with normal development. The zebrafishes were transferred into a 24-well plate, with 12 fishes per well. The blank control group, copper sulfate model group, and compounds group (10 μmol/L, 20 μmol/L, and 40 μmol/L) were set up, and each concentration group was equipped with three repeated wells. Copper sulfate was added to all groups except the blank group after 24 h of culturing, with a concentration of 40 μmol/L. The migration of inflammatory cells was observed under a fluorescence microscope after two hours of culturing, and the number of inflammatory cells migrating to the lateral line behind the cloaca was calculated.
3.8. Statistical Analysis
All statistical analyses were conducted using SPSS 10.0 software. Data are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using Student’s t-test. All the data were generated from at least three independent experiments; p-value of <0.05 was considered statistically significant.
4. Conclusions
In summary, the use of the OSMAC strategy can effectively change the secondary metabolites of polar fungus Eutypella sp. D-1 [21]. In this study, seven diterpenes of pimarane-type were obtained from strain fermentation broth by adding ethanol as a promoter in the medium, including three new compounds eutypellenone F, libertellenone Y, and libertellenone Z, and libertellenone Y possess a rare tetrahydrofuran-fused pimarane diterpene skeleton. The anti-inflammatory activity of all compounds was evaluated using the RAW264.7 inflammation model and zebrafish model, and the results showed that compounds 3–6 possess significant anti-inflammatory activity. This work indicated that the adding of a precursor in the medium leads to the production of new secondary metabolites with biological activity in the fungus.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md21100541/s1. Figures S1–S36 spectrums of compounds 1–7. Figure S37 The colony and mycelium characteristics of Eutypella sp. D-1
Author Contributions
Conceptualization, methodology, and writing—original draft preparation, Y.N. and S.Z.; software and validation, Y.X. and S.L.; methodology, T.Z. and Y.Z.; investigation and resources, J.Z. and Z.M.; writing—review and editing, B.J.; writing—review and editing, funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (82373786).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. De. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Boshtam, M.; Asgary, S.; Kouhpayeh, S. Aptamers Against Pro- and Anti-Inflammatory Cytokines: A Review. Inflammation 2017, 40, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 123–131. [Google Scholar] [CrossRef]
- Hsemian, M.; Owlia, S.; Owlia, M.B. Review of Anti-Inflammatory Herbal Medicines. Adv. Pharmacol. Sci. 2016, 5, 9130979. [Google Scholar]
- Butler, M.S. Natural products to drugs: Natural product derived compounds in clinical trials. Nat. Prod. Rep. 2005, 22, 162–195. [Google Scholar] [CrossRef]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Pape, P.L.; Ganesan, A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, S.Y.; Cao, S.G. Antimicrobial compounds from marine fungi. Phytochem. Rev. 2021, 20, 85–117. [Google Scholar] [CrossRef]
- Yang, A.G.; Si, L.L.; Shi, Z.P.; Tian, L.; Liu, D.; Zhou, D.M.; Proksch, P.; Lin, W.H. Nitrosporeusines A and B, unprecedented thioester-bearing alkaloids from the Arctic Streptomyces nitrosporeus. Org. Lett. 2013, 15, 5366–5369. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.L.; Zhao, F.C. Secondary Metabolites from Polar Organisms. Mar. Drugs 2017, 15, 28. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.X.; Zhang, J.P.; Yu, H.B.; Liu, X.Y.; Lu, X.L. A new sesquiterpene lactone from fungus Eutypella sp. D-1. Nat. Prod. Res. 2017, 31, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Yu, H.B.; Xu, W.H.; Hu, B.; Guild, A.; Zhang, J.P. Eutypellacytosporins A-D, meroterpenoids from the Arctic fungus Eutypella sp. D-1. J. Nat. Prod. 2019, 82, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Liu, J.T.; Liu, X.Y.; Gao, Y.; Zhang, J.P.; Jiao, B.H. Pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. J. Antibiot. 2014, 67, 171–174. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, X.L.; Zhang, Y.X.; Xu, W.H.; Zhang, J.P.; Zhou, X.Y. Libertellenones O-S and Eutypellenones A and B, pimarane diterpene derivatives from the Arctic fungus Eutypella sp. D-1. J. Nat. Prod. 2018, 81, 1553–1560. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, X.L.; Xu, W.H.; Zhang, Y.X.; Qian, Y.S.; Zhang, J.P.; Lu, X.L.; Liu, X.Y. Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Mar. Drugs 2018, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.R.; Zhu, Y.P.; Yu, H.B.; Liu, X.Y.; Jiao, B.H.; Lu, X.L. Libertellenone H, a natural pimarane diterpenoid, inhibits thioredoxin system and induces ROS-mediated apoptosis in human pancreatic cancer cells. Molecules 2021, 26, 315. [Google Scholar] [CrossRef]
- Shen, C.; Xu, N.; Gao, Y.Y.; Sun, X.Y.; Yin, Y.; Cai, M.H. Stimulatory effect of ethanol on libertellenone H biosynthesis by Arctic fungus Eutypella sp. D-1. Bioprocess Biosyst. Eng. 2016, 39, 353–360. [Google Scholar] [CrossRef]
- Oh, D.C.; Jensen, P.R.; Kauffman, C.A. Libertellenones A-D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorgan. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Prathumpai, W.; Laksanacharoen, P. Pimarane diterpenes from the endophytic fungus Eutypella sp. BCC 13199. Chem. Pharm. Bull. 2011, 59, 1157–1159. [Google Scholar] [CrossRef]
- Fan, M.M.; Xiang, G.; Chen, J.W. Libertellenone M, a diterpene derived from an endophytic fungus Phomopsis sp. S12, protects against DSS-induced colitis via inhibiting both nuclear translocation of NF-κB and NLRP3 inflammasome activation. Int. Immunopharmacol 2020, 80, 106144. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.L.; Chen, J.W.; Zhang, H.W.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).