Abstract
In reviewing a selection of recent case studies from our laboratory, we revealed some lessons learned and benefits accrued from the application of mass spectrometry (MS/MS) molecular networking in the field of marine sponge natural products. Molecular networking proved pivotal to our discovery of many new natural products and even new classes of natural product, some of which were opaque to alternate dereplication and prioritization strategies. Case studies included the discovery of: (i) trachycladindoles, an exceptionally rare class of bioactive indole alkaloid previously only known from a single southern Australia sample of Trachycladus laevispirulifer; (ii) dysidealactams, an unprecedented class of sesquiterpene glycinyl-lactam and glycinyl-imide from a Dysidea sp., a sponge genera often discounted as having been exhaustively studied; (iii) cacolides, an unprecedented family of sesterterpene α-methyl-γ-hydroxybutenolides from a Cacospongia sp., all too easily mischaracterized and deprioritized during dereplication as a well-known class of sponge sesterterpene tetronic acids; and (iv) thorectandrins, a new class of indole alkaloid which revealed unexpected insights into the chemical and biological properties of the aplysinopsins, one of the earliest and more extensively reported class of sponge natural products.
1. Introduction
Marine sponges are a well-established source of structurally diverse natural products, many with no biosynthetic counterparts in other life forms, whether marine or terrestrial, plant, animal, or microbial [1]. Over a period spanning several decades, our laboratory investigated the chemistry of many southern Australian marine sponges, leading to the discovery of numerous new natural products. Notwithstanding past successes, in recent years the field of marine sponge natural products research experienced a slow-down in reports of new natural products. There are several factors that might contribute to this decline, a key one of which is the challenge of dereplication–where an inability to rapidly and cost effectively detect and prioritize new over known and rare over common natural products results in the rediscovery of known chemistry and an overall decrease in productivity. Over the years, natural products researchers relied on a number of dereplication strategies with varying levels of success. For example, in the early days, thin layer chromatography (TLC) was used extensively, evolving over time to normal and then reversed phase high-performance liquid chromatography (HPLC) and, in time, to ultra-high performance liquid chromatography (UPLC), with columns featuring ever smaller particle sizes and resolving power. Chromatographic methods also benefited from an evolution in detectors ranging from refractive index (RI) to single wavelength ultra-violet (UV-vis), diode array (DAD), evaporative light scattering (ELS), and electro-spray mass spectrometry (ESI-MS). In time, mass spectrometric detectors advanced in both sensitivity and accuracy (UPLC-DAD-QTOF), with data analysis further enabled by such techniques as single ion extraction (SIE) and tandem mass spectrometry (MSn). With dereplication based on sophisticated mass spectrometric analysis generating an almost bewildering wealth of data, it was a logical (albeit challenging) next step to develop innovative computational tools to process and visualise these data to more easily and rapidly interrogate very large data sets incorporating many 1000s even 10,000s of compounds. Importantly, these mass spectrometric approaches required only a few μg of crude extract (or fractions or pure compounds) with individual data acquisitions taking only a few minutes and at minimal cost. In recent years, one of the more popular mass-spectrometry-based dereplication methodologies is that of Global Natural Products Social (GNPS) molecular networking [2].
Since its emergence in 2016 [3], GNPS molecular networking has been widely used by marine natural products researchers; for instance, in 2017 Crusemann et al. applied GNPS molecular networking to prioritize 146 marine Salinispora and Streptomyces strains, which revealed 15 natural products families and enabled identification of new analogues including metabolite production patterns and efficient growth and extraction conditions [4]; in 2020 Mangoni et al. applied GNPS to discover a new proline-rich cyclic heptapeptide stylissamide L from a well-studied marine sponge Stylissa caribica [5], while Keyzers et al. applied GNPS molecular networking to isolate new rubrolide analogues from the New Zealand marine tunicate Synoicum kuranui [6]. Our own laboratory also used GNPS on multiple occasions, including during investigations into mullet fish gut-derived fungi which lead to: new lipodepsipeptide scopularides from Scopulariopsis spp. CMB-F458 and CMB-F115, and Beauveria sp. CMB-F585 [7]; new P-glycoprotein inhibitory phenyl propanoid piperazine alkaloid chrysosporazines from Aspergillus sp. CMB-F661, Spiromastix sp. CMB-F455, Chrysosporium sp. CMB-F214, and Chrysosporium sp. CMB-F294 [8,9,10]; and new polyketide amaurones from Amauroascus sp. CMB-F713 [11].
In this perspective we seek to demonstrate through a selection of case studies how we successfully applied GNPS molecular networking to breathe new life into a library of 960 southern Australian marine extracts (predominantly sponges). This library was the focus of extensive study in our group for over 30 years, but in recent years it was largely sidelined due to a perception it was a near-exhausted resource. As demonstrated below, the acquisition and analysis of a GNPS molecular network on this library revealed a wealth of new opportunities, with an investigation of just a few of these newly revealed opportunities prompting discoveries that might otherwise have been overlooked. These include, for example, an alternate source and new exemplars of the exceptionally rare class of highly selective kinase inhibitory alkaloid, trachycladindoles from Geodia sp. (CMB-001063) [12]; an unprecedented class of sesquiterpene glycinyl lactam and imide, dysidealactams from Dysidea sp. (CMB-01171) [13]; a new class of sesterterpene featuring an unprecedented α-methyl-γ-hydroxybutenolide, cacolides from Cacospongia sp. [14]; and a new class of indole alkaloid, thorectandrin A from Thorectandra choanoides (CMB-01889) [15].
2. Acquiring a GNPS Molecular Network of a Marine Library
2.1. Preparing a Marine Extract Library
Over a period of 35+ years our laboratory assembled a marine natural products biodiscovery resource consisting of >3000 marine invertebrates and macroalgae sourced from locations across southern Australia and Antarctica. This marine collection proved to be a valuable resource for marine natural products research, informed by an array of bioassays across human and animal health, and crop protection (i.e., targeting such indications as anti-infective and neurodegenerative diseases, cancer, pain, etc.), as well as unusual chemical parameters detected using an evolving array of dereplication technologies (i.e., TLC, HPLC-RI-UV, HPLC-DAD, HPLC-ESI(±)MS, and NMR spectroscopy). In total ~15% of the library was subjected to detailed chemical analysis, resulting in the discovery of many hundreds of novel marine natural products, some with promising (even commercially significant) biological properties. Notwithstanding this success, this left ~85% of the collection designated of lesser (no?) interest. Given the propensity of marine organisms to produce chemical defences this deprioritization was likely an artifact of less than optimal dereplication methods. We were therefore intrigued to observe the emergence of GNPS molecular networking, rationalising that this methodology may hold the means to better assess and hopefully reprioritize some of our stranded marine extracts. Once we had secured access to a suitable UPLC-QTOF mass spectrometer we elected to carry out a pilot study to better understand this dereplication paradigm, applying GNPS molecular networking to a selection of southern Australian marine extracts.
A library of 960 marine extracts (predominantly sponges) was chosen, as it could be conveniently accommodated in 10 × 96 well plates. Our selection also included several marine sponge extracts that we previously studied and for which we had knowledge of their natural products (Table 1). To support dereplication of these known natural product classes we also cross-correlated with a separate library of 95 pure authentic marine natural products. In total our library of 960 extracts were derived from 409 sponges with taxonomy to at least the level of genus (Table 2), along with a further 551 taxonomically unidentified samples, comprising 384 sponges, 49 tunicates, and 118 macroalgae. All samples were collected over the last 35 years by either intertidal (0–1 m), near shore SCUBA (5–20 m), or commercial trawling or scientific benthic sleds operations (20–500 m), from locations across the southern Australian coast, south to Antarctic waters. Soon after collection all samples were individually bagged and frozen (or placed on ice) for transport to the laboratory, where they were diced and stored (extracted) in sealed Nalgene bottles in 10% water/EtOH at −20 °C (for up to 35 years).

Table 1.
Marine sponges known to producing different classes of natural product.

Table 2.
Taxonomically identified sponges (409/960 specimens).
In order to carry out our GNPS analyses, fresh individual aliquots (7 mL) dispensed from the 10% water/EtOH extracts of 960 samples were concentrated to dryness in vacuo at 45 °C, after which they were partitioned between n-BuOH (2 mL) and water (2 mL). Aliquots (1 mL) of both the n-BuOH and water phases were transferred to deep 96-well plates to generate a set of stock extract plates, which were stored at –30 °C. Aliquots (100 μL) of the n-BuOH solubles were transferred to a second set of deep 96-well plates, after which they were dried under nitrogen gas at 40 °C and redissolved in DMSO (1 mL) to prepare a 10-fold dilution daughter plates to be used for GNPS analysis. All plates were stored at –30 °C in the dark until required.
2.2. Preparing a GNPS Molecular Network
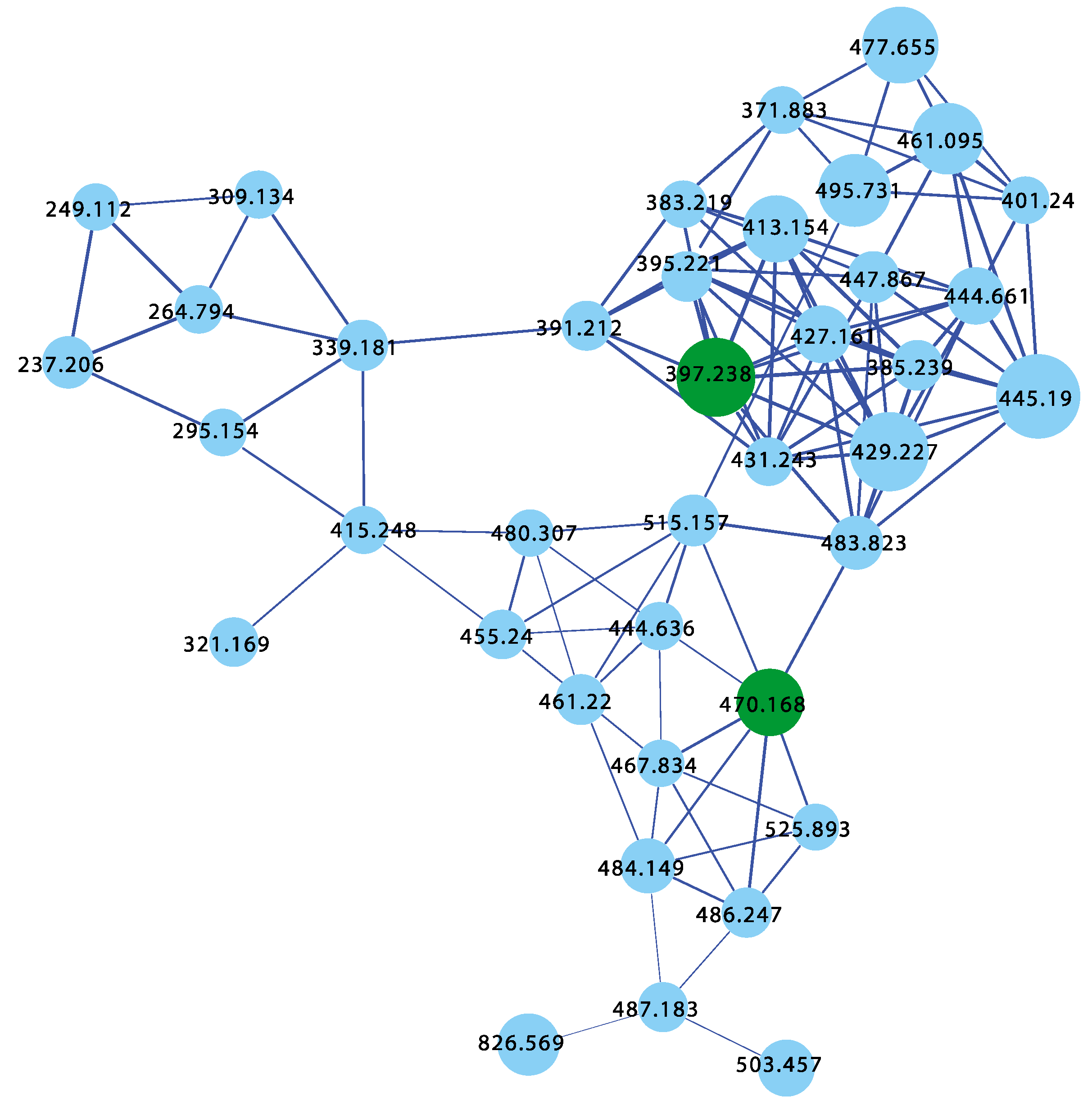
Aliquots (0.1 μL) of the daughter plates (960 extracts and 95 authentic pure marine natural products) were analysed on an Agilent 6545 QTOF LC/MS equipped with an Agilent 1290 infinity II UHPLC system, utilising an Agilent SB-C8 1.8 μm, 2.1 × 50 mm column, with a 0.5 mL min−1 gradient elution over 4.5 min from 90% H2O/CH3CN to CH3CN, followed by isocratic elution with CH3CN for 1 min, with a constant isocratic 0.1% formic acid/CH3CN modifier. The UPLC-QTOF(+)MS/MS data acquired for all samples at a fixed collision energy of 40 eV were converted from Agilent MassHunter data files (.d) into mzXML file format, and transferred to the GNPS server (gnps.ucsd.edu). Molecular networking was performed using the GNPS data analysis workflow using the spectroscopic clustering algorithm, and a cosine score of 0.7 and a minimum of six matched peaks. The resulting spectroscopic networks were imported into Cytoscape version 3.5.1, where nodes represented parent m/z and edge thickness corresponded to cosine scores, for a network featuring ~43,000 nodes, and hundreds of clusters (Figure 1). Analysis of the GNPS cluster revealed the presence of new analogues of known chemical scaffolds, as well as unprecedented new classes of compounds.

Figure 1.
GNPS molecular network for 960 marine extracts (blue nodes) including marine sponge extracts containing known chemistry (red nodes), superimposed over 95 authentic pure natural product standards (green nodes). See the Supplementary Materials for a higher resolution image.
3. Detecting New Analogues of Known Chemistry
Analysis of GNPS molecular networking (Figure 1) revealed several molecular families (Figures 2–8), where the pure authentic samples co-clustered with the original producing organisms, validating the use of GNPS to detect the presence of known compounds and their analogues in a large collection of extracts. Exemplars of these molecular families are discussed herein, with a brief introduction to their original isolations from the producing organisms and their biological activities.
