Unveiling the Assembly of Neutral Marine Polysaccharides into Electrostatic-Driven Layer-by-Layer Bioassemblies by Chemical Functionalization
Abstract
1. Introduction
2. Results and Discussion
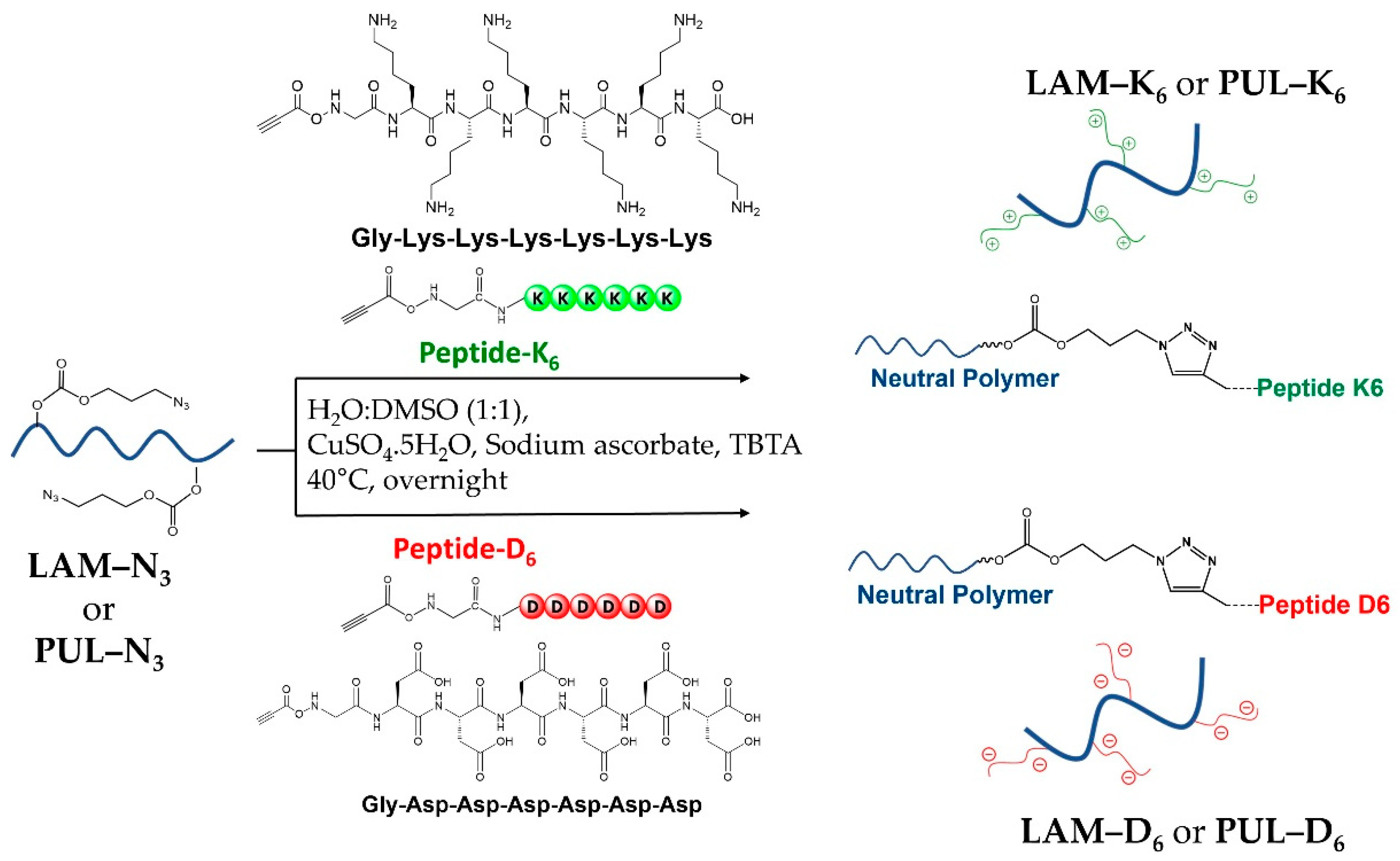
2.1. Synthesis and Physicochemical Characterization
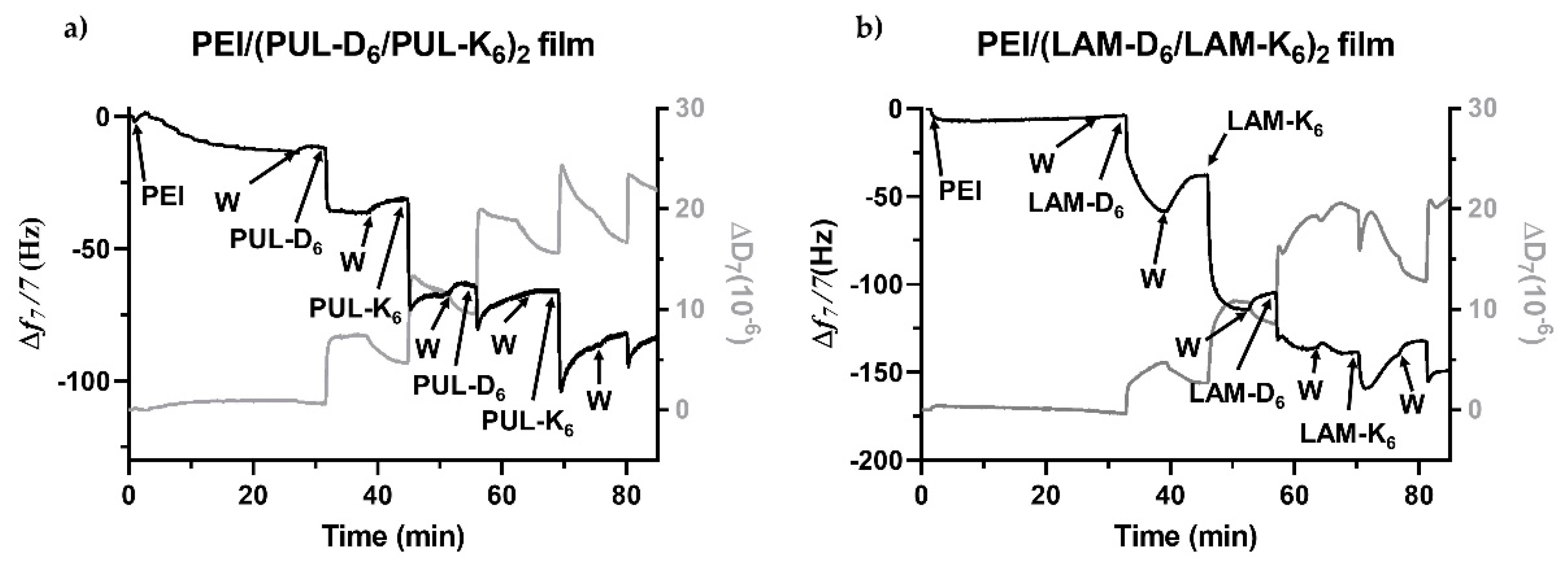
2.2. Build-up of Multilayered Thin Films
2.3. Scanning Electron Microscopy (SEM)
2.4. In Vitro Live/Dead Assays
3. Materials and Methods
3.1. Synthesis of 3-azidopropanol (AP) and 3-azidopropyl Carbonylimidazole (AP-CI)
3.1.1. Synthesis of 3-azidopropanol (AP)
3.1.2. Synthesis of 3-azidopropyl Carbonylimidazole (AP-CI)
3.2. Synthesis of Polysaccharide-azidopropylcarbonate (LAM-N3 and PUL-N3)
3.3. Synthesis of Polysaccharide–Peptide Conjugates
3.3.1. LAM-Peptide Conjugation: LAM-D6 and LAM-K6
3.3.2. PUL-Peptide Conjugation: PUL-D6 and PUL-K6
3.4. Zeta-Potential Measurements
3.5. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
3.6. Preparation of Multilayered Thin Films onto Gold-Coated Glass Substrates
3.7. Scanning Electron Microscopy (SEM)
3.8. In Vitro Assays: Cell Seeding and Live-Dead Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borges, J.; Mano, J.F. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sun, J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Costa, R.R.; Mano, J.F. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 2014, 43, 3453–3479. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Ono, S.S.; Voegel, J.C.; Schaaf, P.; Decher, G. Dipping versus spraying: Exploring the deposition conditions for speeding up layer-by-layer assembly. Langmuir 2005, 21, 7558–7567. [Google Scholar] [CrossRef]
- Kolasinska, M.; Krastev, R.; Gutberlet, T.; Warszynski, P. Layer-by-layer deposition of polyelectrolytes. Dipping versus spraying. Langmuir 2009, 25, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Mjahed, H.; Voegel, J.-C.; Senger, B.; Chassepot, A.; Rameau, A.; Ball, V.; Schaaf, P.; Boulmedais, F. Hole formation induced by ionic strength increase in exponentially growing multilayer films. Soft Matter 2009, 5, 2269–2276. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Kozlovskaya, V.; Chan, J.; Ankner, J.F.; Tsukruk, V.V. Spin-assisted layer-by-layer assembly: Variation of stratification as studied with neutron reflectivity. Langmuir 2009, 25, 14017–14024. [Google Scholar] [CrossRef]
