An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts
Abstract
:1. Introduction
2. Causes of Antimicrobial Resistance
3. An Overview of Terrestrial Plants’ Antibacterial Activity
| Terrestrial Plant | Extract Type | Microbes | Minimum Inhibitory Concentration (MIC) | Inhibition Zone Diameter (mm) | Reference |
|---|---|---|---|---|---|
| Anacardium occidentale | Ethanolic extract | Staphylococcusaureus | 1.56 mg/mL | 16.00 | [60] |
| Bacillus subtilis | 1.56 mg/mL | 15.00 | |||
| Escherichia coli | 1.56 mg/mL | 12.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 14.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 1.56 mg/mL | 14.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 15.00 | |||
| MAE | Staphylococcusaureus | 0.78 mg/mL | 17.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 1.56 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 16.00 | |||
| Careya sphaerica | Ethanolic extract | Staphylococcusaureus | <1.56 mg/mL | 17.00 | [60] |
| Bacillus subtilis | <1.56 mg/mL | 17.00 | |||
| Escherichia coli | 3.12 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 13.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 17.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 3.12 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 14.00 | |||
| MAE | Staphylococcusaureus | <1.56 mg/mL | 18.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 17.00 | |||
| Escherichia coli | 1.56 mg/mL | 16.00 | |||
| Pseudomonas aeruginosa | 1.56 mg/mL | 16.00 | |||
| Garcinia cowa | Ethanolic extract | Staphylococcusaureus | 3.12 mg/mL | 14.00 | [60] |
| Bacillus subtilis | 1.56 mg/mL | 11.00 | |||
| Escherichia coli | 12.5 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 12.5 mg/mL | 12.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 12.5 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 12.5 mg/mL | 13.00 | |||
| MAE | Staphylococcusaureus | 0.78 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 16.00 | |||
| Escherichia coli | 12.5 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 12.5 mg/mL | 12.00 | |||
| Glochidion wallichianum | Ethanolic extract | Staphylococcusaureus | <1.56 mg/mL | 16.00 | [60] |
| Bacillus subtilis | <1.56 mg/mL | 14.00 | |||
| Escherichia coli | 6.25 mg/mL | 10.00 | |||
| Pseudomonas aeruginosa | 3.12 mg/mL | 13.00 | |||
| UAE | Staphylococcusaureus | <1.56 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 15.00 | |||
| Escherichia coli | 3.12 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 6.25 mg/mL | 13.00 | |||
| MAE | Staphylococcusaureus | 0.78 mg/mL | 14.00 | ||
| Bacillus subtilis | <1.56 mg/mL | 15.00 | |||
| Escherichia coli | 3.12 mg/mL | 15.00 | |||
| Pseudomonas aeruginosa | 3.12 mg/mL | 13.00 | |||
| Glycyrrhiza glabra | Ethanolic extract | Mycobacteriumtuberculosis H37Ra | 500 mg/mL | - | [56] |
| Mycobacteriumtuberculosis H37Rv | 500 mg/mL | - | |||
| Glycyrrhiza glabra | Methanolic extract | Staphylococcusaureus | 6.25 mg/mL | 10 ± 1.34 | [57] |
| Bacilluscereus | 12.5 mg/mL | 7 ± 1 | |||
| Escherichiacoli | 50 mg/mL | 6 ± 1.22 | |||
| Pseudomonas aeruginosa | 100 mg/mL | - | |||
| Gnetum gnemon var. temerum | Ethanolic extract | Staphylococcusaureus | 50.00 mg/mL | 10.00 | [60] |
| Bacillus subtilis | 25.00 mg/mL | - | |||
| Escherichia coli | 50.00 mg/mL | 9.00 | |||
| Pseudomonas aeruginosa | 50.00 mg/mL | 9.00 | |||
| UAE | Staphylococcusaureus | 50 mg/mL | 10.00 | ||
| Bacillus subtilis | 12.5 mg/mL | - | |||
| Escherichia coli | 50 mg/mL | 10.00 | |||
| Pseudomonas aeruginosa | 25 mg/mL | 10.00 | |||
| MAE | Staphylococcusaureus | 12.5 mg/mL | 11.00 | ||
| Bacillus subtilis | 6.25 mg/mL | - | |||
| Escherichia coli | 50 mg/mL | 11.00 | |||
| Pseudomonas aeruginosa | 25 mg/mL | 10.00 | |||
| Hedypnois cretica | Methanolic extract | Bacilluscereus | 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 2GP: 0.60 mg/mL | - | |||
| Listeria monocytogenes | 2GP: 0.45 mg/mL | - | |||
| Escherichiacoli | 2GP: 0.20 mg/mL | - | |||
| Enterobactercloacae | 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 2GP: 0.30 mg/mL | - | |||
| Hibiscus sabdariffa | Ethanolic extract | Bacillus cereus | 5 (% w/v) | 22.2 + 0.8 | [49] |
| Water extract | 0.625 (% w/v) | 17.0 + 1.1 | |||
| Ethanolic extract | Staphylococcus aureus | 2.5 (% w/v) | 21.5 + 2.1 | ||
| Water extract | 2.5 (% w/v) | 15.7 + 1.0 | |||
| Ethanolic extract | Escherichia coli | 5 (% w/v) | 21.1 + 1.3 | ||
| Water extract | 5 (% w/v) | 15.6 + 1.2 | |||
| Ethanolic extract | Salmonella enteritidis | 5 (% w/v) | 20.2 + 1.7 | ||
| Water extract | 10 (% w/v) | 14.0 + 1.9 | |||
| Ethanolic extract | Vibrio parahaemolyticus | 2.5 (% w/v) | 20.3 + 1.8 | ||
| Water extract | 5 (% w/v) | 15.9 + 1.7 | |||
| Ethanolic extract | Pseudomonas aeruginosa | 2.5 (% w/v) | 23.4 + 1.4 | ||
| Water extract | 5 (% w/v) | 13.9 + 1.9 | |||
| Hymenonema graecum | Methanolic extract | Bacilluscereus | 1GP: 0.20 mg/mL 2GP: 0.20 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.60 mg/mL 2GP: 0.60 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.60 mg/mL 2GP: 0.60 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.60 mg/mL 2GP: 0.60 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.45 mg/mL 2GP: 0.60 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Morus alba | Ethanolic extract | Staphylococcusaureus | - | 10.5 ± 1.15 | [58] |
| Bacilluscereus | 2500 µg/mL | 14.75 ± 0.15 | |||
| Escherichiacoli | - | 7.5 ± 0.15 | |||
| Pasteurellamultocida | 1250 µg/mL | 15.42 ± 0.15 | |||
| Salmonella enteritidis | 625 µg/mL | 12.02 ± 0.05 | |||
| Olea europaea | Ethanolic extract | Staphylococcusaureus | 625 µg/mL | 12.02 ± 2.05 | [58] |
| Bacilluscereus | 5000 µg/mL | 16.62 ± 1.05 | |||
| Escherichiacoli | 2500 µg/mL | 16.72 ± 0.55 | |||
| Pasteurellamultocida | 625 µg/mL | 9.12 ± 0.05 | |||
| Salmonella enteritidis | 5000 µg/mL | 18.02 ± 0.05 | |||
| Picris echioides | Methanolic extract | Bacilluscereus | 1GP: 0.075 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.60 mg/mL 2GP: 0.30 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.45 mg/mL 2GP: 0.15 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.30 mg/mL 2GP: 0.20 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.60 mg/mL 2GP: 0.20 mg/mL | - | |||
| Psidium guajava | Ethanolic extract | Staphylococcusaureus | 1250 µg/mL | 15.62 ± 1.15 | [58] |
| Bacilluscereus | - | 10.05 ± 0.15 | |||
| Escherichiacoli | 625 µg/mL | 10.55 ± 0.15 | |||
| Pasteurellamultocida | 5000 µg/mL | 18.02 ± 0.95 | |||
