Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo
Abstract
1. Introduction
2. Results
2.1. Molecular Characterization of Jellyfish Collagen
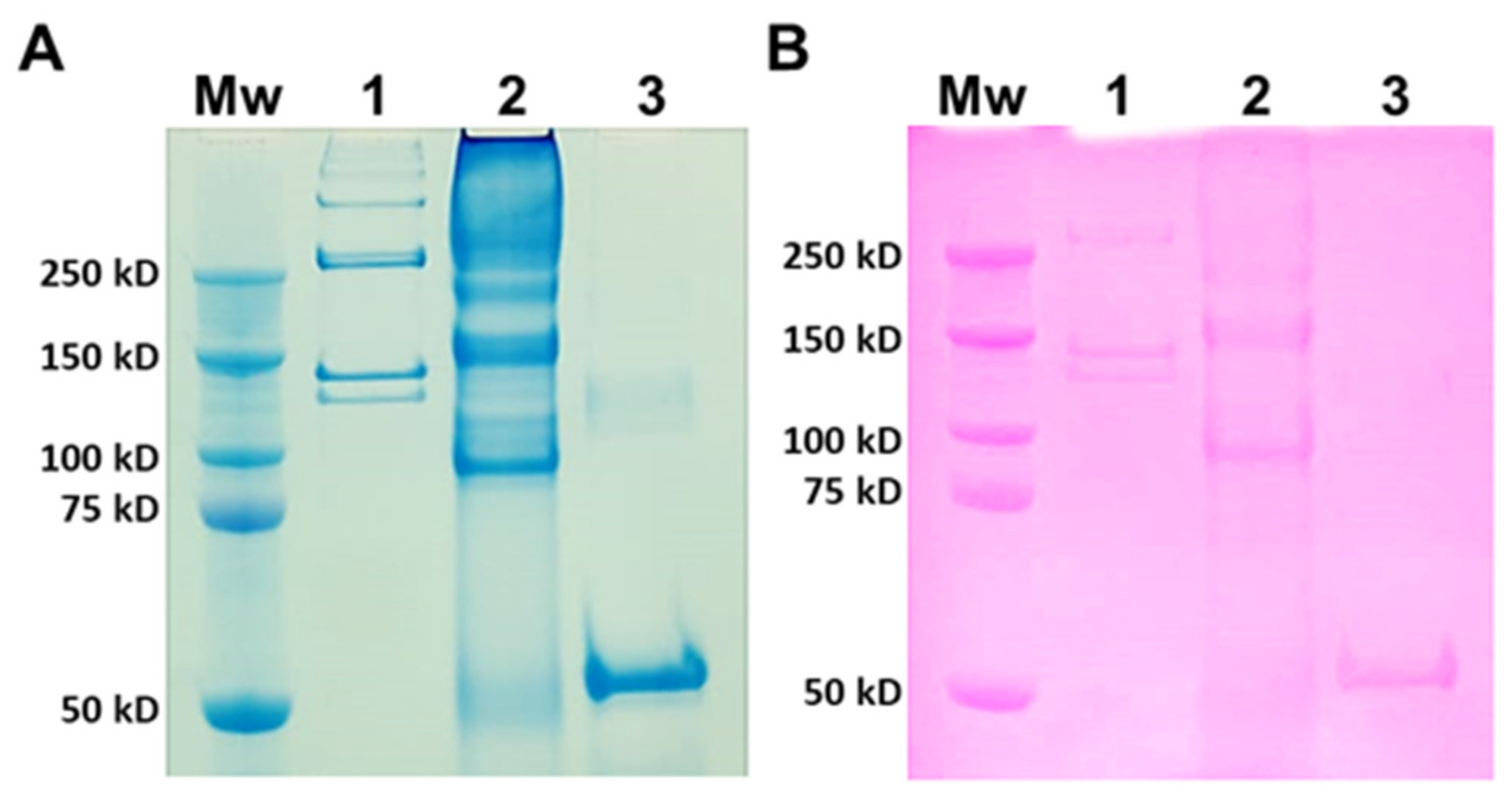
2.1.1. SDS-PAGE
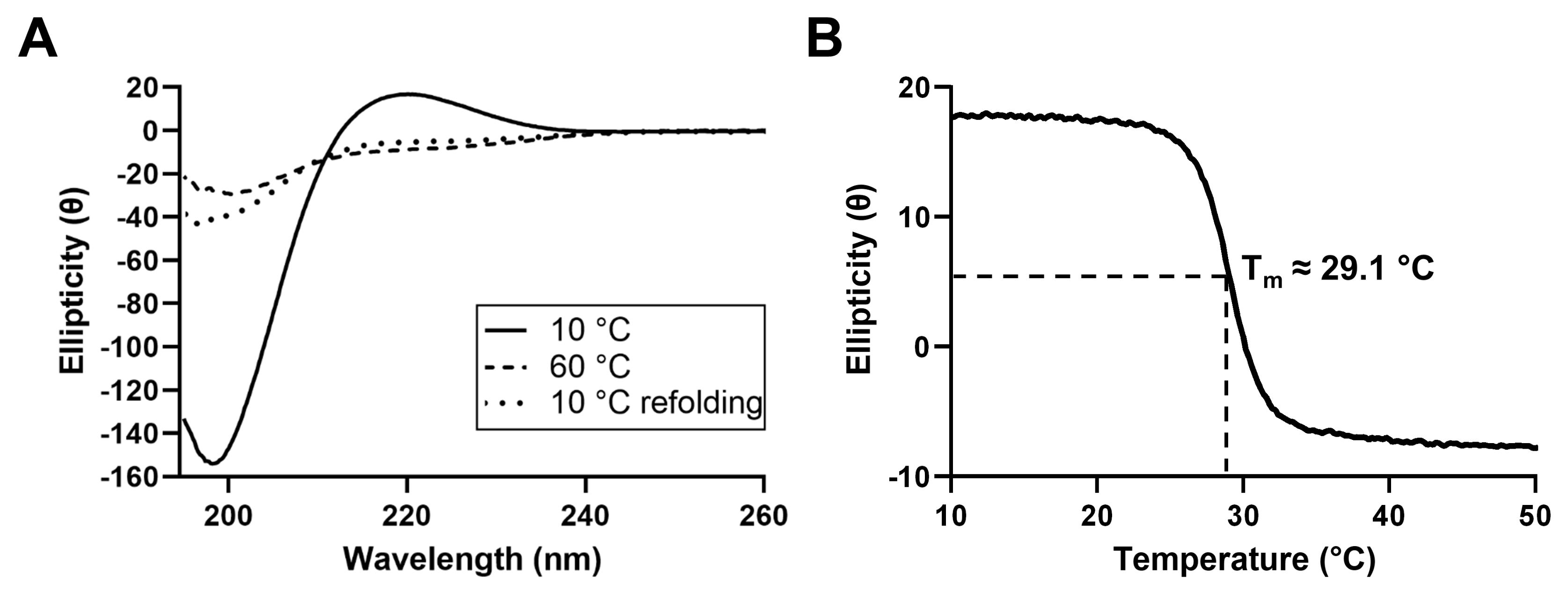
2.1.2. Circular Dichroism Spectroscopy
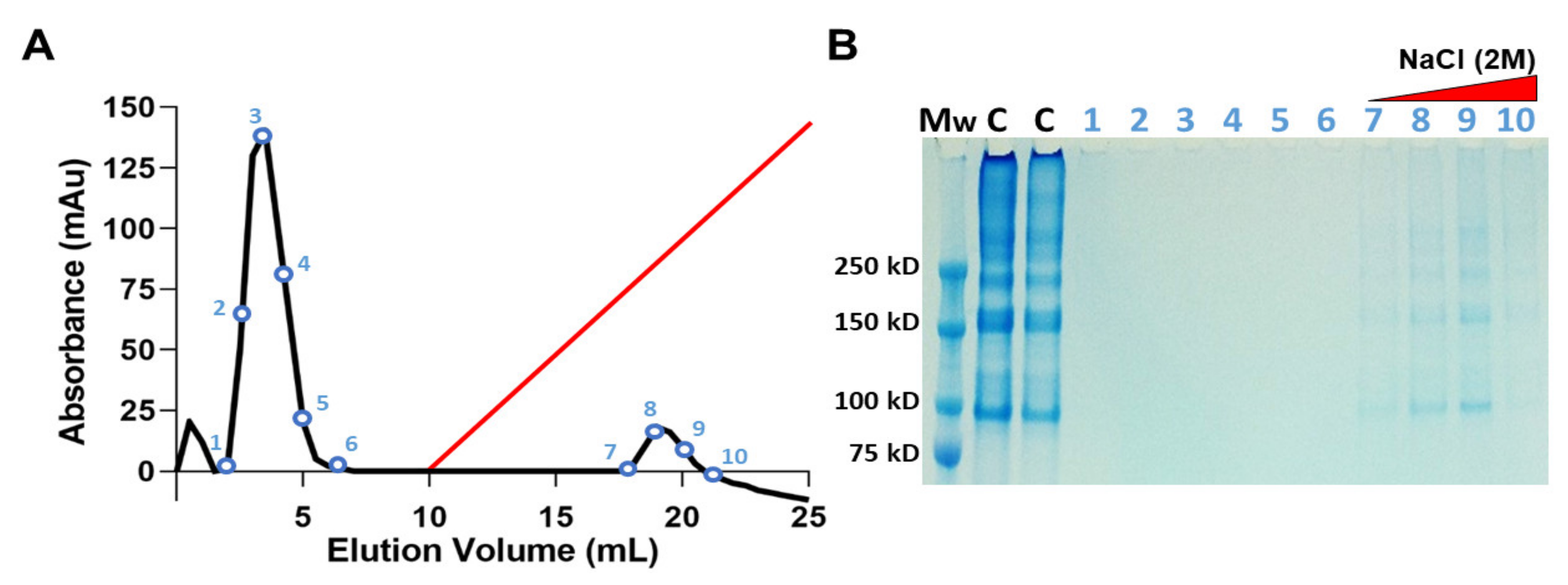
2.1.3. Heparin-Affinity Chromatography
2.2. Jellyfish Collagen Fibrillogenesis
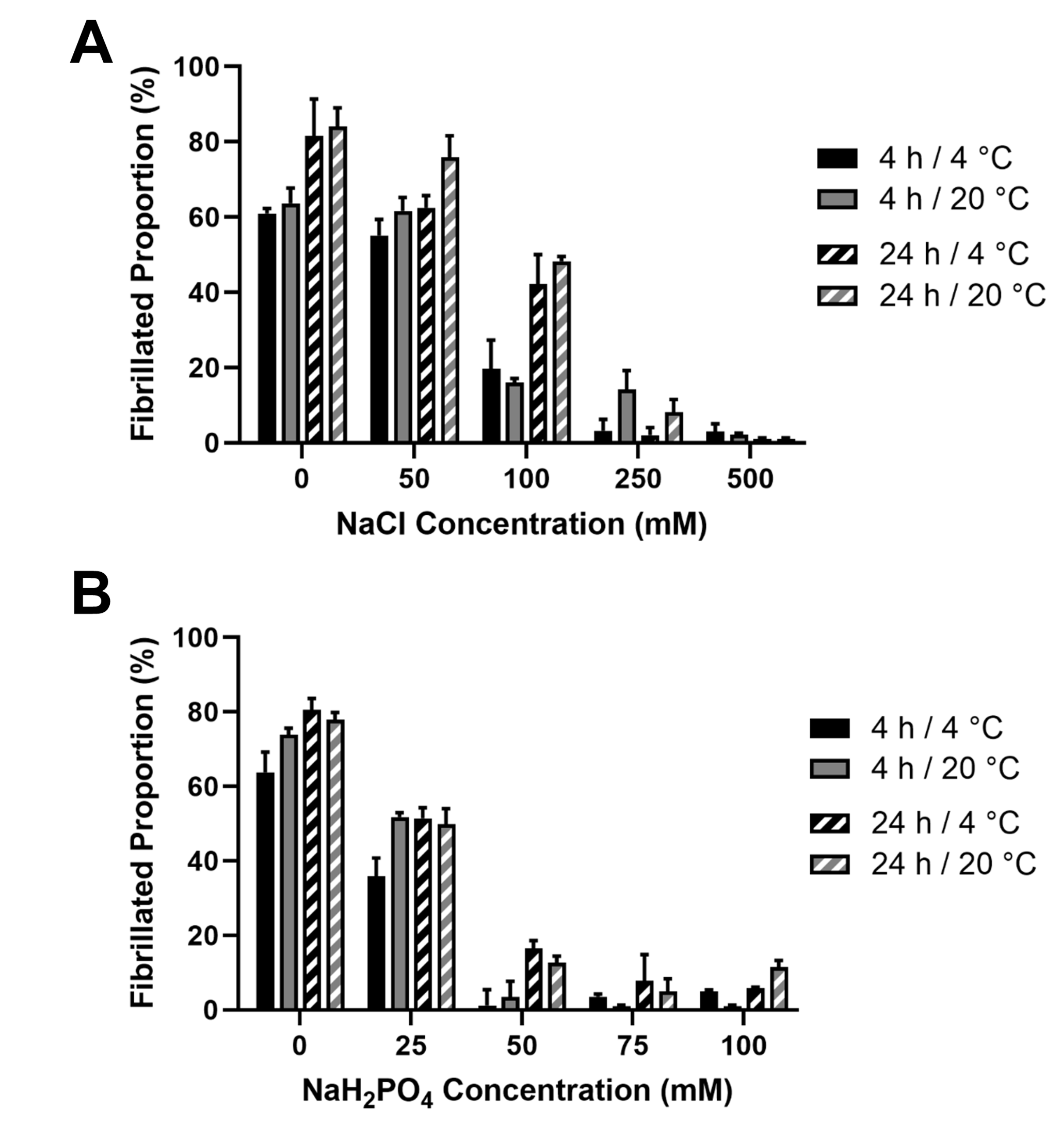
2.2.1. Fibrillogenesis Assay
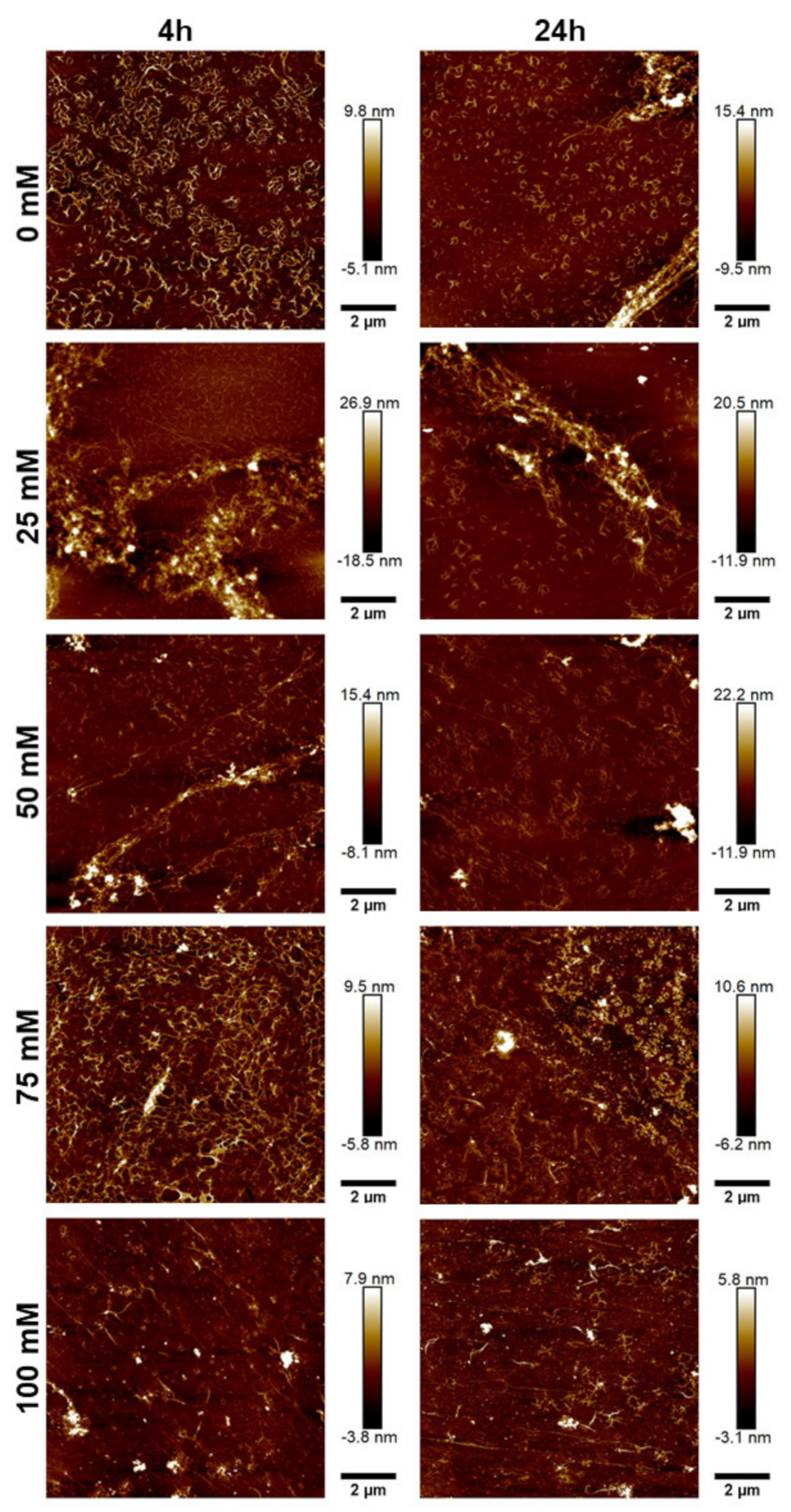
2.2.2. Atomic Force Microscopy
2.3. In Vitro Culture of Human Fibroblasts and MSCs on Jellyfish Collagen
2.3.1. Proliferation and Spreading Assay
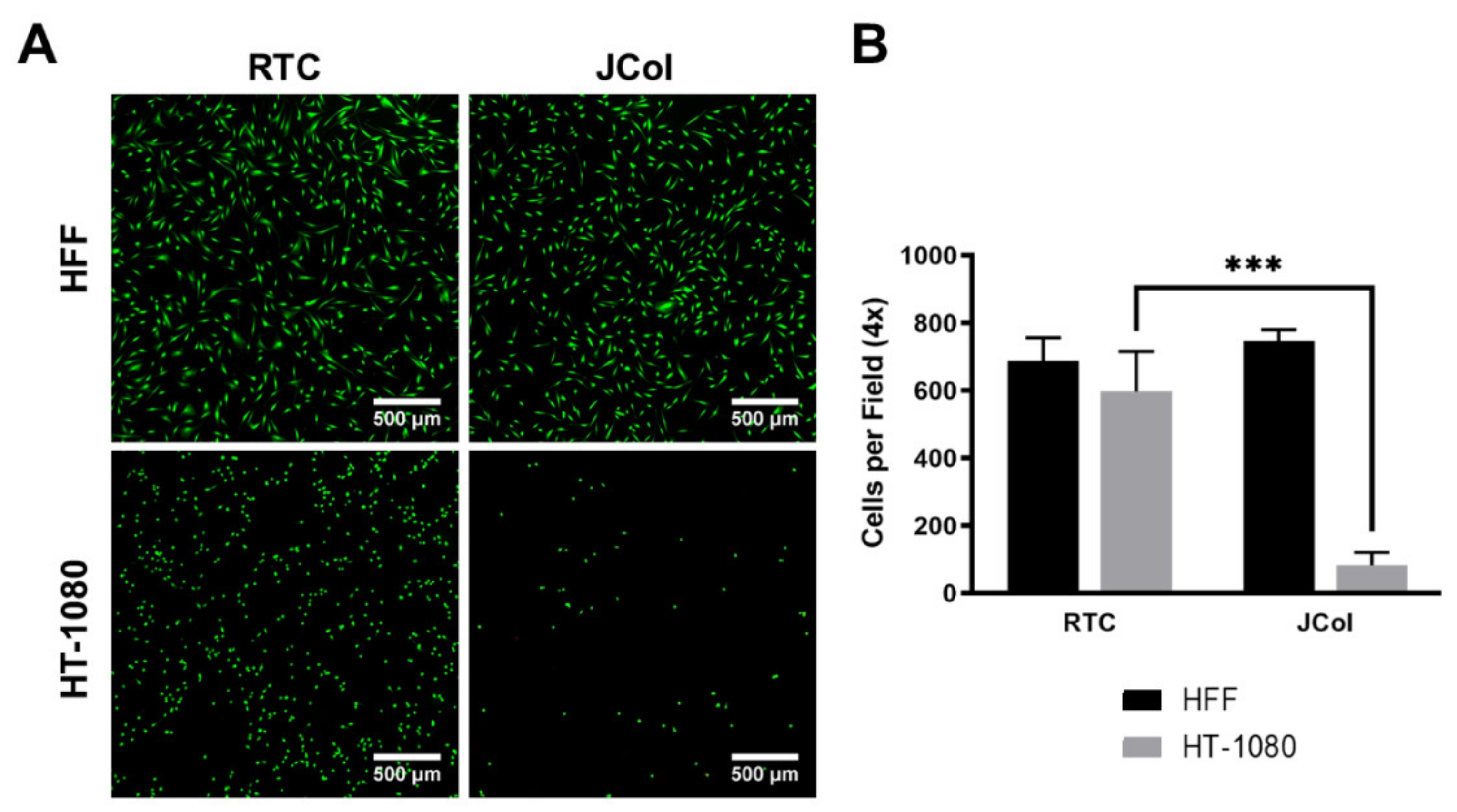
2.3.2. Adhesion Assay
2.3.3. Integrin and Heparan Sulfate Chain Adhesion Interactions of Jellyfish Collagen
3. Discussion
3.1. SDS-PAGE Analysis of JCol
3.2. JCol Secondary Structure and Thermal Stability
3.3. JCol Fibrillogenesis
3.4. Mammalian Cell Adhesion to JCol
3.5. Jellyfish Collagenomes
4. Materials and Methods
4.1. Collagen
4.2. SDS-PAGE
4.3. Circular Dichroism Spectroscopy
4.4. Heparin Chromatography
4.5. Collagen Fibrillogenesis Assay
4.6. Atomic Force Microscopy
4.7. Cell Culture
4.8. Proliferation and Spreading Assay
4.9. Immunofluorescence Staining and Quantitative Imaging
4.10. Adhesion Assay
