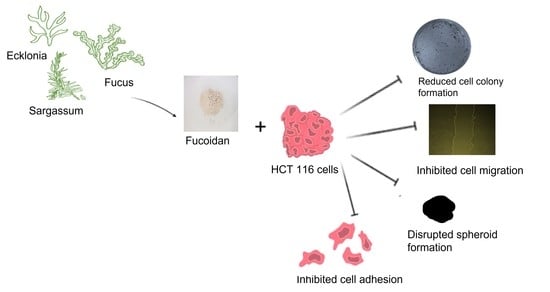
Comparative Analyses of Fucoidans from South African Brown Seaweeds That Inhibit Adhesion, Migration, and Long-Term Survival of Colorectal Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Fucoidan Yield
2.2. Structural Analysis of Fucoidans
2.2.1. FTIR Analysis
2.2.2. Proton NMR Analysis of Extracted Fucoidans
2.2.3. Thermogravimetric Analysis of Fucoidans
2.3. Composition of Fucoidans
2.4. Fucoidans’ Cytotoxicity to HCT116 Cancer Cells
2.4.1. The Effect of Fucoidans on HCT116 Colony Formation
2.4.2. Fucoidans Inhibit the 2D Migration of HCT116 Cancer Cells
2.4.3. Fucoidans Inhibit HCT116 3D Spheroid Migration
2.4.4. Fucoidans Disrupt Cancer Cell Sphere Formation
2.4.5. Fucoidans Inhibit HCT116 Cell Adhesion
3. Materials and Methods
3.1. Sampling and Seaweed Processing
3.2. Hot Water Extraction
3.3. Structural Validation of Extracted Fucoidans
3.3.1. Fourier Transform Infrared Spectrometry (FTIR) Analysis
3.3.2. NMR Spectroscopy Analysis
3.3.3. Thermogravimetric Analysis
3.4. Chemical Characterisation of Fucoidans
3.5. Determination of Fucoidans Molecular Weights by HPLC
3.6. Cell Culture
3.7. Cytotoxicity Screening
3.8. Clonogenic Assay
3.9. Wound Healing Assay
3.10. Sphere-Based Tumour Migration Assays
3.11. Cell Adhesion Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Marudhupandi, T.; Kumar, T.T.A.; Lakshmanasenthil, S.; Suja, G.; Vinothkumar, T. In vitro anti-cancer activity of fucoidan from Turbinaria conoides against A549 cell lines. Int. J. Biol. Macromol. 2015, 72, 919–923. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 5 January 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Motsuku, L.; Chen, W.C.; Muchengeti, M.M.; Naidoo, M.; Quene, T.M.; Kellett, P.; Mohlala, M.I.; Chu, K.M.; Singh, E. Colorectal cancer incidence and mortality trends by sex and population group in South Africa: 2002–2014. BMC Cancer 2021, 21, 129. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Manivasagan, R.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signalling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Siegel, R.L.; Jakubowski, C.D.; Fedewa, S.A.; Davis, A.; Azad, N.S. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am. Soc. Clin. Oncol Educ. Book 2020, 40, e75–e88. [Google Scholar] [CrossRef]
- Jin, J.O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anti-cancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Product. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Fernando, S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kim, E.A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; de Silva, E.D.; et al. FTIR characterisation and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. ALGAE 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural Characterisation of Fucoidan from Laminaria hyperborea: Assessment of Coagulation and Inflammatory Properties and Their Structure−Function Relationship. ACS Appl. Bio Mater. 2018, 1, 1880–1892. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. A Combination Approach in Inhibiting Type 2 Diabetes-Related Enzymes Using Ecklonia radiata Fucoidan. Pharmaceutics 2021, 13, 1979. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 2, 3446–3452. [Google Scholar] [CrossRef]
- Park, H.Y.; Park, S.H.; Jeong, J.W.; Yoon, D.; Han, M.H.; Lee, D.S.; Choi, G.; Yim, M.-J.; Lee, J.M.; Kim, D.-H.; et al. Induction of p53-Independent Apoptosis and G1 Cell Cycle Arrest by FuFucoidan in HCT116 Human Colorectal Carcinoma Cells. Mar. Drugs 2017, 15, 154. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling. Biomol. Ther. 2015, 23, 225–232. [Google Scholar] [CrossRef]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anti-cancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556–563. [Google Scholar] [CrossRef]
