Fatty Acids in Waste Tissues: The Nutraceutical Value of Gonads and Livers from the Moroccan Hypophthalmichthys molitrix and Cyprinus carpio Fishes
Abstract
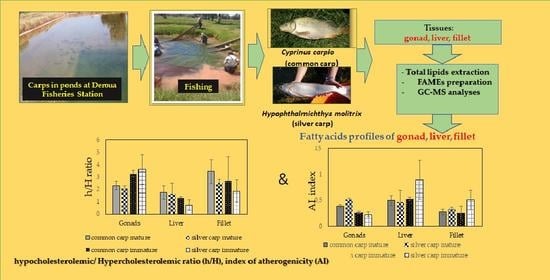
:1. Introduction
2. Results
2.1. Fatty Acids Profiles of Common and Silver Carp
2.1.1. Saturated Fatty Acids Profile of Common and Silver Carp
2.1.2. Monounsaturated Fatty Acids Profile of Common and Silver Carp
2.1.3. Polyunsaturated Fatty Acids Profile of Common and Silver Carp
2.2. Fat Quality Indices of Common and Silver Carp
3. Discussion
3.1. Fatty Acid Profile of Gonads of Common and Silver Carps
3.2. Fatty Acid Profile of Livers of Common and Silver Carps
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Lipid and Fatty Acid Analysis by Gas Chromatography-Mass Spectrometry (GC-MS)
4.3. Determination of Fat Quality Indices
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina-Vega, M.; Gómez-Pérez, A.M.; Tinahones, F.J. Fish in the Mediterranean diet. In The Mediterranean Diet: An Evidence-Based Approach, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 275–284. [Google Scholar]
- Parisi, C.; De Marco, G.; Labar, S.; Hasnaoui, M.; Grieco, G.; Caserta, L.; Inglese, S.; Vangone, R.; Madonna, A.; Alwany, M.; et al. Biodiversity Studies for Sustainable Lagoon: Thermophilic and Tropical Fish Species vs. Endemic Commercial Species at Mellah Lagoon (Mediterranean, Algeria). Water 2022, 14, 635. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Adnan, M.; Patel, M.; Siddiqui, A.J.; Sachidanandan, M.; Snoussi, M.; Hadi, S. Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Mar. Drugs 2020, 18, 265. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation. 2022. Available online: www.fao.org (accessed on 4 July 2022).
- Moroccan-Aquaculture-Sector. 2022. Available online: www.moroccoworldnews.com (accessed on 7 December 2022).
- Aba, M.; Belghyt, D.; Benabid, M. The main species of freshwater fish aquaculture interest in Morocco, current status and prospects. Int. J. Fish. Aquat. Stud. 2014, 2, 216–218. [Google Scholar]
- Majdoubi, F.-Z.; Ouizgane, A.; Droussi, M.; Hasnaoui, M. The Effect of the Spawning Period on the Viability of Silver Carp (Hypophthalmichthys molitrix) Eggs. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions. EMCEI 2017. Advances in Science, Technology & Innovation (IEREK Interdisciplinary Series for Sustainable Development); Kallel, A., Ksibi, M., Ben Dhia, H., Khélifi, N., Eds.; Springer: Cham, Switzerland, 2018; pp. 1409–1411. [Google Scholar]
- Jawdhari, A.; Mihăilescu, D.F.; Fendrihan, S.; Jujea, V.; Stoilov-Linu, V.; Negrea, B.-M. Silver Carp (Hypophthalmichthys molitrix) (Asian Silver Carp) Presence in Danube Delta and Romania—A Review with Data on Natural Reproduction. Life 2022, 12, 1582. [Google Scholar] [CrossRef] [PubMed]
- Guilhermino, L.; Martins, A.; Lopes, C.; Raimundo, J.; Vieira, L.R.; Barboza, L.G.A.; Costa, J.; Antunes, C.; Caetano, M.; Vale, C. Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Mar. Pollut. Bull. 2021, 173, 113008. [Google Scholar] [CrossRef]
- Roy, K.; Vrba, J.; Kaushik, S.J.; Mraz, J. Nutrient footprint and ecosystem services of carp production in European fishponds in contrast to EU crop and livestock sectors. J. Clean. Prod. 2020, 270, 122268. [Google Scholar] [CrossRef]
- Fishbase. Trophic Ecosystem of Hypophthalmichthys Molitrix. Available online: www.fishbase.se/TrophicEco/EcosysList.php?ID=274&GenusName=Hypophthalmichthys&SpeciesName=molitrix (accessed on 17 November 2022).