4.11. Binding Inhibition Assay
4.12. Statistical Calculations
4.13. Preliminary Analysis of Collagen Sequences in Jellyfish Genomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M. Biomedical applications of collagens. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 665–675. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Bella, J.; Hulmes, D.J.S. Fibrillar Collagens. In Fibrous Proteins: Structures and Mechanisms; Springer: Cham, Switzerland, 2017; pp. 457–490. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; De Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Silva, T.H.; Alves, A.; Ferreira, B.M.; Oliveira, J.M.; Reys, L.L.; Ferreira, R.J.F.; Sousa, A.R.; Silva, S.S.; Mano, J.F.; Reis, R.L. Materials of marine origin: A review on polymers and ceramics of biomedical interest. Int. Mater. Rev. 2012, 57, 276–306. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2016, 28, 4–9. [Google Scholar] [CrossRef]
- Hashim, P.; Mohd Ridzwan, M.S.; Bakar, J.; Mat Hashim, D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass Valorization 2019, 11, 5687–5698. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Bishop, C. Verbals in -tos in Sophocles. Am. J. Philol. 2009, 20, 214–226. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Kumar, K.K.; Bhat, A.R.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Felician, F.F.; Yu, R.-H.; Li, M.-Z.; Li, C.-J.; Chen, H.-Q.; Jiang, Y.; Tang, T.; Qi, W.-Y.; Xu, H.-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Frazão, B.; Antunes, A. Jellyfish Bioactive Compounds: Methods for Wet-Lab Work. Mar. Drugs 2016, 14, 75. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, A.; Osaki, T.; Matahira, Y.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Evaluation of the Chondroprotective Effects of Glucosamine and Fish Collagen Peptide on a Rabbit ACLT Model Using Serum Biomarkers. J. Veter. Med. Sci. 2013, 75, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A. Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Rizzo, L.; Basso, L.; Marzano, M.; Fosso, B.; Pesole, G.; Piraino, S. The Microbial Community Associated with Rhizostoma pulmo: Ecological Significance and Potential Consequences for Marine Organisms and Human Health. Mar. Drugs 2020, 18, 437. [Google Scholar] [CrossRef]
- Purcell, J.E.; Uye, S.-I.; Lo, W.-T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- De Donno, A.; Idolo, A.; Bagordo, F.; Grassi, T.; Leomanni, A.; Serio, F.; Guido, M.; Canitano, M.; Zampardi, S.; Boero, F.; et al. Impact of Stinging Jellyfish Proliferations along South Italian Coasts: Human Health Hazards, Treatment and Social Costs. Int. J. Environ. Res. Public Health 2014, 11, 2488–2503. [Google Scholar] [CrossRef]
