Natural Products from Chilean and Antarctic Marine Fungi and Their Biomedical Relevance
Abstract
:1. Introduction
2. Secondary Metabolites Isolated from Chilean Marine Fungi in Continental Coasts
3. Secondary Metabolites Isolated from Antarctic Marine Fungi
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tong, Y.; Deng, Z. An Aurora of Natural Products-Based Drug Discovery Is Coming. Synth. Syst. Biotechnol. 2020, 5, 92–96. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Clardy, J.; Walsh, C. Lessons from Natural Molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.-H. A Genome Tree of Life for the Fungi Kingdom. Proc. Natl. Acad. Sci. USA 2017, 114, 9391–9396. [Google Scholar] [CrossRef] [Green Version]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between Secondary Metabolism and Fungal Development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.C.; Grijseels, S.; Prigent, S.; Ji, B.; Dainat, J.; Nielsen, K.F.; Frisvad, J.C.; Workman, M.; Nielsen, J. Global Analysis of Biosynthetic Gene Clusters Reveals Vast Potential of Secondary Metabolite Production in Penicillium Species. Nat. Microbiol. 2017, 2, 17044. [Google Scholar] [CrossRef]
- Schueffler, A.; Anke, T. Fungal Natural Products in Research and Development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef]
- Montuori, E.; De Pascalle, D.; Lauritano, C. Recent Discoveries on Marine Organism Immunomodulatory Activities. Mar. Drugs. 2022, 20, 422. [Google Scholar] [CrossRef]
- Durães, F.; Szemerédi, N.; Kumla, D.; Pinto, M.; Kijjoa, A.; Spengler, G.; Sousa, E. Metabolites from Marine-Derived Fungi as Potential Antimicrobial Adjuvants. Mar. Drugs. 2021, 19, 475. [Google Scholar] [CrossRef]
- De la Calle, F. Fármacos de Origen Marino. Treballs de la SCB. 2007, 58, 141–155. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary Metabolites of Fungi from Marine Habitats. Nat. Prod. Rep. 2011, 28, 290. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Kijjoa, A. Marine-Derived Compounds with Anti-Alzheimer’s Disease Activities. Mar. Drugs. 2021, 19, 410. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A Review of Terpenes from Marine-Derived Fungi: 2015–2019. Mar. Drugs. 2020, 18, 321. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Kumla, D.; Kijjoa, A. Chemical Diversity and Biological Activities of Meroterpenoids from Marine Derived-Fungi: A Comprehensive Update. Mar. Drugs. 2020, 18, 317. [Google Scholar] [CrossRef]
- Nuestro País. Available online: https://www.gob.cl/nuestro-pais/ (accessed on 24 October 2022).
- Masas de Agua En El Mar Chileno. Available online: http://www7.uc.cl/sw_educ/geo_mar/html/h43.html (accessed on 24 October 2022).
- Biblioteca del Congreso Nacional | SIIT | Chile Nuestro País. Available online: https://www.bcn.cl/siit/nuestropais/index_html (accessed on 24 October 2022).
- Núñez-Pons, L.; Shilling, A.; Verde, C.; Baker, B.J.; Giordano, D. Marine Terpenoids from Polar Latitudes and Their Potential Applications in Biotechnology. Mar. Drugs 2020, 18, 401. [Google Scholar] [CrossRef]
- Yasukawa, K.; Akihisa, T.; Kanno, H.; Kaminaga, T.; Izumida, M.; Sakoh, T.; Tamura, T.; Takido, M. Inhibitory Effects of Sterols Isolated from Chlorella Vulgaris on 12-O-Tetradecanoylphorbol-13-Acetate-Induced Inflammation and Tumor Promotion in Mouse Skin. Biol. Pharm. Bull. 1996, 19, 573–576. [Google Scholar] [CrossRef] [Green Version]
- San Martin, A.; Painemal, K.; Diaz, Y.; Martinez, C.; Rovirosa, J. Metabolites from the marine fungus Cladosporium cladosporioides. J. Chil. Chem. Soc. 2005, 93, 247–251. [Google Scholar]
- Rovirosa, J.; Diaz-Marrero, A.; Darias, J.; Painemal, K.; San Martin, A. Secondary metabolites from the marine Penicillium brevicompactum. J. Chil. Chem. Soc. 2006, 51, 775–778. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mangoni, A.; Pansini, M. A Novel Conjugated Ketosteroid from the Marine Sponge Dictyonella Incisa. J. Nat. Prod. 1989, 52, 1331–1333. [Google Scholar] [CrossRef]
- San-Martín, A.; Orejarena, S.; Gallardo, C.; Silva, M.; Becerra, J.; Reinoso, R.; Chamy, M.C.; Vergara, K.; Rovirosa, J. Steroids from the marine fungus Geotrichum sp. J. Chil. Chem. Soc. 2008, 53, 1377–1378. [Google Scholar] [CrossRef] [Green Version]
