Abstract
Marine macroalgae (seaweeds) are important primary global producers, with a wide distribution in oceans around the world from polar to tropical regions. Most of these species are exposed to variable environmental conditions, such as abiotic (e.g., light irradiance, temperature variations, nutrient availability, salinity levels) and biotic factors (e.g., grazing and pathogen exposure). As a result, macroalgae developed numerous important strategies to increase their adaptability, including synthesizing secondary metabolites, which have promising biotechnological applications, such as UV-absorbing Mycosporine-Like Amino Acid (MAAs). MAAs are small, water-soluble, UV-absorbing compounds that are commonly found in many marine organisms and are characterized by promising antioxidative, anti-inflammatory and photoprotective properties. However, the widespread use of MAAs by humans is often restricted by their limited bioavailability, limited success in heterologous expression systems, and low quantities recovered from the natural environment. In contrast, bloom-forming macroalgal species from all three major macroalgal clades (Chlorophyta, Phaeophyceae, and Rhodophyta) occasionally form algal blooms, resulting in a rapid increase in algal abundance and high biomass production. This review focuses on the bloom-forming species capable of producing pharmacologically important compounds, including MAAs, and the application of proteomics in facilitating macroalgal use in overcoming current environmental and biotechnological challenges.
1. Introduction
Organisms are exposed to diverse levels of ultraviolet radiation (UVR: 280–400 nm) depending on the geographic location. In areas near the equator, the detected UVR levels are extremely high, while UVR levels measured at both poles are very low. Beyond latitude, the seasonal viability in UVR levels also needs to be considered regarding organisms’ capacity to adapt to the periods of high UVR that can be much higher during summer times compared to winter [1]. Additional environmental factors, such as altitude and clouds, could also impact UV levels [2].
Prolonged exposure to UVR may lead to DNA damage, resulting in cellular mutations and long-lasting negative impacts. Humans are especially sensitive to prolonged UVR exposure, leading to more incidences of skin cancers [3,4,5]. Current sunscreens are missing the sufficient sustainable features that are needed for environmental protection. Therefore, natural products (NPs) that could be isolated from species exposed to high levels of UVR are currently very attractive options with clear industrial interest. Potentially promising sources of UV-absorbing NPs come from abundant marine species that are naturally exposed to high UVR, like algae (including macroalgae and microalgae), and can be used to generate sustainable and environmentally friendly sunscreens.
Marine macroalgae (seaweeds) form the base of many marine and estuarine food webs worldwide. Most members of this polyphyletic grouping belong to one of three main clades: Chlorophyta (green algae), Phaeophyceae (brown algae), and Rhodophyta (red algae). Macroalgae can be found from polar regions to the tropics and range from inhabiting the intertidal zone to nearly 300 m in depth [6,7] in tropical waters (primarily rhodoliths, a group of coralline red algae), although most live in shallower waters of 100 m or less [6]. Macroalgae are all dependent upon specific physical parameters (including light, temperature, salinity, and nutrients) for survival, growth, and reproduction. Macroalgae serve as habitats and/or food sources for a wide variety of marine organisms, modify wave action in coastal areas and serve in blue carbon sequestration pathways [8,9,10,11]. They are also part of a >USD 13 billion dollar global aquaculture industry for human uses, including direct consumption and biomedical and pharmaceutical industries, among others [12,13,14,15]. Macroalgae have evolved to occupy a diverse suite of ecological and environmental niches, with some capable of forming algal blooms. While some species are primarily adapted to cold temperate to polar regions, other groups thrive in tropical locations. Some species have adapted to live in highly stochastic intertidal environments, with diel swings in temperature, salinity and UV exposure, while others occupy much more constant environments in subtidal habitats. Species living in more stochastic environments have evolved with a wide array of defensive compounds and mechanisms. Species in intertidal environments subject to freezing temperatures have evolved to survive the freeze–thaw cycles [16], and most brown macroalgal species have phenolic compounds that protect against a variety of biotic and abiotic stressors [17,18]. Some intertidal species can reduce photosynthetic activity when emersed, which may reduce damage from excess light [19], even though they have increased access to CO2 [20].
Many macroalgal species living in high-light environments contain mycosporine-like amino acids (MAAs), which are small, temperature- and light-stable, and water-soluble UV-absorbing compounds, with maximal absorption within the range of ~310–360 nm [12,21,22,23]. Strong photoprotective properties and the capacity to absorb light in the UV-A (315–400 nm) and UV-B (280–315 nm) ranges without the generation of harmful free radicals have been confirmed for various MAAs [24,25,26]. Dominant UV-A, which makes ~95% of UV energy reaching the Earth’s surface at moderate levels, has a stimulating role in macroalgal growth and photosynthesis, while UV-B usually has a more harmful impact on marine macroalgae [27,28]. Although UV-A can enhance algal photosynthesis in moderate doses, high levels can reduce quantum yield [29] and inhibit photosynthesis [30], which can be particularly damaging to microscopic stages [31]. Although many species thrive in physiologically stressful habitats, there is still limited understanding and ability to predict the interactive impacts of multiple abiotic stressors on macroalgae [32,33].
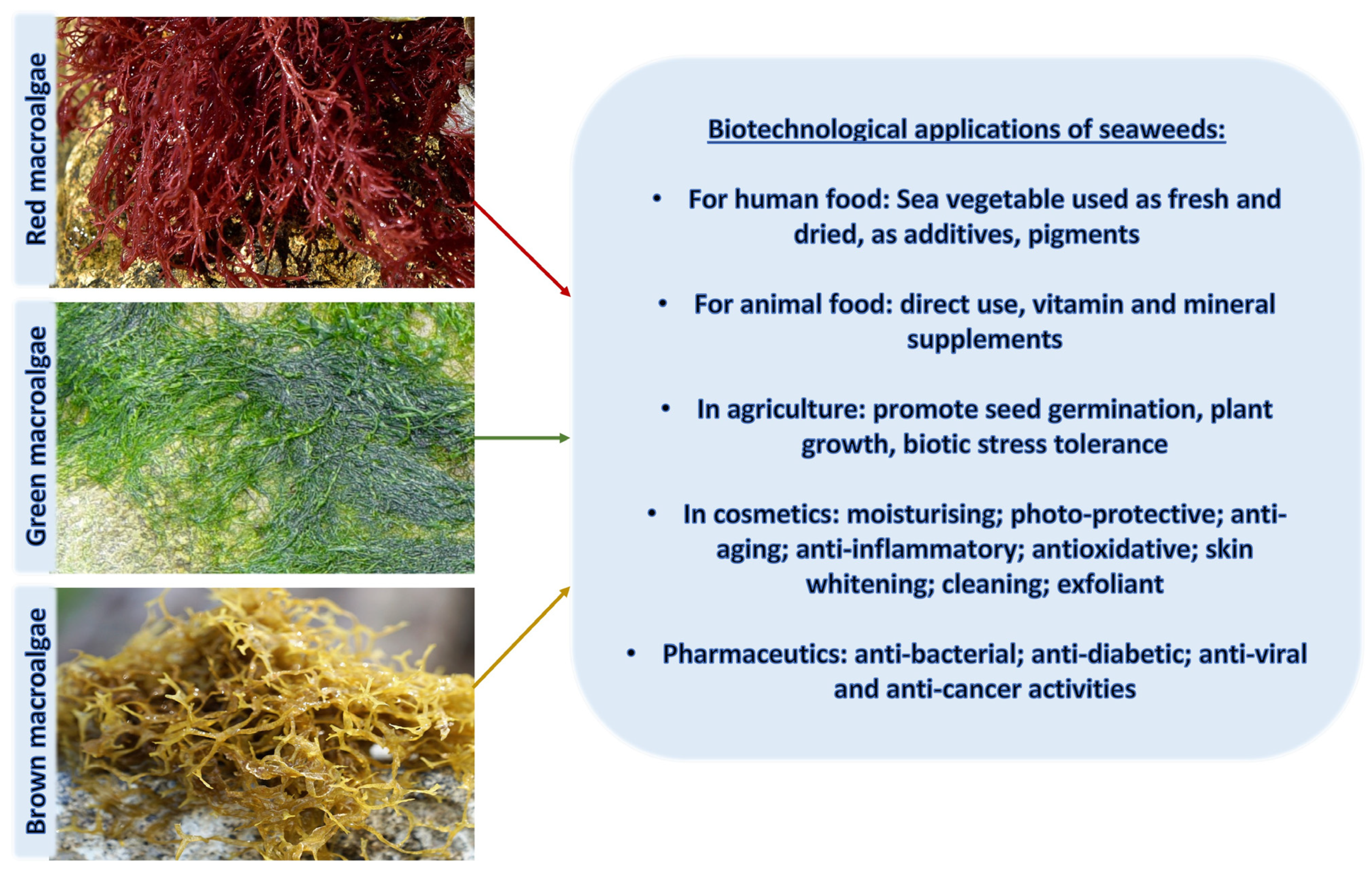
In ancient times, human uses of macroalgae started independently across the globe; Romans used it as food for animals [34], macroalgal remnants have been found in hearths in southern Chile from 12,000 BCE, and medicinal uses of macroalgae were documented nearly 5000 years ago in traditional Chinese herbal medicine [35], as well as many other cultures. Since then, a wide range of applications have been developed, and in modern times, macroalgae have been used in human and animal food, pharmaceutical and other industries, and cosmetics for skin protection due to the presence of components with anti-aging properties, photoprotective, and specifically UV-absorbing capacities (Figure 1 and Figure 2).
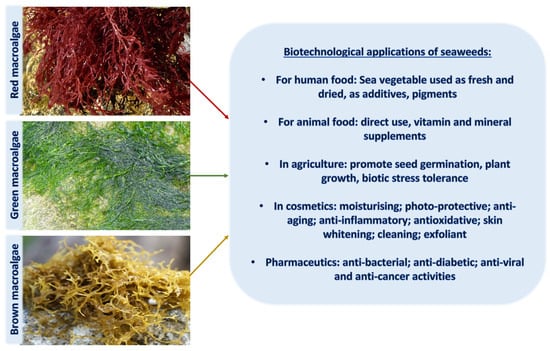
Figure 1.
An overview of biotechnological applications of macroalgae in various industries.
