New l-Rhamnose-Binding Lectin from the Bivalve Glycymeris yessoensis: Purification, Partial Structural Characterization and Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
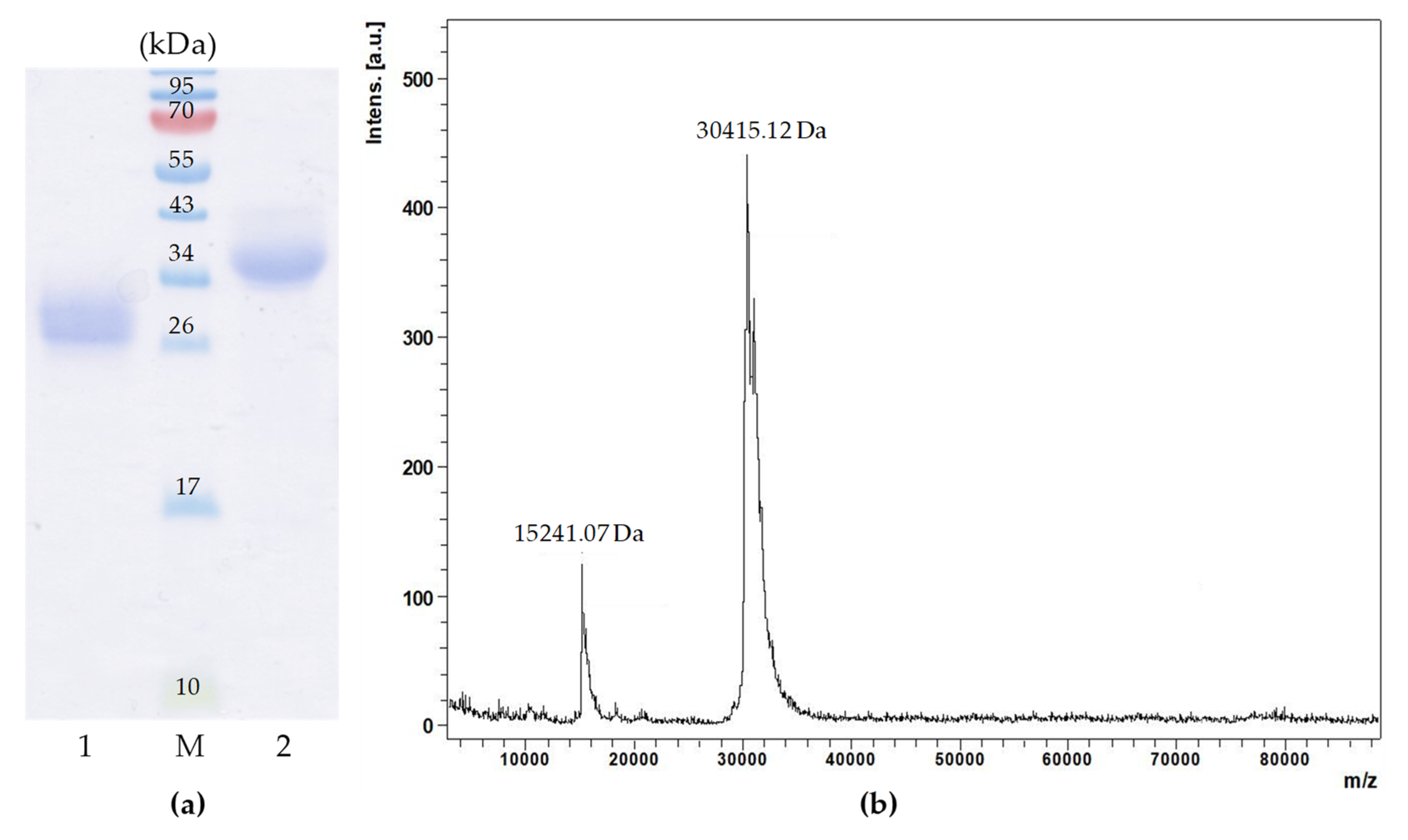
2.1. Purification of G. yessoensis Lectin
2.2. Hemagglutinating Activity and the Carbohydrate Specificity
2.3. Physico Chemical Characterization of Lectin
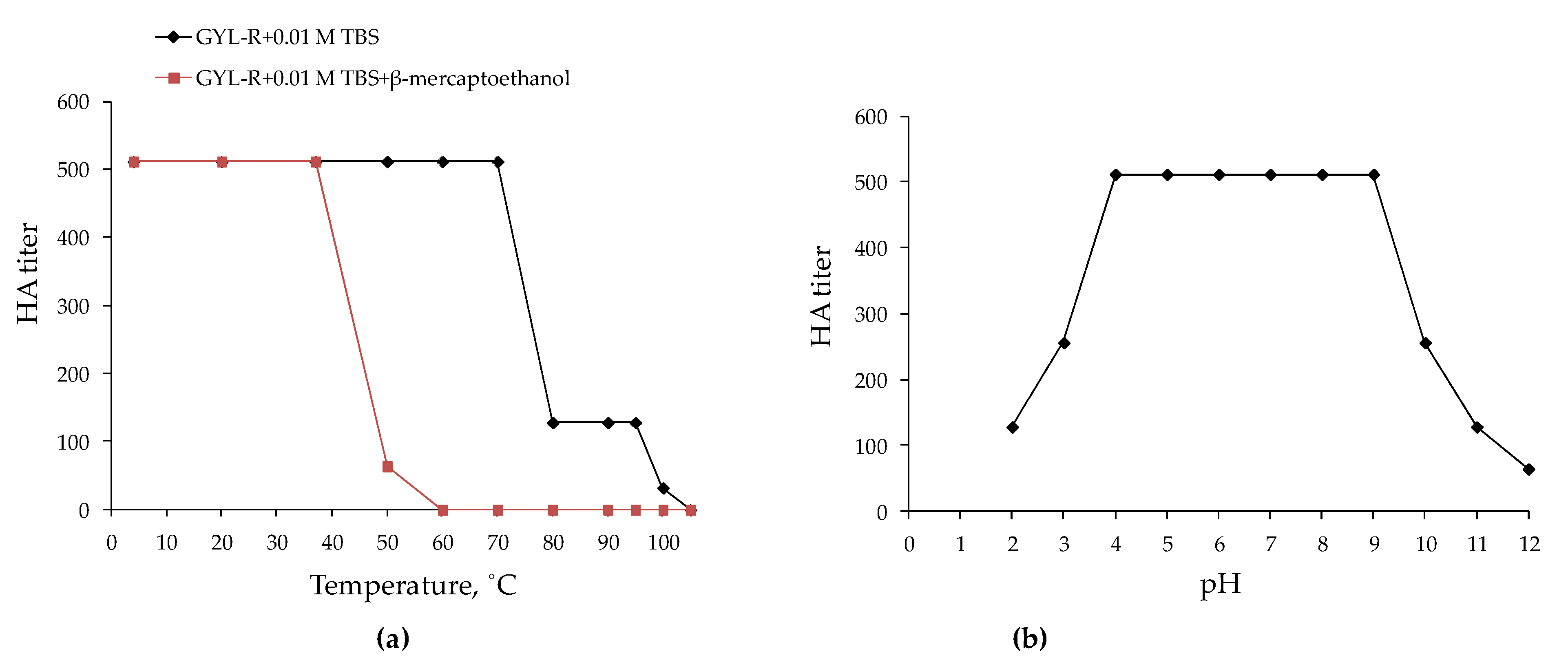
2.3.1. The Thermal and pH Stability of GYL-R
2.3.2. Dependence of the Lectin Activity on the Concentration of Ca2+ Ions
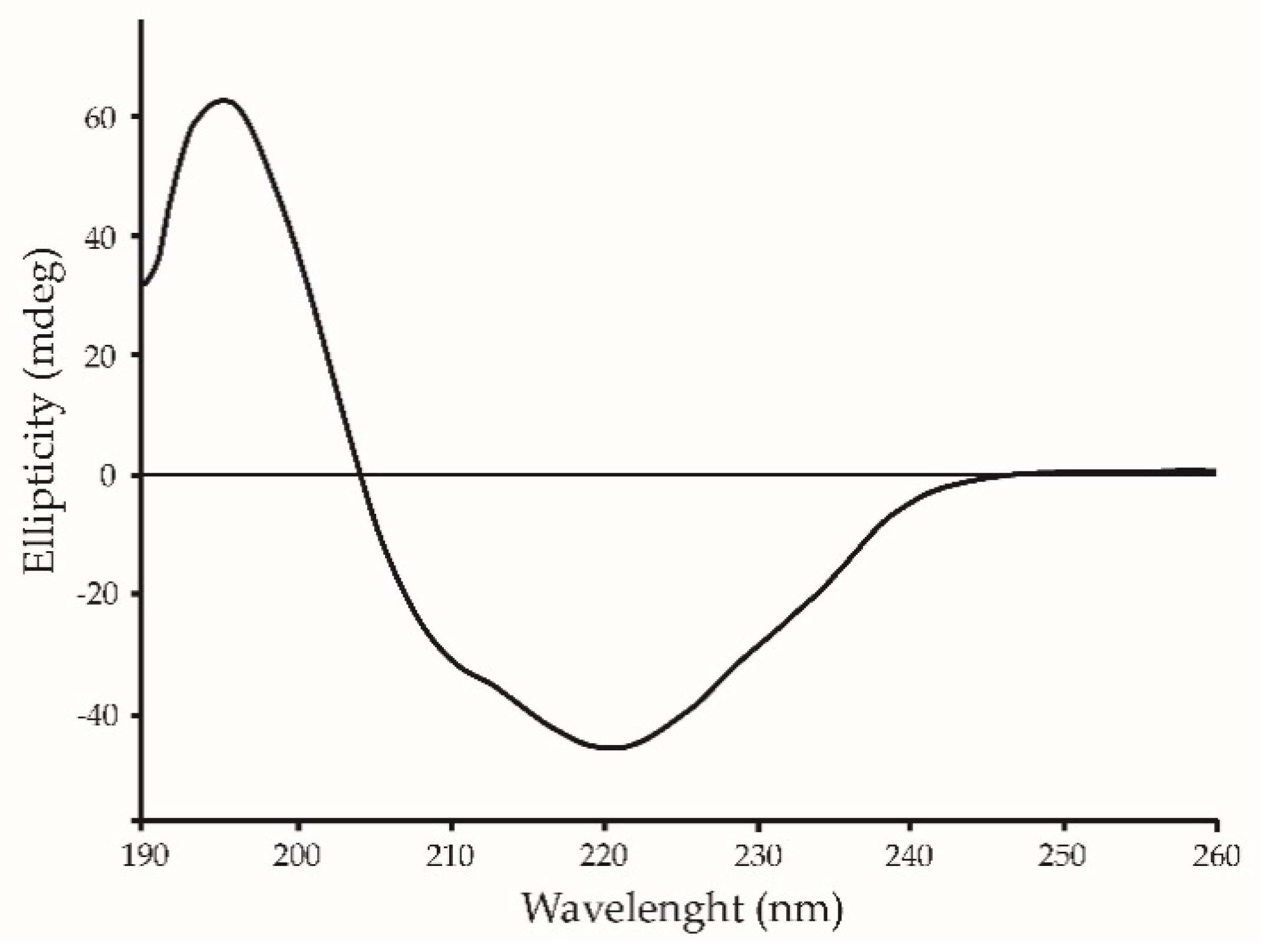
2.4. Structural Studies by Circular Dichroism (CD)
2.5. Amino Acid Sequencing of GYL-R
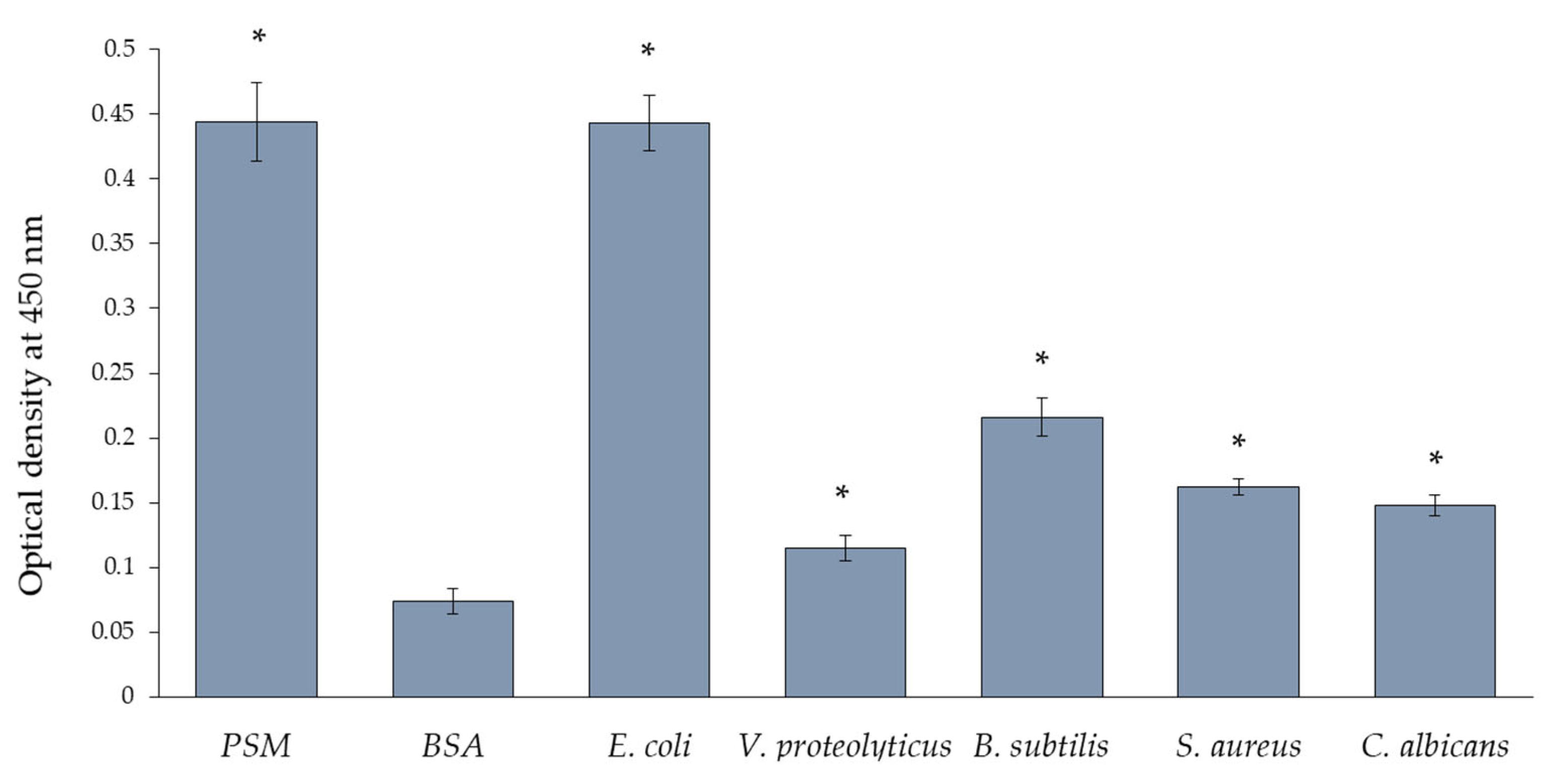
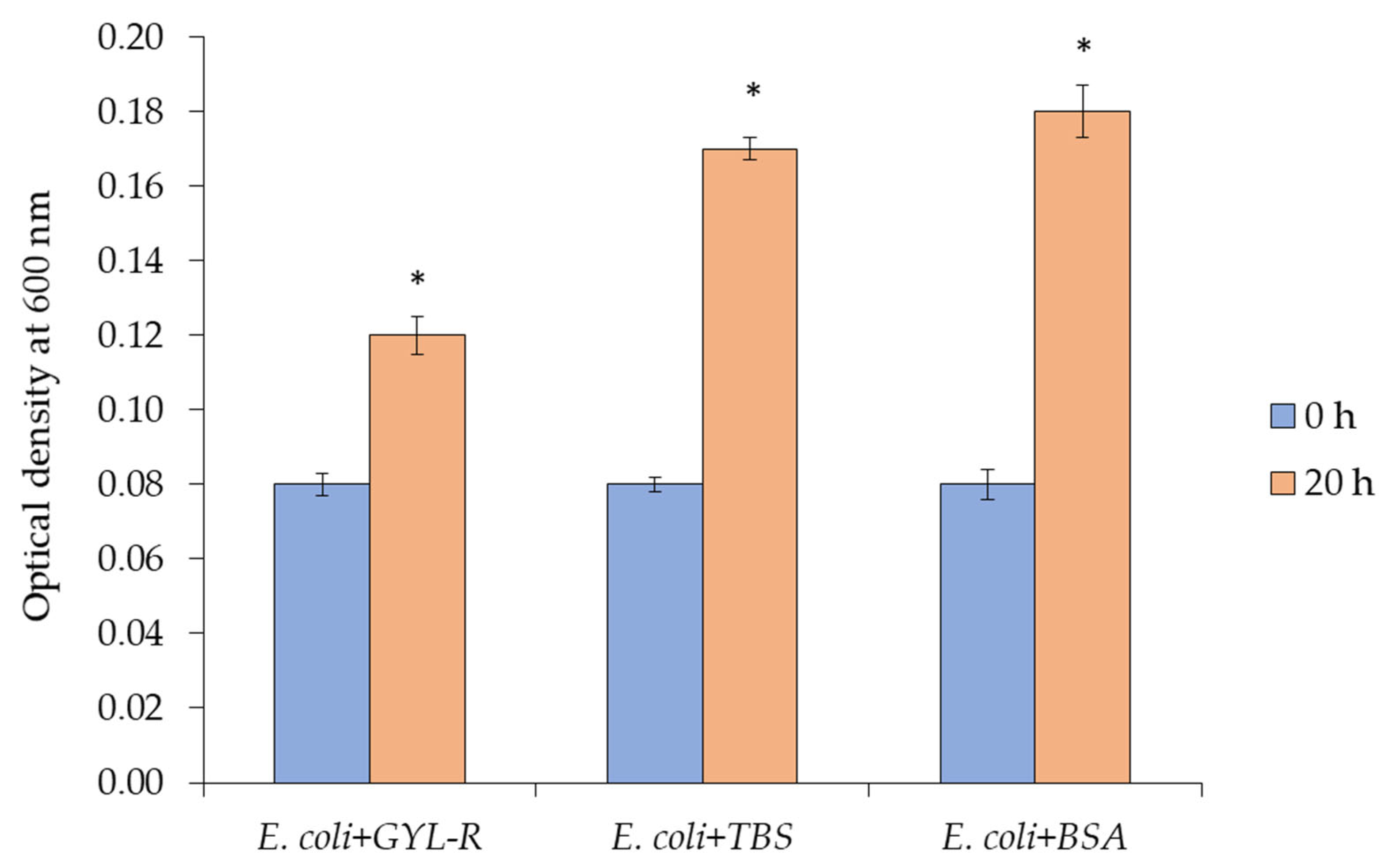
2.6. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Collection of Hemolymph
3.3. Isolation and Purification of Lectin from G. yessoensis (GYL-R)
3.4. Molecular Mass Determination
3.5. Preparation of 2% Suspension of Native or Enzyme-Treated Erythrocytes
3.6. Hemagglutinating Activity (HA) and Inhibition Assay
3.7. Determination of Fine Carbohydrate Specificity
3.8. Protein Content Measurements
3.9. Physical and Chemical Treatments
3.10. Circular Dichroism
3.11. Amino Acid Sequencing of Lectin
3.11.1. N-Terminal Sequence of GYL-R
3.11.2. Liquid Chromatography and Mass Spectrometry Data Analysis
3.11.3. Data Analysis
3.12. Biological Activity of GYL-R
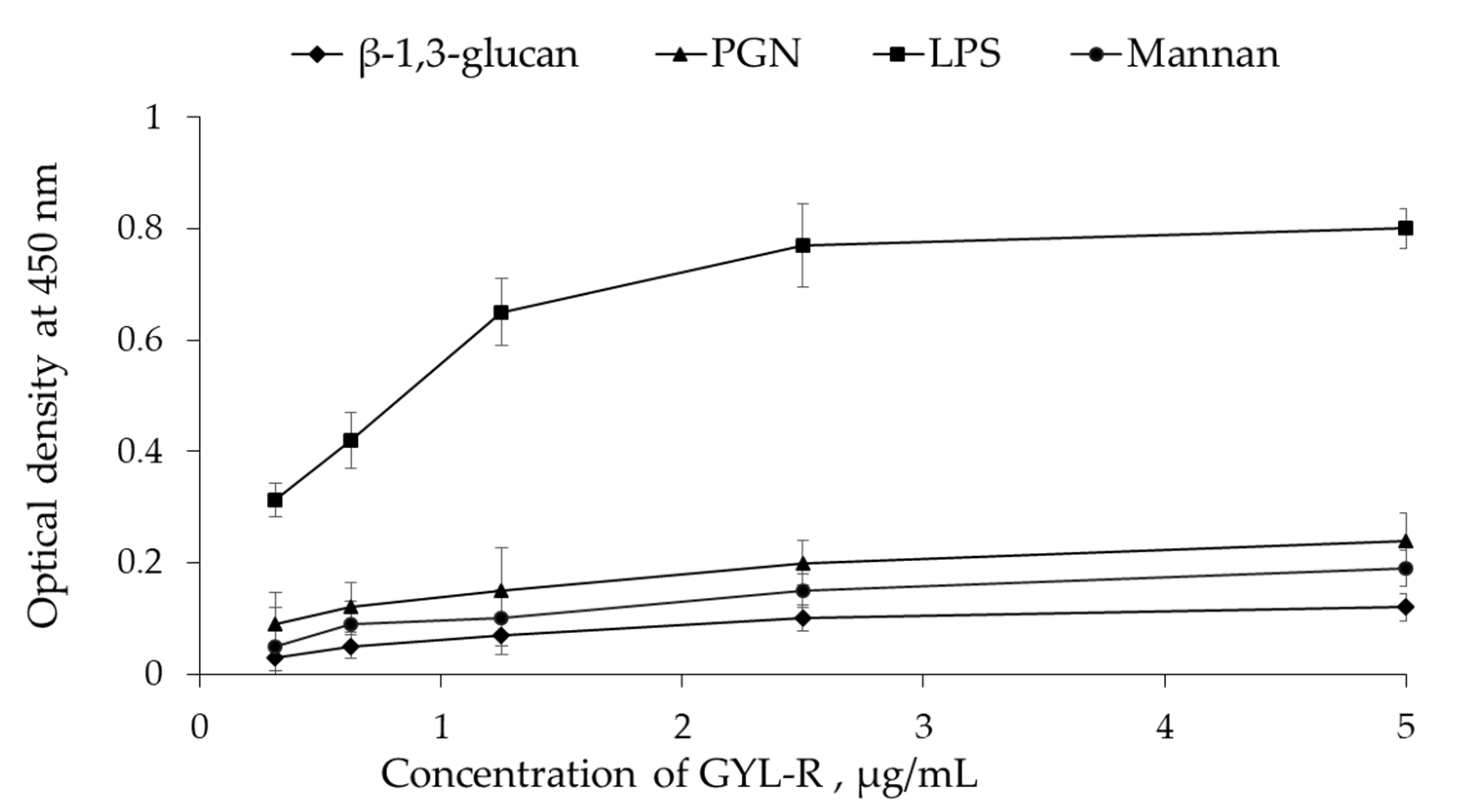
3.12.1. PAMP-Binding Assay
3.12.2. Interaction of GYL-R with Microorganisms
3.12.3. Inhibition of GYL-R Binding to Microorganisms
3.12.4. Growth of Bacterial Cells
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmmed, M.K.; Bhowmik, S.; Giteru, S.G.; Zilani, M.N.H.; Adadi, P.; Islam, S.S.; Kanwugu, O.N.; Haq, M.; Ahmmed, F.; Ng, C.C.W.; et al. An Update of Lectins from Marine Organisms: Characterization, Extraction Methodology, and Potential Biofunctional Applications. Mar. Drugs 2022, 20, 430. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.J.A.; Braga, A.L.; Filho, J.R.; Teixeira, C.S.; da Hora, G.C.A.; Morais-Braga, M.F.B. A review on the antimicrobial properties of lectins. Int. J. Biol. Macromol. 2022, 195, 163–178. [Google Scholar] [CrossRef]