- Manríquez, V.; Galdámez, A.; Veliz, B.; Rovirosa, J.; Díaz-Marrero, A.R.; Cueto, M.; Darias, J.; Martínez, C.; San-Martín, A. N-methyl-1H-indole-2-carboxamide from the marine fungus Cladosporium cladosporioides. J. Chil. Chem. Soc. 2009, 54, 314–316. [Google Scholar] [CrossRef] [Green Version]
- San-Martín, A.; Rovirosa, J.; Vaca, I.; Vergara, K.; Acevedo, L.; Viña, D.; Orallo, F.; Chamy, M.C. New butyrolactone from a marine-derived fungus Aspergillus sp. J. Chil. Chem. Soc. 2011, 56, 625–627. [Google Scholar] [CrossRef] [Green Version]
- Wilson, Z.E.; Brimble, M.A. Molecules Derived from the Extremes of Life. Nat. Prod. Rep. 2009, 26, 44–71. [Google Scholar] [CrossRef] [PubMed]
- Rusman, Y.; Held, B.W.; Blanchette, R.A.; He, Y.; Salomon, C.E. Cadopherone and Colomitide Polyketides from Cadophora Wood-Rot Fungi Associated with Historic Expedition Huts in Antarctica. Phytochemistry 2018, 148, 1–10. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.-L.; Zhao, F.-C. Secondary Metabolites from Polar Organisms. Mar. Drugs. 2017, 15, 28. [Google Scholar] [CrossRef] [Green Version]
- Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications; Rosa, L.H. (Ed.) Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-18366-0. [Google Scholar]
- Cong, M.; Pang, X.; Zhao, K.; Song, Y.; Liu, Y.; Wang, J. Deep-Sea Natural Products from Extreme Environments: Cold Seeps and Hydrothermal Vents. Mar. Drugs. 2022, 20, 404. [Google Scholar] [CrossRef]
- Li, Y.; Sun, B.; Liu, S.; Jiang, L.; Liu, X.; Zhang, H.; Che, Y. Bioactive Asterric Acid Derivatives from the Antarctic Ascomycete Fungus Geomyces Sp. J. Nat. Prod. 2008, 71, 1643–1646. [Google Scholar] [CrossRef]
- Ren, J.; Xue, C.; Tian, L.; Xu, M.; Chen, J.; Deng, Z.; Proksch, P.; Lin, W. Asperelines A−F, Peptaibols from the Marine-Derived Fungus Trichoderma Asperellum. J. Nat. Prod. 2009, 72, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, H.; Zhu, T.; Li, J.; Gu, Q.; Li, D. Penilactones A and B, Two Novel Polyketides from Antarctic Deep-Sea Derived Fungus Penicillium Crustosum PRB-2. Tetrahedron. 2012, 68, 9745–9749. [Google Scholar] [CrossRef]
