Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum
Abstract
:1. Introduction
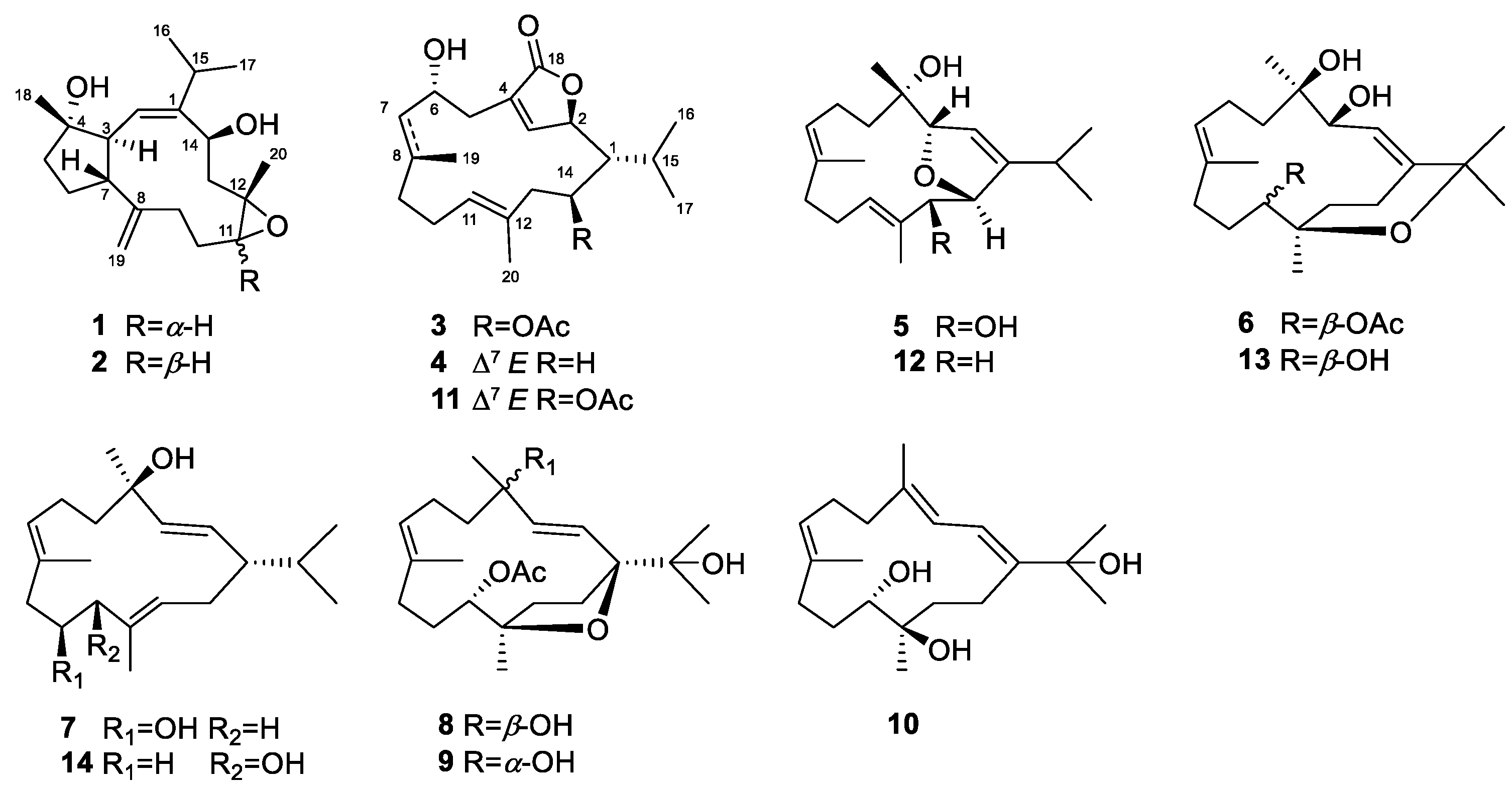
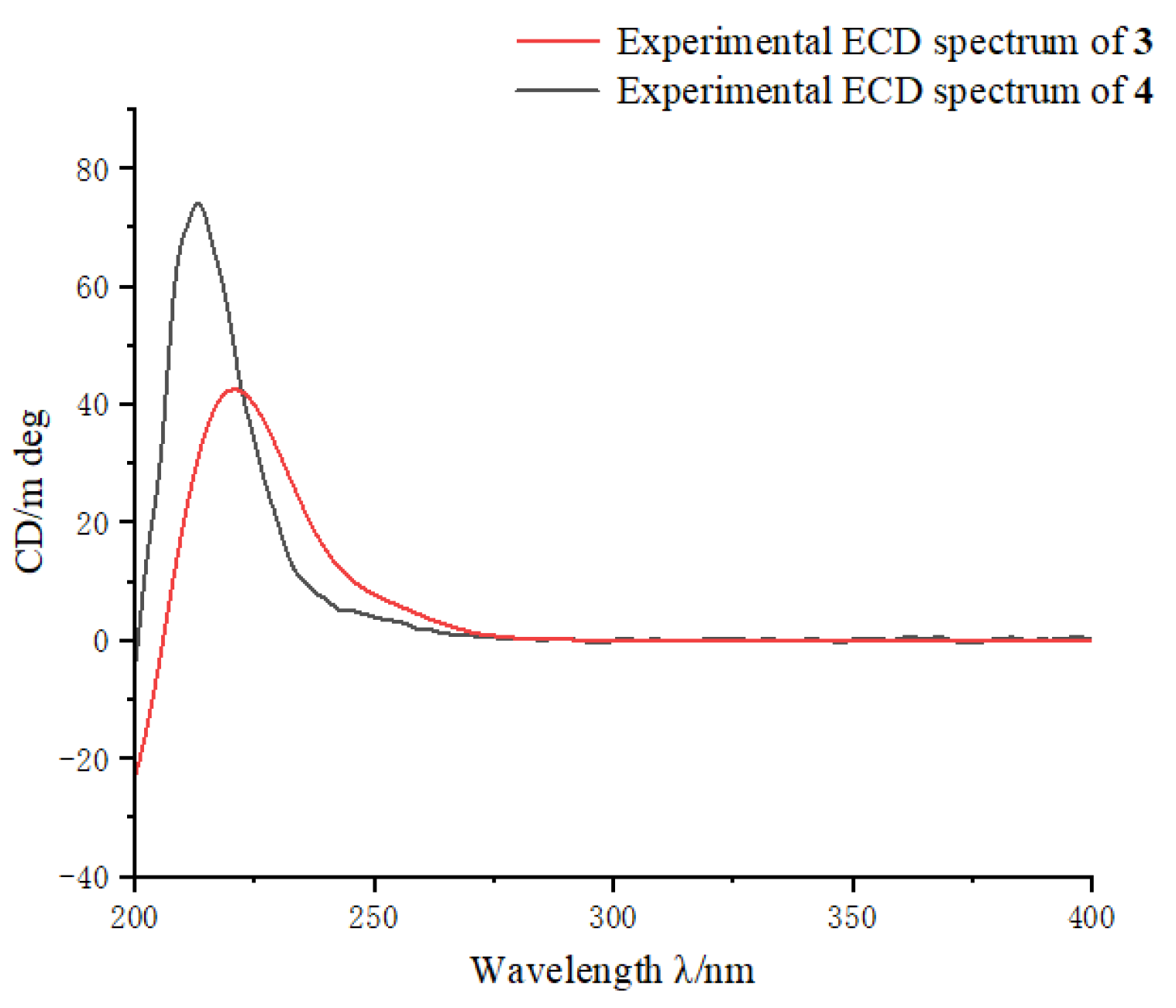
2. Results and Discussion
3. Materials and Methods
3.1. Subsection
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of Compounds
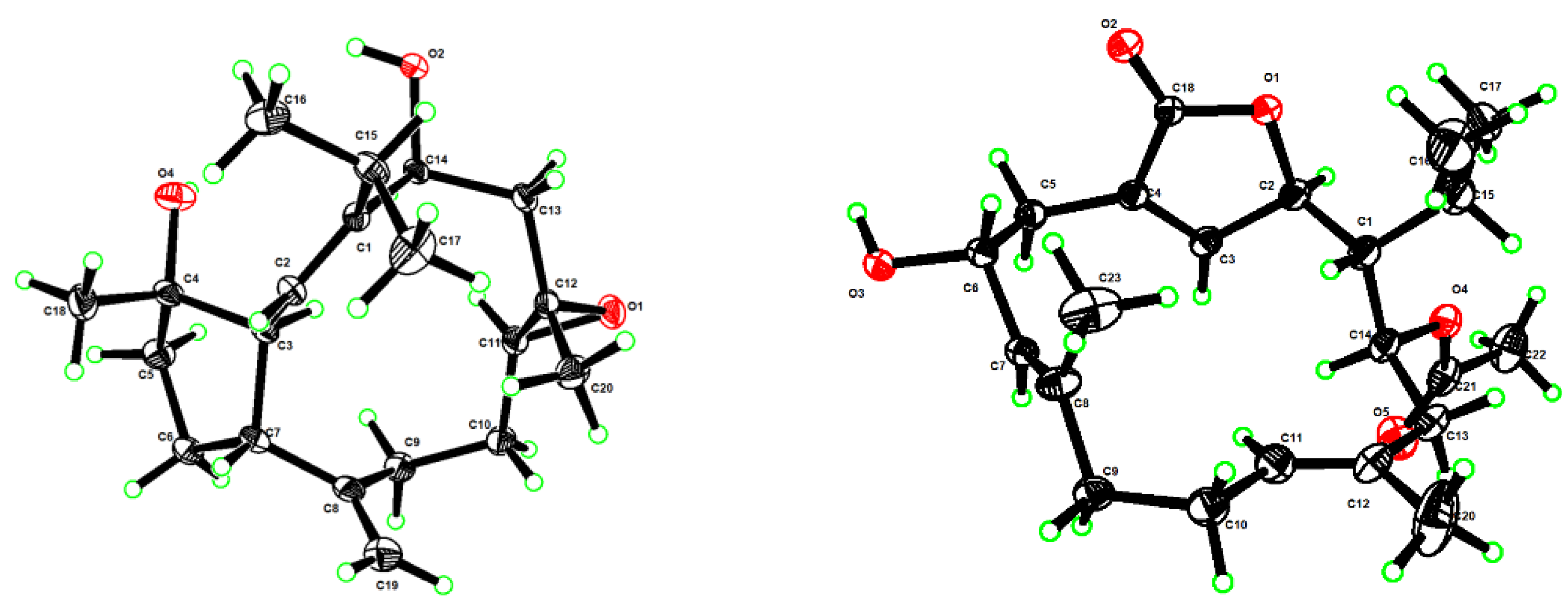
3.5. X-ray Crystallographic Analysis for Compounds 1 and 4
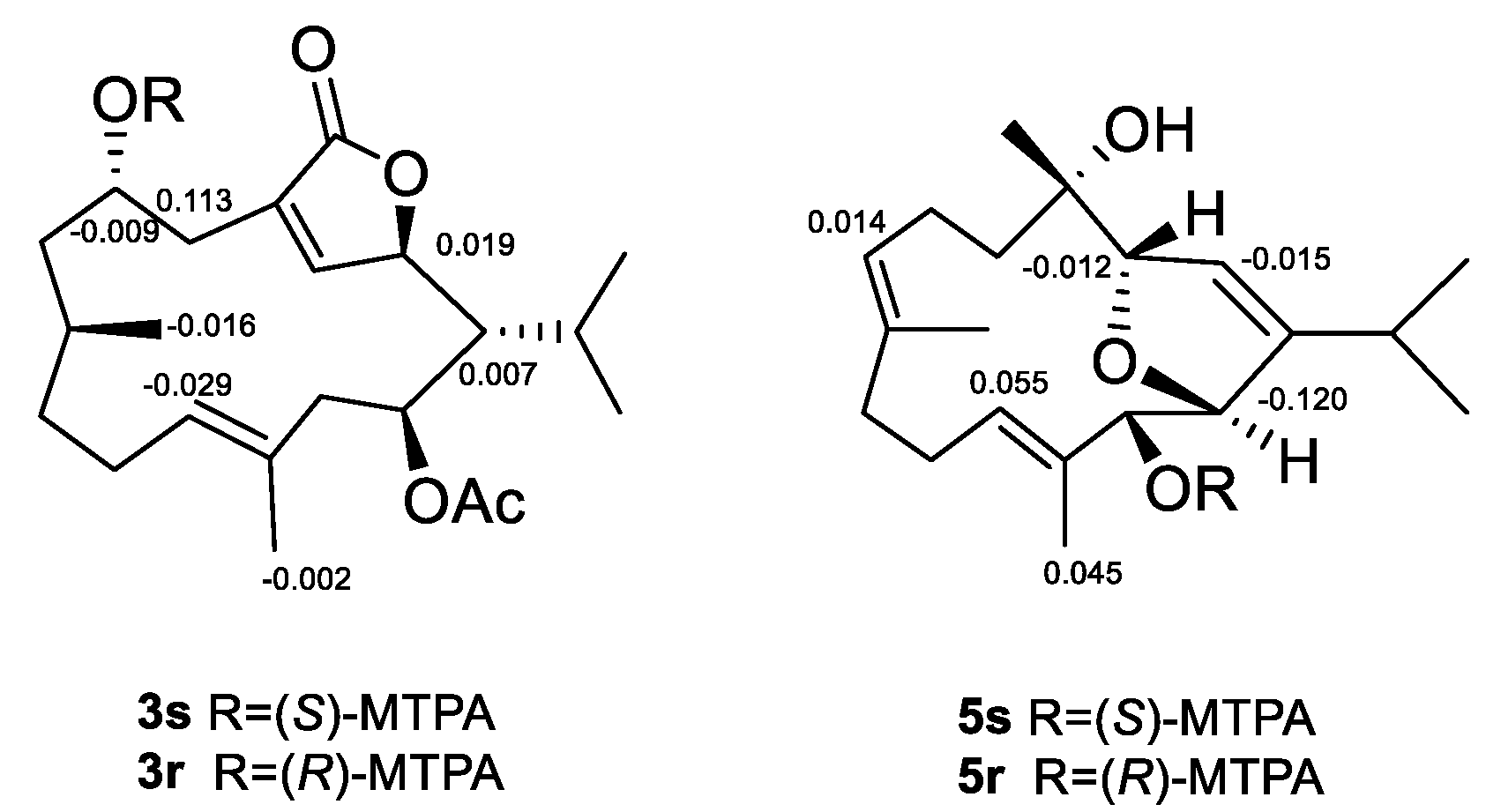
3.6. Esterification of Compounds 3 and 5 with MTPA Chlorides
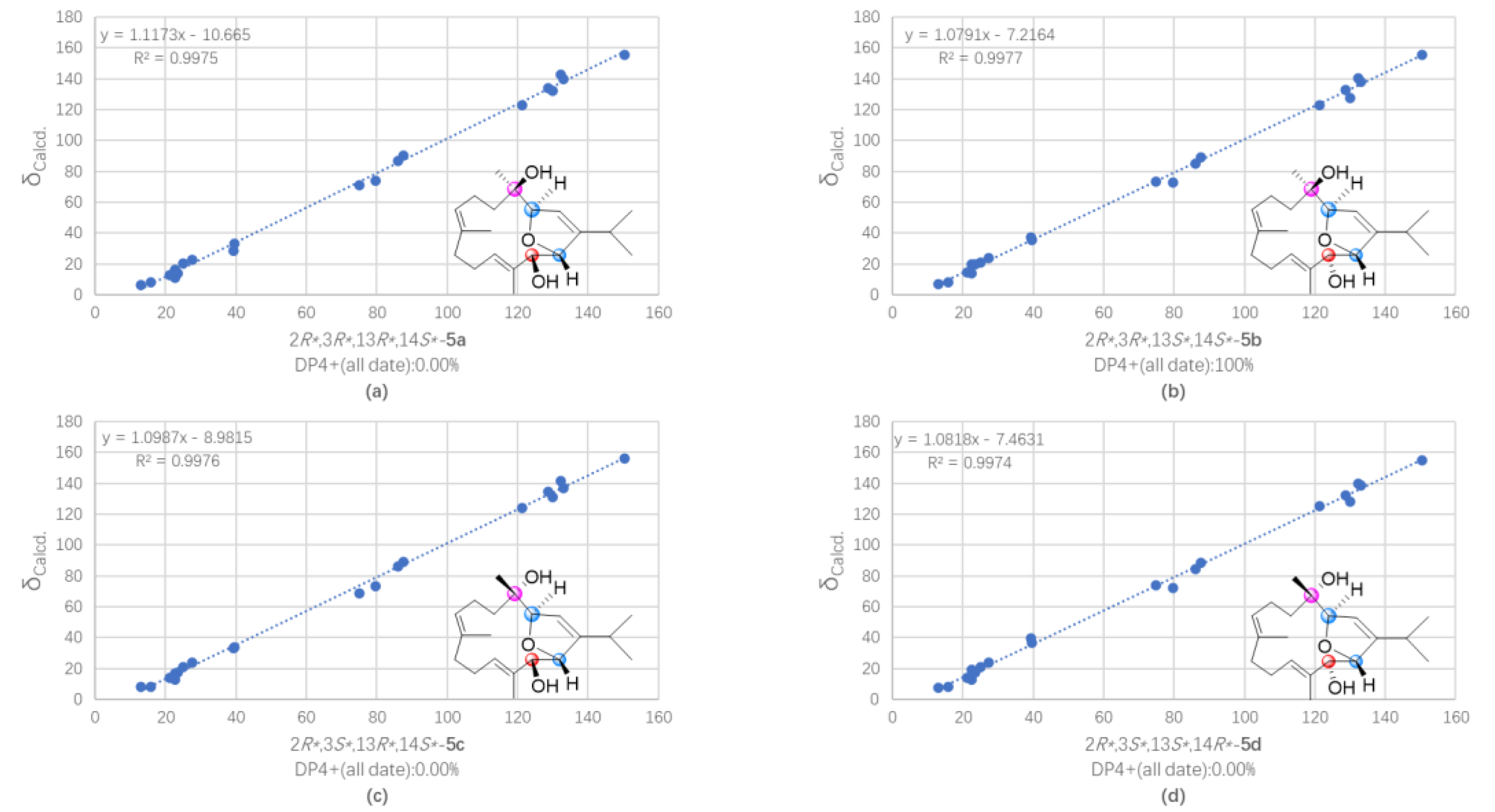
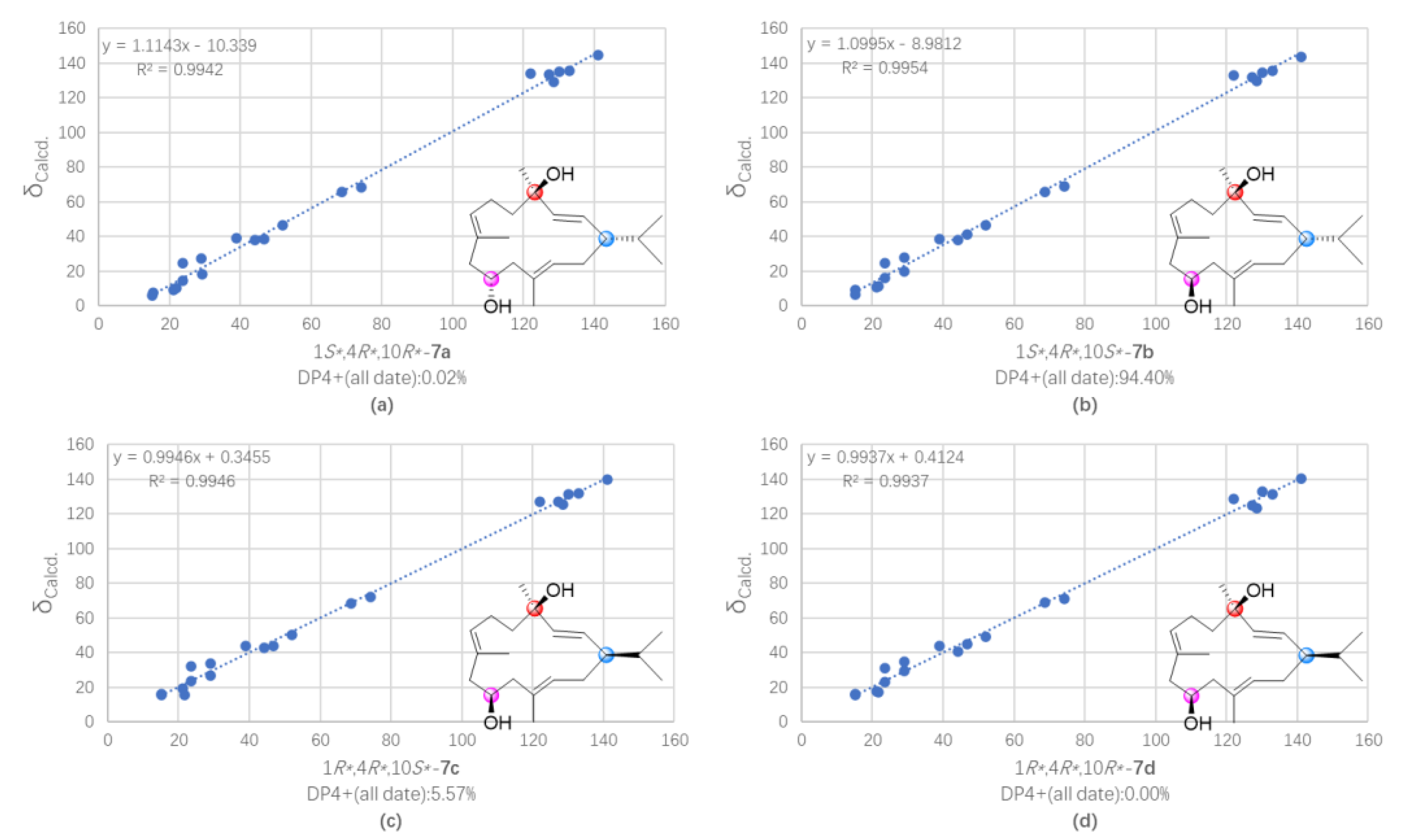
3.7. QM-NMR Calculation of Compounds 5 and 7
3.8. Cytotoxic Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anjaneyulu, A.S.R.; Rao, G.V. Chemical constituents of the soft coral species of Sarcophyton genus: A review. J. Indian Chem. Soc. 1997, 74, 272–278. [Google Scholar]
- Liang, L.-F.; Guo, Y.-W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013, 10, 2161–2196. [Google Scholar] [CrossRef]
- Zubair, M.S.; Al-Footy, K.O.; Ayyad, S.-E.N.; Al-Lihaibi, S.S.; Alarif, W.M. A review of steroids from Sarcophyton species. Nat. Prod. Res. 2016, 30, 869–879. [Google Scholar] [CrossRef]
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Al-Sayed, E.; Bishr, M.M.; Singab, A.N.B.; Salama, O.M. Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: A review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tang, H.; Wang, P.; Gong, W.; Xue, M.; Zhang, H.; Liu, T.; Liu, B.; Yi, Y.; Zhang, W. Bioactive polyoxygenated steroids from the South China Sea soft coral, Sarcophyton sp. Mar. Drugs 2013, 11, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-T.; Liu, H.-L.; Yao, L.-G.; Guo, Y.-W. 9,11-Secosteroids and polyhydroxylated steroids from two South China Sea soft corals Sarcophyton trocheliophorum and Sinularia flexibilis. Steroids 2014, 92, 56–61. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Hanh, T.T.H.; Quang, T.H.; Cuong, N.X.; Nam, N.H.; Thao, D.T.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Polyhydroxylated steroids from the Vietnamese soft coral Sarcophyton ehrenbergi. Steroids 2021, 176, 108932. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, A.-N.; Shao, C.-L.; Li, L.; Xu, Y.; Qian, P.-Y. Chemical constituents of soft coral Sarcophyton infundibuliforme from the South China Sea. Biochem. Syst. Ecol. 2011, 39, 853–856. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Huang, C.-Y.; Chen, S.-R.; Weng, J.-R.; Tu, T.-H.; Cheng, Y.-B.; Wu, S.-H.; Sheu, J.-H. New hydroquinone monoterpenoid and cembranoid-related metabolites from the soft coral Sarcophyton tenuispiculatum. Mar. Drugs 2021, 19, 8. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Murthy, M.V.R.K.; Gowri, P.M.; Venugopal, M.; Laatsch, H. A rare prostaglandin from the soft coral Sarcophyton crassocaule of the Indian Ocean. J. Nat. Prod. 2000, 63, 1425–1426. [Google Scholar] [CrossRef]
- Cheng, Z.-B.; Deng, Y.-L.; Fan, C.-Q.; Han, Q.-H.; Lin, S.-L.; Tang, G.-H.; Luo, H.-B.; Yin, S. Prostaglandin derivatives: Nonaromatic phosphodiesterase-4 inhibitors from the soft coral Sarcophyton ehrenbergi. J. Nat. Prod. 2014, 77, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Wen, Z.-H.; Chiou, S.-F.; Tsai, C.-W.; Wang, S.-K.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Wang, W.-H.; Duh, C.-Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009, 72, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; Zaitone, S.A.; Gomaa, M.; ElSohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Mechanism of action of antiepileptic ceramide from Red Sea soft coral Sarcophyton auritum. Bioorg. Med. Chem. Lett. 2015, 25, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Dembitsky, V.M. γ-Lactones from the soft corals Sarcophyton trocheliophorum and Lithophyton arboreum. Tetrahedron 2001, 57, 8743–8749. [Google Scholar] [CrossRef]
- Shaaban, M.; Ghani, M.A.; Issa, M.Y. New naturally occurring compounds from Sarcophyton trocheliophorum. Biointerface Res. App. Chem. 2022, 12, 2285–2331. [Google Scholar] [CrossRef]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, M.; Yassin, F.Y.; Soltan, M.M. Calamusins J-K: New anti-angiogenic sesquiterpenes from Sarcophyton glaucum. Nat. Prod. Res. 2021, 35, 5720–5731. [Google Scholar] [CrossRef]