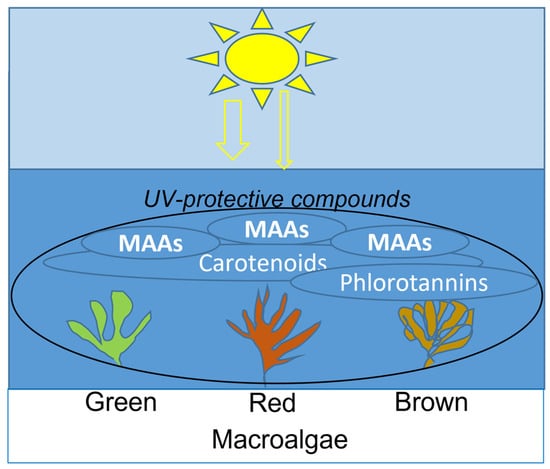
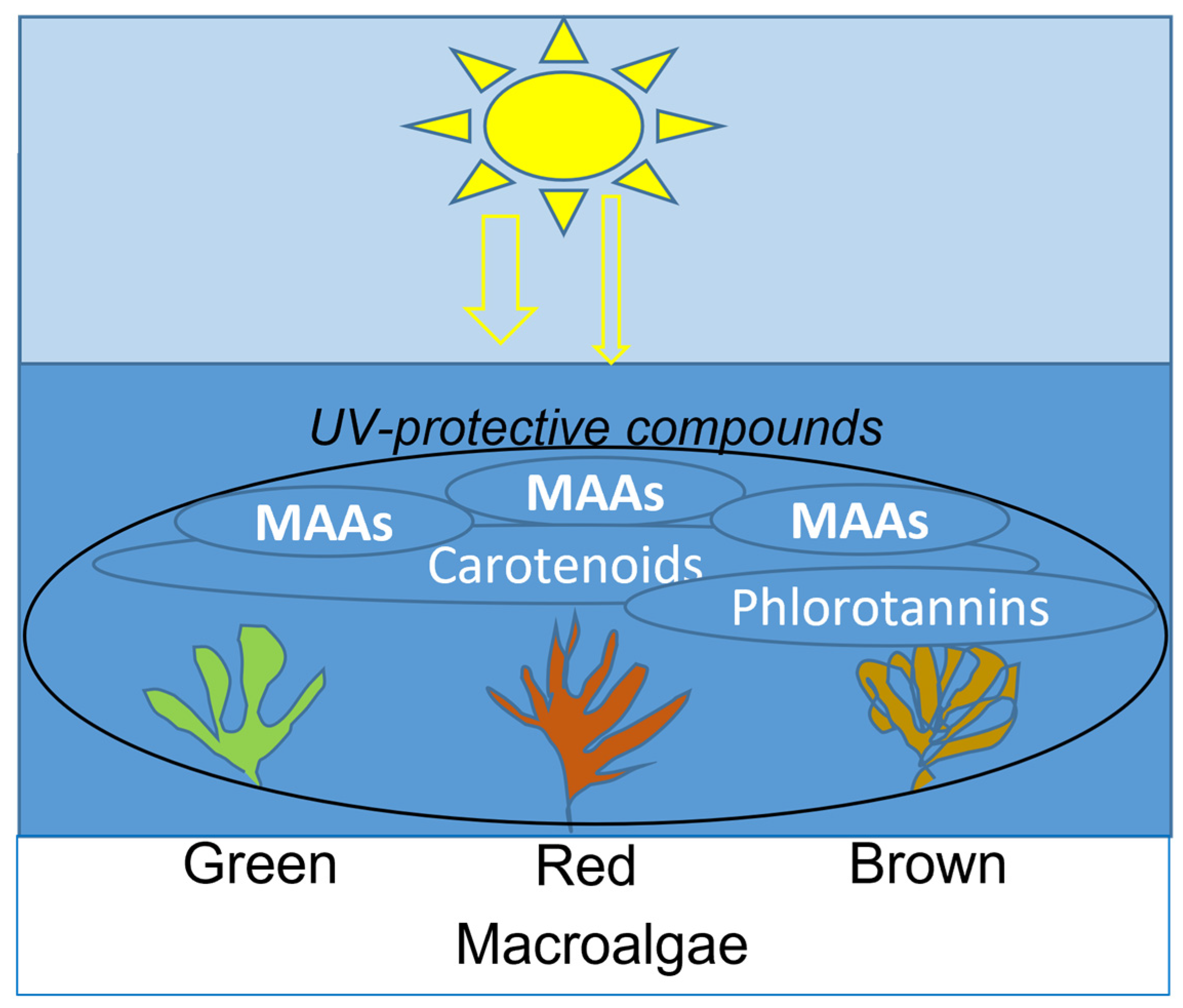
Figure 2.
Distribution of main UV-absorbing compounds in macroalgae.
Macroalgae also have an ecologically important role as bioindicators of water quality and have been utilized for bioremediation strategies and the removal of waste products, including heavy metals [36,37]. Pesticides used in local agriculture negatively impact water quality and aquatic ecosystems [38,39], and various macroalgae, such as the red macroalga, Gracilaria lemaneiformis, were successfully used for the reduction in insecticide cypermethrin concentration [40], or the brown alga Saccharina japonica, for the removal of the herbicide glyphosate in saline waters [41].
Human uses of macroalgae have typically focused on either cultivated macroalgae or those harvested from naturally growing populations. Relatively little research has focused on the vast pools of bloom-forming macroalgae, which are sometimes washed ashore (i.e., beach cast or wrack algae), as potential sources of compounds for human usage. Because some bloom-forming species are known to produce UV-absorbing compounds such as MAAs [12], blooms may represent an additional resource for the isolation and purification of these compounds. The aim of this review is to (1) provide an overview of macroalgae forming algal blooms, including the factors triggering these events; (2) determine the biotechnological capacity of selected macroalgal species; (3) evaluate the potential of using these species as a source of UV-absorbing compounds. Furthermore, this review assesses the current application of proteomics for evaluating and utilizing macroalgae capable of forming algal blooms as a sustainable resource for future sunscreens.
2. Macroalgal Blooms
2.1. Bloom Overview
Algal blooms are naturally occurring events described as a rapid increase in algal abundance in both microalgae and/or macroalgae, and usually lasting for weeks to months [42,43]. This review is focused specifically on blooms of macroalgae, as bloom-forming species can be found in all three major groups of marine macroalgae [44,45,46]. While many species can occur in both benthic (attached) and pelagic (drift) states, a subset of species typically reach sufficient quantities of drift biomass, characterized as harmful macroalgal blooms. Unlike benthic populations of macroalgae, blooms are characterized by large floating or drifting mats, leading to increased biomass via fragmentation and/or reproduction processes. While some environmental parameters, including light, temperature, and salinity, are well understood to trigger macroalgal booms [47], the exact initiations for a particular species (or group of species) to form a bloom are frequently dependent upon a complex interaction of these abiotic and other biotic factors [48].
Harmful macroalgal blooms can have significant adverse environmental and economic impacts on their surroundings. Blooms can impact coral reefs, reduce solar radiation for deeper-dwelling species, hamper gas exchange, and outcompete seagrass [49,50,51,52]. Blooms frequently occur in coastal areas, impeding marine aquaculture, fishing, recreation, and tourism activities. Bloom biomass can foul beaches, ruin fishing gear, and impede fishing, deter tourism, and impede coastal use by a variety of human stakeholders [53,54]. As the location of a particular bloom is impacted by tidal motion, wave energy, and wind direction, their distribution can shift rapidly, making sustained monitoring and/or removal efforts more challenging. In addition, blooms frequently deposit large amounts of biomass on and near coastlines (called beach-cast algae) and subsequently decompose over a period of days to weeks [10,55,56,57]. This decomposition, due to microbial activity, creates hypoxic conditions, harming fish and benthic marine invertebrate communities [58]. In addition, some species release hydrogen sulfide upon decomposition [59] and have a negative impact on carbon sediments [60]. Rather than only serving as a nuisance (or worse) to coastal communities, these beach-cast macroalgae can represent an important source of biomass for human usage. While current efforts typically focus on the removal of beach-cast algae and its subsequent deposition in landfills, the opportunity to use the algae for one (or more) technological applications remains understudied [57].
Most bloom-forming species are characterized as being able to survive increased levels of physical stress due to their presence at immediately above or below the waterline. In these environments, they are subjected to increased fluctuations in temperature and salinity (both high and low), as well as higher UV radiation exposure compared to their deeper-dwelling counterparts. Many species of red, green, and brown macroalgae with these characteristics have been documented in blooms, and these blooms are frequently deposited on shorelines, representing a potential for their harvest and utilization. Some of the most prevalent and well-studied blooms include those of the green sea lettuces (Ulva spp.), the brown algae Sargassum spp. (sometimes referred to as gulfweed), and the red algae Gracilaria spp. [45,48,61,62,63]. All three of these taxonomic groups have significant promise for the commercial utilization of bloom biomass [14,64,65,66,67]. Although not covered explicitly in this review, we recognize that many other macroalgal species can form blooms and/or be deposited on beaches in mass quantities, including the genera Asparagopsis, Ecklonia, Dasysiphonia [68,69,70], among many others (see review [57]), which indicates the widespread availability of bloom tissue for potential biotechnological uses.
2.2. Ulva Blooms
The genus Ulva contains approximately 100 taxonomically accepted species worldwide, which form thin blades and/or tubes and live predominately in shallow marine and estuarine environments (algaebase.org accessed on 7 June 2023). Blooms of several species of Ulva (also known as green tides) occur worldwide in predominantly temperate and subtropical coastal systems. Ulva blooms gained worldwide notoriety in 2008 during the Summer Olympics, when their presence threatened the Olympic sailing events [71]. In addition to the Yellow Sea and East China Sea, Ulva blooms have been reported near Brittany, France [72], Venice, Italy [73], California and Washington, USA [74], the New England region, USA; [46,75,76], South Africa [77], and the Gulf of California, Mexico [78].
Ulva blooms are frequently triggered by increases in nutrients on either localized or regional scales [79,80,81], and co-occurring Ulva species may react differently to the same environmental triggers [82]. U. prolifera blooms in the south Yellow Sea have been linked to the cultivation of nori (the red alga Pyropia—previously identified as Porphyra) [79,80]. In these blooms, the predominant mechanism of spread originates from attached U. prolifera on the floating Pyropia aquaculture rafts. These patches then dislodge and drift with prevailing surface currents into the northern Yellow Sea (see summary in Zhang et al. 2017 [80]), and their growth is enhanced by a combination of inorganic and organic nitrogen sources [83]. In Narragansett Bay, Rhode Island USA, Ulva blooms frequently contain both U. compressa and U. lacinulata (as U. rigida), and their growth rates are positively impacted by increases in dissolved inorganic nitrogen due, in part, to outputs from sewage treatment plants and changing rainfall patterns [82,84]. Although U. lacinulata and U. compressa co-occur in blooms, they vary in their growth rates, thermal tolerances, production of allelopathic chemicals, and susceptibility to herbivory [75,85].
Ulva can reproduce both sexually and vegetatively through fragmentation, with few top-down controls on its spread [75,86]. Most of the environments where Ulva blooms are found are in the intertidal/shallow subtidal, which are high-light (and coupled UV-A and UV-B) conditions [87]. In many ecosystems, Ulva bloom biomass is deposited on shorelines due to changes in wind, water currents, and/or tidal patterns [48,80], thereby exacerbating its impacts on coastal communities.
Ulva’s ability to withstand high irradiance has been well documented [87,88], although DNA and photosystem II damage can result [89]. Ulva has physiological protective mechanisms that limit the accrual of DNA damage due to UV-B [90], and Ulva can undergo photoinhibition during periods of increased UV-B [91]. In addition, some Ulva generates higher concentrations of UV-B absorbing pigments than other genera [92]. However, they lack high levels of UV-absorbing compounds like mycosporine-like amino acids and phlorotannins that are found in red and brown macroalgae [93,94].
2.3. Sargassum Blooms
The brown algal genus Sargassum contains many species found in temperate and tropical systems across the globe. However, large floating mats of pelagic Sargassum are typically found in the North Atlantic Ocean. There are two species that are most abundant in this region: Sargassum fluitans and S. natans [66]. The aptly named Sargasso Sea has been reported from the 15th century onwards [45]; it occupies a wide swath of ocean in the North Atlantic sub-tropical gyre. Although the Sargassum in the Sargasso Sea is not typically considered to be a bloom due to its longevity, it is characterized by the same qualities of having drifting, large quantities of biomass similar to those found in macroalgal blooms. The Sargassum species in the Sargasso Sea are well recognized for their importance as a habitat for numerous fish and invertebrate species as well as food sources [95]. As a genus, Sargassum can reproduce sexually or asexually; pelagic S. fluitans and S. natans reproduce via the latter mechanism, with vegetative growth and division [66], which enhances their potential rate of biomass increase.