- Bermueller, C.; Schwarz, S.; Elsaesser, A.F.; Sewing, J.; Baur, N.; von Bomhard, A.; Scheithauer, M.; Notbohm, H.; Rotter, N. Marine Collagen Scaffolds for Nasal Cartilage Repair: Prevention of Nasal Septal Perforations in a New Orthotopic Rat Model Using Tissue Engineering Techniques. Tissue Eng. Part A 2013, 19, 2201–2214. [Google Scholar] [CrossRef]
- Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and its Suitability for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- Paradiso, F.; Fitzgerald, J.; Yao, S.; Barry, F.; Taraballi, F.; Gonzalez, D.; Conlan, R.S.; Francis, L. Marine Collagen Substrates for 2D and 3D Ovarian Cancer Cell Systems. Front. Bioeng. Biotechnol. 2019, 7, 343. [Google Scholar] [CrossRef]
- Sun, X.-D.; Yan, H.; Shan, C.; Ding, S.-S.; Li, C.; Dong, J.; Cheng, X.; Qian, W.-P. Biomimetic Double-Layered Scaffolds Composed of Jellyfish Collagen and Chitosan for Cartilage Tissue Engineering. J. Biomater. Tissue Eng. 2014, 4, 1080–1086. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Salamone, M.; Barbarino, E.; Barbarino, M.; Nicosia, A.; Ghersi, G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 5798. [Google Scholar] [CrossRef] [PubMed]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J. Tissue