- Spence, J.T.J.; George, J.H. Biomimetic Total Synthesis of ent-Penilactone A and Penilactone B. Org. Lett. 2013, 15, 3891–3893. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic Metabolites from the Antarctic Psychrophilic Fungus Oidiodendron Truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef]
- Henríquez, M.; Vergara, K.; Norambuena, J.; Beiza, A.; Maza, F.; Ubilla, P.; Araya, I.; Chávez, R.; San-Martín, A.; Darias, J.; et al. Diversity of Cultivable Fungi Associated with Antarctic Marine Sponges and Screening for Their Antimicrobial, Antitumoral and Antioxidant Potential. World J. Microbiol. Biotechnol. 2014, 30, 65–76. [Google Scholar] [CrossRef]
- Figueroa, L.; Jiménez, C.; Rodríguez, J.; Areche, C.; Chávez, R.; Henríquez, M.; de la Cruz, M.; Díaz, C.; Segade, Y.; Vaca, I. 3-Nitroasterric Acid Derivatives from an Antarctic Sponge-Derived Pseudogymnoascus Sp. Fungus. J. Nat. Prod. 2015, 78, 919–923. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; Carvalho, C.R.; Johann, S.; Mendes, G.; Alves, T.M.A.; Zani, C.L.; Junior, P.A.S.; Murta, S.M.F.; Romanha, A.J.; Cantrell, C.L.; et al. Antibacterial, Antifungal and Antiprotozoal Activities of Fungal Communities Present in Different Substrates from Antarctica. Polar Biol. 2015, 38, 1143–1152. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.-H.; Yu, H.-B.; Liu, X.-Y.; Lu, X.-L.; Jiao, B.-H. Furanone Derivative and Sesquiterpene from Antarctic Marine-Derived Fungus Penicillium Sp. S-1-18. J. Asian Nat. Prod. Res. 2018, 20, 1108–1115. [Google Scholar] [CrossRef]
- Wentzel, L.C.P.; Inforsato, F.J.; Montoya, Q.V.; Rossin, B.G.; Nascimento, N.R.; Rodrigues, A.; Sette, L.D. Fungi from Admiralty Bay (King George Island, Antarctica) Soils and Marine Sediments. Microb. Ecol. 2019, 77, 12–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Sun, Z.; Peng, J.; Zhu, M.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Secondary Metabolites Produced by Combined Culture of Penicillium crustosum and a Xylaria Sp. J. Nat. Prod. 2019, 82, 2013–2017. [Google Scholar] [CrossRef]
