A Novel Anticancer Peptide Derived from Bryopsis plumosa Regulates Proliferation and Invasion in Non-Small Cell Lung Cancer Cells
Abstract
:1. Introduction
2. Results
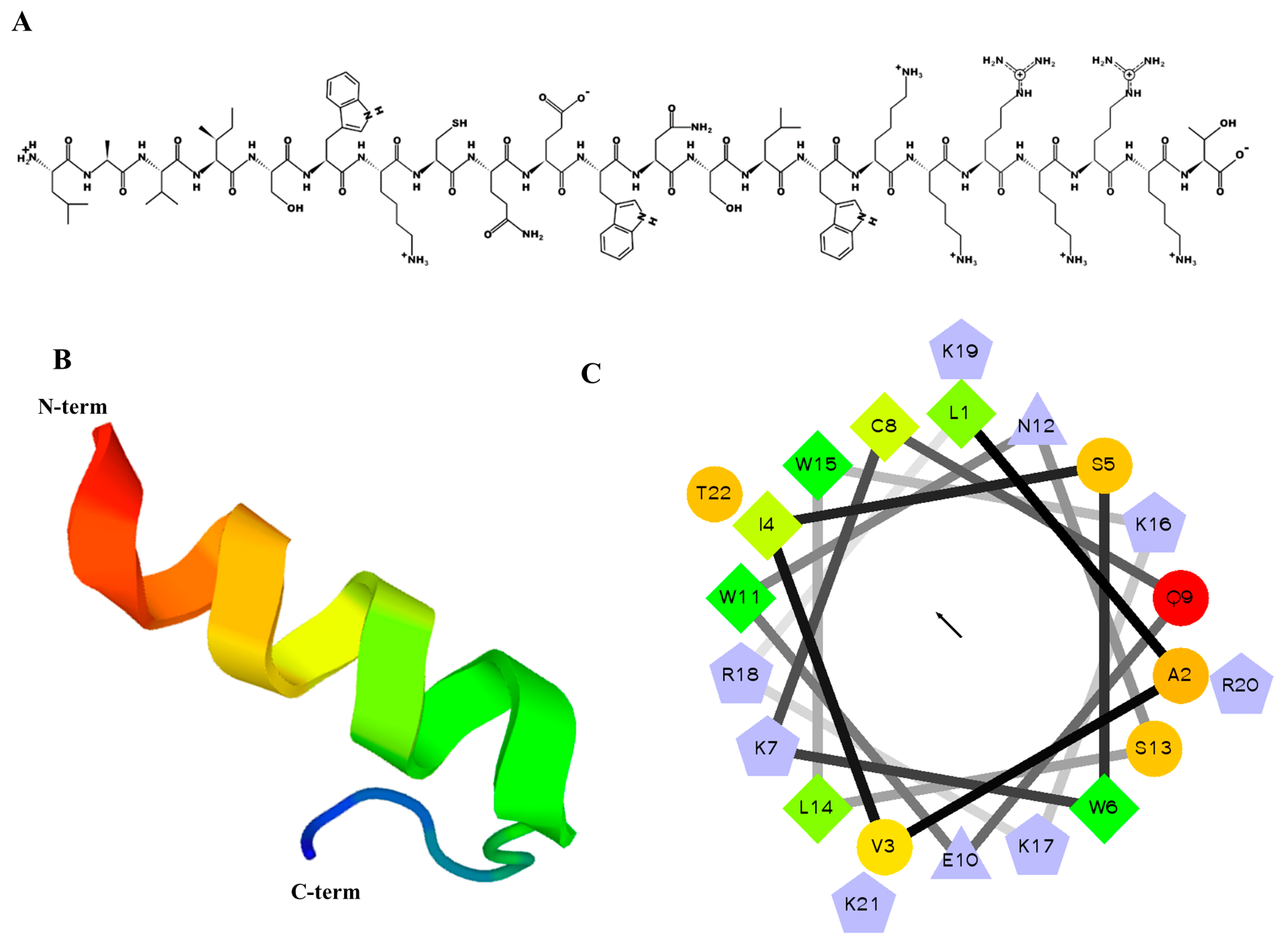
2.1. Designing, Synthesis, and Characteristics of MP06
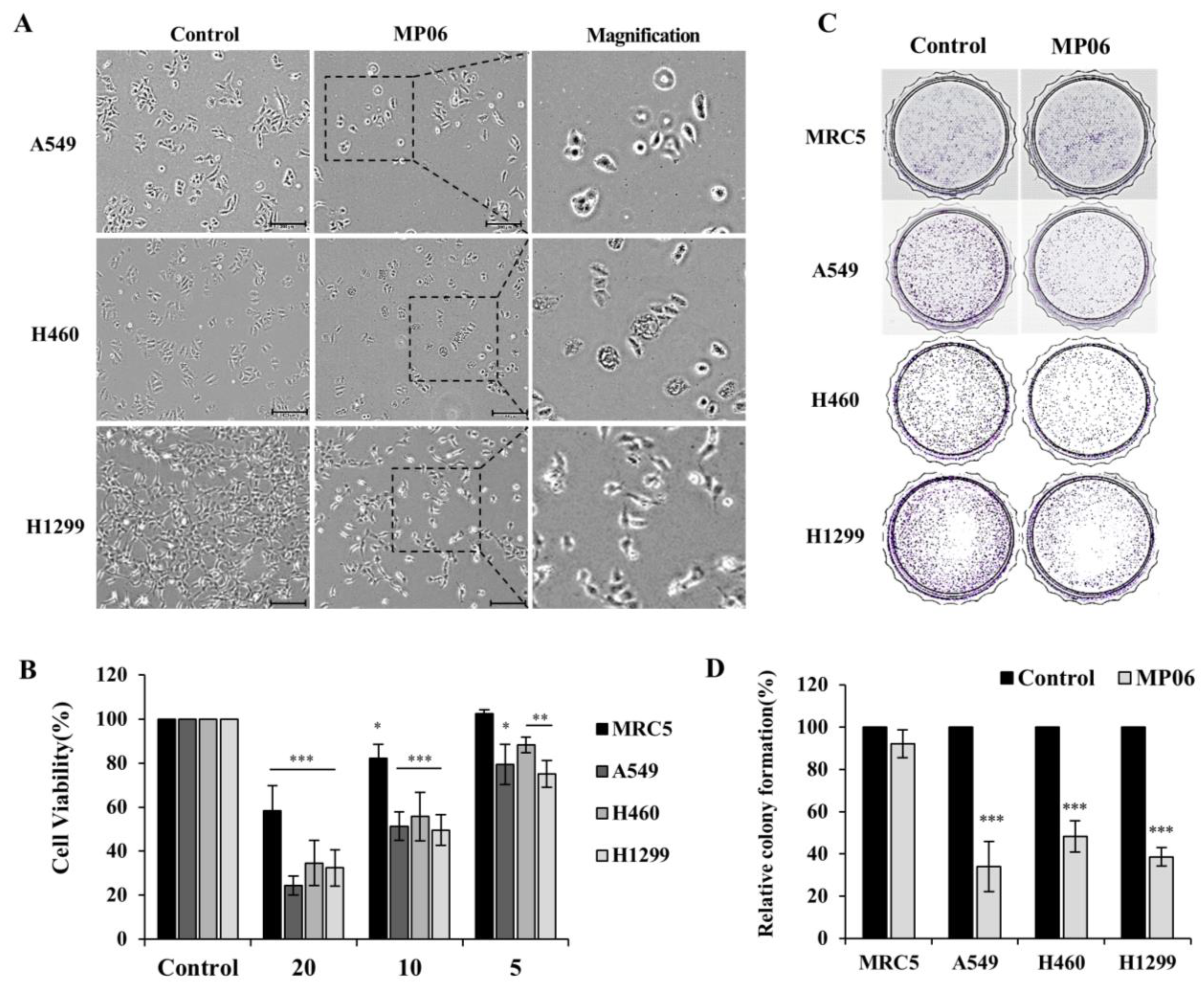
2.2. Cellular Viability in Lung Cancer Cells
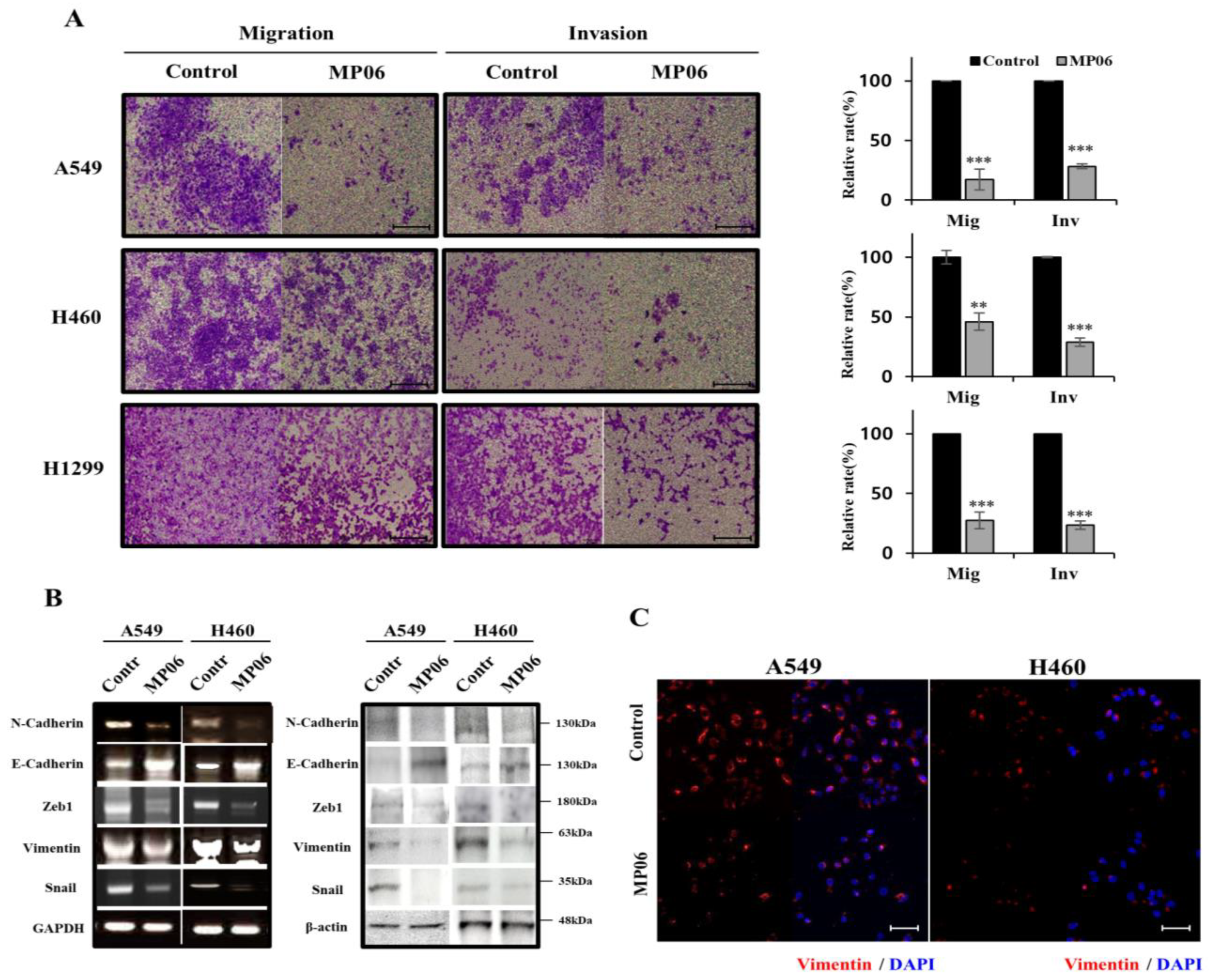
2.3. Effect of MP06 on EMT
2.4. Effect of MP06 on Apoptosis and Signaling in NSCLC Line
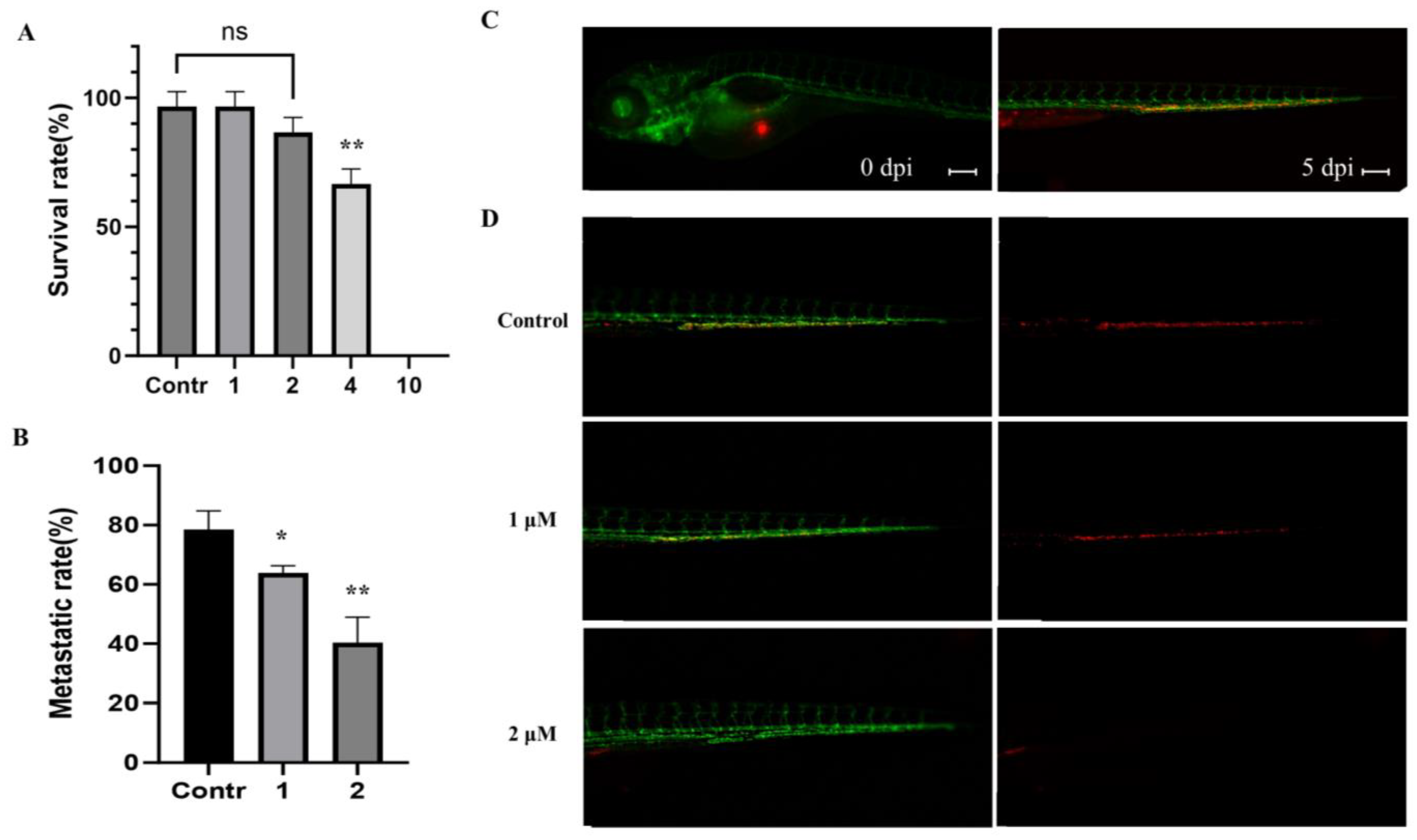
2.5. Effect of MP06 in Zebrafish Xenograft
3. Discussion
4. Materials and Methods
4.1. Peptides Design, Synthesis and Purification
4.2. Cell Culture and Cytotoxic Assay
4.3. Colony-Forming Assay
4.4. Reverse Transcription PCR (RT-PCR)
4.5. Western Blot Analysis and Immunofluorescence
4.6. Invasion and Migration Assay
4.7. Annexin V/PI Double Staining
4.8. In Vivo Test of Zebrafish Embryos and Xenograft
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bade, B.C.; Cruz, C.S.D. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Dhahbi, J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci. Rep. 2021, 11, 13323. [Google Scholar] [CrossRef] [PubMed]
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J. Hematol. Oncol. 2021, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Plygawko, A.T.; Kan, S.; Campbell, K. Epithelial–mesenchymal plasticity: Emerging parallels between tissue morphogenesis and cancer metastasis. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20200087. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, Q.