Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds
Abstract
:1. Introduction
2. Aspergillus sp. from Various Marine Sources and Their Antimicrobial Activities
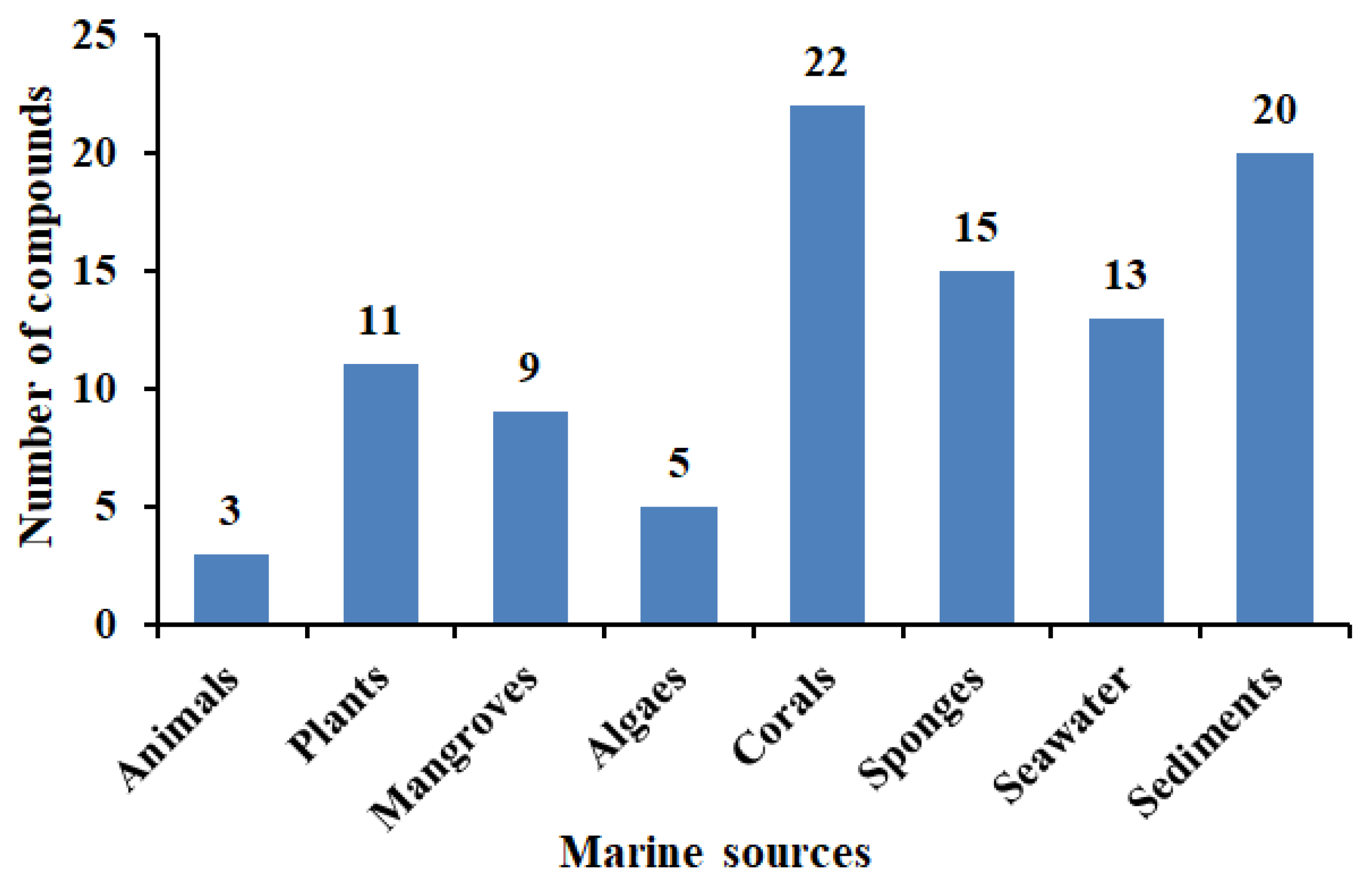
2.1. Aspergillus sp. from Marine Animals and Their Antimicrobial Activities
2.2. Aspergillus sp. from Marine Plants and Their Antimicrobial Activities
2.3. Aspergillus sp. from Mangroves and Their Antimicrobial Activities
2.4. Aspergillus sp. Derived from Algae and Their Antimicrobial Activities
2.5. Aspergillus sp. from Corals and Their Antimicrobial Activities
2.6. Aspergillus sp. Derived from Sponges and Their Antimicrobial Activities
2.7. Aspergillus sp. from Seawater and Their Antimicrobial Activities
2.8. Aspergillus sp. from Marine Sediments and Their Antimicrobial Activities
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.S.; Liu, L.T.; Yang, L.; Li, J.; Dong, X. Chemistry and bioactivity of marine-derived bisabolane sesquiterpenoids: A review. Front. Chem. 2022, 10, 881767. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Liu, L.L.; Wu, C.H.; Qian, P.Y. Marine natural products as antifouling molecules—A mini-review (2014–2020). Biofouling 2020, 36, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.; Nakayama, D.G.; Sousa, E.; Pinto, E. Marine-derived compounds and prospects for their antifungal application. Molecules 2020, 25, 5856. [Google Scholar] [CrossRef]
- Bian, C.; Wang, J.; Zhou, X.; Wu, W.; Guo, R. Recent advances on marine alkaloids from sponges. Chem. Biodivers. 2020, 17, e2000186. [Google Scholar] [CrossRef]
- Ge, X.; Wang, Y.; Sun, C.; Zhang, Z.; Song, L.; Tan, L.; Li, D.; Yang, S.; Yu, G. Secondary metabolites produced by coculture of Pleurotus ostreatus SY10 and Pleurotus eryngii SY302. Chem. Biodivers. 2022, 19, e202100832. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef]
- Wiese, J.; Imhoff, J.F. Marine bacteria and fungi as promising source for new antibiotics. Drug Dev. Res. 2019, 80, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.N.; Sun, S.S.; Liu, M.Z.; Yan, M.C.; Liu, Y.F.; Zhu, Z.; Zhang, Z. Natural bioactive compounds from marine fungi (2017–2020). J. Asian Nat. Prod. Res. 2022, 24, 203–230. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.C.; Bast, F.; Varjani, S.; Mehariya, S.; Bhatia, S.K.; Sharma, N.; Funk, C. A review on microbial products and their perspective application as antimicrobial agents. Biomolecules 2021, 11, 1860. [Google Scholar] [CrossRef]
- Wang, W.; Gao, M.; Luo, Z.; Liao, Y.; Zhang, B.; Ke, W.; Shao, Z.; Li, F.; Chen, J. Secondary metabolites isolated from the deep sea-derived fungus Aspergillus sydowii C1-S01-A7. Nat. Prod. Res. 2019, 33, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, H.F.; Pei, Y.H. Secondary metabolites from marine-derived microorganisms. J. Asian Nat. Prod. Res. 2014, 16, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Julianti, E.; Abrian, I.A.; Wibowo, M.S.; Azhari, M.; Tsurayya, N.; Izzati, F.; Juanssilfero, A.B.; Bayu, A.; Rahmawati, S.I.; Putra, M.Y. Secondary metabolites from marine-derived fungi and actinobacteria as potential sources of novel colorectal cancer drugs. Mar. Drugs 2022, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2021, 39, 560–595. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, X.; He, Y.; Lin, X.; Zhou, X.; Liu, Y.; Yang, B. Secondary metabolites from coral-associated fungi: Source, chemistry and bioactivities. J. Fungi 2022, 8, 1043. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.-Y.; Sun, S.-F.; Li, Y.; Liu, Y.-B. Fungi: Outstanding source of novel chemical scaffolds. J. Asian Nat. Prod. Res. 2018, 22, 99–120. [Google Scholar] [CrossRef]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Rastelli, E.; Buschi, E.; Barone, G.; Beolchini, F.; Dell’Anno, A. Fungi can be more effective than bacteria for the bioremediation of marine sediments highly contaminated with heavy metals. Microorganisms 2022, 10, 993. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef]
- Qadri, H.; Shah, A.H.; Ahmad, S.M.; Alshehri, B.; Almilaibary, A.; Mir, M.A. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 103376. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A comprehensive review of bioactive peptides from marine fungi and their biological significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Chen, C.; He, Y.; Wang, Y. Genomic analysis and antimicrobial components of M7, an Aspergillus terreus strain derived from the south china sea. J. Fungi 2022, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.M.; Hasan, M.; Miah, M.M.; Hossain, M.A.S.; Islam, S.; Shanta, V. Inhibitory potentiality of secondary metabolites extracted from marine fungus target on avian influenza virus-a subtype H5N8 (Neuraminidase) and H5N1 (Nucleoprotein): A rational virtual screening. Vet. Anim. Sci. 2022, 15, 100231. [Google Scholar]
- Fan, M.; Nath, A.K.; Tang, Y.; Choi, Y.-J.; Debnath, T.; Choi, E.-J.; Kim, E.-K. Investigation of the anti-prostate cancer properties of marine-derived compounds. Mar. Drugs 2018, 16, 160. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ji, R.; Zha, X.; Xu, Z.; Lin, Y.; Zhou, S. Investigation of the bactericidal mechanism of Penicilazaphilone C on Escherichia coli based on 4D label-free quantitative proteomic analysis. Eur. J. Pharm. Sci. 2022, 179, 106299. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, J.; Zhang, X.; Xu, W.; Yang, J.; Song, F. Investigation on the chemical constituents of the marine-derived fungus strain Aspergillus brunneoviolaceus MF180246. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Sheng, R.; Sohail, M.; Jahromi, S.T.; Babich, O.; Sukhikh, S.; Nahavandi, R. Alginate lyases from marine bacteria: An enzyme ocean for sustainable future. Molecules 2022, 27, 3375. [Google Scholar] [CrossRef] [PubMed]
- Willems, T.; De Mol, M.L.; De Bruycker, A.; De Maeseneire, S.L.; Soetaert, W.K. Alkaloids from marine fungi: Promising antimicrobials. Antibiotics 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Lin, R.; Yang, N.; Jia, J.; Wei, S.; Han, J.; Li, J.; Bi, H.; Xu, X. Antibacterial secondary metabolites from marine-derived fungus Aspergillus sp. IMCASMF180035. Antibiotics 2021, 10, 377. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Huang, G.; Li, Y.; Yang, L.; Zhang, Y.; Huang, S.; Pan, L.; Yang, D. Antibiotics development and the potentials of marine-derived compounds to stem the tide of multidrug-resistant pathogenic bacteria, fungi, and protozoa. Mar. Drugs 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.C.; Chang, C.H.; Liao, H.R.; Fu, S.L.; Chen, J.J. Anti-cancer and anti-inflammatory activities of three new chromone derivatives from the marine-derived Penicillium citrinum. Mar. Drugs 2021, 19, 408. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.M.; Swaileh, Z.; Ammar, M.; Jaghama, W.; Yousef, M.; Karaman, R.; Bufo, S.A.; Scrano, L. Antifungal and antibacterial activities of isolated marine compounds. Toxins 2023, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.-H.; Liang, X.; Cheng, X.; Ling, J.; Dong, J.-D.; Qi, S.-H. Antifungal peptides from the marine gorgonian-associated fungus Aspergillus sp. SCSIO41501. Phytochemistry 2021, 192, 112967. [Google Scholar] [CrossRef]
