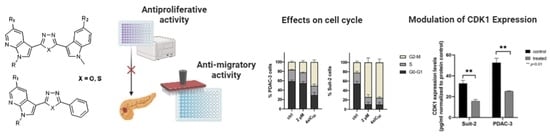
3.1. Chemistry
Analytical thin-layer chromatography (TLC) was performed on silica gel 60 F254 plates (0.25 mm thickness) and the developed plates were examined under ultraviolet (UV) light. All melting points were taken on a Buchi-Tottoly capillary apparatus and were uncorrected. IR spectra were determined in bromoform with a Shimadzu FT/IR 8400S spectrophotometer and peaks were reported in wavenumber (cm−1). 1H and 13C NMR spectra were measured at 200 and 50 MHz, respectively, on DMSO-d6 solution, using a Bruker Avance II series 200 MHz spectrometer. The chromatography column was performed with MERK silica gel 230–400 mesh ASTM or FLASH40i Biotage chromatography or with a Buchi Sepacore chromatography module (prepacked cartridge reference). Elementary analyses (C, H, N) were within ± 0.4% of the theoretical values and were performed with a VARIO EL III elemental analyzer (Elementar, Langenselbold, Germany). Compounds 3–6,7c,8b were characterized only by 1H NMR spectra, for their poor solubility.
3.1.1. General Procedure for the Synthesis of 5-Bromo-1-methyl-7-azaindole-3-carbohydrazide (9c)
Appropriate carboxylic acid 10 (3.1 mmol) was stirred at room temperature with thionyl chloride (68 mmol), then the reaction mixture was heated under reflux for three hours. The reaction mixture was cooled and the excess of thionyl chloride (SOCl2) was distilled off. The resulting solid was recrystallized from aqueous ethanol, dissolved in absolute alcohol and heated under reflux with hydrazine hydrate to give 7-azaindole-3-carbohydrazide. The product was then recrystallized from aqueous ethanol.
Yield: 70%; white solid; mp: 130 °C; IR (cm−1): 3310 (NH2), 3148 (NH), 1684 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 9.40 (bs, 1H, NH), 8.58 (d, J = 2.3 Hz, 1H), 8.42 (d, J = 2.2 Hz, 1H), 8.17 (s, 1H), 4.40 (s, 2H, NH2), 3.84 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 164.7 (s), 148.5 (s), 144.6 (d), 134.3 (d), 130.1 (d), 120.0 (s), 111.3 (s), 109.4 (s), 31.6 (q); Anal. Calculated for C9H9BrN4O (MW: 269,10): C, 40.17; H, 3.37; N, 20.82%. Found: C, 40.17; H, 3.37; N, 20.82%.
3.1.2. General Procedure for the Synthesis of N’-(1-Methyl-1H-indole-3-carbonyl)-7-azaindole-3-carbohydrazides (7a–h)
To a solution of the proper acid 10a–d (2 mmol) in dimethylformamide (10 mL), 1-ethyl-3(3-dimethylaminopropyl)carbodiimide (2 mmol), hydroxylbenzotriazole (2 mmol) and diisopropylethylamine (4 mmol) were added. The reaction was stirred for 1–4 h at room temperature. Then, the proper hydrazine 9a–c (4 mmol) was added, and the reaction mixture was heated at 60° for 2–24 h or under reflux for 24 h. Once cooled, ice was added, and the formed precipitate was filtered off and recrystallized in diethyl ether to afford the pure compounds.
N’-(1-methyl-1H-indole-3-carbonyl)-7-azaindole-3-carbohydrazide (7a). Conditions: r.t. for 1 h, then reflux for 24 h. Yield: 50%; brown solid; mp: 182 °C; IR (cm−1): 3447 (NH), 3366 (NH), 3229 (NH), 1558 (CO), 1541 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 12.21 (bs, 1H, NH), 9.94 (bs, 1H, NH), 9.83 (bs, 1H, NH), 8.44 (d, J = 6.7 Hz, 1H), 8.32–8.25 (m, 2H), 8.15 (d, J = 7.9 Hz, 1H), 8.11 (s, 1H), 7.53 (d, J = 8.2 Hz, 1H), 7.28–7.22 (m, 1H), 7.22– 7.19 (m, 1H), 7.18 (dd, J = 10.0, 5.2 Hz, 1H), 3.87 (s, 3H, CH3); 13C NMR (101 MHz, DMSO-d6) δ: 148.8 (s), 144.2 (d), 137.1 (s), 132.6 (d), 129.7 (d), 129.0 (d), 127.1 (s), 126.2 (s), 122.6 (d), 121.5 (d), 121.3 (d), 119.8 (s), 119.1 (s), 117.6 (d), 110.8 (d), 107.9 (s), 107.2 (s), 33.6 (q); Anal. Calculated for C18H15N5O2 (MW: 333,35): C, 64.86; H, 4.54; N, 21.01%. Found C, 64.71; H, 4.50; N, 21.22%.