-Z.; Zhang, Y.-F.; Wang, X.-R.; Cao, L.-M.; Li, N.; Zhao, L.-X.; Zhang, S.-X.; Zhuang, X.-F. Identifying the EMT-related signature to stratify prognosis and evaluate the tumor microenvironment in lung adenocarcinoma. Front. Genet. 2022, 13, 1008416. [Google Scholar] [CrossRef] [PubMed]
- Tièche, C.C.; Gao, Y.; Bührer, E.D.; Hobi, N.; Berezowska, S.A.; Wyler, K.; Froment, L.; Weis, S.; Peng, R.-W.; Bruggmann, R.; et al. Tumor initiation capacity and therapy resistance are differential features of EMT-related subpopulations in the NSCLC cell line A549. Neoplasia 2018, 21, 185–196. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Liu, L.; Niu, X. EMT-mediated acquired EGFR-TKI resistance in NSCLC: Mechanisms and strategies. Front. Oncol. 2019, 9, 1044. [Google Scholar] [CrossRef]
- Bronte, G.; Puccetti, M.; Crinò, L.; Bravaccini, S. Epithelial-to-mesenchymal transition and EGFR status in NSCLC: The role of vimentin expression. Ann. Oncol. 2019, 30, 339–340. [Google Scholar] [CrossRef]
- Farmakovskaya, M.; Khromova, N.; Rybko, V.; Dugina, V.; Kopnin, B.; Kopnin, P. E-Cadherin repression increases amount of cancer stem cells in human A549 lung adenocarcinoma and stimulates tumor growth. Cell Cycle 2016, 15, 1084–1092. [Google Scholar] [CrossRef]
- Esposito, M.; Ganesan, S.; Kang, Y. Emerging strategies for treating metastasis. Nat. Cancer 2021, 2, 258–270. [Google Scholar] [CrossRef]