- Sawant, S.S.; Youssef, D.T.A.; Sylvester, P.W.; Wali, V.; Sayed, K.A.E. Antiproliferative sesquiterpenes from the Red Sea soft coral Sarcophyton glaucum. Nat. Prod. Commun. 2007, 2, 117–119. [Google Scholar] [CrossRef] [Green Version]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G.; Rao, D.V. Trocheliophorin: A novel rearranged sesquiterpenoid from the Indian Ocean soft coral Sarcophyton trocheliophorum. J. Asian Nat. Prod. Res. 2008, 10, 597–601. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Wu, Y.; Zhu, Z.-D.; Mollo, E.; Gavagnin, M.; Gu, Y.-C.; Zhu, W.-L.; Li, X.-W.; Guo, Y.-W. Sarinfacetamides A and B, nitrogenous diterpenoids with tricyclo [6.3.1.01,5] dodecane scaffold from the South China Sea soft coral Sarcophyton infundibuliforme. Org. Lett. 2018, 20, 2637–2640. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lin, Y.-C.; Huang, C.-Y.; Tseng, Y.-J.; Chen, S.-R.; Cheng, Y.-B.; Hwang, T.-L.; Wang, S.-Y.; Chen, H.-Y.; Dai, C.-F.; et al. Cembranoid-related diterpenes, novel secoditerpenes, and an unusual bisditerpene from a Formosan soft coral Sarcophyton tortuosum. Bull. Chem. Soc. Jpn. 2021, 94, 2774–2783. [Google Scholar] [CrossRef]
- Bu, Q.; Yang, M.; Yan, X.-Y.; Yao, L.-G.; Guo, Y.-W.; Liang, L.-F. New flexible cembrane-type macrocyclic diterpenes as TNF-α inhibitors from the South China Sea soft coral Sarcophyton mililatensis. Int. J. Biol. Macromol. 2022, 222, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Shi, X.; Li, K.; Li, F.; Tang, X.; Li, G.; Li, P. Sarcoeleganolides C–G, five new cembranes from the South China Sea soft coral Sarcophyton elegans. Mar. Drugs 2022, 20, 574. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.A.; Elshamy, A.I.; Abd El-Razek, M.H.; Abdel-Tawab, A.M.; Ali, S.K.; Aboelmagd, M.; Suenaga, M.; Pare, P.W.; Umeyama, A.; Hegazy, M.-E.F. Sarcoconvolutums F and G: Polyoxygenated cembrane-type diterpenoids from Sarcophyton convolutum, a Red Sea soft coral. Molecules 2022, 27, 5835. [Google Scholar] [CrossRef]