- Fishbase. Trophic Ecosystem of Cyprinus Carpio. Available online: www.fishbase.se/TrophicEco/EcosysList.php?ID=1450&GenusName=Cyprinus&SpeciesName=carpio (accessed on 17 November 2022).
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [Green Version]
- Yeganeh, S.; Shabanpour, B.; Hosseini, H.; Imanpour, M.R.; Shabani, A. Comparison of farmed and wild common carp (Cyprinus carpio): Seasonal variations in chemical composition and fatty acid profile. Czech J. Food Sci. 2012, 30, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Tokur, B.; Ozkütük, S.; Atici, E.; Ozyurt, G.; Ozyurt, C.E. Chemical and sensory quality changes of fish fingers, made from mirror carp (Cyprinus carpio L., 1758), during frozen storage (−18 °C). Food Chem. 2006, 99, 335–341. [Google Scholar] [CrossRef]
- Buchtová, H.; Ježek, F. A new look at the assessment of the silver carp (Hypophthalmichthys molitrix Val.) as a food fish. Czech J. Food Sci. 2011, 29, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Organic Facts. Available online: www.organicfacts.net/health-benefits/animal-product/health-benefits-of-carp.html#comments-container (accessed on 7 December 2022).
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Crexi, V.; Monte, M.L.; Soares, L.A.D.S.; Pinto, L.A.D.A. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chem. 2009, 119, 945–950. [Google Scholar] [CrossRef]
- Majdoubi, F.-Z.; Ouizgane, A.; Farid, S.; Mossetti, L.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Fry Survival Rate as a Predictive Marker of Optimal Production of Silver Carp (Hypophthalmichthys molitrix, Valenciennes 1844): A Biostatistical Study in Deroua Fish Farm, Morocco. Proc. Zool. Soc. 2021, 75, 152–160. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tramice, A.; Trifuoggi, M.; Ahmad, M.F.; Lam, S.S.; Iodice, C.; Velotto, G.; Giarra, A.; Inglese, S.; Cupo, A.; Guerriero, G.; et al. Comparative Fatty Acid Profiling of Edible Fishes in Kuala Terengganu, Malaysia. Foods 2021, 10, 2456. [Google Scholar] [CrossRef]
- Sobczak, M.; Panicz, R.; Eljasik, P.; Sadowski, J.; Tórz, A.; Żochowska-Kujawska, J.; Barbosa, V.; Dias, J.; Marques, A. Nutritional value and sensory properties of common carp (Cyprinus carpio L.) fillets enriched with sustainable and natural feed ingredients. Food Chem. Toxicol. 2021, 152, 112197. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion of the Panel on Dietetic products, Nutrition and Allergies on a request from European Commission related to labelling reference intake values for n-3 and n-6 polyunsaturated fatty acids. EFSA J. 2009, 1176, 1–11. [Google Scholar]
- Corsolini, S.; Borghesi, N. A comparative assessment of fatty acids in Antarctic organisms from the Ross Sea: Occurrence and distribution. Chemosphere 2017, 174, 747–753. [Google Scholar] [CrossRef]
- Chapkin, R.S. Reappraisal of the essential fatty acids. In Fatty Acids in Foods and Their Health Implications, 3rd ed.; Chow, C.K., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 689–706. [Google Scholar]
- Hwang, D. Dietary fatty acids and eicosanoids. In Fatty Acids in Foods and Their Health Implications, 1st ed.; Dekker, M., Chow, C.K., Eds.; CRC: Boca Raton, FL, USA, 1992; pp. 545–558. [Google Scholar]
- Fei, S.; Chen, Z.; Xia, Y.; Liu, H.; Han, D.; Jin, J.; Zhu, X.; Xie, S. Effects of dietary arachidonic acid on reproduction performance, tissue fatty acid profile and gonadal steroidogenesis in female yellow catfish Pelteobagrus fulvidraco. Aquac. Nutr. 2021, 27, 700–711. [Google Scholar] [CrossRef]