- Nelson, D.B.; Mehran, R.J.; Mitchell, K.G.; Correa, A.M.; Sepesi, B.; Antonoff, M.B.; Rice, D.C. Enhanced recovery after thoracic surgery is associated with improved adjuvant chemotherapy completion for non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 279–286.e1. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Yu, W.-D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, M. DaiCee: A database for anti-cancer compounds with targets and side effect profiles. Bioinformation 2020, 16, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Hilchie, A.; Hoskin, D.; Power Coombs, M. Anticancer activities of natural and synthetic peptides. Antimicrob. Pept. Basics Clin. Appl. 2019, 1117, 131–147. [Google Scholar]
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014, 351, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Contreras, N.; Alvíz, A.; Torres, J.; Uribe, S. Bryopsis spp.: Generalities, Chemical and Biological Activities. Pharmacogn. Rev. 2019, 13, 63–70. [Google Scholar] [CrossRef]
- Ghariani, M.; Ben Saad, H.; Hamzaoui, A.; Ajela, M.; Hilali, A.; Ben Amara, I. Effect of the incorporation of polysaccharides from green alga Bryopsis plumosa on beef sausages quality. Cell. Mol. Biol. 2022, 68, 30–37. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.B.; Kim, H.; Shin, J.M.; Yoon, M.; An, H.S.; Han, J.W. Anticancer Activity of Mannose-Specific Lectin, BPL2, from Marine Green Alga Bryopsis plumosa. Mar. Drugs 2022, 20, 776. [Google Scholar] [CrossRef] [PubMed]