1-methyl-N’-(1-methyl-1H-indole-3-carbonyl)-7-azaindole-3-carbohydrazide (7b). Conditions: r.t. for 4 h, then 60 °C.for 2 h. Yield: 80%; brown solid; mp: 159 °C; IR (cm−1): 3228 (2 NH), 1653 (CO), 1636 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 9.98 (bs, 1H, NH), 9.87 (bs, 1H, NH), 8.49–8.45 (dd, J = 1.4, 7.9 Hz, 1H), 8.39–8.36 (dd, J = 1.4, 4.6 Hz, 1H), 8.31 (s, 1H), 8.18–8.15 (d, J = 7.3 Hz, 1H), 8.13 (s, 1H), 7.56–7.52 (d, J = 8 Hz, 1H), 7.29–7.15 (m, 3H), 3.91 (s, 3H, CH3), 3.88 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 163.6 (s), 147.3 (s), 143.5 (d), 136.6 (s), 132.1 (d), 129.4 (d), 126.6 (s), 122.1 (d), 121.0 (d), 120.9 (dx2), 118.8 (s), 117.2 (d), 110.3 (d), 107.3 (sx2), 106.2 (s), 33.1 (q), 31.4 (q); Anal. Calculated for C19H17N5O2 (MW: 347,38): C, 65.69; H, 4.93; N, 20.16%. Found C, 65.57; H, 4.81; N, 20.10%.
N’-(5-fluoro-1-methyl-1H-indole-3-carbonyl)-1-methyl-7-azaindole-3-carbohydrazide (7c). Conditions: r.t. for 2 h, then 60 °C for 24 h. Yield: 85%; brown solid; mp: 172 °C; IR (cm−1): 3184 (NH), 3119 (NH), 1576 (CO), 1526 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 9.99 (bs, 1H, NH), 9.93 (bs, 1H, NH), 8.48–8.44 (d, J = 7.5 Hz, 1H), 8.38–8.36 (d, J = 4.6 Hz, 1H), 8.31 (s, 1H), 8.19 (s, 1H), 7.86–7.80 (dd, J = 2.3, 10 Hz, 1H), 7.61–7.54 (dd, J = 4.3, 8.9 Hz, 1H), 7.29–7.22 (dd, J = 4.7, 7.9 Hz, 1H), 7.17–7.07 (td, J = 2.4, 9.2 Hz, 1H), 3.91 (s, 3H, CH3), 3.89 (s, 3H, CH3); Anal. Calculated for C19H16FN5O2 (MW: 365,37): C, 62.46; H, 4.41; N, 19.17%. Found: C, 62.40; H, 4.37; N, 19.22%.
N’-(5-bromo-1-methyl-1H-indole-3-carbonyl)-1-methyl-7-azaindole-3-carbohydrazide (7d). Conditions: r.t. for 1 h, then 60 °C for 24 h. Yield: 88%; white solid; mp: 169 °C; IR (cm−1): 3184–3199 (2 NH), 1647 (CO), 1578 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 9.99 (bs, 1H, NH), 9.96 (bs, 1H, NH), 8.48–8.44 (d, J = 7.8 Hz, 1H), 8.39–8.36 (d, J = 4.6 Hz, 1H), 8.31 (s, 2H), 8.17 (s, 1H), 7.57–7.52 (d, J = 8.7 Hz, 1H), 7.41–7.36 (dd, J = 1.7, 8.8 Hz, 1H), 7.29–7.22 (dd, J = 4.7, 7.8 Hz, 1H), 3.91 (s, 3H, CH3), 3.88 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 163.7 (s), 163.5 (s), 147.3 (s), 143.5 (d), 135.4 (s), 133.3 (d), 132.2 (d), 129.4 (d), 128.3 (s), 124.7 (d), 123.1 (d), 118.8 (s), 117.3 (d), 113.8 (s), 112.6 (d), 106.9 (s), 106.1 (s), 33.3 (q), 31.3 (q); Anal. Calculated for C19H16BrN5O2 (MW: 426,27): C, 53.54; H, 3.78; N, 16.43%. Found: C, 53.50; H, 3.74; N, 16.33%.
5-bromo-1-methyl-N’-(1-methyl-1H-indole-3-carbonyl)-7-azaindole-3-carbohydrazide (7e). Conditions: r.t. for 4 h, then 60 °C.for 2 h. Yield: 80%; white solid; mp: 175 °C; IR (cm−1): 3354 (NH), 3254 (NH), 1558 (CO), 1541 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 9.91–9.96 (bs, 2H, 2NH), 8.61–8.60 (d, J = 2.2 Hz, 1H), 8.46–8.45 (d, J = 2.2 Hz, 1H), 8.37 (s, 1H), 8.18–8.13 (d, J = 9.0 Hz, 1H), 8.13 (s, 1H), 7.56–7.52 (d, J = 7.6 Hz, 1H), 7.30–7.15 (m, 2H), 3.91 (s, 3H, CH3), 3.88 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 163.1 (s), 162.3 (s), 145.8 (s), 143.6 (d), 136.6 (s), 133.8 (d), 132.1 (d), 131.1 (d), 126.6 (s), 122.1 (d), 121.0 (d), 120.9 (d), 120.3 (s), 112.7 (s), 110.3 (d), 107.2 (s), 105.8 (s), 33.1 (q), 31.6 (q); Anal. Calculated for C19H16BrN5O2 (MW: 426,27): C, 53.54; H, 3.78; N, 16.43%. Found: C, 53.50; H, 3.81; N, 16.39%.