- Jahan, I.; Tiwari, V.; Verma, A.; Ranjan, A. Effect of salinity on lipid profile of Cyprinus carpio reared in inland saline water. J. Environ. Biol. 2020, 41, 228–233. [Google Scholar] [CrossRef]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: Are marine phospholipids the answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In Vitro Antithrombotic Properties of Salmon (Salmo salar) Phospholipids in a Novel Food-Grade Extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef] [Green Version]
- Hussain, B.; Mahboob, S.; Hassan, M.; Nadeem, S.; Sultana, T. Effect of maturation degree on fatty acid profile of different tissues in wild and farmed rohu (Labeo rohita). Grasas Aceites 2011, 62, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Nowosad, J.; Kucharczyk, D.; Łuczyńska, J.; Targońska, K.; Czarkowski, T.; Biłas, M.; Krejszeff, S.; Horváth, L.; Müller, T. Changes in European eel ovary development and body and ovary chemistry during stimulated maturation under controlled conditions: Preliminary data. Aquac. Int. 2014, 23, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Dhurmeea, Z.; Pethybridge, H.; Appadoo, C.; Bodin, N. Lipid and Fatty Acid Dynamics in Mature Female Al-Bacore Tuna (Thunnus alalunga) in the Western Indian Ocean. PLoS ONE 2018, 13, e0194558. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Zhou, Y.; Wu, H.; Luo, Y.; Shen, H. Lipid Content and Fatty Acid Profile of Muscle, Brain and Eyes of Seven Freshwater Fish: A Comparative Study. J. Am. Oil Chem. Soc. 2014, 91, 795–804. [Google Scholar] [CrossRef]
- Böhm, M.; Schultz, S.; Koussoroplis, A.-M.; Kainz, M.J. Tissue-Specific Fatty Acids Response to Different Diets in Common Carp (Cyprinus carpio L.). PLoS ONE 2014, 9, e94759. [Google Scholar] [CrossRef]
- Sobczak, M.; Panicz, R.; Eljasik, P.; Sadowski, J.; Tórz, A.; Żochowska-Kujawska, J.; Barbosa, V.; Domingues, V.; Marques, A.; Dias, J. Quality improvement of common carp (Cyprinus carpio L.) meat fortified with n-3 PUFA. Food Chem. Toxicol. 2020, 139, 111261. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hong, H.; Luo, Y.; Desk, S. The seasonal fatty acids composition in different tissues of farmed common carp (Cyprinus carpio). SDRP J. Food Sci. Technol. 2016, 1, 11–18. [Google Scholar]
- Jorjani, S.; Ghelichi, A.; Jorjani, H. Comparison of Chemical Compositions and Fatty Acid Profile of Cultured Common Carp (Cyprinus carpio) and Silver Carp (Hypophthalmichthys molitrix). In Biology Education and Research in a Changing Planet; Gnanamalar, S.D.E., Ed.; Springer: Singapore, 2015; pp. 167–172. [Google Scholar] [CrossRef]
- Kmínková, M.; Winterová, R.; Kučera, J. Fatty acids in lipids of carp (Cyprinus carpio) tissues. Czech J. Food Sci. 2001, 19, 177–181. [Google Scholar] [CrossRef] [Green Version]
- WHO/FAO Expert Consultation. Available online: www.foodpolitics.com/wp-content/uploads/FFA_summary_rec_conclsion.pdf (accessed on 8 December 2022).
- Nutritional Aspects of Cardiovascular Disease. Report of the Cardiovascular Review Group Committee on Medical Aspects of Food Policy. Report on Health and Social Subjects 46. Lessons from DHA status regulation, our ancient diet, epidemiology and randomized controlled trials. Rep. Health Soc. Subj. 1994, 46, 1–186. [Google Scholar] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2009, 3, 107. [Google Scholar]
- WHO/FAO Expert Consultation. Available online: https://www.who.int/publications/i/item/924120916X (accessed on 7 December 2022).
