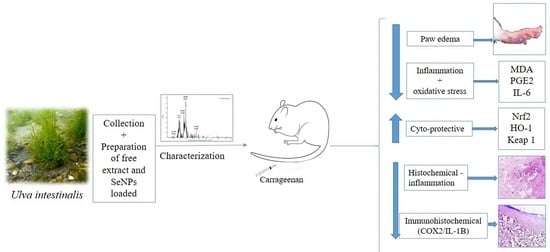
New Insight on the Cytoprotective/Antioxidant Pathway Keap1/Nrf2/HO-1 Modulation by Ulva intestinalis Extract and Its Selenium Nanoparticles in Rats with Carrageenan-Induced Paw Edema
Abstract
1. Introduction
2. Results
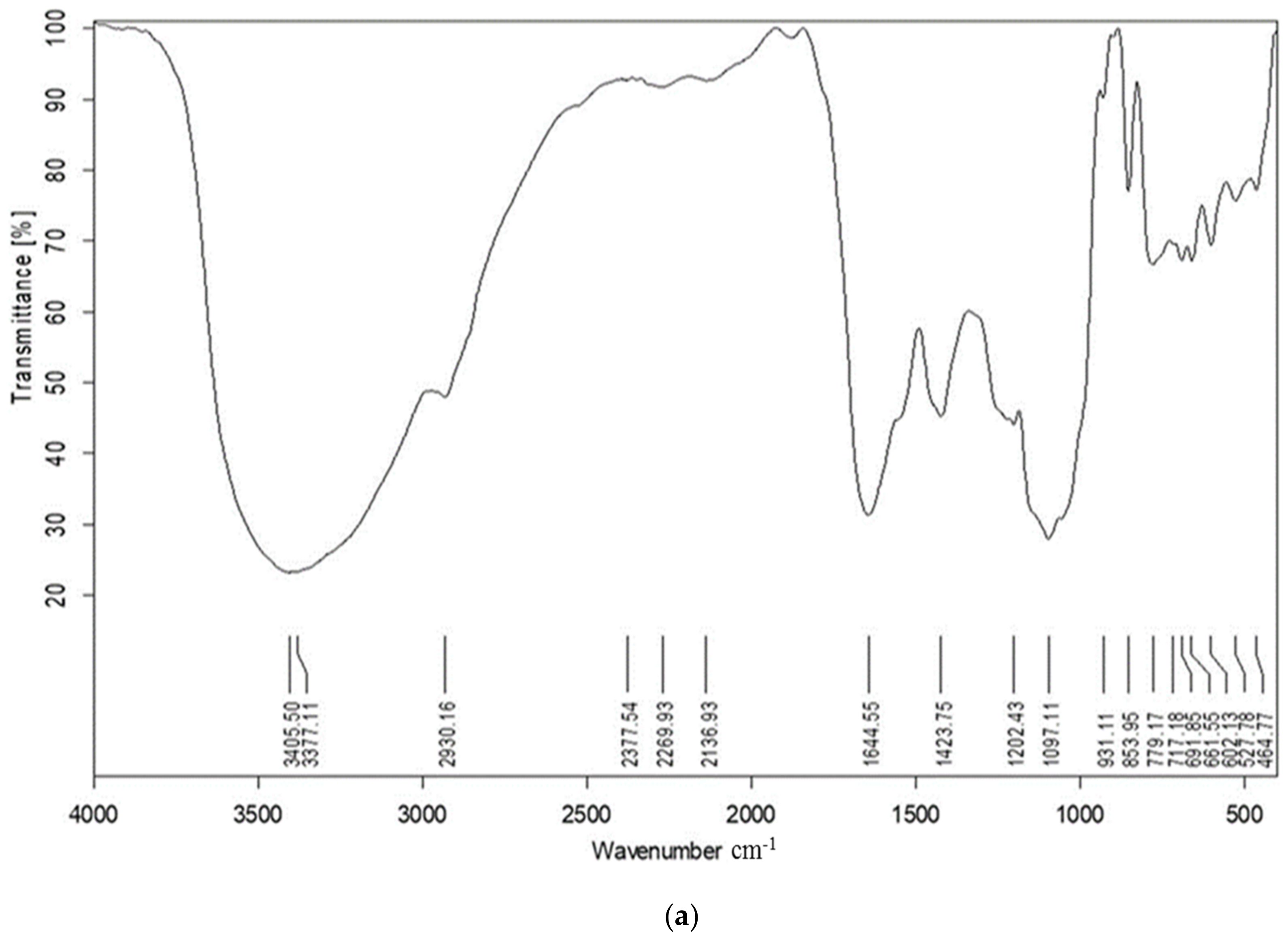
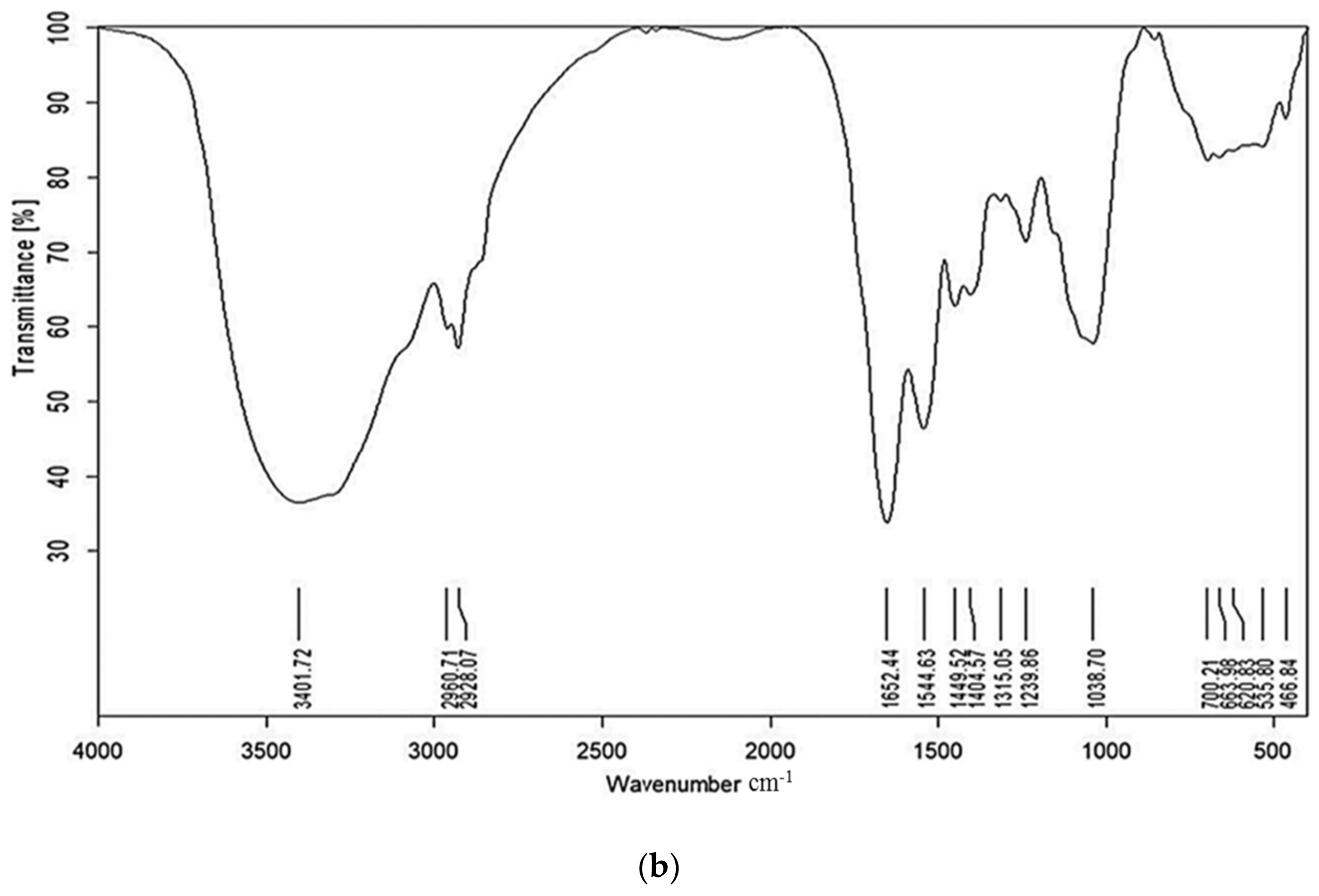
2.1. FT-IR
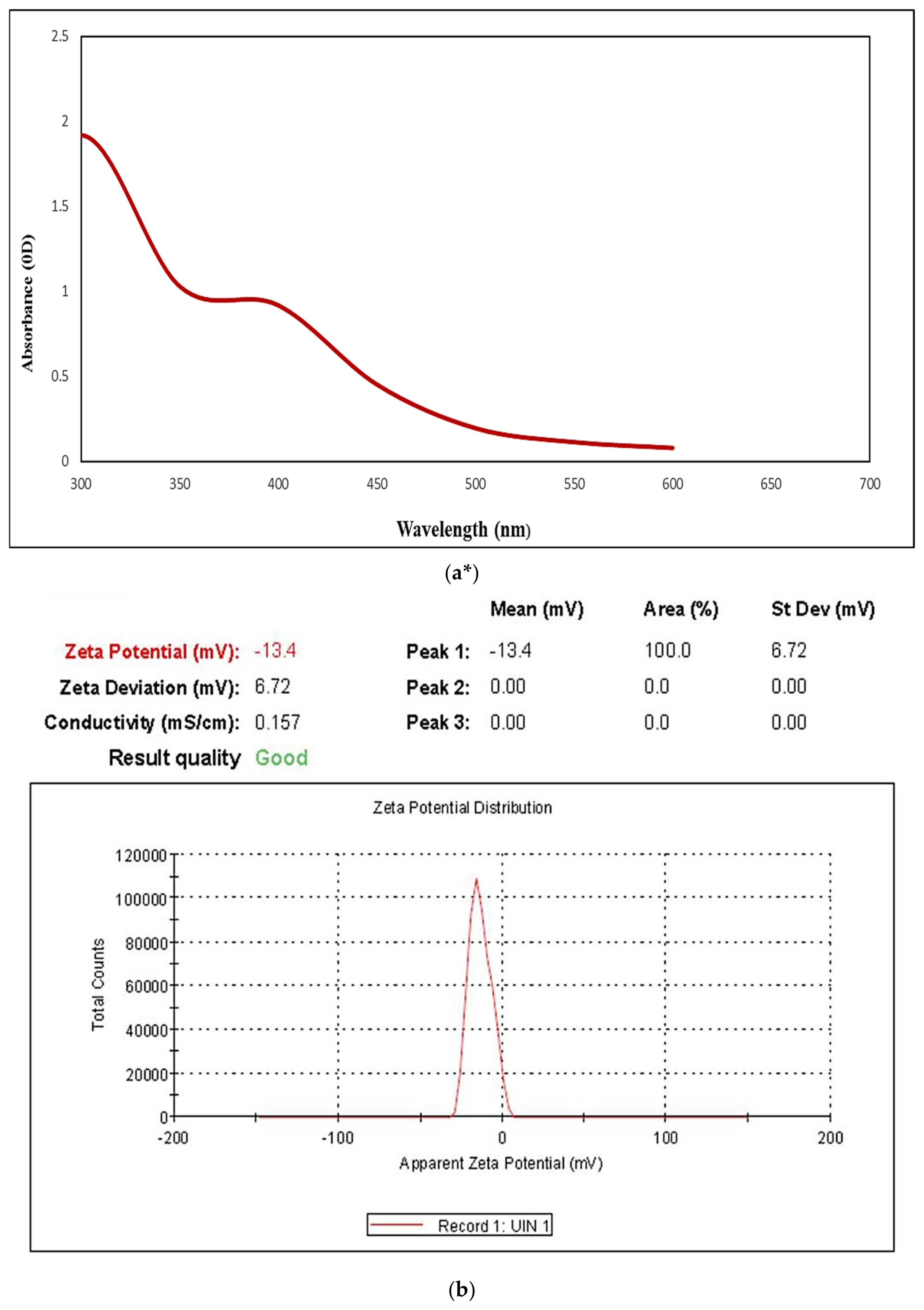
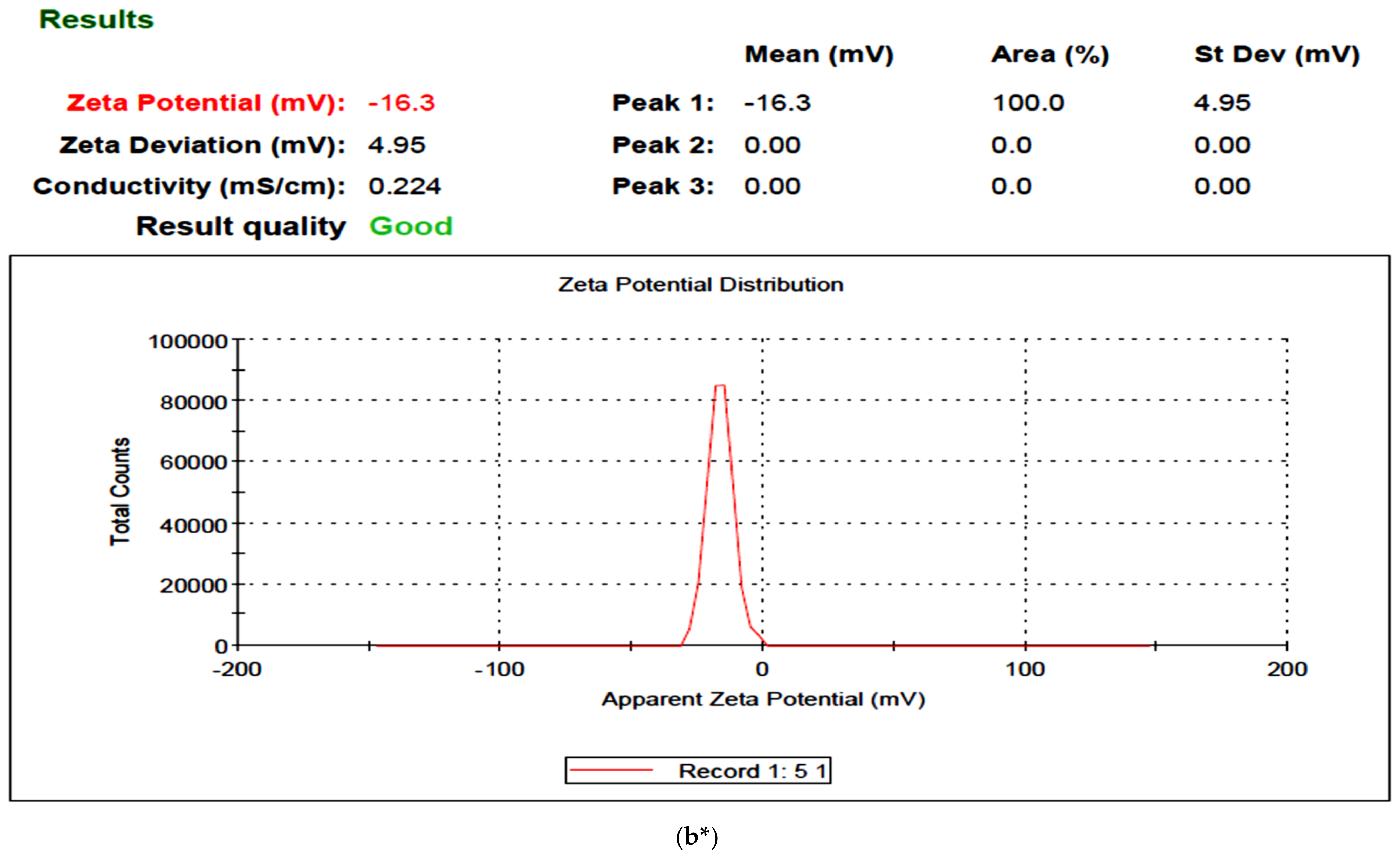
2.2. UISeNPs UV Spectra and Zeta Potential
2.3. XRD
2.4. TEM Imaging
2.5. SEM Imaging
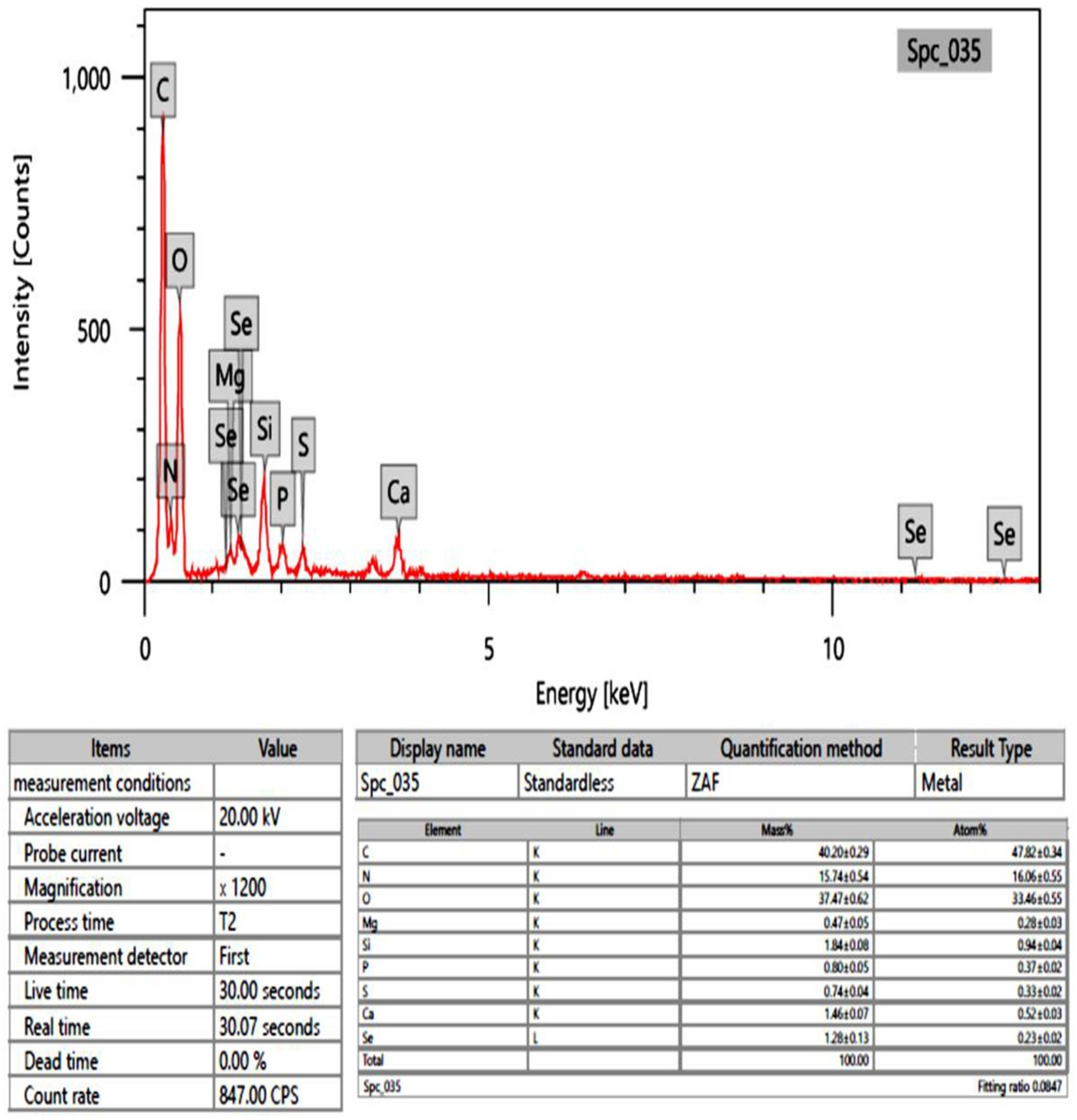
2.6. EDX Analysis
2.7. Effect of Treatments on the Weight of Paw Edema
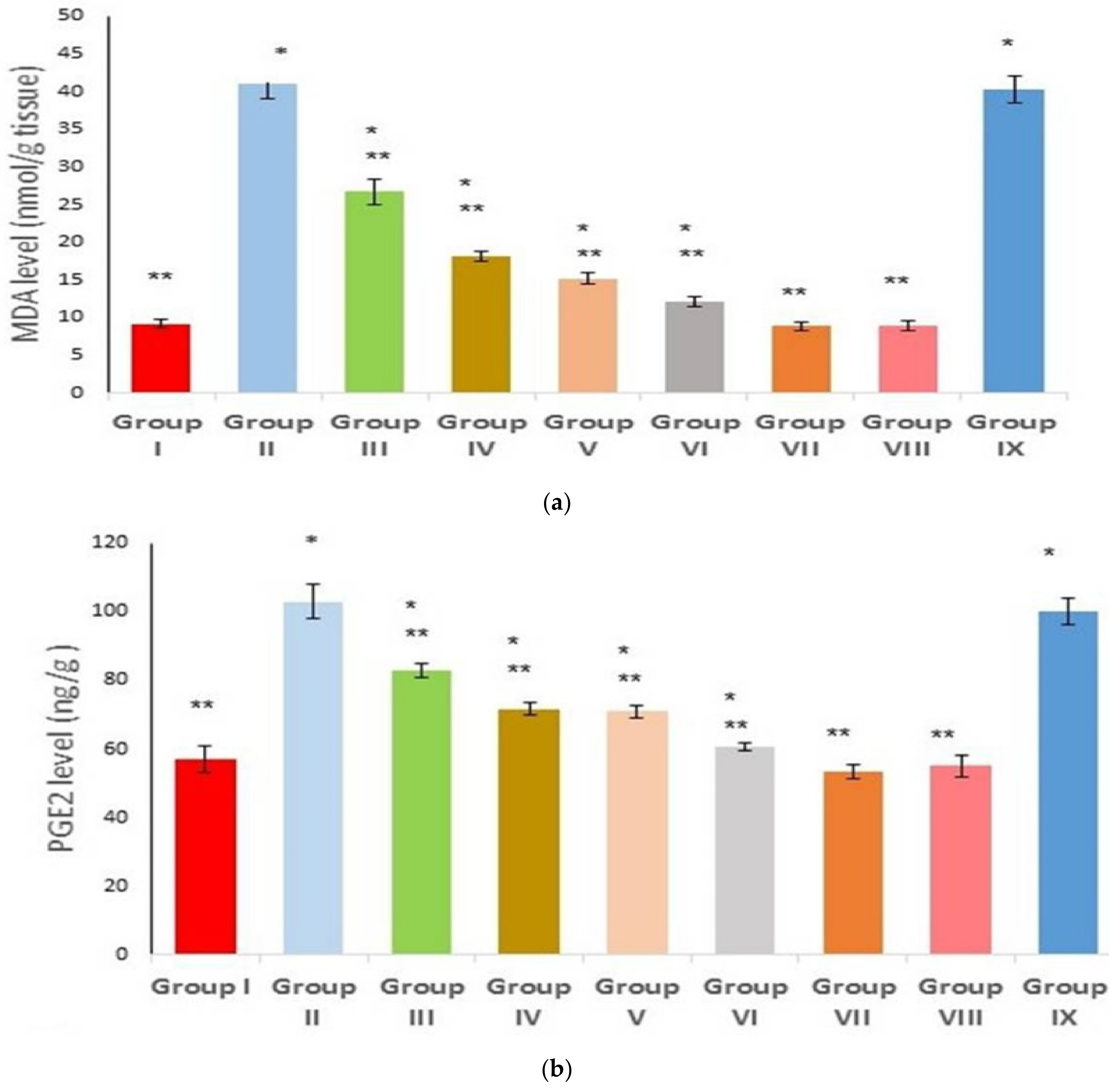
2.8. Effect on Inflammatory and Oxidative Stress Markers
2.9. Effect on IL-6, Keap 1, Nrf2, and HO-1 Gene Expression
2.10. Effect on Keap 1, Nrf2, and HO-1 Protein Level
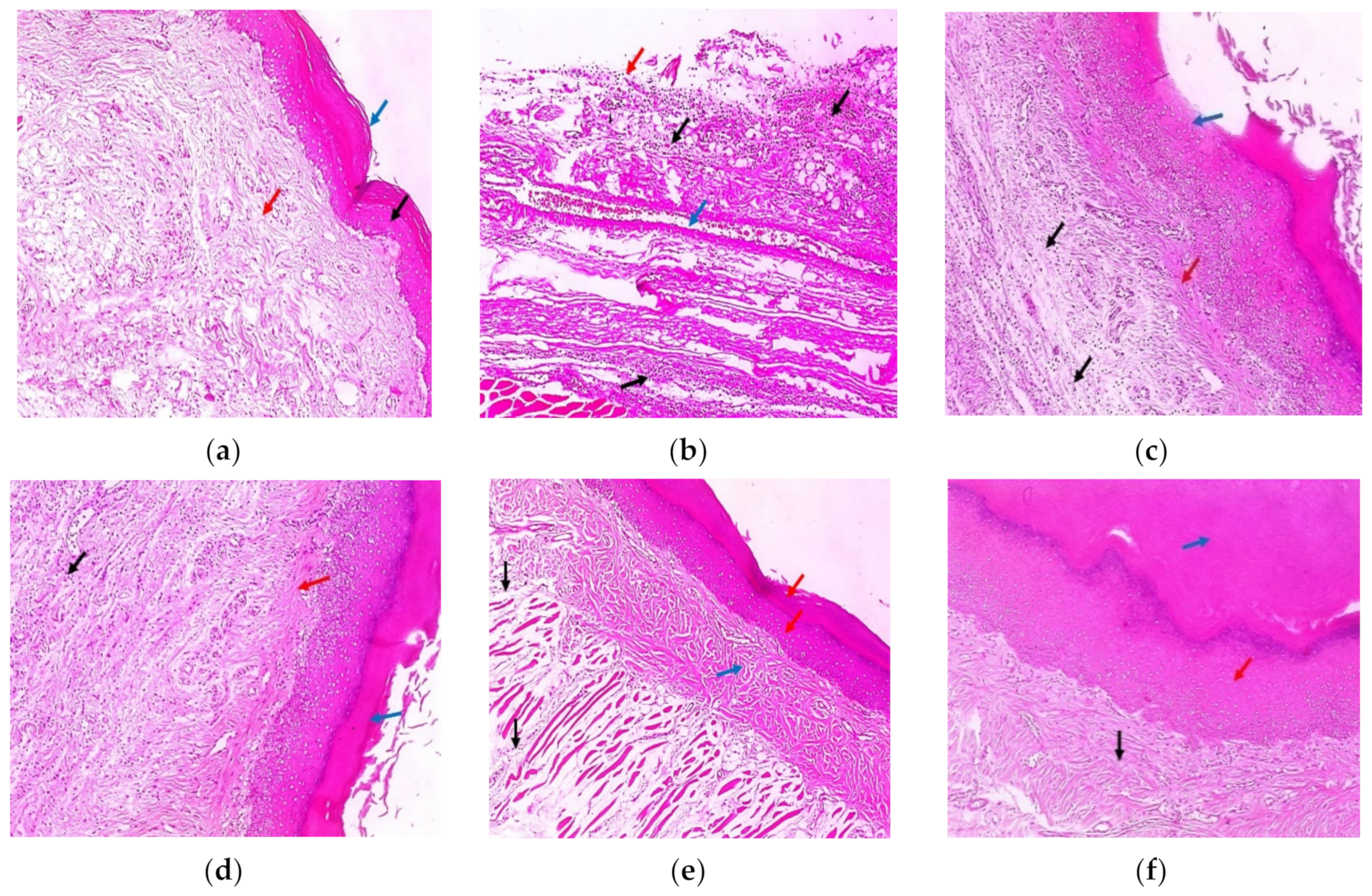
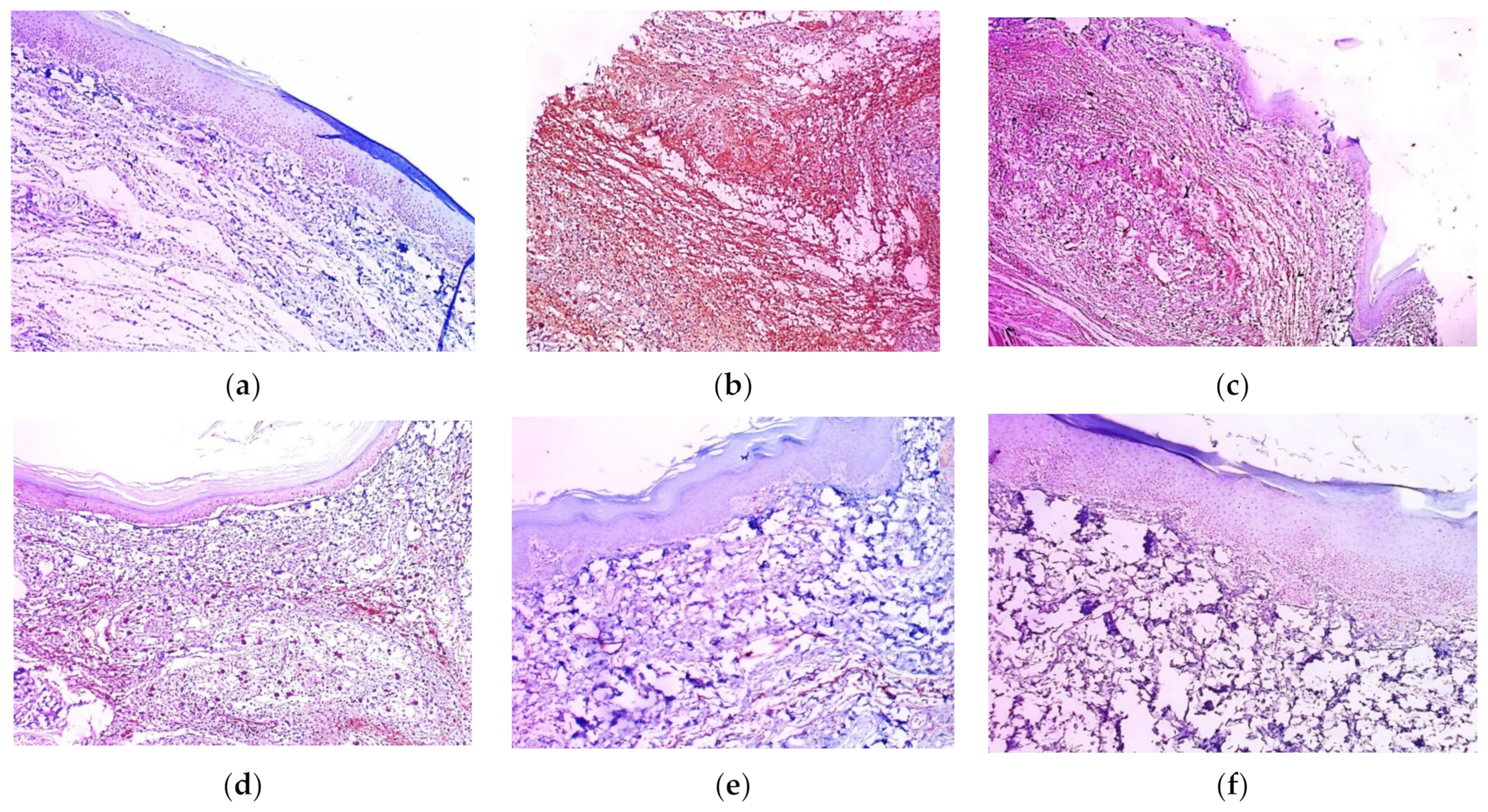
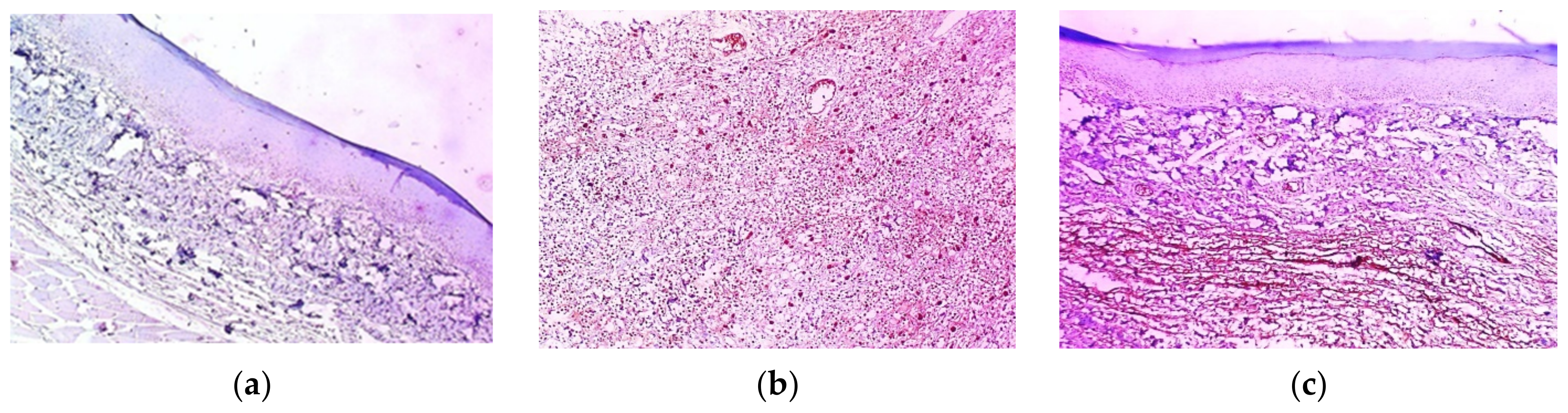
2.11. Histopathological Examination
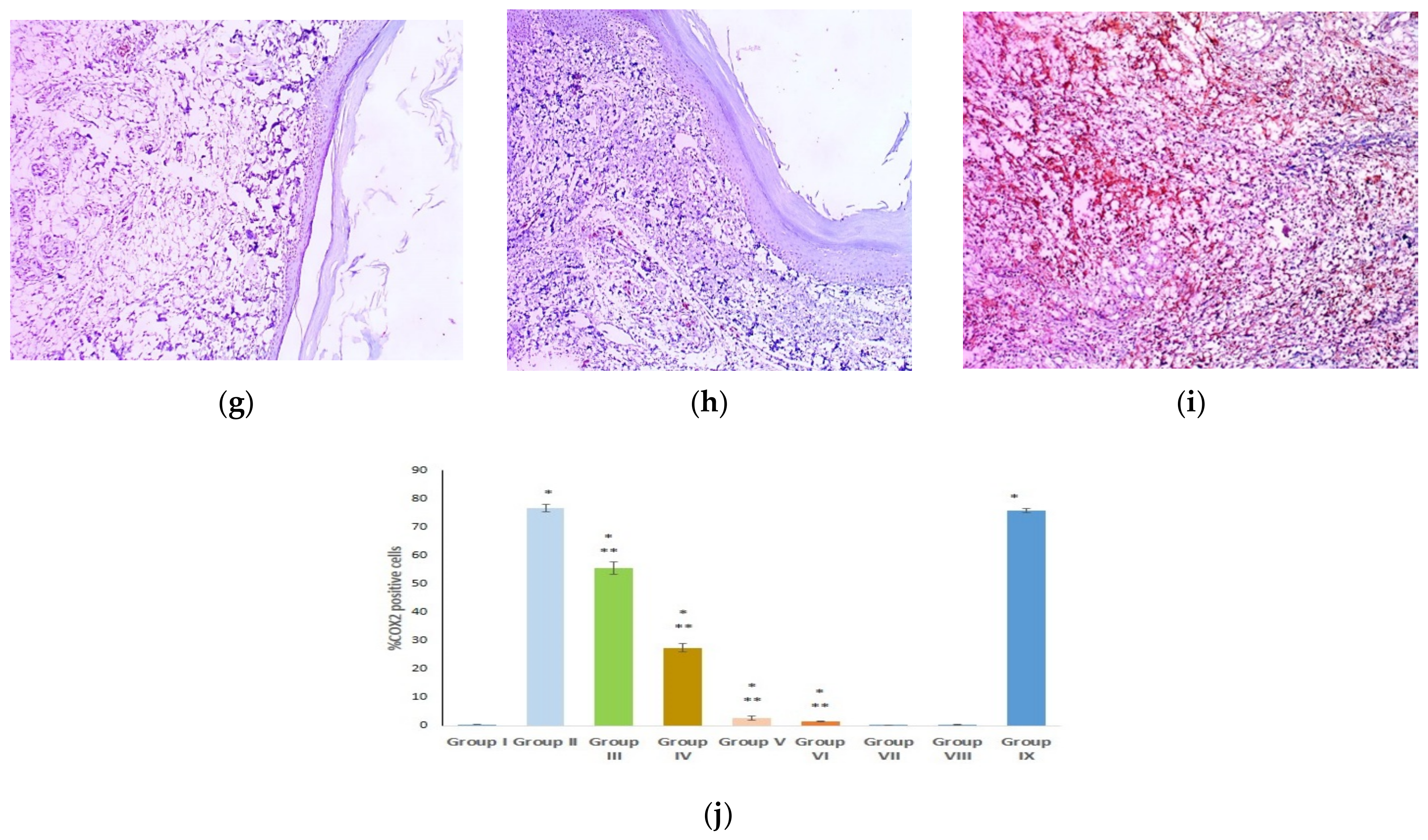
2.12. Immunohistochemical Examination
3. Discussion
4. Materials and Methods
4.1. Ulva Intestinalis Collection, Processing, and Aqueous Extract Preparation
4.2. Preparation of Ulva-Intestinalis-Mediated Selenium Nanoparticles (UISeNPs)
4.3. Characterization of Phycosynthesized SeNPs
4.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
4.3.2. X-ray Diffraction (XRD) Analysis
4.3.3. Transmission Electron Microscopy (TEM)
4.3.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX)