- Sun, P.; Cai, F.-Y.; Lauro, G.; Tang, H.; Su, L.; Wang, H.-L.; Li, H.H.; Mándi, A.; Kurtán, T.; Riccio, R.; et al. Immunomodulatory biscembranoids and assignment of their relative and absolute configurations: Data set modulation in the density functional theory/nuclear magnetic resonance approach. J. Nat. Prod. 2019, 82, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Cuadrado, C.; Gao, C.; Wu, Q.; Li, X.; Pang, T.; Daranas, A.H.; Guo, Y.; Li, X. Polyoxygenated anti-inflammatory biscembranoids from the soft coral Sarcophyton tortuosum and their stereochemistry. Chin. Chem. Lett. 2021, 32, 271–276. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Huang, J.; Wang, Y.; Leng, X.; Li, T.; Ouyang, H.; Yan, X.; He, S. Bistrochelides H−L: Biscembranoids from the South China Sea soft coral Sarcophyton serenei. Phytochemistry 2022, 204, 113438. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Chen, L.-Y.; Chen, P.-J.; El-Shazly, M.; Hwang, T.-L.; Su, J.-H.; Su, C.-H.; Yen, P.-T.; Peng, B.-R.; Lai, K.-H. MS/MS molecular networking unveils the chemical diversity of biscembranoid derivatives, neutrophilic inflammatory mediators from the cultured soft coral Sarcophyton trocheliophorum. Int. J. Mol. Sci. 2022, 23, 15464. [Google Scholar] [CrossRef]
- Al-Footy, K.O.; Alarif, W.M.; Asiri, F.; Aly, M.M.; Ayyad, S.-E.N. Rare pyrane-based cembranoids from the Red Sea soft coral Sarcophyton trocheliophorum as potential antimicrobial–antitumor agents. Med. Chem. Res. 2015, 24, 505–512. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Elshamy, A.I.; Abdel-Tawab, A.M.; AbdelMohsen, M.M.; Ohta, S.; Pare, P.W.; Hegazy, M.-E.F. Oxygenated cembrene diterpenes from Sarcophyton convolutum: Cytotoxic sarcoconvolutum A–E. Mar. Drugs 2021, 19, 519. [Google Scholar] [CrossRef]
- Li, J.-F.; Zeng, Y.-B.; Li, W.-S.; Luo, H.; Zhang, H.-Y.; Guo, Y.-W. Xishaglaucumins A—J, new cembranoids with anti-inflammatory activities from the South China Sea soft coral Sarcophyton glaucum. Chin. J. Chem. 2022, 40, 79–90. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Han, X.; Feng, D.; Luo, X.; Ofwegen, L.v.; Li, P.; Li, G. Sarcoglaucins A-I, new antifouling cembrane-type diterpenes from the South China Sea soft coral Sarcophyton glaucum. Org. Chem. Front. 