- Kilpatrick, D.C. Animal lectins: A historical introduction and overview. Biochim. Biophys. Acta 2002, 1572, 187–197. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Unno, H. Functional Diversity of Novel Lectins with Unique Structural Features in Marine Animals. Cells 2023, 12, 1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, X.; Wang, L.; Song, L. Pathogen-Derived Carbohydrate Recognition in Molluscs Immune Defense. Int. J. Mol. Sci. 2018, 19, 721. [Google Scholar] [CrossRef]
- Chatterjee, B.P.; Adhya, M. Lectins with Varying Specificity and Biological Activity from Marine Bivalves. In Marine Proteins and Peptides; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 41–68. ISBN 9781118375082. [Google Scholar]
- Ogawa, T.; Watanabe, M.; Naganuma, T.; Muramoto, K. Diversified carbohydrate-binding lectins from marine resources. J. Amino Acids 2011, 2011, 838914. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M. First Insights into the Repertoire of Secretory Lectins in Rotifers. Mar. Drugs 2022, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Bah, C.S.F.; Fang, E.F.; Ng, T.B.; Mros, S.; McConnell, M.; El-Din Ahmed Bekhit, A. Purification and characterization of a rhamnose-binding chinook salmon roe lectin with antiproliferative activity toward tumor cells and nitric oxide-inducing activity toward murine macrophages. J. Agric. Food Chem. 2011, 59, 5720–5728. [Google Scholar] [CrossRef]
- Cheung, R.C.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.; Dan, X.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773. [Google Scholar] [CrossRef]
- Fu, T.K.; Ng, S.K.; Chen, Y.E.; Lee, Y.C.; Demeter, F.; Herczeg, M.; Borbás, A.; Chiu, C.H.; Lan, C.Y.; Chen, C.L.; et al. Rhamnose Binding Protein as an Anti-Bacterial Agent-Targeting Biofilm of Pseudomonas aeruginosa. Mar. Drugs 2019, 17, 355. [Google Scholar] [CrossRef]