- Chiarelli, P.A.; Johal, M.S.; Holmes, D.J.; Casson, J.L.; Robinson, J.M.; Wang, H.-L. Polyelectrolyte Spin-Assembly. Langmuir 2002, 18, 168–173. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Wong, K.C.; Mamah, S.C.; Ismail, A.F.; Zulhairun, A.K. Accelerated spraying-assisted layer by layer assembly of polyethyleneimine/titania nanosheet on thin film composite membrane for reverse osmosis desalination. Desalination 2022, 529, 115645–115657. [Google Scholar] [CrossRef]
- Krogman, K.C.; Cohen, R.E.; Hammond, P.T.; Rubner, M.F.; Wang, B.N. Industrial-scale spray layer-by-layer assembly for production of biomimetic photonic systems. Bioinspir. Biomim. 2013, 8, 45005–45017. [Google Scholar] [CrossRef]
- Dierendonck, M.; De Koker, S.; De Rycke, R.; De Geest, B.G. Just spray it-LbL assembly enters a new age. Soft Matter 2014, 10, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, P.; Voegel, J.C.; Jierry, L.; Boulmedais, F. Spray-assisted polyelectrolyte multilayer buildup: From step-by-step to single-step polyelectrolyte film constructions. Adv. Mater. 2012, 24, 1001–1016. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Factors Controlling the Growth of Polyelectrolyte Multilayers. Macromolecules 1999, 32, 8153–8160. [Google Scholar] [CrossRef]
- Elizarova, I.S.; Luckham, P.F. Layer-by-layer adsorption: Factors affecting the choice of substrates and polymers. Adv. Colloid Interface Sci. 2018, 262, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Garcia, J.R.; Reis, R.L.; Garcia, A.J.; Mano, J.F. Tuning cell adhesive properties via layer-by-layer assembly of chitosan and alginate. Acta Biomater. 2017, 51, 279–293. [Google Scholar] [CrossRef]
- Moon, H.C.; Choi, H.; Kikionis, S.; Seo, J.; Youn, W.; Ioannou, E.; Han, S.Y.; Cho, H.; Roussis, V.; Choi, I.S. Fabrication and Characterization of Neurocompatible Ulvan-Based Layer-by-Layer Films. Langmuir 2020, 36, 11610–11617. [Google Scholar] [CrossRef]
- Rochin-Wong, S.; Rosas-Durazo, A.; Zavala-Rivera, P.; Maldonado, A.; Martinez-Barbosa, M.E.; Velaz, I.; Tanori, J. Drug Release Properties of Diflunisal from Layer-By-Layer Self-Assembled kappa-Carrageenan/Chitosan Nanocapsules: Effect of Deposited Layers. Polymers 2018, 10, 760. [Google Scholar] [CrossRef]
- Borges, J.; Sousa, M.P.; Cinar, G.; Caridade, S.G.; Guler, M.O.; Mano, J.F. Nanoengineering Hybrid Supramolecular Multilayered Biomaterials Using Polysaccharides and Self-Assembling Peptide Amphiphiles. Adv. Funct. Mater. 2017, 27, 1605122–1605136. [Google Scholar] [CrossRef]
- Kopach, O.; Pavlov, A.M.; Sindeeva, O.A.; Sukhorukov, G.B.; Rusakov, D.A. Biodegradable Microcapsules Loaded with Nerve Growth Factor Enable Neurite Guidance and Synapse Formation. Pharmaceutics 2020, 13, 25. [Google Scholar] [CrossRef]