In contrast to the long-documented Sargasso Sea, it is only within the past fifteen years that the presence of a ‘Great Atlantic Sargassum Belt’ has been identified; this Sargassum belt originates in the equatorial Atlantic, not the Sargasso Sea [96]. The annual mega bloom (‘golden tide’) extends from West Africa to the Gulf of Mexico and contains over 20 million tons of Sargassum [45,97]; when it reaches coastlines in the Caribbean and Florida (USA) and is deposited on beaches, it can wreak havoc on the environment and economies of local communities [98,99]. Nutrient enrichment is most likely the cause of these blooms, including the introduction of nutrients from increased flooding in the Amazon basin as well as periodic upwelling along the western coast of Africa; as such, golden tides can be viewed as indicators of large-scale eutrophication [100,101]. Another species, Sargassum horneri, has been reported to form blooms in the Yellow Sea, indicating that the spread of blooms by this cosmopolitan genus is occurring [61].
By living at or close to the ocean’s surface, Sargassum is subject to high levels of UV radiation. Many brown algae contain protective antioxidants, including phenolics, carotenoids, and/or isoprenoids [102] to combat the impacts of UV stressors. As a genus, Sargassum is no exception to this pattern [103,104]. S. filipendula, like other Sargassum species, has high antioxidant activity [102]. Species can also undergo structural changes due to UV exposure; S. cymosum increases the abundance of phenolic compounds and thickens its cell walls in response to UVR exposure [105]. However, assessments of the physiological properties of pelagic Sargassum can be challenging due to logistical constraints, as pelagic Sargassum does not grow well in traditional culturing conditions [106]. A recent study of S. horneri in the Yellow Sea [107] found that its photosynthetic activity is decreased by exposure to UVR, with higher tissue concentrations of malondialdehyde (MDA) in specimens exposed to UVR. At the same time, the production of carotenoids and UV-absorbing compounds was increased, indicating photoprotective mechanisms that allow Sargassum existence in high-light, high-UV conditions at the ocean’s surface. Similarly, pelagic S. natans and S. fluitans can increase carotenoid production as a result of increased light exposure [108]. In addition, pelagic S. natans and S. fluitans can release large amounts of dissolved inorganic carbon (DOC), with a high concentration of phlorotannins, a class of polyphenolics [109].
2.4. Gracilaria Blooms
The red algal genus Gracilaria is typically found in intertidal and shallow subtidal estuarine and rocky habitats in tropical and temperate zones and is well known for its bloom-forming capabilities. This highly branched genus can fragment easily and persist while floating in nearshore habitats, increasing its potential for forming large-scale blooms in coastal systems. Two species, Gracilaria tikvahiae and G. vermiculophylla, have frequently been documented in blooms in the Atlantic Ocean, ranging from Florida to Maine USA [84,110,111], Portugal [112], northern Europe [113] and the Gulf of California, Mexico [63], among other locations. These Gracilaria blooms are frequently deposited on shorelines in large amounts [48,114]. Although most studies of Gracilaria blooms have focused on these two species, other species, such as G. tenuistipitata in Shenzhen Bay, China, have also been documented as forming blooms [115]. Like their bloom-forming counterparts in the green and brown algae, these species live at or near the ocean’s surface and are thus subjected to high light and UV radiation levels. Some species of Gracilaria are also cultivated extensively, primarily for agar production or direct human consumption [116].
3. Macroalgae as a Source of UV-Absorbing Compounds
The biotechnological potential of macroalgae includes the range of molecules from polysaccharides, lipids, proteins, pigments and phenolic composites to various halogenated derivatives [117]. Macroalgae, like many other marine organisms, have been exposed to severe variations in environmental conditions that forced them to adjust, adapt and survive under various external pressures [118]. Macroalgae, like other sessile organisms, experience variable abiotic conditions, including temperature, light irradiance, salinity, and water turbidity, impacting their physiological performance [19,32,51,119,120,121,122,123,124,125]. These stressful conditions especially require adaptability to high levels of UVR, specifically ultraviolet A (UVA; 320–400 nm) and ultraviolet B (UVB; 280–315 nm) [34]. Macroalgal adaptability to extremely high light conditions is facilitated via a range of secondary metabolites, such as different photoprotective pigments (i.e., chlorophyll and carotenoids), as well as UV-absorbing compounds, such as mycosporine-like amino acids (MAAs). Compared to terrestrial plants, macroalgal species have the advantageous capacity to produce biofuels and chemicals due to the renewable nature of these resources [126]. The presence of MAAs in macroalgae was confirmed in 486 species of red algae, 45 species of green algae, and 41 species of brown algae [127]. However, the variability in MAA content and profiles in macroalgae and other marine species are noted to be strongly influenced by the environmental conditions, symbiosis, nutrient bioavailability (e.g., ammonium availability), as well as seasonal changes, especially variation in irradiance levels [128,129,130,131,132,133]. MAAs biotechnological potential is well recognized due to their pharmacological properties, including antioxidant capacities, the ability to suppress singlet oxygen-induced damage [127,129,134,135], anti-inflammatory and anti-aging properties [136,137,138]. To be able to improve the biotechnological application and use of MAAs in cosmetics and for medical purposes, a number of challenges need to be overcome, including the lack of sufficient research regarding the steps necessary for obtaining purified MAA standards, as well as overcoming strong water solubility issues and the low yields of MAAs coming from natural resources. These challenges are in some parts overpowered by an application of various methods for MAA isolation and characterizations [139] and via a use of heterologous expression systems [127,140,141,142] and/or by stimulation of MAA synthesis via specific nutrient and UVR stimulative conditions [90,128,143,144,145,146]. Recovered MAA quantities isolated from different red algae including bloom-forming Gracilaria sp. were found to be significantly impacted by extraction solvent used, with the highest yield (increased up to 32.34%) obtained when 25% of ethanol was used compared to other extraction solvents [139]. In addition, there are still significant challenges in harvesting bloom biomass from beach cast areas as well as the water, rapidly separating species of interest from harvested blooms, purifying algal tissues for the extraction of compounds of interest, and conducting all steps with an economically viable approach [57,147,148].
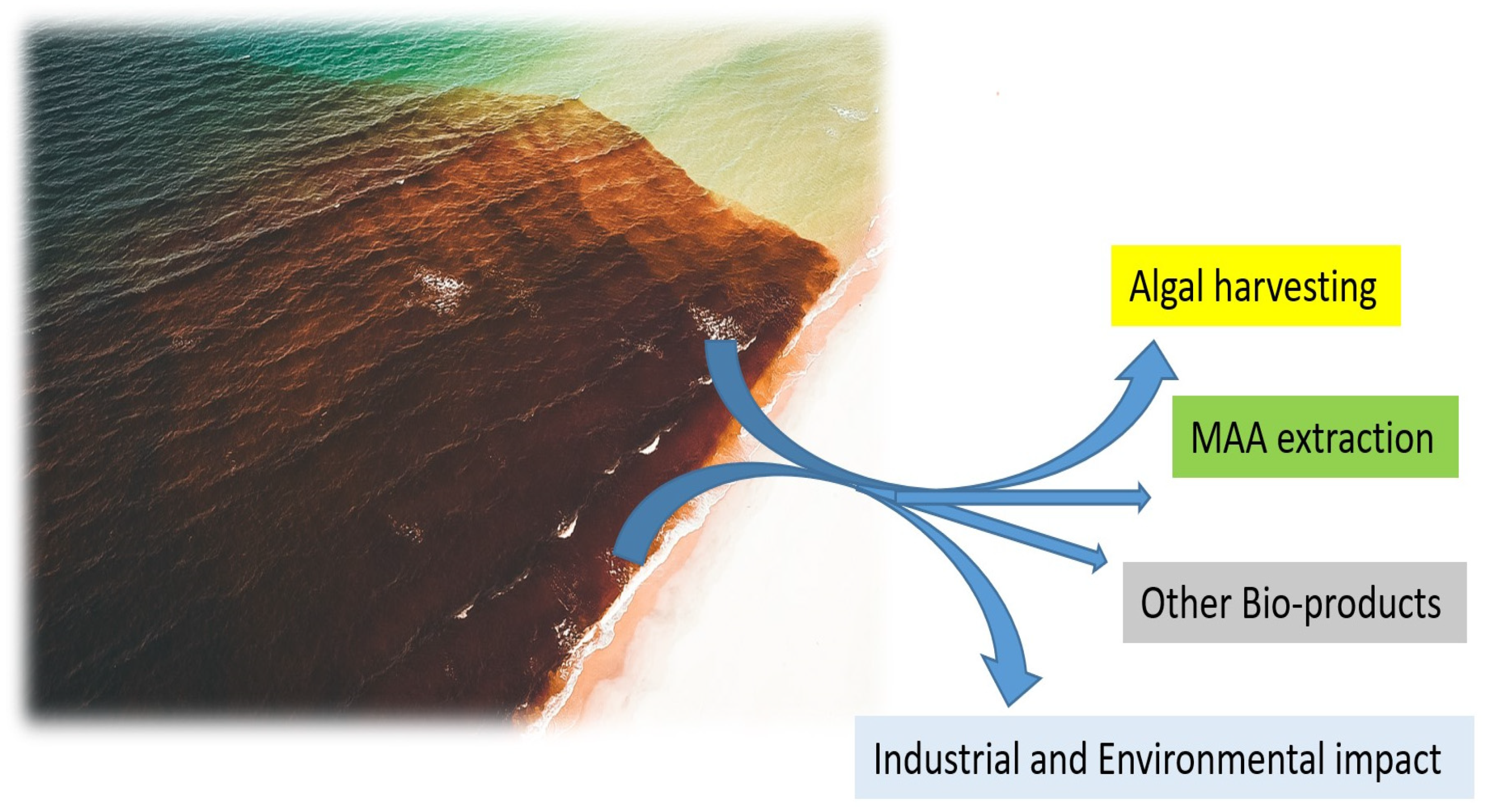
Macroalgal species capable of forming algal blooms are especially attractive for biotechnological applications due to the potential economic benefits resulting from their high biomass (Figure 3). Various bioactive compounds can be utilized for different applications ranging from UV protection, food sources, and cosmetics to eco-friendly biopesticides (Table 1). MAA production is confirmed in all three major groups of macroalgae at different profiles and compositions [127]. In green algae, 45 species report detectable levels of MAAs, with the highest quantities identified in species from the class Prasiolales, with MAA content of more than 3.5 mg/g DW, such as reported in Prasiola crispa [149], while lower or no detectable MAA quantities are reported in some Ulva species [127]. Many macroalgal blooms are dominated by green macroalgae, with the majority of blooms resulting from Ulva species [150]. In the case of the bloom-forming genus Ulva [151] that leads to overwhelming green tides across the world, mycosporine-glycine and porphyra-334 are confirmed in detectable levels [127]. Biotechnological applications of ulvan beyond MAAs also includes other sulfated polysaccharide with promising anti-cancer, anti-viral, antioxidant and other pharmacological activities [152].
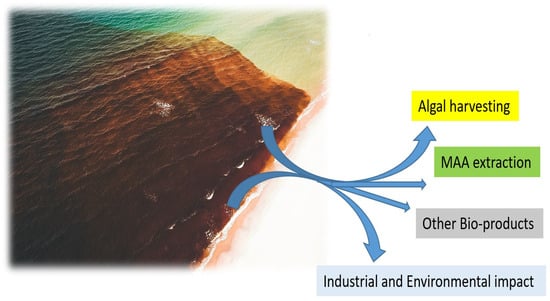
Figure 3.
The most economically beneficial source of biomass for massive MAA and other bio-products manufacturing.