Eng. Regen. Med. 2015, 11, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish collagen and alginate: Combined marine materials for superior chondrogenesis of hMSC. Mater. Sci. Eng. C 2016, 64, 190–198. [Google Scholar] [CrossRef]
- Mearns-Spragg, A.; Tilman, J.; Tams, D.; Barnes, A. The Biological Evaluation of Jellyfish Collagen as a New Research Tool for the Growth and Culture of iPSC Derived Microglia. Front. Mar. Sci. 2020, 689. [Google Scholar] [CrossRef]
- Keller, L. Combined Jellyfish Collagen Type II, Human Stem Cells and Tgf-β3 as a Therapeutic Implant for Cartilage Repair. J. Stem Cell Res. Ther. 2017, 7, 382. [Google Scholar] [CrossRef]
- Ahmed, Z.; Powell, L.; Matin, N.; Mearns-Spragg, A.; Thornton, C.; Khan, I.; Francis, L. Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity. Mar. Drugs 2021, 19, 405. [Google Scholar] [CrossRef]
- Khalturin, K.; Shinzato, C.; Khalturina, M.; Hamada, M.; Fujie, M.; Koyanagi, R.; Kanda, M.; Goto, H.; Anton-Erxleben, F.; Toyokawa, M.; et al. Medusozoan genomes inform the evolution of the jellyfish body plan. Nat. Ecol. Evol. 2019, 3, 811–822. [Google Scholar] [CrossRef]