- Sugawara, S.; Im, C.; Kawano, T.; Tatsuta, T.; Koide, Y.; Yamamoto, D.; Ozeki, Y.; Nitta, K.; Hosono, M. Catfish rhamnose-binding lectin induces G0/1 cell cycle arrest in Burkitt’s lymphoma cells via membrane surface Gb3. Glycoconj. J. 2017, 34, 127–138. [Google Scholar] [CrossRef]
- Ozeki, Y.; Yokota, Y.; Kato, K.H.; Titani, K.; Matsui, T. Developmental expression of D-galactoside-binding lectin in sea urchin (Anthocidaris crassispina) eggs. Exp. Cell Res. 1995, 216, 318–324. [Google Scholar] [CrossRef]
- Cammarata, M.; Parisi, M.G.; Benenati, G.; Vasta, G.R.; Parrinello, N. A rhamnose-binding lectin from sea bass (Dicentrarchus labrax) plasma agglutinates and opsonizes pathogenic bacteria. Dev. Comp. Immunol. 2014, 44, 332–340. [Google Scholar] [CrossRef]
- Gao, C.; Su, B.; Zhang, D.; Yang, N.; Song, L.; Fu, Q.; Zhou, S.; Tan, F.; Li, C. l-rhamnose-binding lectins (RBLs) in turbot (Scophthalmus maximus L.): Characterization and expression profiling in mucosal tissues. Fish Shellfish Immunol. 2018, 80, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Yin, X.; Qi, W.; Li, J.; Bai, H.; Chen, N.; Yang, Y.; Wang, B.; Ye, J. An l-rhamnose-binding lectin from Nile tilapia (Oreochromis niloticus) possesses agglutination activity and regulates inflammation, phagocytosis and respiratory burst of monocytes/macrophages. Dev. Comp. Immunol. 2022, 126, 104256. [Google Scholar] [CrossRef] [PubMed]
- Franchi, N.; Schiavon, F.; Carletto, M.; Gasparini, F.; Bertoloni, G.; Tosatto, S.C.E.; Ballarin, L. Immune roles of a rhamnose-binding lectin in the colonial ascidian Botryllus schlosseri. Immunobiology 2011, 216, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, L.; Cammarata, M.; Franchi, N.; Parrinello, N. Routes in Innate Immunity Evolution: Galectins and Rhamnose-Binding Lectins in Ascidians. In Marine Proteins and Peptides; Kim, S.-K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 185–205. ISBN 9781118375068. [Google Scholar]