3.1. Franklinolides
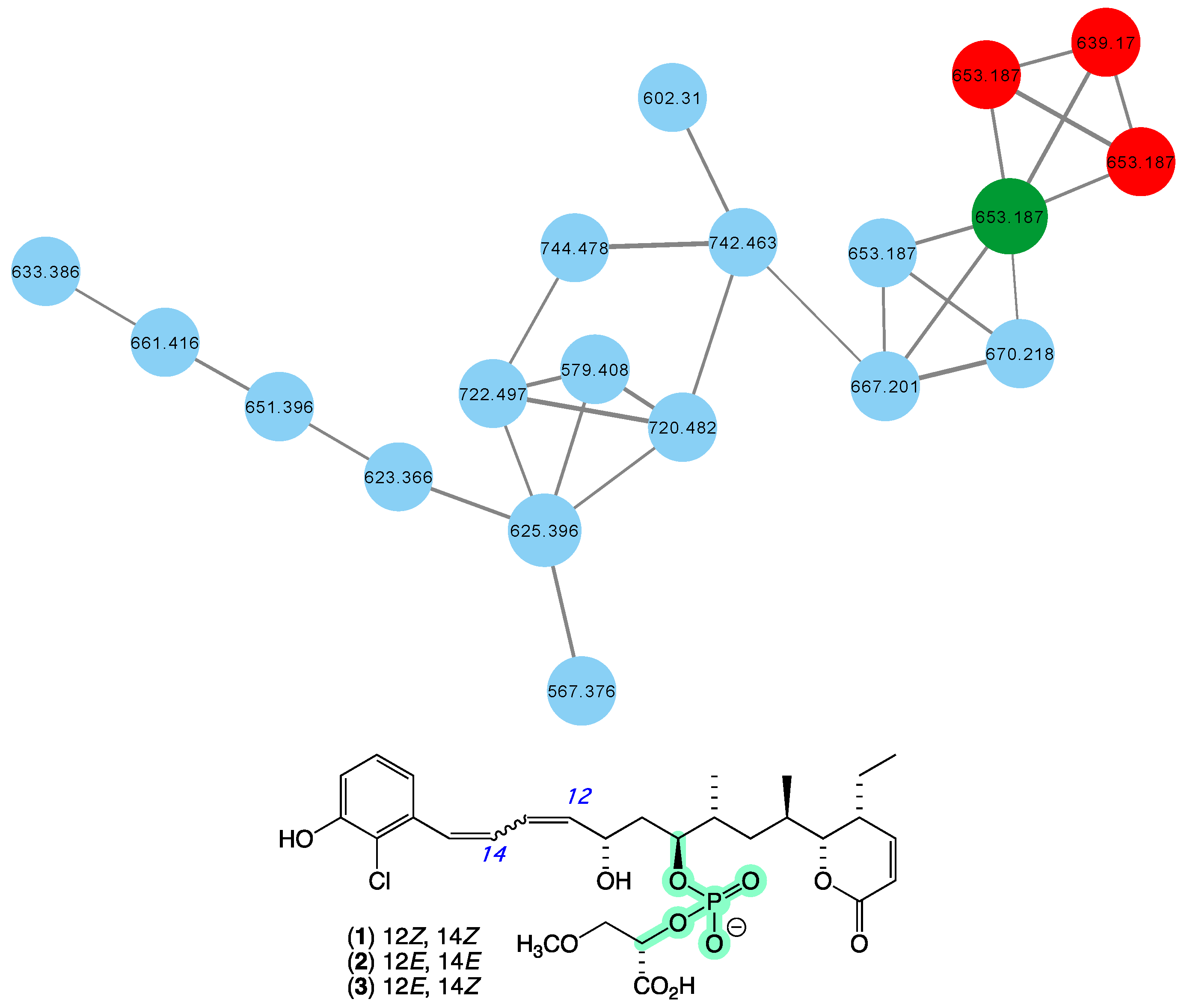
In 2010 [16], we reported franklinolides A–C (1–3) (Figure 2) from a marine sponge-complex (CMB-01989) consisting of a massive Geodia sp. thinly encrusted with a Halichondria sp., collected in 1995 during deep water (−105 m) scientific trawling operations in the Great Australian Bight. The franklinolides were the first reported natural occurrence of polyketide phosphodiesters, and inhibited the growth of a range of human carcinoma cells, including colon (HT-29), prostate (DU145), ovary (JAM and C180-13S), and lung (A549). With the exception of a 2016 re-isolation of franklinolide A (1) from a Caribbean sponge Plakina jamaicensis [27], no other members of this structure class have been reported, with a 2022 review on natural phosphate esters reinforced the rarity of natural phosphodiesters [28]. While traditional dereplication approaches failed to detect alternate sources of franklinolides in our marine library, the GNPS molecular network shown in Figure 2 revealed a family of nodes clustered around the authentic natural product franklinolide A (1) (dark green) and analogues in the original CMB-01989 extract (red), associated with new nodes (blue) present in two unidentified sponges (CMB-01451 and CMB-01518) collected in 1993 by SCUBA (−15 m) in Port Phillip Bay, Victoria. Based on this analysis, these latter sponges are worthy of more detailed chemical analysis, and are expected to provide access to new franklinolides.
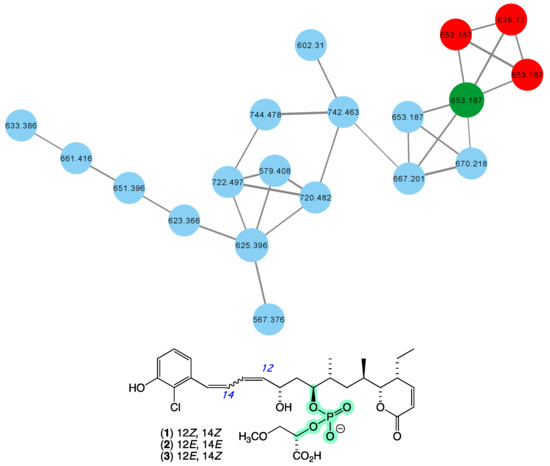
Figure 2.
GNPS molecular family and structures for franklinolides. Highlights: authentic sample of 1 (dark green), franklinolides in the original CMB-01989 extract (red), potential new franklinolides in CMB-01451 and CMB-01518 extracts (blue), and the rare phosphodiester moiety in 1 (light green).
3.2. Bistellettazines
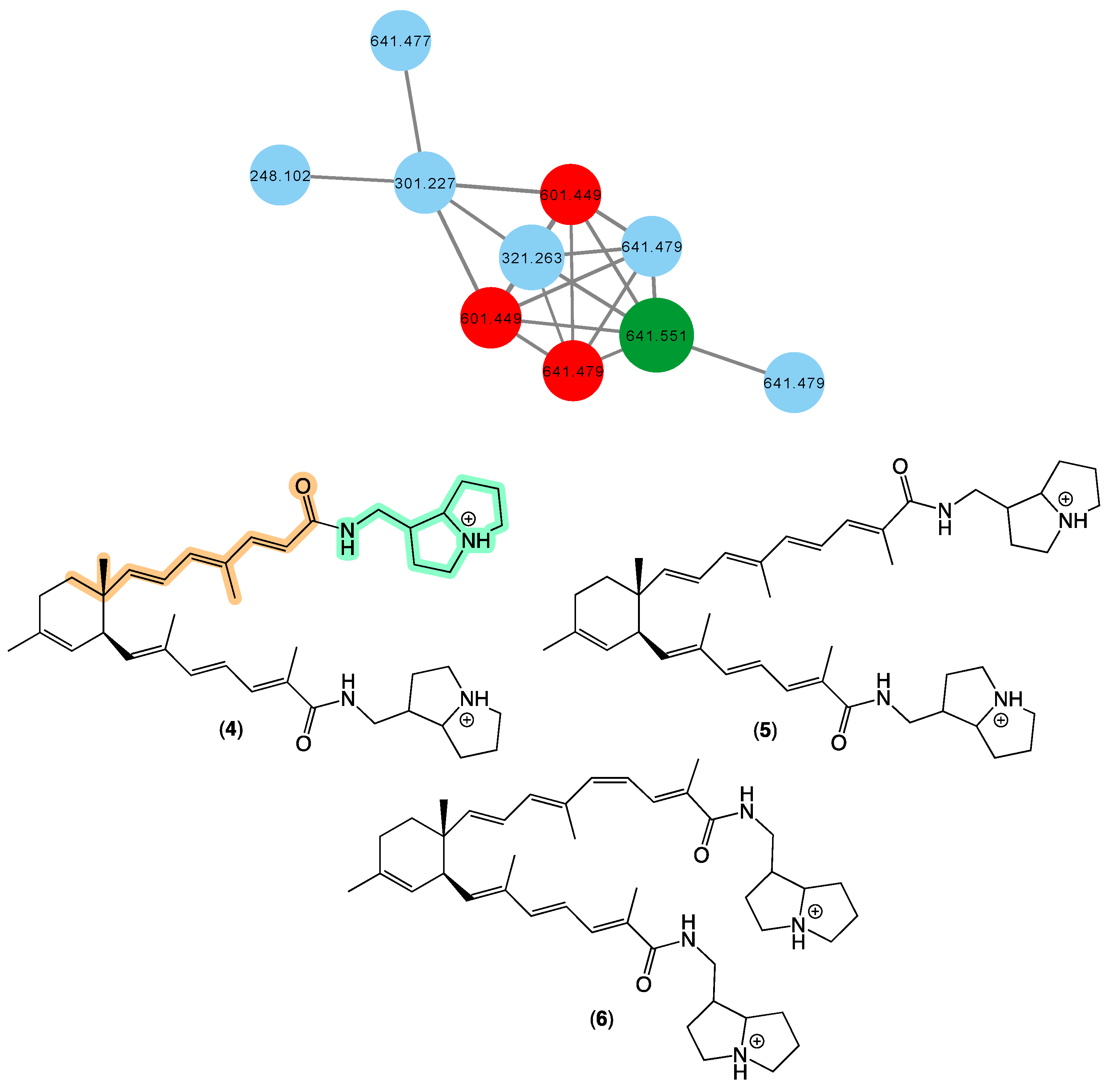
In 2008 [17], we reported bistellettazines A–C (4–6) from a Stelletta sp. (CMB-01936) collected in 1995 during scientific trawling in the Great Australian Bight. The bistellettazines were then, and remain, the only reported examples of terpenyl-pyrrolizidine conjugates, and their biosynthesis was speculated to proceed via a Diels–Alder-like addition between two hypothetical polyenyl norsesquiterpene precursors. The GNPS molecular network shown in Figure 3 revealed a family of nodes clustered around the authentic natural product bistellettazine A (4) (dark green) and analogues in the original CMB-01986 extract (red), and new nodes (blue) associated with the unidentified sponge CMB-01021 collected in 1989 during scientific trawling operations in Bass Strait, Victoria. Based on this analysis, CMB-01021 is worthy of more detailed chemical analysis, and could provide access to new bistellettazines, including hypothetical biosynthetic precursor monomers.
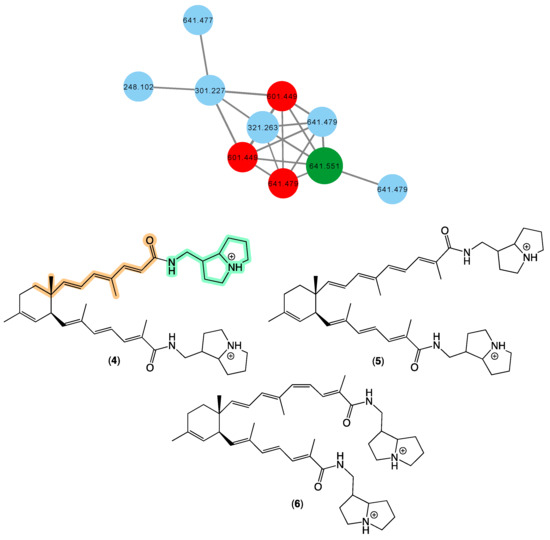
Figure 3.
GNPS molecular family and structures for bistellettazines. Highlights: authentic sample of 4 (dark green), bistellettazines in CMB-01986 extract (red), potential new bistellettazines in the CMB-01021 extract (blue), and the pyrrolizidine (light green) and terpene (tan) sub-structures in 1.
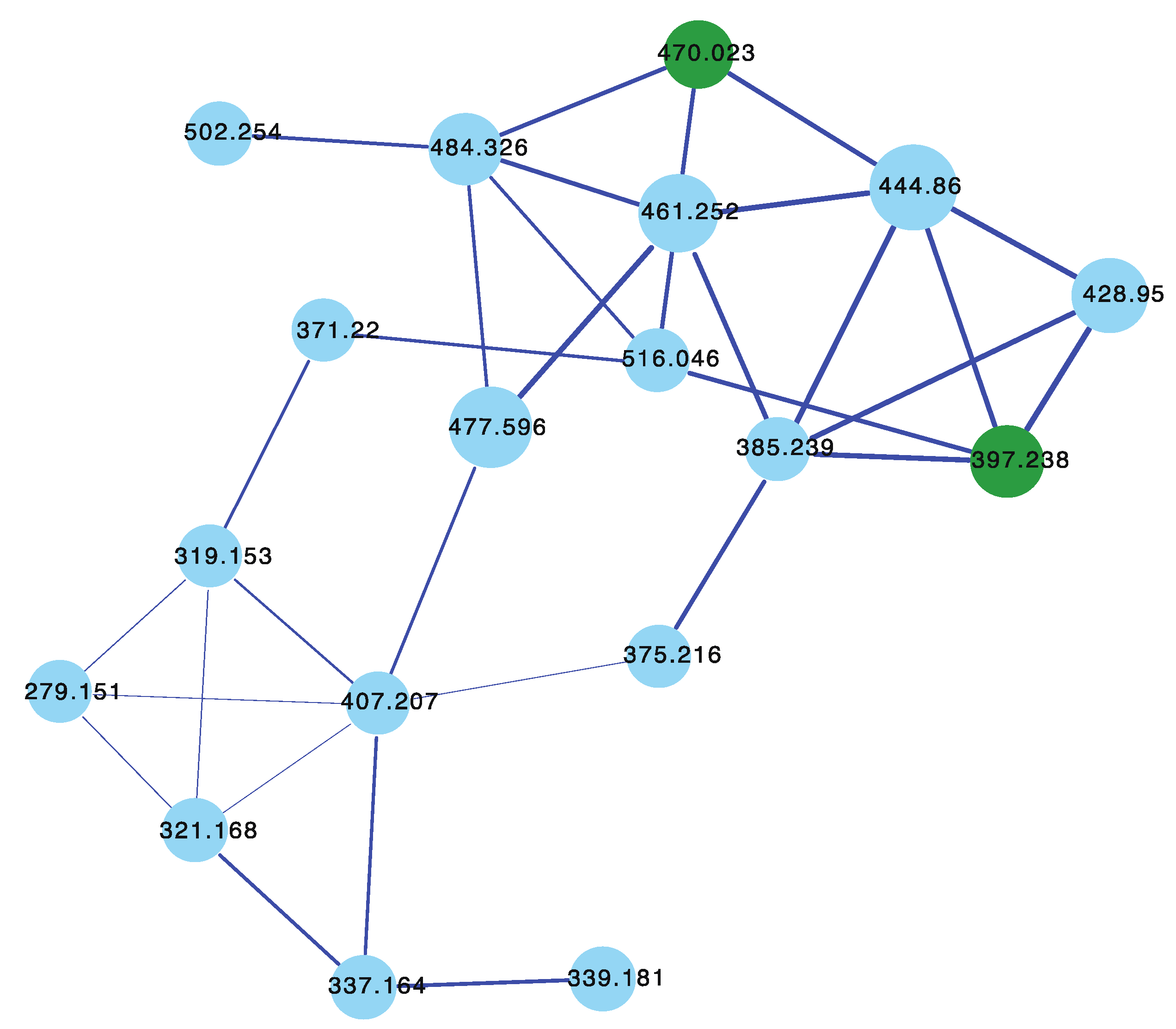
3.3. Dragmacidins
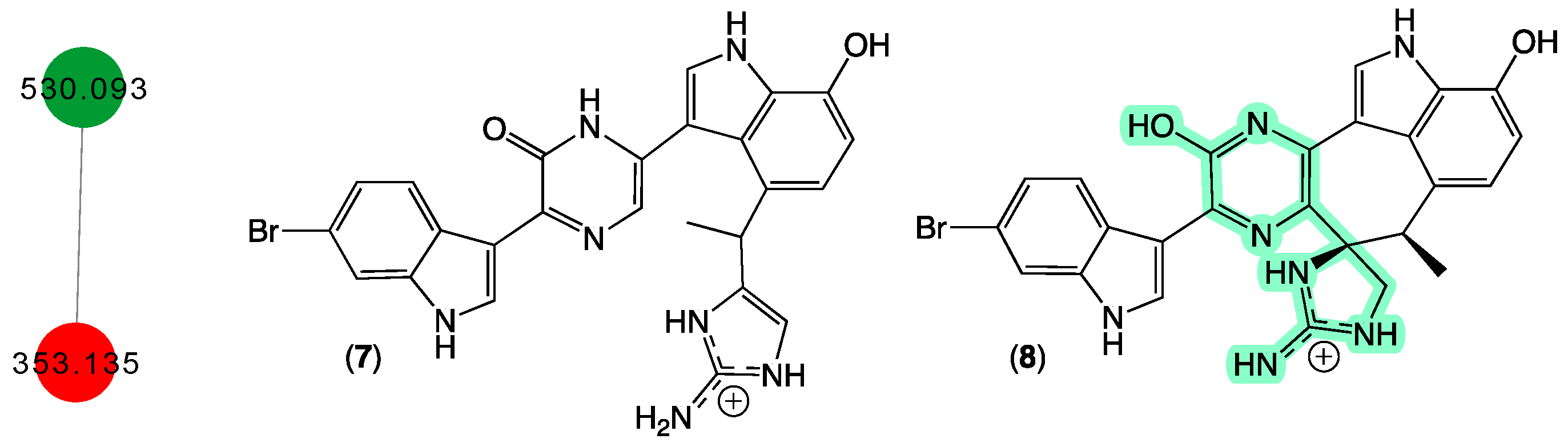
In 1998 [18], we reported two new protein phosphatase inhibitors, dragmacidins D–E (7–8) from a Spongosorites sp. (CMB-01931) collected in 1991 during scientific trawling operations (−90 m) in the Great Australian Bight. Curiously, while both 7 and 8 were yellow pigments, 8 with its unique heterocyclic scaffold was an especially brilliant fluorescent yellow and proved tenacious at staining laboratory glassware. Although our CMB-01931 extract had been fully consumed in earlier studies, we were aware of another sponge sample in our collection that produced dragmacidins; CMB-02782 identified as a Leiosella sp. encrusted with a Halichondria sp. was collected in 1998 during scientific trawling operations in the Great Australian Bight. The GNPS molecular network shown in Figure 4 revealed a family of nodes clustered around the authentic natural product dragmacidin E (8) (dark green) and nodes associated with the CMB-01986 extract (red). Significantly, despite screening 960 specimens, we did not detect any other sponge extracts with nodes co-clustering with 8, suggesting that this class of dragmacidin are quite rare.