4.3.5. Zeta Potential (ζ) and Polydispersity Index (PDI)
4.4. Experimental Model for Inflammation Induction
4.4.1. Animals
4.4.2. Experimental Design and Animal Groups
4.4.3. Sample Collection
4.4.4. Measurement of Prostaglandin E2 (PGE2)
4.4.5. Measurement of Malondialdehyde (MDA)
4.4.6. Measurement of IL-6, HO-1, Keap1, and Nrf2 Gene Expression
4.4.7. Measurement of HO-1, Keap1, and Nrf2 Protein Level
4.5. Histological and Immuno-Histochemical Examination
4.6. Statistical Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sychrová, A.; Koláriková, I.; Žemlička, M.; Šmejkal, K. Natural compounds with dual antimicrobial and anti-inflammatory effects. Phytochem. Rev. 2020, 19, 1471–1502. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Khan, I.; Khan, W.; Khan, I.; Khan, A.; Halim, S.A.; Khan, H.; Hussain, J.; Al-Harrasi, A. Anti-nociceptive and Anti-inflammatory Activities of Asparacosin A Involve Selective Cyclooxygenase 2 and Inflammatory Cytokines Inhibition: An in-vitro, in-vivo, and in-silico Approach. Front. Immunol. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Alam, B.; Kwon, Y.-G.; Simu, S.Y.; Shahriyar, S.A.; Lee, S.H. Attenuation of Inflammatory Symptoms by Icariside B2 in Carrageenan and LPS-Induced Inflammation Models via Regulation of MAPK/NF-κB Signaling Cascades. Biomolecules 2020, 10, 1037. [Google Scholar] [CrossRef]