5-bromo-N’-(5-bromo-1-methyl-1H-indole-3-carbonyl)-1-methyl-7-azaindole-3-carbohydrazide (7f). Conditions: r.t. for 1 h, then 60 °C for 6 h; Yield: 82%; white solid; mp: 168 °C; IR (cm−1): 3244 (2 NH), 1730 (2 CO); 1H NMR (200 MHz, DMSO-d6) δ: 10.0 (bs, 1H, NH), 9.93 (bs, 1H, NH), 8.49–8.44 (dd, J = 1.5, 7.9 Hz, 1H), 8.38–8.35 (dd, J = 1.5, 4.7 Hz, 1H), 8.20 (s, 1H), 7.87–7.81 (dd, J = 2.5, 10 Hz, 1H), 7.61–7.54 (dd, J = 4.5, 8.9 Hz, 1H), 7.29–7.23 (dd, J = 4.7, 7.9 Hz, 1H), 7.17–7.07 (td, J = 2.6, 9.2 Hz, 1H), 3.91 (s, 3H, CH3), 3.89 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 163.3 (s), 162.4 (s), 145.7 (s), 143.5 (d), 133.61 (d), 132.2 (d), 130.7 (s), 129.4 (d), 127.3 (s), 120.1 (s), 117.3 (d), 112.4 (s), 110.1 (s), 108.9 (s), 105.9 (d), 105.4 (d), 104.8 (s), 33.4 (q), 31.4 (q); Anal. Calculated for C19H15Br2N5O2 (MW: 505,17): C, 45.17; H, 2.99; N, 13.86%. Found C, 45.12; H, 2.88; N, 13.80%.
5-bromo-N’-(5-fluoro-1-methyl-1H-indole-3-carbonyl)-1-methyl-7-azaindole-3-carbohydrazide (7g). Conditions: r.t. for 3 h, then 60 °C for 24 h. Yield: 85%; brown solid; mp: 189 °C; IR (cm−1): 3420 (NH), 3312 (NH), 1653 (CO), 1616 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 10.08 (bs, H, NH), 9.96 (bs, H, NH), 8.60–8.59 (d, J = 2.1 Hz, 1H), 8.46–8.45 (d, J = 2.2 Hz, 1H), 8.36 (s, 1H), 8.19 (s, 1H), 7.86–7.79 (dd, J = 2.4, 10.0 Hz, 1H), 7.61–7.55 (dd, J = 4.5, 8.9 Hz, 1H), 7.17–7.07 (td, J = 2.5, 9.2 Hz, 1H), 3.90 (s, 3H, CH3), 3.89 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 164.3 (s), 163.6 (s), 159.8 (d, JC-F =114.2 Hz), 146.3 (s), 144.1 (d), 134.4 (s), 134.3 (d, JC-F =20.7 Hz), 133.9 (s), 131.6 (s), 120.3 (s), 120.8 (s), 113.2 (s), 112.4 (d), 112.3 (d), 110.9 (d, JC-F =26.4 Hz), 106.2 (d, JC-F =24.5 Hz), 106.1 (s), 33.9 (q), 32.1 (q); Anal. Calculated for C19H15BrFN5O2 (MW: 444,26): C, 51.37; H, 3.40; N, 15.76%. Found: C, 51.30; H, 3.47; N, 15.79%.
N’-(5-methoxy-1-methyl-1H-indole-3-carbonyl)-1-methyl-7-azaindole-3-carbohydrazide (7h). Conditions: r.t. for 4 h, then 60 °C for 24 h. Yield: 88%; white solid; mp: 198 °C; IR (cm−1): 3231–3101 (2 NH), 1624 (CO), 1541 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 9.94 (bs, 1H, NH), 9.82 (bs, 1H, NH), 8.48–8.45 (d, J = 6.9 Hz, 1H), 8.38–8.36 (d, J = 4.5 Hz, 1H), 8.30 (s, 1H), 8.06 (s, 1H), 7.67–7.66 (d, J = 2.3 Hz, 1H), 7.46–7.42 (d, J = 8.9 Hz, 1H), 7.29–7.23 (dd, J = 4.7, 7.8 Hz, 1H), 6.91–6.85 (dd, J = 2.4, 8.9 Hz, 1H), 3.91 (s, 3H, OCH3), 3.84 (s, 3H, CH3), 3.77 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 162.2 (s), 150.1 (s), 143.5 (d), 132.2 (d), 132.1 (d), 130.2 (s), 129.4 (d), 124.4 (s), 120.1 (s), 117.3 (d), 115.4 (s), 112.2 (d), 111.2 (d), 110.2 (s), 105.6 (s), 102.5 (d), 101.9 (s), 55.2 (q), 32.2 (q), 31.4 (q); Anal. Calculated for C20H19N5O3 (MW: 377,40): C, 63.65; H, 5.07; N, 18.56%. Found: C, 63.55; H, 5.21; N, 18.60%.