- Sweilam, S.H.; Alqarni, M.H.; Youssef, F.S.; Noureini, S.K. Antimicrobial alkaloids from marine-derived fungi as drug leads versus COVID-19 infection: A computational approach to explore their anti-COVID-19 activity and ADMET properties. Evid.-Based Complement. Altern. Med. 2022, 2022, 1–19. [Google Scholar] [CrossRef]
- Abuhijjleh, R.K.; Shabbir, S.; Al-Abd, A.M.; Jiaan, N.H.; Alshamil, S.; El-labbad, E.M.; Khalifa, S.I. Bioactive marine metabolites derived from the Persian Gulf compared to the Red Sea: Similar environments and wide gap in drug discovery. PeerJ 2021, 9, e11778. [Google Scholar] [CrossRef]
- Giri, A.; Ohshima, T. Bioactive marine peptides: Nutraceutical value and novel approaches. Adv. Food Nutr. Res. 2012, 65, 73–105. [Google Scholar] [CrossRef]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Chen, S.; Li, J.; Liu, L. The biological and chemical diversity of tetramic acid compounds from marine-derived microorganisms. Mar. Drugs 2020, 18, 114. [Google Scholar] [CrossRef] [Green Version]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufosse, L. Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef] [Green Version]
- Hafez Ghoran, S.; Taktaz, F.; Ayatollahi, S.A.; Kijjoa, A. Anthraquinones and their analogues from marine-derived fungi: Chemistry and biological activities. Mar. Drugs 2022, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Himaya, S.W.; Kim, S.K. Triterpenoids of marine origin as anti-cancer agents. Molecules 2013, 18, 7886–7909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.W.; Ding, P. New bioactive metabolites from the marine-derived fungi Aspergillus. Mini-Rev. Med. Chem. 2018, 18, 1072–1094. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yuan, X.L.; Li, C.; Li, A.X. Recent discovery of heterocyclic alkaloids from marine-derived Aspergillus species. Mar. Drugs 2020, 18, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rajhi, A.M.H.; Mashraqi, A.; Al Abboud, M.A.; Shater, A.M.; Al Jaouni, S.K.; Selim, S.; Abdelghany, T.M. Screening of bioactive compounds from endophytic marine-derived fungi in saudi arabia: Antimicrobial and anticancer potential. Life 2022, 12, 1182. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhang, Z.Z.; Feng, Y.Y.; Gu, Q.Q.; Li, D.H.; Zhu, T.J. Secondary metabolites from antarctic marine-derived fungus Penicillium crustosum HDN153086. Nat. Prod. Res. 2019, 33, 414–419. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Z.L.; Wu, J.S.; Shao, C.L.; Yao, G.S.; Wang, C.Y. Induction of secondary metabolite biosynthesis by deleting the histone deacetylase HdaA in the marine-derived fungus Aspergillus terreus RA2905. J. Fungi 2022, 8, 1024. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Lin, R.; Yang, H.; Song, F.; Xu, X.; Wang, L. New secondary metabolites from the marine-derived fungus Talaromyces mangshanicus BTBU20211089. Mar. Drugs 2022, 20, 79. [Google Scholar] [CrossRef]
- Zaman, K.A.U.; Wu, X.; Sarotti, A.M.; Cao, S. New and bioactive polyketides from Hawaiian marine-derived fungus Trichoderma sp. FM652. Nat. Prod. Res. 2022, 36, 5984–5990. [Google Scholar] [CrossRef]