- El-Ashmawy, N.; El-Zamarany, E.; Khedr, E.G.; Selim, H.M.; Khedr, N.F. Blocking of The Prostaglandin E2 Receptor as a Therapeutic Strategy for Treatment of Breast Cancer: Promising Findings in a Mouse Model. Asian Pac. J. Cancer Prev. 2022, 23, 3763–3770. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; El-Zamarany, E.A.; Khedr, N.F.; Selim, H.M.; Khedr, E.G. Inhibition of PKC/MEK pathway suppresses β1-integrin and mitigates breast cancer cells proliferation. Toxicol. Rep. 2021, 8, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Shen, P.; Song, Y.; Huang, Y.; Tu, S. Reactive Oxygen Species in Autoimmune Cells: Function, Differentiation, and Metabolism. Front. Immunol. 2021, 12, 635021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Younis, N.S.; Abduldaium, M.S.; Mohamed, M.E. Protective Effect of Geraniol on Oxidative, Inflammatory and Apoptotic Alterations in Isoproterenol-Induced Cardiotoxicity: Role of the Keap1/Nrf2/HO-1 and PI3K/Akt/mTOR Pathways. Antioxidants 2020, 9, 977. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Rahman, S.; Alam, B.; Kim, Y.K.; Madina, M.H.; Fliss, I.; Lee, S.H.; Yoo, J.C. Activation of Nrf2/HO-1 by Peptide YD1 Attenuates Inflammatory Symptoms through Suppression of TLR4/MYyD88/NF-κB Signaling Cascade. Int. J. Mol. Sci. 2021, 22, 5161. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.M.; El-Sherbeni, S.A.; El-Kadem, A.H.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Negm, W.A. Elucidation of the Metabolite Profile of Yucca gigantea and Assessment of Its Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2022, 27, 1329. [Google Scholar] [CrossRef] [PubMed]