3.1.3. General Procedure for the Synthesis of N’-Benzoyl-7-azaindole-3-carbohydrazides (8a–d)
To a solution of the proper acid 12 (2 mmol) in dimethylformamide (10 mL), 1-ethyl-3(3-dimethylaminopropil)carbodiimide (2 mmol), hydroxylbenzotriazole (2 mmol) and diisopropylethylamine (4 mmol) were added. The reaction was stirred for 1 h at room temperature. Then, the proper hydrazine 11 (4 mmol) was added and the reaction mixture was heated at room temperature for 24 h. Once cooled, ice was added and the formed precipitate was filtered off and recrystallized in diethyl ether to afford the pure compounds.
N’-benzoyl-7-azaindole-3-carbohydrazide (8a). Yield: 92%; white solid; mp: 170 °C; IR (cm−1): 3454 (NH), 3377 (NH), 3221 (NH), 1636 (CO), 1558 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 12.27 (bs, 1H, NH), 10.44 (bs, 1H, NH), 10.11 (bs, 1H, NH), 8.45 (d, J = 7.6 Hz, 1H), 8.30 (s, 2H), 7.95 (d, J = 6.8 Hz, 2H), 7.57 (t, J = 8.0 Hz, 3H), 7.22 (dd, J = 7.5, 4.8 Hz, 1H); 13C NMR (50 MHz, DMSO-d6) δ: 166.1 (s), 163.5 (s), 148.3 (s), 143.7 (d), 132.7 (s), 131.8 (d), 129.1 (d), 128.6 (d), 128.5 (dx2), 127.4 (dx2), 118.5 (s), 117.2 (d), 107.1 (s); Anal. Calculated for C15H12N4O2 (MW: 280,29): C, 64.28; H, 4.32; N, 19.99%. Found C, 64.32; H, 4.45; N, 19.80%.
N’-benzoyl-1-methyl-7-azaindole-3-carbohydrazide (8b). Yield: 94%; white solid; mp: 174 °C; IR (cm−1): 3219–3125 (2 NH), 1663 (CO), 1636 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 10.44 (bs, 1H, NH), 10.16 (bs, 1H, NH), 8.58 (d, J = 1.5 Hz, 1H), 8.34 (s, 1H), 7.94 (s, 1H), 7.91 (d, J = 1.5 Hz, 2H), 7.60–7.58 (m, 1H), 7.54–7.51 (m, 3H), 3.89 (s, 3H, CH3); Anal. Calculated for C16H14N4O2 (MW: 294,31): C, 65.30; H, 4.79; N, 19.04%. Found C, 65.41; H, 4.82; N, 19.00%.
N’-benzoyl-5-bromo-7-azaindole-3-carbohydrazide (8c). Yield: 92%; white solid; mp: 205 °C; IR (cm−1): 3458 (NH), 3184 (NH), 3098 (NH), 1628 (CO), 1558 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 12.52 (bs, 1H, NH), 10.43 (bs, 1H, NH), 10.16 (bs, 1H, NH), 8.57 (d, J = 2.0 Hz, 1H), 8.39 (d, J = 2.0 Hz, 1H), 8.34 (s, 1H), 7.93 (d, J = 7.4 Hz, 2H), 7.60–7.51 (m, 3H); 13C NMR (50 MHz, DMSO-d6) δ: 166.6 (s), 147.2 (s), 144.4 (s), 133.2 (s), 132.3 (d), 131.4 (d), 130.8 (d), 129.0 (dx2), 127.9 (dx2), 127.8 (d), 120.7 (s), 113.0 (s), 107.3 (s); Anal. Calculated for C15H11BrN4O2 (MW: 359,18): C, 50.16; H, 3.09; N, 15.60%. Found C, 50.26; H, 3.21; N, 15.64%.
N’-benzoyl-5-bromo-1-methyl-7-azaindole-3-carbohydrazide (8d). Yield: 95%; white solid; mp: 180 °C; IR (cm−1): 3221 (NH), 3098 (NH), 1707 (CO), 1629 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 1H NMR (200 MHz, DMSO-d6) δ: 10.47 (bs, 1H, NH), 10.21 (bs, 1H, NH), 8.59 (s, 1H), 8.47 (s, 1H), 8.36 (s, 1H), 7.94 (d, J = 7.4 Hz, 3H), 7.56 (d, J = 7.5 Hz, 2H), 3.91 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d6) δ: 166.5 (s), 163.4 (s), 146.3 (s), 144.2 (d), 138.9 (s), 134.5 (d), 132.3 (d), 131.6 (d), 129.0 (dx2), 127.9 (dx2), 120.7 (s), 113.3 (s), 106.1 (s), 32.1 (q); Anal. Calculated for C16H13BrN4O2 (MW: 373,21): C, 51.49; H, 3.51; N, 15.01%. Found C, 51.55; H, 3.61; N, 15.23%.
3.1.4. General Procedure for the Synthesis of 3-[5-(1-Methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-7-azaindoles (3a–g)
The solution of the proper intermediate 7 (0.81 mmol) in phosphoryl chloride (5.4 mL) was heated at 45 °C for 24 or 48 h or under reflux for 3–6 h. Once cooled, the reaction mixture was evaporated in vacuo and extracted with a saturated solution of sodium hydrogen carbonate (3 × 30 mL) and ethyl acetate (3 × 30 mL). The organic layers were evaporated in vacuo and dried with sodium sulphate. The resulting crude material was purified in a column using dichloromethane/methanol 99:1 as eluent.