| Salmonella enteritidis | 625 µg/mL | 10.12 ± 0.55 | |||
| Reichardia picroides | Methanolic extract | Bacilluscereus | 1GP: 0.15 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.15 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.30 mg/mL 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.30 mg/mL 2GP: 0.60 mg/mL | - | |||
| Rosmarinus officinalis | Ethanolic extract | Bacillus cereus | 5 (% w/v) | 19.8 ± 0.8 | [49] |
| Water extract | 1.25 (% w/v) | 13.9 ± 1.2 | |||
| Ethanolic extract | Staphylococcus aureus | 1.25 (% w/v) | 19.8 ± 0.4 | ||
| Water extract | 20 (% w/v) | 12.7 ± 0.4 | |||
| Ethanolic extract | Escherichia coli | 5 (% w/v) | 21.1 ± 0.9 | ||
| Water extract | 20 (% w/v) | 12.5 ± 0.7 | |||
| Ethanolic extract | Salmonella enteritidis | 2.5 (% w/v) | 20.7 ± 1.2 | ||
| Ethanolic extract | Vibrio parahaemolyticus | - | - | ||
| Water extract | - | - | |||
| Ethanolic extract | Pseudomonas aeruginosa | - | - | ||
| Water extract | - | - | |||
| Salvia officinalis | Ethanolic extract | Staphylococcusaureus | 5000 µg/mL | 17.05 ± 1.05 | [58] |
| Bacilluscereus | 625 µg/mL | 16.45 ± 1.05 | |||
| Escherichiacoli | 2500 µg/mL | 19.25 ± 0.65 | |||
| Pasteurellamultocida | - | 9.05 ± 1.05 | |||
| Salmonella enteritidis | 2500 µg/mL | 16.25 ± 0.75 | |||
| Scolymus hispanicus | Methanolic extract | Bacilluscereus | 2GP: 0.10 mg/mL | - | [59] |
| Staphylococcusaureus | 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 2GP: 0.20 mg/mL | - | |||
| Escherichiacoli | 2GP: 0.10 mg/mL | - | |||
| Enterobactercloacae | 2GP: 0.15 mg/mL | - | |||
| Salmonella typhimurium | 2GP: 0.15 mg/mL | - | |||
| Sonchus oleraceus | Methanolic extract | Bacilluscereus | 1GP: 0.20 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.45 mg/mL 2GP: 0.60 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.60 mg/mL 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Syzygium aromaticum | Ethanolic extract | Bacillus cereus | 2.5 (% w/v) | 18.2 ± 3.2 | [49] |
| Water extract | 0.313 (% w/v) | 15.1 ± 0.9 | |||
| Ethanolic extract | Staphylococcus aureus | 2.5 (% w/v) | 16.7 ± 1.0 | ||
| Water extract | 5 (% w/v) | 13.6 ± 1.3 | |||
| Ethanolic extract | Escherichia coli | 2.5 (% w/v) | 17.4 ± 0.8 | ||
| Water extract | 5 (% w/v) | 13.2 ± 1.6 | |||
| Ethanolic extract | Salmonella enteritidis | 5 (% w/v) | 15.1 ± 1.4 | ||
| Water extract | 5 (% w/v) | 12.2 ± 1.1 | |||
| Ethanolic extract | Vibrio parahaemolyticus | 0.625 (% w/v) | 14.7 ± 2.0 | ||
| Water extract | 2.5 (% w/v) | 13.1 ± 1.8 | |||
| Ethanolic extract | Pseudomonas aeruginosa | 5 (% w/v) | 17.0 ± 0.5 | ||
| Water extract | 10 (% w/v) | 13.2 ± 1.4 | |||
| Taraxacum officinale | Methanolic extract | Bacilluscereus | 1GP: 0.037 mg/mL 2GP: 0.20 mg/mL | - | [59] |
| Staphylococcus aureus | 1GP: 0.30 mg/mL 2GP: 0.90 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.30 mg/mL 2GP: 0.90 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.15 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.15 mg/mL 2GP: 0.30 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.15 mg/mL 2GP: 0.60 mg/mL | - | |||
| Taraxacum sp. | Methanolic extract | Bacilluscereus | 1GP: 0.075 mg/mL 2GP: 0.075 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.60 mg/mL 2GP: 0.30 mg/mL | - | |||
| Enterobactermonocytogenes | 1GP: 0.45 mg/mL 2GP: 0.45 mg/mL | - | |||
| Escherichiacoli | 1GP: 0.20 mg/mL 2GP: 0.90 mg/mL | - | |||
| Enterobactercloacae | 1GP: 0.20 mg/mL 2GP: 0.20 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.20 mg/mL 2GP: 0.30 mg/mL | - | |||
| Thymus vulgaris | Ethanolic extract | Bacillus cereus | 5 (% w/v) | 17.3 ± 0.7 | [49] |
| Water extract | 5 (% w/v) | 13.8 ± 1.1 | |||
| Ethanolic extract | Staphylococcus aureus | 5 (% w/v) | 15.9 ± 0.3 | ||
| Water extract | 2.5 (% w/v) | 12.2 ± 0.7 | |||
| Ethanolic extract | Escherichia coli | 10 (% w/v) | 15.9 ± 0.3 | ||
| Water extract | 5 (% w/v) | 12.2 ± 0.7 | |||
| Water extract | Salmonella enteritidis | 5 (% w/v) | 11.8 ± 1.4 | ||
| Water extract | Vibrio parahaemolyticus | 2.5 (% w/v) | 13.9 ± 1.3 | ||
| Ethanolic extract | 10 (% w/v) | 14.3 ± 0.1 | |||
| Water extract | Pseudomonas aeruginosa | - | - | ||
| Ethanolic extract | - | - | |||
| Urospermum picroides | Methanolic extract | Bacilluscereus | 1GP: 0.15 mg/mL 2GP: 0.15 mg/mL | - | [59] |
| Staphylococcusaureus | 1GP: 0.90 mg/mL 2GP: 0.90 mg/mL | - | |||
| Listeria monocytogenes | 1GP: 0.90 mg/mL 2GP: 0.30 mg/mL | - | |||
| Escherichiacoli | 1GP: 0. 90 mg/mL 2GP: 0.45 mg/mL | - | |||
| Enterobacter cloacae | 1GP: 0.45 mg/mL 2GP: 0.60 mg/mL | - | |||
| Salmonella typhimurium | 1GP: 0.45 mg/mL 2GP: 0.30 mg/mL | - | |||
| Ziziphusspina christi | Ethanolic extract | Staphylococcusaureus | 625 µg/mL | 11.82 ± 2.5 | [58] |
| Bacilluscereus | 625 µg/mL | 13.52 ± 2.1 | |||
| Escherichiacoli | - | 10.02 ± 0.05 | |||
| Pasteurellamultocida | - | 8.52 ± 2.5 | |||
| Salmonella enteritidis | 625 µg/mL | 12.82 ± 2.5 |
4. Antimicrobial Activity from Rhodophyta and Chlorophyta Extracts
4.1. Rhodophyta
| Rhodophyta | Extract Type | Microbes | Minimum Inhibitory Concentration (MIC) | Inhibition Zone Diameter (mm) | Reference |
|---|---|---|---|---|---|
| Asparagopsistaxiformis | Methanolic extract | Staphylococcus aureus | 0.5 mg/mL | >15 | [75] |
| Serratia sp. | 0.5 mg/mL | - | |||
| Klebseilla sp. | 0.5 mg/mL | >1 | |||
| Salmonella sp. | 0.5 mg/mL | - | |||
| Escherichia coli | 0.5 mg/mL | >10 | |||
| Klebseilla pneumonia | 0.5 mg/mL | >1 | |||
| Pseudomonas aeruginosa | 0.5 mg/mL | - | |||
| Pseudomonas fluorescens | 0.5 mg/mL | >10 | |||
| Vibrio proteolyticus | 0.5 mg/mL | >1 | |||
| Streptococcus sp. | 0.5 mg/mL | 10 | |||
| Bacillussubtilis | 0.5 mg/mL | 10 | |||
| Chondrus crispus | Bacillus subtilis | 12.5 mg/mL | - | [72] | |
| Gelidium sp. | Water extract | Salmonella enterica | 12.5 mg/mL | >10 | [76] |
| Klebsiella pneumoniae | 50 mg/mL | >11 | |||
| Listeria monocytogenes | 50 mg/mL | 11 | |||
| Enterobacter aerogenes | 25 mg/mL | >11 | |||
| Proteus mirabilis | 50 mg/mL | >11 | |||
| Vibrio parahaemolyticus | nd | >11 | |||
| Vibrio alginolyticus | nd | 13 | |||
| Bacillus licheniformis | 25 mg/mL | 11 | |||
| Bacillus cereus | 0.625 mg/mL | >11 | |||
| Bacillus subtilis | 3.125 mg/mL | >10 | |||
| Escherichia coli | 50 mg/mL | >13 | |||
| Pseudomonas putida | - | - | |||
| Pseudomonas fluorescens | - | - | |||
| Gracilaria corticata | Methanolic extract | Escherichia coli | 100 µg/mL | 7 ± 0.01 | [67] |