Increasing levels of golden algal blooms are reported, especially for Sargassum blooms [150]. Brown algae (the class Phaeophyceae) contain the pigments chlorophyll and carotenoids, including fucoxanthin, which is important for photo and antioxidant protection, as well as polyphenolic compounds phlorotannins and complex carbohydrates laminarin and mannitol [153,154]. MAAs are reported in over 40 species coming from brown algae, including some Saragasumm species, such as Sargassum oligocystum and S. fluitans [127]. The invasive species from the genus Sargassum (F. Sargassaceae) contain bioactive compounds demonstrating anti-bacterial, anti-inflammatory, antioxidant, anti-tumor, anti-viral, and other pharmacologically promising activities [155], while UVB absorption is confirmed in ethanol extract demonstrating [156] and UVA photoprotective properties [157]. Sargassum species are well distributed in tropical and subtropical climate regions and have been used as a source of food for a long time due to their nutrient values (a rich source of vitamins, proteins, and minerals). In addition, pharmacological bioactivities recognized in Sargassum species include antioxidant, anti-fouling, anti-microbial, and anti-tumor activities [14]. For example, UV protective, anti-microbial and anti-inflammatory activities are demonstrated in Sargassum cristaefolium with confirmed presence of MAA palythene [157,158]. Multiple other MAAs are confirmed in another Saragassum species (Table 2).
Red algae can produce promising bioactive compounds and pigments [14]. Therefore, red macroalgae capable of forming algal blooms are especially attractive due to the high growth and biomass available [117]. On the other hand, the species from the order Bonnemaisoniales, Asparagopsis armata, recognized as one of the most aggressive invasive macroalgal species and able to form blooms [159], has very promising biotechnology potential containing various secondary metabolites, including MAAs [117]. Asparagopsis armata is becoming very abundant in some countries, presenting a huge negative environmental and economic impact on local communities [160,161]. Although MAA levels are the highest in species coming from the genera Porphyra and Bangia, the species from the genus Asparagopsis are also prosperous with MAA [162], with MAA concentration and profile being directly influenced by nitrogen status [163]. Strong capacity for synthesis of UV-absorbing molecules is reported for Asparagopsis armata [163], but not for another invasive bloom-forming species Dasysiphonia japonica [44] (order Ceramiales), with species with low content of MAAs (1–2 mg g−1 DW). Rhodophyta orders characterized by the highest levels of MAAs of >2 mg g−1 DW include Bangiales, Gelidiales and Gracilariales [162]. In 23 red algal species analyzed, the most common MAAs are shinorine, palythine, asterina-330 and porphyra-334 [164]. However, even in red algae, the MAA content may vary from low levels (<1 mg g−1 of DW) to higher levels above 2 mg g−1 of DW in some species [165]. While the bloom-forming G. vermiculophylla contains various MAAs (Table 1 and Table 2), the MAA content and profile can vary seasonally [166]. For cultivated G. vermiculophylla, the highest levels of porphyra-334 and shinorine are reported in November–January, while palythine and asterina-330 are highest from April to August. Similarly, when grown in seawater with elevated nutrient concentrations (150 uM of NH4+ and 15 uM of PO43−) and increased UVA and UVB levels, MAA production can increase by 50% in G. cornea (old name Hydropuntia cornea) [167]. The targeted UV radiation also induces a substantial increase in the MAA levels in macroalga Gracilaria gracilis compared to control [143]. Similarly, elevated levels of NO3− can increase levels of MAAs in G. tenuistipitata [162,168]. Thus, as blooms of Gracilaria frequently occur during the summer months when UV exposure is higher and nutrient levels are frequently elevated as well [169], they may represent a high-quality source for obtaining the high biomass levels needed for MAA extraction in the future (Figure 3).

Table 1.
Macroalgal species forming algal blooms were reported to accumulate high levels of UV-absorbing compounds.
Table 1.
Macroalgal species forming algal blooms were reported to accumulate high levels of UV-absorbing compounds.
| Macroalgal Species | Biotechnology Use [Ref.] | MAAs |
|---|---|---|
| Green algae Order: Ulvales Ulva spp. | Human and animal nutrients; preservatives; pharmaceuticals; cosmeceuticals | MG, PR [127] |
| Brown algae Order: Fucales Sargassum cristaefolium Sargassum oligocystum | Photoprotective activity against UVR; Inhibited proinflammatory TNF-α and IL-6 expression while increasing IL-10 production in the BALB/c mice skin [157,158] | PE dominant MAAs PR, PI, SH 5 [127,170] |
| Red algae Order: Bonnemaisoniales Asparagopsis armata | High biofiltration capacity of nutrients; UV photoprotection [163] Exudate cocktail as a biopesticide for eco-friendly weed control [171] Preservatives, cosmeceuticals, biopharmaceuticals [117] | MAAs (accumulated only under a high ammonium-N availability) [163] AS, PR, PE, SH, UN [127] |
| Red algae Order: Gracilariales Gracilaria vermiculophylla | Increase in MAAs in freshly released spores increased under UVR 8 h [172] | AS, PE, PR, PI, SH, US, UN [127,166] |
Abbreviations: Asterina-330 (AS), Mycosporine-glycine (MG), Palythine (PI), Palythene (PE), Porphyra-334 (PR), Shinorine (SH), Unidentified MAAs (UN), Usujirene (US). Red algae—Rhodophyta; Green algae—Chlorophyta; brown algae—Phaeophyceae.

Table 2.
Chemical structure of common MAAs found in bloom forming macroalgal species.
Table 2.
Chemical structure of common MAAs found in bloom forming macroalgal species.
| UV-Protective Natural Products | Chemical Structure | Key Properties (Ref) | ʎ Max (nm) ε Coefficient (M−1 cm−1) Molecular Mass (g/mol) |
|---|---|---|---|
| Mycosporine-glycine (C10H15NO6) | 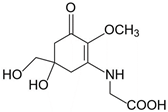 | UV-absorbing, antioxidants [134] | 310 nm 28,100 M−1 cm−1 245 g/mol |
| Shinorine (C13H20N2O8) | 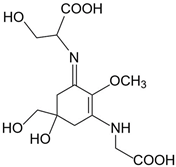 | UV-absorbing, antioxidants [135,173] | 334 nm 44,668 M−1 cm−1 332 g/mol |
| Usujirene (C13H20N2O5) | 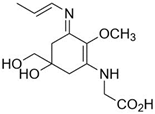 | UV-absorbing, antioxidants [174] | 357 nm 45,070 M−1 cm−1 284 g/mol |
| Asterina-330 (C12H20N2O6) | 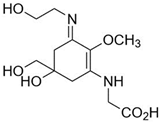 | UV-absorbing, antioxidants [175] | 330 nm 43,800 M−1 cm−1 288 g/mol |
| Porphyra-334 (C14H22N2O8) | 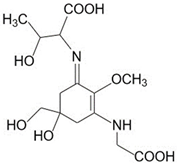 | UV-absorbing, antioxidants [135,175] | 334 nm 42,300 M−1 cm−1 346 g/mol |
| Palythene (C13H20N2O5) | 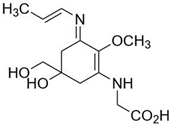 | UV-absorbing, antioxidants [176] | 360 nm 50,000 M−1 cm−1 284 g/mol |
| Palythine (C10H16N2O5) | 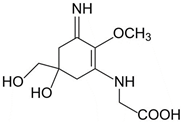 | UV-absorbing, antioxidants [175,177] | 320 nm 35,500–36,200 M−1 cm−1 244 g/mol |
4. Proteomics for Monitoring Macroalgal Blooms and Discovery of MAA Profiles
The use of transcriptomics and proteomics in bioinformatic pipelines enables faster discovery and functional characterization of novel marine natural products [178]. The exploration of the biosynthesis of MAAs employed genomic mining techniques [142,179], confirming that MAA production occurs via the shikimate pathway [180,181] and the pentose phosphate pathway [179]. Proteomic data analyses demonstrated that UV-induced MAA production mainly occurs via the shikimate pathway and is, therefore, more critical in photoprotection [182]. Four genes making a core of the MAA pathway (e.g., so-called mys cluster genes) were identified to be dehydroquinate synthase (DHQS), O-methyltransferase (O-MT), adenosine triphosphate (ATP) grasp, and nonribosomalpeptide synthetase (NRPS) in the cyanobacterium Anabaena variabilis [142]. Some of these genes are duplicated or have additional mys-cluster genes in different organisms [129,179,183,184]. Similar observations were reported in other algal groups with mechanisms such as horizontal gene transfer and acquisition of diverse MAA gene clusters playing a driving role in the development of species with high-temperature resilience [185], plus epigenetics mechanisms influencing gene expression patterns [186].
Various proteomic techniques can be applied to explore MAA synthesis and monitor macroalgal blooms that could be utilized for the biotechnological application of MAAs. These include high-throughput methods such as top-down proteomic methodologies that separate proteins and then complete individual characterization, such as mass spectrometry (MS)-based proteomics [187]. In bottom-up proteomics (also called ‘shotgun’ proteomics), the proteins first undergo the digestion process, producing a mixture of peptides that are analyzed using MS or LC/MS and compared to existing databases via automated analyses [188]. Most MAA analyses include the purification step, identification and quantification using high-performance liquid chromatography (HPLC) separation and identification based on retention times and UV spectra [189,190,191]. In addition to HPLC chromatography, the confirmatory analyses for improved MAA characterization and quantification also included the implementation of mass spectrometry, including various types of liquid chromatography (LC/MS) methodologies [139,192,193,194,195,196]. The different HPLC and MS techniques improved the discovery and characterization of MAAs, especially when purified MAAs were characterized by nuclear magnetic resonance (NMR) [173,197]. The use of ultrahigh-performance liquid chromatography (UHPLC) was also applied in MAA analyses [198], combined with hyphenated to orbitrap high-resolution tandem mass spectroscopy for feature-based molecular networking characterization and classification of MAAs, which is one of the most recent advancements in proteomics [199]. This approach incorporates the published MAA fragmentation patterns and uses in silico annotation tools that allow for more accurate identification, discovery, and classification of MAAs [199].
Furthermore, predicting algal bloom events could be critical so that the biotechnological industry can utilize these natural events and harvest a large amount of algal biomass as a source of bioproducts. Proteomic studies of differential expression of specific proteins involved in growth processes and stress response can be potentially useful as biomarkers for the prediction of future algal blooms [200]. In China’s coastal waters, distinct genetics patterns were linked to harmful macroalgal blooms (HMBs) involving green and gold tides [201]. Due to the increased negative ecological impact of these HMBs, monitoring these changes and identifying driving regulatory mechanisms is becoming critically important to allow improved scientific forecasting of future algal blooms. Consequently, utilizing other omics datasets was recognized as a promising way to increase the modeling strength for predicting climate-driven algal blooms [202,203].
5. Conclusions
Macroalgal blooms are spontaneous and frequently brief occurrences usually triggered by anthropogenic factors; they are characterized by a sharp rise in drift macroalgal abundance. Consequently, large amounts of macroalgal biomass can be harvested from these blooms and utilized for biotechnological purposes, although there may be technological challenges associated with the proper harvesting of bloom-forming species. Several macroalgal species that have a strong capacity for both algal blooms and large MAA production were identified in this review. Environmental conditions, including seasonal periods of high UVR exposure and nutrient enrichment, which enhance algal growth, also enhance the accumulation of MAAs, which hold excessive biotechnological potential due to their enhanced photoprotective and other pharmacological properties. Advancing new trends, such as applying a novel molecular networking approach, presents a promising field that combines in silico tools with modern high-throughput chemistry methodologies for analyzing the clusters of MAAs chemistry based on their fragmentation patterns for improved characterization and classifications. Finally, advancements in proteomic techniques can empower our understanding of MAA structural diversity and functional significance for better utilization of natural phenomena of algal blooms and future biotechnological developments (i.e., sunscreens) in an environmentally sustainable manner.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable, as this study did not involve humans or animals.