3-[5-(1-methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-7-azaindole (3a). Conditions: reflux for 4 h. Yield 75%; white solid; mp: 302 °C; IR (cm−1): 3117 (NH); 1H NMR (200 MHz, DMSO-d6) δ: 12.57 (s, 1H, NH), 8.53 (dd, J = 7.9, 1.6 Hz, 1H, Ar-H), 8.41 (dd, J = 4.7, 1.6 Hz, 1H, Ar-H), 8.37 (d, J = 2.4 Hz, 1H, Ar-H), 8.29 (s, 1H, Ar-H), 8.23–8.20 (m, 1H, Ar-H), 7.37–7.32 (m, 2H, Ar-H), 7.32–7.28 (m, 2H, Ar-H), 3.93 (s, 3H, CH3); Anal. Calculated for C18H13N5O (MW: 315,34): C, 68.56; H, 4.16; N, 22.21%. Found: C, 68.59; H, 4.21; N, 22.15%.
1-methyl-3-[5-(1-methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-7-azaindole (3b). Conditions: reflux for 6 h. Yield 86%; white solid, mp: 239 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.55–8.45 (m, 3H, 3 Ar-H), 8.30 (s, 1H, Ar-H), 8.23–8.19 (d, J = 7.3 Hz, 1H, Ar-H), 7.65–7.61 (d, J = 8.1 Hz, 1H, Ar-H), 7.51–7.37 (m, 3H, 3 Ar-H), 3.96 (s, 3H, CH3), 3.94 (s, 3H, CH3); Anal. Calculated for C19H15N5O (MW: 329,36): C, 69.29; H, 4.59; N, 21.26%. Found: C, 71.29; H, 4.69; N, 21.20%.
3-[5-(5-fluoro-1-methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-1-methyl-7-azaindole (3c). Conditions: 45 °C for 48 h. Yield 85%; white solid; mp: 268 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.56–8.53 (d, J = 7.1 Hz, 2H, 2 Ar-H), 8.48–8.46 (d, J = 4.0 Hz, 1H, Ar-H), 8.39 (s, 1H, Ar-H), 7.90–7.85 (d, J = 9.4 Hz, 1H, Ar-H), 7.71–7.65 (dd, J = 4.4, 8.9 Hz, 1H, Ar-H), 7.42–7.35 (dd, J = 5.1, 7.6 Hz, 1H, Ar-H), 7.28–7.24 (d, J = 8.8 Hz, 1H, Ar-H), 3.97 (s, 6H, 2 CH3); Anal. Calculated for C19H14FN5O (MW: 347,35): C, 65.70; H, 4.06; N, 20.16%. Found: C, 65.75; H, 4.13; N, 20.26%.
3-[5-(5-bromo-1-methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-1-methyl-7-azaindole (3d). Conditions: reflux for 3 h. Yield 86%; white solid; mp: 277 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.54 (dd, J = 7.9, 1.2 Hz, 1H, Ar-H), 8.47 (d, J = 2.9 Hz, 2H, 2 Ar-H), 8.37 (s, 1H, Ar-H), 8.31 (d, J = 1.7 Hz, 1H, Ar-H), 7.64 (d, J = 8.8 Hz, 1H, Ar-H), 7.49 (dd, J =1.8, 8.7 Hz, 1H, Ar-H), 7.38 (dd, J = 4.7, 7.8 Hz, 1H, Ar-H), 3.97 (s, 3H, CH3), 3.95 (s, 3H, CH3); Anal. Calculated for C19H14BrN5O (MW: 408,26): C, 55.90; H, 3.46; N, 17.15%. Found: C, 55.87; H, 3.51; N, 17.27%.
5-bromo-1-methyl-3-[5-(1-methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-7-azaindole (3e). Conditions: 45 °C for 24 h. Yield 78%; white solid; mp:244 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.62–8.61 (d, J = 2.2 Hz, 1H, Ar-H), 8.54–8.53 (d, J = 2.3 Hz, 2H, 2 Ar-H), 8.29 (s, 1H, Ar-H), 8.22–8.17 (m, 1H, Ar-H), 7.65–7.61 (dd, J = 2.1, 6.1 Hz, 1H, Ar-H), 7.40–7.32 (m, 2H, 2 Ar-H), 3.95 (s, 6H, 2 CH3); Anal. Calculated for C19H14BrN5O (MW: 408,26): C, 55.90; H, 3.46; N, 17.15%. Found: C, 55.81; H, 3.57; N, 17.23%.
5-bromo-3-[5-(5-bromo-1-methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-1-methyl-7-azaindole (3f). Conditions: reflux for 5 h. Yield 90%; white solid; mp: 275 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.62 (s, 1H, Ar-H), 8.55 (s, 2H, 2 Ar-H), 8.37 (s, 1H, Ar-H), 8.31 (s, 1H, Ar-H), 7.65 (d, J = 8.6 Hz, 1H, Ar-H), 7.50 (d, J = 8.6 Hz, 1H, Ar-H), 3.96 (s, 6H, 2CH3); Anal. Calculated for C19H13Br2N5O (MW: 487,16): C, 46.85; H, 2.69; N, 14.38%. Found: 46.93; H, 2.75; N, 14.52%.