- Xie, M.M.; Jiang, J.Y.; Zou, Z.B.; Xu, L.; Zhang, Y.; Wang, C.F.; Liu, C.B.; Yan, Q.X.; Liu, Z.; Yang, X.W. Chemical constituents of the deep-sea-derived fungus Cladosporium oxysporum 170103 and their antibacterial effects. Chem. Biodivers. 2022, 19, e202200963. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Xu, Z.; Bai, Q.; Zhou, X.; Zheng, C.; Bai, M.; Chen, G. Biological secondary metabolites from the lumnitzera littorea-derived fungus Penicillium oxalicum HLLG-13. Mar. Drugs 2022, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, S.; Chen, H.; Zhang, Z.; Li, X.; Wang, W.; Guo, L. Bioassay-guided isolation and characterization of antibacterial compound from Aspergillus fumigatus HX-1 associated with Clam. 3 Biotech 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Shaala, L.A.; Genta-Jouve, G. Asperopiperazines A and B: Antimicrobial and cytotoxic dipeptides from a tunicate-derived fungus Aspergillus sp. DY001. Mar. Drugs 2022, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhu, Y.; Chen, J.; Chen, J.; Li, C.; Gao, Z.; Li, J.; Liu, L. Sesquiterpenoids with phytotoxic and antifungal activities from a pathogenic fungus Aspergillus alabamensis. J. Agric. Food Chem. 2022, 70, 12065–12073. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, Y.; Chen, J.; Chen, J.; Li, C.; Gao, Z.; Li, J.; Liu, L. Discovery of novel bactericides from Aspergillus alabamensis and their antibacterial activity against fish pathogens. J. Agric. Food Chem. 2023, 71, 4298–4305. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, X.; Shah, M.; Che, Q.; Zhang, G.; Gu, Q.; Zhu, T.; Li, D. Polyhydroxy p-terphenyls from a mangrove endophytic fungus Aspergillus candidus LDJ-5. Mar. Drugs 2021, 19, 82. [Google Scholar] [CrossRef]
- Zang, Z.; Yang, W.; Cui, H.; Cai, R.; Li, C.; Zou, G.; Wang, B.; She, Z. Two antimicrobial heterodimeric tetrahydroxanthones with a 7,7′-linkage from mangrove endophytic fungus Aspergillus flavus QQYZ. Molecules 2022, 27, 2691. [Google Scholar] [CrossRef]
- Lin, L.-B.; Gao, Y.-Q.; Han, R.; Xiao, J.; Wang, Y.-M.; Zhang, Q.; Zhai, Y.-J.; Han, W.-B.; Li, W.-L.; Gao, J.-M. Alkylated salicylaldehydes and prenylated indole alkaloids from the endolichenic fungus Aspergillus chevalieri and their bioactivities. J. Agric. Food Chem. 2021, 69, 6524–6534. [Google Scholar] [CrossRef]
- Li, H.-L.; Yang, S.-Q.; Li, X.-M.; Li, X.; Wang, B.-G. Structurally diverse alkaloids produced by Aspergillus creber EN-602, an endophytic fungus obtained from the marine red alga Rhodomela confervoides. Bioorg. Chem. 2021, 110, 104822. [Google Scholar] [CrossRef]
- Fang, S.-T.; Liu, X.-H.; Yan, B.-F.; Miao, F.-P.; Yin, X.-L.; Li, W.-Z.; Ji, N.-Y. Terpenoids from the marine-derived fungus Aspergillus sp. RR-YLW-12, associated with the red alga Rhodomela confervoides. J. Nat. Prod. 2021, 84, 1763–1771. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Y.; Wang, J.; Shi, X.; Che, Y.; Chen, X.; Zhong, W.; Zhang, W.; Wei, X.; Wang, F.; et al. Diverse secondary metabolites from the coral-derived fungus Aspergillus hiratsukae SCSIO 5Bn1003. Mar. Drugs 2022, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Zeng, Q.; Chen, Y.C.; Zhong, W.M.; Xiang, Y.; Wang, J.F.; Shi, X.F.; Zhang, W.M.; Zhang, S.; Wang, F.Z. Chevalones H-M: Six new alpha-pyrone meroterpenoids from the gorgonian coral-derived fungus Aspergillus hiratsukae SCSIO 7S2001. Mar. Drugs 2022, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Chen, W.; Lin, X.; Xiao, J.; Liu, Y.; Zhou, X. Butenolides