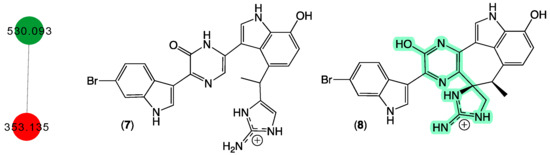
Figure 4.
GNPS molecular family and structures for dragmacidins. Highlights: authentic 8 (dark green), 7 in CMB-02782 extract (red), and unusual chromaphore associated with 8 (light green).
3.4. Lamellarins
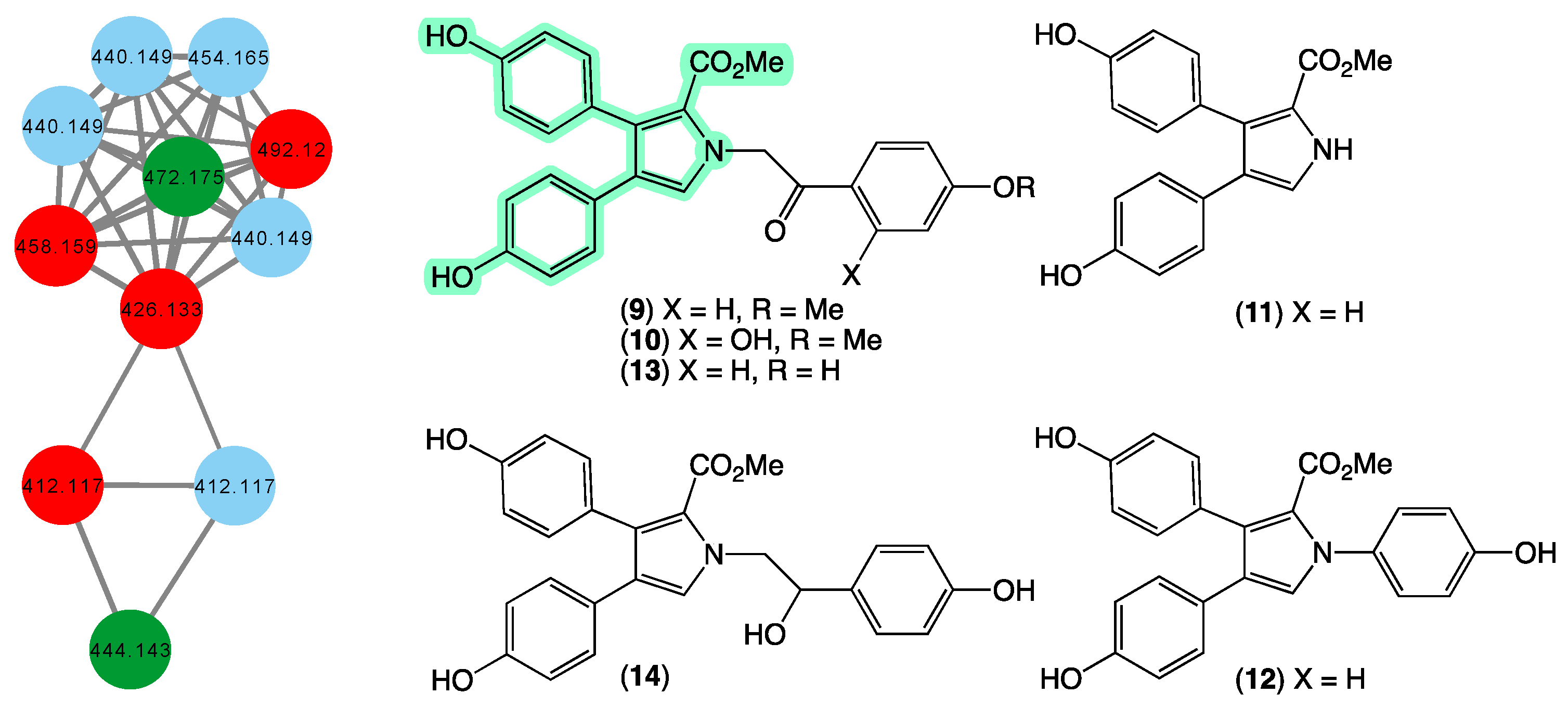
In 1994 [29], we reported on the first example of pyrrolo-alkaloid lamellarins from a marine sponge, namely lamellarins O–P (9–10) from a specimen of Dendrilla cactos collected during scientific trawling operations in Bass Strait, Victoria. This was followed in 1995 [30] with a report on lamellarins Q–R (11–12) from a second specimen of Dendrilla cactos collected by SCUBA off Durras, New South Wales, and in 2012 [31] by a report on lamellarins O1–O2 (13–14) from an Ianthella sp. (CMB-01245) collected in 1991 during scientific trawling operations in Bass Strait, Victoria. In 2014 [19], we reported on the ability of 9 to reverse efflux mediated (BCRP) drug resistance in cancer cells, prompting widespread interest in the scientific community. In addition to sponges, we reported on new lamellarins from Australian collections of marine tunicates [32,33,34], including from Didemnum sp. (CMB-01656) collected in 1994 by SCUBA (−20 m) off Wasp Island, Durras, New South Wales, and Didemnum sp. (CMB-02127) collected in 1996 during scientific trawling operations off the Northern Rottnest Shelf, Western Australia. To date, our reports of 9–14 remain the only accounts of lamellarins from marine sponges, prompting us to speculate whether our collection contained other lamellarin-producing sponges. The GNPS molecular network shown in Figure 5 revealed a family of nodes clustered around the authentic natural product 13 and the monomethyl ether derivative of lamellarin O (dark green) and analogues in the original CMB-01245 extract (red), as well as new nodes (blue) associated with another Ianthella sp. (CMB-01311) also collected in 1991 during scientific trawling operations in Bass Strait, Victoria, Australia. This latter specimen contains nodes with unique molecular weights (molecular formula) that warrant more detailed chemical analysis.
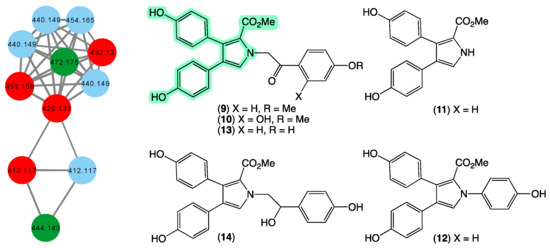
Figure 5.
GNPS molecular family and structures for lamellarins. Highlights: authentic samples of 13 and the mono methyl ether of 9 (dark green), related lamellarins in the original CMB-01245 extract (red), new lamellarins in CMB-01311 (blue), and the pyrrole core common across 9–14 (light green).
3.5. Ircinialactams
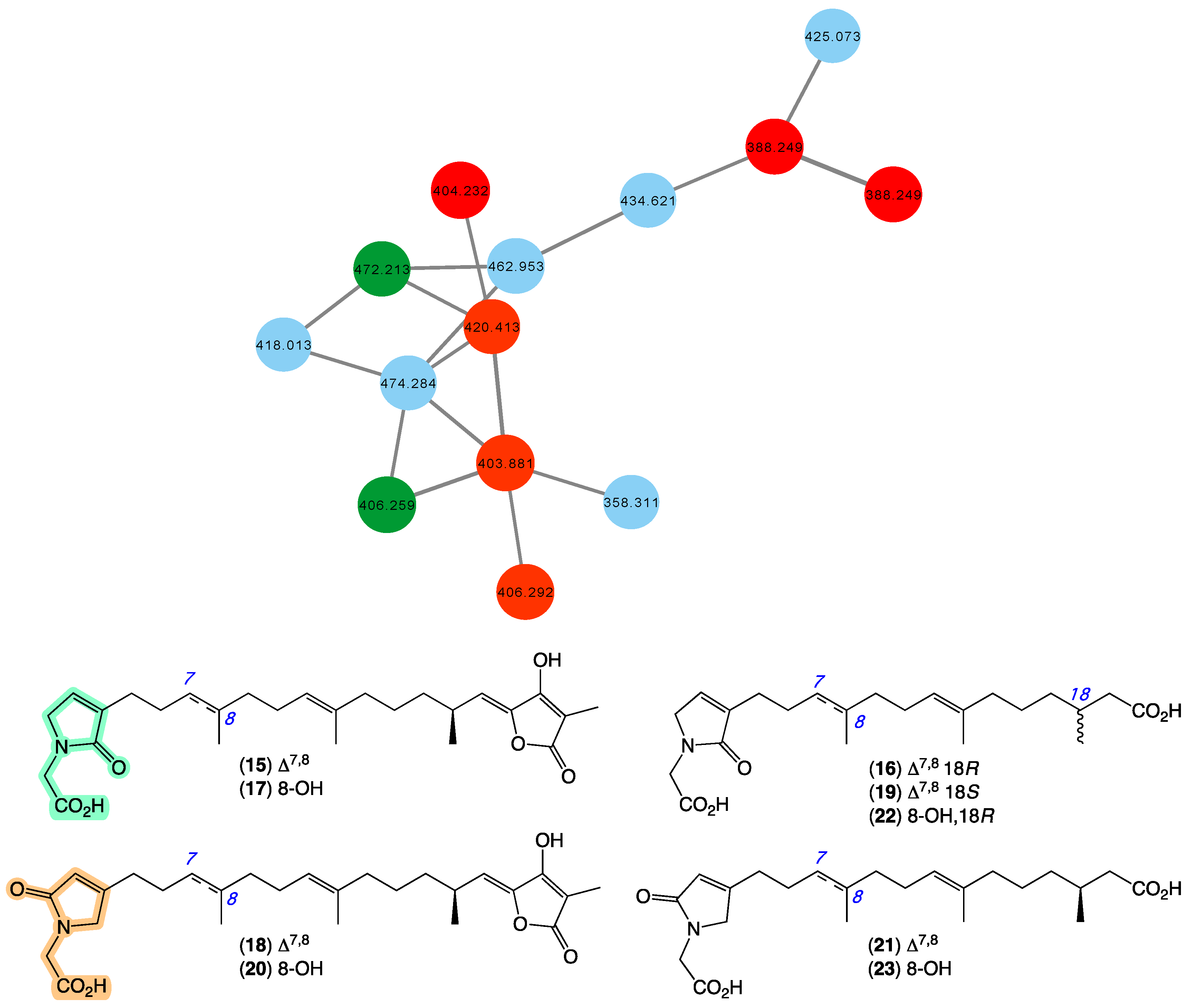
In 2010 [20], we reported a series of sesterterpene glycinyl-lactams, including ircinialactams A (15) and C (16), 8-hydroxyircinialactams A (17) and B (18), and ent-ircinialactam C (19) from a selection of sponges, including Ircinia sp. (CMB-01064) collected in 1990 during commercial trawling operations in the Great Australian Bight, and Ircinia sp. (CMB-03363) and Psammocinia sp. (CMB-03321)) collected in 2001 by SCUBA near Port Phillip Heads, Victoria, Australia. In 2018 [35], we went on to report additional sesterterpene glycinyl-lactams including ircinialactams B (20) and G (21), and 8-hydroxyircinialactams C (22) and G (23) from a series of sponges, including Sarcotragus sp. (CMB-01012) and Psammocinia sp. (CMB-01018) collected in 1986 and 1988, respectively, by SCUBA (−15 m) off Durras on the mid-south coast of New South Wales. Importantly, selected ircinialactams exhibited promising isoform selective potentiation of glycine gated chloride channel receptors (GlyRs), a unique pharmacology that could inform development of a new first-in-class treatment for chronic inflammatory pain. The GNPS molecular network shown in Figure 6 revealed a family of nodes clustered around the authentic natural products 15–16 (dark green) and analogues in the known ircinialactam producer CMB-01064 (red), as well as potentially new ircinialactams (blue) associated with an unidentified sponge CMB-01017 collected in 1986 by SCUBA (−15 m) off Durras on the mid-south coast of New South Wales. Some 35 years after being collected, CMB-01017 would appear to be worthy of further investigation.
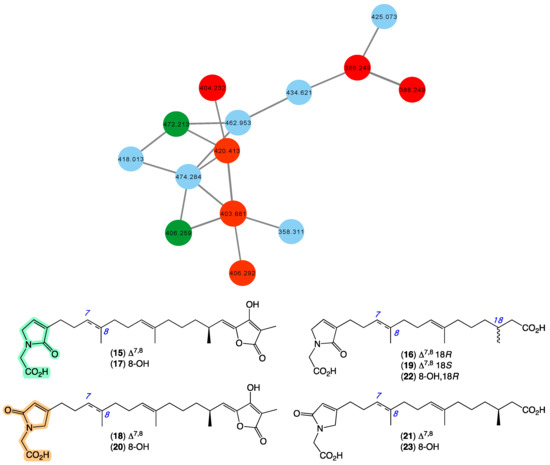
Figure 6.
GNPS molecular family and structures for ircinialactams. Highlights: authentic samples of 15–16 (dark green), related ircinialactams in the original CMB-01064 extract (red), new lamellarins in CMB-01017 (blue), and the glycinyl-lactam regioisomeric sub-structures common across 9–14 (light green and tan).
3.6. Discorhabdins
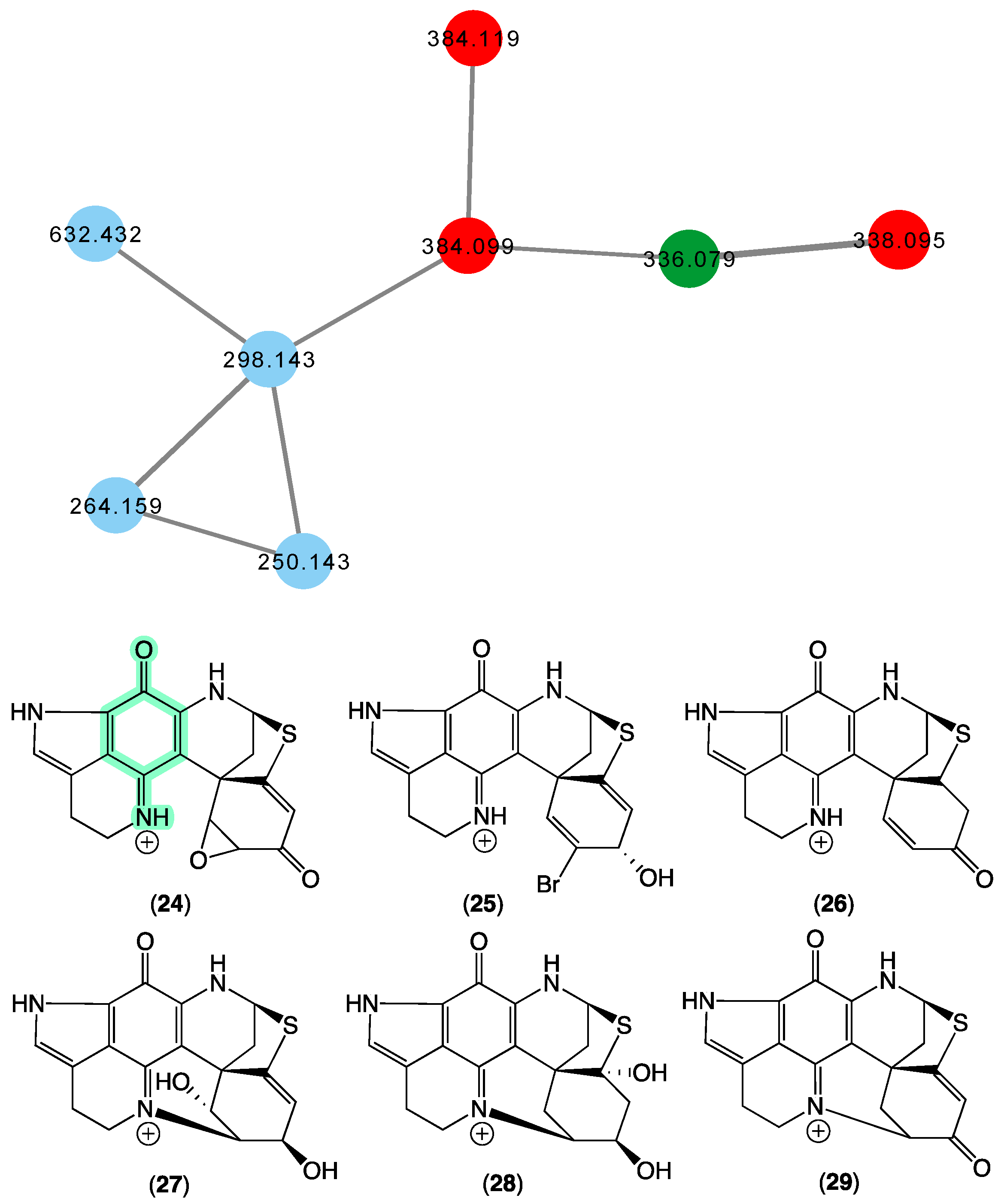
In 2000 [21], we reported on the new pyrroloiminoquinone discorhabdin R (24) from a Latrunculia sp. (CMB-02255) collected in 1997 during deep-sea scientific trawling operations (−540 m) off Prydz Bay, Antarctica, and a Negombata sp. (CMB-02644) collected in 1998 by SCUBA (−20 m) off Port Campbell, Victoria, Australia. In 2009 [36,37], we reported on the new (+)-dihydrodiscorhabdin A (25), (+)-debromodiscorhabdin A (26), (+)-dihydrodiscorhabdin L (27), and (+)-discorhabdin X (28) from a Higginsia sp. (CMB-02720) collected in 1998 by SCUBA (−20 m) off Deal island, Bass Strait, Victoria, and a Spongosorites sp. (CMB-02523) collected in 1998 by SCUBA (−15 m)off Port Campbell, Victoria. The GNPS molecular network shown in Figure 7 revealed a family of nodes clustered around the authentic natural product discorhabdin D (29) (dark green) and analogues in the known discorhabdin producers CMB-02720 and CMB-02523 (red), as well as potentially new discorhabdins (blue) associated with an unidentified sponge CMB-01879 collected in 1995 during scientific trawling operations (−45 m) in the Great Australian Bight.