5-bromo-3-[5-(5-fluoro-1-methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-yl]-1-methyl-7-azaindole (3g). Conditions: r.t. for 24 h. Yield 45%; beige solid mp: 310 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.69–8.51 (m, 1H, Ar-H); 8.50–8.28 (m, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 7.65 (dd, J = 10.0, 2.4 Hz, 2H, 2 Ar-H), 7.56 (dd, J = 8.9, 4.4 Hz, 1H, Ar-H), 7.11 (td, J = 9.2, 2.3 Hz, 1H, Ar-H), 3.95 (s, 3H, CH3), 3.85 (s, 3H, CH3); Anal. Calculated for C19H13BrFN5O (MW: 426,25): C, 53.54; H, 3.07; N, 16.43%. Found: C, 53.45; H, 3.16; N, 16.51%.
3.1.5. General Procedure for the Synthesis of 3-(5-Phenyl-1,3,4-oxadiazol-2-yl)-7-azaindoles (4a–d)
The solution of the proper intermediate 8 (0.81 mmol) in phosphoryl chloride (5.4 mL) was heated under reflux for 3–6 h. Once cooled, the reaction mixture was evaporated in vacuo and extracted with a saturated solution of sodium hydrogen carbonate (3 × 30 mL) and ethyl acetate (3 × 30 mL). The organic layers were evaporated in vacuo and dried with sodium sulfate. The resulting crude material was purified in column using dichloromethane/methanol 99:1 ad eluent.
3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-azaindole (
4a). Conditions: reflux for 3 h. Yield 70%; white solid; mp272 °C; Spectroscopic data in accordance with [
24].
Anal. Calculated for C
15H
10N
4O (MW: 262,27): C, 68.69; H, 3.84; N, 21.36%. Found C, 68.78; H, 3.90; N, 21.61%.
1-methyl-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-azaindole (4b). Conditions: reflux for 4 h. Yield 75%; white solid; mp: 201 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.56 (s, 1H, Ar-H), 8.52 (dd, J = 7.9, 1.4 Hz, 1H, Ar-H), 8.47 (d, J = 4.7 Hz, 1H, Ar-H), 8.15 (d, J = 2.2 Hz, 1H, Ar-H), 8.11 (d, J = 3.4 Hz, 1H, Ar-H), 7.67–7.62 (m, 3H, Ar-H), 7.38 (dd, J = 4.7, 7.9 Hz, 1H, Ar-H), 3.96 (s, 3H, CH3); Anal. Calculated for C16H12N4O (MW: 276,30): C, 69.55; H, 4.38; N, 20.28%. Found C, 69.66; H, 4.51; N, 20.41%.
5-bromo-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-azaindole (4c). Conditions: reflux for 3 h. Yield 55%; beige solid; mp: 275 °C; IR (cm−1): 3112 (NH); 1H NMR (200 MHz, DMSO-d6) δ: 12.91 (s, 1H, NH), 8.53 (dd, J = 10.2, 7.7 Hz, 3H, 3 Ar-H), 8.12 (d, J = 3.6 Hz, 2H, 2 Ar-H), 7.64 (d, J = 2.2 Hz, 3H, 3 Ar-H); Anal. Calculated for C15H9BrN4O (MW: 341,17): C, 52.81; H, 2.66; N, 16.42%. Found C, 52.85; H, 2.60; N, 16.40%.
5-bromo-1-methyl-3-(5-phenyl-1,3,4-oxadiazol-2-yl)-7-azaindole (4d). Conditions: reflux for 6 h. Yield 60%; white solid; mp: 236 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.62 (d, J = 5.0 Hz, 2H), 8.56 (s, 1H, Ar-H), 8.13 (s, 2H, 2 Ar-H), 7.67 (s, 3H, 3 Ar-H), 3.95 (s, 3H, CH3); Anal. Calculated for C16H11BrN4O (MW: 355,20): C, 54.10; H, 3.12; N, 15.77%. Found C, 54.10; H, 3.12; N, 15.77%.
3.1.6. General Procedure for the Synthesis of 3-[5-(1-Methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-7-azaindoles (5a–h)
To a solution of the proper intermediate 7 (0.38 mmol) in pyridine (15 mL), Lawesson’s reagent (0.41 mmol) was added and the reaction mixture was heated under reflux for 4–48 h. The mixture was evaporated in vacuo and the crude material was purified in a chromatography column using dichloromethane/methanol 99:1 as eluent.
3-[5-(1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-7-azaindole (5a). Conditions: reflux for 24 h. Yield 78%; beige solid; mp: 246 °C; IR (cm−1): 3171 (NH); 1H NMR (200 MHz, DMSO-d6) δ: 12.58 (s, 1H, NH), 8.53 (dd, J = 7.9, 1.5 Hz, 1H, Ar-H), 8.42–8.36 (m, 2H, 2 Ar-H), 8.30 (s, 1H, Ar-H), 8.23–8.18 (m, 1H, Ar-H), 7.60 (t, J = 9.3 Hz, 1H, Ar-H), 7.39–7.28 (m, 3H, 3 Ar-H), 3.94 (s, 3H, CH3); Anal. Calculated for C18H13N5S (MW: 331,40): C, 65.24; H, 3.95; N, 21.13%. Found: C, 65.41; H, 3.71; N, 21.32%.