from the coral-derived fungus Aspergillius terreus SCSIO41404. Mar. Drugs 2022, 20, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Huang, B.; Liu, K.; Peng, S.; Liu, X.; Gao, C.; Liu, Y.; Tan, Y.; Luo, X. Anti-osteoclastogenic and antibacterial effects of chlorinated polyketides from the Beibu gulf coral-derived fungus Aspergillus unguis GXIMD 02505. Mar. Drugs 2022, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Buttachon, S.; Ramos, A.A.; Inacio, A.; Dethoup, T.; Gales, L.; Lee, M.; Costa, P.M.; Silva, A.M.S.; Sekeroglu, N.; Rocha, E.; et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA. Mar. Drugs 2018, 16, 119. [Google Scholar] [CrossRef] [Green Version]
- Machado, F.P.; Kumla, D.; Pereira, J.A.; Sousa, E.; Dethoup, T.; Freitas-Silva, J.; Costa, P.M.; Mistry, S.; Silva, A.M.S.; Kijjoa, A. Prenylated phenylbutyrolactones from cultures of a marine sponge-associated fungus Aspergillus flavipes KUFA1152. Phytochemistry 2021, 185, 112709. [Google Scholar] [CrossRef]
- Liu, J.; Yu, R.; Jia, J.; Gu, W.; Zhang, H. Assignment of absolute configurations of two promising anti-helicobacter pylori agents from the marine sponge-derived fungus Aspergillus niger L14. Molecules 2021, 26, 5061. [Google Scholar] [CrossRef]
- Guo, C.; Wang, P.; Pang, X.; Lin, X.; Liao, S.; Yang, B.; Zhou, X.; Wang, J.; Liu, Y. Discovery of a dimeric zinc complex and five cyclopentenone derivatives from the sponge-associated fungus Aspergillus ochraceopetaliformis. ACS Omega 2021, 6, 8942–8949. [Google Scholar] [CrossRef]
- Li, J.X.; Xu, Q.H.; Shang, R.Y.; Liu, Q.; Luo, X.C.; Lin, H.W.; Jiao, W.H. Aspergetherins A-D, new chlorinated biphenyls with anti-MRSA activity from the marine sponge symbiotic fungus Aspergillus terreus 164018. Chem. Biodivers. 2023, e202300010. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, L.; He, J.; Zhang, Z.; Deng, Y.; He, S.; Yan, X. A new antibacterial chromone from a marine sponge-associated fungus Aspergillus sp. LS57. Fitoterapia 2021, 154, 105004. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, L.; Shi, Y.; Yan, X.; Wu, B.; He, S. Molecular networking-driven discovery of antibacterial perinadines, new tetracyclic alkaloids from the marine sponge-derived fungus Aspergillus sp. ACS Omega 2022, 7, 9909–9916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, H.; Chen, B.; Dai, H.; Sun, J.; Han, J.; Liu, H. Discovery of anti-MRSA secondary metabolites from a marine-derived fungus Aspergillus fumigatus. Mar. Drugs 2022, 20, 302. [Google Scholar] [CrossRef] [PubMed]
- Anh, C.V.; Kwon, J.-H.; Kang, J.S.; Lee, H.-S.; Heo, C.-S.; Shin, H.J. Antibacterial and cytotoxic phenolic polyketides from two marine-derived fungal strains of Aspergillus unguis. Pharmaceuticals 2022, 15, 74. [Google Scholar] [CrossRef]
- Wu, J.; Shui, H.; Zhang, M.; Zeng, Y.; Zheng, M.; Zhu, K.K.; Wang, S.B.; Bi, H.; Hong, K.; Cai, Y.S. Aculeaxanthones A-E, new xanthones from the marine-derived fungus Aspergillus aculeatinus WHUF0198. Front. Microbiol. 2023, 14, 1138830. [Google Scholar] [CrossRef]
- Xiang, Y.; Zeng, Q.; Mai, Z.-M.; Chen, Y.-C.; Shi, X.-F.; Chen, X.-Y.; Zhong, W.-M.; Wei, X.-Y.; Zhang, W.-M.; Zhang, S.; et al. Asperorydines N-P, three new cyclopiazonic acid alkaloids from the marine-derived fungus Aspergillus flavus SCSIO F025. Fitoterapia 2021, 150, 104839. [Google Scholar] [CrossRef]
- Yan, L.-H.; Li, X.-M.; Chi, L.-P.; Li, X.; Wang, B.-G. Six new antimicrobial metabolites from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406. Mar. Drugs 2021, 20, 4. [Google Scholar] [CrossRef]