- Kolb, N.; Vallorani, L.; Milanović, N.; Stocchi, V. Evaluation of marine algae Wakame (Undaria pinnatifida) and kombu (Laminariadigitata japonica) as food supplements. Food Technol. Biotechnol. 2004, 42, 57–61. [Google Scholar]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Diego-González, L.; Lastra-Valdor, M.; Rodríguez-Argüelles, M.C.; Grimaldi, M.; Cavazza, A.; Bigi, F.; Simón-Vázquez, R. Immunostimulant and biocompatible gold and silver nanoparticles synthesized using the Ulva intestinalis L. aqueous extract. J. Mater. Chem. B 2019, 7, 4677–4691. [Google Scholar] [CrossRef]
- Rahimi, Z.; Yousefzadi, M.; Noori, A.; Akbarzadehl, A. Green Synthesis of Silver Nanoparticles using Ulva flexousa from the Persian Gulf, Iran. J. Persian Gulf. Mar. Sci. 2015, 5, 9–16. [Google Scholar]
- Fernández-Díaza, C.; Costea, O.; Maltab, E.-J. Polymer chitosan nanoparticles functionalized with Ulva ohnoi extracts boost in vitro ulvan immune-stimulant effect in Solea senegalensis macrophages. Algal Res. 2016, 26, 135–142. [Google Scholar] [CrossRef]
- Ktari, L. Pharmacological Potential of Ulva Species: A Valuable Resource. J. Anal. Pharm. Res. 2017, 6, 1–4. [Google Scholar] [CrossRef][Green Version]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- Touliabah, H.E.; El-Sheekh, M.M.; Makhlof, M.E.M. Evaluation of Polycladia myrica mediated selenium nanoparticles (PoSeNPS) cytotoxicity against PC-3 cells and antiviral activity against HAV HM175 (Hepatitis A), HSV-2 (Herpes simplex II), and Adenovirus strain 2. Front. Mar. Sci. 2022, 9, 1092343. [Google Scholar] [CrossRef]