| Photobacterium sp. | 100 µg/mL | 6 ± 0.04 | |||
| Pseudomonas fluorescens | 100 µg/mL | 8 ± 0.1 | |||
| Staphylococcus aureus | 100 µg/mL | 4 ± 0.10 | |||
| Bacillus subtilis | 100 µg/mL | 8 ± 0.01 | |||
| Dimethyl sulfoxide (DMSO) extract | Escherichia coli | 100 µg/mL | 5 ± 0.10 | ||
| Photobacterium sp. | 100 µg/mL | 4 ± 0.30 | |||
| Pseudomonas fluorescens | 100 µg/mL | 4 ± 0.05 | |||
| Staphylococcus aureus | 100 µg/mL | 6 ± 0.05 | |||
| Bacillus subtilis | 100 µg/mL | 5 ± 0.12 | |||
| Gracilaria edulis | Methanolic extract | Escherichia coli | 100 µg/mL | 3 ± 0.01 | [67] |
| Photobacterium sp. | 100 µg/mL | 1 ± 0.00 | |||
| Pseudomonas fluorescens | 100 µg/mL | 3 ± 0.05 | |||
| Staphylococcus aureus | 100 µg/mL | 3 ± 0.05 | |||
| Bacillus subtilis | 100 µg/mL | 3 ± 0.03 | |||
| Dimethyl sulfoxide (DMSO) extract | Escherichia coli | 100 µg/mL | 4.5 ± 0.01 | ||
| Photobacterium sp. | 100 µg/mL | 4 ± 0.01 | |||
| Pseudomonas fluorescens | 100 µg/mL | 4 ± 0.10 | |||
| Staphylococcus aureus | 100 µg/mL | 3 ± 0.00 | |||
| Gracilaria edulis | Methanolic extracts | Klebsiella oxytoca | 0.3 mg/mL | 21 | [71] |
| Escherichia coli | 0.3 mg/mL | 19 | |||
| Staphylococcus aureus | 0.3 mg/mL | 18 | |||
| Pseudomonas aeruginosa | 0.3 mg/mL | 16 | |||
| Bacillus subtilis | 0.3 mg/mL | 23 | |||
| Serratia sp. | 0.3 mg/mL | 20 | |||
| Salmonella sp. | 0.3 mg/mL | 22 | |||
| Grateloupia turuturu | Ethanolic extract | Staphylococcus aureus | 10 mg/mL | - | [70] |
| Escherichia coli | 10 mg/mL | - | |||
| Polysaccharides (carrageenan) | Staphylococcus aureus | 7.5 mg/mL | - | ||
| Escherichia coli | 7.5 mg/mL | - | |||
| Hypnea valentiae | Methanolic extract | Klebsiella oxytoca | 0.3 mg/mL | 17 | [71] |
| Escherichia coli | 0.3 mg/mL | 12 | |||
| Staphylococcus aureus | 0.3 mg/mL | 14 | |||
| Pseudomonas aeruginosa | 0.3 mg/mL | 11 | |||
| Bacillus subtilis | 0.3 mg/mL | 15 | |||
| Serratia sp. | 0.3 mg/mL | 13 | |||
| Salmonella sp. | 0.3 mg/mL | 16 | |||
| Kappaphycus alvarezii | Ethanolic extract | Escherichia coli | - | - | [73] |
| Bacillus cereus | 0.5 mg/mL | <10 | |||
| Hot water extract | Escherichia coli | - | - | ||
| Bacillus cereus | 0.5 mg/mL | <10 | |||
| Osmundea pinnatifida | Bacillus subtilis | 1.56 mg/mL | - | [72] | |
| Porphyra umbilicalis | Aqueous extract | Bacillus subtilis | 3.13 mg/mL | - | [72] |
| Pyropiaorbicularis | Methanolic extract | Staphylococcus aureus | 250 mg/mL | nd | [74] |
| Escherichia coli | 500 mg/mL |
4.2. Chlorophyta
| Chlorophyta | Extract Type | Microbes | Minimum Inhibitory Concentration (MIC) | Inhibition Zone Diameter (mm) | Reference |
|---|---|---|---|---|---|
| Caulerpa cupressoides | Benzene | Escherichia coli | nd | 6 | [81] |
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 5 | |||
| Streptococcus pyogens | nd | 6 | |||
| Staphylococcus aureus | nd | 6 | |||
| Butanol | Escherichia coli | nd | 7 | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 7 | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | 6 | |||
| Propanol | Escherichia coli | nd | 7 | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 8 | |||
| Streptococcus pyogens | nd | 7 | |||
| Staphylococcus aureus | nd | 6 | |||
| Acetone | Escherichia coli | nd | 9 | ||
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 5 | |||
| Streptococcus pyogens | nd | 8 | |||
| Staphylococcus aureus | nd | 7 | |||
| Water | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Caulerpa lentillifera | Water extract | Methicillin-resistant Staphylococcus aureus, Escherichia coli | 5 µg/mL | nd | [78] |
| Caulerpa lentillifera | Methanolic extract | Escherichia coli | 136.50 ± 0.85 mg/mL | nd | [80] |
| Staphylococcus aureus | 125.25 ± 3.78 mg/mL | nd | |||
| Streptococcus sp. | 175.25 ± 0.23 mg/mL | nd | |||
| Salmonella sp. | 140.50 ± 0.55 mg/mL | nd | |||
| Caulerpa racemosa | Water extract | Methicillin-resistant Staphylococcus aureus, Escherichia coli | 5 µg/mL | nd | [78] |
| Caulerpa racemosa | Methanolic extract | Vibrio fluvialis | nd | 9 ± 0.50 | [79] |
| Caulerpa racemosa var. clavifera f. microphysa | Methanolic extract | Escherichia coli | 245.25 ± 2.11 mg/mL | nd | [80] |
| Staphylococcus aureus | 225.50 ± 0.45 mg/mL | nd | |||
| Streptococcus sp. | 450.75 ± 1.09 mg/mL | nd | |||
| Salmonella sp. | 275. 20 ± 0.66 mg/mL | nd | |||
| Caulerpa racemosa var. laetevirens | Methanolic extract | Escherichia coli | 360.50 ± 2.14 mg/mL | nd | [80] |
| Staphylococcus aureus | 375.75 ± 0.07 mg/mL | nd | |||
| Streptococcus sp. | 450. 25 ± 0.42 mg/mL | nd | |||
| Salmonella sp. | 345. 25 ± 0.35 mg/mL | nd | |||
| Caulerpa taxifolia | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | - | 10.00–11.17 | |||
| Chaetomorpha anteninna | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | 640 µg/mL | 10.00–11.17 | |||
| Chaetomorpha linum | Chloroform/methanol extract | Escherichia coli | >640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | - | 10.00–11.17 | |||
| Cladophora vagabunda | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | nd | 10.00–11.17 | |||
| Codium dichotomum | Methanolic extract | Staphylococcus aureus | nd | ≥20 | [90] |
| Escherichia coli | nd | <10 | |||
| Klebliella pneumoniae | nd | <10 | |||
| Enterobacter faecalis | nd | <10 | |||
| Codium fragile | Methanolic extract | Staphylococcus aureus | nd | ≥20 | [90] |
| Escherichia coli | nd | <10 | |||
| Klebliella pneumoniae | nd | <10 | |||
| Enterobacter faecalis | nd | <10 | |||
| Codium fragile | Hexane extract | Bacillus subtilis | 250 µg/mL | 6.5 | [89] |
| Bacillus cereus | 1000 µg/mL | - | |||
| Staphylococcus epidermidis | - | - | |||
| Staphylococcus aureus | - | - | |||
| Methicillin-resistant Staphylococcus aureus | - | 6.5 | |||
| Enterobacter cloacae | 1000 µg/mL | 7 | |||
| Enterobacter cloacae | - | - | |||
| Escherichia coli | 500 µg/mL | - | |||
| Escherichia coli (Hemorrhagic, O157:H7) | 500 µg/mL | - | |||
| Pseudomonas aeruginosa | <50 µg/mL | - | |||
| Proteus vulgaris | 250 µg/mL | - | |||
| Salmonella typhimurium | - | - | |||
| Candida albicans | - | ||||
| Methanol extract | Bacillus subtilis | 250 µg/mL | 6.5 | ||
| Bacillus cereus | 500 µg/mL | - | |||
| Staphylococcus epidermidis | 500 µg/mL | - | |||
| Staphylococcus aureus | 500 µg/mL | - | |||
| Methicillin-resistant Staphylococcus aureus | - | 7.5 | |||
| Enterobacter cloacae | - | 7 | |||
| Escherichia coli | - | - | |||
| Escherichia coli (Hemorrhagic, O157:H7) | - | - | |||
| Pseudomonas aeruginosa | 250 µg/mL | - | |||