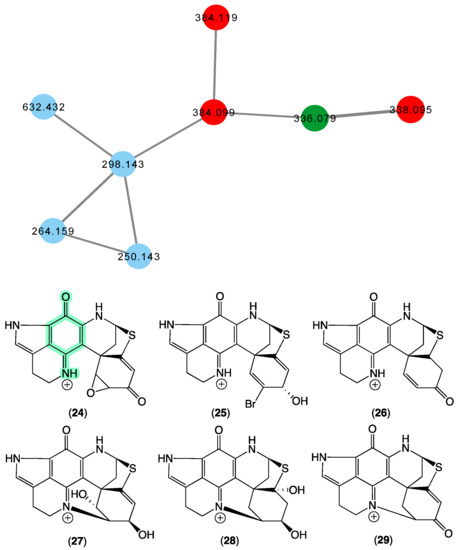
Figure 7.
GNPS molecular family and structures for discorhabdins. Highlights: authentic sample of 29 (dark green), related discorhabdins in CMB-02720 and CMB-02523 (red), new discorhabdins in CMB-01879 (blue), and the pyrroloiminoquinone sub-structure common to 24–29 (light green).
3.7. Trunculins
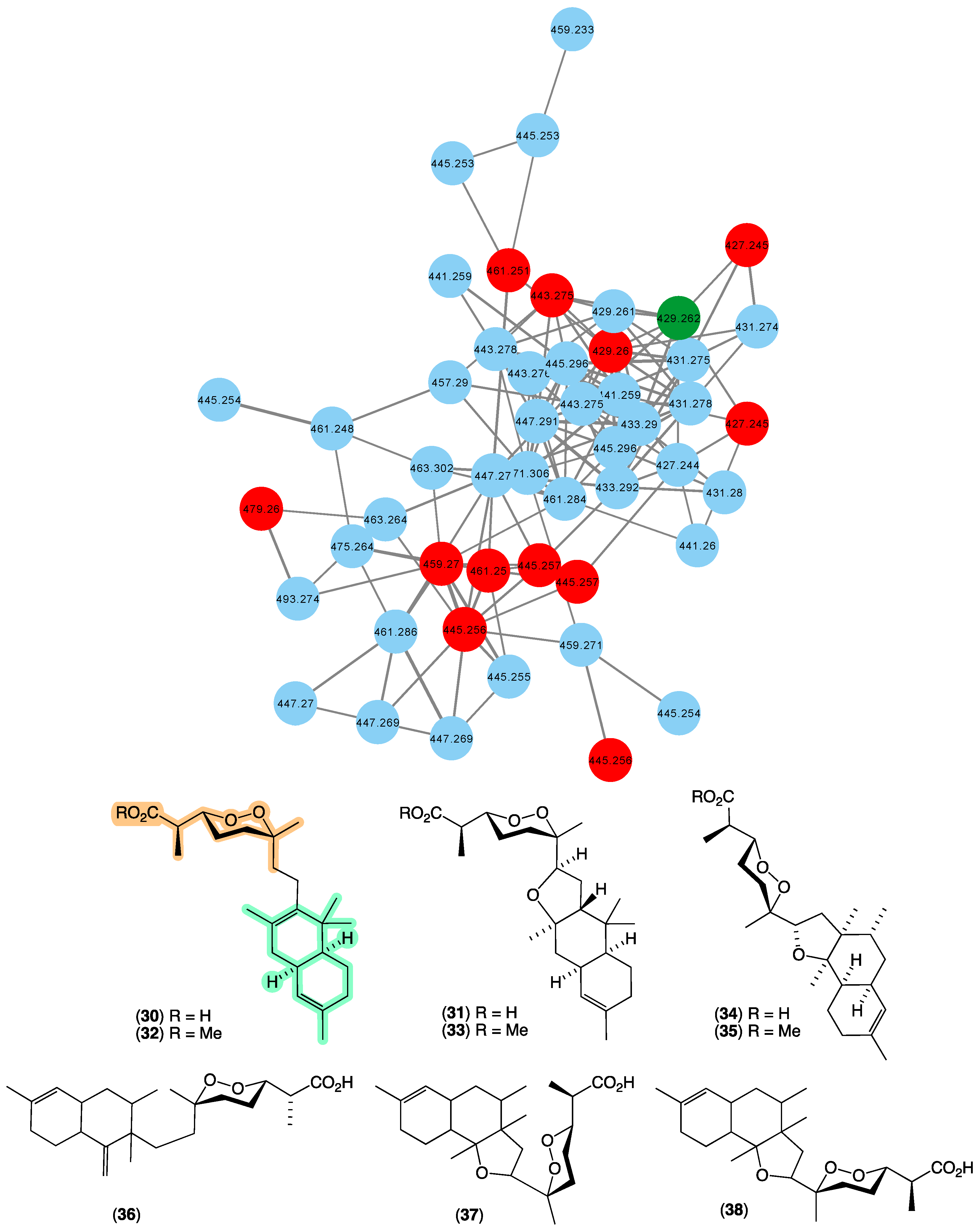
In 1987 [22], we reported the first occurrence of a family of norsesterterpene cyclic peroxides bearing an unprecedented carbon skeleton, trunculins A–B (30–31), and their methyl esters 32–33 from Latrunculia brevis (CMB-01738) collected by SCUBA (−20 m) off South Durras, New South Wales. This was followed in 1993 [38] with a report of trunculin F (34) and its methyl ester 35 from Latrunculia conulosa collected by SCUBA (−5 m) off Flinders, Victoria, and in 1998 [39], trunculins G–I (36–38) from a Latrunculia sp. collected in 1995 by SCUBA (−30 m) off a World War II submarine scuttled near Port Phillip Heads, Victoria. Terpene cyclic peroxides are a class of natural product unique to marine sponges, with empirical rules for assignment of peroxide configurations based on NMR chemical shifts proposed by Capon in 1985 [40], allowing for rapid identification of natural stereo diversity. The GNPS molecular network shown in Figure 8 revealed a family of nodes clustered around the authentic natural product trunculin B (30) (dark green) and analogues in the known trunculin producer CMB-01738 (red), as well as potentially new trunculins (blue) associated with three unidentified sponges; CMB-01001 and CMB-01723 collected in 1985 and 1995, respectively, by SCUBA (−20 m) off Durras, New South Wales, and CMB-01179 collected in 1991 by SCUBA (−25 m) off Port Phillip Heads, Victoria. The expansive diversity of new trunculin nodes suggests that these latter three sponge samples offer the prospect of an array of new members of this relatively rare structure class.
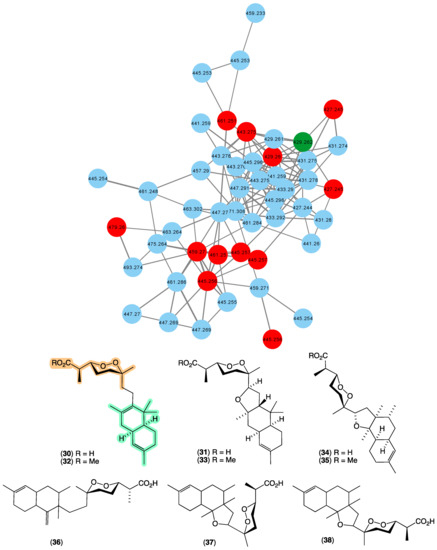
Figure 8.
GNPS molecular family and structures for trunculins. Highlights: authentic sample of 30 (dark green), related trunculins in the original CMB-01738 extract (red), new trunculins in CMB-01001, CMB-01723 and CMB-01179 (blue), and the trunculin carbo/heterocyclic skeleton and cyclic peroxide sub-structure common across 30–38 (light green and tan).
4. Case Studies in the Discovery of Rare and New Natural Product Classes
Analysis of GNPS molecular families in Figure 1 revealed several unique clusters associated with only one producing organism [i.e., Dysidea sp. (CMB-01171), Cacospongia sp. (CMB-03404), and Thorectandra choanoides (CMB-01889)]. Further investigations of these sponges resulted in the isolation of new classes of natural products: dysidealactams and dysidealactones [13], cacolides [14], and thorectandrins [15]. The GNPS analysis also identified another producer of the rare trachycladindoles, curiously from a different taxonomical genera as the original producer [12]. All the case studies are briefly summarised below.
4.1. Trachycladindoles
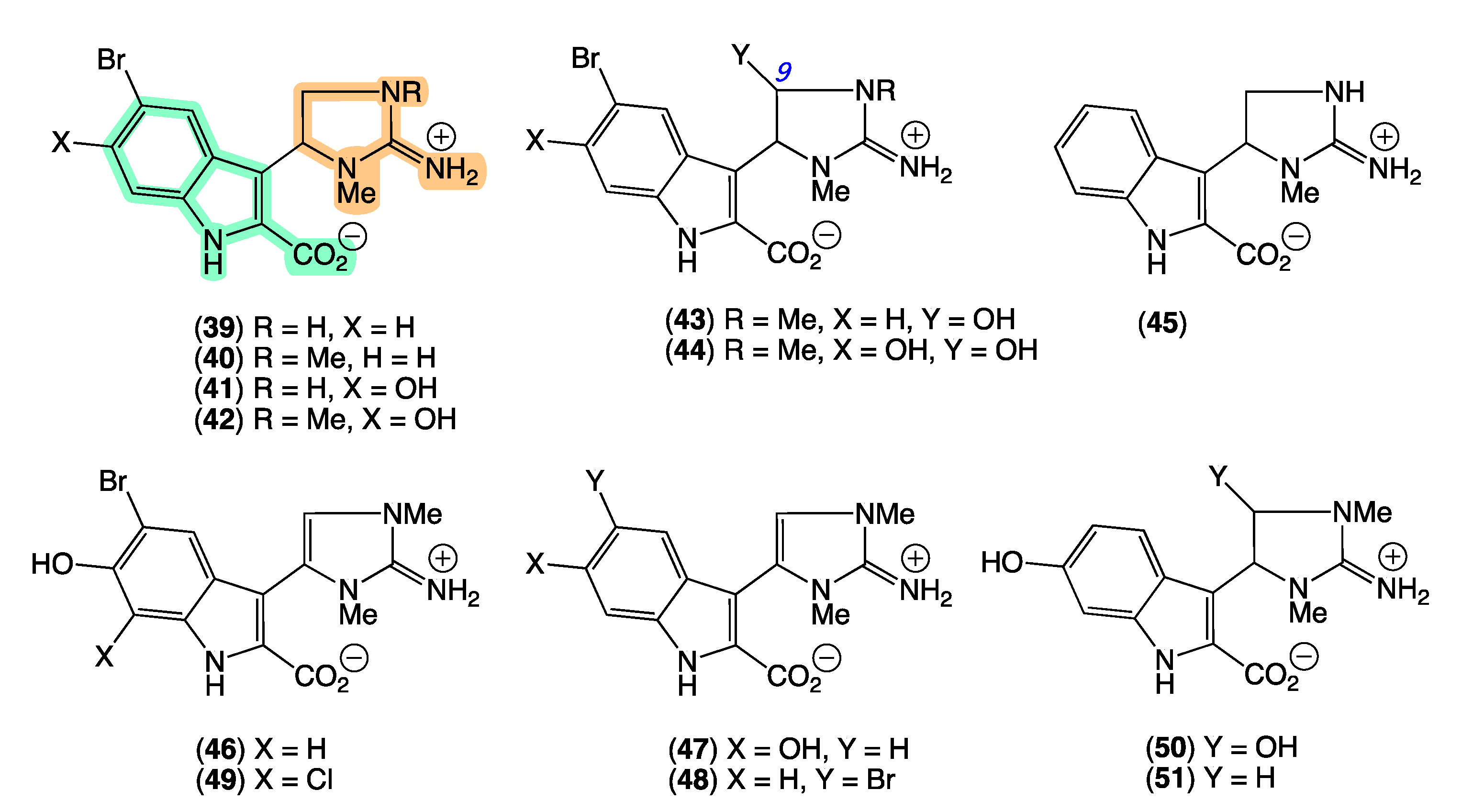
In 2008 [26], we reported the first natural examples of an indolo-2-carboxylate bearing a 2-amino-4,5-dihydroimidazole moiety, trachycladindoles A–G (39–45) from Trachycladus laevispirulifer (CMB-03397) collected in 2001 during commercial trawling operations in the Great Australian Bight. We subsequently patented the trachycladindoles as potent and selective kinase inhibitors with potential application in the treatment of neurodegenerative diseases (i.e., Alzheimer’s and Parkinson’s diseases) [41]. Unfortunately, with available supplies limited to <1 mg and targeted resupply of the Australian deep-water source Trachycladus sponge impractical, combined with no other literature reports of trachycladindoles, further investigations of this promising pharmacophore were stalled. Efforts at investigating structure activity relationships using available material were also compromised by the observation that on storage (−30 °C in DMSO) trachycladindoles E (43) and F (44) underwent quantitative deformylation to trachycladindoles A (39) and C (41) [42].