- Blomster, J.; Hoey, E.M.; Maggs, C.A.; Stanhope, M.J. Species-specific oligonucleotide probes for macroalgae: Molecular discrimination of two marine fouling species of Enteromorpha (Ulvophyceae). Mol. Ecol. 2000, 9, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ruangchuay, R.; Dahamat, S.; Chirapat, A.; Notoya, M. Effects of culture conditions on the growth and reproduction of Gut Weed, Ulva intestinalis Linnaeus (Ulvales, Chlorophyta). Songklankarin J. Sci. Technol. 2012, 34, 501–507. [Google Scholar]
- Neto, A.I.; Titley, I.; Raposeiro, P. Flora Marinha do Litoral dos Açores–Rocky Shore Marine Flora of the Azores; Secretaria Regional do Ambiente e do Mar: Horta, Portugal, 2005; 156p. [Google Scholar]
- Ruangchuay, R.; Lueangthuwapranit, C.; Pianthumdee, N. Apparent characteristics and taxonomic study of macroalgae in Pattani Bay. Songklanakarin J. Sci. Technol. 2007, 29, 893–905. [Google Scholar]
- Van Reine, P.; Trono, G.C., Jr. Plant Resources of South-East Asia No. 15 (1): [Cryptogams: Algae]; Backhuys Publishers: Leiden, The Netherlands, 2001. [Google Scholar]
- Anjali, K.; Sangeetha, B.; Devi, G.; Raghunathan, R.; Dutta, S. Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): The compound characterization and functional applications in medicine-a comparative study. J. Photochem. Photobiol. B Biol. 2019, 200, 111622. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Paulert, R.; Ascrizzi, R.; Malatesta, S.; Berni, P.; Noseda, M.D.; de Carvalho, M.M.; Marchioni, I.; Pistelli, L.; Duarte, M.E.R.; Mariotti, L.; et al. Ulva intestinalis Extract Acts as Biostimulant and Modulates Metabolites and Hormone Balance in Basil (Ocimum basilicum L.) and Parsley (Petroselinum crispum L.). Plants 2021, 10, 1391. [Google Scholar] [CrossRef]