| Proteus vulgaris | 250 µg/mL | - | |||
| Salmonella typhimurium | - | - | |||
| Candida albicans | - | - | |||
| Dichloromethane extract | Bacillus subtilis | - | 6.5 | ||
| Bacillus cereus | - | - | |||
| Staphylococcus epidermidis | - | - | |||
| Staphylococcus aureus | - | - | |||
| Methicillin-resistant Staphylococcus aureus | - | - | |||
| Enterobacter cloacae | - | 7 | |||
| Escherichia coli | - | 7 | |||
| Escherichia coli (Hemorrhagic, O157:H7) | - | - | |||
| Pseudomonas aeruginosa | - | - | |||
| Proteus vulgaris | - | - | |||
| Salmonella typhimurium | - | - | |||
| Candida albicans | - | - | |||
| Codium intricatum | Methanol extract | Methicillin-resistant Staphylococcus aureus | 250 µg/mL | nd | [88] |
| Bacillus cereus | 500 µg/mL | nd | |||
| Listeria monocytogenes | 500 µg/mL | nd | |||
| Streptococcus mutans | - | nd | |||
| Pseudomonas aeruginosa | - | nd | |||
| Escherichia coli | - | nd | |||
| Enterobacter cloacae | - | nd | |||
| Salmonella typhimurium | - | nd | |||
| Aeromonas hydrophila | - | nd | |||
| Codium tomentosum | Methanolic extract | Staphylococcus aureus | nd | ≥20 | [90] |
| Escherichia coli | nd | <10 | |||
| Klebliella pneumoniae | nd | <10 | |||
| Enterobacter faecalis | nd | <10 | |||
| Enteromorpha compressa | Ethanolic extract | Salmonella sp. | nd | 15 | [86] |
| Klebsiella sp. | nd | 10 | |||
| Proteus sp. | nd | 5 | |||
| Staphylococcus aureus | nd | 5 | |||
| Enteromorpha sp. | Methanol:acetone extract | Pseudomonas aeruginosa | 150 g/mL | 11 ± 0.2 | [85] |
| Staphylococcus aureus | 100 g/mL | 10 ± 0.2 | |||
| Escherichia coli | 100 g/mL | 11 ± 0.2 | |||
| Ulva fasciata | Chloroform/methanol extract | Escherichia coli | 640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | >640 µg/mL | 10.00–11.17 | |||
| Ulva intestinalis | Methanolic extract | Vibrio fluvialis | nd | 7 ± 0.56 | [79] |
| Ulva intestinalis | Benzene | Escherichia coli | nd | 6 | [81] |
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | 6 | |||
| Staphylococcus aureus | nd | 6 | |||
| Butanol | Escherichia coli | nd | 7 | ||
| Klebsiella pneumoniae | nd | 7 | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | 7 | |||
| Staphylococcus aureus | nd | 6 | |||
| Propanol | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 7 | |||
| Streptococcus pyogens | nd | 7 | |||
| Staphylococcus aureus | nd | 7 | |||
| Acetone | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Water | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 10 | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Ulva intestinalis | Methanolic extract | Escherichia coli | nd | - | [84] |
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | 1024 µg/mL | 6.85 ± 0.17 | |||
| Enterobacter faecalis | nd | - | |||
| Listeria monocytogenes | - | ||||
| Methicillin-resistant Staphylococcus aureus | >1024 µg/mL | 12.71 ± 0.98 | |||
| Staphylococcus aureus | >1024 µg/mL | 8.41 ± 0.56 | |||
| Ethanolic extract | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | nd | - | |||
| Enterobacter faecalis | nd | - | |||
| Listeria monocytogenes | >1024 µg/mL | 7.96 ± 0.38 | |||
| Methicillin-resistant Staphylococcus aureus | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Dichloromethane extract | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | nd | - | |||
| Enterobacter faecalis | 1024 µg/mL | - | |||
| Listeria monocytogenes | nd | 9.89 ± 0.24 | |||
| Methicillin-resistant Staphylococcus aureus | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Hexane extract | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Proteus mirabilis | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Salmonella typhi | nd | - | |||
| Vibrio alginolyticus | nd | - | |||
| Vibrio harveyi | nd | - | |||
| Vibrio parahaemolyticus | nd | - | |||
| Bacillus cereus | 256 µg/mL | 7.28 ± 0.02 | |||
| Enterobacter faecalis | nd | - | |||
| Listeria monocytogenes | 1024 µg/mL | 10.55 ± 0.29 | |||
| Methicillin-resistant Staphylococcus aureus | 256 µg/mL | 16.4 ± 2.4 | |||
| Staphylococcus aureus | 256 µg/mL | 12.13 ± 0.16 | |||
| Ulva lactuca | Benzene | Escherichia coli | nd | 6 | [81] |
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | 6 | |||
| Staphylococcus aureus | nd | 6 | |||
| Butanol | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 7 | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | 8 | |||
| Propanol | Escherichia coli | nd | 6 | ||
| Klebsiella pneumoniae | nd | 6 | |||
| Pseudomonas aeruginosa | nd | 6 | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | 7 | |||
| Acetone | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | 8 | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Water | Escherichia coli | nd | - | ||
| Klebsiella pneumoniae | nd | - | |||
| Pseudomonas aeruginosa | nd | - | |||
| Streptococcus pyogens | nd | - | |||
| Staphylococcus aureus | nd | - | |||
| Ulva lactuca | Chloroform/methanol extract | Escherichia coli | >640 µg/mL | 7.33–10.67 | [82] |
| Staphylococcus aureus | 640 µg/mL | 10.00–11.17 | |||
| Ulva lactuca | N–hexane extract | Staphylococcus aureus | nd | 10 | [87] |
| Staphylococcus epidermidis | nd | 12 | |||
| Escherichia coli | nd | 11 | |||
| Pseudomonas aeruginosa | nd | 12 | |||
| Chloroform extract | Staphylococcus aureus | nd | 11 | ||
| Staphylococcus epidermidis | nd | 11 | |||
| Escherichia coli | nd | 11 | |||
| Pseudomonas aeruginosa | nd | 10 | |||
| ethanol: water (1:1) extract | Staphylococcus aureus | nd | 9 | ||
| Staphylococcus epidermidis | nd | 10 | |||
| Escherichia coli | nd | 9 | |||
| Pseudomonas aeruginosa | nd | 9 | |||
| Ulva lactuca | Polysaccharide (ulvan) | Staphylococcus aureus | - | - | [102] |
| Enterobacter faecalis | - | - | |||
| Bacillus subtilis | 12.50 ± 0.0 mg/mL | 15 ± 0.50 | |||
| Listeria monocytogenes | - | - | |||
| Pseudomonas aeruginosa | 25.00 ± 0.0 mg/mL | 12 ± 0.10 | |||
| Escherichia coli | 6.25 ± 0.0 mg/mL | 11 ± 0.21 | |||
| Klebsiella pneumoniae | 6.25 ± 0.0 mg/mL | 12 ± 0.00 | |||
| Bordetella pertussis | - | - | |||
| Ulva reticulata | Polysaccharide (ulvan) | Bacillus cereus | nd | - | [101] |
| Enterobacter faecalis | nd | - | |||
| Enterobacter cloacae | nd | 20.00 ± 1.00 | |||
| Staphylococcus aureus | nd | - | |||
| Escherichia coli | nd | 18 ± 0.5 | |||
| Pseudomonas aeruginosa | nd | <18 ± 0.5 | |||
| Vibrio harveyi | nd | - | |||
| Ulva sp. | Methanolic extract | Staphylococcus saprophyticus | 16 µg/mL | 29 ± 0.592 | [83] |
| Staphylococcus epidermidis | 4 µg/mL | 26 ± 0.548 | |||