1-methyl-3-[5-(1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-7-azaindole (5b). Conditions: reflux for 6 h. Yield 99%; white solid; mp: 244 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.61 (d, J = 7.6 Hz, 1H, Ar-H), 8.44 (d, J = 4.5 Hz, 1H, Ar-H), 8.40 (s, 1H, Ar-H), 8.27 (dd, J = 6.1, 2.1 Hz, 1H, Ar-H), 8.22 (s, 1H, Ar-H), 7.60 (d, J = 6.7 Hz, 1H, Ar-H), 7.41–7.25 (m, 3H, 3 Ar-H), 3.93 (s, 3H, CH3), 3.91 (s, 3H, CH3); Anal. Calculated for C19H15N5S (MW: 345,42): C, 66.07; H, 4.38; N, 20.28%. Found C, 66.23; H, 4.42; N, 20.33; S, 9.35%.
3-[5-(5-bromo-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-1-methyl-7-azaindole (5c). Conditions: reflux for 24 h. Yield 78%; beige solid; mp: 292 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.56–8.44 (m, 3H, 3 Ar-H), 8.38 (s, 1H, Ar-H), 8.32–8.29 (m, 1H, Ar-H), 7.71–7.63 (m, 1H, Ar-H), 7.52–7.47 (dd, J =1.8, 8.8 Hz, 1H, Ar-H), 7.42–7.36 (dd, J =4.5, 7.8 Hz, 1H, Ar-H), 3.97 (s, 3H, CH3), 3.96 (s, 3H, CH3); Anal. Calculated for C19H14BrN5S (MW: 424,32): C, 53.78; H, 3.33; N, 16.51%. Found C, 53.81; H, 3.40; N, 16.72%.
3-[5-(5-fluoro-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-1-methyl-7-azaindole (5d). Conditions: reflux for 6 h. Yield 60%; white solid; mp:240 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.62–8.58 (dd, J = 1.5, 7.9 Hz, 1H, Ar-H), 8.46–8.42 (dd, J = 1.5, 4.7 Hz, 1H, Ar-H), 8.41 (s, 1H, Ar-H), 8.29 (s, 1H, Ar-H), 8.00–7.94 (dd, J = 2.5, 9.8 Hz, 1H, Ar-H), 7.67–7.61 (dd, J = 4.5, 8.9 Hz, 1H, Ar-H), 7.39–7.33 (dd, J = 4.7, 7.9 Hz, 1H, Ar-H), 7.26–7.16 (td, J = 2.5, 9.2, 9.2 Hz, 1H, Ar-H), 3.93 (s, 3H, CH3), 3.92 (s, 3H, CH3); Anal. Calculated for C19H14FN5S (MW: 363,41): C, 62.80; H, 3.88; N, 19.27%. Found: C, 62.89; H, 3.67; N, 19.31%.
3-[5-(5-methoxy-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-1-methyl-7-azaindole (5e). Conditions: reflux for 4 h. Yield 60%; white solid; mp: 200 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.63–8.37 (m, 3H, 3 Ar-H), 8.21–8.12 (m, 1H, Ar-H), 7.77–7.64 (m, 1H, Ar-H), 7.54–7.47 (dd, J = 5.8, 8.9 Hz, 1H, Ar-H), 7.40–7.31 (dt, J = 7.9, 3.9 Hz, 1H, Ar-H), 7.00–6.93 (dt, J = 2.9, 5.8 Hz, 1H, Ar-H), 3.95 (s, 3H, OCH3), 3.90 (s, 3H, CH3), 3.87 (s, 3H, CH3); Anal. Calculated for C20H17N5OS (MW: 375,45): C, 63.98; H, 4.56; N, 18.65%. Found C, 63.98; H, 4.56; N, 18.65%.
5-bromo-1-methyl-3-[5-(1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-7-azaindole (5f). Conditions: reflux for 6 h. Yield 70%; beige solid; mp: 233 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.74–8.73 (d, J = 2.2 Hz, 1H, Ar-H), 8.55–8.51 (dd, J = 2.2, 5.4 Hz, 1H, Ar-H), 8.47 (s, 1H, Ar-H), 8.30–8.23 (m, 2H, 2 Ar-H), 7.64–7.58 (m, 1H, Ar-H), 7.38–7.29 (m, 2H, 2 Ar-H), 3.95 (s, 3H, CH3), 3.91 (s, 3H, CH3); Anal. Calculated for C19H14BrN5S (MW: 424,32): C, 53.78; H, 3.33; N, 16.51%. Found C, 53.81; H, 3.40; N, 16.62%.
5-bromo-3-[5-(5-bromo-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-1-methyl-7-azaindole (5g). Conditions: reflux for 48 h. Yield 50%; beige solid; mp: 275 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.60 (d, J = 2.1 Hz, 1H, Ar-H), 8.52 (t, J = 5.6 Hz, 2H, 2 Ar-H), 8.35 (s, 1H, Ar-H), 8.28 (s, 1H, Ar-H), 7.61 (t, J = 7.4 Hz, 1H, Ar-H), 7.52–7.39 (m, 1H, Ar-H), 3.95 (s, 3H, CH3), 3.91 (s, 3H, CH3); Anal. Calculated for C19H13Br2N5S (MW: 503,22): C, 45.35; H, 2.60; N, 13.92%. Found C, 45.45; H, 2.65; N, 13.99%.