- Al-Radadi, N.S.; Adam, S.I. Green biosynthesis of Pt-nanoparticles from Anbara fruits: Toxic and protective effects on CCl4 induced hepatotoxicity in Wister rats. Arab. J. Chem. 2019, 13, 4386–4403. [Google Scholar] [CrossRef]
- Veeraraghavan, V.P.; Periadurai, N.D.; Karunakaran, T.; Hussain, S.; Surapaneni, K.M.; Jiao, X. Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929). Saudi J. Biol. Sci. 2021, 28, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Navid, R.; Sepideh, A.; Siavash, I.; Rajender, S.V. Natural resources for sustainable synthesis of nanomaterials with anticancer applications: A move toward green nanomedicine. Environ. Res. 2023, 216, 0013–9351. [Google Scholar]
- Al-Radadi, N.S. Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arab. J. Chem. 2019, 12, 330–349. [Google Scholar] [CrossRef]
- Abdullah; Al-Radadi, N.S.; Hussain, T.; Faisal, S.; Shah, S.A.R. Novel biosynthesis, characterization and bio-catalytic potential of green algae (Spirogyra hyalina) mediated silver nanomaterials. Saudi J. Biol. Sci. 2021, 29, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jain, A. Algae-mediated synthesis of biogenic nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 043001. [Google Scholar] [CrossRef]
- Horky, P. Selenium Nanoparticles in Animal Nutrition. Encyclopedia. Available online: https://encyclopedia.pub/entry/18555 (accessed on 6 February 2023).