- Baek, H.; Lee, C.; Lim, K.I.; Cho, J. Resistive switching memory properties of layer-by-layer assembled enzyme multilayers. Nanotechnology 2012, 23, 155604–155614. [Google Scholar] [CrossRef]
- Sakr, O.S.; Borchard, G. Encapsulation of enzymes in Layer-by-Layer (LbL) structures: Latest advances and applications. Biomacromolecules 2013, 14, 2117–2135. [Google Scholar] [CrossRef]
- Roh, Y.H.; Lee, J.B.; Shopsowitz, K.E.; Dreaden, E.C.; Morton, S.W.; Poon, Z.; Hong, J.; Yamin, I.; Bonner, D.K.; Hammond, P.T. Layer-by-layer assembled antisense DNA microsponge particles for efficient delivery of cancer therapeutics. ACS Nano 2014, 8, 9767–9780. [Google Scholar] [CrossRef]
- Damanik, F.F.R.; Rothuizen, C.T.; Lalai, R.; Khoenkhoen, S.; van Blitterswijk, C.; Rotmans, J.I.; Moroni, L. Long-Term Controlled Growth Factor Release Using Layer-by-Layer Assembly for the Development of In Vivo Tissue-Engineered Blood Vessels. ACS Appl. Mater. Interfaces 2022, 14, 28591–28603. [Google Scholar] [CrossRef]
- Sacramento, M.M.; Borges, J.; Correia, F.J.; Calado, R.; Rodrigues, J.M.; Patrício, S.G.; Mano, J.F. Green approaches for extraction, chemical modification and processing of marine polysaccharides for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 1041102–1041132. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides and Their Importance for Human Health. In Blue Biotechnology; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Ma, X.; Wu, G.; Dai, F.; Li, D.; Li, H.; Zhang, L.; Deng, H. Chitosan/polydopamine layer by layer self-assembled silk fibroin nanofibers for biomedical applications. Carbohydr. Polym. 2021, 251, 117058–117065. [Google Scholar] [CrossRef]
- Martins, N.I.; Sousa, M.P.; Custodio, C.A.; Pinto, V.C.; Sousa, P.J.; Minas, G.; Cleymand, F.; Mano, J.F. Multilayered membranes with tuned well arrays to be used as regenerative patches. Acta. Biomater. 2017, 57, 313–323. [Google Scholar] [CrossRef]
- Govindharajulu, J.P.; Chen, X.; Li, Y.; Rodriguez-Cabello, J.C.; Battacharya, M.; Aparicio, C. Chitosan-Recombinamer Layer-by-Layer Coatings for Multifunctional Implants. Int. J. Mol. Sci. 2017, 18, 369. [Google Scholar] [CrossRef]
- Li, D.; Dai, F.; Li, H.; Wang, C.; Shi, X.; Cheng, Y.; Deng, H. Chitosan and collagen layer-by-layer assembly modified oriented nanofibers and their biological properties. Carbohydr. Polym. 2021, 254, 117438–117448. [Google Scholar] [CrossRef]
