Mutable Collagenous Tissue: A Concept Generator for Biomimetic Materials and Devices
Abstract
:1. Introduction
2. Structural Organization of MCT
2.1. Extracellular Components
2.2. Cellular Components
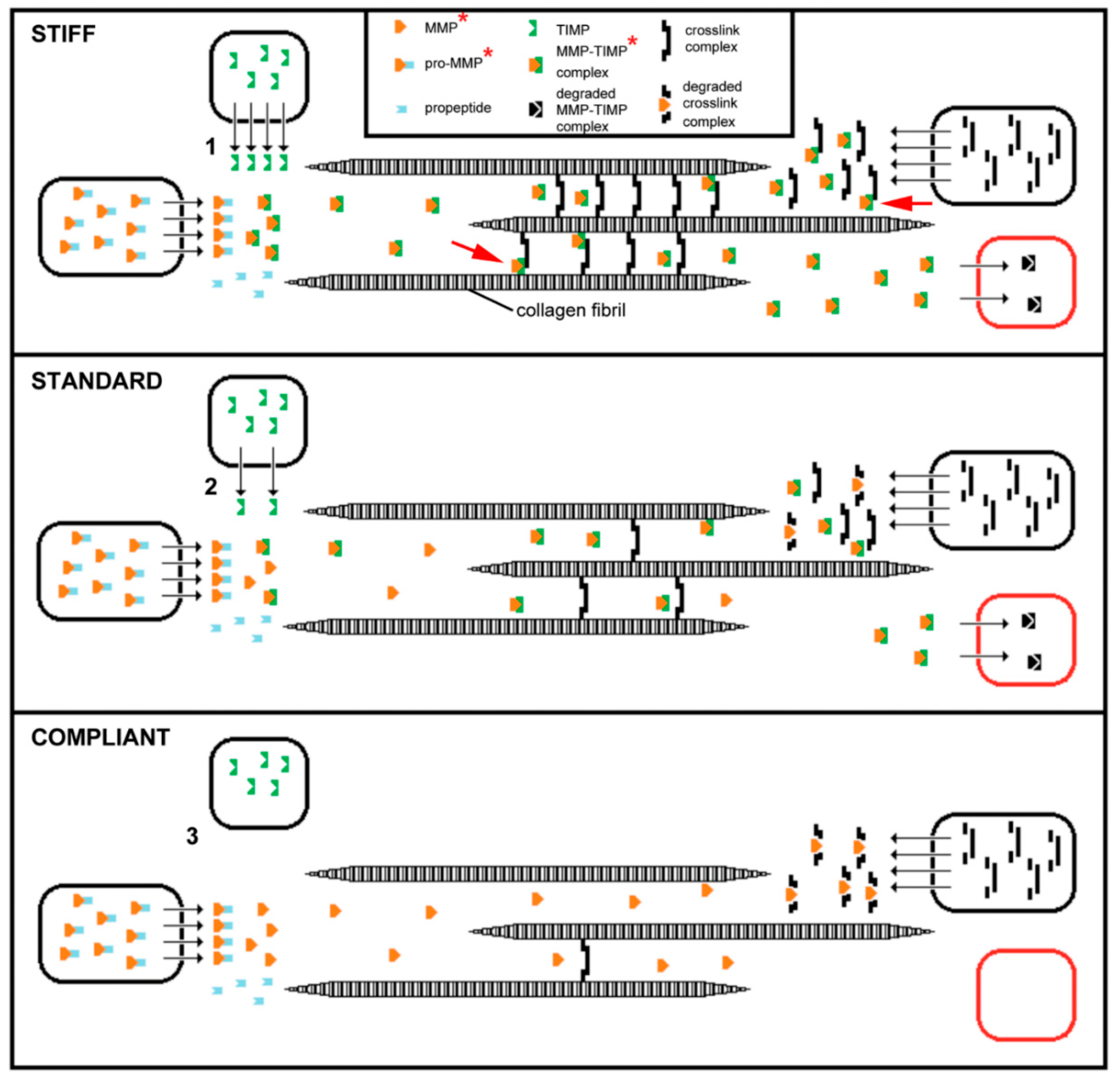
3. Mechanisms of Tensile Change
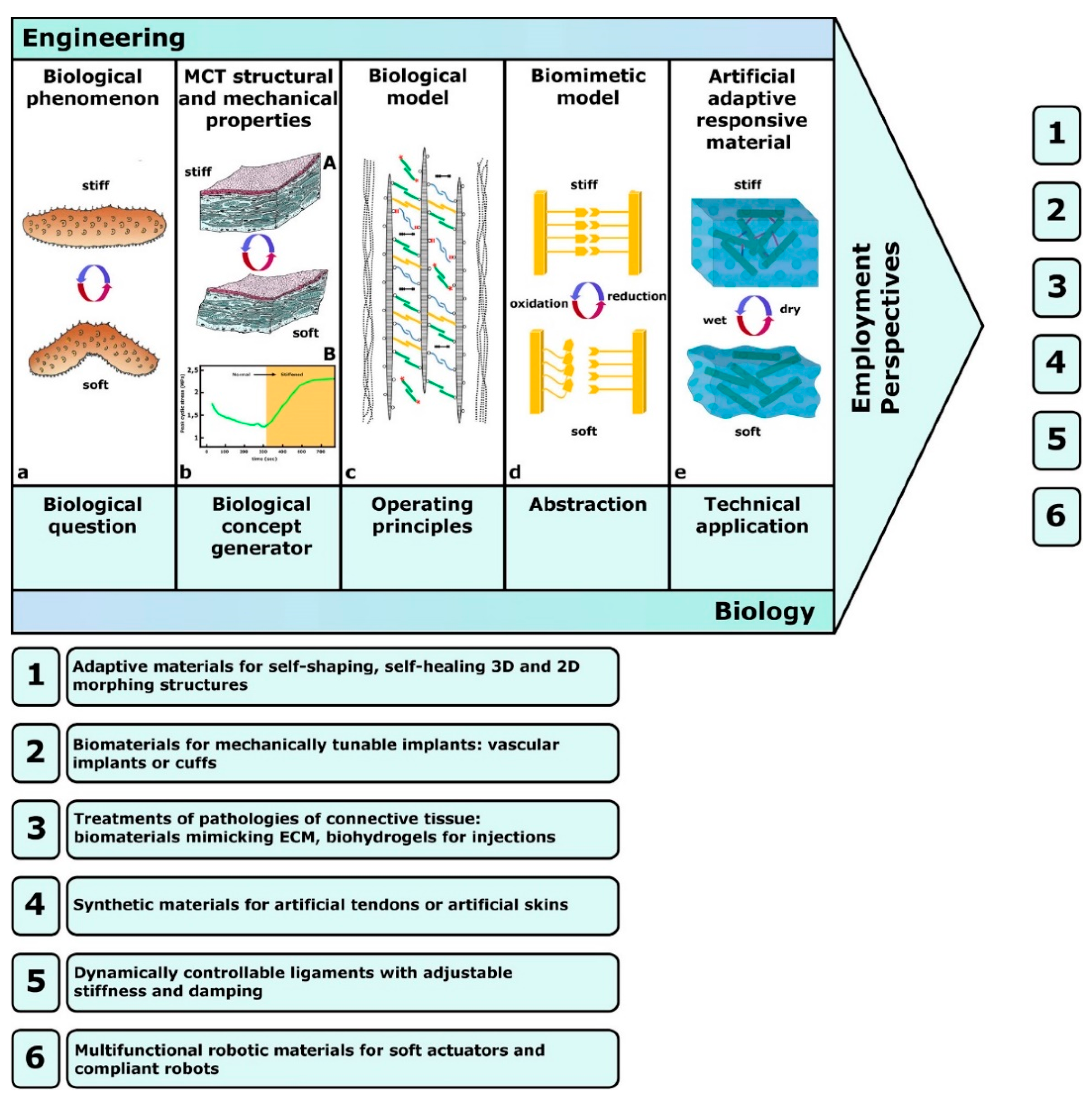
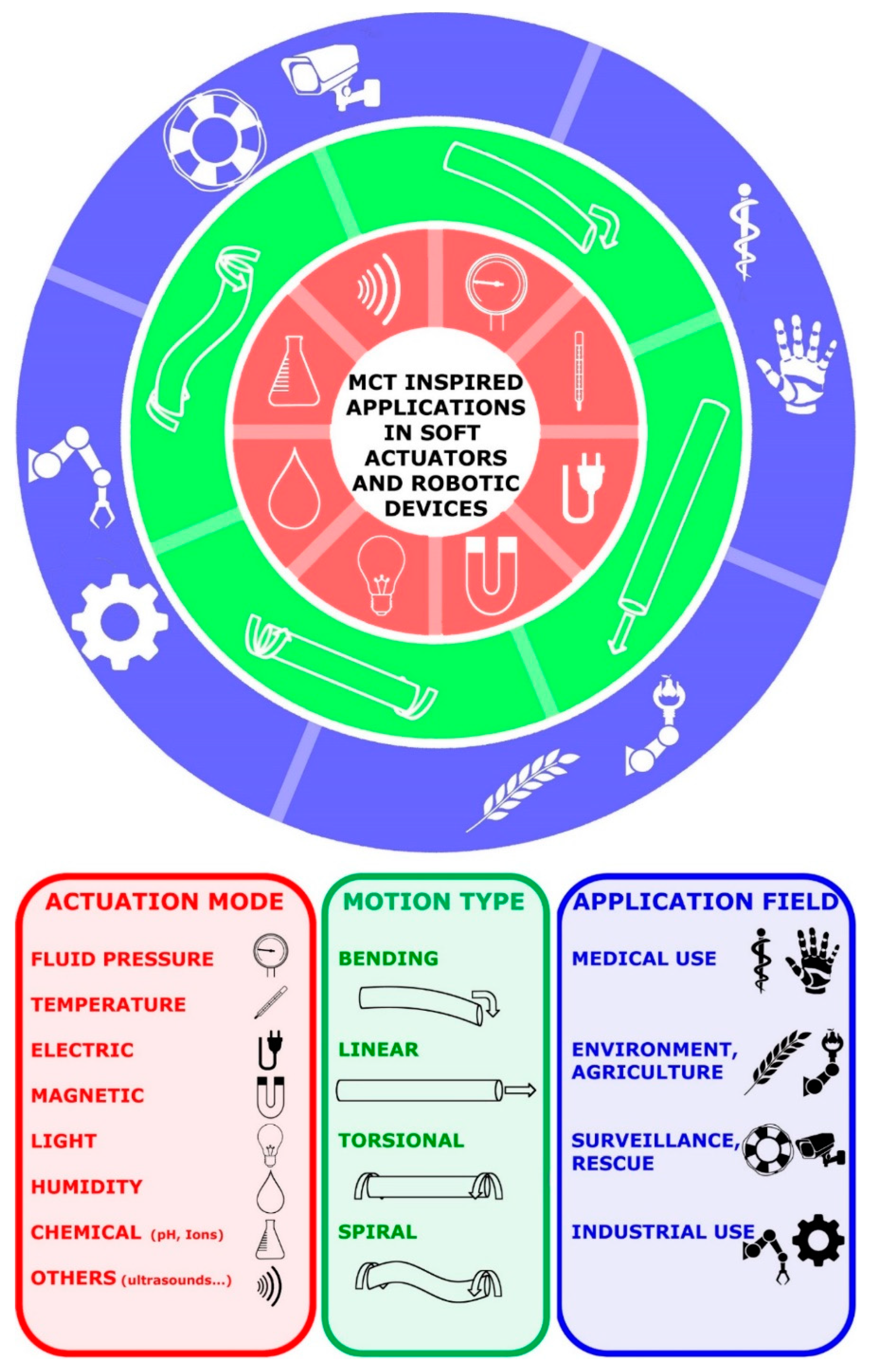
4. MCT-Inspired Biomimetic Systems
4.1. Biology-Inspired Developments: Biology-Push Processes
4.2. Technology-Derived Developments: Technology-Pull Processes
4.3. Advanced Materials
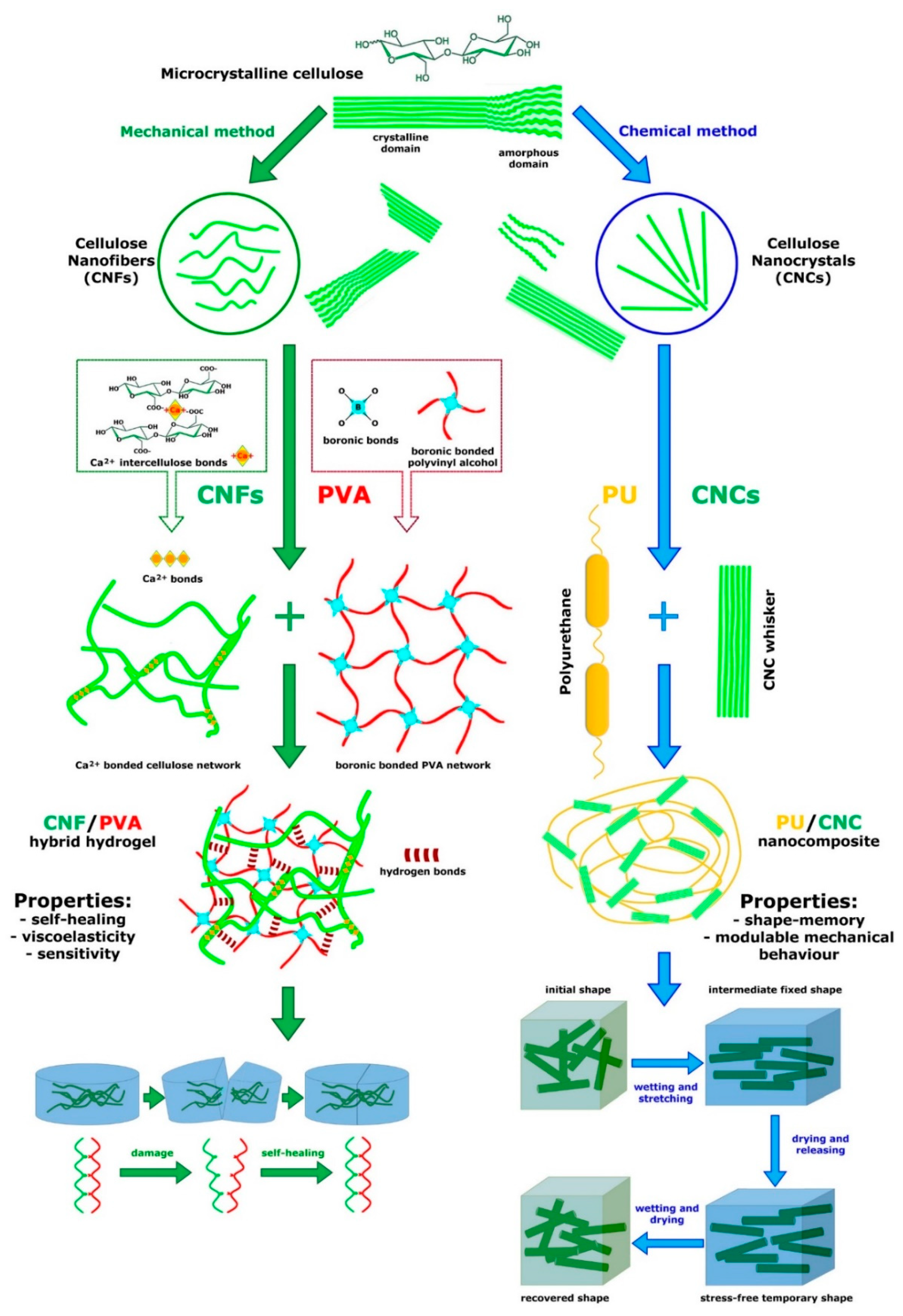
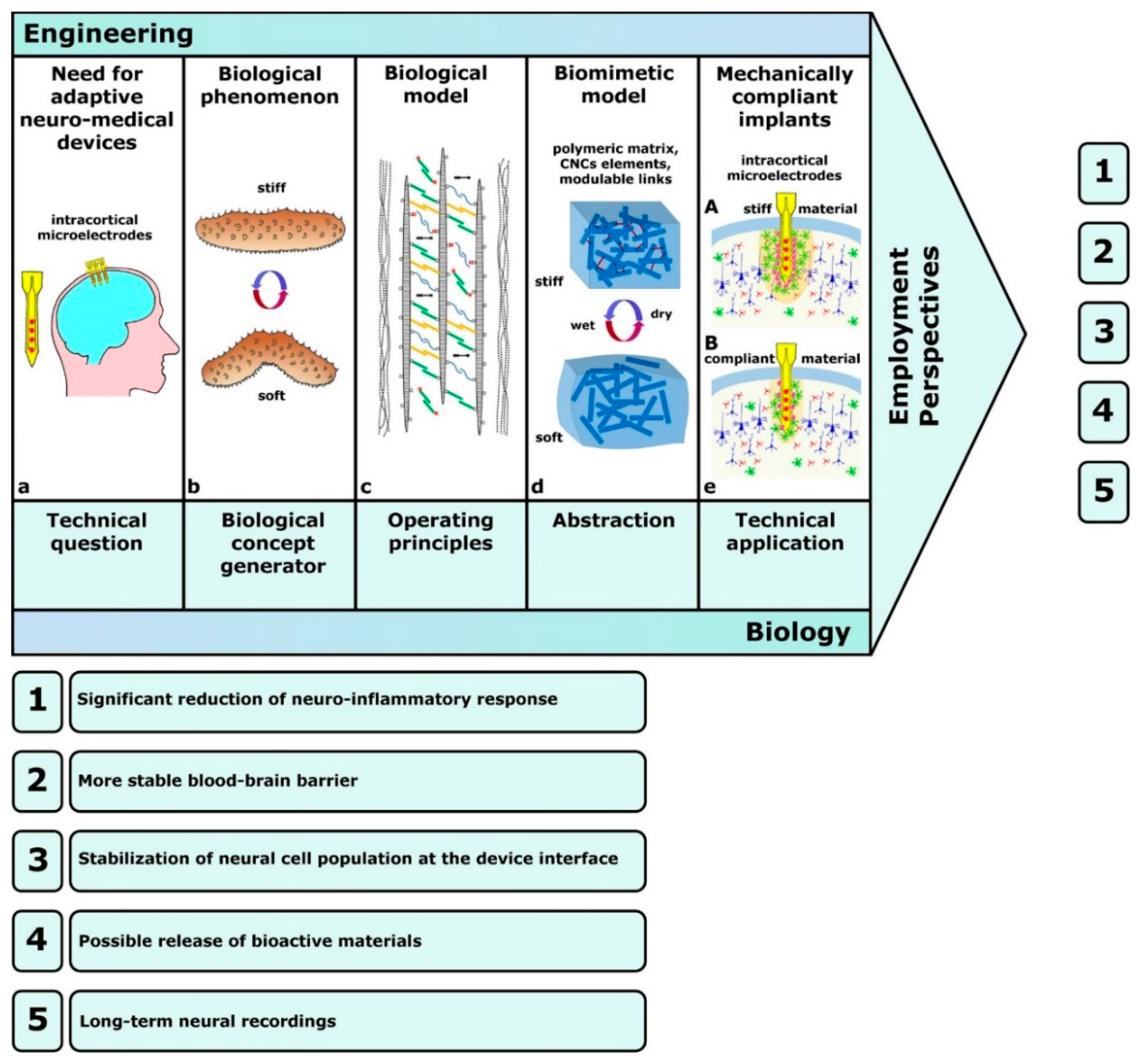
4.4. Biocompatible Substrates
4.5. Soft Actuators and Robotic Tools
5. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | acrylamide |
| BNBM | biomimetic natural biomaterial |
| CFRP | carbon fiber-reinforced polymer |
| CM | carboxymethyl-chitosan |
| CNC | cellulose nanocrystal |
| CNF | cellulose nanofiber |
| DC | direct current |
| ECM | extracellular matrix |
| EO-EPI | ethylene oxide–epichlorohydrin 1:1 copolymer |