- Bolton, J.J.; Stegenga, H. Seaweed species diversity in South Africa. S. Afr. J. Mar. Sci. 2002, 24, 9–18. [Google Scholar] [CrossRef]
- Kumar, T.V.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan—A inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ding, X.; Hou, Y.; Hou, W.; Liu, Y.; Xu, T.; Yang, D. Structural characterisation, immune regulation and antioxidant activity of a new heteropolysaccharide from Cantharellus cibarius Fr. Int. J. Mol. Med. 2018, 41, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of fucoidan extracted from Laminaria japonica using a novel procedure with high yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Gheda, S.F.; Ribeiro-Claro, P.J.A. Analysis by Vibrational Spectroscopy of Seaweed Polysaccharides with Potential Use in Food, Pharmaceutical, and Cosmetic Industrie. Int. J. Carbohydr. Chem. 2013, 7, 537202. [Google Scholar] [CrossRef]
- Alwarsamy, M.; Gooneratne, R.; Ravichandran, R. Effect of fucoidan from Turbinaria conoides on human lung adenocarcinoma epithelial (A549) cells. Carbohydr. Polym. 2016, 152, 207–213. [Google Scholar] [CrossRef]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.L.; Liu, X.X.; Li, C.X.; Yu, G.L. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Cao, H.T.T. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Liu, X.; Yu, W. Evaluating the Thermal Stability of High Performance Fibers by TGA. J. Appl. Polym. Sci. 2006, 99, 937–944. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Carpio, R.B.; Zhang, Y.; Kuo, C.T.; Chen, W.T.; Schideman, L.C.; Leon, R.L. Characterisation and thermal decomposition of demineralised wastewater algae biomass. Algal Res. 2019, 38, 101399. [Google Scholar] [CrossRef]
- Morimoto, M.; Takatori, M.; Hayashi, T.; Mori, D.; Takashima, O.; Yoshida, S.; Sato, K.; Kawamoto, H.; Tamura, J.-I.; Izawa, H.; et al. Depolymerisation of sulfated polysaccharides under hydrothermal conditions. Carbohydr. Res. 2014, 384, 56–60. [Google Scholar] [CrossRef]
- Saravanaa, P.S.; Choa, Y.N.; Patilb, M.P.; Choa, Y.J.; Kimb, G.D.; Park, Y.B.; Woo, H.-C.; Chun, B.-S. Hydrothermal degradation of seaweed polysaccharide: Characterisation and biological activities. Food Chem. 2018, 268, 179–187. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anti-cancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–885. [Google Scholar] [CrossRef]
- January, G.G.; Naidoo, R.K.; Kirby-McCullough, B.; Bauerd, R. Assessing methodologies for fucoidan extraction from South African brown algae. Algal Res. 2019, 40, 101517. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phytochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef]
- Lee, J.J.; Beumer, J.H.; Chu, E. Therapeutic Drug Monitoring of 5-Fluorouracil. Cancer chemotherapy and pharmacology. Cancer Chemother Pharmacol. 2016, 78, 447. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shina, I.S.; Lee, H.; Kim, J.; Lee, Y. Effects of molecular weight and hydrolysis conditions on anti-cancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Bree, C.V. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Jung, W.; Choi, C.; Kim, S.Y.; Son, A.; Kim, H.; Lee, N.; Park, H.C. Fucoidan-Manganese Dioxide Nanoparticles Potentiate Radiation Therapy by Co-Targeting Tumor Hypoxia and Angiogenesis. Mar. Drugs 2018, 16, 510. [Google Scholar] [CrossRef] [PubMed]