- Akkus, O.; Belaney, R.M.; Das, P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J. Orthop. Res. 2005, 23, 838–845. [Google Scholar] [CrossRef]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen structural hierarchy and susceptibility to degradation by ultraviolet radiation. Mater. Sci. Eng. C 2008, 28, 1420–1429. [Google Scholar] [CrossRef]
- Gopinath, A.; Reddy, S.M.M.; Madhan, B.; Shanmguam, G.; Rao, J.R. Effect of aqueous ethanol on the triple helical structure of collagen. Eur. Biophys. J. 2014, 43, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Water and Carbon Dioxide: Green Solvents for the Extraction of Collagen/Gelatin from Marine Sponges. ACS Sustain. Chem. Eng. 2015, 3, 254–260. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically Mineralized Salmon Collagen Scaffolds for Application in Bone Tissue Engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater. Sci. Eng. C 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Schifferli, K. Analysis of Integrin Expression and Function in ht1080 Cells using Inhibitory Anti-Integrin Antibodies. 2020. Available online: https://www.researchgate.net/profile/Kevin-Schifferli/publication/290130839_ANALYSIS_OF_INTEGRIN_EXPRESSION_AND_FUNCTION_IN_HT1080_CELLS_USING_INHIBITORY_ANTI-INTEGRIN_ANTIBODIES/links/5f747a49a6fdcc008648e6c4/ANALYSIS-OF-INTEGRIN-EXPRESSION-AND-FUNCTION-IN-HT1080-CELLS-USING-INHIBITORY-ANTI-INTEGRIN-ANTIBODIES.pdf (accessed on 15 January 2023).