Data Availability Statement
The original data presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The author would like to thank the two anonymous reviewers, as well as Isidora Skrlin, for their critical reviews of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neale, R.E.; Hamilton, A.R.; Janda, M.; Gies, P.; Green, A.C. Seasonal variation in measured solar ultraviolet radiation exposure of adults in subtropical Australia. Photochem. Photobiol. 2010, 86, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Blumthaler, M.; Ambach, W.; Ellinger, R. Increase in solar UV radiation with altitude. J. Photochem. Photobiol. B Biol. 1997, 39, 130–134. [Google Scholar] [CrossRef]
- Olsen, C.M.; Wilson, L.F.; Green, A.C.; Bain, C.J.; Fritschi, L.; Neale, R.E.; Whiteman, D.C. Cancers in Australia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust. N. Z. J. Public Health 2015, 39, 471. [Google Scholar] [CrossRef] [PubMed]
- Climstein, M.; Doyle, B.; Stapelberg, M.; Rosic, N.; Hertess, I.; Furness, J.; Simas, V.; Walsh, J. Point prevalence of non-melanoma and melanoma skin cancers in Australian surfers and swimmers in Southeast Queensland and Northern New South Wales. PeerJ 2022, 10, e13243. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.J.; Stapelberg, M.; Rosic, N.; Hudson, J.; Coxon, P.; Furness, J.; Walsh, J.; Climstein, M. Implementation of artificial intelligence for the detection of cutaneous melanoma within a primary care setting: Prevalence and types of skin cancer in outdoor enthusiasts. PeerJ 2023, 11, e15737. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.S.; Filho, G.M.A.; Kamenos, N.A.; Riosmena-Rodríguez, R.; Steller, D.L. Rhodoliths and Rhodolith Beds. In Research and Discoveries: The Revolution of Science through SCUBA; Lang, M.A., Marinelli, R.L., Roberts, S.J., Taylor, P.R., Eds.; Smithsonian Contributions to the Marine Sciences; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2013; pp. 143–155. [Google Scholar]
- Littler, M.M.; Littler, D.S. The nature of crustose coralline algae and their interactions on reefs. In Research and Discoveries: The Revolution of Science through SCUBA; Lang, M.A., Marinelli, R.L., Roberts, S.J., Taylor, P.R., Eds.; Smithsonian Contributions to the Marine Sciences; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2013; pp. 199–212. [Google Scholar]
- Fulton, C.J.; Berkström, C.; Wilson, S.K.; Abesamis, R.A.; Bradley, M.; Åkerlund, C.; Barrett, L.T.; Bucol, A.A.; Chacin, D.H.; Chong-Seng, K.M.; et al. Macroalgal meadow habitats support fish and fisheries in diverse tropical seascapes. Fish Fish. 2020, 21, 700–717. [Google Scholar] [CrossRef]
- Smale, D.A.; Pessarrodona, A.; King, N.; Burrows, M.T.; Yunnie, A.; Vance, T.; Moore, P. Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Sci. Rep. 2020, 10, 12161. [Google Scholar] [CrossRef]
- Zheng, X.; Como, S.; Huang, L.; Magni, P. Temporal changes of a food web structure driven by different primary producers in a subtropical eutrophic lagoon. Mar. Environ. Res. 2020, 161, 105128. [Google Scholar] [CrossRef]
- Ouyang, X.; Kristensen, E.; Zimmer, M.; Thornber, C.; Yang, Z.; Lee, S.Y. Response of macrophyte litter decomposition in global blue carbon ecosystems to climate change. Glob. Chang. Biol. 2023, 29, 3806–3820. [Google Scholar] [CrossRef]
- Ashkenazi, D.Y.; Figueroa, F.L.; Korbee, N.; García-Sánchez, M.; Vega, J.; Ben-Valid, S.; Paz, G.; Salomon, E.; Israel, Á.; Abelson, A. Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Mar. Drugs 2022, 20, 767. [Google Scholar] [CrossRef]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2022, 14, 5–26. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity—The biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vásquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I.; et al. Seaweeds: An opportunity for wealth and sustainable livelihood for coastal communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Davison, I.R.; Dudgeon, S.R.; Ruan, H.-M. Effect of freezing on seaweed photosynthesis. Mar. Ecol. Prog. Ser. 1989, 58, 123–131. [Google Scholar] [CrossRef]
- van Hees, D.H.; Olsen, Y.S.; Wernberg, T.; Van Alstyne, K.L.; Kendrick, G.A. Phenolic concentrations of brown seaweeds and relationships to nearshore environmental gradients in Western Australia. Mar. Biol. 2017, 164, 74. [Google Scholar] [CrossRef]
- Jormalainen, V.; Honkanen, T. Variation in natural selection for growth and phlorotannins in the brown alga Fucus vesiculosus. J. Evol. Biol. 2004, 17, 807–820. [Google Scholar] [CrossRef]
- Williams, S.L.; Dethier, M.N. High and Dry: Variation in Net Photosynthesis of the Intertidal Seaweed Fucus gardneri. Ecology 2005, 86, 2373–2379. [Google Scholar] [CrossRef]
- Beardall, J.; Beer, S.; Raven, J.A. Biodiversity of Marine Plants in an Era of Climate Change: Some Predictions Based on Physiological Performance. J. Bot. Mar. 1998, 41, 113–124. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Malcolm, S.J. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: A biological and environmental perspective. J. Phycol. 1998, 34, 418–430. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Sinha, R.P.; Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. UV radiation-induced biosynthesis, stability and antioxidant activity of mycosporine-like amino acids (MAAs) in a unicellular cyanobacterium Gloeocapsa sp. CU2556. J. Photochem. Photobiol. B Biol. 2014, 130, 287–292. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Beardall, J.; Stojkovic, S.; Gao, K. Interactive effects of nutrient supply and other environmental factors on the sensitivity of marine primary producers to ultraviolet radiation: Implications for the impacts of global change. Aquat. Biol. 2014, 22, 5–23. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, Z.; Zou, D.; Gao, K. Ecophysiological responses of marine macroalgae to climate change factors. J. Appl. Phycol. 2016, 28, 2953–2967. [Google Scholar] [CrossRef]
- Xu, J.; Gao, K. Use of UV-A Energy for Photosynthesis in the Red Macroalga Gracilaria lemaneiformis. Photochem. Photobiol. 2010, 86, 580–585. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, K. Impacts of solar uv radiation on the photosynthesis, growth, and uv-absorbing compounds in Gracilaria lemaneiformis (rhodophyta) grown at different nitrate concentrations1. J. Phycol. 2009, 45, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Gao, K. Chapter Two—Effects of climate change factors on marine macroalgae: A review. In Advances in Marine Biology; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 88, pp. 91–136. [Google Scholar]
- Wahl, M.; Jormalainen, V.; Eriksson, B.K.; Coyer, J.A.; Molis, M.; Schubert, H.; Dethier, M.; Karez, R.; Kruse, I.; Lenz, M.; et al. Chapter Two—Stress Ecology in Fucus: Abiotic, Biotic and Genetic Interactions. In Advances in Marine Biology; Lesser, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 59, pp. 37–105. [Google Scholar]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects of climate change on global seaweed communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Seaweeds: Edible, Available, and Sustainable; University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Neveux, N.; Bolton, J.J.; Bruhn, A.; Roberts, D.A.; Ras, M. The Bioremediation Potential of Seaweeds: Recycling Nitrogen, Phosphorus, and Other Waste Products. In Blue Biotechnology; Wiley: Hoboken, NJ, USA, 2018; pp. 217–239. [Google Scholar] [CrossRef]
- Henriques, B.; Lopes, C.B.; Figueira, P.; Rocha, L.S.; Duarte, A.C.; Vale, C.; Pardal, M.A.; Pereira, E. Bioaccumulation of Hg, Cd and Pb by Fucus vesiculosus in single and multi-metal contamination scenarios and its effect on growth rate. Chemosphere 2017, 171, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.; Bradbury, J.; Lee, M.; Baltrotsky, K.; Grace, S. The impact of pesticides on local waterways: A scoping review and method for identifying pesticides in local usage. Environ. Sci. Policy 2020, 106, 12–21. [Google Scholar] [CrossRef]
- Mosalaei Rad, S.; Ray, A.; Barghi, S. Water Pollution and Agriculture Pesticide. Clean Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, W. Removal of cypermethrin with seaweed Gracilaria lemaneiformis. J. Ocean Univ. China 2015, 14, 858–864. [Google Scholar] [CrossRef]
- Tang, X.; Shen, L.; Liu, S.; Gao, J. Effective removal of the herbicide glyphosate by the kelp Saccharina japonica female gametophytes from saline waters and its mechanism elucidation. Chemosphere 2021, 274, 129826. [Google Scholar] [CrossRef]
- Lyons, D.A.; Mant, R.C.; Bulleri, F.; Kotta, J.; Rilov, G.; Crowe, T.P. What are the effects of macroalgal blooms on the structure and functioning of marine ecosystems? A systematic review protocol. Environ. Evid. 2012, 1, 7. [Google Scholar] [CrossRef][Green Version]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Young, C.S.; Lee, C.-S.; Sylvers, L.H.; Venkatesan, A.K.; Gobler, C.J. The invasive red seaweed, Dasysiphonia japonica, forms harmful algal blooms: Mortality in early life stage fish and bivalves and identification of putative toxins. Harmful Algae 2022, 118, 102294. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef]
- Valiela, I.; McClelland, J.W.; Hauxwell, J.A.; Behr, P.J.; Hersh, D.; Foreman, K.H. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnol. Oceanogr. 1997, 42, 1105–1118. [Google Scholar] [CrossRef]
- Teichberg, M.; Fox, S.E.; Olsen, Y.S.; Valiela, I.; Martinetto, P.; Iribarne, O.; Muto, E.Y.; Petti, M.A.V.; Corbisier, T.N.; Soto- Jiménez, M.; et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Chang. Biol. 2010, 16, 2624–2637. [Google Scholar] [CrossRef]
- Thornber, C.S.; Guidone, M.; Deacutis, C.; Green, L.; Ramsay, C.N.; Palmisciano, M. Spatial and temporal variability in macroalgal blooms in a eutrophied coastal estuary. Harmful Algae 2017, 68, 82–96. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Xing, Q.; Shang, S. Long-term trend of Ulva prolifera blooms in the western Yellow Sea. Harmful Algae 2016, 58, 35–44. [Google Scholar] [CrossRef]
- Schein, A.; Courtenay, S.C.; Crane, C.S.; Teather, K.L.; van den Heuvel, M.R. The Role of Submerged Aquatic Vegetation in Structuring the Nearshore Fish Community Within an Estuary of the Southern Gulf of St. Lawrence. Estuaries Coasts 2012, 35, 799–810. [Google Scholar] [CrossRef]
- Thomsen, M.S.; McGlathery, K. Effects of accumulations of sediments and drift algae on recruitment of sessile organisms associated with oyster reefs. J. Exp. Mar. Biol. Ecol. 2006, 328, 22–34. [Google Scholar] [CrossRef]