| Streptococcus agalactiae (group B) | 0.5 µg/mL | 14 ± 0.592 | |||
| Enterobacter faecalis | 2 µg/mL | 21 ± 0.592 | |||
| Stenotrophomonas maltophilia | 1 µg/mL | 15 ± 0.592 | |||
| Salmonella enterica | 2 µg/mL | 11 ± 0.592 | |||
| Shigella sonnei | 2 µg/mL | 12 ± 0.592 | |||
| Pproteus vulgaris | 2 µg/mL | 20 ± 0.592 | |||
| Pproteus mirabilis | - | - | |||
| Enterobacter cloacae | - | - | |||
| Haemophilus influenzae | - | - |
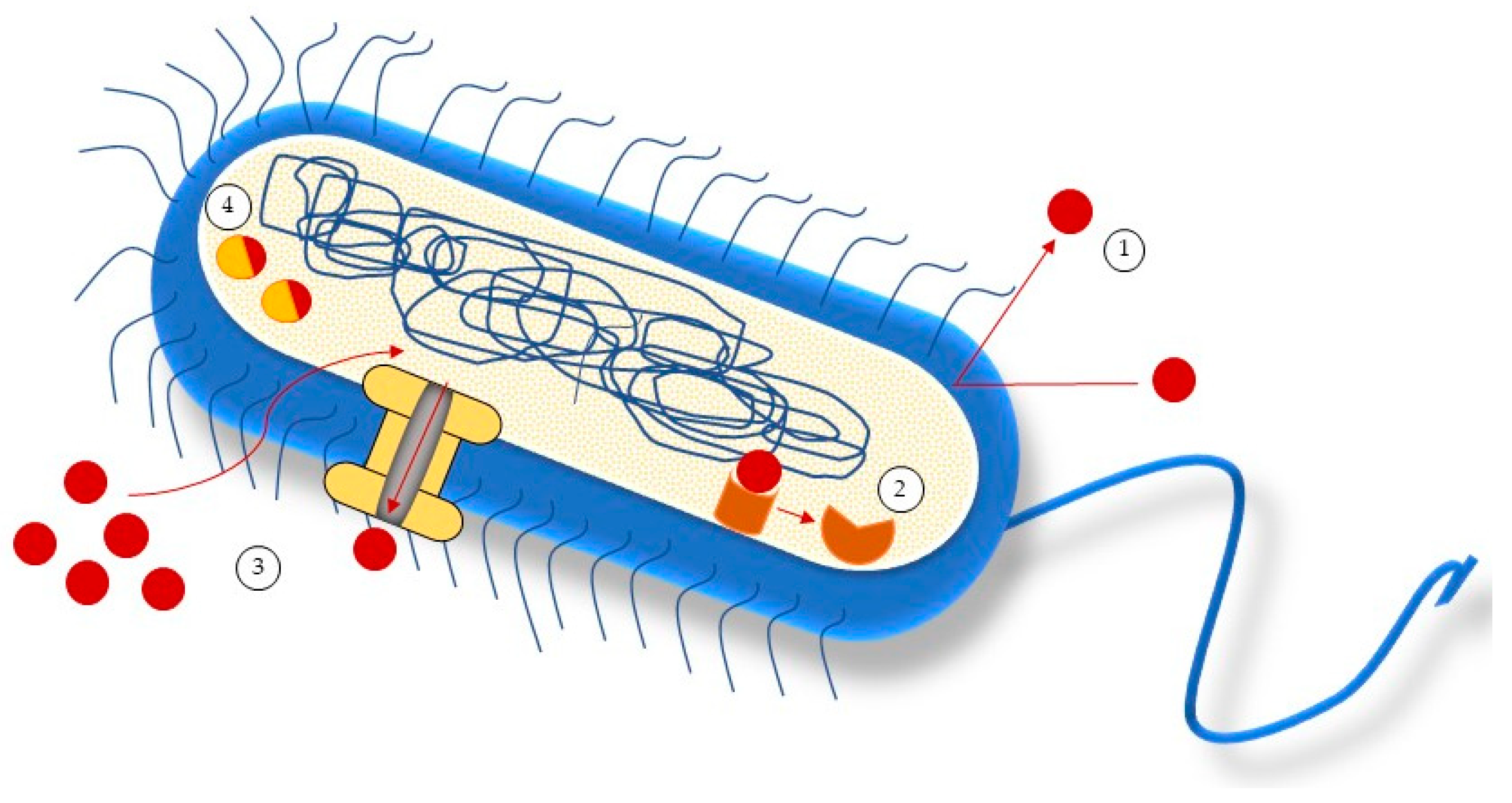
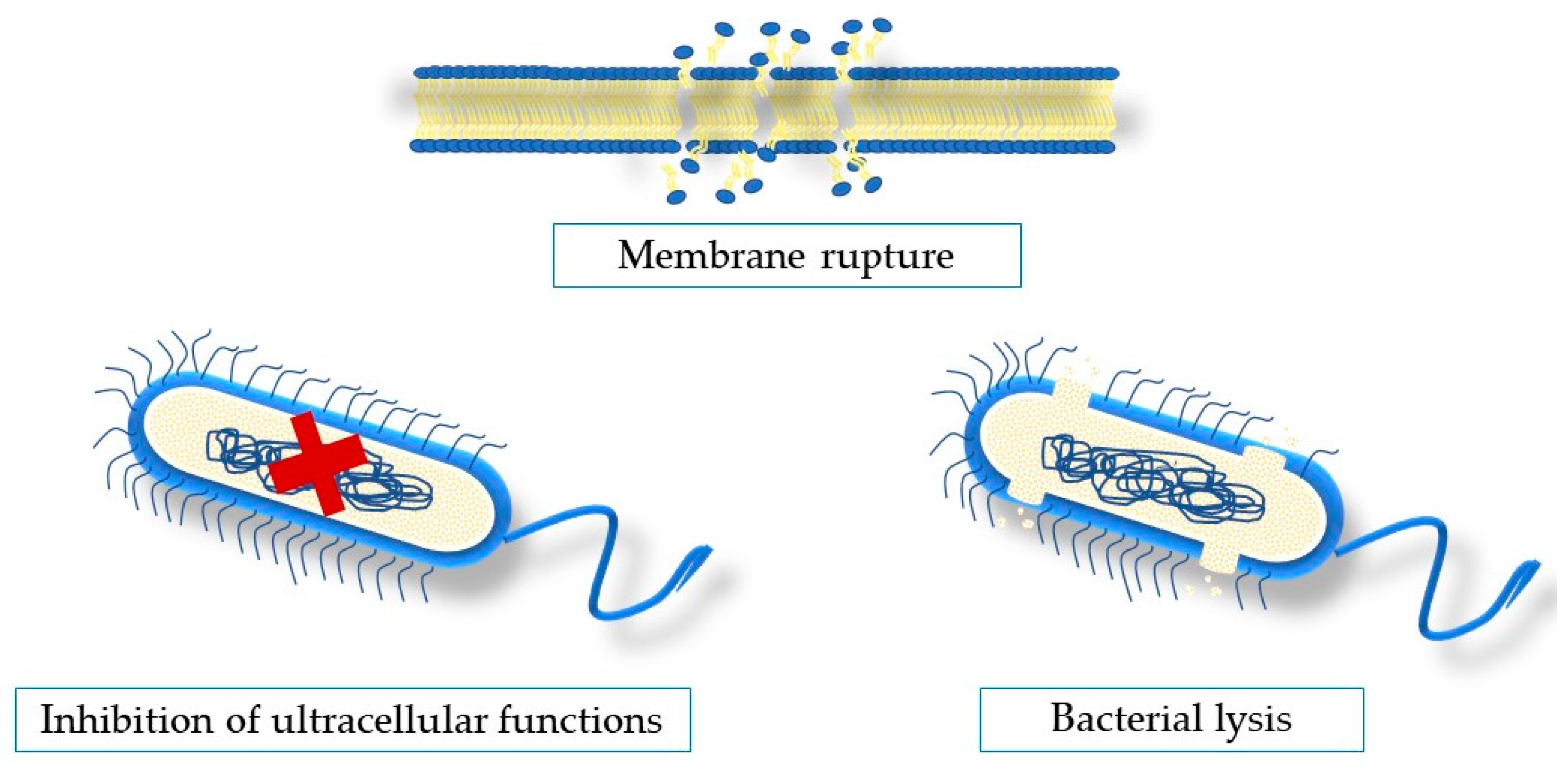
5. Antimicrobial Mechanisms of Action of Seaweeds Compounds
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffman, P.S. Antibacterial Discovery: 21st Century Challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Overcoming Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–17. [Google Scholar] [CrossRef]
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural Products to Prevent Drug Resistance in Cancer Chemotherapy: A Review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; El-Hossary, E.M.; Oelschlaeger, T.A.; Donia, M.S.; Quinn, R.J.; Abdelmohsen, U.R. Potential of Marine Natural Products against Drug-Resistant Bacterial Infections. Lancet Infect. Dis. 2019, 19, e237–e245. [Google Scholar] [CrossRef]
- Qin, Y. Health Benefits of Bioactive Seaweed Substances; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128133125. [Google Scholar]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinheimer, A.J.; Spraggins, R.L. The Occurrence of Two New Prostaglandin Derivatives (15-Epi-PGA2 and Its Acetate, Methyl Ester) in the Gorgonian Plexaura homomalla Chemistry of Coelenterates. XV. Tetrahedron Lett. 1969, 10, 5185–5188. [Google Scholar] [CrossRef]
- Masuda, M.; Abe, T.; Suzuki, T.; Suzuki, M. Morphological and Chemotaxonomic Studies on Laurencia Composita and L. okamurae (Ceramiales, Rhodophyta). Phycologia 1996, 35, 550–562. [Google Scholar] [CrossRef]
- Taylor, R.E. Tedanolide and the Evolution of Polyketide Inhibitors of Eukaryotic Protein Synthesis. Nat. Prod. Rep. 2008, 25, 854–861. [Google Scholar] [CrossRef]
- Santos, J.D.; Vitorino, I.; Reyes, F.; Vicente, F.; Lage, O.M. From Ocean to Medicine: Pharmaceutical Applications of Metabolites from Marine Bacteria. Antibiotics 2020, 9, 455. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in Exploring the Therapeutic Potential of Marine Natural Products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef]
- Singh, S.B.; Barrett, J.F. Empirical Antibacterial Drug Discovery-Foundation in Natural Products. Biochem. Pharmacol. 2006, 71, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Takó, M.; Beáta Kerekes, E.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [Green Version]
- Alzoreky, N.S.; Nakahara, K. Antibacterial Activity of Extracts from Some Edible Plants Commonly Consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Fankam, A.G.; Kuete, V.; Voukeng, I.K.; Kuiate, J.R.; Pages, J. Antibacterial Activities of Selected Cameroonian Spices and Their Synergistic Effects with Antibiotics against Multidrug-Resistant Phenotypes. Complement. Altern. Med. 2011, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial Activity of Plant Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Brazilian J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 17. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of Silver Nanoparticles Using Marine Macroalgae Padina sp. and Its Antibacterial Activity towards Pathogenic Bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Fátima Barroso, M.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef]
- Kizhakkekalam, V.K.; Chakraborty, K. Marine Macroalgae-Associated Heterotrophic Firmicutes and Gamma-Proteobacteria: Prospective Anti-Infective Agents against Multidrug Resistant Pathogens. Arch. Microbiol. 2020, 202, 905–920. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R.; Monsalud, R.G.; Sapin, A.B. Chemical Composition and in Vitro Antioxidant and Antibacterial Activities of Sargassum Vulgare c. Agardh from Lobo, Batangas, Philippines. J. Int. Soc. Southeast Asian Agric. Sci. 2019, 25, 112–122. [Google Scholar]
- Salvador, N.; Gómez Garreta, A.; Lavelli, L.; Ribera, M.A. Antimicrobial Activity of Iberian Macroalgae. Sci. Mar. 2007, 71, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Maschek, J.A.; Baker, B.J. The Chemistry of Algal Secondary Metabolism. In Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–20. ISBN 9783540741800. [Google Scholar]
- Ayyad, S.-E.N.; Al-Footy, K.O.; Alarif, W.M.; Sobahi, T.R.; Bassaif, S.A.; Makki, M.S.; Asiri, A.M.; Al Halawani, A.Y.; Bandria, A.F.; Bandria, F.A.A. Bioactive C15 Acetogenins from the Red Alga Laurencia obtusa. Chem. Pharm. Bull. 2011, 59, 1294–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD, (Organisation for Economic Co-operation and Development). Stemming the Superbug Tide: Just A Few Dollars More. In OECD Health Policy Studies; OECD Publishing: Paris, France, 2018; pp. 1–12. ISBN 9789264307582. [Google Scholar]
- Thompson, T. The Staggering Death Toll of Drug-Resistant Bacteria. Nature, 2022; Epub ahead print. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.H.; Moore, L.S.P.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. V Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet Infect. Dis. 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Read, A.F.; Woods, R.J. Antibiotic Resistance Management. Evol. Med. Public Heal. 2020, 1, 147. [Google Scholar] [CrossRef]
- Lushniak, B.D. Antibiotic Resistance: A Public Health Crisis. Surg. Gen. Perspect. 2014, 129, 314–316. [Google Scholar] [CrossRef] [Green Version]
- World Health Orgnization Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 8 February 2023).