2019, 6, 2004–2013. [Google Scholar] [CrossRef]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.G.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-Q.; Chen, J.; Wu, M.-J.; Zhang, H.-Y.; Liang, L.-F.; Guo, Y.-W. Uncommon capnosane diterpenes with neuroprotective potential from South China Sea soft coral Sarcophyton boettgeri. Mar. Drugs 2022, 20, 602. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef]
- Liu, J.; Gu, Y.-c.; Su, M.-z.; Guo, Y.-w. Chemistry and bioactivity of secondary metabolites from South China Sea marine fauna and flora: Recent research advances and perspective. Acta Pharmacol. Sin. 2022, 43, 3062–3079. [Google Scholar] [CrossRef]
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Yao, L.-G.; Li, J.; Zhang, W.; Guo, Y.-W. Unprecedented diterpenoids as a PTP1B inhibitor from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 2013, 15, 274–277. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.-L.; Wang, J.-R.; Lei, X.; Tang, W.; Li, X.-W.; Sun, H.; Guo, Y.-W. Sarcomililate A, an unusual diterpenoid with tricyclo [11.3.0.02,16] hexadecane carbon skeleton, and its potential biogenetic precursors from the Hainan soft coral Sarcophyton mililatensis. J. Org. Chem. 2019, 84, 2568–2576. [Google Scholar] [CrossRef]
- Yang, Q.-B.; Wu, Q.; Chen, J.-K.; Liang, L.-F. The soft coral Sarcophyton trocheliophorum: A warehouse of terpenoids with structural and pharmacological diversity. Mar. Drugs 2023, 21, 30. [Google Scholar] [CrossRef]
- Yao, L.-G.; Zhang, H.-Y.; Liang, L.-F.; Guo, X.-J.; Mao, S.-C.; Guo, Y.-W. Yalongenes A and B, two new cembranoids with cytoprotective effects from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Helv. Chim. Acta 2012, 95, 235–239. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Gao, T.-R.; Yang, M.; Yao, L.-G.; Guo, Y.-W. Further new cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Fitoterapia 2021, 151, 104902. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Sarcophyolides B–E, new cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2013, 11, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Guo, Y.-W.; Mollo, E.; Gavagnin, M.; Cimino, G. Sarcophytonolides E−H, cembranolides from the Hainan soft coral Sarcophyton latum. J. Nat. Prod. 2006, 69, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-F.; Chen, W.-T.; Li, X.-W.; Wang, H.-Y.; Guo, Y.-W. New bicyclic cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Sci. Rep. 2017, 7, 46584. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Ding, Y.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Sinulaflexiolides A-K, cembrane-type diterpenoids from the Chinese soft coral Sinularia flexibilis. J. Nat. Prod. 2008, 71, 1133–1140. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kondo, K.; Osabe, K.; Mitsuhashi, H. Marine terpenes and terpenoids. V. Oxidation of sarcophytol A, a potent anti-tumor-promoter from the soft coral Sarcophyton glaucum. Chem. Pharm. Bull. 1988, 36, 2331–2341. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-W.; Cuadrado, C.; Yao, L.-G.; Daranas, A.H.; Guo, Y.-W. Quantum mechanical–NMR-aided configuration and conformation of two unreported macrocycles isolated from the soft coral Lobophytum sp.: Energy calculations versus coupling constants. Org. Lett. 2020, 22, 4093–4096. [Google Scholar] [CrossRef]
- Raldugin, V.A.; Pleshkov, I.G.; Gatilov, Y.V.; Yaroshenko, N.I.; Salenko, V.L.; Shevtsov, S.A.; Pentegova, V.A. Photosensitized oxidation of isocembrol. VII. Products of reaction at the C11 double bond. Chem. Nat. Compd. 1984, 20, 45–52. [Google Scholar] [CrossRef]


| No. | 1 a | 3 b | 5 b | 6 b | 7 b | 8 a |
|---|---|---|---|---|---|---|
| 1 | 1.55 (d, 10.7) | 1.54 (br s) | ||||
| 2 | 5.14 (d, 10.6) | 4.95 (d, 10.7) | 5.51 (d, 1.4) | 5.42 (m) | 5.51 (dd, 15.6, 9.6) | 5.56 (d, 15.6) |
| 3 | 2.84 (t, 10.6) | 7.44 (t, 1.8) | 4.66 (dq, 5.5, 1.4) | 4.40 (br s) | 5.63 (d, 15.6) | 6.13 (d, 15.9) |
| 4 | ||||||
| 5 | 1.84 (m) | 2.75 (dt, 13.2, 1.2) | 1.83 (m) | 1.94 (m) | 1.89 (ddd, 13.4, 8.6, 1.9) | 1.87 (m) |
| 1.78 (m) | 2.45 (dd, 13.3, 10.5) | 1.53 (m) | 1.76 (m) | 1.58 (m) | 1.69 (m) | |