- Olea-Flores, M.; Zuñiga-Eulogio, M.D.; Mendoza-Catalán, M.A.; Rodríguez-Ruiz, H.A.; Castañeda-Saucedo, E.; Ortuño-Pineda, C.; Padilla-Benavides, T.; Navarro-Tito, N. Extracellular-signal regulated kinase: A central molecule driving epithelial–mesenchymal transition in cancer. Int. J. Mol. Sci. 2019, 20, 2885. [Google Scholar] [CrossRef] [PubMed]
- Kordi, M.; Borzouyi, Z.; Chitsaz, S.; Asmaei, M.H.; Salami, R.; Tabarzad, M. Antimicrobial peptides with anticancer activity: Today status, trends and their computational design. Arch. Biochem. Biophys. 2022, 733, 109484. [Google Scholar] [CrossRef]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. ACPred: A computational tool for the prediction and analysis of anticancer peptides. Molecules 2019, 24, 1973. [Google Scholar] [CrossRef] [PubMed]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the epithelial–mesenchymal transition (EMT) with cisplatin resistance. Int. J. Mol. Sci. 2020, 21, 4002. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, S.; Kim, I.-G.; Kim, R.-K.; Kahm, Y.-J.; Jung, S.-H.; Lee, J.H. Effect of Copper Chelators via the TGF-β Signaling Pathway on Glioblastoma Cell Invasion. Molecules 2022, 27, 8851. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Shin, M.K.; Jang, B.-Y.; Bu, K.-B.; Lee, S.-H.; Han, D.-H.; Oh, J.W.; Sung, J.-S. De Novo Design of AC-P19M, a Novel Anticancer Peptide with Apoptotic Effects on Lung Cancer Cells and Anti-Angiogenic Activity. Int. J. Mol. Sci. 2022, 23, 15594. [Google Scholar] [CrossRef]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Rationally designed short cationic α-helical peptides with selective anticancer activity. J. Colloid Interface Sci. 2021, 607, 488–501. [Google Scholar] [CrossRef]