- Ozeki, Y.; Matsui, T.; Suzuki, M.; Titani, K. Amino acid sequence and molecular characterization of a D-galactoside-specific lectin purified from sea urchin (Anthocidaris crassispina) eggs. Biochemistry 1991, 30, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.F.; Teixeira, C.S.; de Melo, A.A.; de Almeida, A.S.; Cavada, B.S.; de Sousa, O.V.; da Rocha, B.A.M.; Nagano, C.S.; Sampaio, A.H. L-Rhamnose-binding lectin from eggs of the Echinometra lucunter: Amino acid sequence and molecular modeling. Int. J. Biol. Macromol. 2015, 78, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.A.C.; Pacheco, J.P.F.; Waniek, P.J.; Geraldo, R.B.; Sibajev, A.; Dos Santos, A.L.; Evangelho, V.G.O.; Dyson, P.J.; Azambuja, P.; Ratcliffe, N.A.; et al. A rhamnose-binding lectin from Rhodnius prolixus and the impact of its silencing on gut bacterial microbiota and Trypanosoma cruzi. Dev. Comp. Immunol. 2021, 114, 103823. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H. SUEL-related lectins, a lectin family widely distributed throughout organisms. Biosci. Biotechnol. Biochem. 2010, 74, 1141–1144. [Google Scholar] [CrossRef]
- Naganuma, T.; Ogawa, T.; Hirabayashi, J.; Kasai, K.; Kamiya, H.; Muramoto, K. Isolation, characterization and molecular evolution of a novel pearl shell lectin from a marine bivalve, Pteria penguin. Mol. Divers. 2006, 10, 607–618. [Google Scholar] [CrossRef]
- Mizgina, T.O.; Chikalovets, I.V.; Molchanova, V.I.; Kokoulin, M.S.; Filshtein, A.P.; Sidorin, E.V.; Chernikov, O.V. Lectin of the Bivalve Glycymeris yessoensis as a Pattern Recognition Receptor. Russ. J. Bioorganic Chem. 2020, 46, 1187–1197. [Google Scholar] [CrossRef]
- Mizgina, T.O.; Chikalovets, I.V.; Molchanova, V.I.; Ziganshin, R.H.; Chernikov, O.V. Identification and Characterization of a Novel Lectin from the Clam Glycymeris yessoensis and Its Functional Characterization under Microbial Stimulation and Environmental Stress. Mar. Drugs 2021, 19, 474. [Google Scholar] [CrossRef] [PubMed]