| GAG | glycosaminoglycan |
| JLC | juxtaligamental cell |
| LDCV | large dense-core vesicle |
| MCT | mutable collagenous tissue |
| MMP | metalloproteinase |
| NSF | novel stiffening factor |
| PBS | polyborosiloxane |
| PU | polyurethane |
| PVAc | polyvinyl acetate |
| SAXS | small-angle X-ray scattering |
| SELP | silk–elastin-like protein |
| SCIM | sea-cucumber inspired material |
| SMA | shape memory alloy |
| SP | supramolecular polymer |
| SSG | shear stiffening gel |
| SURF | sea-urchin fibrillar |
| TE | tissue engineering |
| TEM | transmission electron microscope |
| TER | tissue engineering and regenerative |
| TIMP | tissue inhibitor of metalloproteinase |
| UPy | 2-ureido-4[1H]-pyrimidinone |
References
- Socha, M.W.; Flis, W.; Pietrus, M.; Wartęga, M.; Stankiewicz, M. Signaling pathways regulating human cervical ripening in preterm and term delivery. Cells 2022, 11, 3690. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.B.; Heinemeier, K.M.; Couppé, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. 2016, 121, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Sugni, M.; Fassini, D.; Barbaglio, A.; Biressi, A.; Di Benedetto, C.; Tricarico, S.; Bonasoro, F.; Wilkie, I.C.; Candia Carnevali, M.D. Comparing dynamic connective tissue in echinoderms and sponges: Morphological and mechanical aspects and environmental sensitivity. Mar. Environ. Res. 2014, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Takemae, N.; Nakaya, F.; Motokawa, T. Low Oxygen consumption and high body content of catch connective tissue contribute to low metabolic rate of sea cucumbers. Biol. Bull. 2009, 216, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, T.; Sato, E.; Umeyama, K. Energy expenditure associated with softening and stiffening of echinoderm connective tissue. Biol. Bull. 2012, 222, 150–157. [Google Scholar] [CrossRef]
- Wilkie, I.C. Autotomy as a prelude to regeneration in echinoderms. Microsc. Res. Tech. 2001, 55, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, I.C.; Emson, R.H.; Mladenov, P.V. Morphological