- Li, L.; Vorobyov, I.; Allen, T.W. The different interactions of lysine and arginine side chains with lipid membranes. J. Phys. Chem. B 2013, 117, 11906–11920. [Google Scholar] [CrossRef] [PubMed]
- Rybczyńska-Tkaczyk, K.; Grenda, A.; Jakubczyk, A.; Krawczyk, P. Natural Bacterial and Fungal Peptides as a Promising Treatment to Defeat Lung Cancer Cells. Molecules 2023, 28, 4381. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application. Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kang, Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Podhorodecka, Z.; McCulloch, C.A. Vimentin regulates the assembly and function of matrix adhesions. Wound Repair Regen. 2021, 29, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yu, J.; Zhang, M.; Qin, F.; Lan, X. ZEB1 promotes tumorigenesis and metastasis in hepatocellular carcinoma by regulating the expression of vimentin. Mol. Med. Rep. 2019, 19, 2297–2306. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT factors and metabolic pathways in cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Shull, A.Y.; Hu, C.-A.A.; Teng, Y. Zebrafish as a model to evaluate peptide-related cancer therapies. Amino Acids 2017, 49, 1907–1913. [Google Scholar] [CrossRef]
- Gamble, J.; Elson, D.; Greenwood, J.; Tanguay, R.; Kolluri, S. The zebrafish xenograft models for investigating cancer and cancer therapeutics. Biology 2021, 10, 252. [Google Scholar] [CrossRef]