- Ivanova, A.; Hadzhinikolova, L. Evaluation of nutritional quality of common carp (Cyprinus carpio L.) lipidsthrough fatty acid ratios and lipid indices. Bulg. J. Agric. Sci. 2015, 21, 180–185. [Google Scholar]
- Grigorakis, K.; Alexis, M.N.; Taylor, K.D.A.; Hole, M. Comparison of wild and cultured gilthead sea bream (Sparus aurata); composition, appearance and seasonal variations. Int. J. Food Sci. Technol. 2002, 37, 477–484. [Google Scholar] [CrossRef]
- Fehily, A.M.; Pickering, J.E.; Yarnell, J.W.G.; Elwood, P.C. Dietary indices of atherogenicity and thrombogenicity and ischemic heart disease risk: The Caerphilly Prospective Study. Br. J. Nutr. 1994, 71, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Bušová, M.; Kouřimská, L.; Tuček, M. Fatty acids profile, atherogenic and thrombogenic indices in freshwater fish common carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss) from market chain. Cent. Eur. J. Public Health 2020, 28, 313–319. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Fernandes, C.E.; Vasconcelos, M.A.D.S.; Ribeiro, M.D.A.; Sarubbo, L.A.; Andrade, S.A.C.; Filho, A.B.D.M. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, X.; He, X.; Sun, X.; Yu, X.; Cheng, Y.; Yu, R.-Q.; Wu, Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS ONE 2020, 15, e0228276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargent, J. Origins and functions of egg lipids: Nutritional implications. In Broodstock Management and Egg and Larval Quality; Bromage, N.R., Roberts, R.J., Eds.; Blackwell Science: Oxford, UK, 1995; pp. 353–372. [Google Scholar]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, Ó.; Díaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Andrade, A.D.; Rubira, A.F.; Matsushita, M.; Souza, N.E. θ3 fatty acids in freshwater fish from south brazil. J. Am. Oil Chem. Soc. 1995, 72, 1207–1210. [Google Scholar] [CrossRef]
- Aprodu, I.; Vasile, A.; Gurau, G.; Ionescu, A.; Paltenea, E. Evaluation of nutritional quality of the common carp (Cyprinus carpio) enriched in fatty acids. Ann. Univ. Dunarea Jos Galati Fasc. VI Food Technol. 2012, 36, 61–73. [Google Scholar]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Institute of Medicine; National Research Council. Annex 1. Dietary recommendations for fish consumption. In A Framework for Assessing Effects of the Food System; National Academies Press: Washington, DC, USA, 2015; pp. 287–302. [Google Scholar]
- Connor, W.E. n-3 fatty acids in health and disease. In Nutrition and Disease Update: Heart Disease; Kritchevski, D., Carroll, K.K., Eds.; American Oil Chemists’ Society: Champaign, IL, USA, 1994; pp. 7–42. [Google Scholar]
- Sargent, J.; Tocher, D.; Bell, J. The lipids. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 181–257. [Google Scholar]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2018, 87, 187–254. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.; Ouzgane, A.; Droussi, M.; Hasnaoui, M. Evolution des parametres zootechniques de la carpe argentee (Hypophthalmichthys molitrix) elevee sous climat semi-aride a la station de pisciculture deroua, Maroc. J. Water Environ. Sci. 2017, 1, 115–122. [Google Scholar]