- Tripathi, V.C.; Satish, S.; Horam, S.; Raj, S.; lal, A.; Arockiaraj, J.; Pasupuleti, M.; Dikshit, D.K. Natural Products from Polar Organisms: Structural Diversity, Bioactivities and Potential Pharmaceutical Applications. Polar Sci. 2018, 18, 147–166. [Google Scholar] [CrossRef]
- Teixeira, T.R.; Santos, G.S.d.; Armstrong, L.; Colepicolo, P.; Debonsi, H.M. Antitumor Potential of Seaweed Derived-Endophytic Fungi. Antibiotics. 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Zhang, H.; Liu, W.; Zhang, L.; Peng, F.; Chen, Y.; Zhang, Q.; Zhang, G.; Zhang, W.; Zhang, C. Identification and Bioactivity Evaluation of Secondary Metabolites from Antarctic-Derived Penicillium chrysogenum CCTCC M 2020019. RSC Adv. 2020, 10, 20738–20744. [Google Scholar] [CrossRef]
- Teixeira, T.R.; Rangel, K.C.; Tavares, R.S.N.; Kawakami, C.M.; dos Santos, G.S.; Maria-Engler, S.S.; Colepicolo, P.; Gaspar, L.R.; Debonsi, H.M. In Vitro Evaluation of the Photoprotective Potential of Quinolinic Alkaloids Isolated from the Antarctic Marine Fungus Penicillium echinulatum for Topical Use. Mar. Biotechnol. 2021, 23, 357–372. [Google Scholar] [CrossRef]
- Vieira, G.; Khalil, Z.G.; Capon, R.J.; Sette, L.D.; Ferreira, H.; Sass, D.C. Isolation and Agricultural Potential of Penicillic Acid against Citrus Canker. J. Appl. Microbiol. 2022, 132, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, Q.; Shah, M.; Che, Q.; Zhang, G.; Zhu, T.; Zhou, J.; Rong, X.; Li, D. Talaverrucin A, Heterodimeric Oxaphenalenone from Antarctica Sponge-Derived Fungus Talaromyces Sp. HDN151403, Inhibits Wnt/β-Catenin Signaling Pathway. Org. Lett. 2022, 24, 3993–3997. [Google Scholar] [CrossRef]
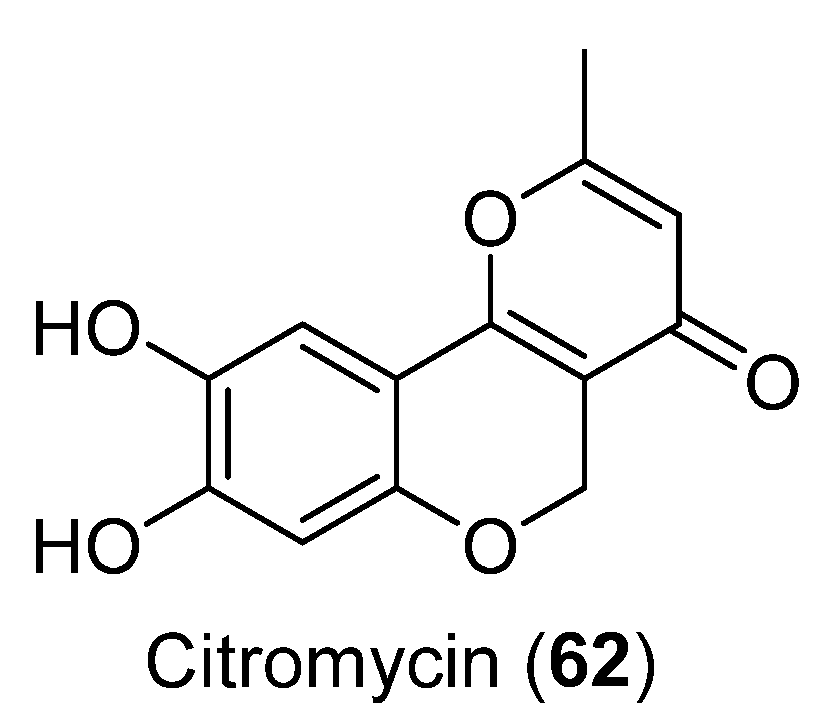
- Choi, H.Y.; Ahn, J.-H.; Kwon, H.; Yim, J.H.; Lee, D.; Choi, J.-H. Citromycin Isolated from the Antarctic Marine-Derived Fungi, Sporothrix Sp., Inhibits Ovarian Cancer Cell Invasion via Suppression of ERK Signaling. Mar. Drugs. 2022, 20, 275. [Google Scholar] [CrossRef]