- Singh, P.; Lim, B. Targeting apoptosis in cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Podhorodecka, Z.; Ding, I.; Norouzi, M.; McCulloch, C.A. Impact of vimentin on regulation of cell signaling and matrix remodeling. Front. Cell Dev. Biol. 2022, 10, 869069. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, H.-T.; Jung, S.-H.; Han, J.W.; Jo, S.; Kim, I.-G.; Kim, R.-K.; Kahm, Y.-J.; Choi, T.-I.; Kim, C.-H.; et al. A Novel Anticancer Peptide Derived from Bryopsis plumosa Regulates Proliferation and Invasion in Non-Small Cell Lung Cancer Cells. Mar. Drugs 2023, 21, 607. https://doi.org/10.3390/md21120607
Kim H, Kim H-T, Jung S-H, Han JW, Jo S, Kim I-G, Kim R-K, Kahm Y-J, Choi T-I, Kim C-H, et al. A Novel Anticancer Peptide Derived from Bryopsis plumosa Regulates Proliferation and Invasion in Non-Small Cell Lung Cancer Cells. Marine Drugs. 2023; 21(12):607. https://doi.org/10.3390/md21120607
Chicago/Turabian StyleKim, Heabin, Hyun-Taek Kim, Seung-Hyun Jung, Jong Won Han, Seonmi Jo, In-Gyu Kim, Rae-Kwon Kim, Yeon-Jee Kahm, Tae-Ik Choi, Cheol-Hee Kim, and et al. 2023. "A Novel Anticancer Peptide Derived from Bryopsis plumosa Regulates Proliferation and Invasion in Non-Small Cell Lung Cancer Cells" Marine Drugs 21, no. 12: 607. https://doi.org/10.3390/md21120607
APA StyleKim, H., Kim, H. -T., Jung, S. -H., Han, J. W., Jo, S., Kim, I. -G., Kim, R. -K., Kahm, Y. -J., Choi, T. -I., Kim, C. -H., & Lee, J. H. (2023). A Novel Anticancer Peptide Derived from Bryopsis plumosa Regulates Proliferation and Invasion in Non-Small Cell Lung Cancer Cells. Marine Drugs, 21(12), 607. https://doi.org/10.3390/md21120607