- Tuckwell, D.S.; Reid, K.B.M.; Barnes, M.J.; Humphries, M.J. The A-Domain of Integrin alpha2 Binds Specifically to a Range of Collagens but is not a General Receptor for the Collagenous Motif. JBIC J. Biol. Inorg. Chem. 1996, 241, 732–739. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Derkus, B.; Arslan, Y.E.; Bayrac, A.T.; Kantarcioglu, I.; Emregul, K.C.; Emregul, E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sensors Actuators B Chem. 2016, 228, 725–736. [Google Scholar] [CrossRef]
- Arslan, Y.E.; Arslan, T.S.; Derkus, B.; Emregul, E.; Emregul, K.C. Fabrication of human hair keratin/jellyfish collagen/eggshell-derived hydroxyapatite osteoinductive biocomposite scaffolds for bone tissue engineering: From waste to regenerative medicine products. Colloids Surf. B Biointerfaces 2017, 154, 160–170. [Google Scholar] [CrossRef]
- Carvalho, D.N.; López-Cebral, R.; Sousa, R.O.; Alves, A.L.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Reis, R.L.; Silva, T.H. Marine collagen-chitosan-fucoidan cryogels as cell-laden biocomposites envisaging tissue engineering. Biomed. Mater. 2020, 15, 055030. [Google Scholar] [CrossRef]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In vivocomparison of jellyfish and bovine collagen sponges as prototype medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xue, C.; Chang, Y.; Shen, J.; Zhang, Y.; Li, Z.; Wang, Y. Collagen fibrils of sea cucumber (Apostichopus japonicus) are heterotypic. Food Chem. 2020, 316, 126272. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Heino, J. Cellular Signaling by Collagen-Binding Integrins. In I Domain Integrins; Springer: Berlin/Heidelberg, Germany, 2014; Volume 819, pp. 143–155. [Google Scholar] [CrossRef]
- Tulla, M.; Pentikäinen, O.T.; Viitasalo, T.; Käpylä, J.; Impola, U.; Nykvist, P.; Nissinen, L.; Johnson, M.S.; Heino, J. Selective Binding of Collagen Subtypes by Integrin α1I, α2I, and α10I Domains. J. Biol. Chem. 2001, 276, 48206–48212. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Hamaia, S.; Farndale, R.W. Integrin Recognition Motifs in the Human Collagens. In I Domain Integrins; Springer: Berlin/Heidelberg, Germany, 2014; Volume 819, pp. 127–142. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2009, 339, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Meneghetti, M.C.Z.; Hughes, A.; Rudd, T.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1−SMAD1 interaction. Bone Res. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Thromb. Haemost. 2010, 12, 216. [Google Scholar] [CrossRef]
- Zhang, X.; Boot-Handford, R.; Huxley-Jones, J.; Forse, L.N.; Mould, P.; Robertson, D.L.; Li, L.; Athiyal, M.; Sarras, M.P. The Collagens of Hydra Provide Insight into the Evolution of Metazoan Extracellular Matrices. J. Biol. Chem. 2007, 282, 6792–6802. [Google Scholar] [CrossRef]
- Leclère, L.; Horin, C.; Chevalier, S.; Lapébie, P.; Dru, P.; Peron, S.; Jager, M.; Condamine, T.; Pottin, K.; Romano, S.; et al. The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat. Ecol. Evol. 2019, 3, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.A.; Katsuki, T.; Li, Y.; Yan, X.; Regulski, M.; Ibberson, D.; Holstein, T.; Steele, R.E.; Jacobs, D.K.; Greenspan, R.J. The genome of the jellyfish Aurelia and the evolution of animal complexity. Nat. Ecol. Evol. 2018, 3, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Hwang, D.-S.; Lee, J.-S.; Song, J.-I.; Seo, T.-K.; Won, Y.-J. Estimation of divergence times in cnidarian evolution based on mitochondrial protein-coding genes and the fossil record. Mol. Phylogenetics Evol. 2012, 62, 329–345. [Google Scholar] [CrossRef]
- Li, Y.; Gao, L.; Pan, Y.; Tian, M.; Li, Y.; He, C.; Dong, Y.; Sun, Y.; Zhou, Z. Chromosome-level reference genome of the jellyfish Rhopilema esculentum. Gigascience 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Deutzmann, R.; Fowler, S.; Zhang, X.; Boone, K.; Dexter, S.; Boot-Handford, R.; Rachel, R.; Sarras, M. Molecular, biochemical and functional analysis of a novel and developmentally important fibrillar collagen (Hcol-I) in hydra. Development 2000, 127, 4669–4680. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2017, 46, D493–D496. [Google Scholar] [CrossRef]