- Cai, K.; Hu, Y.; Wang, Y.; Yang, L. Build up of multilayered thin films with chitosan/DNA pairs on poly(D,L-lactic acid) films: Physical chemistry and sustained release behavior. J. Biomed. Mater. Res. A 2008, 84, 516–522. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, L.; Li, Y.; Jiang, S.; Sun, Q.; Xie, E.; Chen, H.; Zhao, Z.; Qiao, W.; Xu, J.; et al. Structure of a laminarin-type beta-(1-->3)-glucan from brown algae Sargassum henslowianum and its potential on regulating gut microbiota. Carbohydr. Polym. 2021, 255, 117389–117401. [Google Scholar] [CrossRef]
- Feng, L.; Hao, Y.; Zhu, M.; Zhai, Y.; Yang, L.; Liu, Y.; Cheng, G. Incorporation of Laminarin-Based Hydrogel with Graphene Foam To Enhance the Toughness of Scaffold and Regulate the Stem Cell Behavior. ACS Biomater. Sci. Eng. 2019, 5, 5295–5304. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Rodrigues, J.M.M.; Perez-Madrigal, M.M.; Dove, A.P.; Mano, J.F. Modular Functionalization of Laminarin to Create Value-Added Naturally Derived Macromolecules. J. Am. Chem. Soc. 2020, 142, 19689–19697. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Silva, A.S.; Nunes, C.; Coimbra, M.A.; Custodio, C.A.; Mano, J.F. Self-glucose feeding hydrogels by enzyme empowered degradation for 3D cell culture. Mater. Horiz. 2022, 9, 694–707. [Google Scholar] [CrossRef]
- Castanheira, E.J.; Correia, T.R.; Rodrigues, J.M.M.; Mano, J.F. Novel Biodegradable Laminarin Microparticles for Biomedical Applications. BCSJ 2020, 93, 713–719. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Chi, Z.; Zhang, L.; Zhang, D. Intraspecific diversity of Aureobasidium pullulans strains from different marine environments. J. Ocean Univ. China 2009, 8, 241–246. [Google Scholar] [CrossRef]
- Autissier, A.; Letourneur, D.; Le Visage, C. Pullulan-based hydrogel for smooth muscle cell culture. J. Biomed. Mater. Res. A 2007, 82, 336–342. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Wang, J.; Tang, R.; Wei, B.; Jiang, Q. Succinyl pullulan-crosslinked carboxymethyl chitosan sponges for potential wound dressing. Int. J. Polym. Mater. 2016, 66, 61–70. [Google Scholar] [CrossRef]
- Della Giustina, G.; Gandin, A.; Brigo, L.; Panciera, T.; Giulitti, S.; Sgarbossa, P.; D’Alessandro, D.; Trombi, L.; Danti, S.; Brusatin, G. Polysaccharide hydrogels for multiscale 3D printing of pullulan scaffolds. Mater. Des. 2019, 165, 107566–107575. [Google Scholar] [CrossRef]
- Li, X.; Xue, W.; Liu, Y.; Li, W.; Fan, D.; Zhu, C.; Wang, Y. HLC/pullulan and pullulan hydrogels: Their microstructure, engineering process and biocompatibility. Mater. Sci. Eng. C 2016, 58, 1046–1057. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Ju, J.; Sun, X.; Deng, N.; Li, Z.; Kang, W.; Cheng, B. Preparation and characterization of crosslinked electrospun pullulan nanofiber membrane as a potential for biomaterials. J. Text. Inst. 2017, 109, 750–756. [Google Scholar] [CrossRef]