- Okeke, I.N.; Lamikanra, A.; Edelman, R. Socioeconomic and Behavioral Factors Leading to Acquired Bacterial Resistance to Antibiotics in Developing Countries. Emerg. Infect. Dis. 1999, 5, 18–27. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Malmir, S.; Bahreinian, M.; Zahiri Yeganeh, S.; Mirnejad, R.; Moosazadeh Moghaddam, M.; Saberi, F. Molecular Mechanisms of Resistance to Conventional Antibiotics in Bacteria. Int. J. Med. Rev. 2018, 5, 118–129. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Lambert, T. Antibiotics That Affect the Ribosome. OIE Rev. Sci. Technol. 2012, 31, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sales, A.J.; Shadbad, N.N.; Kaleybar, V.P. The Investigation of the Antibacterial Effects of Ethanol Extract of Cichorium The Investigation of the Antibacterial Effects of Ethanol Extract of Cichorium intybus L. on Antibiotic-Resistant Staphylococcus aureus Strains. Bull. Environ. Pharmacol. Life Sci. 2018, 4, 161–164. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Bereksi, M.S.; Hassaïne, H.; Bekhechi, C.; Abdelouahid, D.E. Evaluation of Antibacterial Activity of Some Medicinal Plants Extracts Commonly Used in Algerian Traditional Medicine against Some Pathogenic Bacteria. Pharmacogn. J. 2018, 10, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Zaynab, M.; Fatima, M.; Sharif, Y.; Zafar, M.H.; Ali, H.; Ali, K. Microbial Pathogenesis Role of Primary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2019, 137, 103728. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S. Glycyrrhiza Glabra: Medicine over the Millennium. Nat. Prod. Radiance 2005, 4, 358–367. [Google Scholar]
- Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Kobayashi, M.; Kumada, H. The Long Term Efficacy of Glycyrrhizin in Chronic Hepatitis C Patients. Am. Cancer Soc. 1997, 79, 1494–1500. [Google Scholar]
- Baba, M.; Shigeta, S. Antiviral Activity of Glycyrrhizin against Varicella-Zoster Virus in Vitro. Antiviral Res. 1987, 7, 99–107. [Google Scholar] [CrossRef]
- Thirugnanam, S.; Xu, L.; Ramaswamy, K.; Gnanasekar, M. Glycyrrhizin Induces Apoptosis in Prostate Cancer Cell Lines DU-145 and LNCaP. Oncol. Rep. 2008, 20, 1387–1392. [Google Scholar] [CrossRef]
- Lin, D.; Zhong, W.; Li, J.; Zhang, B.; Song, G.; Hu, T.; Lin, D.; Zhong, W.; Li, J.; Zhang, B. Involvement of BID Translocation in Glycyrrhetinic Acid and 11-Deoxy Glycyrrhetinic Acid-Induced Attenuation of Gastric Cancer Growth Involvement of BID Translocation in Glycyrrhetinic Acid and 11-Deoxy Glycyrrhetinic Acid-Induced Attenuation of Gastric C. Nutr. Cancer 2014, 66, 463–473. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.K.; Rahuja, N.; Luqman, S.; Sisodia, B.S.; Saikia, D.; et al. Antimicrobial Potential of Glycyrrhiza glabra Roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Jafari-sales, A.; Bolouri, P. Evaluation of the Antimicrobial Effects of Glycyrrhiza glabra L. on Some Gram Positive and Gram Negative Pathogenic Bacteria in Laboratory. Jorjani Biomed. J. 2018, 6, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Saudi Journal of Biological Sciences Antimicrobial Effect of Different Herbal Plant Extracts against Different Microbial Population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive Compounds Content and Antimicrobial Activities of Wild Edible Asteraceae Species of the Mediterranean Flora under Commercial Cultivation Conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Junsathian, P.; Nakamura, S.; Katayama, S.; Rawdkuen, S. Antioxidant and Antimicrobial Activities of Thai Edible Plant Extracts Prepared Using Different Extraction Techniques. Molecules 2022, 27, 6489. [Google Scholar] [CrossRef]
- Zazharskyi, V.V.; Davydenko, P.; Kulishenko, O.; Borovik, I.V.; Brygadyrenko, V.V. Antimicrobial Activity of 50 Plant Extracts. Biosyst. Divers. 2019, 27, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kishore, Y.; Padhi, L.; Luyten, W. Antimicrobial Activity of Select Edible Plants from Odisha, India against Food-Borne Pathogens. LWT Food Sci. Technol. 2019, 113, 108246. [Google Scholar] [CrossRef]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2018, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Rosaline, X.D.; Sakthivelkumar, S.; Rajendran, K.; Janarthanan, S. Screening of Selected Marine Algae from the Coastal Tamil Nadu, South India for Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2012, 2, S140–S146. [Google Scholar] [CrossRef]
- Vallinayagam, K.; Arumugam, R.; Kannan, R.R.R.; Thirumaran, G.; Anantharaman, P. Antibacterial Activity of Seaweeds of Pudumadam Coast Antibacterial Activity of Some Selected Seaweeds from Pudumadam Coastal Regions. Glob. J. Pharmacol. 2016, 3, 50–52. [Google Scholar]
- Arulkumar, A.; Rosemary, T.; Paramasivam, S. Phytochemical Composition, In Vitro Antioxidant, Antibacterial Potential and GC-MS Analysis of Red Seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatal. Agric. Biotechnol. 2018, 15, 63–71. [Google Scholar] [CrossRef]
- Ivanovic, J.; Dimitrijevic-brankovic, S.; Misic, D.; Ristic, M.; Zizovic, I. Evaluation and Improvement of Antioxidant and Antibacterial Activities of Supercritical Extracts from Clove Buds. J. Funct. Foods 2012, 5, 416–423. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and Analysis of Compounds with Antibacterial Potential from the Red Alga Grateloupia turuturu. J. Mar. Sci. Eng. 2019, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J.J.; Pandiarajan, J. In Vitro Antioxidant Study of Polyphenol from Red Seaweeds Dichotomously Branched Gracilaria edulis and Robust Sea Moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.V.; Inácio, L.G.; Ruas, A.; Silva, I.A.; Mouga, T.; Pereira, L.; Afonso, C. Antioxidant and Antimicrobial Properties of Selected Red Seaweeds from Central Portugal. Appl. Sci. 2022, 13, 157. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and Antibacterial Activity of Red Seaweed: Kappaphycus alvarezii against Pathogenic Bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar]
- García, V.; Uribe, E.; Vega-Gálvez, A.; Delporte, C.; Valenzuela-Barra, G.; López, J.; Pastén, A. Health-Promoting Activities of Edible Seaweed Extracts from Chilean Coasts: Assessment of Antioxidant, Anti-Diabetic, Anti-Inflammatory and Antimicrobial Potential. Rev. Chil. Nutr. 2020, 47, 792–800. [Google Scholar] [CrossRef]
- Saim, S.; Sahnouni, F.; Bouhadi, D.; Kharbouche, S. The Antimicrobial Activity of Two Marine Red Algae Collected from Algerian West Coast. Trends Pharmacol. Sci. 2021, 7, 233–242. [Google Scholar] [CrossRef]
- Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage. Foods 2022, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Oliviera, A.; Veiga-santos, P.; Prado, B.; Filho, D.; Sudatti, D.B.; Pereira, R.C.; Nakamura, C.V.; De Londrina, U.E.; Celso, R.; Cid, G.; et al. Effect of Elatol, Isolated from Red Seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef] [Green Version]
- Yap, W.-F.; Tay, V.; Tan, S.-H.; Yow, Y.-Y.; Chew, J. Decoding Antioxidant and Antibacterial Potentials of Malaysian Green Seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Aftabuddin, S.; Akter, S.; Hossen, S.; Rahman, M.A. Antioxidant, Antibacterial and Cytotoxic Activity of Caulerpa racemosa (Forsskål) J. Agardh and Ulva (Enteromorpha) intestinalis L. Bangladesh J. Sci. Ind. Res. 2020, 55, 237–244. [Google Scholar]