- Miura, S.; Kimura, S. Jellyfish mesogloea collagen. Characterization of molecules as alpha 1 alpha 2 alpha 3 heterotrimers. J. Biol. Chem. 1985, 260, 15352–15356. [Google Scholar] [CrossRef]
- Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of edible jellyfish exumbrella, J. Sci. Food Agric. 1999, 79, 855–858. [Google Scholar] [CrossRef]
- Riacci, L.; Sorriento, A.; Ricotti, L. Genipin-Based Crosslinking of Jellyfish Collagen 3D Hydrogels. Gels 2021, 7, 238. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- James, S.; Fox, J.; Afsari, F.; Lee, J.; Clough, S.; Knight, C.; Ashmore, J.; Ashton, P.; Preham, O.; Hoogduijn, M.; et al. Multiparameter Analysis of Human Bone Marrow Stromal Cells Identifies Distinct Immunomodulatory and Differentiation-Competent Subtypes. Stem Cell Rep. 2015, 4, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Torre, A.G.; Shaw, J.E.; Wood, A.; Gilbert, H.T.J.; Dobre, O.; Genever, P.; Brennan, K.; Richardson, S.M.; Swift, J. An immortalised mesenchymal stem cell line maintains mechano-responsive behaviour and can be used as a reporter of substrate stiffness. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda–Carrera, I.; Frise, E.; Verena, K.; Mark, L.; Tobias, P.; Stephan, P.; Curtis, R.; Stephan, S.; Benjamin, S.; et al. Fiji-an open platform for biological image analysis. Nat. Methods 2009, 9, 10–38. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A.; Kirkness, E.F.; Simakov, O.; Hampson, S.E.; Mitros, T.; Weinmaier, T.; Rattei, T.; Balasubramanian, P.G.; Borman, J.; Busam, D.; et al. The dynamic genome of Hydra. Nature 2010, 464, 592–596. [Google Scholar] [CrossRef]
- Ohdera, A.; Ames, C.L.; Dikow, R.B.; Kayal, E.; Chiodin, M.; Ben Busby, B.; La, S.; Pirro, S.; Collins, A.G.; Medina, M.; et al. Box, stalked, and upside-down? Draft genomes from diverse jellyfish (Cnidaria, Acraspeda) lineages: Alatina alata (Cubozoa), Calvadosia cruxmelitensis (Staurozoa), and Cassiopea xamachana (Scyphozoa). Gigascience 2019, 8, giz069. [Google Scholar] [CrossRef]
- Ryu, J.-C.; Kim, H.-M.; Weber, J.A.; Lee, N.; Park, S.G.; Cho, Y.S.; Bhak, Y.; Lee, N.; Jeon, Y.; Jeon, S.; et al. The jellyfish genome sheds light on the early evolution of active predation. BioRxiv 2018, 1–12. [Google Scholar] [CrossRef]
- Xia, W.; Li, H.; Cheng, W.; Li, H.; Mi, Y.; Gou, X.; Liu, Y. High-Quality Genome Assembly of Chrysaora quinquecirrha Provides Insights Into the Adaptive Evolution of Jellyfish. Front. Genet. 2020, 11. [Google Scholar] [CrossRef]




| Genome | Class | HCol1, HCol2, HCol5 | Hcol3 | Other fibrillar | Hcol4 | Hcol6 |
|---|---|---|---|---|---|---|
| Hydra vulgaris | Hydrozoa | 3 | 1 | 2 | 1 | 1 |
| Clytia hemisphaerica | Hydrozoa | 5 | 1 | 2 | 1 | 4 |
| Aurelia aurita | Scyphozoa | 4 | 1 | 2 | 1 | 1 |
| Nepomilema nomurai | Scyphozoa | 4 | 1 | 2 | 1 | 1 |
| Rhopilema esculentum | Scyphozoa | 4 | 1 | 3 | 2 | 1 |
| Chrysaora quinquecirrha | Scyphozoa | 4 | 1 | 2 | 1 | 1 |
| Cassiopea xamachana | Scyphozoa | 4 | 1 | 2 | 1 | 1 |
| Cassiopea andromeda | Scyphozoa | 4 | 1 | 2 | 1 | 1 |
| Alatina alata | Cubozoa | 2 | 1 | 2 | 1 | 1 |
| Morbakka virulenta | Cubozoa | 2 | 1 | 2 | 1 | 2 |
| Calvadoxia cruxmellitensis | Staurozoa | 2 | 1 | 2 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, I.P.; Domingos, M.; Richardson, S.M.; Bella, J. Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo. Mar. Drugs 2023, 21, 59. https://doi.org/10.3390/md21020059
Smith IP, Domingos M, Richardson SM, Bella J. Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo. Marine Drugs. 2023; 21(2):59. https://doi.org/10.3390/md21020059
Chicago/Turabian StyleSmith, Ian P., Marco Domingos, Stephen M. Richardson, and Jordi Bella. 2023. "Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo" Marine Drugs 21, no. 2: 59. https://doi.org/10.3390/md21020059
APA StyleSmith, I. P., Domingos, M., Richardson, S. M., & Bella, J. (2023). Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo. Marine Drugs, 21(2), 59. https://doi.org/10.3390/md21020059