- Mizgina, T.O.; Baldaev, S.N.; Likhatskaya, G.N.; Molchanova, V.I.; Kokoulin, M.S.; Filshtein, A.P.; Rogozhin, E.A.; Chikalovets, I.V.; Isaeva, M.P.; Chernikov, O.V. Molecular Cloning and Characteristics of a Lectin from the Bivalve Glycymeris yessoensis. Mar. Drugs 2023, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, K.S.; Cunha, A.I.; Nascimento, K.S.; Cavada, B.S.; Azevedo, A.M.; Aires-Barros, M.R. An overview of lectins purification strategies. J. Mol. Recognit. 2012, 25, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Shiina, N.; Shinozaki, F.; Yokoyama, H.; Kominami, J.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Sugahara, K.; Kamiya, H.; Matsubara, H.; et al. Isolation and characterization of l-rhamnose-binding lectin, which binds to microsporidian Glugea plecoglossi, from ayu (Plecoglossus altivelis) eggs. Dev. Comp. Immunol. 2008, 32, 487–499. [Google Scholar] [CrossRef]
- Tateno, H.; Saneyoshi, A.; Ogawa, T.; Muramoto, K.; Kamiya, H.; Saneyoshi, M. Isolation and characterization of rhamnose-binding lectins from eggs of steelhead trout (Oncorhynchus mykiss) homologous to low density lipoprotein receptor superfamily. J. Biol. Chem. 1998, 273, 19190–19197. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Ichise, A.; Yonekura, T.; Unno, H.; Goda, S.; Nakagawa, H. cDNA cloning and characterization of a rhamnose-binding lectin SUL-I from the toxopneustid sea urchin Toxopneustes pileolus venom. Toxicon 2015, 94, 8–15. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef]
- Siukstaite, L.; Imberty, A.; Römer, W. Structural Diversities of Lectins Binding to the Glycosphingolipid Gb3. Front. Mol. Biosci. 2021, 8, 704685. [Google Scholar] [CrossRef]
- Shirai, T.; Watanabe, Y.; Lee, M.-s.; Ogawa, T.; Muramoto, K. Structure of rhamnose-binding lectin CSL3: Unique pseudo-tetrameric architecture of a pattern recognition protein. J. Mol. Biol. 2009, 391, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Chikalovets, I.V.; Mizgina, T.O.; Molchanova, V.I.; Ovcharenko, Y.S.; Chernikov, O.V. Isolation and Characterization of Lectin from the Scallop Patinopecten yessoensis. Chem. Nat. Compd. 2017, 53, 717–721. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of Protein Secondary Structure from Circular Dichroism Spectra: Comparison of CONTIN, SELCON, and CDSSTR Methods with an Expanded Reference Set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Ogawa, T.; Muramoto, K.; Kamiya, H.; Saneyoshi, M. Rhamnose-binding lectins from steelhead trout (Oncorhynchus mykiss) eggs recognize bacterial lipopolysaccharides and lipoteichoic acid. Biosci. Biotechnol. Biochem. 2002, 66, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.C.; White, D.; Orskov, F.; Orskov, I.; Rick, P.D.; Lewis, M.S.; Bhattacharjee, A.K.; Leive, L. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J. Bacteriol. 1982, 151, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.; Neal, B.; Liu, D.; Hobbs, M.; Packer, N.H.; Batley, M.; Redmond, J.W.; Lindquist, L.; Reeves, P. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J. Bacteriol. 1994, 176, 4144–4156. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Yen, C.H.; Yeh, M.S.; Huang, C.J.; Liu, T.Y. Biochemical properties and cDNa cloning of two new lectins from the plasma of Tachypleus tridentatus: Tachypleus plasma lectin 1 and 2+. J. Biol. Chem. 2001, 276, 9631–9639. [Google Scholar] [CrossRef]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- Kapteyn, J.C.; Montijn, R.C.; Dijkgraaf, G.J.P.; Van den Ende, H.; Klis, F.M. Covalent association of beta-1,3-glucan with beta-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J. Bacteriol. 1995, 177, 3788–3792. [Google Scholar] [CrossRef]
- Ng, S.K.; Huang, Y.T.; Lee, Y.C.; Low, E.L.; Chiu, C.H.; Chen, S.L.; Mao, L.C.; Chang, M.D.T. A recombinant horseshoe crab plasma lectin recognizes specific pathogen-associated molecular patterns of bacteria through rhamnose. PLoS ONE 2014, 9, e115296. [Google Scholar] [CrossRef] [PubMed]
- Mistou, M.Y.; Sutcliffe, I.C.; Van Sorge, N.M. Bacterial glycobiology: Rhamnose-containing cell wall polysaccharides in Gram-positive bacteria. FEMS Microbiol. Rev. 2016, 40, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Lutaenko, K.A.; Volvenko, I.E.; Adrianov, A.V.; Vladivostok, R. Atlas of Common Bivalve Mollusks of Peter the Great Bay (Sea of Japan); Far Eastern Federal University: Vladivostok, Russia, 2017; p. 140. [Google Scholar]