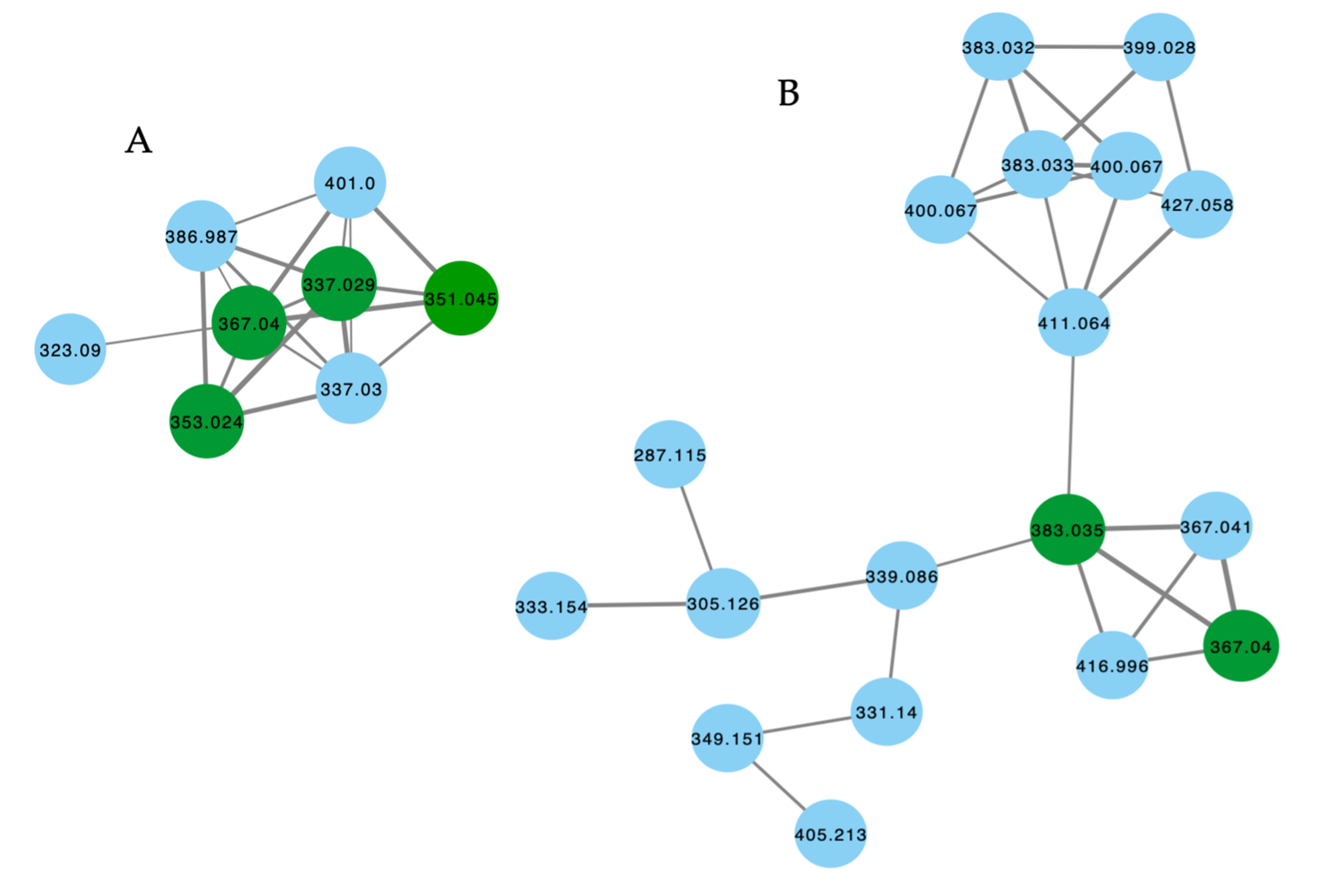
The GNPS molecular network shown in Figure 9 revealed two families of nodes: Family A clustered around authentic samples of trachycladindoles A–C (39–41) and E (43) (dark green), and Family B clustered around authentic samples of trachycladindoles D (42) and F (44) (dark green), with both families featuring additional nodes (blue) attributed to new trachycladindoles associated with a Geodia sp. (CMB-01063) collected in 1990 during commercial trawling operations in the Great Australian Bight. Encouraged by this discovery, in 2020 [12], we reported on the re-isolation of trachycladindoles A–G (39–44) and an array of new trachycladindoles H–M (46–51) from CMB-01063. As an interesting aside, the discovery of trachycladindoles in two taxonomically unrelated sponge genera (Trachycladus and Geodia), and only from Great Australian Bight specimens of these sponges, does fuel speculation that the trachycladindoles may not be sponge natural products but may be microbial in origin.
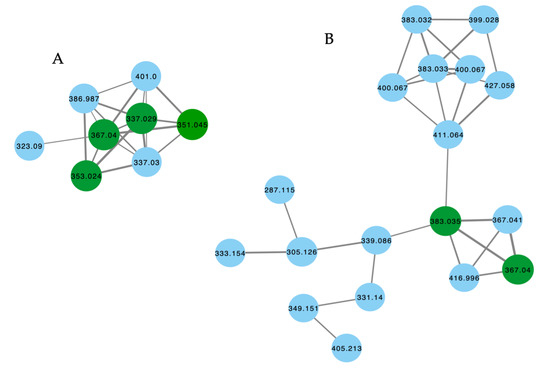
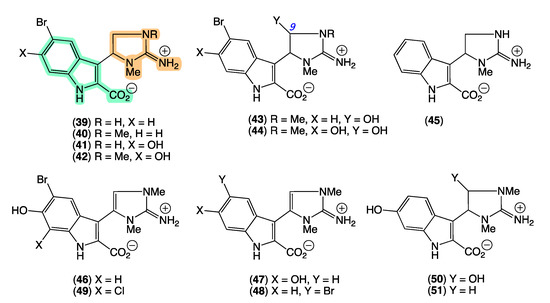
Figure 9.
GNPS molecular family and structures for trachycladindoles. Highlights: authentic samples of 39–41 and 43 (cluster A, dark green); 42 and 44 (cluster B, dark green) and putative new trachycladindoles in CMB-01063 (blue), and the indolo-2-carboxylate and 2-amino-4,5-dihydroimidazole sub-structures in common across all trachycladindoles (light green and tan).
4.2. Dysidealactams
Australian sponges of the genus Dysidea have a history of producing structurally diverse natural products, many featuring novel chemical scaffolds and functionality. For example, as early as the 1970s, Dysidea specimens collected off the Great Barrier Reef near Townsville and Cooktown, Queensland, were reported to yield brominated diphenyl ethers [43,44] and the chlorinated dysidin and dysidenin [45,46], while specimens collected further south off Gladstone yielded sesquiterpenes spirodysin and trichloroleucine diketopiperazines [47], and those off Cronulla, New South Wales, yielded sesquiterpene furans such as furodysin and furodysinin [48]. Since the 1970s, many other natural products have been reported from Australian Dysidea, including sesquiterpenes [49,50,51,52,53], furanosesquiterpenes [54], thiosesquiterpenes [55], chlorinated N-acyl amino esters [56], sesterterpene tetronic acids [57], mycosporines [58], chloroleucines [59,60,61], brominated diphenyl ethers [53,62,63,64], dioxanes [65], 9,11-secosterols [66], and polyoxygenated sterols [67,68]. Given this historic productivity, it is perhaps understandable that there has been a decline in the discovery of new classes of natural products from Australian Dysidea.
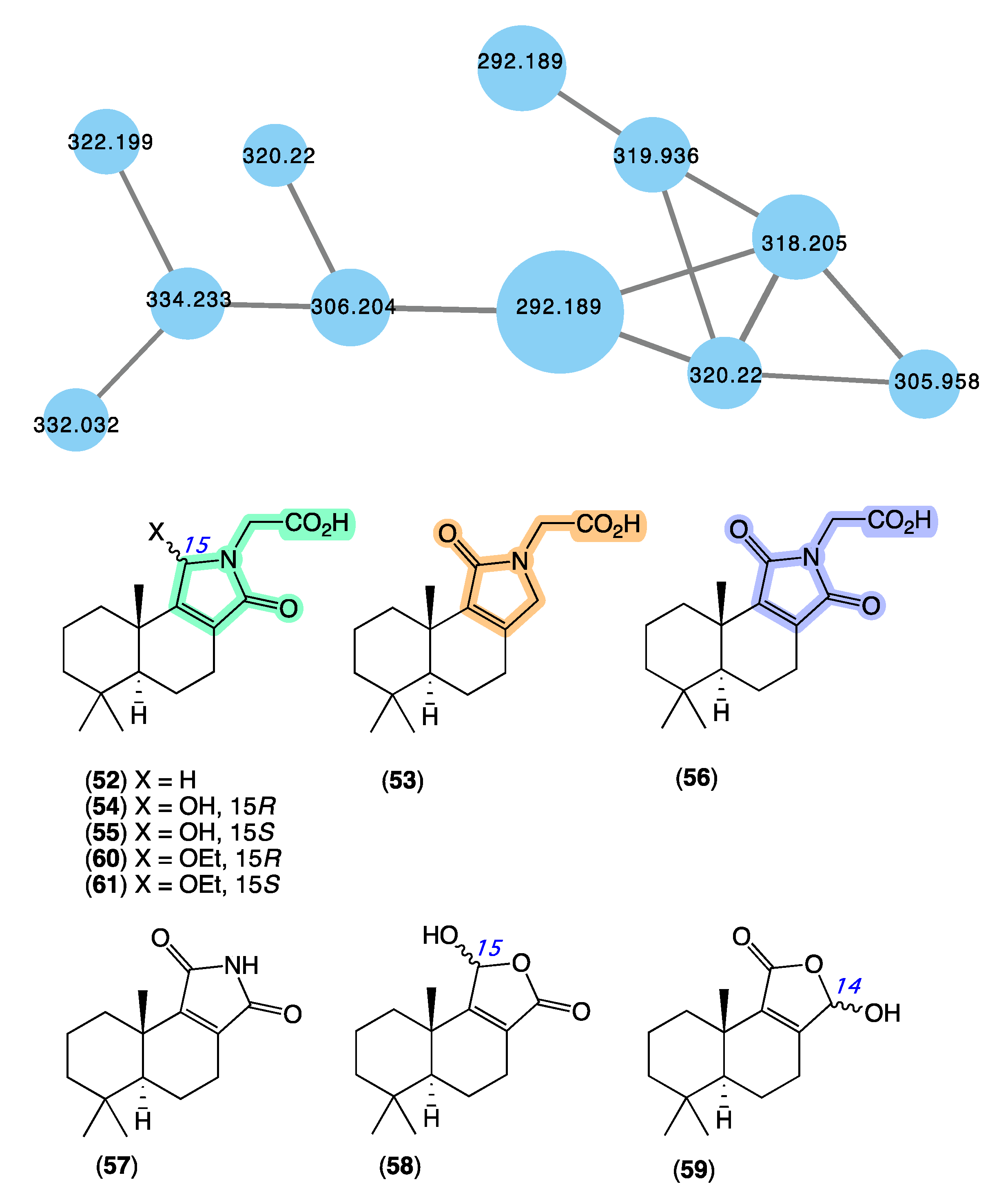
The GNPS molecular network shown in Figure 10 revealed a molecular family uniquely associated with Dysidea sp. (CMB-01171) collected in 1991 by SCUBA (−25 m) off Port Phillip Heads, Victoria. Although this specimen had been in our collection for over 30 years, it had failed to be noticed. Consequently, we were intrigued to have the GNPS analysis reveal natural product nodes unique to this Dysidea sp., especially given the extensive history of Australian Dysidea sp. chemistry (see above). Following a chemical investigation, in 2020 [13], we reported on a suite of new sesquiterpenes from Dysidea sp. (CMB-01171), including dysidealactams A–F (52–57), dysidealactones A–B (58–59), and two solvolysis artifacts 60–61. Of particular note, dysidealactams A–D (52–55) incorporate a rare glycinyl lactam moiety (see ircinialactams above), and 56 incorporates an unprecedented glycinyl imide moiety. Due to a prolonged storage in EtOH, it is quite common to observe the esterifaction of carboxylic acid groups to the corresponding ethyl esters [42]. The dysidealactams and dysidealactones immediately attracted the attention of synthetic chemists, with successful synthesis reported in 2023 [69].
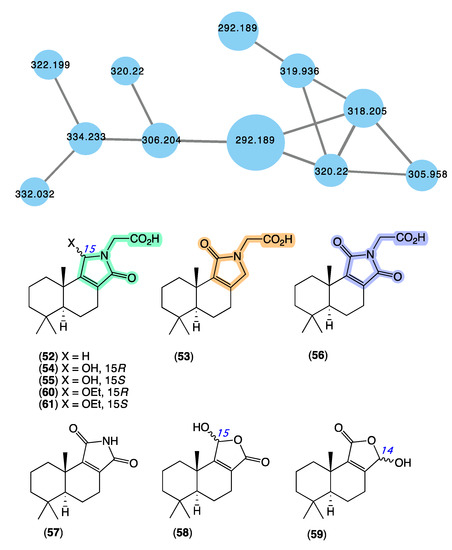
Figure 10.
GNPS molecular family (blue) and structures for dysidealactams and dysidealactones. Highlights: the regioisomeric glycinyl-lactam and glycinyl-imide sub-structures (green, tan and purple).
4.3. Cacolides
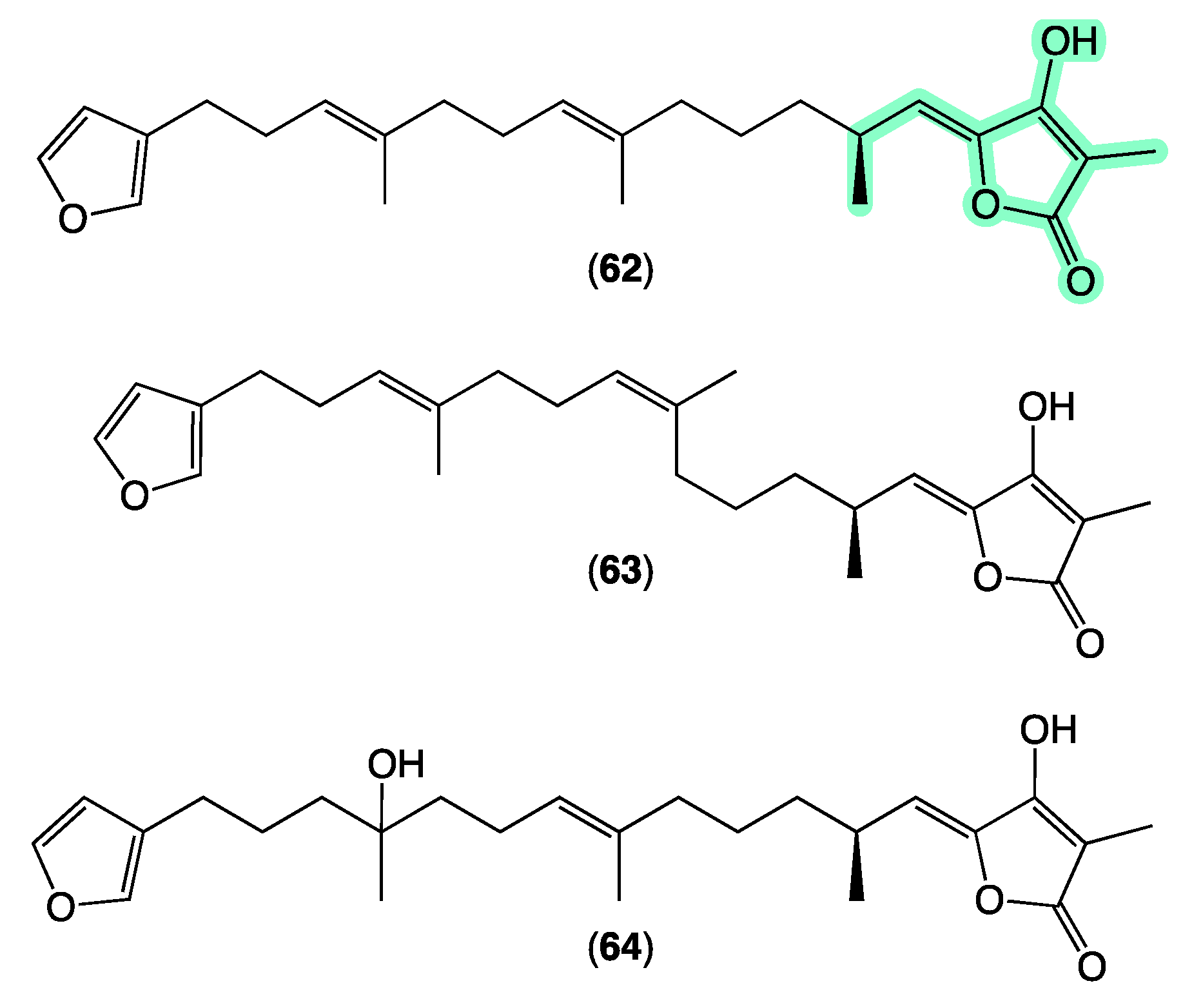
Sesterterpene tetronic acids are a family of natural products unique to selected genera of marine sponges, with numerous examples reported over the last 50 years. The earliest accounts, for example, include a 1972 [70] report of ircinin-1 and ircinin-2 from Ircinia oros followed shortly after in 1973 [71] by variabilin from Ircinia variabilis. Following re-isolation from Australian Ircinia spp., absolute configurations were assigned to all three of these earliest exemplars in 1994 [72]. The last four decades of marine natural products research saw many reports of sesterterpene tetronic acids from Irciniidae sponges, including from Australian Ircinia [20,73], Psammocinia [20,35,74,75,76], and Sarcotragus spp. [71]. As the number of known tetronic acids increased, so did the incidence of re-isolation, driving the need for rapid, reliable, and cost-effective methods for dereplication, with many researchers becoming adept at detecting and deprioritizing sesterterpene tetronic acids in crude sponge extracts. Some tell-tales included the observation that extracts/fractions rich in sesterterpene tetronic acids exhibited significant antibacterial activity and diagnostic 1H NMR resonances, particularly for the furan and tetronic acid termini. As our experience with the ircinialactams revealed (described above), there is still scope for discovery within the sesterterpene tetronic acid motif. Hence, we were keen to determine if a GNPS molecular network approach could provide a more reliable means of dereplication. To this end, we interrogated our GNPS molecular network of 960 marine extracts using authentic standards of (7E,12E,20Z,18S)-variabilin (62) (Figure 11) and ircinialactam A (15) (Figure 6). Unsurprisingly for such a common structure class, a great many marine extracts co-clustered with the authentic standards. To better understand these data, we elected to further refine the visualization into three separate analyses based around specimens belonging to each of the genera Ircinia, Psammocinia and Sarcotragus.