- Cen, S.; Jia, J.; Ge, Y.; Ma, Y.; Li, X.; Wei, J.; Bai, Y.; Wu, X.; Song, J.; Bi, H.; et al. A new antibacterial 3,5-dimethylorsellinic acid-based meroterpene from the marine fungus Aspergillus sp. CSYZ-1. Fitoterapia 2021, 152, 104908. [Google Scholar] [CrossRef]
- Yang, J.; Gong, L.; Guo, M.; Jiang, Y.; Ding, Y.; Wang, Z.; Xin, X.; An, F. Bioactive indole diketopiperazine alkaloids from the marine endophytic fungus Aspergillus sp. YJ191021. Mar. Drugs 2021, 19, 157. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, J.; Xue, Y.; Li, S.; Sun, X.; Jia, J.; Bi, H.; Wang, S.; Su, H.; Zhu, M.; et al. Two new austocystin analogs from the marine-derived fungus Aspergillus sp. WHUF05236. Chem. Biodivers. 2022, 19, e202200207. [Google Scholar] [CrossRef]
- Quang, T.H.; Phong, N.V.; Anh, L.N.; Hanh, T.T.H.; Cuong, N.X.; Ngan, N.T.T.; Trung, N.Q.; Nam, N.H.; Minh, C.V. Secondary metabolites from a peanut-associated fungus Aspergillus niger IMBC-NMTP01 with cytotoxic, anti-inflammatory, and antimicrobial activities. Nat. Prod. Res. 2022, 36, 1215–1223. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y. A review on bioactive compounds from marine-derived chaetomium species. J. Microbiol. Biotechnol. 2022, 32, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yi, M.; Ding, L.; He, S. A review of anti-inflammatory compounds from marine fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Wang, H.; Yan, M.; Sai, C.; Zhang, Z. Research advances of bioactive sesquiterpenoids isolated from marine-derived Aspergillus sp. Molecules 2022, 27, 7376. [Google Scholar] [CrossRef] [PubMed]
- Arockianathan, P.M.; Mishra, M.; Niranjan, R. Recent status and advancements in the development of antifungal agents: Highlights on plant and marine based antifungals. Curr. Top. Med. Chem. 2019, 19, 812–830. [Google Scholar] [CrossRef]
- Sharma, D.; Bisht, G.S. Recent updates on antifungal peptides. Mini-Rev. Med. Chem. 2020, 20, 260–268. [Google Scholar] [CrossRef]
- Montuori, E.; de Pascale, D.; Lauritano, C. Recent discoveries on marine organism immunomodulatory activities. Mar. Drugs 2022, 20, 422. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Xue, M.; Zhao, Z.; Zhang, H.; Xu, D.; Lai, D.; Zhou, L. Recent advances in sorbicillinoids from fungi and their bioactivities (Covering 2016–2021). J. Fungi 2022, 8, 62. [Google Scholar] [CrossRef]
- Lima, R.N.; Porto, A.L.M. Recent advances in marine enzymes for biotechnological processes. Adv. Food Nutr. Res. 2016, 78, 153–192. [Google Scholar]
- Wang, Y.-N.; Meng, L.-H.; Wang, B.-G. Progress in research on bioactive secondary metabolites from deep-sea derived microorganisms. Mar. Drugs 2020, 18, 614. [Google Scholar] [CrossRef]
- Hang, S.; Chen, H.; Wu, W.; Wang, S.; Fang, Y.; Sheng, R.; Tu, Q.; Guo, R. Progress in isoindolone alkaloid derivatives from marine microorganism: Pharmacology, preparation, and mechanism. Mar. Drugs 2022, 20, 405. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Mei, J.; Jiang, R.; Tu, S.; Deng, H.; Liu, J.; Yang, S.; Li, J. Origins, structures, and bioactivities of secondary metabolites from marine-derived Penicillium fungi. Mini-Rev. Med. Chem. 2021, 21, 2000–2019. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Wang, P.; Ding, W. Novel indole diketopiperazine stereoisomers from a marine-derived fungus Aspergillus sp. Mycology 2023, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lin, X.; Fu, J.; Zhang, J.; Tang, W.; He, Z. A novel bi-functional fibrinolytic enzyme with anticoagulant and thrombolytic activities from a marine-derived fungus Aspergillus versicolor ZLH-1. Mar. Drugs 2022, 20, 356. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, P.; Li, X.; Gan, L.; Wang, P. Novel stemphol derivatives from a marine fungus Pleospora sp. Nat. Prod. Res. 2018, 33, 367–373. [Google Scholar] [CrossRef]