- Farid, S.; Ouizgane, A.; Majdoubi, F.-Z.; Hasnaoui, M.; Droussi, M. Diet of Hypophthalmichthys molitrix, Ctenopharyngodon idella and Cyprinus carpio in Ponds of Deroua Fisheries Station, Morocco. In Proceedings of the 4th Edition of International Conference on Geo-IT and Water Resources 2020, Geo-IT and Water Resources 2020, Al Hoceima, Morocco, 11–12 March 2020; pp. 1–6. [Google Scholar]
- Sadouni, S.; Iguer-Ouada, M. Factors limiting successful reproduction in wild silver carp, Hypophthalmichtys molitrix, in Kherrata Reservoir, Algeria. Fish. Aquat. Life 2019, 27, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Mutethya, E.; Yongo, E.; Laurent, C.; Waithaka, E.; Lomodei, E. Population biology of common carp, Cyprinus carpio (Linnaeus, 1758), in Lake Naivasha, Kenya. Lakes Reserv. Res. Manag. 2020, 25, 326–333. [Google Scholar] [CrossRef]
- Guerriero, G.; Ferro, R.; Ciarcia, G. Correlations between plasma levels of sex steroids and spermatogenesis during the sexual cycle of the chub, Leuciscus cephalus L. (Pisces: Cyprinidae). Zool. Stud. Taipei 2005, 44, 228–233. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Cladis, D.P.; Kleiner, A.C.; Freiser, H.H.; Santerre, C.R. Fatty Acid Profiles of Commercially Available Finfish Fillets in the United States. Lipids 2014, 49, 1005–1018. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv. Lipid Methodol. 1993, 2, 69–111. [Google Scholar]
- Tvrzická, E. Analysis of Fatty Acids in Plasma Lipoproteins by Gas Chromatography–Flame Ionization Detec-Tion: Quantitative Aspects. Anal. Chim. Acta 2002, 465, 337–350. [Google Scholar] [CrossRef]
- Ackman, R.G.; Sipos, J.C. Application of Specific Response Factors in the Chromatographic Analysis of Methyl Esters of Fatty Acids with Flame Ionization Detectors. J. Am. Oil Chem. Soc. 1964, 41, 377. [Google Scholar] [CrossRef]
- Craske, J.D.; Bannon, C.D. Gas liquid chromatography analysis of the fatty acid composition of fats and oils: A total system for high accuracy. J. Am. Oil Chem. Soc. 1987, 64, 1413–1417. [Google Scholar] [CrossRef]
- Eder, K.; Reichlmayr-Lais, A.; Kirchgessner, M. Gas chromatographic analysis of fatty acid methyl esters: Avoiding discrimination by programmed temperature vaporizing injection. J. Chromatogr. A 1991, 588, 265–272. [Google Scholar] [CrossRef]
- Fernández, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; de la Hoz, L. Fatty acid compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]



| Total Lipids (g/100 g) | Total FA a (g/100 g) | SFA b (mg/100 g) | MUFA c (mg/100 g) | PUFA d (mg/100 g) | ω-3 e FA (mg/100 g) | ω-6 f FA (mg/100 g) | PUFA/SFA | MUFA/SFA | |
|---|---|---|---|---|---|---|---|---|---|
| common carp immature | |||||||||
| G | 0.88 ± 0.68 | 0.109 ± 0.006 | 28.10 ± 1.22 | 41.14 ± 3.45 | 40.36 ± 2.14 | 17.06 ± 0.80 | 23.30 ± 1.34 | 1.44 ± 0.10 | 1.46 ± 0.14 |
| L | 0.83 ± 0.08 | 0.412 ± 0.006 | 187.80 ± 13.84 | 209.72 ± 10.01 | 14.56 ± 2.31 | n.d. | 14.56 ± 2.31 | 0.08 ± 0.01 | 1.12 ± 0.10 |
| F | 0.14 ± 0.07 | 0.059 ± 0.033 | 17.09 ± 9.05 | 29.64 ± 19.08 | 12.81 ± 5.66 | 4.70 ± 1.54 | 8.11 ± 4.12 | 0.75 ± 0.52 | 1.73 ± 1.44 |
| silver carp immature | |||||||||
| G | 0.25 ± 0.12 | 0.109 ± 0.033 | 26.43 ± 5.06 | 57.72 ± 16.45 | 25.05 ± 5.58 * | 7.13 ± 0.97 ** | 17.92 ± 4.61 | 0.95 ± 0.28 * | 2.18 ± 0.75 |
| L | 0.44 ± 0.18 | 0.135 ± 0.067 | 73.20 ± 27.91 ** | 52.62 ± 22.00 * | 9.33 ± 5.00 | n.d | 9.33 ± 5.00 | 0.13 ± 0.08 | 0.72 ± 0.41 |
| F | 0.25 ± 0.06 | 0.021 ± 0.008 | 10.23 ± 3.61 * | 6.50 ± 2.47 | 4.20 ± 1.65 | 1.60 ± 0.52 * | 2.60 ± 1.13 | 0.41 ± 0.22 | 0.64 ± 0.33 |