- Bulgakov, A.A.; Eliseikina, M.G.; Petrova, I.Y.; Nazarenko, E.I.; Kovalchuk, S.N.; Kozhemyako, V.B.; Rasskazov, V.A. Molecular and biological characterization of a mannan-binding lectin from the holothurian Apostichopus japonicus. Glycobiology 2007, 17, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kulak, N.A.; Pichler, G.; Paron, I.; Nagaraj, N.; Mann, M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods 2014, 11, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, S.I.; Jensen, O.N.; Rogowska-Wrzesinska, A. FlashPack: Fast and Simple Preparation of Ultrahigh-performance Capillary Columns for LC-MS. Mol. Cell. Proteom. 2019, 18, 383–390. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: Powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef]
- Nakane, P.K.; Kawaoi, A. Peroxidase labeled antibody. A new method of conjugation. J. Histochem. Cytochem. 1974, 22, 1084–1091. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, Y.; Huang, H.; Feng, K.; Pan, M.; Yuan, S.; Huang, S.; Wu, T.; Guo, L.; Dong, M.; et al. A short-form C-type lectin from amphioxus acts as a direct microbial killing protein via interaction with peptidoglycan and glucan. J. Immunol. 2007, 179, 8425–8434. [Google Scholar] [CrossRef]

| Type of Erythrocytes | Titer of Agglutination a | |
|---|---|---|
| Native | Trypsinized | |
| Human O | 128 | 512 |
| Human A | Na b | 2 |
| Human B | Na | 2 |
| Human AB | Na | 2 |
| Rabbit | 128 | 2048 |
| Saccharides | MIC b mg/mL | MIC b mM | Relative Potency c |
|---|---|---|---|
| l-Rhamnose | 0.002 | 0.12 | 1.0 |
| d-Galactose | 0.156 | 0.87 | 78.0 |
| Rafinose | 0.625 | 1.24 | 312.5 |
| Lactose | 0.313 | 0.91 | 156.2 |
| PSM d | 0.016 | - | 8.0 |
| dsPSM e | 0.063 | - | 31.5 |
| # | Glycan Structure | Average RFU |
|---|---|---|
| 20 | l-Rhaα | 64,931 |
| 365 | Galα1-4(Fucα1-2)Galβ1-4GlcNAcβ | 47,673 |
| 266 | Galα1-4Galβ1-4GlcNAcβ | 24,853 |
| 81 | Galα1-4GlcNAcβ | 17,157 |