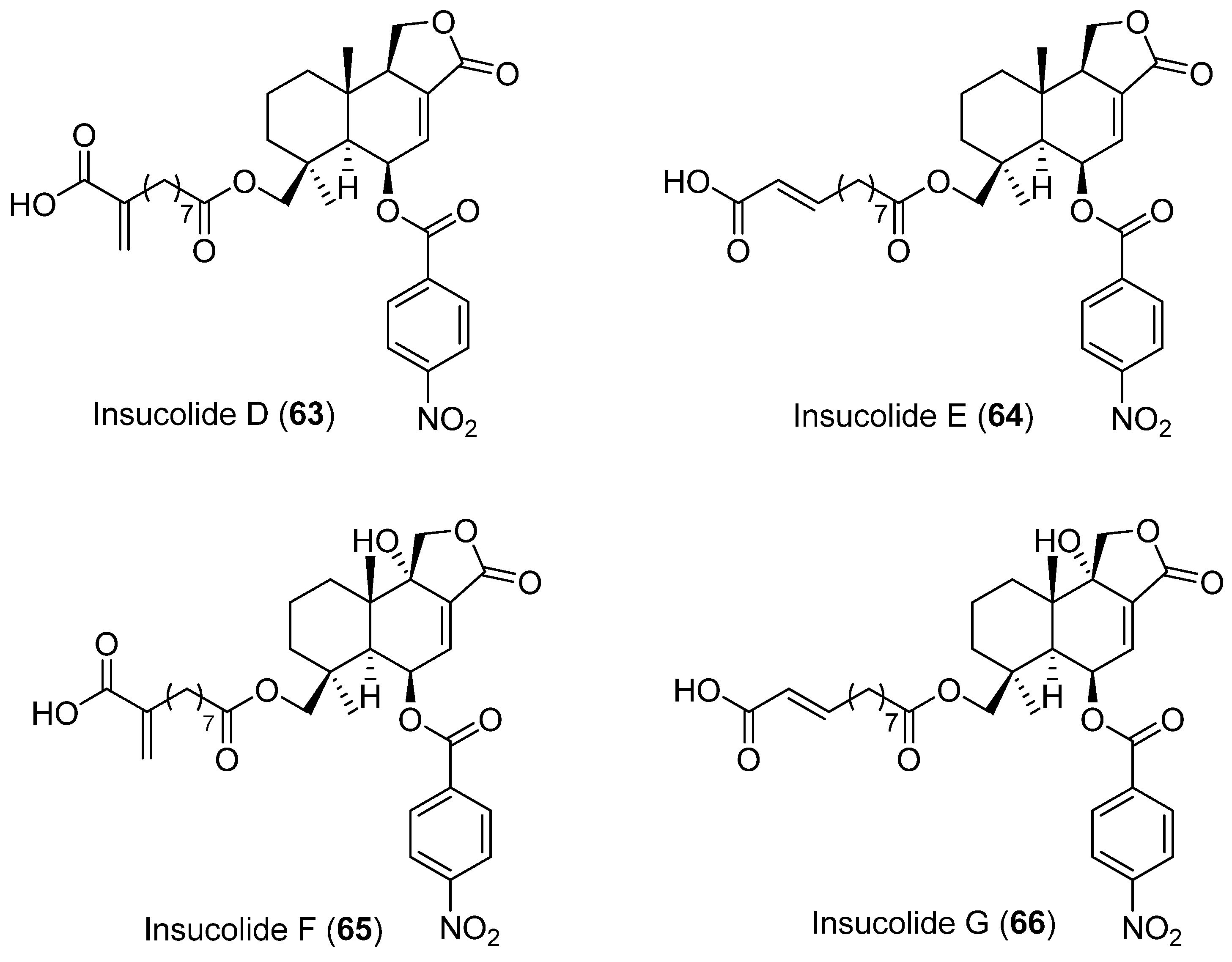
- Sun, C.; Liu, X.; Sun, N.; Zhang, X.; Shah, M.; Zhang, G.; Che, Q.; Zhu, T.; Li, J.; Li, D. Cytotoxic Nitrobenzoyl Sesquiterpenoids from an Antarctica Sponge-Derived Aspergillus Insulicola. J. Nat. Prod. 2022, 85, 987–996. [Google Scholar] [CrossRef]
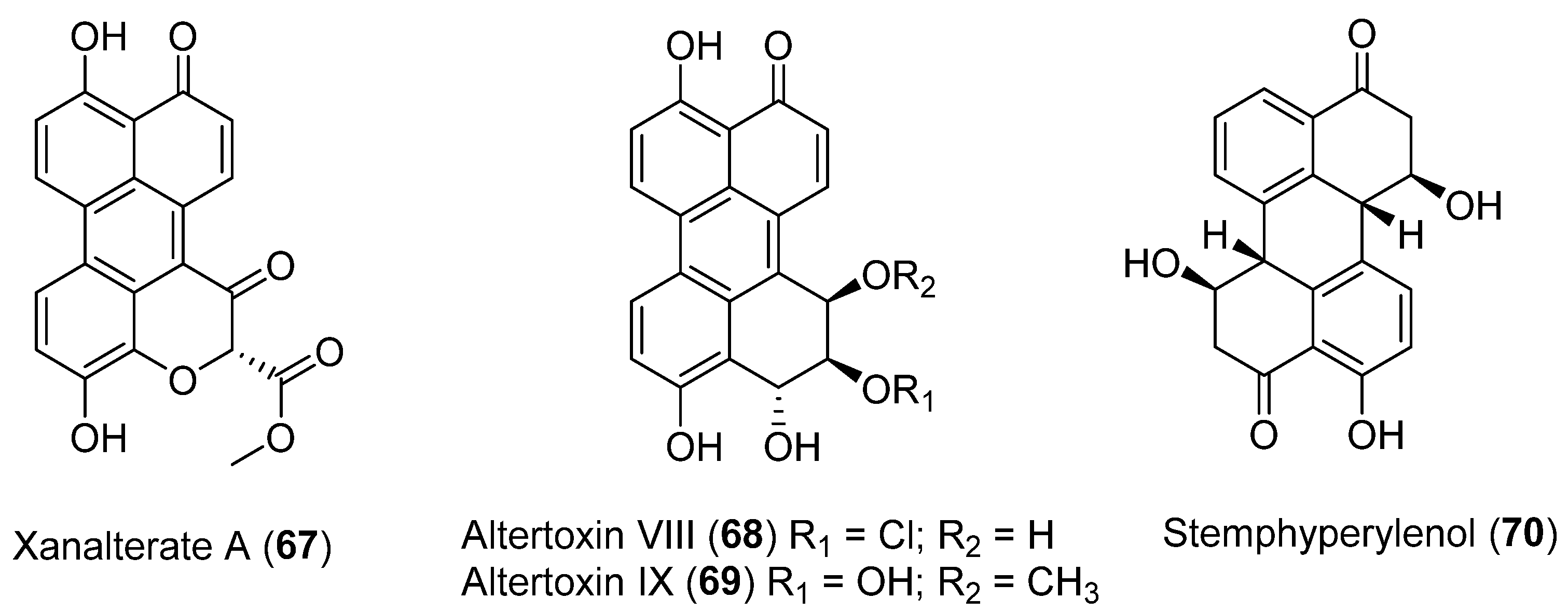
- Hou, Z.; Sun, C.; Chen, X.; Zhang, G.; Che, Q.; Li, D.; Zhu, T. Xanalterate A, Altertoxin VIII and IX, Perylenequinone Derivatives from Antarctica-Sponge-Derived Fungus Alternaria Sp. HDN19-690. Tetrahedron Lett. 2022, 96, 153778. [Google Scholar] [CrossRef]

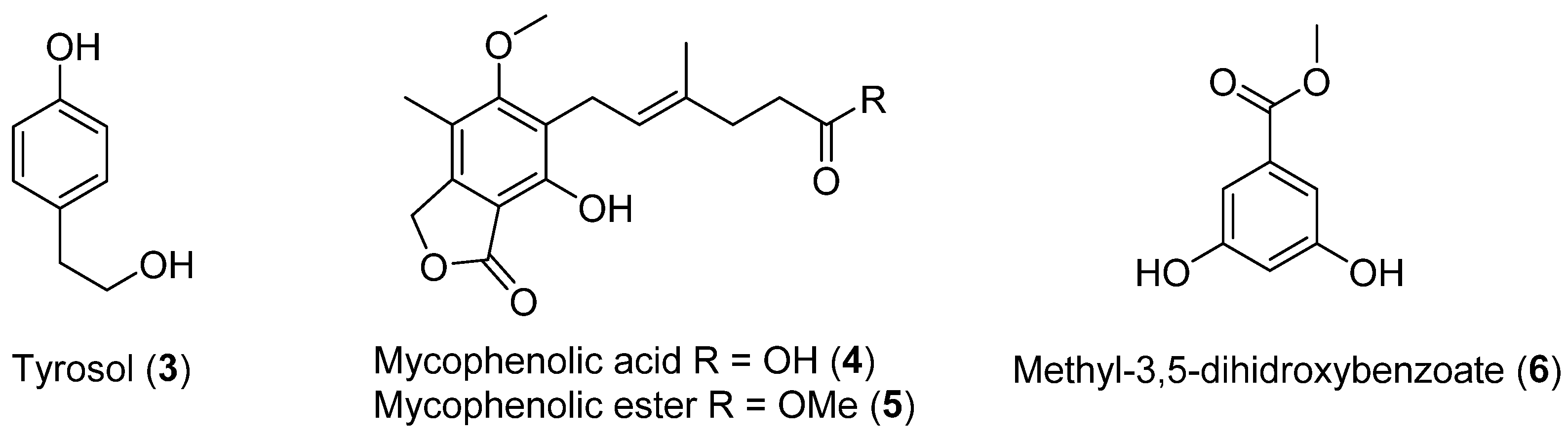



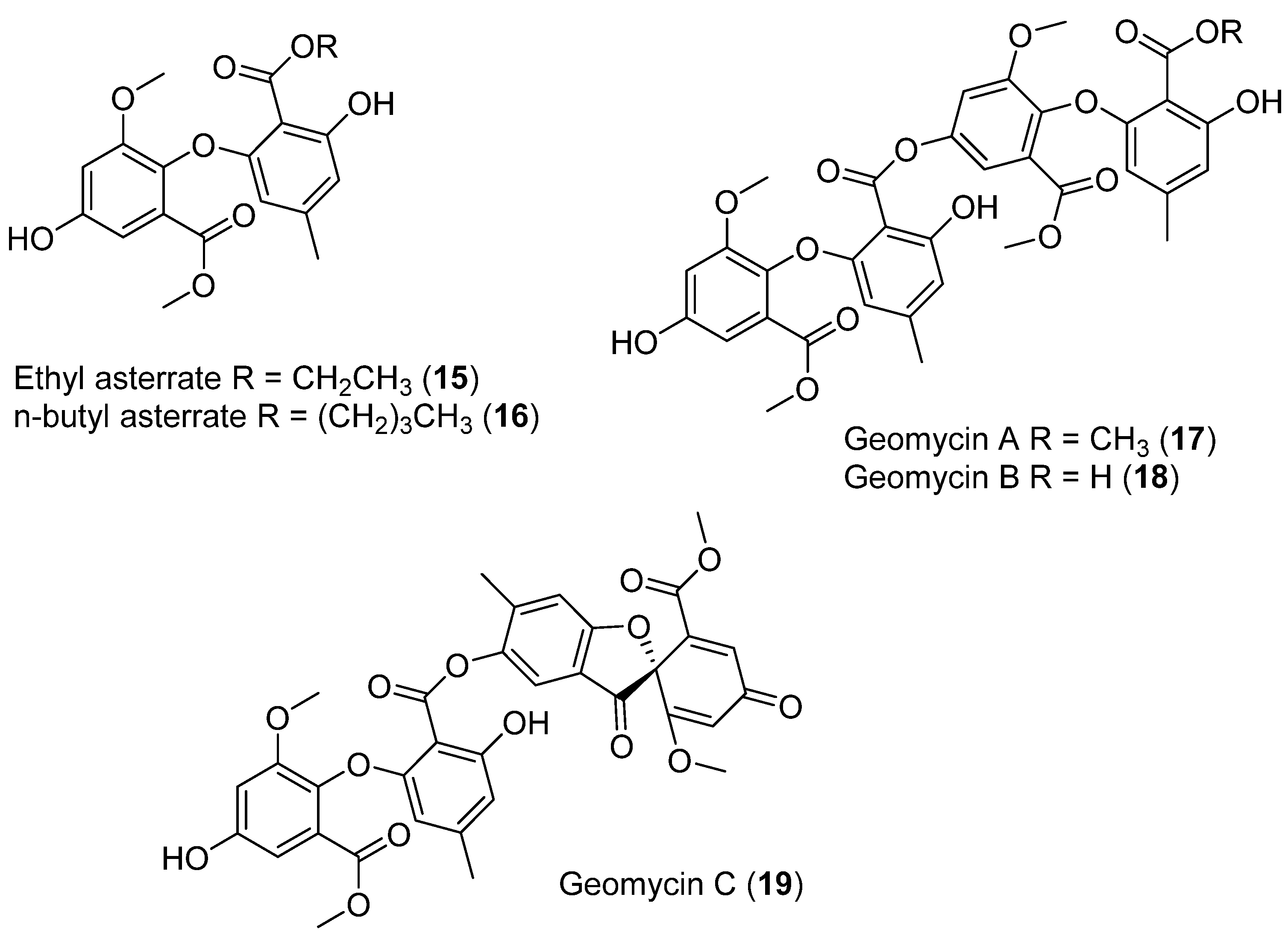
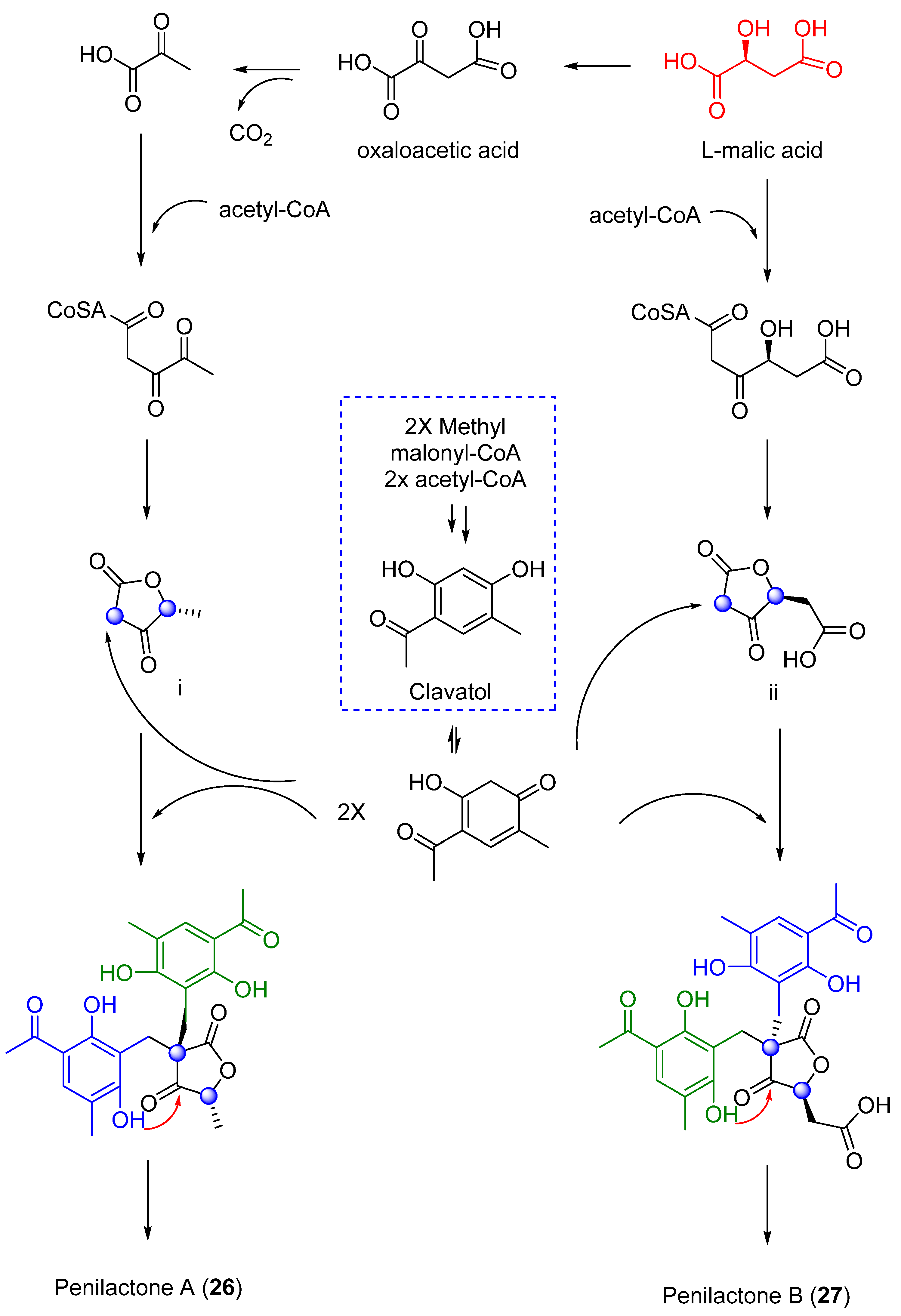


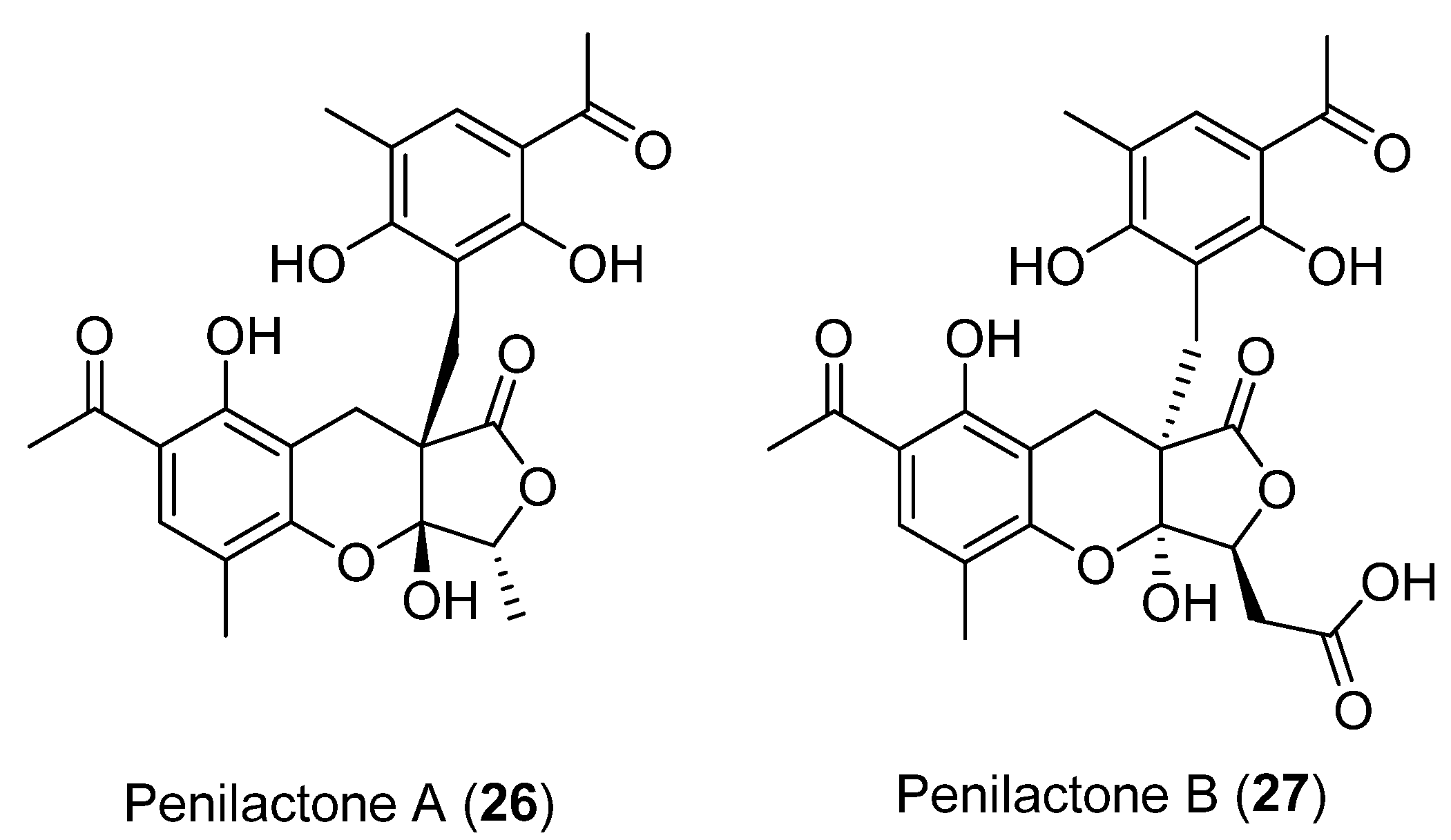




| Compounds | Fungi | Region | Bioactivity | References |
|---|---|---|---|---|
| 1 | Cladosporium cladosporioides | Chilean coasts | Not antimicrobial activity showed | [23] |
| 2 | Inonotus obliquus Geotrichum sp. | Cytotoxic activity, Lipid-peroxidation, Antioxidant activity | [22,23,26] | |
| 3 | Penicillium brevicompactum | Antibacterial activity | [24] | |
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | Geotrichum sp. | Not tested | [26] | |
| 8 | Scleroderma polyrizum Geotrichum sp. Lampteromyces japonicus Fomes officinalis | Not tested | [25,26] | |
| 9 | Geotrichum sp. | Not tested | [26] | |
| 10 | Geomyces sp. | Not tested | [27] | |
| 11 | Aspergillus sp. | Antibacterial activity | [28] | |
| 12 | Antitumor activity | |||
| 13 | Vasorelaxant activity | |||
| 14 | Aspergillus sp. | Not tested | [28] | |
| 15 | Geomyces sp. | Antarctic | Not antimicrobial, and antifungal activity showed | [31,32,33,34] |
| 16 | Not antimicrobial, and antifungal activity showed | |||
| 17 | Not antimicrobial, and antifungal activity showed | |||
| 18 | Antifungal activity | |||
| 19 | Antibacterial activity | |||
| 20 | Tridocherma asperellum | Not tested | [35] | |
| 21 | Not tested | |||