- Song, Z.; Gao, J.; Hu, J.; He, H.; Huang, P.; Zhang, L.; Song, F. One new xanthenone from the marine-derived fungus Aspergillus versicolor MF160003. Nat. Prod. Res. 2020, 34, 2907–2912. [Google Scholar] [CrossRef]
- Wu, J.S.; Shi, X.H.; Yao, G.S.; Shao, C.L.; Fu, X.M.; Zhang, X.L.; Guan, H.S.; Wang, C.Y. New thiodiketopiperazine and 3,4-dihydroisocoumarin derivatives from the marine-derived fungus Aspergillus terreus. Mar. Drugs 2020, 18, 132. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Huang, R.; Liu, H.; Yan, T.; Ding, W.; Jiang, Y.; Wang, P.; Zheng, D.; Xu, J. New polyketides from the marine-derived fungus Letendraea Sp. 5XNZ4-2. Mar. Drugs 2019, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Li, J.; Zhang, K.; Wei, S.; Lin, R.; Polyak, S.W.; Yang, N.; Song, F. New isocoumarin analogues from the marine-derived fungus Paraphoma sp. CUGBMF180003. Mar. Drugs 2021, 19, 313. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Gao, J.; He, H.; Dai, H.; Xia, X.; Liu, C.; Zhang, L.; Song, F. New diketopiperazines from a marine-derived fungus strain Aspergillus versicolor MF180151. Mar. Drugs 2019, 17, 262. [Google Scholar] [CrossRef] [Green Version]
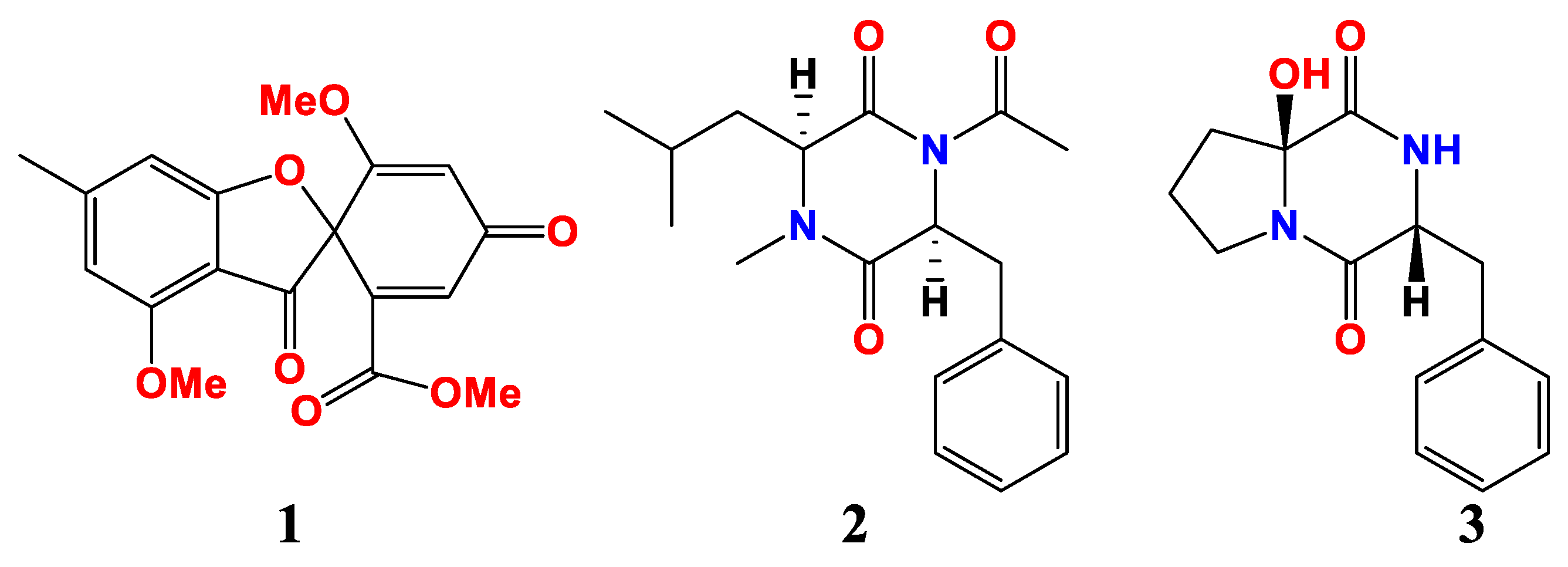


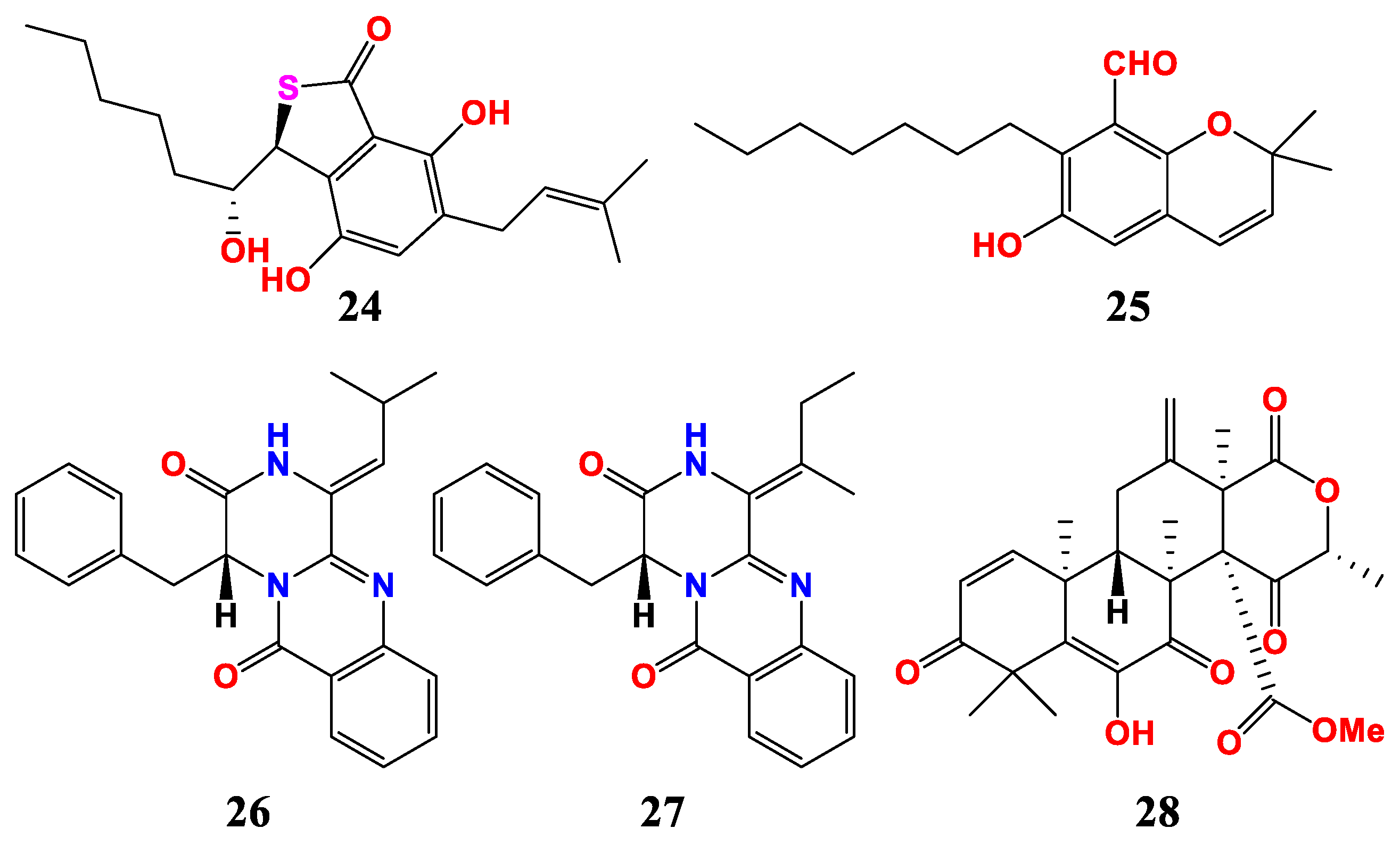


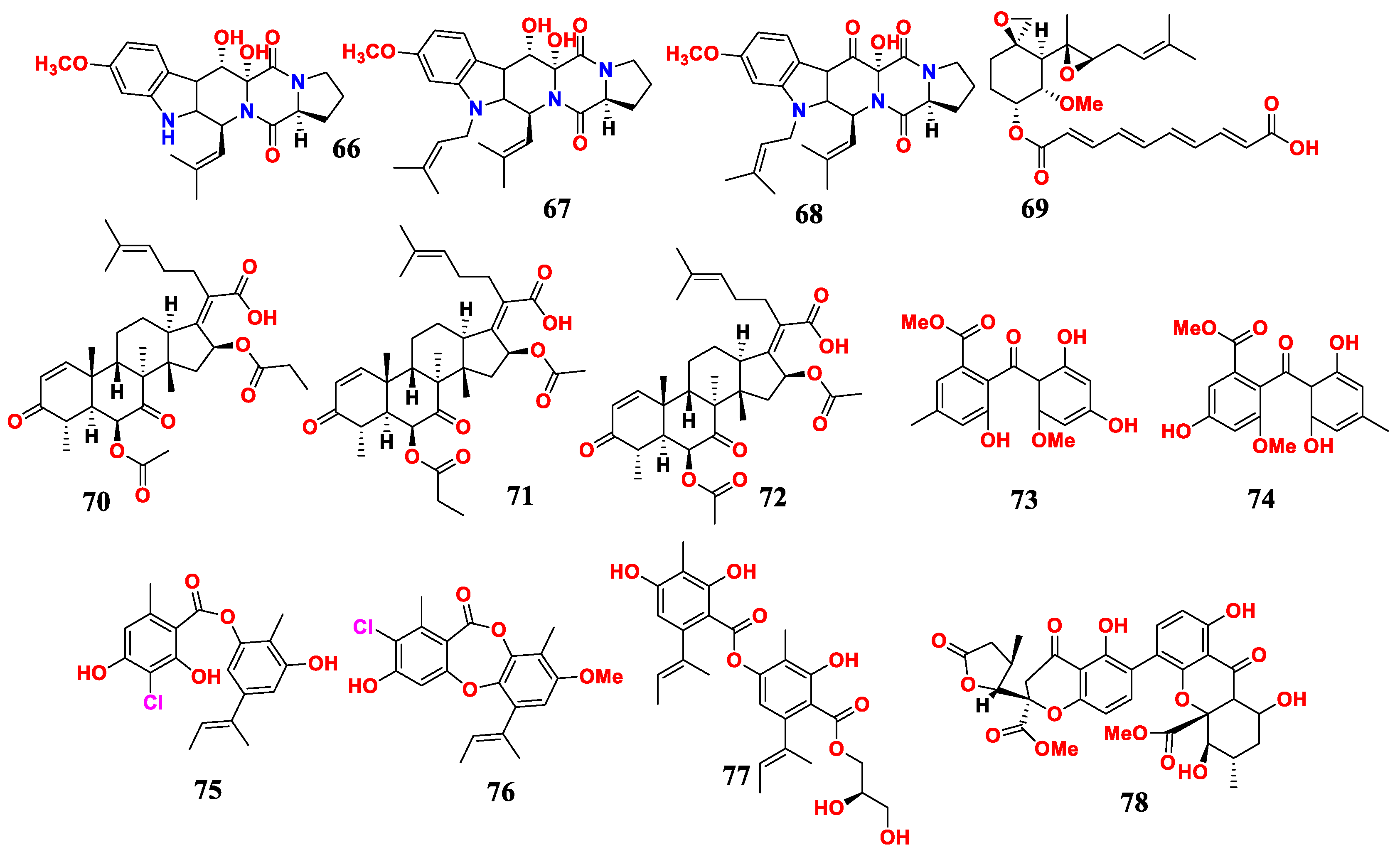
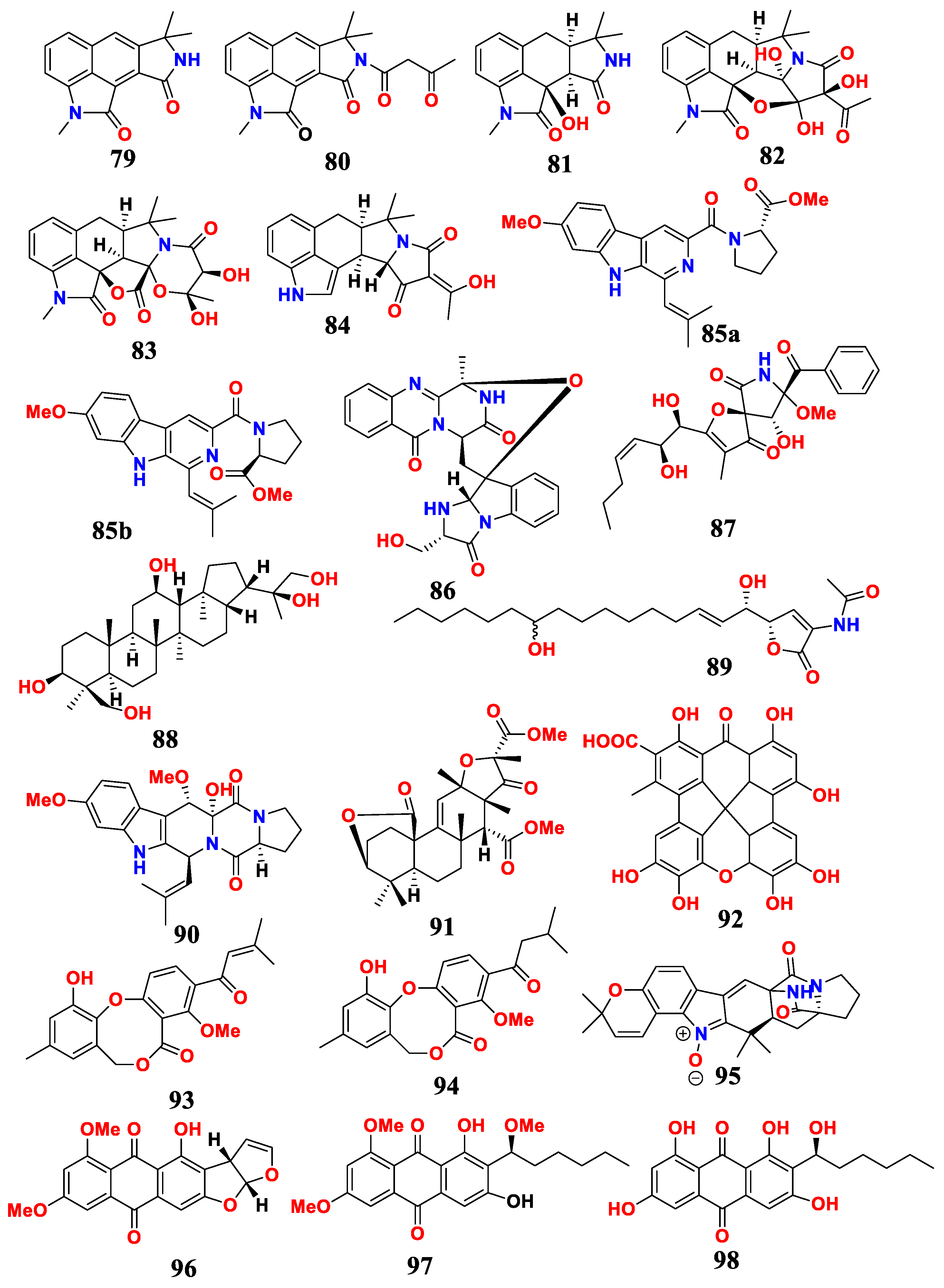

| Sources and Aspergillus | Compounds | Activities | References |
|---|---|---|---|
| Marine animals | |||
| A. fumigatus HX-1 | Trypacidin (1) | MIC (anti-V. harveyi) was 31.25 μg/mL | [52] |
| Aspergillus sp. DY001 | Asperopiperazines A, B (2, 3) | MIC (anti-E. coli) were 8 and 4 μM MIC (anti-S. aureus) were 8 and 8 μM | [53] |
| Marine plants | |||
| A. alabamensis | 4-hydroxy-5(6)-dihydroterrecyclic acid A (4), asperalacids A–D (5–8) | MIC (anti-plant pathogens) was 25–200 μg/mL | [54] |
| A. alabamensis | asperalins A–F (9–14) | MIC (anti-fish pathogens) was 2.2–87.3 μM | [55] |
| Mangroves | |||
| A. brunneoviolaceus MF180246 | asperbrunneo acid (15), secalonic acids H, F1 (16, 18), chrysoxanthone C (17), asperdichrome (19), penicillixanthone A (20) | MIC (anti-S. aureus) were 200, 50, 50, 25, 25, 6.25 μg/mL | [27] |
| A. candius LDJ-5 | asperterphenyllin C (21) | MIC (anti-Proteus sp.) was 19 μg/mL | [56] |
| A. flavus QQYZ | aflatoxones A, B (22, 23) | MIC (anti-pathogens) was 3.13–50 μM | [57] |
| Marine algaes | |||
| A. chevalieri SQ-8 | asperglaucins A, B (24, 25) | MIC (anti-plant pathogens) was 6.25 μM | [58] |
| A. creber EN-602 | versiamide A (26), 3, 15-dehydroprotuboxepin K (27) | MIC (anti-bacteria) was 8–64 μg/mL | [59] |
| Aspergillus sp. RR-YLW12 | terretonin F (28) | IC50 (anti-three microalgae) were 3.1, 5.2, 10.5 μg/mL | [60] |
| Marine corals | |||
| A. hiratsukae SCSIO 5Bn1003 | demethylincisterol A2 (29), asperophiobolin E (30), butyrolactone I (31) | MIC (anti-B. subtilis) were 10.26 ± 0.76, 17.00 ± 1.25 and 5.30 ± 0.29 μM | [61] |