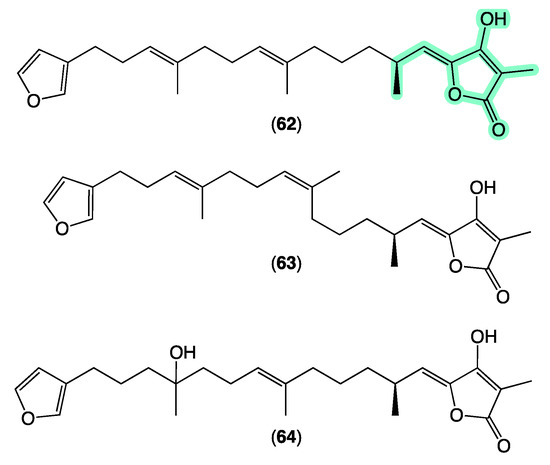
Figure 11.
Selected known sesterterpene tetronic acids. Highlights: tetronic acid termini common to all sesterterpene tetronic acids (light green).
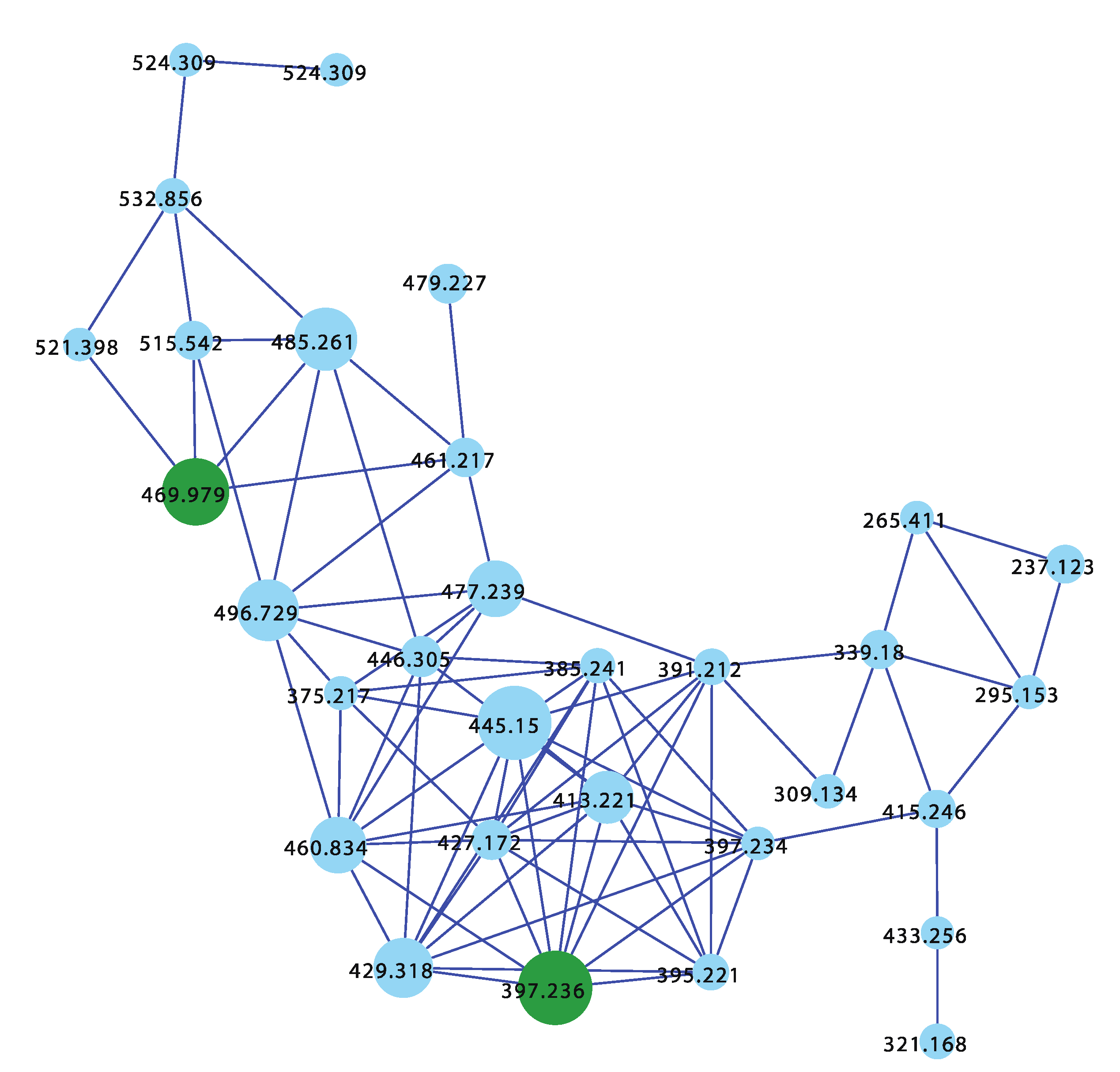
The sponge Ircinia sp. (CMB-01064) collected in 1990 during commercial trawling operations in the Great Australian Bight was known to produce (7E,12E,20Z,18S)-variabilin (62), (7E,12Z,20Z,18S)-variabilin (63), (12E,20Z,18S)-8-hydroxy-variabilin (64), ircinialactam A (15), and 8-hydroxyircinialactams A (17) and B (18) (Figure 6 and Figure 11); while Ircinia sp. (CMB-03363) collected in 2001 by SCUBA near Port Phillip Heads, Victoria, was known to produce 62, 63, 64, and 15. Three additional Ircinia spp CMB-01058 were collected in 1990 by SCUBA near Flinders, Victoria; CMB-01693 collected in 1994 by benthic grab (−71 m) during a scientific operations off Port Lincoln, South Australia, and CMB-02014 collected in 1995 by SCUBA in Port Phillip Bay, Victoria, had not been previously subjected to chemical analysis. The GNPS molecular network shown in Figure 12 on these five Ircinia spp. revealed a common molecular family co-clustering with authentic samples of 62 and 15 (dark green)—confirming that GNPS could be used to detect/dereplicate Ircinia sesterterpene tetronic acids.
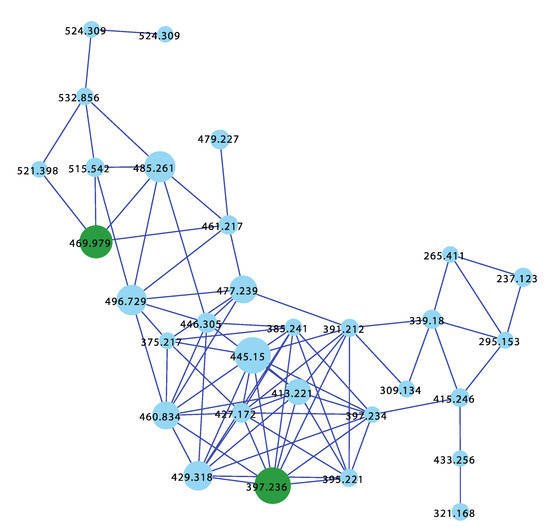
Figure 12.
GNPS molecular family for Ircinia spp. CMB-01064, CMB-03363, CMB-01058, CMB-01693, and CMB-02014. Highlights: authentic standards for 62 and 15 (dark green), sesterterpene tetronic acids common to CMB-01064, CMB-03363, CMB-01058, CMB-01693, and CMB-02014 (blue).
The sponges Psammocinia sp. (CMB-03231) collected in 2000 by SCUBA off Lonsdale, Victoria, Psammocinia sp. (CMB-01018) collected in 1988 by SCUBA off Durras, New South Wales, and Psammocinia sp. (CMB-03344) collected in 2001 by SCUBA off Port Phillip Heads, Victoria, were all known to produce sesterterpene teronic acids; while Psammocinia sp. (CMB-01757) collected in 1995 by SCUBA (−30 m) off Barwon Heads, Victoria, and Psammocinia sp. (CMB-02026) collected in 1995 during scientific trawling operations (−100 m) in the Great Australian Bight, was not subjected to chemical analysis. The GNPS molecular network shown in Figure 13 on these five Psammocinia spp. revealed a common molecular family co-clustering with authentic samples of 62 and 15 (dark green)—confirming that GNPS could be used to detect/dereplicate Psammocinia sesterterpene tetronic acids.
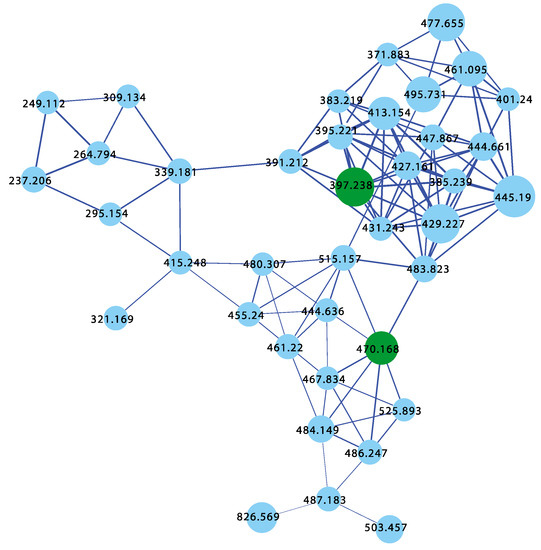
Figure 13.
GNPS molecular family for Psammocinia spp. CMB-03231, CMB-01018, CMB-03344, CMB-01757, and CMB-02026. Highlights: authentic standards for 62 and 15 (dark green), sesterterpene tetronic acids common to CMB-03231, CMB-01018, CMB-03344, CMB-01757, and CMB-02026 (blue).
The sponges Sarcotragus sp. (CMB-01788) and Sarcotragus sp. (CMB-01848) collected in 1995 during scientific trawling operations (−30 m and −85 m, respectively) in the Great Australian Bight, Sarcotragus sp. (CMB-02707) and Sarcotragus sp. (CMB-02717) collected in 1998 by SCUBA (−10 m) off Deal Island, Bass Strait, and Sarcotragus sp. (CMB-03390) collected in 2001 during commercial trawling operations in the Great Australian Bight, had not been subjected to chemical analysis. The GNPS molecular network shown in Figure 14 on these five Sarcotragus spp. revealed a common molecular family co-clustering with authentic samples of 62 and 15 (dark green)—confirming that GNPS could be used to detect/dereplicate Sarcotragus sesterterpene tetronic acids.
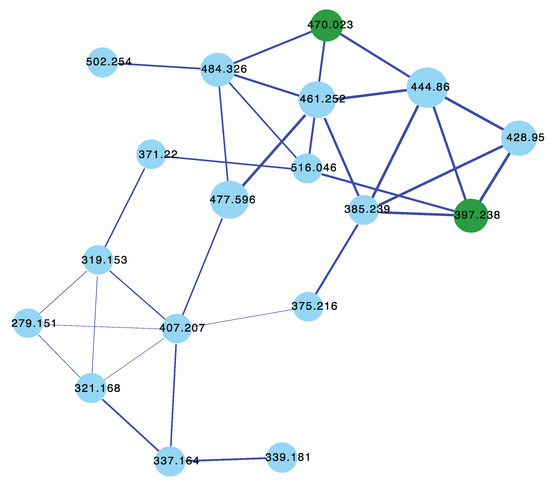
Figure 14.
GNPS molecular family for Sarcotragus spp. CMB-01788, CMB-01848, CMB-02707, CMB-02717, and CMB-03390. Highlights: authentic standards for 62 and 15 (dark green), sesterterpene tetronic acids common to CMB-01788, CMB-01848, CMB-02707, CMB-02717, and CMB-03390 (blue).
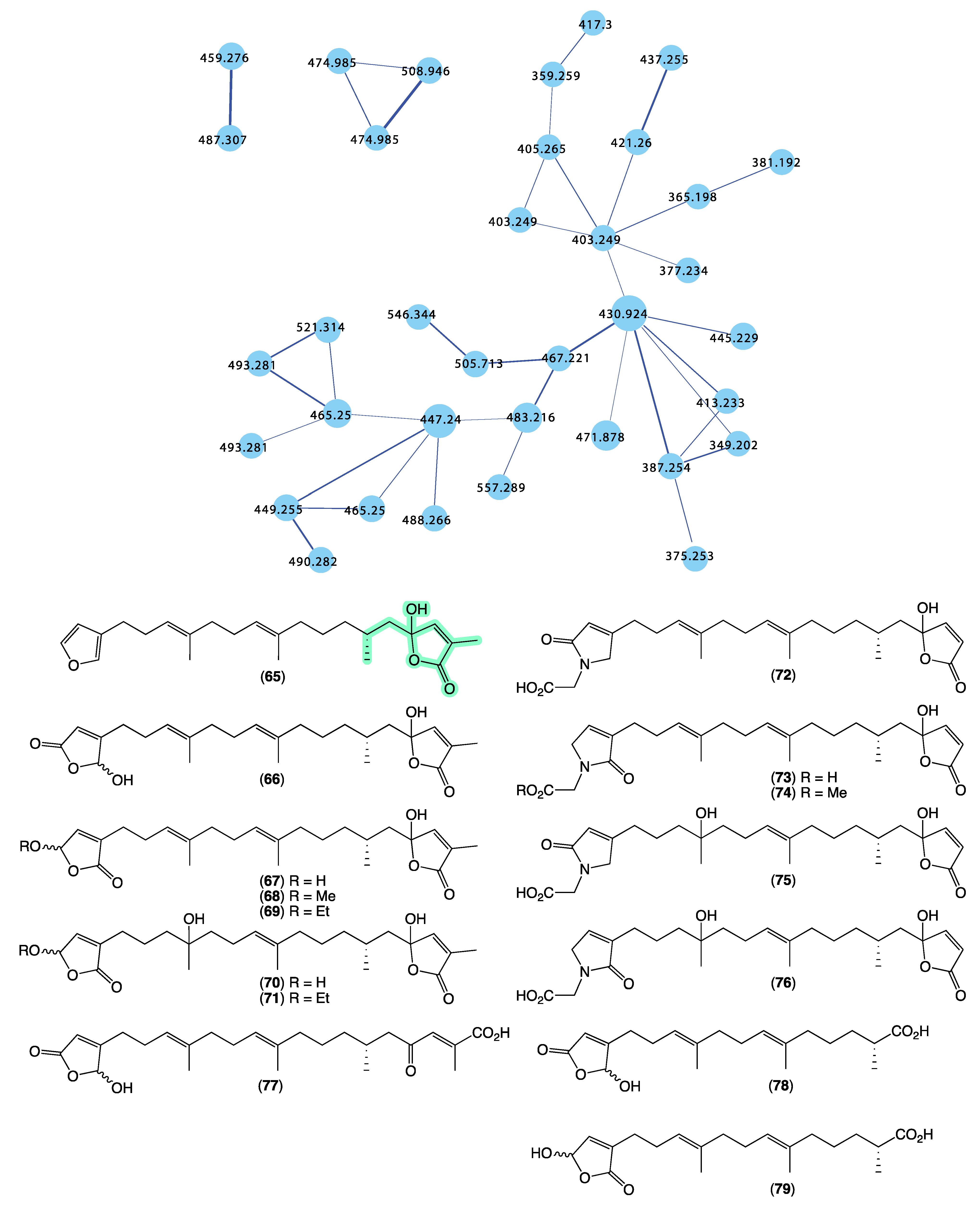
Returning to the GNPS molecular network, we noted that Cacospongia sp. (CMB-03404) collected in 2001 during commercial trawling operations in the Great Australian Bight featured a unique molecular family (Figure 15) that did not share any nodes with authentic sesterterpene tetronic acids, or any of the Ircinia, Psammocinia, or Sarcotragus spp. extracts noted above. This was initially perplexing as at an earlier time, and using more traditional UPLC-DAD and 1H NMR dereplication approaches, we dismissed this extract as containing known sesterterpene tetronic acids. Clearly, our initial assessment was incorrect, with the GNPS analysis prompting a more detailed chemical investigation. In 2018 [14], we reported on an unprecedented family of sesterterpene α-methyl-γ-hydroxybutenolides, cacolides A–L (65–76), together with the biosynthetically related cacolic acids A–C (77–79) from Cacospongia sp. (CMB-03404) (Figure 15). Had it not been for the detection of a unique GNPS molecular family for CMB-03404, it is likely that our original dereplication assessment would had held, and this extract would have remained dormant in our collection for many more years. This experience reinforces the need to be cautious in all dereplication, and be willing to revise determinations when contradictory data emerge.