- Nagappan, T.; Vairappan, C.S. Nutritional and Bioactive Properties of Three Edible Species of Green Algae, Genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Ravikumar, S.; Anburajan, L.; Ramanathan, G.; Kaliaperumal, N. Screening of Seaweed Extracts against Antibiotic Resistant Post Operative Infectious Pathogens. Seaweed Res. Util. 2002, 24, 95–99. [Google Scholar]
- Agbaje-Daniels, F.; Adeleye, A.; Nwankwo, D.; Adeniyi, B.; Seku, F.; Beukes, D. Antibacterial Activities of Selected Green Seaweeds from West African Coast. EC Pharmacol. Toxicol. 2020, 4, 84–92. [Google Scholar]
- Al-Zahrani, A.; Al-Haj, N.; Omer, H.; Al-Judaibi, A. Impact of Extracts of Marine Macroalgae on Multidrug-Resistant Bacteria. J. Microbiol. Res. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Srikong, W.; Bovornreungroj, N.; Mittraparparthorn, P.; Bovornreungroj, P. Antibacterial and Antioxidant Activities of Differential Solvent Extractions from the Green Seaweed Ulva intestinalis. ScienceAsia 2017, 43, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Swathi, N.; Kumar, A.G.; Parthasarathy, V.; Sankarganesh, P. Isolation of Enteromorpha Species and Analyzing Its Crude Extract for the Determination of in Vitro Antioxidant and Antibacterial Activities. Biomass Convers. Biorefinery 2022, 3, 1–10. [Google Scholar] [CrossRef]
- Priya, N.; Kokila, M.; Janani, J. Comparative Phytochemical Studies and Antibacterial Activity of Green and Brown Seaweeds Extract of Enteromorpha compressa and Padina pavonica. Int. J. Res. Eng. Sci. Manag. 2021, 4, 136–139. [Google Scholar]
- Cadar, E.; Negreanu-Pirjol, T.; Negreanu-Pirjo, B.-S. Antioxidant and Antibacterial Potential of Ulva lactuca Species from Romanian Black Sea Coast. Eur. J. Nat. Sci. Med. 2022, 8705, 26–38. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R. Evaluation of Nutritional Composition and In Vitro Antioxidant and Antibacterial Activities of Codium intricatum Okamura from Ilocos Norte (Philippines). Jordan J. Biol. Sci. 2020, 13, 375–382. [Google Scholar]
- Koz, F.F.Y.; Yavasoglu, N.U.; Demirel, Z.; Sukatar, A.; Ozdemir, G. Antimicrobial Activities of Some Macroalgal Essential Oil and Extracts from Antioxidant and Antimicrobial Activities of Codium fragile (Suringar) Hariot (Chlorophyta) Essential Oil and Extracts. Asian J. Chem. 2009, 21, 1197–1209. [Google Scholar]
- Ibtissam, C.; Hassane, R.; Jose, M.; Francisco, D.S.; Antonio, G.V.; Hassan, B.; Mohamed, K. Screening of Antibacterial Activity in Marine Green and Brown Macroalgae from the Coast of Morocco. Afr. J. Biotechnol. 2009, 8, 1258–1262. [Google Scholar] [CrossRef]
- Kaeffer, B.; Benard, C.; Lahaye, M.; Herve, M.B.; Cherbut, C. Biological Properties of Ulvan, a New Source of Green Seaweed Sulfated Polysaccharides, on Cultured Normal and Cancerous Colonic Epithelial Cells. Planta Med. 1999, 65, 527–531. [Google Scholar] [CrossRef]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated Polysaccharides from Marine Green Algae Ulva conglobata and Their Anticoagulant Activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Sousa, R.A.; Reis, R.L. In Vitro Cytotoxicity Assessment of Ulvan, a Polysaccharide Extracted from Green Algae. Phyther. Res. 2013, 27, 1143–1148. [Google Scholar] [CrossRef]
- Quemener, B.; Lahaye, M.; Bobin-Dubigeon, C. Sugar Determination in Ulvans by a Chemical-Enzymatic Method Coupled to High Performance Anion Exchange Chromatography. J. Appl. Phycol. 1997, 9, 179–188. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A Practical Perspective on Ulvan Extracted from Green Algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Ray, B.; Lahaye, M. Cell-Wall Polysaccharides from the Marine Green Alga Ulva “rigida” (Ulvales, Chlorophyta). Extraction and Chemical Composition. Carbohydr. Res. 1995, 274, 251–261. [Google Scholar] [CrossRef]
- Lahaye, M.; Inizan, F.; Vigouroux, J. Carbohydrate Polymers NMR Analysis of the Chemical Structure of Ulvan and of Ulvan-Boron Complex Formation. Carbohydr. Polym. 1998, 36, 239–249. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Food Hydrocolloids Effect of Extraction Conditions on the Yield and Purity of Ulvan Extracted from Ulva lactuca. Food Hydrocoll. 2020, 31, 375–382. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Van Structure and Cytotoxic Activity of Ulvan Extracted from Green Seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, T.T.; Truong, H.B.; Ha, N.; Tran, V.; Thu, T.M. Structure, Conformation in Aqueous Solution and Antimicrobial Activity of Ulvan Extracted from Green Seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.A.; Amer, M.S.; Ibrahim, H.A.H.; Zaghloul, E.H. Considerable Production of Ulvan from Ulva lactuca with Special Emphasis on Its Antimicrobial and Anti-Fouling. Appl. Biochem. Biotechnol. 2022, 194, 3097–3118. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Kraan, S. Seaweed Resources, Collection, and Cultivation with Respect to Sustainability; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128179437. [Google Scholar]
- Hayashi, L.; Cantarino, S.D.J.; Critchley, A.T. Challenges to the Future Domestication of Seaweeds as Cultivated Species: Understanding Their Physiological Processes for Large-Scale Production; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 95, ISBN 9780081027103. [Google Scholar]
- Freile-Pelegŕn, Y.; Morales, J.L. Antibacterial Activity in Marine Algae from the Coast of Yucatan, Mexico. Bot. Mar. 2004, 47, 140–146. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal Variation in Phenolic Composition, Antibacterial and Antioxidant Activities of Ulva rigida (Chlorophyta) and Assessment of Antiacetylcholinesterase Potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Kandhasamy, M.; Arunachalam, K.D. Evaluation of in Vitro Antibacterial Property of Seaweeds of Southeast Coast of India. Afr. J. Biotechnol. 2008, 7, 1958–1961. [Google Scholar]
- Garcia-Vaquero, M.; Hayes, M. Red and Green Macroalgae for Fish and Animal Feed and Human Functional Food Development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pereira, R.C.; Costa, E.d.S.; Sudatti, D.B.; da Gama, B.A.P. Inducible Defenses against Herbivory and Fouling in Seaweeds. J. Sea Res. 2017, 122, 25–33. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae Polyphenolic Compounds and Modern Antibacterial Strategies: Current Achievements and Immediate Prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef]