and mechanical aspects of fission in Ophiocomella ophiactoides (Echinodermata, Ophiuroida). Zoomorphology 1984, 104, 310–322. [Google Scholar] [CrossRef]
- Rubilar, T.; Pastor, C.; Díaz de Vivar, E. Timing of fission in the starfish Allostichaster capensis (Echinodermata: Asteroidea) in laboratory. Rev. Biol. Trop. 2005, 53 (Suppl. 3), 299–303. [Google Scholar]
- Dolmatov, I.Y.; Afanasyev, S.V.; Boyko, A.V. Molecular mechanisms of fission in echinoderms: Transcriptome analysis. PLoS ONE 2018, 13, e0195836. [Google Scholar] [CrossRef]
- Zhu, B.; Zheng, J.; Zhang, Z.; Dong, X.; Zhao, L.; Tada, M. Autophagy plays a potential role in the process of sea cucumber body wall “melting” induced by UV irradiation. Wuhan Univ. J. Nat. Sci. 2008, 13, 232–238. [Google Scholar] [CrossRef]
- Sun, L.-M.; Wang, T.-T.; Zhu, B.-W.; Niu, H.-L.; Zhang, R.; Hou, H.-M.; Zhang, G.-L.; Murata, Y. Effect of matrix metalloproteinase on autolysis of sea cucumber Stichopus japonicus. Food Sci. Biotechnol. 2013, 22, 1–3. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhou, D.; Liu, X.; Dong, X.; Li, D.; Shahidi, F. The role of matrix metalloprotease (MMP) to the autolysis of sea cucumber (Stichopus japonicus). J. Sci. Food Agric. 2019, 99, 5752–5759. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K. The catch apparatus of the sea-urchin spine I. Gross histology. J. Fac. Sci., Univ. Tokyo Sec. IV 1967, 11, 110–120. [Google Scholar]
- Takahashi, K. The catch apparatus of the sea-urchin spine II. Responses to stimuli. J. Fac. Sci., Univ. Tokyo Sec. IV 1967, 11, 122–130. [Google Scholar]
- Takahashi, K. The catch apparatus of the sea-urchin spine III. The ball-and-socket joint of the sea-urchin spine: Geometry and its functional implications. J. Fac. Sci., Univ. Tokyo Sec. IV 1967, 11, 131–135. [Google Scholar]
- Trotter, J.A.; Tipper, J.; Lyons-Levy, G.; Chino, K.; Heuer, A.H.; Liu, Z.; Mrksich, M.; Hodneland, C.; Dillmore, W.S.; Koob, T.J.; et al. Towards a fibrous composite with dynamically controlled stiffness: Lessons from echinoderms. Biochem. Soc. Trans. 2000, 28, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Moatsou, D.; Weder, C. Mechanically adaptive nanocomposites inspired by sea cucumbers. In Bio-Inspired Polymers; Bruns, N., Kilbinger, A.F.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 402–428. ISBN 978-1-78262-413-4. [Google Scholar]
- Choi, A.; Han, H.; Kim, D.S. A programmable powerful and ultra-fast water-driven soft actuator inspired by the mutable collagenous tissue of the sea cucumber. J. Mater. Chem. A 2021, 9, 15937–15947. [Google Scholar] [CrossRef]
- Myronidis, K.; Kopec, M.; Meo, M.; Pinto, F. A novel, bioinspired, non-Newtonian energy absorption medium for the protection of composite laminates under low velocity impact (LVI). In Bioinspiration, Biomimetics, and Bioreplication XII; Lakhtakia, A., Martín-Palma, R.J., Knez, M., Eds.; SPIE: Long Beach, CA, USA, 2022; p. 27. [Google Scholar]