| 6 | 1.66 (m) | 4.22 (t, 9.8) | 2.34 (m) | 2.06 (m) | 2.24 (m) | 2.64 (m) |
| 1.92 (m) | ||||||
| 7 | 2.48 (t, 7.1) | 1.63 (m) | 5.22 (m) | 5.24 (t, 6.3) | 5.11 (d, 7.8) | 5.43 (dd, 10.6, 4.0) |
| 1.35 (m) | ||||||
| 8 | 1.29 (m) | |||||
| 9 | 2.33 (m) | 1.36 (m) | 2.10 (m) | 1.96 (m) | 2.25 (m) | 2.00 (m) |
| 2.00 (t, 13.5) | 1.26 (m) | 1.67 (m) | 2.12 (m) | |||
| 10 | 2.21 (m) | 2.08 (m) | 2.26 (m) | 1.86 (m) | 4.07 (d, 10,9) | 1.90 (m) |
| 1.46 (m) | 2.11 (m) | 1.43 (m) | 1.55 (m) | |||
| 11 | 2.89 (dd, 10.6, 2.9) | 5.05 (t, 7.7) | 5.32 (m) | 5.04 (d, 10.3) | 2.09 (m) | 5.08 (d, 9.4) |
| 12 | ||||||
| 13 | 2.25 (m) | 2.26 (dd, 13.5, 3.0) | 4.29 (t, 4.3) | 1.94 (m) | 5.24 (t, 7.7) | 1.78 (m) |
| 1.65 (m) | 2.20 (dd, 13.5, 10.9) | 1.61 (m) | 1.59 (m) | |||
| 14 | 4.88 (dd, 11.2, 5.1) | 5.02 (ddd, 10.8, 4.2, 1.1) | 4.84 (td, 5.0, 1.6) | 3.04 (m) | 2.07 (m) | 2.33 (td, 11.8, 7.4) |
| 2.38 (m) | 1.68 (m) | |||||
| 15 | 2.48 (m) | 2.22 (m) | 2.65 (m) | 1.70 (m) | ||
| 16 | 1.12 (d, 6.5) | 1.11 (d, 6.6) | 1.16 (d, 6.8) | 1.37 (s) | 0.96 (d, 6.6) | 1.11 (s) |
| 17 | 1.16 (d, 6.5) | 1.12 (d, 6.6) | 1.08 (d, 6.9) | 1.30 (s) | 0.84 (d, 6.7) | 1.13 (s) |
| 18 | 1.16 (s) | 1.00 (s) | 1.20 (s) | 1.30 (s) | 1.35 (s) | |
| 19 | 4.88 (s) | 0.77 (d, 6.0) | 1.56 (s) | 1.56 (s) | 1.54 (s) | 1.71 (s) |
| 4.69 (s) | ||||||
| 20 | 1.12 (s) | 1.67 (s) | 1.56 (s) | 1.10 (s) | 1.57 (m) | 1.17 (s) |
| OAc | 2.09 (s) | 2.08 (s) | 2.05 (s) |
| No. | 1 | 3 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|
| 1 | 149.90, qC | 49.67, CH | 150.34, qC | 146.95, qC | 51.96, CH | 91.52, qC |
| 2 | 125.85, CH | 81.19, CH | 121.20, CH | 121.34, CH | 122.10, CH | 129.84, CH |
| 3 | 51.08, CH | 151.06, CH | 87.63, CH | 73.16, CH | 141.04, CH | 137.41, CH |
| 4 | 81.64, qC | 131.39, qC | 74.93, qC | 75.86, qC | 74.20, qC | 72.47, qC |
| 5 | 41.04, CH2 | 37.42, CH2 | 39.37, CH2 | 39.18, CH2 | 44.21, CH2 | 41.64, CH2 |
| 6 | 25.90, CH2 | 66.40, CH | 22.57, CH2 | 21.75, CH2 | 23.66, CH2 | 22.90, CH2 |
| 7 | 54.09, CH | 47.19, CH2 | 128.74, CH | 127.40, CH | 127.31, CH | 130.06, CH |