| 22 | Not tested | |||
| 23 | Not tested | |||
| 24 | Not tested | |||
| 25 | Not tested | |||
| 26 | Penicillium crustosum | Not cytotoxic activity showed | [36,37] | |
| 27 | Inhibit nuclear factor-κB (NF-κB) | |||
| 28 | Oidiodendron truncatum GW3-13 | Cytotoxic activity | [38] | |
| 29 | ||||
| 30 | ||||
| 31 | No significant cytotoxic activity showed | |||
| 32 | No significant cytotoxic activity showed | |||
| 33 | No significant cytotoxic activity showed | |||
| 34 | No significant cytotoxic activity showed | |||
| 35 | Pseudogymnoascus sp. | Not antimicrobial and antifungal activity showed | [40] | |
| 36 | Not antimicrobial and antifungal activity showed | |||
| 37 | Not antimicrobial and antifungal activity showed | |||
| 38 | Not antimicrobial and antifungal activity showed | |||
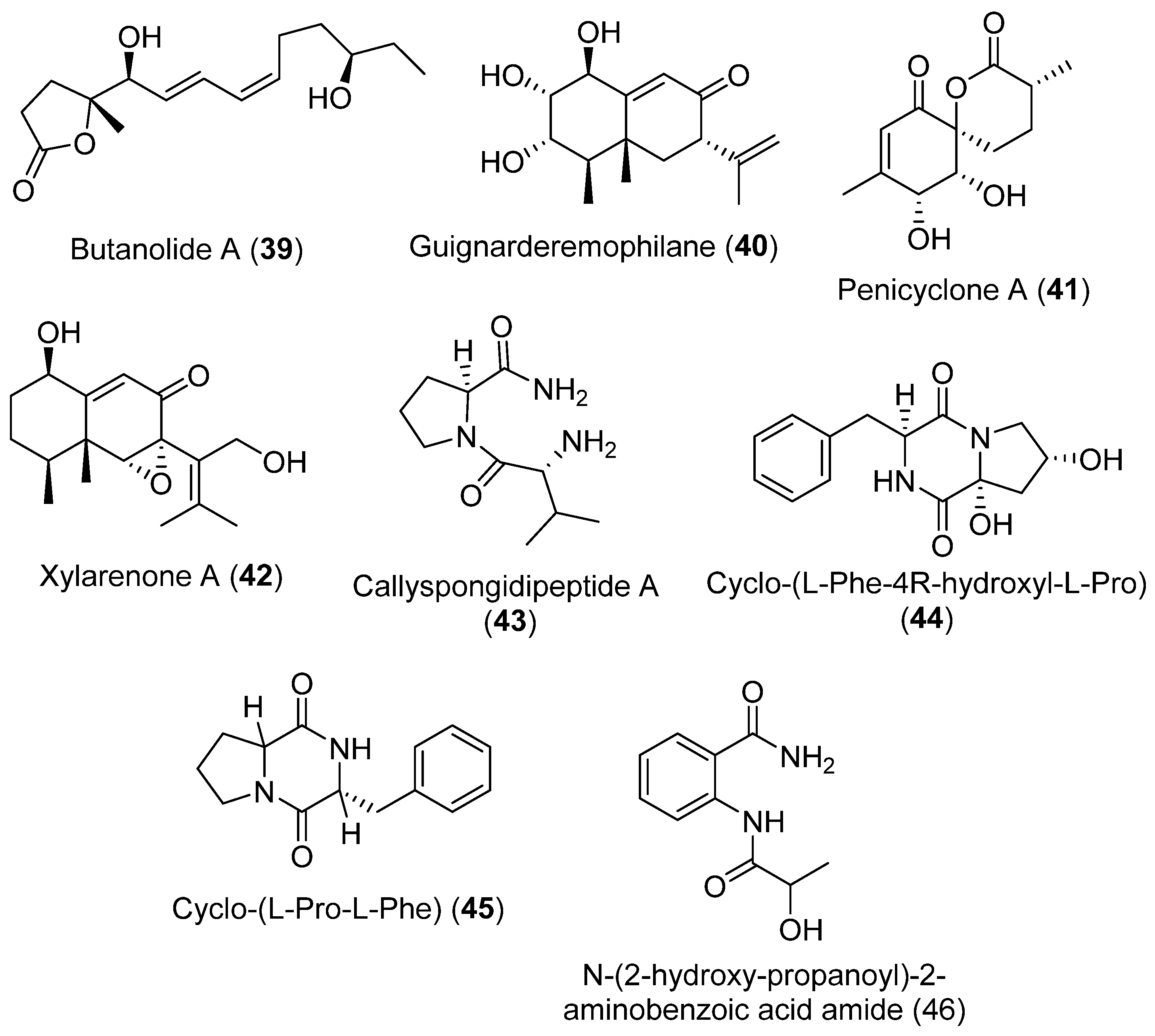
| 39 | Penicillium sp. | Antarctic | Antiproliferative effect | [42] |
| 40 | Not antiproliferative effect showed | |||
| 41 | Not antiproliferative effect showed | |||
| 42 | Not antiproliferative effect showed | |||
| 43 | Not antiproliferative effect showed | |||
| 44 | Not antiproliferative effect showed | |||
| 45 | Not antiproliferative effect showed | |||
| 46 | Not antiproliferative effect showed | |||
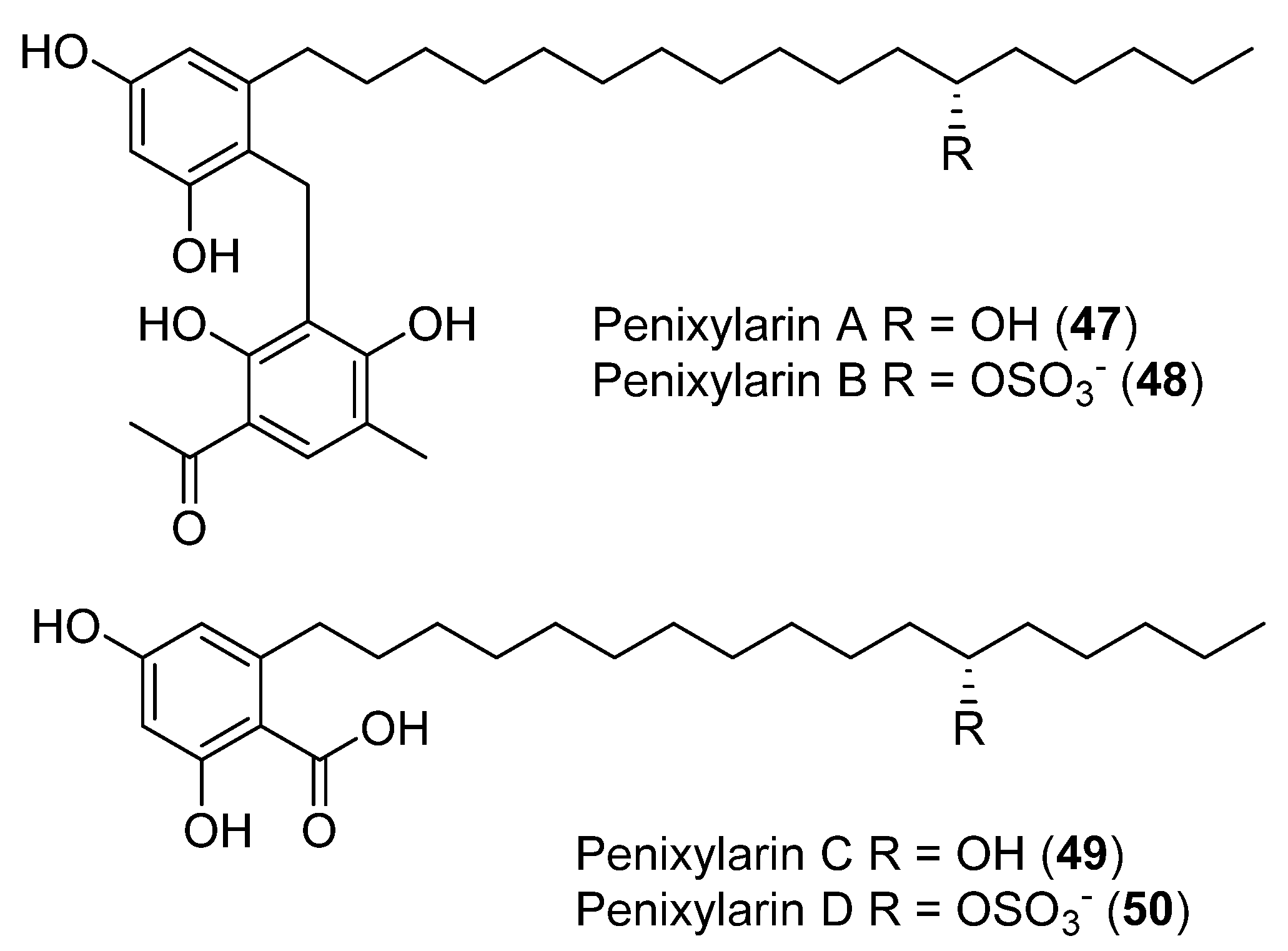
| 47 | Penicillium crestosum (PRB-2) | No cytotoxic and antibacterial activity showed | [44] | |
| 48 | Antibacterial activity | |||
| 49 | Antibacterial activity | |||
| 50 | No cytotoxic, and antibacterial activity showed | |||
| 51 | Penicillium chrysogenum | Moderate alpha glucosidase inhibition, no cytotoxic and antibacterial activity showed | [47] | |
| 51a | Moderate alpha glucosidase inhibition, no cytotoxic and antibacterial activity showed | |||
| 51b | Moderate alpha glucosidase inhibition, no cytotoxic and antibacterial activity showed | |||
| 52a | Moderate alpha glucosidase inhibition, no cytotoxic and antibacterial activity showed | |||
| 52b | Moderate alpha glucosidase inhibition, no cytotoxic and antibacterial activity showed | |||