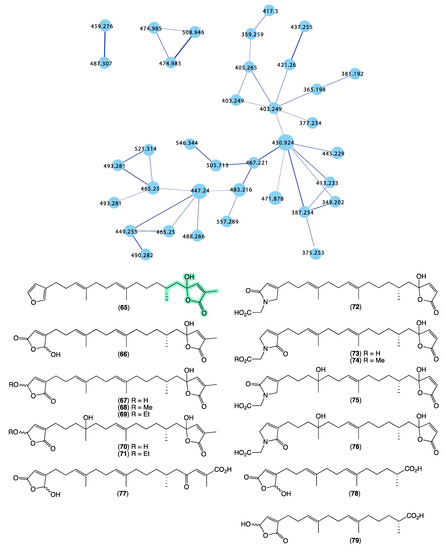
Figure 15.
GNPS molecular family and structures for cacolides A–L (65–76) and cacolic acids A–C (77–79). Highlights: cacolides and cacolic acids in CMB-03404 (blue), and the unique α-methyl-γ-hydroxybutenolide sub-structures in common across many cacolides (light green).
4.4. Thorectandrins
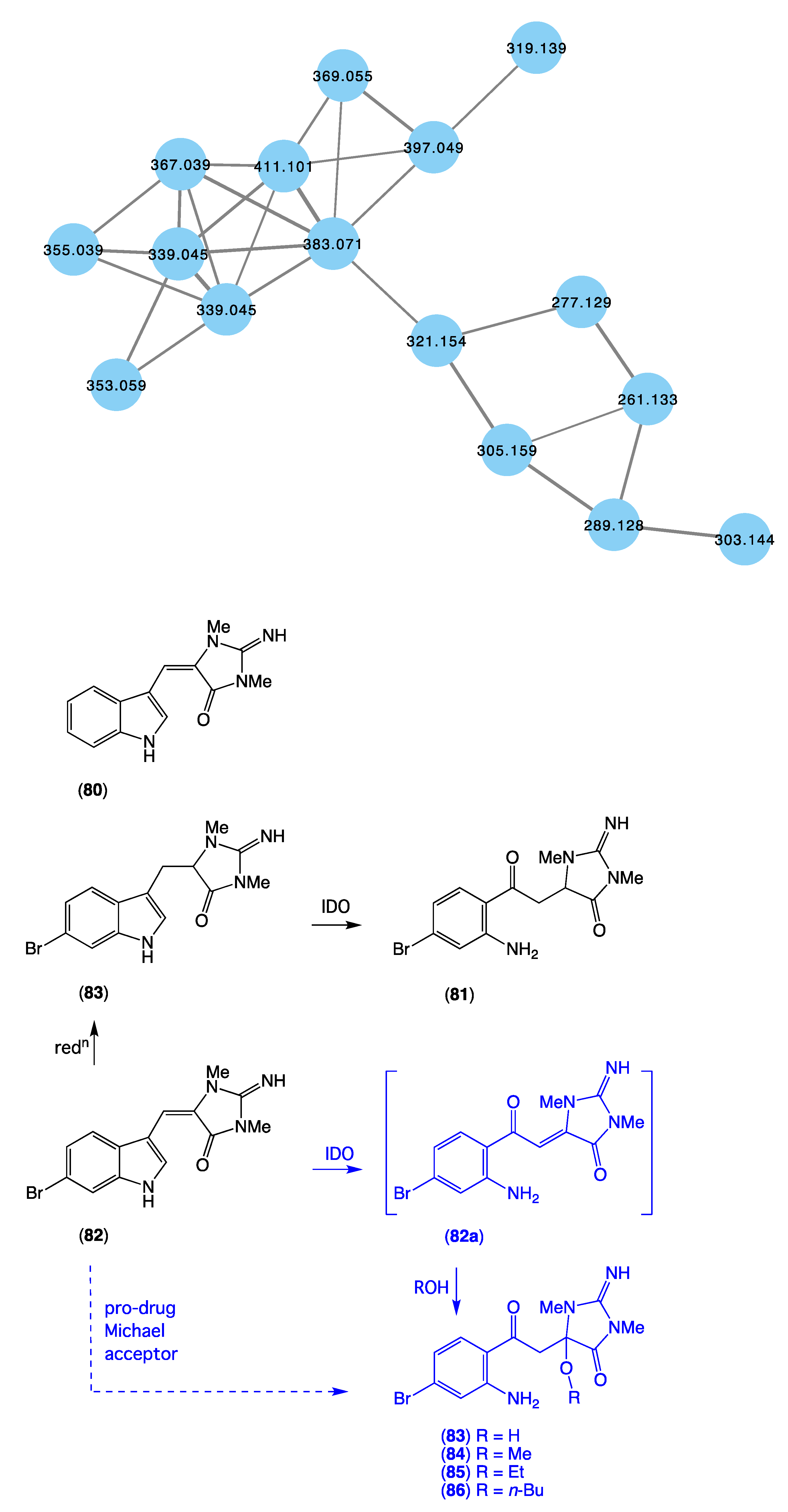
The bright yellow tryptophan alkaloid aplysinopsin (80), first reported in 1977 [77] from a Great Australian Bight sponge of the genus Thorecta sp., heralded the discovery of a great many related and uniquely sponge-derived natural products, including from the genera Verongia, Dercitus, Smenospongia, Dictyoceratida, Aplysina, Hyrtios, and Thorectandra [78]. Many examples of the aplysinopsin family of natural products were reported to exhibit prominent biological properties ranging across antimicrobial, antiviral, antimalarial, anticancer, anti-cholinesterase, and anti-depressant activity [79]. Indeed, some 36 years after first being reported from a northern Australian sponge, we returned to the aplysinopsin scaffold and, in 2013 [80], reported on a series of new analogues from the southern Australia sponge Ianthella cf. flabelliformis (CMB-03322)—examples of which exhibited promising isoform selective GlyRs potentiation with possible application in the development of a new class of analgesic for the treatment of chronic inflammatory pain (see ircinialactams above). Nevertheless, with such an extensive literature on aplysinopsins, the prospect of new discoveries seemed slight—that is, until we reviewed our GNPS data.
The GNPS molecular network shown in Figure 16 revealed a unique molecular family inclusive of multiple nodes associated with Thorectandra choanoides (CMB-01889) collected in 1995 during deep water (−45 m) scientific trawling operations in the Great Australian Bight. As a result, this sponge was targeted for detailed chemical analysis and, in 2021 [15], we reported on a new tryptophan alkaloid, thorectandrin A (81), along with an array of known analogues, including 6-bromoaplysinopsin (82), 6-bromo-1′,8-dihydroaplysinospin (83), and an array of solvolysis adducts 84–87. The GNPS thorectandrin molecular family clusters were particularly useful at revealing solvolysis artifacts. Close examination of these compounds led us to speculate that 82 (and 80) could serve as a substrate for an indoleamine 2,3-dioxygenase-like (IDO) enzyme, resulting in transformation to the intermediate ring opened produce 82a, which, as a potent Michael acceptor, could act as a cellular toxin (explaining some of the observed “aplysinopsin” biology). Alternatively, the intermediate Michael acceptor 82a could form solvolysis adducts during handling, such as 83–86. By contrast, the reduced aplysinopsin analogue 83 might also be viewed as a prospective IDO substrate, being transformed to the thorectandin 81 which was not a Michael acceptor and, hence, not toxic. As a result, the discovery of 81 shed light on a previously unappreciated “aplysinopsin” chemical reactivity and ecology, where some aplysinopsins (i.e., 80 and 82) can be viewed as pro-drug Michael acceptors that are activated by IDO’s in the tissues of species that prey on sponges, allowing them to act as potent Michael acceptor toxins. This same mechanism of action likely plays out in mammalian and microbial cells, explaining the anticancer and anti-infective properties of selected aplysinopsins that are pro-drug Michael acceptors.
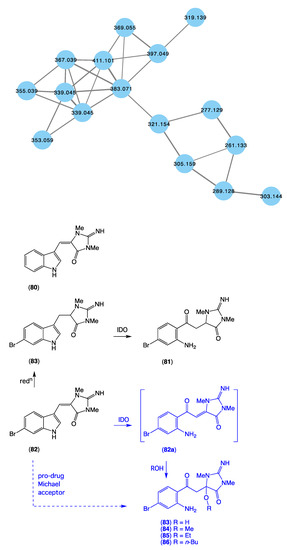
Figure 16.
GNPS molecular cluster uniquely associated with Thorectandra choanoides (CMB-01889) (blue), and structures of aplysinopsin (8), and selected CMB-01889 metabolites 81–83 and solvolysis artifacts 83–86.
5. Conclusions
Hopefully the case studies summarized above revealed how GNPS molecular networking can extract new value from a library of 960 southern Australian marine extracts including;
- Disclosing the rarity of a structure class (i.e., dragmacidins);
- Finding new sources/examples of rare chemistry (i.e., trachycladindoles);
- Prioritizing otherwise stranded extracts to help find new analogues of known structure classes (i.e., franklinolides, bistellettazines, lamellarins, ircinialactams, discorhabdins, trunculins);
- Finding entirely new structure classes (i.e., dysidealactams, cacolides); and
- Discovering new natural products that shed light on ecological properties and pharmacological mechanisms of action (i.e., thorectandrins).
In conclusion, we would like to share observations on some challenges/limitations of GNPS molecular networking:
- Access to UPLC-MS/MS technology: While UPLC-MS/MS technology is increasingly accessible, the levels of access (and cost) can vary greatly between laboratories. To best apply GNPS as a routine biodiscovery dereplication tool requires hands on and reliable access to appropriate instrumentation.
- Access to HRESIMS: In our hands, the accuracy of the m/z values acquired for each GNPS node was insufficient to assign definitive molecular formula (MF). As MF assignments are critical to online database searches (i.e., SciFinder) that are the key to rapidly discriminating known from new natural products, this necessitates separate measurements on targeted molecular families and individual nodes. In due course this technological limitation may be solved by better instrumentation.
- Clusters versus singletons: In any GNPS molecular network, what stands out most are the molecular families (i.e., the clusters of nodes linked by solid lines of varying thickness). That said, all GNPS molecular networks feature a large number of singletons—nodes that are not clustered. While it may be tempting to disregard singletons during any GNPS analysis, this runs the risk of overlooking interesting (even remarkable) natural products—that simply are so unique in their MS/MS fragment that they have no cluster partners. Singletons can (on occasion) count!
- Molecular families: Molecular families are clusters of natural products (nodes) that share a common/related MS/MS fragmentation. As such, members of any given structure class can be incorporated into one or more molecular families, depending on the nature of structure diversity and its impact on MS/MS fragmentation. Do not assume all members of the same structure class will co-cluster.
- Molecular adducts: Some natural products classes can exist as multiple adducts (i.e., M+H, M+Na, M+K, M+NH4), which can cluster separately into different molecular families. This can create the illusion of more structure diversity than is actually the case.
- Ionization: Some natural products do not ionize well under ESI(+) conditions, and as such, would not feature prominently (or at all) in a +ve mode GNPS molecular network. Depending on the structure class you are looking for, you might consider reacquiring a –ve mode GNPS molecular network.
- Structure Proof: Finally, and arguably of greatest importance, there are occurrences in the literature where researchers seemed to defer to GNPS as a definitive proof of structure assignment. This is unwise, especially for chiral natural products, as MS/MS fragmentation is silent on matters of stereochemistry. GNPS can be a valuable guide for dereplication and prioritization, but unless you have access to an authentic natural product standard, it is not a substitute for isolation and more traditional structure elucidation based on spectroscopic analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md21070413/s1, Figure S1: GNPS molecular network for 960 marine extracts (high resolution pdf file).
Author Contributions
Conceptualized the research and assembled the marine sponge collection, R.J.C.; GNPS analysis, S.K.; writing—original draft preparation, S.K., A.A.S. and R.J.C.; writing—review and editing, A.A.S. and R.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The full MS/MS data of the 980 sponge extracts can be accessed from ftp://massive.ucsd.edu/MSV000086621/ (accessed on 16 July 2023).
Acknowledgments
We thank The University of Queensland, Institute for Molecular Bioscience for supporting our research, and to the many students, staff, and visiting researchers that have, over the years, contributed to refining and optimizing our methods in marine biodiscovery.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hong, L.-L.; Ding, Y.-F.; Zhang, W.; Lin, H.-W. Chemical and biological diversity of new natural products from marine sponges: A review (2009–2018). Mar. Life Sci. Technol. 2022, 4, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible Molecular Networking of Untargeted Mass Spectrometry Data Using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Crüsemann, M.; O’Neill, E.C.; Larson, C.B.; Melnik, A.V.; Floros, D.J.; da Silva, R.R.; Jensen, P.R.; Dorrestein, P.C.; Moore, B.S. Prioritizing natural product diversity in a collection of 146 bacterial strains based on growth and extraction protocols. J. Nat. Prod. 2017, 80, 588–597. [Google Scholar] [CrossRef]
- Scarpato, S.; Teta, R.; Della Sala, G.; Pawlik, J.R.; Costantino, V.; Mangoni, A. New tricks with an old sponge: Feature-based molecular networking led to fast identification of new stylissamide L from Stylissa caribica. Mar. Drugs 2020, 18, 443. [Google Scholar] [CrossRef]
- Bracegirdle, J.; Stevenson, L.J.; Page, M.J.; Owen, J.G.; Keyzers, R.A. Targeted Isolation of rubrolides from the New Zealand marine tunicate Synoicum kuranui. Mar. Drugs 2020, 18, 337. [Google Scholar] [CrossRef]
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Scopularides revisited: Molecular networking guided exploration of lipodepsipeptides in Australian marine fish gastrointestinal tract-derived fungi. Mar. Drugs 2019, 17, 475. [Google Scholar] [CrossRef]
- Elbanna, A.H.; Agampodi Dewa, A.; Khalil, Z.G.; Capon, R.J. Precursor-directed biosynthesis mediated amplification of minor aza phenylpropanoid piperazines in an Australian marine fish-gut-derived fungus, Chrysosporium sp. CMB-F214. Mar. Drugs 2021, 19, 478. [Google Scholar] [CrossRef]
- Agampodi Dewa, A.; Khalil, Z.G.; Elbanna, A.H.; Capon, R.J. Chrysosporazines revisited: Regioisomeric phenylpropanoid piperazine P-glycoprotein inhibitors from Australian marine fish-derived fungi. Molecules 2022, 27, 3172. [Google Scholar] [CrossRef]
- Dewa, A.A.; Elbanna, A.H.; Khalil, Z.G.; Capon, R.J. Neochrysosporazines: Precursor-directed biosynthesis defines a marine-derived fungal natural product P-glycoprotein inhibitory pharmacophore. J. Med. Chem. 2022, 65, 2610–2622. [Google Scholar] [CrossRef]
- Wu, T.; Salim, A.A.; Bernhardt, P.V.; Capon, R.J. Amaurones A–K: Polyketides from the fish gut-derived fungus Amauroascus sp. CMB-F713. J. Nat. Prod. 2021, 84, 474–482. [Google Scholar] [CrossRef]
- Khushi, S.; Nahar, L.; Salim, A.A.; Capon, R.J. Trachycladindoles H–M: Molecular networking guided exploration of a library of Southern Australian marine sponges. Aust. J. Chem. 2020, 73, 338–343. [Google Scholar] [CrossRef]
- Khushi, S.; Salim, A.A.; Elbanna, A.H.; Nahar, L.; Bernhardt, P.V.; Capon, R.J. Dysidealactams and dysidealactones: Sesquiterpene glycinyl-lactams, imides, and lactones from a Dysidea sp. marine sponge collected in southern Australia. J. Nat. Prod. 2020, 83, 1577–1584. [Google Scholar] [CrossRef]
- Khushi, S.; Nahar, L.; Salim, A.A.; Capon, R.J. Cacolides: Sesterterpene butenolides from a Southern Australian marine sponge, Cacospongia sp. Mar. Drugs 2018, 16, 456. [Google Scholar] [CrossRef]
- Khushi, S.; Salim, A.A.; Elbanna, A.H.; Nahar, L.; Capon, R.J. New from old: Thorectandrin alkaloids in a Southern Australian marine sponge, Thorectandra choanoides (CMB-01889). Mar. Drugs 2021, 19, 97. [Google Scholar] [CrossRef]
- Zhang, H.; Conte, M.M.; Capon, R.J. Franklinolides A–C from an Australian marine sponge complex: Phosphodiesters strongly enhance polyketide cytotoxicity. Angew. Chem. 2010, 122, 10100–10102. [Google Scholar] [CrossRef]
- El-Naggar, M.; Piggott, A.M.; Capon, R.J. Bistellettazines A−C and bistellettazole A: New terpenyl− pyrrolizidine and terpenyl− imidazole alkaloids from a Southern Australian marine sponge, Stelletta sp. Org. Lett. 2008, 10, 4247–4250. [Google Scholar] [CrossRef]
- Capon, R.J.; Rooney, F.; Murray, L.M.; Collins, E.; Sim, A.T.R.; Rostas, J.A.P.; Butler, M.S.; Carroll, A.R. Dragmacidins: New protein phosphatase inhibitors from a southern australian deep-water marine sponge, Spongosorites sp. J. Nat. Prod. 1998, 61, 660–662. [Google Scholar] [CrossRef]
- Huang, X.-C.; Xiao, X.; Zhang, Y.-K.; Talele, T.T.; Salim, A.A.; Chen, Z.-S.; Capon, R.J. Lamellarin O, a pyrrole alkaloid from an Australian marine sponge, Ianthella sp., reverses BCRP mediated drug resistance in cancer cells. Mar. Drugs 2014, 12, 3818–3837. [Google Scholar] [CrossRef]
- Balansa, W.; Islam, R.; Fontaine, F.; Piggott, A.M.; Zhang, H.; Webb, T.I.; Gilbert, D.F.; Lynch, J.W.; Capon, R.J. Ircinialactams: Subunit-selective glycine receptor modulators from Australian sponges of the family Irciniidae. Bioorg. Med. Chem. 2010, 18, 2912–2919. [Google Scholar] [CrossRef]