- Young, C.S.; Peterson, B.J.; Gobler, C.J. The Bloom-Forming Macroalgae, Ulva, Outcompetes the Seagrass, Zostera marina, Under High CO2 Conditions. Estuaries Coasts 2018, 41, 2340–2355. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Xing, Q.; Shi, P. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef]
- Teichberg, M.; Fricke, A.; Bischof, K. Increased physiological performance of the calcifying green macroalga Halimeda opuntia in response to experimental nutrient enrichment on a Caribbean coral reef. Aquat. Bot. 2013, 104, 25–33. [Google Scholar] [CrossRef]
- Castaldelli, G.; Welsh, D.T.; Flachi, G.; Zucchini, G.; Colombo, G.; Rossi, R.; Fano, E.A. Decomposition dynamics of the bloom forming macroalga Ulva rigida C. Agardh determined using a 14C-carbon radio-tracer technique. Aquat. Bot. 2003, 75, 111–122. [Google Scholar] [CrossRef]
- Conover, J.; Green, L.A.; Thornber, C.S. Biomass decay rates and tissue nutrient loss in bloom and non-bloom-forming macroalgal species. Estuar. Coast. Shelf Sci. 2016, 178, 58–64. [Google Scholar] [CrossRef]
- Harb, T.B.; Chow, F. An overview of beach-cast seaweeds: Potential and opportunities for the valorization of underused waste biomass. Algal Res. 2022, 62, 102643. [Google Scholar] [CrossRef]
- Lyons, D.A.; Arvanitidis, C.; Blight, A.J.; Chatzinikolaou, E.; Guy-Haim, T.; Kotta, J.; Orav-Kotta, H.; Queirós, A.M.; Rilov, G.; Somerfield, P.J.; et al. Macroalgal blooms alter community structure and primary productivity in marine ecosystems. Glob. Chang. Biol. 2014, 20, 2712–2724. [Google Scholar] [CrossRef]
- Lomstein, B.A.; Guldberg, L.B.; Neubauer, A.-T.A.; Hansen, J.; Donnelly, A.; Herbert, R.A.; Viaroli, P.; Giordani, G.; Azzoni, R.; de Wit, R.; et al. Benthic decomposition of Ulva lactuca: A controlled laboratory experiment. Aquat. Bot. 2006, 85, 271–281. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Z.; Wu, Y.; Deng, Y.; Chen, Q.; Zhao, C.; Cui, L.; Huang, X. Macroalgae bloom decay decreases the sediment organic carbon sequestration potential in tropical seagrass meadows of the South China Sea. Mar. Pollut. Bull. 2019, 138, 598–603. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.; Song, H.; Fan, S.; Yuan, C.; Fu, M.; Miao, X.; Zhang, X.; Su, R.; Hu, C. An anomalous bi-macroalgal bloom caused by Ulva and Sargassum seaweeds during spring to summer of 2017 in the western Yellow Sea, China. Harmful Algae 2020, 93, 101760. [Google Scholar] [CrossRef]
- Fidai, Y.A.; Dash, J.; Tompkins, E.L.; Tonon, T. A systematic review of floating and beach landing records of Sargassum beyond the Sargasso Sea. Environ. Res. Commun. 2020, 2, 122001. [Google Scholar] [CrossRef]
- Piñón-Gimate, A.; Soto-Jiménez, M.F.; Ochoa-Izaguirre, M.J.; García-Pagés, E.; Páez-Osuna, F. Macroalgae blooms and δ15N in subtropical coastal lagoons from the Southeastern Gulf of California: Discrimination among agricultural, shrimp farm and sewage effluents. Mar. Pollut. Bull. 2009, 58, 1144–1151. [Google Scholar] [CrossRef]
- Joniver, C.F.H.; Photiades, A.; Moore, P.J.; Winters, A.L.; Woolmer, A.; Adams, J.M.M. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal Res. 2021, 58, 102407. [Google Scholar] [CrossRef]
- López-Contreras, A.; Núñez, P.; García, B.; Driegen, J.; Lwanga, E.; Domin, P.; Gurrola, M.P.; Rosas-Luis, R.; Verde-Gómez, Y.; Vrije, T. Sargassum in Mexico: From Environmental Problem to Valuable Resource; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Marx, U.C.; Roles, J.; Hankamer, B. Sargassum blooms in the Atlantic Ocean—From a burden to an asset. Algal Res. 2021, 54, 102188. [Google Scholar] [CrossRef]
- Oxenford, H.A.; Cox, S.-A.; van Tussenbroek, B.I.; Desrochers, A. Challenges of Turning the Sargassum Crisis into Gold: Current Constraints and Implications for the Caribbean. Phycology 2021, 1, 27–48. [Google Scholar] [CrossRef]
- Lorbeer, A.J.; Tham, R.; Zhang, W. Potential products from the highly diverse and endemic macroalgae of Southern Australia and pathways for their sustainable production. J. Appl. Phycol. 2013, 25, 717–732. [Google Scholar] [CrossRef]
- Newton, C.; Bracken, M.E.; McConville, M.; Rodrigue, K.; Thornber, C.S. Invasion of the red seaweed Heterosiphonia japonica spans biogeographic provinces in the Western North Atlantic Ocean. PLoS ONE 2013, 8, e62261. [Google Scholar] [CrossRef] [PubMed]
- Manilal, A.; Sujith, S.; Sabarathnam, B.; Kiran, G.S.; Selvin, J.; Shakir, C.; Lipton, A.P. Bioactivity of the red algae Asparagopsis taxiformis collected from the Southwestern coast of India. Braz. J. Oceanogr. 2010, 58, 93–100. [Google Scholar] [CrossRef]
- Shimada, S.; Nagano, M.; Hiraoka, M.; Ichihara, K.; Mineur, F.; Zhu, W. Phylogeographic analysis of the genus Ulva (Ulvales, Chlorophyta), including bloom sample in Qingdao, China. Coast. Mar. Sci. 2010, 34, 117–122. [Google Scholar]
- Quillien, N.; Nordström, M.C.; Guyonnet, B.; Maguer, M.; Le Garrec, V.; Bonsdorff, E.; Grall, J. Large-scale effects of green tides on macrotidal sandy beaches: Habitat-specific responses of zoobenthos. Estuar. Coast. Shelf Sci. 2015, 164, 379–391. [Google Scholar] [CrossRef]
- Viaroli, P.; Bartoli, M.; Azzoni, R.; Giordani, G.; Mucchino, C.; Naldi, M.; Nizzoli, D.; Tajé, L. Nutrient and iron limitation to Ulva blooms in a eutrophic coastal lagoon (Sacca di Goro, Italy). Hydrobiologia 2005, 550, 57–71. [Google Scholar] [CrossRef]
- Nelson, T.A.; Nelson, A.V.; Tjoelker, M. Seasonal and Spatial Patterns of “Green Tides” (Ulvoid Algal Blooms) and Related Water Quality Parameters in the Coastal Waters of Washington State, USA. Bot. Mar. 2003, 46, 263–275. [Google Scholar] [CrossRef]
- Guidone, M.; Thornber, C.S.; Van Alstyne, K.L. Herbivore impacts on two morphologically similar bloom-forming Ulva species in a eutrophic bay. Hydrobiologia 2015, 753, 175–188. [Google Scholar] [CrossRef][Green Version]
- Fox, S.E.; Teichberg, M.; Valiela, I.; Heffner, L. The Relative Role of Nutrients, Grazing, and Predation as Controls on Macroalgal Growth in the Waquoit Bay Estuarine System. Estuaries Coasts 2012, 35, 1193–1204. [Google Scholar] [CrossRef]
- Human, L.R.D.; Adams, J.B.; Allanson, B.R. Insights into the cause of an Ulva lactuca Linnaeus bloom in the Knysna Estuary. S. Afr. J. Bot. 2016, 107, 55–62. [Google Scholar] [CrossRef]
- Chávez-Sánchez, T.; Piñón-Gimate, A.; Serviere-Zaragoza, E.; López-Bautista, J.M.; Casas-Valdez, M. Ulva blooms in the southwestern Gulf of California: Reproduction and biomass. Estuar. Coast. Shelf Sci. 2018, 200, 202–211. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Chen, L.; Hu, M.; Yu, K.; Chen, Q.; He, Q.; He, P. Green algae blooms caused by Ulva prolifera in the southern Yellow Sea: Identification of the original bloom location and evaluation of biological processes occurring during the early northward floating period. Limnol. Oceanogr. 2013, 58, 2206–2218. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, P.; Huo, Y.; Yu, K.; He, P. The fast expansion of Pyropia aquaculture in “Sansha” regions should be mainly responsible for the Ulva blooms in Yellow Sea. Estuar. Coast. Shelf Sci. 2017, 189, 58–65. [Google Scholar] [CrossRef]
- Ober, G.T.; Thornber, C.S. Divergent responses in growth and nutritional quality of coastal macroalgae to the combination of increased pCO2 and nutrients. Mar. Environ. Res. 2017, 131, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Green-Gavrielidis, L.A.; Thornber, C.S. Will Climate Change Enhance Algal Blooms? The Individual and Interactive Effects of Temperature and Rain on the Macroalgae Ulva. Estuaries Coasts 2022, 45, 1688–1700. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Han, X.; Shi, X.; Rivkin, R.B.; Legendre, L. Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China. Sci. Rep. 2016, 6, 26498. [Google Scholar] [CrossRef] [PubMed]
- Thornber, C.S.; DiMilla, P.; Nixon, S.W.; McKinney, R.A. Natural and anthropogenic nitrogen uptake by bloom-forming macroalgae. Mar. Pollut. Bull. 2008, 56, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Green-Gavrielidis, L.A.; MacKechnie, F.; Thornber, C.S.; Gomez-Chiarri, M. Bloom-forming macroalgae (Ulva spp.) inhibit the growth of co-occurring macroalgae and decrease eastern oyster larval survival. Mar. Ecol. Prog. Ser. 2018, 595, 27–37. [Google Scholar] [CrossRef]
- Young, C.S.; Lowell, A.; Peterson, B.; Gobler, C.J. Ocean acidification and food limitation combine to suppress herbivory by the gastropod Lacuna vincta. Mar. Ecol. Prog. Ser. 2019, 627, 83–94. [Google Scholar] [CrossRef]
- Beer, S. Photosynthetic traits of the ubiquitous and prolific macroalga Ulva (Chlorophyta): A review. Eur. J. Phycol. 2022, 58, 390–398. [Google Scholar] [CrossRef]
- Cruces, E.; Rautenberger, R.; Cubillos, V.M.; Ramírez-Kushel, E.; Rojas-Lillo, Y.; Lara, C.; Montory, J.A.; Gómez, I. Interaction of Photoprotective and Acclimation Mechanisms in Ulva rigida (Chlorophyta) in Response to Diurnal Changes in Solar Radiation in Southern Chile. J. Phycol. 2019, 55, 1011–1027. [Google Scholar] [CrossRef]
- Pescheck, F.; Campen, H.; Nichelmann, L.; Bilger, W. Relative sensitivity of DNA and photosystem II in Ulva intestinalis (Chlorophyta) under natural solar irradiation. Mar. Ecol. Prog. Ser. 2016, 555, 95–107. [Google Scholar] [CrossRef]
- Bischof, K.; Peralta, G.; Kräbs, G.; van de Poll, W.H.; Pérez-Lloréns, J.L.; Breeman, A.M. Effects of solar UV-B radiation on canopy structure of Ulva communities from southern Spain. J. Exp. Bot. 2002, 53, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-S.; Han, T. UV-B induction of uv-b protection in Ulva pertusa (chlorophyta). J. Phycol. 2005, 41, 523–530. [Google Scholar] [CrossRef]
- Pescheck, F.; Bilger, W. Compensation of lack of UV screening by cellular tolerance in green macroalgae (Ulvophyceae) from the upper eulittoral. Mar. Biol. 2018, 165, 132. [Google Scholar] [CrossRef]
- Pescheck, F.; Bischof, K.; Bilger, W. Screening of ultraviolet-a and ultraviolet-b radiation in marine green macroalgae (chlorophyta). J. Phycol. 2010, 46, 444–455. [Google Scholar] [CrossRef]
- Laffoley, D.; Roe, H.; Angel, M.V.; Ardron, J.; Bates, N.; Boyd, L.L.; Brooke, S.; Buck, K.; Carlson, C.; Causey, B.; et al. The Protection and Management of the Sargasso Sea: The Golden Floating Rainforest of the Atlantic Ocean: Summary Science and Supporting Evidence Case; Sargasso Sea Alliance, Government of Bermuda: Bermuda, UK, 2011.