| 53 | Antibacterial activity | |||
| 54 | Cytotoxic activity | |||
| 55 | Alpha glucosidase inhibition | |||
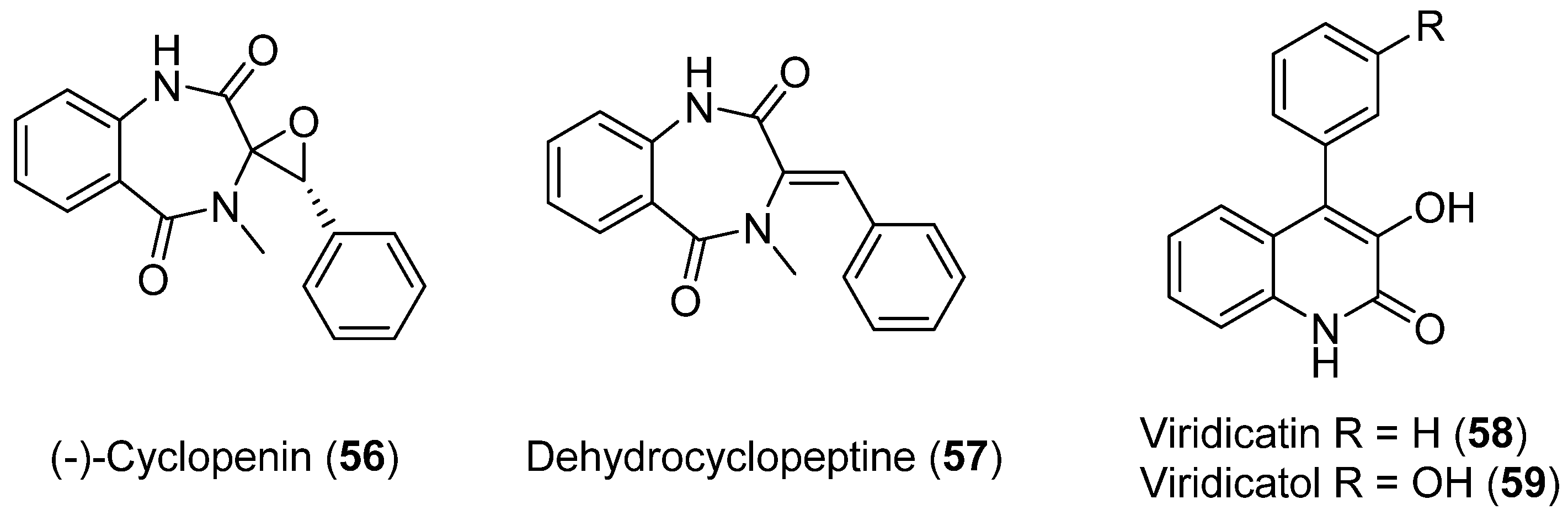
| 56 | Penicillium echinulatum | Photoprotective and antioxidant activity | [48] | |
| 57 | No cytotoxic and antibacterial activity showed | |||
| 58 | Cytotoxic activity | |||
| 59 | Cytotoxic activity | |||
| 60 | Penicillium sp. CRM-1540 | Antibacterial activity | [49] | |
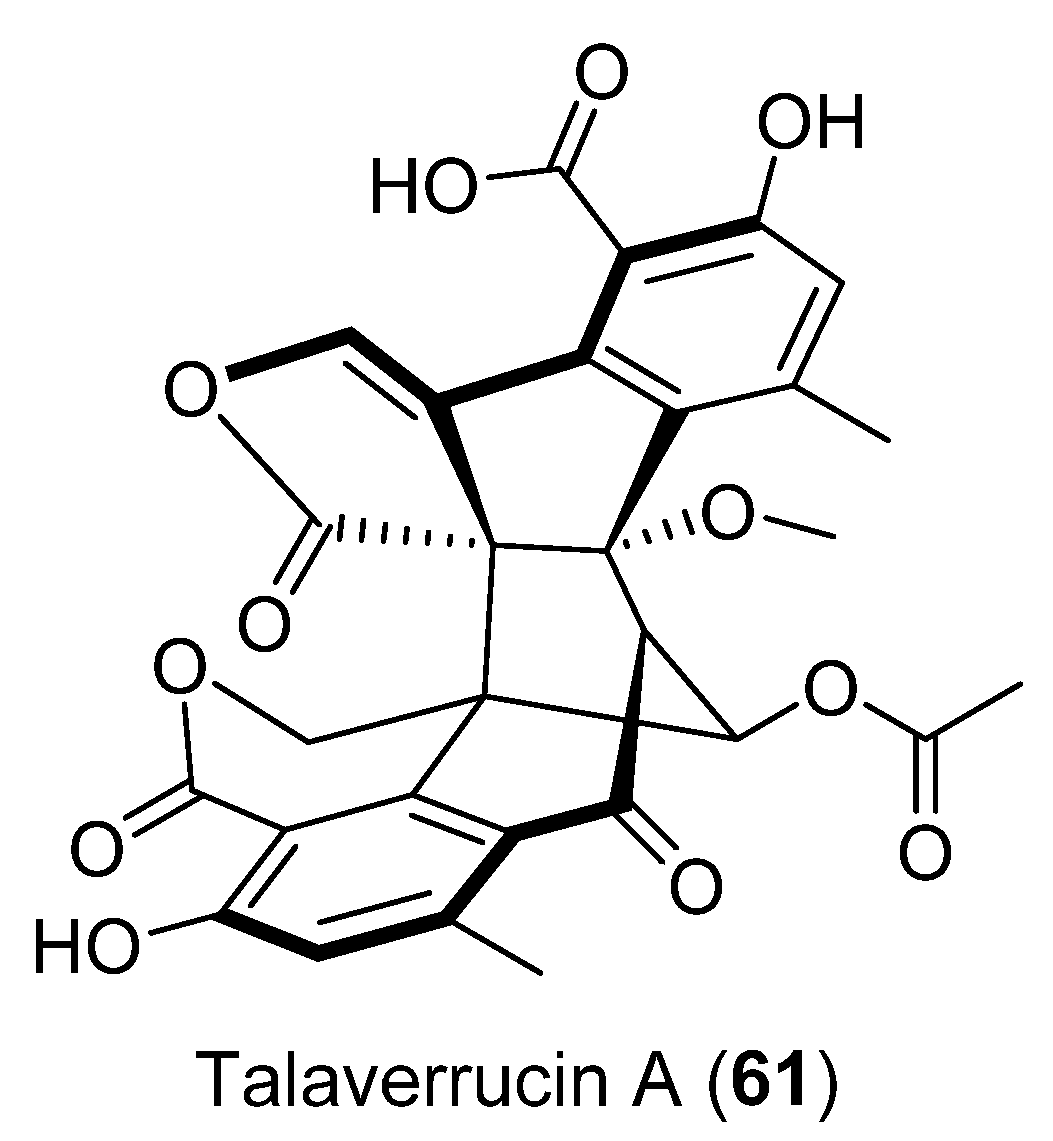
| 61 | Talaromyces sp. HDN151403 | Cytotoxic activity | [50] | |
| 62 | Sporothrix sp. | Cytotoxic activity | [51] | |
| 63 | Aspergillus insulicola HDN151418 | No cytotoxic activity showed | [52] | |
| 64 | No cytotoxic activity showed | |||
| 65 | Cytotoxic activity | |||
| 66 | Cytotoxic activity | |||
| 67 | Alternaria sp. HDN19-690 | Antibacterial activity, no cytotoxic activity showed | [53] | |
| 68 | No cytotoxic and antibacterial activity showed | |||
| 69 | No cytotoxic and antibacterial activity showed | |||
| 70 | No cytotoxic and antibacterial activity showed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieche, D.; Cabrera-Pardo, J.R.; San-Martin, A.; Carrasco, H.; Taborga, L. Natural Products from Chilean and Antarctic Marine Fungi and Their Biomedical Relevance. Mar. Drugs 2023, 21, 98. https://doi.org/10.3390/md21020098
Arrieche D, Cabrera-Pardo JR, San-Martin A, Carrasco H, Taborga L. Natural Products from Chilean and Antarctic Marine Fungi and Their Biomedical Relevance. Marine Drugs. 2023; 21(2):98. https://doi.org/10.3390/md21020098
Chicago/Turabian StyleArrieche, Dioni, Jaime R. Cabrera-Pardo, Aurelio San-Martin, Héctor Carrasco, and Lautaro Taborga. 2023. "Natural Products from Chilean and Antarctic Marine Fungi and Their Biomedical Relevance" Marine Drugs 21, no. 2: 98. https://doi.org/10.3390/md21020098
APA StyleArrieche, D., Cabrera-Pardo, J. R., San-Martin, A., Carrasco, H., & Taborga, L. (2023). Natural Products from Chilean and Antarctic Marine Fungi and Their Biomedical Relevance. Marine Drugs, 21(2), 98. https://doi.org/10.3390/md21020098