- Cherian, C.; Jannet Vennila, J.; Sharan, L. Marine Bromophenols as an Effective Inhibitor of Virulent Proteins (Peptidyl Arginine Deiminase, Gingipain R and Hemagglutinin A) in Porphyromas Gingivalis. Arch. Oral Biol. 2019, 100, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentão, P. Antifungal Activity of Phlorotannins against Dermatophytes and Yeasts: Approaches to the Mechanism of Action and Influence on Candida albicans Virulence Factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The Impact and Mode of Action of Phenolic Compounds Extracted from Brown Seaweed on Mixed Anaerobic Microbial Cultures. J. Appl. Microbiol. 2012, 114, 964–973. [Google Scholar] [CrossRef]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal Metabolites: An Inevitable Substitute for Antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia Coli to Seaweed (Ascophyllum nodosum) Phlorotannins and Terrestrial Tannins. Asian-Australasian J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the Membrane Permeability and Cell Death of Vibrio parahaemolyticus Caused by Phlorotannins with Low Molecular Weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Dobrodeeva, L.; Druzhinina, A.; Ovchinnikov, D.; Parshina, A.; Shulgina, E. Biological Activity of a Polyphenolic Complex of Arctic Brown Algae. J. Appl. Phycol. 2019, 31, 3341–3348. [Google Scholar] [CrossRef]
- Cabral, E.M.; Oliveira, M.; Mondala, J.R.M.; Curtin, J.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobials from Seaweeds for Food Applications. Mar. Drugs 2021, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K. Lectins from Red Algae and Their Biomedical Potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef] [Green Version]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, G.; Shoubaky, E. Active Ingredients Fatty Acids as Antibacterial Agent from the Brown Algae Padina pavonica and Hormophysa triquetra. J. Coast. Life Med. 2014, 2, 535–542. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.P.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R.; et al. Valorization of Lipids from Gracilaria sp. through Lipidomics and Decoding of Antiproliferative and Anti-Inflammatory Activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabili, L.; Acquaviva, M.I.; Biandolino, F.; Cavallo, R.A.; De Pascali, S.A.; Fanizzi, F.P.; Narracci, M.; Cecere, E.; Petrocelli, A. Biotechnological Potential of the Seaweed Cladophora rupestris (Chlorophyta, Cladophorales) Lipidic Extract. N. Biotechnol. 2014, 31, 436–444. [Google Scholar] [CrossRef]
- Patra, J.K.; Kim, S.H.; Baek, K.H. Antioxidant and Free Radical-Scavenging Potential of Essential Oil from Enteromorpha linza L. Prepared by Microwave-Assisted Hydrodistillation. J. Food Biochem. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.H. Chemical Composition and Antioxidant and Antibacterial Activities of an Essential Oil Extracted from an Edible Seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Lee, S.W.; Park, J.G.; Baek, K.H. Antioxidant and Antibacterial Properties of Essential Oil Extracted from an Edible Seaweed Undaria pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on Antibacterial Activity and Antibacterial Mechanism of a Novel Polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Jha, B. 24-Branched Δ5 Sterols from Laurencia papillosa Red Seaweed with Antibacterial Activity against Human Pathogenic Bacteria. Microbiol. Res. 2014, 169, 301–306. [Google Scholar] [CrossRef]
- Ikekawa, N.; Morisaki, N.; Tsuda, K.; Yoshida, T. Sterol Compositions in Some Green Algae and Brown Algae. Steroids 1968, 12, 41–48. [Google Scholar] [CrossRef]
- Prakash, S.; Sasikala, S.L.; Aldous, V.H.J. Isolation and Identification of MDR-Mycobacterium tuberculosis and Screening of Partially Characterised Antimycobacterial Compounds from Chosen Marine Micro Algae. Asian Pac. J. Trop. Med. 2010, 3, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Wächter, G.A.; Franzblau, S.G.; Montenegro, G.; Hoffmann, J.J.; Maiese, W.M.; Timmermann, B.N. Inhibition of Mycobacterium tuberculosis Growth by Saringosterol from Lessonia nigrescens. J. Nat. Prod. 2001, 64, 1463–1464. [Google Scholar] [CrossRef]
- Kawakami, L.M.; Yoon, B.K.; Jackman, J.A.; Knoll, W.; Weiss, P.S.; Cho, N.J. Understanding How Sterols Regulate Membrane Remodeling in Supported Lipid Bilayers. Langmuir 2017, 33, 14756–14765. [Google Scholar] [CrossRef]
- Eng, R.H.K.; Padberg, F.T.; Smith, S.M.; Tan, E.N.; Cherubin, C.E. Bactericidal Effects of Antibiotics on Slowly Growing and Nongrowing Bacteria. Antimicrob. Agents Chemother. 1991, 35, 1824–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y.; Andoh, A. Modification of Intestinal Flora in the Treatment of Inflammatory Bowel Disease. Curr. Pharm. Des. 2003, 9, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Takesue, Y.; Yokoyama, T.; Akagi, S.; Ohge, H.; Imamura, Y.; Murakami, Y.; Seuda, T. Changes in the Intestinal Flora After the Administration of Prophylactic Antibiotics to Patients Undergoing a Gastrectomy. Surg. Today 2002, 35, 581–586. [Google Scholar] [CrossRef]
- Basappa, K.; Gopal, J.V. Natural Alternatives to Antibiotic Agents. Asian J. Biomed. Pharm. Sci. 2013, 25, 1–4. [Google Scholar]
- Setty, A.R.; Sigal, L.H. Herbal Medications Commonly Used in the Practice of Rheumatology: Mechanisms of Action, Efficacy, and Side Effects. Semin. Arthritis Rheum. 2005, 34, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, B.; Gruber, C. Side-Effects of Complementary and Alternative Medicine. Allergy 2003, 58, 707–716. [Google Scholar] [CrossRef]
- Tattelman, E. Health Effects of Garlic. Complement. Altern. Med. 2005, 72, 103–106. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomartire, S.; Gonçalves, A.M.M. An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts. Mar. Drugs 2023, 21, 163. https://doi.org/10.3390/md21030163
Lomartire S, Gonçalves AMM. An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts. Marine Drugs. 2023; 21(3):163. https://doi.org/10.3390/md21030163
Chicago/Turabian StyleLomartire, Silvia, and Ana M. M. Gonçalves. 2023. "An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts" Marine Drugs 21, no. 3: 163. https://doi.org/10.3390/md21030163
APA StyleLomartire, S., & Gonçalves, A. M. M. (2023). An Overview on Antimicrobial Potential of Edible Terrestrial Plants and Marine Macroalgae Rhodophyta and Chlorophyta Extracts. Marine Drugs, 21(3), 163. https://doi.org/10.3390/md21030163