| 815 | Galα1-4GalNAcα | 12,517 |
| 821 | Galα1-4Galβ | 2404 |
| 387 | Galβ1-4GlcNAcβ1-6Galβ1-4GlcNAcβ | 1829 |
| 223 | Galα1-4Galβ1-4Glcβ | 1245 |
| 78 | Galα1-3GalNAcα | 1061 |
| 97 | Galβ1-4GlcNAcβ | 735 |
| Peptide | m/z a | z | Mass b |
|---|---|---|---|
| LEYASYGR | 479.7357 | 2 | 957.4556 |
| YLSVVYTCK | 566.7903 | 2 | 1131.5635 |
| LEAVNSVFGDPCVGTYK | 928.4499 | 2 | 1854.8821 |
| EDTLVCELNTDLLSCEER | 732.6665 | 3 | 2194.9722 |
| VNNCQNVDCDSWPFEYK | 725.6359 | 3 | 2173.8833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizgina, T.O.; Chikalovets, I.V.; Bulanova, T.A.; Molchanova, V.I.; Filshtein, A.P.; Ziganshin, R.H.; Rogozhin, E.A.; Shilova, N.V.; Chernikov, O.V. New l-Rhamnose-Binding Lectin from the Bivalve Glycymeris yessoensis: Purification, Partial Structural Characterization and Antibacterial Activity. Mar. Drugs 2024, 22, 27. https://doi.org/10.3390/md22010027
Mizgina TO, Chikalovets IV, Bulanova TA, Molchanova VI, Filshtein AP, Ziganshin RH, Rogozhin EA, Shilova NV, Chernikov OV. New l-Rhamnose-Binding Lectin from the Bivalve Glycymeris yessoensis: Purification, Partial Structural Characterization and Antibacterial Activity. Marine Drugs. 2024; 22(1):27. https://doi.org/10.3390/md22010027
Chicago/Turabian StyleMizgina, Tatyana O., Irina V. Chikalovets, Tatyana A. Bulanova, Valentina I. Molchanova, Alina P. Filshtein, Rustam H. Ziganshin, Eugene A. Rogozhin, Nadezhda V. Shilova, and Oleg V. Chernikov. 2024. "New l-Rhamnose-Binding Lectin from the Bivalve Glycymeris yessoensis: Purification, Partial Structural Characterization and Antibacterial Activity" Marine Drugs 22, no. 1: 27. https://doi.org/10.3390/md22010027
APA StyleMizgina, T. O., Chikalovets, I. V., Bulanova, T. A., Molchanova, V. I., Filshtein, A. P., Ziganshin, R. H., Rogozhin, E. A., Shilova, N. V., & Chernikov, O. V. (2024). New l-Rhamnose-Binding Lectin from the Bivalve Glycymeris yessoensis: Purification, Partial Structural Characterization and Antibacterial Activity. Marine Drugs, 22(1), 27. https://doi.org/10.3390/md22010027