- Putman, N.F.; Goni, G.J.; Gramer, L.J.; Hu, C.; Johns, E.M.; Trinanes, J.; Wang, M. Simulating transport pathways of pelagic Sargassum from the Equatorial Atlantic into the Caribbean Sea. Prog. Oceanogr. 2018, 165, 205–214. [Google Scholar] [CrossRef]
- Oviatt, C.A.; Huizenga, K.; Rogers, C.S.; Miller, W.J. What nutrient sources support anomalous growth and the recent Sargassum mass stranding on Caribbean beaches? A review. Mar. Pollut. Bull. 2019, 145, 517–525. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R.; et al. Massive Influx of Pelagic Sargassum spp. on the Coasts of the Mexican Caribbean 2014–2020: Challenges and Opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, R.; Muñiz-Castillo, A.I.; Euán-Avila, J.I.; Hernández-Núñez, H.; Valdés-Lozano, D.S.; Collí-Dulá, R.C.; Arias-González, J.E. Assessing temporal dynamics on pelagic Sargassum influx and its relationship with water quality parameters in the Mexican Caribbean. Reg. Stud. Mar. Sci. 2021, 48, 102005. [Google Scholar] [CrossRef]
- Aquino, R.; Noriega, C.; Mascarenhas, A.; Costa, M.; Monteiro, S.; Santana, L.; Silva, I.; Prestes, Y.; Araujo, M.; Rollnic, M. Possible Amazonian contribution to Sargassum enhancement on the Amazon Continental Shelf. Sci. Total Environ. 2022, 853, 158432. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Brewton, R.A.; Herren, L.W.; Wang, M.; Hu, C.; McGillicuddy, D.J.; Lindell, S.; Hernandez, F.J.; Morton, P.L. Nutrient content and stoichiometry of pelagic Sargassum reflects increasing nitrogen availability in the Atlantic Basin. Nat. Commun. 2021, 12, 3060. [Google Scholar] [CrossRef] [PubMed]
- Polo, L.K.; Chow, F. Variation of antioxidant capacity and antiviral activity of the brown seaweed Sargassum filipendula (Fucales, Ochrophyta) under UV radiation treatments. Appl. Phycol. 2022, 3, 260–273. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Lann, K.L.; Ferret, C.; VanMee, E.; Spagnol, C.; Lhuillery, M.; Payri, C.; Stiger-Pouvreau, V. Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: Spatial and specific variability. Phycol. Res. 2012, 60, 37–50. [Google Scholar] [CrossRef]
- Polo, L.K.; de L. Felix, M.R.; Kreusch, M.; Pereira, D.T.; Costa, G.B.; Simioni, C.; Ouriques, L.C.; Chow, F.; Ramlov, F.; Maraschin, M.; et al. Photoacclimation Responses of the Brown Macroalga Sargassum cymosum to the Combined Influence of UV Radiation and Salinity: Cytochemical and Ultrastructural Organization and Photosynthetic Performance. Photochem. Photobiol. 2014, 90, 560–573. [Google Scholar] [CrossRef]
- Magaña-Gallegos, E.; García-Sánchez, M.; Graham, C.; Olivos-Ortiz, A.; Siuda, A.N.S.; van Tussenbroek, B.I. Growth rates of pelagic Sargassum species in the Mexican Caribbean. Aquat. Bot. 2023, 185, 103614. [Google Scholar] [CrossRef]
- Xu, Z.; Li, L.; Jiang, H.; Yan, F.; Liu, L.; Zang, S.; Ma, Y.; Wu, H. Photosynthetic responses of a golden tide alga (Sargassum horneri) to ultraviolet radiation. Front. Mar. Sci. 2022, 9, 978376. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef]
- Powers, L.C.; Hertkorn, N.; McDonald, N.; Schmitt-Kopplin, P.; Del Vecchio, R.; Blough, N.V.; Gonsior, M. Sargassum sp. Act as a Large Regional Source of Marine Dissolved Organic Carbon and Polyphenols. Glob. Biogeochem. Cycles 2019, 33, 1423–1439. [Google Scholar] [CrossRef]
- Whitehouse, L.N.A.; Lapointe, B.E. Comparative ecophysiology of bloom-forming macroalgae in the Indian River Lagoon, Florida: Ulva lactuca, Hypnea musciformis, and Gracilaria tikvahiae. J. Exp. Mar. Biol. Ecol. 2015, 471, 208–216. [Google Scholar] [CrossRef]
- Tyler, A.C.; McGlathery, K.J. Uptake and release of nitrogen by the macroalgae Gracilaria vermiculophylla (Rhodophyta). J. Phycol. 2006, 42, 515–525. [Google Scholar] [CrossRef]
- Cacabelos, E.; Engelen, A.H.; Mejia, A.; Arenas, F. Comparison of the assemblage functioning of estuary systems dominated by the seagrass Nanozostera noltii versus the invasive drift seaweed Gracilaria vermiculophylla. J. Sea Res. 2012, 72, 99–105. [Google Scholar] [CrossRef]
- Thomsen, M.; Staehr, P.; Nyberg, C.; Schwaerter, S.; Krause-Jensen, D.; Silliman, B. Gracilaria vermiculophylla (Ohmi) Papenfuss, 1967 (Rhodophyta, Gracilariaceae) in northern Europe, with emphasis on Danish conditions, and what to expect in the future. ECU Publ. 2007, 2, 83–94. [Google Scholar] [CrossRef]
- Gómez, M.; Barreiro, F.; López, J.; Lastra, M.; de la Huz, R. Deposition patterns of algal wrack species on estuarine beaches. Aquat. Bot. 2013, 105, 25–33. [Google Scholar] [CrossRef]
- Wang, C.; Lei, A.; Zhou, K.; Hu, Z.; Hao, W.; Yang, J. Growth and nitrogen uptake characteristics reveal outbreak mechanism of the opportunistic macroalga Gracilaria tenuistipitata. PLoS ONE 2014, 9, e108980. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y.A.C. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Félix, R.; Dias, P.; Félix, C.; Cerqueira, T.; Andrade, P.B.; Valentão, P.; Lemos, M.F.L. The biotechnological potential of Asparagopsis armata: What is known of its chemical composition, bioactivities and current market? Algal Res. 2021, 60, 102534. [Google Scholar] [CrossRef]
- Rosic, N. Molecular Mechanisms of Stress Tolerance in Cyanobacteria. In Ecophysiology and Biochemistry of Cyanobacteria; Rastogi, R.P., Ed.; Springer Nature: Singapore, 2021; pp. 131–153. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Negara, B.F.; Lee, J.-H.; Cho, M.-G.; Kim, H.K.; Choi, Y.-S.; Lee, S.-H.; Choi, J.-S. The Influence of Abiotic Factors on the Induction of Seaweed Callus. J. Mar. Sci. Eng. 2022, 10, 513. [Google Scholar] [CrossRef]
- Rosic, N.; Remond, C.; Mello-Athayde, M.A. Differential impact of heat stress on reef-building corals under different light conditions. Mar. Environ. Res. 2020, 158, 104947. [Google Scholar] [CrossRef]
- Rosic, N.N.; Pernice, M.; Dove, S.; Dunn, S.; Hoegh-Guldberg, O. Gene expression profiles of cytosolic heat shock proteins Hsp70 and Hsp90 from symbiotic dinoflagellates in response to thermal stress: Possible implications for coral bleaching. Cell Stress Chaperones 2011, 16, 69–80. [Google Scholar] [CrossRef]
- Rosic, N.N.; Pernice, M.; Dunn, S.; Dove, S.; Hoegh-Guldberg, O. Differential regulation by heat stress of novel cytochrome P450 genes from the dinoflagellate symbionts of reef-building corals. Appl. Environ. Microbiol. 2010, 76, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.P. Effects of abiotic stressors on synthesis of the mycosporine-like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. Photochem. Photobiol. 2008, 84, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Voolstra, C.; Miller, D.; Ragan, M.; Hoffmann, A.; Hoegh-Guldberg, O.; Bourne, D.; Ball, E.; Ying, H.; Foret, S.; Takahashi, S.; et al. The ReFuGe 2020 Consortium—Using “omics” approaches to explore the adaptability and resilience of coral holobionts to environmental change. Front. Mar. Sci. 2015, 2, 68. [Google Scholar] [CrossRef]
- Ewere, E.E.; Rosic, N.; Bayer, P.E.; Ngangbam, A.; Edwards, D.; Kelaher, B.P.; Mamo, L.T.; Benkendorff, K. Marine heatwaves have minimal influence on the quality of adult Sydney rock oyster flesh. Sci. Total Environ. 2021, 795, 148846. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and Types of Mycosporine-Like Amino Acids (MAAs) in Marine Macroalgae and a Database for MAAs Based on These Characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-like amino acids from coral dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef] [PubMed]
- Navarro, N.P.; Figueroa, F.L.; Korbee, N. Mycosporine-like amino acids vs carrageenan yield in Mazzaella laminarioides (Gigartinales; Rhodophyta) under high and low UV solar irradiance. Phycologia 2017, 56, 570–578. [Google Scholar] [CrossRef]
- Korbee, N.; Huovinen, P.; Figueroa, F.L.; Aguilera, J.; Karsten, U. Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Mar. Biol. 2005, 146, 645–654. [Google Scholar] [CrossRef]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Avilés, A.; Chaves, P.; Horta, P.A.; Korbee, N.; Bonomi-Barufi, J. Physiological and biochemical responses driven by different UV-visible radiation in Osmundea pinnatifida (Hudson) Stackhouse (Rhodophyta). Photochem. Photobiol. Sci. 2020, 19, 1650–1664. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Rosic, N. Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid. Mar. Drugs 2022, 20, 478. [Google Scholar] [CrossRef]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory Effects of the Mycosporine-Like Amino Acids Shinorine and Porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Hu, Z.; Cheng, T.; Tang, Q.; Wang, H.; Deng, X.; Han, X. Extraction, Isolation and Characterization of Mycosporine-like Amino Acids from Four Species of Red Macroalgae. Mar. Drugs 2021, 19, 615. [Google Scholar] [CrossRef]
- Rosic, N.; Climstein, M.; Boyle, G.M.; Thanh Nguyen, D.; Feng, Y. Exploring Mycosporine-like Amino Acid UV-Absorbing Natural Products for a New Generation of Environmentally Friendly Sunscreens. Mar. Drugs 2023, 21, 253. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Ghedifa, A.B.; Vega, J.; Korbee, N.; Mensi, F.; Figueroa, F.L.; Sadok, S. Effects of light quality on the photosynthetic activity and biochemical composition of Gracilaria gracilis (Rhodophyta). J. Appl. Phycol. 2021, 33, 3413–3425. [Google Scholar] [CrossRef]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Avilés, A.; Horta, P.A.; Korbee, N.; Bonomi-Barufi, J. Effects of UV–visible radiation on growth, photosynthesis, pigment accumulation and UV-absorbing compounds in the red macroalga Gracilaria cornea (Gracilariales, Rhodophyta). Algal Res. 2022, 64, 102702. [Google Scholar] [CrossRef]
- Franklin, L.A.; Kräbs, G.; Kuhlenkamp, R. Blue light and UV-A radiation control the synthesis of mycosporine-like amino acids in Chondrus crispus (Florideophyceae). J. Phycol. 2001, 37, 257–270. [Google Scholar] [CrossRef]
- Kräbs, G.; Bischof, K.; Hanelt, D.; Karsten, U.; Wiencke, C. Wavelength-dependent induction of UV-absorbing mycosporine-like amino acids in the red alga Chondrus crispus under natural solar radiation. J. Exp. Mar. Biol. Ecol. 2002, 268, 69–82. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pacheco, D.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L.; Figueirinha, A.; Dias, J.M. Valorisation of marine macroalgae waste using a cascade biorefinery approach: Exploratory study. J. Clean. Prod. 2023, 385, 135672. [Google Scholar] [CrossRef]
- Mandalka, A.; Cavalcanti, M.I.; Harb, T.B.; Toyota Fujii, M.; Eisner, P.; Schweiggert-Weisz, U.; Chow, F. Nutritional Composition of Beach-Cast Marine Algae from the Brazilian Coast: Added Value for Algal Biomass Considered as Waste. Foods 2022, 11, 1201. [Google Scholar] [CrossRef]