- Bercea, M.; Constantin, M.; Plugariu, I.-A.; Oana Daraba, M.; Luminita Ichim, D. Thermosensitive gels of pullulan and poloxamer 407 as potential injectable biomaterials. J. Mol. Liq. 2022, 362, 119717–119728. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.U. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Aflak, N.; Ben El Ayouchia, H.; Bahsis, L.; El Mouchtari, E.M.; Julve, M.; Rafqah, S.; Anane, H.; Stiriba, S.E. Sustainable Construction of Heterocyclic 1,2,3-Triazoles by Strict Click [3+2] Cycloaddition Reactions Between Azides and Alkynes on Copper/Carbon in Water. Front. Chem. 2019, 7, 81–94. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Marx, K.A. Quartz crystal microbalance: A useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromolecules 2003, 4, 1099–1120. [Google Scholar] [CrossRef]
- Rodahl, M.; Hook, F.; Kasemo, B. QCM Operation in Liquids: An Explanation of Measured Variations in Frequency and Q Factor with Liquid Conductivity. Anal. Chem. 1996, 68, 2219–2227. [Google Scholar] [CrossRef]
- Xu, L.; Selin, V.; Zhuk, A.; Ankner, J.F.; Sukhishvili, S.A. Molecular Weight Dependence of Polymer Chain Mobility within Multilayer Films. ACS Macro Lett. 2013, 2, 865–868. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, L.P.G.; Borges, J.; Rodrigues, J.M.M.; Mano, J.F. Unveiling the Assembly of Neutral Marine Polysaccharides into Electrostatic-Driven Layer-by-Layer Bioassemblies by Chemical Functionalization. Mar. Drugs 2023, 21, 92. https://doi.org/10.3390/md21020092
Monteiro LPG, Borges J, Rodrigues JMM, Mano JF. Unveiling the Assembly of Neutral Marine Polysaccharides into Electrostatic-Driven Layer-by-Layer Bioassemblies by Chemical Functionalization. Marine Drugs. 2023; 21(2):92. https://doi.org/10.3390/md21020092
Chicago/Turabian StyleMonteiro, Luís P. G., João Borges, João M. M. Rodrigues, and João F. Mano. 2023. "Unveiling the Assembly of Neutral Marine Polysaccharides into Electrostatic-Driven Layer-by-Layer Bioassemblies by Chemical Functionalization" Marine Drugs 21, no. 2: 92. https://doi.org/10.3390/md21020092
APA StyleMonteiro, L. P. G., Borges, J., Rodrigues, J. M. M., & Mano, J. F. (2023). Unveiling the Assembly of Neutral Marine Polysaccharides into Electrostatic-Driven Layer-by-Layer Bioassemblies by Chemical Functionalization. Marine Drugs, 21(2), 92. https://doi.org/10.3390/md21020092