- Makhlof, M.E.M.; Albalwe, F.M.; Al-Shaikh, T.M.; El-Sheekh, M.M. Suppression Effect of Ulva lactuca Selenium Nanoparticles (USeNPs) on HepG2 Carcinoma Cells Resulting from Degradation of Epidermal Growth Factor Receptor (EGFR) with an Evaluation of Its Antiviral and Antioxidant Activities. Appl. Sci. 2022, 12, 11546. [Google Scholar] [CrossRef]
- Castejón, N.; Thorarinsdottir, K.A.; Einarsdóttir, R.; Kristbergsson, K.; Marteinsdóttir, G. Exploring the Potential of Icelandic Seaweeds Extracts Produced by Aqueous Pulsed Electric Fields-Assisted Extraction for Cosmetic Applications. Mar. Drugs 2021, 19, 662. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, H.; Ameen, F.; AlYahya, S.; Almansob, A.; Alwakeel, S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef]
- Padalia, H.; Moteriya, P.; Chanda, S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2015, 8, 732–741. [Google Scholar] [CrossRef]
- El-Rafie, H.; El-Rafie, M.; Zahran, M. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Alipour, H.J.; Rezaei, M.; Shabanpour, B.; Tabarsa, M. Effects of sulfated polysaccharides from green alga Ulva intestinalis on physicochemical properties and microstructure of silver carp surimi. Food Hydrocoll. 2018, 74, 87–96. [Google Scholar] [CrossRef]
- Nahar, K.N.; Rahaman, H.; Khan, G.A.; Islam, K.; Al-Reza, S.M. Green synthesis of silver nanoparticles from Citrus sinensis peel extract and its antibacterial potential. Asian J. Green Chem. 2021, 5, 135–150. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Vikneshan, M.; Saravanakumar, R.; Mangaiyarkarasi, R.; Rajeshkumar, S.; Samuel, S.; Suganya, M.; Baskar, G. Algal biomass as a source for novel oral nano-antimicrobial agent. Saudi J. Biol. Sci. 2020, 27, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Tiloke, C.; Phulukdaree, A.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera Gold Nanoparticles Modulate Oncogenes, Tumor Suppressor Genes, and Caspase-9 Splice Variants in A549 Cells. J. Cell. Biochem. 2016, 117, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, G.; Subramanian, R.; Ahamed, M. Fish mucus mediated biosynthesis of copper oxide nanoparticles: Spectral characterization, morphology and biological activity. Mater. Res. Express 2020, 7, 125012. [Google Scholar] [CrossRef]
- Kumar, D.A.; Palanichamy, V.; Roopan, S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.; Premkumar, C.; Perumal, S.; Saraswathi, N.T. Biosynthesis of silver nanoparticles using CalotropisN. Afr. J. Basic Appl. Sci. 2011, 3, 265. [Google Scholar]
- Salih, A.H.; Elshehabi, T.A.; Bilgesu, H.I. Impact of Nanomaterials on the Rheological and Filtration Properties of Water-Based Drilling Fluids. In Proceedings of the SPE Eastern Regionalmeeting, Canton, OH, USA, 13–15 September 2016; p. SPE-184067-MS. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Zamorano, M.; Castillo, R.L.; Beltran, J.F.; Herrera, L.; Farias, J.A.; Antileo, C.; Aguilar-Gallardo, C.; Pessoa, A.; Calle, Y.; Farias, J.G. Tackling Ischemic Reperfusion Injury with the Aid of Stem Cells and Tissue Engineering. Front. Physiol. 2021, 12, 705256. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H. Pathophysiology of tissue fluid accumulation in inflammation. J. Physiol. 2011, 589, 2945–2953. [Google Scholar] [CrossRef] [PubMed]
- Guay, J.; Bateman, K.; Gordon, R.; Mancini, J.; Riendeau, D. Carrageenan-induced Paw Edema in Rat Elicits a Predominant Prostaglandin E2 (PGE2) Response in the Central Nervous System Associated with the Induction of Microsomal PGE2 Synthase-1. J. Biol. Chem. 2004, 279, 24866–24872. [Google Scholar] [CrossRef]
- Margret, R.J.; Kumaresan, S.; Ravikumar, S. A preliminary study on the anti-inflammatory activity of methanol extract of Ulva lactuca in rat. J. Environ. Biol. 2009, 30, 899–902. [Google Scholar] [PubMed]
- Ahmed, O.M.; Soliman, H.A.; Mahmoud, B.; Gheryany, R.R. Ulva lactuca hydroethanolic extract suppresses experimental arthritis via its anti-inflammatory and antioxidant activities. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 394–408. [Google Scholar] [CrossRef]
- Seutter, S.; Winfield, J.; Esbitt, A.; Snyder, S.; Magner, A.; Kim, K.; Carcuffe, C.; Schmoyer, J.; Kamrani, P.; Mercando, J.; et al. Interleukin 1β and Prostaglandin E2 affect expression of DNA methylating and demethylating enzymes in human gingival fibroblasts. Int. Immunopharmacol. 2019, 78, 105920. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Selim, H.M. Nebivolol prevents indomethacin-induced gastric ulcer in rats. J. Immunotoxicol. 2016, 13, 580–589. [Google Scholar] [CrossRef]
- Ghorbanzadeh, B.; Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G.; et al. Cashew (Anacardium occidentale L.) Nuts Counteract Oxidative Stress and Inflammation in an Acute Experimental Model of Carrageenan-Induced Paw Edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Z.; Wang, Y.; Guo, C.; Cai, X.; Zhang, Y.; Liu, L.; Xue, H.; Tang, J. Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp. Ther. Med. 2021, 22, 1473. [Google Scholar] [CrossRef]
- Chekaoui, A.; Lahmar, K.; Belguendouz, H.; Mazari, F.; Terahi, M.; Hakem, D.; Youinou, P.; Touil-Boukoffa, C. Increased IL-1β levels are associated with an imbalance of “oxidant/antioxidant” status during Behçet’s disease. Eur. Cytokine Netw. 2018, 29, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Cha, H.-J.; Choi, E.O.; Kim, C.H.; Kim, G.-Y.; Yoo, Y.H.; Hwang, H.-J.; Park, H.T.; Yoon, H.M.; Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int. J. Med. Sci. 2019, 16, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, L.; Lv, C.; Liu, L.; Miao, S.; Xu, Y.; Li, K.; Zhao, Y.; Zhao, J. PGE2 pathway mediates oxidative stress-induced ferroptosis in renal tubular epithelial cells. FEBS J. 2022, 290, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.A. Contibution to the Study of the Marine Algae of the Red Sea. I-The Algae in the Neighborhood of Al-Ghardaqa, Egypt (Cyanophyceae, Chlorophyceae and Phaeophyceae; Boll Faculty Science, King Abdul Aziz University: Jeddah, Saudi Arabia, 1978; Volume 2, pp. 73–88. [Google Scholar]
- Aleem, A.A. Marine Algae in Alexandria, Egypt; Alexandria Privately Published: Alexandria, Egypt, 1993; pp. 1–135. [Google Scholar]
- Lipkin, Y.S. Marine algae and seagrasses of the Dahlak Archipelago, southern Red Sea. Nova Hedwig. 2002, 75, 1–90. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, M.D.G. AlgaeBase; Worldwide Electronic Publication; National University of Ireland: Galway, Ireland, 2018; Available online: http://www.algaebase.org (accessed on 18 January 2022).