| A. hiratsukae SCSIO 7S2001 | methterpenoids H-L (32–36) neoechinulin A (37) | MIC (anti-bacteria) was 6.25–100 μg/mL | [62] |
| A. terreus SCSIO41404 | versicolactone B (38), butyrolactone VI (39) | IC50 (anti-E. faecalis, K. pneumoniae) were 25 and 50 μg/mL | [63] |
| A. unguis GXIMD 02505 | 40–45 | MIC (anti-bacteria) was 2–64 μg/mL | [64] |
| Aspergillus sp. SCSIO 41501 | maribasins C–E,A,B (46–50) | MIC (anti-plant pathogens) was 3.12–50 μg/disc | [34] |
| Sponges | |||
| A. candius KUFA 0062 | preussin (51) | anti-pathogens | [65] |
| A. flavipes KUFA1152 | aspulvinones B’, H, R and S (52–55) | MIC (anti-pathogens) was 16–64 μg/mL | [66] |
| A. niger L14 | fonsecinone A (56), isoaurasperone A (57) | MIC (anti-H. pylori) was ≤4 μg/mL | [67] |
| A. ochraceopetaliformis SCSIO 41018 | hydroxy-neotriamycin (58) | MIC (anti-pathogens) was 0.45–7.8 μg/mL μM | [68] |
| A. terreus 164018 | aspergetherins A, C (59, 60) 3, 5-dichloroasterric acid (61), methyl chloroasterrate (62) | MIC (anti-MRSA) was 1.0–128 μg/mL | [69] |
| Aspergillus sp. LS57 | aspergilluone A (63) | MIC (anti-pathogens) was 32–128 μg/mL | [70] |
| Aspergillus sp. LS116 | perinadines B, C (64, 65) | MIC (anti-B. subtilis) were 32 and 64 μg/mL | [71] |
| Seawater | |||
| A. fumigatus H22 | 12,13-dihydroxyfumitremorgin C (66), fumitremorgin B (67) | MIC(anti-M. Bovis, C. albicans) were 25 and 50 μM | [72] |
| A. fumigatus H22 | (66),13-oxofumitremorgin B (68) | antibacterial activity | [72] |
| A. fumigatus H22 | fumagillin (69), helvolic acid (70), 6-O-propionyl-16-O-deacetylhelvolic acid (71), 16-O-propionyl-6-O-deacetylhelvolic acid (72), penibenzophenone E (73), sulochrin (74) | MIC (anti-MRSA) were 1.25 and 2.5 | [72] |
| A. unguis | unguidepside C (75), aspersidone B (76), agonodepside C (77) | MIC (anti-bacteria) was 5.3 to 22.1 μM | [73] |
| A. aculeatinus WHUF0198 | aculeaxanthone A (78) | MIC (anti-bacteria) was 1.0 to 4.0 μM | [74] |
| Marine sediments | |||
| A. flavus SCSIO F025 | cyclopiamide (79), speradines G,H,B,C (80–83), CPA (84) | weak anti-bacteria | [75] |
| A. fumigatus SD-406 | 85–90 | MIC (anti-bacteria and plant pathogens) were 4–64 μg/mL | [76] |
| Aspergillus sp. CSYZ-1 | meroterpenoid (91) | MIC (anti-S. aureus, H. pylori) were 2–16 and 1–4 μg/mL | [77] |
| Aspergillus sp. IMCASMF180035 | aspergiloxathene A (92) | MIC (anti-MRSA, S. aureus) were 22.40 and 5.60 μM | [30] |
| Aspergillus sp. IMCASMF180035 | Δ2′-1′-dehydropenicillide (93), dehydropenicillide (94) | MIC (anti-H. pylori) were 21.61 and 21.73 μM | [30] |
| Aspergillus sp. YJ191021 | asperthrins A (95) | MIC (anti-plant pathogens) was 8–25μg/mL | [78] |
| Aspergillus sp. WHUF05236 | 6, 8-di-O-methylversicolorin A (96), 6,8,1′-tri-O-methylaverantin (97), 6,8-di-O-methylaverantin (98) | MIC (anti-H. pylori) was 20.00 to 43.47 μM | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Fu, Y.; Song, F. Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds. Mar. Drugs 2023, 21, 277. https://doi.org/10.3390/md21050277
Li H, Fu Y, Song F. Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds. Marine Drugs. 2023; 21(5):277. https://doi.org/10.3390/md21050277
Chicago/Turabian StyleLi, Honghua, Yanqi Fu, and Fuhang Song. 2023. "Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds" Marine Drugs 21, no. 5: 277. https://doi.org/10.3390/md21050277
APA StyleLi, H., Fu, Y., & Song, F. (2023). Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds. Marine Drugs, 21(5), 277. https://doi.org/10.3390/md21050277