5-bromo-3-[5-(5-fluoro-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-1-methyl-7-azaindole (5h). Conditions: reflux for 6 h. Yield 80%; white solid; mp: 290 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.59–8.45 (m, 3H, 3 Ar-H), 8.28 (s, 1H, Ar-H), 7.97–7.81 (qd, J = 2.3, 9.5 Hz, 1H, Ar-H), 7.68–7.59 (m, 1H, Ar-H), 7.25–7.16 (m, 1H, Ar-H), 3.94 (s, 3H, CH3), 3.90 (s, 3H, CH3); Anal. Calculated for C19H13BrFN5S (MW: 442,31): C, 51.59; H, 2.96; N, 15.83%. Found C, 51.78; H, 2.85; N, 15.89%.
3.1.7. General Procedure for the Synthesis of 3-[5-(1-Methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-yl]-7-azaindoles (6a–d)
To a solution of the proper intermediate 8 (0.38 mmol) in pyridine (15 mL), Lawesson’s reagent (0.41 mmol) was added, and the reaction mixture was heated under reflux for 24–48 h. The mixture was evaporated in vacuo and the crude material was purified in a chromatography column using dichloromethane/methanol 99:1 as eluent.
3-(5-phenyl-1,3,4-thiadiazol-2-yl)-7-azaindole (6a). Conditions: reflux for 24 h. Yield: 78%; white solid; mp: 249 °C; IR (cm−1): 3111 (NH); 1H NMR (200 MHz, DMSO-d6) δ: 12.56 (s, 1H, NH), 8.61–8.57 (dd, J = 1.4, 7.9 Hz, 1H, Ar-H), 8.41 (s, 1H, Ar-H), 8.39–8.38 (d, J = 1.4 Hz, 1H, Ar-H), 8.03–7.98 (m, 2H, 2 Ar-H), 7.60 (s, 1H, Ar-H), 7.58–7.57 (d, J = 2.7 Hz, 2H, 2 Ar-H), 7.35–7.29 (dd, J = 4.7, 7.9 Hz, 1H, Ar-H); Anal. Calculated for C15H10N4S (MW: 278,33): C, 64.73; H, 3.62; N, 20.13%. Found C, 64.80; H, 3.91; N, 20.25%.
1-methyl-3-(5-phenyl-1,3,4-thiadiazol-2-yl)-7-azaindole (6b). Conditions: reflux for 24 h. Yield: 95%; white solid; mp: 191 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.71–8.70 (d, J = 2.2 Hz, 1H, Ar-H); 8.54 (s, 1H, Ar-H), 8.52–8.51 (d, J = 2.2 Hz, 1H, Ar-H), 8.06–7.99 (m, 2H, 2 Ar-H), 7.70–7.69 (d, J = 2.1 Hz, 1H, Ar-H), 7.60–7.57 (t, J = 3.2 Hz, 3H, 3 Ar-H), 3.91 (s, 3H, CH3); Anal. Calculated for C16H12N4S (MW: 292,36): C, 65.73; H, 4.14; N, 19.16%. Found C, 65.65; H, 4.20; N, 19.31%.
5-bromo-3-(5-phenyl-1,3,4-thiadiazol-2-yl)-7-azaindole (6c). Conditions: reflux for 24 h. Yield: 60%; white solid; mp: 308 °C; IR (cm−1): 3111 (NH); 1H NMR (200 MHz, DMSO-d6) δ: 12.68 (s, 1H, NH), 8.73–8.72 (d, J = 2.2 Hz, 1H, Ar-H), 8.49 (s, 1H, Ar-H), 8.47–8.46 (d, J = 2.2 Hz, 1H, Ar-H), 8.02–8.01 (d, J = 3.5 Hz, 1H, Ar-H), 7.99–7.98 (d, J = 2.5 Hz, 1H, Ar-H), 7.60–7.57 (t, J = 3.2 Hz, 3H, 3 Ar-H); Anal. Calculated for C15H9BrN4S (MW: 357,23): C, 50.43; H, 2.54; N, 15.68%. Found C, 50.49; H, 2.40; N, 15.77%.
5-bromo-1-methyl-3-(5-phenyl-1,3,4-thiadiazol-2-yl)-7-azaindole (6d). Conditions: reflux for 48 h. Yield: 82%; beige solid; mp: 188 °C; 1H NMR (200 MHz, DMSO-d6) δ: 8.73–8.72 (d, J = 2.2 Hz, 1H, Ar-H), 8.57 (s, 1H, Ar-H), 8.54–8.53 (d, J = 2.3 Hz, 1H, Ar-H), 8.04–8.03 (d, J = 3.3 Hz, 1H, Ar-H), 8.00–7.99 (d, J = 2.5 Hz, 1H, Ar-H), 7.61–7.58 (t, J = 3.3 Hz, 3H, 3 Ar-H), 3.92 (s, 3H, CH3); Anal. Calculated for C16H11BrN4S (MW: 371,26): C, 51.76; H, 2.99; N, 15.09%. Found C, 51.70; H, 2.79; N, 15.25%.