| common carp mature | |||||||||
| G | 2.56 ± 0.48 | 0.581 ± 0.056 | 207.63 ± 16.61 | 193.25 ± 18.80 | 180.36 ± 26.92 | 119.84 ± 23.40 | 60.52 ± 3.52 | 0.87 ± 0.15 | 0.93 ± 0.12 |
| L | 1.27 ± 0.22 | 0.182 ± 0.058 | 79.79 ± 13.49 | 88.41 ± 23.37 | 14.64 ± 3.61 | 8.89 ± 2.01 | 5.74 ± 1.60 | 0.18 ± 0.05 | 1.11 ± 0.35 |
| F | 0.28 ± 0.12 | 0.067 ± 0.014 | 21.37 ± 4.45 | 22.38 ± 4.28 | 22.75 ± 5.35 | 13.57 ± 3.04 | 9.19 ± 2.31 | 1.06 ± 0.33 | 1.05 ± 0.30 |
| silver carp mature | |||||||||
| G | 0.98 ± 0.27 | 0.553 ± 0.055 | 170.30 ± 8.52 | 252.41 ± 19.79 | 130.96 ± 12.98 * | 13.47 ± 0.67 ** | 117.48 ± 12.31 * | 0.77 ± 0.08 | 1.48 ± 0.1 * |
| L | 1.10 ± 0.47 | 0.215 ± 0.091 | 80.72 ± 39.48 | 124.79 ± 19.89 * | 9.45 ± 5.03 | n.d | 9.45 ± 5.03 * | 0.12 ± 0.08 | 1.55 ± 0.79 |
| F | 0.50 ± 0.08 | 0.210 ± 0.008 | 65.03 ± 5.91 * | 119.43 ± 3.67 ** | 25.73 ± 3.33 | 12.01 ± 1.36 | 13.72 ± 1.98 | 0.40 ± 0.06 | 1.84 ± 0.1 |
| Female Fish (n = 10 Species) | Total Weight (g) | Length (cm) | Body Circumference (cm) | Gonadal Weight (g) | Liver Weight (g) | Fillet Weight (g) | GSI (%) |
|---|---|---|---|---|---|---|---|
| Immature Hypophthalmichthys molitrix (silver carp) | 456.80 ± 70.20 | 36.01 ± 2.59 | 16.66 ± 1.13 | 0.52 ± 0.20 | 2.54 ± 0.54 | 19.62 ± 3.29 | 0.08 ± 0.03 |
| Mature Hypophthalmichthys molitrix (silver carp) | 1845.23 ± 573.06 | 53.50 ± 5.18 | 26.75 ± 3.73 | 8.23 ± 1.18 | 13.60 ± 4.87 | 53.08 ± 16.60 | 0.67 ± 0.29 |
| Immature Cyprinus carpio (common carp) | 804.28 ± 61.94 | 39.75 ± 0.78 | 24.13 ± 0.83 | 74.03 ± 6.59 | 11.58 ± 1.74 | 26.93 ± 4.65 | 7.08 ± 1.94 |
| Mature Cyprinus carpio (common carp) | 2800.07 ± 258.80 | 52.53 ± 2.48 | 39.33 ± 0.33 | 519.37 ± 80.10 | 12.07 ± 0.55 | 56.87 ± 8.03 | 18.34 ± 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommonaro, G.; Paris, D.; Guerriero, G.; Majdoubi, F.-Z.; Grieco, G.; Iodice, C.; Caso, L.; Ouizgane, A.; El Moujtahid, A.; El Ghizi, S.; et al. Fatty Acids in Waste Tissues: The Nutraceutical Value of Gonads and Livers from the Moroccan Hypophthalmichthys molitrix and Cyprinus carpio Fishes. Mar. Drugs 2023, 21, 188. https://doi.org/10.3390/md21030188
Tommonaro G, Paris D, Guerriero G, Majdoubi F-Z, Grieco G, Iodice C, Caso L, Ouizgane A, El Moujtahid A, El Ghizi S, et al. Fatty Acids in Waste Tissues: The Nutraceutical Value of Gonads and Livers from the Moroccan Hypophthalmichthys molitrix and Cyprinus carpio Fishes. Marine Drugs. 2023; 21(3):188. https://doi.org/10.3390/md21030188
Chicago/Turabian StyleTommonaro, Giuseppina, Debora Paris, Giulia Guerriero, Fatima-Zahra Majdoubi, Gaetano Grieco, Carmine Iodice, Lucio Caso, Anouar Ouizgane, Aziz El Moujtahid, Sara El Ghizi, and et al. 2023. "Fatty Acids in Waste Tissues: The Nutraceutical Value of Gonads and Livers from the Moroccan Hypophthalmichthys molitrix and Cyprinus carpio Fishes" Marine Drugs 21, no. 3: 188. https://doi.org/10.3390/md21030188
APA StyleTommonaro, G., Paris, D., Guerriero, G., Majdoubi, F.-Z., Grieco, G., Iodice, C., Caso, L., Ouizgane, A., El Moujtahid, A., El Ghizi, S., Bousseba, M., Hasnaoui, M., Iodice, A., & Tramice, A. (2023). Fatty Acids in Waste Tissues: The Nutraceutical Value of Gonads and Livers from the Moroccan Hypophthalmichthys molitrix and Cyprinus carpio Fishes. Marine Drugs, 21(3), 188. https://doi.org/10.3390/md21030188