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-Anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef]
- ElSaied, B.E.; Diab, A.M.; Tayel, A.A.; Alghuthaymi, M.A.; Moussa, S.H. Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract. Green Process. Synth. 2021, 10, 49–60. [Google Scholar] [CrossRef]
- Abdelkader, D.H.; Abosalha, A.K.; Khattab, M.A.; Aldosari, B.N.; Almurshedi, A.S. A Novel Sustained Anti-Inflammatory Effect of Atorvastatin—Calcium PLGA Nanoparticles: In Vitro Optimization and In Vivo Evaluation. Pharmaceutics 2021, 13, 1658. [Google Scholar] [CrossRef]
- Kammoun, I.; Bkhairia, I.; Ben Abdallah, F.; Jaballi, I.; Ktari, N.; Boudawara, O.; Nasri, M.; Gharsallah, N.; Hakim, A.; Ben Amara, I. Potential protective effects of polysaccharide extracted from Ulva lactuca against male reprotoxicity induced by thiacloprid. Arch. Physiol. Biochem. 2017, 123, 334–343. [Google Scholar] [CrossRef]
- Helal, A.M.; Abdel-Latif, M.S.; Abomughaid, M.M.; Ghareeb, D.A.; El-Sayed, M.M. Potential therapeutic effects of Ulva lactuca water fraction on monosodium glutamate-induced testicular and prostatic tissue damage in rats. Environ. Sci. Pollut. Res. 2021, 28, 29629–29642. [Google Scholar] [CrossRef]
- Eissa, A.A.H.M.; Soliman, G.A.E.-H.; Khataibeh, M.H. Design, Synthesis and Anti-inflammatory Activity of Structurally Simple Anthranilic Acid Congeners Devoid of Ulcerogenic Side Effects. Chem. Pharm. Bull. 2012, 60, 1290–1300. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; El-Bastawissy, E.A.; El-Desoky, K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014, 19, 290–299. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Cui, J.-B.; Xu, X.-M.; Huang, Z.-H.; Jiao, H.-T. Expression of Nrf2-Keap1-ARE signal pathway in traumatic lung injury and functional study. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 1402–1408. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Yin, X.-P.; Zhou, J.; Wu, D.; Bao, B. Mechanisms underlying the perifocal neuroprotective effect of the Nrf2–ARE signaling pathway after intracranial hemorrhage. Drug Des. Dev. Ther. 2015, 9, 5973–5986. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhang, L.; Wang, G.; Zhang, M.; Yu, Y. Neuroprotection of dexmedetomidine against cerebral ischemia-reperfusion injury in rats: Involved in inhibition of NF-κB and inflammation response. Biomol. Ther. 2017, 25, 383–389. [Google Scholar] [CrossRef]
- Miotto, A.; Lins, T.A.; Montero, E.F.; Oshima, C.T.; Alonso, L.G.; Perfeito, J.A. Immunohistochemical analysis of the COX-2 marker in acute pulmonary injury in rats. Ital. J. Anat. Embryol. 2009, 114, 193–199. [Google Scholar]









| Target Gene | Primer Sequence | Reference | |
| Keap1 | Forward Reverse | TAAGAGGAACGGAATG ACATCATCTATTCTCT | [79] |
| Nrf2 | Forward Reverse | CTCATCGTAAGCGAACAATGG GCACCGTTCTTAGCG | [79] |
| HO-1 | Forward Reverse | CTATCGTGCTCGCATGAAC CAGCTCCTCAAACAGCTCAA | [80] |
| IL-6 | Forward Reverse | GCCCTTCAGGAACAGCTATGA TGTCAACAACATCAGTCCCAAGA | [81] |
| GAPDH | Forward Reverse | AGTGCCAGCCTCGTCTCATAG ACTTGCAACTTGCCGTGGGTAG | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almukainzi, M.; El-Masry, T.A.; Selim, H.; Saleh, A.; El-Sheekh, M.; Makhlof, M.E.M.; El-Bouseary, M.M. New Insight on the Cytoprotective/Antioxidant Pathway Keap1/Nrf2/HO-1 Modulation by Ulva intestinalis Extract and Its Selenium Nanoparticles in Rats with Carrageenan-Induced Paw Edema. Mar. Drugs 2023, 21, 459. https://doi.org/10.3390/md21090459
Almukainzi M, El-Masry TA, Selim H, Saleh A, El-Sheekh M, Makhlof MEM, El-Bouseary MM. New Insight on the Cytoprotective/Antioxidant Pathway Keap1/Nrf2/HO-1 Modulation by Ulva intestinalis Extract and Its Selenium Nanoparticles in Rats with Carrageenan-Induced Paw Edema. Marine Drugs. 2023; 21(9):459. https://doi.org/10.3390/md21090459
Chicago/Turabian StyleAlmukainzi, May, Thanaa A. El-Masry, Hend Selim, Asmaa Saleh, Mostafa El-Sheekh, Mofida E. M. Makhlof, and Maisra M. El-Bouseary. 2023. "New Insight on the Cytoprotective/Antioxidant Pathway Keap1/Nrf2/HO-1 Modulation by Ulva intestinalis Extract and Its Selenium Nanoparticles in Rats with Carrageenan-Induced Paw Edema" Marine Drugs 21, no. 9: 459. https://doi.org/10.3390/md21090459
APA StyleAlmukainzi, M., El-Masry, T. A., Selim, H., Saleh, A., El-Sheekh, M., Makhlof, M. E. M., & El-Bouseary, M. M. (2023). New Insight on the Cytoprotective/Antioxidant Pathway Keap1/Nrf2/HO-1 Modulation by Ulva intestinalis Extract and Its Selenium Nanoparticles in Rats with Carrageenan-Induced Paw Edema. Marine Drugs, 21(9), 459. https://doi.org/10.3390/md21090459