- Karsten, U.; Escoubeyrou, K.; Charles, F. The effect of re-dissolution solvents and HPLC columns on the analysis of mycosporine-like amino acids in the eulittoral macroalgae Prasiola crispa and Porphyra umbilicalis. Helgol. Mar. Res. 2009, 63, 231–238. [Google Scholar] [CrossRef]
- Bermejo, R.; Green-Gavrielidis, L.; Gao, G. Editorial: Macroalgal blooms in a global change context. Front. Mar. Sci. 2023, 10, 1204117. [Google Scholar] [CrossRef]
- Guidone, M.; Thornber, C.S. Examination of Ulva bloom species richness and relative abundance reveals two cryptically co-occurring bloom species in Narragansett Bay, Rhode Island. Harmful Algae 2013, 24, 1–9. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Schmitz, C.; Ramlov, F.; de Lucena, L.A.F.; Uarrota, V.; Batista, M.B.; Sissini, M.N.; Oliveira, I.; Briani, B.; Martins, C.D.L.; Nunes, J.M.d.C.; et al. UVR and PAR absorbing compounds of marine brown macroalgae along a latitudinal gradient of the Brazilian coast. J. Photochem. Photobiol. B Biol. 2018, 178, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Haugan, J.A.; Liaaen-Jensen, S.v. Algal carotenoids 54. Carotenoids of brown algae (Phaeophyceae). Biochem. Syst. Ecol. 1994, 22, 31–41. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Chen, B.; Chen, H.; Qu, H.; Qiao, K.; Xu, M.; Wu, J.; Su, Y.; Shi, Y.; Liu, Z.; Wang, Q. Photoprotective effects of Sargassum thunbergii on ultraviolet B-induced mouse L929 fibroblasts and zebrafish. BMC Complement. Med. Ther. 2022, 22, 144. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Syafitri, S.M.; Geraldine, B.; Hamdin, C.D.; Frediansyah, A.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. UVA Photoprotective Activity of Brown Macroalgae Sargassum cristafolium. Biomedicines 2019, 7, 77. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Martyasari, N.W.R.; Abidin, A.S.; Pebriani, S.A.; Ilhami, B.T.K.; Frediansyah, A.; Sunarwidhi, A.L.; Widyastuti, S.; Sunarpi, H. Macroalgae Sargassum cristaefolium Extract Inhibits Proinflammatory Cytokine Expression in BALB/C Mice. Science 2020, 2020, 9769454. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Félix, R.; Soares, A.; Barata, C.; Novais, S.C.; Lemos, M.F.L. Asparagopsis armata Exudate Cocktail: The Quest for the Mechanisms of Toxic Action of an Invasive Seaweed on Marine Invertebrates. Biology 2021, 10, 223. [Google Scholar] [CrossRef]
- Silva, C.O.; Lemos, M.F.L.; Gaspar, R.; Gonçalves, C.; Neto, J.M. The effects of the invasive seaweed Asparagopsis armata on native rock pool communities: Evidences from experimental exclusion. Ecol. Indic. 2021, 125, 107463. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien Marine Species in the Mediterranean—The 100 ‘Worst Invasives’ and their Impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Lopez Figueroa, F.; Bueno, A.; Korbee, N.; Santos, R.; Mata, L.; Schuenhoff, A. Accumulation of Mycosporine-like Amino Acids in Asparagopsis armata Grown in Tanks with Fishpond Effluents of Gilthead Sea Bream, Sparus aurata. J. World Aquac. Soc. 2008, 39, 5. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Miladinovic, H.; Nguyen Ngoc, H.; Karsten, U.; Ganzera, M. Bostrychines A-F, Six Novel Mycosporine-Like Amino-Acids and a Novel Betaine from the Red Alga Bostrychia scorpioides. Mar. Drugs 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The banned sunscreen ingredients and their impact on human health: A systematic review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of Mycosporine-Like Amino Acids from Gracilaria vermiculophylla (Rhodophyta) Cultured Through One Year in an Integrated Multi-trophic Aquaculture (IMTA) System. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Effects of UV Radiation on Photosynthesis, Antioxidant Capacity and the Accumulation of Bioactive Compounds in Gracilariopsis longissima, Hydropuntia cornea and Halopithys incurva (Rhodophyta). J. Phycol. 2019, 55, 1258–1273. [Google Scholar] [CrossRef]
- Barufi, J.B.; Mata, M.T.; Oliveira, M.C.; Figueroa, F.L. Nitrate reduces the negative effect of UV radiation on photosynthesis and pigmentation in Gracilaria tenuistipitata (Rhodophyta): The photoprotection role of mycosporine-like amino acids. Phycologia 2012, 51, 636–648. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, G.; Sun, J.; Leng, X.; Xu, W.; Wu, C.; Li, X.; Pujari, L. Seasonal responses of nutrient to hydrology and biology in the southern Yellow Sea. Cont. Shelf Res. 2020, 206, 104207. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar] [CrossRef]
- Duarte, B.; Carreiras, J.; Feijão, E.; de Carvalho, R.C.; Matos, A.R.; Fonseca, V.F.; Novais, S.C.; Lemos, M.F.L. Potential of Asparagopsis armata as a Biopesticide for Weed Control under an Invasive Seaweed Circular-Economy Framework. Biology 2021, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Roleda, M.Y.; Nyberg, C.D.; Wulff, A. UVR defense mechanisms in eurytopic and invasive Gracilaria vermiculophylla (Gracilariales, Rhodophyta). Physiol. Plant 2012, 146, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ngoennet, S.; Nishikawa, Y.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. A Method for the Isolation and Characterization of Mycosporine-Like Amino Acids from Cyanobacteria. Methods Protoc. 2018, 1, 46. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an Integrated Multi-Trophic Aquaculture system. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef]
- Xie, C.-L.; Liu, Q.; Xia, J.-M.; Gao, Y.; Yang, Q.; Shao, Z.-Z.; Liu, G.; Yang, X.-W. Anti-Allergic Compounds from the Deep-Sea-Derived Actinomycete Nesterenkonia flava MCCC 1K00610. Mar. Drugs 2017, 15, 71. [Google Scholar] [CrossRef]
- Miyamoto, K.T.; Komatsu, M.; Ikeda, H. Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. Appl. Environ. Microbiol. 2014, 80, 5028–5036. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pagès, C.; Gattuso, J.P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef]
- Pope, M.A.; Spence, E.; Seralvo, V.; Gacesa, R.; Heidelberger, S.; Weston, A.J.; Dunlap, W.C.; Shick, J.M.; Long, P.F. O-methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413. ChemBioChem 2015, 16, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Phylogenetic analysis of genes involved in mycosporine-like amino acid biosynthesis in symbiotic dinoflagellates. Appl. Microbiol. Biotechnol. 2012, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, P.M.; Woodhouse, J.N.; Liew, H.T.; Sehnal, L.; Pickford, R.; Wong, H.L.; Burns, B.P.; Neilan, B.A. Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Environ. Microbiol. 2019, 21, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Shoguchi, E. Gene clusters for biosynthesis of mycosporine-like amino acids in dinoflagellate nuclear genomes: Possible recent horizontal gene transfer between species of Symbiodiniaceae (Dinophyceae). J. Phycol. 2022, 58, 1–11. [Google Scholar] [CrossRef]
- de Mendoza, A.; Bonnet, A.; Vargas-Landin, D.B.; Ji, N.; Li, H.; Yang, F.; Li, L.; Hori, K.; Pflueger, J.; Buckberry, S.; et al. Recurrent acquisition of cytosine methyltransferases into eukaryotic retrotransposons. Nat. Commun. 2018, 9, 1341. [Google Scholar] [CrossRef]
- Angel, T.E.; Aryal, U.K.; Hengel, S.M.; Baker, E.S.; Kelly, R.T.; Robinson, E.W.; Smith, R.D. Mass spectrometry-based proteomics: Existing capabilities and future directions. Chem. Soc. Rev. 2012, 41, 3912–3928. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R., III. Protein Analysis by Shotgun/Bottom-up Proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef]
- Callone, A.I.; Carignan, M.; Montoya, N.G.; Carreto, J.I. Biotransformation of mycosporine like amino acids (MAAs) in the toxic dinoflagellate Alexandrium tamarense. J. Photochem. Photobiol. B Biol. 2006, 84, 204–212. [Google Scholar] [CrossRef]
- Jeffrey, S.; MacTavish, H.; Dunlap, W.; Vesk, M.; Groenewoud, K. Occurrence of UVA-and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Mar. Ecol. Prog. Ser. 1999, 189, 35–51. [Google Scholar] [CrossRef]
- Yamamoto, R.; Takizawa, K.; Miyabe, Y.; Mune Mune, M.A.; Kishimura, H.; Kumagai, Y. Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea inkyuleei): Monthly Variation and Improvement in Extraction. Phycology 2023, 3, 394–404. [Google Scholar] [CrossRef]
- Rosic, N.N.; Braun, C.; Kvaskoff, D. Extraction and Analysis of Mycosporine-Like Amino Acids in Marine Algae. In Natural Products from Marine Algae: Methods and Protocols; Stengel, D.B., Connan, S., Eds.; Springer: New York, NY, USA, 2015; pp. 119–129. [Google Scholar] [CrossRef]
- Whitehead, K.; Hedges, J.I. Analysis of mycosporine-like amino acids in plankton by liquid chromatography electrospray ionization mass spectrometry. Mar. Chem. 2002, 80, 27–39. [Google Scholar] [CrossRef]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of mycosporine-like amino acids in selected algae and cyanobacteria by hydrophilic interaction liquid chromatography and a novel MAA from the red alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, V.; de Medeiros, L.S.; Jacinavicius, F.R.; Long, P.F.; Pinto, E. Development and validation of a rapid LC-MS/MS method for the quantification of mycosporines and mycosporine-like amino acids (MAAs) from cyanobacteria. Algal Res. 2020, 46, 101796. [Google Scholar] [CrossRef]
- Parailloux, M.; Godin, S.; Fernandes, S.C.M.; Lobinski, R. Untargeted Analysis for Mycosporines and Mycosporine-Like Amino Acids by Hydrophilic Interaction Liquid Chromatography (HILIC)—Electrospray Orbitrap MS2/MS3. Antioxidants 2020, 9, 1185. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Zwerger, M.; Ganzera, M. Fast and Efficient Separation of Eleven Mycosporine-like Amino Acids by UHPLC-DAD and Their Quantification in Diverse Red Algae. Mar. Drugs 2022, 20, 395. [Google Scholar] [CrossRef]
- Zwerger, M.J.; Hammerle, F.; Siewert, B.; Ganzera, M. Application of feature-based molecular networking in the field of algal research with special focus on mycosporine-like amino acids. J. Appl. Phycol. 2023, 35, 1377–1392. [Google Scholar] [CrossRef]
- Chan, L.L.; Hodgkiss, I.J.; Wan, J.M.; Lum, J.H.; Mak, A.S.; Sit, W.H.; Lo, S.C. Proteomic study of a model causative agent of harmful algal blooms, Prorocentrum triestinum II: The use of differentially expressed protein profiles under different growth phases and growth conditions for bloom prediction. Proteomics 2004, 4, 3214–3226. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef] [PubMed]
- Hennon, G.M.M.; Dyhrman, S.T. Progress and promise of omics for predicting the impacts of climate change on harmful algal blooms. Harmful Algae 2020, 91, 101587. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tang, Y.; Gobler, C.J. Harmful algal blooms in China: History, recent expansion, current status, and future prospects. Harmful Algae 2023, 129, 102499. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).