- Ford, J.; Capon, R.J. Discorhabdin R: A new antibacterial pyrroloiminoquinone from two latrunculiid marine sponges, Latrunculia sp. and Negombata sp. J. Nat. Prod. 2000, 63, 1527–1528. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K.; Willis, A.C. Trunculins A and B, norsesterterpene cyclic peroxides from a marine sponge, Latrunculia brevis. J. Org. Chem. 1987, 52, 339–342. [Google Scholar] [CrossRef]
- Vuong, D.; Capon, R.J. Phorbasin A: A novel diterpene from a southern Australian marine sponge, Phorbas species. J. Nat. Prod. 2000, 63, 1684–1685. [Google Scholar] [CrossRef]
- Salim, A.A.; Rae, J.; Fontaine, F.; Conte, M.M.; Khalil, Z.; Martin, S.; Parton, R.G.; Capon, R.J. Heterofibrins: Inhibitors of lipid droplet formation from a deep-water southern Australian marine sponge, Spongia (Heterofibria) sp. Org. Biomol. Chem. 2010, 8, 3188–3194. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Capon, R.J.; Lacey, E.; Gill, J.H.; Friedel, T.; Wadsworth, D. Amphilactams A−D: Novel nematocides from Southern Australian Marine sponges of the Genus Amphimedon. J. Org. Chem. 1999, 64, 1140–1144. [Google Scholar] [CrossRef]
- Capon, R.J.; Peng, C.; Dooms, C. Trachycladindoles A–G: Cytotoxic heterocycles from an Australian marine sponge, Trachycladus laevispirulifer. Org. Biomol. Chem. 2008, 6, 2765–2771. [Google Scholar] [CrossRef]
- Jamison, M.T.; Molinski, T.F. Jamaicensamide A, a peptide containing β-amino-α-keto and thiazole-homologated η-amino acid residues from the sponge Plakina jamaicensis. J. Nat. Prod. 2016, 79, 2243–2249. [Google Scholar] [CrossRef]
- Della-Felice, F.; de Andrade Bartolomeu, A.; Pilli, R.A. The phosphate ester group in secondary metabolites. Nat. Prod. Rep. 2022, 39, 1066–1107. [Google Scholar] [CrossRef]
- Urban, S.; Butler, M.S.; Capon, R.J. Lamellarins O and P: New aromatic metabolites from the Australian marine sponge Dendrilla cactos. Aust. J. Chem. 1994, 47, 1919–1924. [Google Scholar] [CrossRef]
- Urban, S.; Hobbs, L.; Hooper, J.N.A.; Capon, R.J. Lamellarins Q and R: New aromatic metabolites from an Australian marine sponge, Dendrilla cactos. Aust. J. Chem. 1995, 48, 1491–1494. [Google Scholar] [CrossRef]
- Zhang, H.; Conte, M.M.; Huang, X.-C.; Khalil, Z.; Capon, R.J. A search for BACE inhibitors reveals new biosynthetically related pyrrolidones, furanones and pyrroles from a southern Australian marine sponge, Ianthella sp. Org. Biomol. Chem. 2012, 10, 2656–2663. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R.J. Lamellarin-S: A new aromatic metabolite from an Australian tunicate, Didemnum sp. Aust. J. Chem. 1996, 49, 711–713. [Google Scholar] [CrossRef]
- Plisson, F.; Huang, X.-C.; Zhang, H.; Khalil, Z.; Capon, R.J. Lamellarins as Inhibitors of P-Glycoprotein-Mediated Multidrug Resistance in a Human Colon Cancer Cell Line. Chem. Asian J. 2012, 7, 1616–1623. [Google Scholar] [CrossRef]
- Plisson, F.; Conte, M.; Khalil, Z.; Huang, X.-C.; Piggott, A.M.; Capon, R.J. Kinase lnhibitor scaffolds against neurodegenerative diseases from a Southern Australian ascidian, Didemnum sp. Chem. Med. Chem. 2012, 7, 983–990. [Google Scholar] [CrossRef]
- Prasad, P.; Zhang, A.; Salim, A.A.; Capon, R.J. Pursuing sesterterpene lactams in Australian Irciniidae sponges. Fitoterapia 2018, 126, 83–89. [Google Scholar] [CrossRef]
- El-Naggar, M.; Capon, R.J. Discorhabdins revisited: Cytotoxic alkaloids from Southern Australian marine sponges of the genera Higginsia and Spongosorites. J. Nat. Prod. 2009, 72, 1368, Erratum in J. Nat. Prod. 2009, 72, 460–464. [Google Scholar] [CrossRef]
- El-Naggar, M.; Capon, R.J. Discorhabdins revisited: Cytotoxic alkaloids from Southern Australian marine sponges of the genera Higginsia and Spongosorites. J. Nat. Prod. 2009, 72, 460–464. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. Trunculin-F and contrunculin-A and -B: Novel oxygenated norterpenes from a southern Australian marine sponge, Latrunculia conulosa. Aust. J. Chem. 1993, 46, 1363–1374. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Capon, R.J. Trunculins G-I: New norsesterterpene cyclic peroxides from a southern Australian marine sponge, Latrunculia sp. Aust. J. Chem. 1998, 51, 573–579. [Google Scholar] [CrossRef]
- Capon, R.J.; Macleod, J.K. Structural and stereochemical studies on marine norterpene cyclic peroxides. Tetrahedron 1985, 41, 3391–3404. [Google Scholar] [CrossRef]
- Medina Padilla, M.; Orozco Munoz, L.; Capon, R. Therapeutic Use of Indole-Dihydro-Imidazole Derivatives. WO2013167635, 13 November 2013. [Google Scholar]
- Capon, R.J. Extracting value: Mechanistic insights into the formation of natural product artifacts—Case studies in marine natural products. Nat. Prod. Rep. 2020, 37, 55–79. [Google Scholar] [CrossRef]
- Sharma, G.M.; Vig, B. Studies on the antimicrobial substances of sponges. VI. Structures of two antibacterial substances isolated from the marine sponge Dysidea herbacea. Tetrahedron Lett. 1972, 13, 1715–1718. [Google Scholar] [CrossRef]
- Norton, R.S.; Wells, R.J. Use of 13C spin-lattice relaxation measurements to determine the structure of a tetrabromo diphenyl ether from the sponge Dysidea herbacea. Tetrahedron Lett. 1980, 21, 3801–3804. [Google Scholar] [CrossRef]
- Hofheinz, W.; Oberhänsli, W.E. Dysidin, a novel chlorine containing natural product from the sponge Dysidea herbacea. Helv. Chim. Acta 1977, 60, 660–669. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Lidgard, R.O.; Wells, R.J.; Vetter, W. A novel hexachloro-metabolite from the sponge Dysidea herbacea. Tetrahedron Lett. 1977, 18, 3183–3186. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Wells, R.J. A new sesquiterpene from the sponge Dysidea herbacea. Tetrahedron Lett. 1978, 19, 4949–4950. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Wells, R.J.; Daly, J.J.; Schönholzer, P. Two sesquiterpene furans with new carbocyclic ring systems and related thiol acetates from a species of the sponge genus Dysidea. Tetrahedron Lett. 1978, 19, 4951–4954. [Google Scholar] [CrossRef]
- Dunlop, R.W.; Kazlauskas, R.; March, G.; Murphy, P.T.; Wells, R.J. New furano-sesquiterpenes from the sponge Dysidea herbacea. Aust. J. Chem. 1982, 35, 95–103. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. Beyond polygodial: New drimane sesquiterpenes from a Southern Australian marine sponge, Dysidea sp. Aust. J. Chem. 1993, 46, 1255–1267. [Google Scholar] [CrossRef]
- Garson, M.J.; Dexter, A.F.; Lambert, L.K.; Liokas, V. Isolation of the bioactive terpene 7-deacetoxy-olepupuane from the temperate marine sponge Dysidea sp. J. Nat. Prod. 1992, 55, 364–367. [Google Scholar] [CrossRef]
- Flowers, A.E.; Garson, M.J.; Byriel, K.A.; Kennard, C.H.L. Two new isonakafurans from the Great Barrier Reef sponge Dysidea sp. nov. Aust. J. Chem. 1998, 51, 195–200. [Google Scholar] [CrossRef]
- Cameron, G.M.; Stapleton, B.L.; Simonsen, S.M.; Brecknell, D.J.; Garson, M.J. New sesquiterpene and brominated metabolites from the tropical marine sponge Dysidea sp. Tetrahedron 2000, 56, 5247–5252. [Google Scholar] [CrossRef]
- Searle, P.A.; Jamal, N.M.; Lee, G.M.; Moliski, T.F. Configurational analysis of new furanosesquiterpenes from Dysidea herbacea. Assignment of absolute stereochemistry. Tetrahedron 1994, 50, 3879–3888. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K. Thiofurodysinin, a sulfur-containing furanosesquiterpene from the marine sponge Dysidea avara. J. Nat. Prod. 1987, 50, 1136–1137. [Google Scholar] [CrossRef]
- Lee, G.M.; Molinski, T.F. Herbaceamide, a chlorinated N-acyl amino ester from the marine sponge, Dysidea herbacea. Tetrahedron Lett. 1992, 33, 7671–7674. [Google Scholar] [CrossRef]
- Murray, L.; Sim, A.; Rostas, J.; Capon, R. Isopalinurin: A mild protein phosphatase inhibitor from a Southern Australian marine sponge, Dysidea sp. Aust. J. Chem. 1993, 46, 1291–1294. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Bemis, J.E.; Bourne, D.J. Ultraviolet absorbing pigments from the marine sponge Dysidea herbacea: Isolation and structure of a new mycosporine. Comp. Biochem. Phys. C 1996, 115, 281–286. [Google Scholar] [CrossRef]
- Dumdei, E.J.; Simpson, J.S.; Garson, M.J.; Byriel, K.A.; Kennard, C.H.L. New chlorinated metabolites from the tropical marine sponge Dysidea herbacea. Aust. J. Chem. 1997, 50, 139–144. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Molinski, T.F. Herbacic acid, a simple prototype of 5, 5, 5-trichloroleucine metabolites from the sponge Dysidea herbacea. J. Nat. Prod. 2000, 63, 155–157. [Google Scholar] [CrossRef]
- Stapleton, B.L.; Cameron, G.M.; Garson, M.J. New chlorinated peptides from the tropical marine sponge Dysidea sp. Tetrahedron 2001, 57, 4603–4607. [Google Scholar] [CrossRef]
- Norton, R.S.; Croft, K.D.; Wells, R.J. Polybrominated oxydiphenol derivatives from the sponge Dysidea herbacea: Structure determination by analysis of 13C spin-lattice relaxation data for quaternary carbons and 13C-1H coupling constants. Tetrahedron 1981, 37, 2341–2349. [Google Scholar] [CrossRef]
- Bowden, B.F.; Towerzey, L.; Junk, P.C. A new brominated diphenyl ether from the marine sponge Dysidea herbacea. Aust. J. Chem. 2000, 53, 299–301. [Google Scholar] [CrossRef]
- Agrawal, M.S.; Bowden, B.F. Marine sponge Dysidea herbacea revisited: Another brominated diphenyl ether. Mar. Drugs 2005, 3, 9–14. [Google Scholar] [CrossRef]
- Utkina, N.K.; Denisenko, V.A.; Scholokova, O.V.; Virovaya, M.V.; Gerasimenko, A.V.; Popov, D.Y.; Krasokhin, V.B.; Popov, A.M. Spongiadioxins A and B, two new polybrominated dibenzo-p-dioxins from an Australian marine sponge Dysidea dendyi. J. Nat. Prod. 2001, 64, 151–153. [Google Scholar] [CrossRef]
- Capon, R.J.; Faulkner, D.J. Herbasterol, an ichthyotoxic 9, 11-secosterol from the sponge Dysidea herbacea. J. Org. Chem. 1985, 50, 4771–4773. [Google Scholar] [CrossRef]
- De Almeida Leone, P.; Redburn, J.; Hooper, J.N.A.; Quinn, R.J. Polyoxygenated Dysidea sterols that inhibit the binding of [I125] IL-8 to the human recombinant IL-8 receptor type A. J. Nat. Prod. 2000, 63, 694–697. [Google Scholar] [CrossRef]
- Jacob, M.R.; Hossain, C.F.; Mohammed, K.A.; Smillie, T.J.; Clark, A.M.; Walker, L.A.; Nagle, D.G. Reversal of fluconazole resistance in multidrug efflux-resistant fungi by the Dysidea arenaria sponge sterol 9α,11α-epoxycholest-7-ene-3β,5α, 6α,19-tetrol 6-acetate. J. Nat. Prod. 2003, 66, 1618–1622. [Google Scholar] [CrossRef]
- Chen, Y.; Lan, P.; White, L.V.; Yang, W.; Banwell, M.G. Total syntheses of dysidealactams E and F and dysidealactone B, drimane-type sesquiterpenes derived from a Dysidea sp. of marine sponge. Synlett 2023, 34, 1529–1533. [Google Scholar] [CrossRef]
- Cimino, G.; De Stefano, S.; Minale, L.; Fattorusso, E. Ircinin-1 and -2, linear sesterterpenes from the marine sponge Ircinia oros. Tetrahedron 1972, 28, 333–341. [Google Scholar] [CrossRef]
- Faulkner, J.D. Variabilin, an antibiotic from the sponge, Ircinia variabilis. Tetrahedron Lett. 1973, 14, 3821–3822. [Google Scholar] [CrossRef]
- Capon, R.J.; Dargaville, T.R.; Davis, R. The absolute stereochemistry of variabilin and related sesterterpene tetronic acids. Nat. Prod. Lett. 1994, 4, 51–56. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K. A new sesterterpene tetronic acid from an Australian sponge, Ircinia sp. Aust. J. Chem. 1987, 40, 1327–1330. [Google Scholar] [CrossRef]
- Balansa, W.; Islam, R.; Fontaine, F.; Piggott, A.M.; Zhang, H.; Xiao, X.; Webb, T.I.; Gilbert, D.F.; Lynch, J.W.; Capon, R.J. Sesterterpene glycinyl-lactams: A new class of glycine receptor modulator from Australian marine sponges of the genus Psammocinia. Org. Biomol. Chem. 2013, 11, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.; Hamit, H.; Hooper, J.N.A.; Hobbs, L.; Capon, R.J. A new sesterterpene tetronic acid from an Australian marine sponge, Psammocinia sp. Aust. J. Chem. 1995, 48, 1899–1902. [Google Scholar] [CrossRef]
- Davis, R.; Capon, R.J. Two for one: Structure revision of the marine sesterterpene tetronic acid strobilinin to (8Z,13E,20Z)-strobilinin and (8E,13Z,20Z)-strobilinin. Aust. J. Chem. 1994, 47, 933–936. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. Aplysinopsin, a new tryptophan derivative from a sponge. Tetrahedron Lett. 1977, 18, 61–64. [Google Scholar] [CrossRef]
- Bialonska, D.; Zjawiony, J.K. Aplysinopsins-marine indole alkaloids: Chemistry, bioactivity and ecological significance. Mar. Drugs 2009, 7, 166–183. [Google Scholar] [CrossRef]
- El-Sawy, E.R.; Kirsch, G. An overview of aplysinopsins: Synthesis and biological activities. Mar. Drugs 2023, 21, 268. [Google Scholar] [CrossRef]
- Balansa, W.; Islam, R.; Gilbert, D.F.; Fontaine, F.; Xiao, X.; Zhang, H.; Piggott, A.M.; Lynch, J.W.; Capon, R.J. Australian marine sponge alkaloids as a new class of glycine-gated chloride channel receptor modulator. Bioorg. Med. Chem. 2013, 21, 4420–4425. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).