- Wild, T.; Rahbarnia, A.; Kellner, M.; Sobotka, L.; Eberlein, T. Basics in nutrition and wound healing. Nutrition 2010, 26, 862–866. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Box, C.; Zimmermann, M.; Eccles, S.A. Tumor Spheroid-Based Migration Assays for Evaluation of Therapeutic Agents. In Target Identification and Validation in Drug Discovery: Methods and Protocols, Methods in Molecular Biology; Moll, J., Colombo, R., Eds.; Springer Science & Business Media: New York, NY, USA, 2013; pp. 253–266. [Google Scholar]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. Cell-Based Adhesion Assays for Isolation of Snake Venom’s Integrin Antagonists. In Snake and Spider Toxins: Methods and Protocols, Methods in Molecular Biology; Priel, A., Ed.; Springer Science+Business Media: New York, NY, USA, 2020; pp. 205–220. [Google Scholar]
- Liu, J.M.; Bignon, J.; Haroun-Bouhedja, F.; Bittoun, P.; Vassy, J. Inhibitory Effect of Fucoidan on the Adhesion of Adenocarcinoma Cells to Fibronectin. Anti-Cancer Res. 2005, 25, 2129–2134. [Google Scholar]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R. Separation, purification and preliminary characterisation of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anti-cancer and antioxidant activity. Process Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilising the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–252. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on determination of ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assay. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Keller, G.M. In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 1995, 7, 862–869. [Google Scholar] [CrossRef]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. 2020, 151, 412–420. [Google Scholar] [CrossRef]










| w/w % ± SD | ||||
|---|---|---|---|---|
| Component | E. maxima | E. radiata | S. elegans | F. vesiculosus |
| Total carbohydrates a | 72.8 ± 5.2 | 88 ± 7.4 | 44.4 ± 6.2 | 41.3 ± 9.5 |
| L-fucose b | 4.56 ± 0.8 | 3.7 ± 0.1 | 4.9 ± 0.9 | 8.2 ± 0.4 |
| D-glucose b | 8.1 ± 3.4 | 7.1 ± 2.3 | 5.7 ± 1.7 | 5.1 ± 2.1 |
| D-galactose b | 4.8 ± 0.1 | 4.9 ± 1.2 | 5.7 ± 0.5 | 7.1 ± 1.83 |
| D-mannose b | 3.0 ± 0.5 | 4.2 ± 0.2 | 7.1 ± 1.8 | 4.5 ± 0.8 |
| Total sulphates c | 7.2 ± 1.2 | 8.8 ± 1.4 | 9.7 ± 1.8 | 14.7 ± 2.3 |
| Total phenolics d | 1.9 ± 0.6 | 1.9 ± 0.4 | 2.8 ± 0.8 | 0 ± 0.04 |
| Uronic acids e | 2.6 ± 1.2 | 2.2 ± 0.7 | 4.8 ± 0.6 | 2.2 ± 0.8 |
| Total protein f | 2.1 ± 0.6 | 2.4 ± 0.9 | 4.6 ± 2.4 | 1.9 ± 0.6 |
| Total ash g | 19.7 ± 0.6 | 16.0 ± 2.1 | 23.1 ± 3.5 | 20.4 ± 2.8 |
| MW h | 27.4 kDa | 8.5 kDa | 74.9 kDa | 84.4 kDa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabate, B.; Daub, C.D.; Pletschke, B.I.; Edkins, A.L. Comparative Analyses of Fucoidans from South African Brown Seaweeds That Inhibit Adhesion, Migration, and Long-Term Survival of Colorectal Cancer Cells. Mar. Drugs 2023, 21, 203. https://doi.org/10.3390/md21040203
Mabate B, Daub CD, Pletschke BI, Edkins AL. Comparative Analyses of Fucoidans from South African Brown Seaweeds That Inhibit Adhesion, Migration, and Long-Term Survival of Colorectal Cancer Cells. Marine Drugs. 2023; 21(4):203. https://doi.org/10.3390/md21040203
Chicago/Turabian StyleMabate, Blessing, Chantal Désirée Daub, Brett Ivan Pletschke, and Adrienne Lesley Edkins. 2023. "Comparative Analyses of Fucoidans from South African Brown Seaweeds That Inhibit Adhesion, Migration, and Long-Term Survival of Colorectal Cancer Cells" Marine Drugs 21, no. 4: 203. https://doi.org/10.3390/md21040203
APA StyleMabate, B., Daub, C. D., Pletschke, B. I., & Edkins, A. L. (2023). Comparative Analyses of Fucoidans from South African Brown Seaweeds That Inhibit Adhesion, Migration, and Long-Term Survival of Colorectal Cancer Cells. Marine Drugs, 21(4), 203. https://doi.org/10.3390/md21040203