| 8 | 148.54, qC | 28.31, CH | 133.16, qC | 134.03, qC | 130.05, qC | 132.11, qC |
| 9 | 24.65, CH2 | 37.25, CH2 | 39.64, CH2 | 33.90, CH2 | 46.88, CH2 | 34.86, CH2 |
| 10 | 28.07, CH2 | 24.31, CH2 | 25.00, CH2 | 25.03, CH2 | 68.82, CH | 27.37, CH2 |
| 11 | 62.28, CH | 129.20, CH | 130.08, CH | 72.70, CH | 38.96, CH2 | 77.36, CH |
| 12 | 59.06, qC | 130.80, qC | 132.19, qC | 74.15, qC | 132.94, qC | 84.58, qC |
| 13 | 45.46, CH2 | 41.43, CH2 | 79.67, CH | 29.33, CH2 | 128.47, CH | 35.80, CH2 |
| 14 | 69.34, CH | 73.92, CH | 86.05, CH | 20.95, CH2 | 23.68, CH2 | 30.76, CH2 |
| 15 | 27.09, CH | 25.81, CH | 27.43, CH | 74.60, qC | 29.13, CH | 72.84, qC |
| 16 | 21.23, CH3 | 24.93, CH3 | 21.11, CH3 | 30.42, CH3 | 21.15, CH3 | 26.01, CH3 |
| 17 | 23.55, CH3 | 18.74, CH3 | 22.57, CH3 | 28.85, CH3 | 21.85, CH3 | 24.62, CH3 |
| 18 | 27.70, CH3 | 172.97, qC | 23.36, CH3 | 21.72, CH3 | 29.17, CH3 | 29.00, CH3 |
| 19 | 112.26, CH2 | 18.25, CH3 | 15.84, CH3 | 17.41, CH3 | 15.52, CH3 | 16.33, CH3 |
| 20 | 23.05, CH3 | 18.47, CH3 | 12.89, CH3 | 23.63, CH3 | 15.09, CH3 | 20.69, CH3 |
| OAc | 171.13, qC | 170.47, qC | 170.17, qC | |||
| 21.35, CH3 | 21.75, CH3 | 21.42, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-T.; Yu, D.-D.; Su, M.-Z.; Luo, H.; Cao, J.-G.; Liang, L.-F.; Yang, F.; Guo, Y.-W. Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum. Mar. Drugs 2023, 21, 69. https://doi.org/10.3390/md21020069
Song Y-T, Yu D-D, Su M-Z, Luo H, Cao J-G, Liang L-F, Yang F, Guo Y-W. Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum. Marine Drugs. 2023; 21(2):69. https://doi.org/10.3390/md21020069
Chicago/Turabian StyleSong, Yu-Ting, Dan-Dan Yu, Ming-Zhi Su, Hui Luo, Jian-Guo Cao, Lin-Fu Liang, Fan Yang, and Yue-Wei Guo. 2023. "Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum" Marine Drugs 21, no. 2: 69. https://doi.org/10.3390/md21020069
APA StyleSong, Y.-T., Yu, D.-D., Su, M.-Z., Luo, H., Cao, J.-G., Liang, L.-F., Yang, F., & Guo, Y.-W. (2023). Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum. Marine Drugs, 21(2), 69. https://doi.org/10.3390/md21020069