- Wilkie, I.C.; Sugni, M.; Gupta, H.; Candia Carnevali, M.D.; Elphick, M.R. The mutable collagenous tissue of echinoderms: From biology to biomedical applications. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–33. ISBN 978-1-78801-757-2. [Google Scholar]
- Wilkie, I.C. Arm autotomy in brittlestars (Echinodermata: Ophiuroidea). J. Zool. 1978, 186, 311–330. [Google Scholar] [CrossRef]
- Wilkie, I.C. Functional morphology of the arm spine joint and adjacent structures of the brittlestar Ophiocomina nigra (Echinodermata: Ophiuroidea). PLoS ONE 2016, 11, e0167533. [Google Scholar] [CrossRef]
- Blowes, L.M.; Egertová, M.; Liu, Y.; Davis, G.R.; Terrill, N.J.; Gupta, H.S.; Elphick, M.R. Body wall structure in the starfish Asterias rubens. J. Anat. 2017, 231, 325–341. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Barbaglio, A.; Benedetto, C.D.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. New insights into mutable collagenous tissue: Correlations between the microstructure and mechanical state of a sea-urchin ligament. PLoS ONE 2011, 6, e24822. [Google Scholar] [CrossRef]
- Cluzel, C.; Lethias, C.; Garrone, R.; Exposito, J.-Y. Distinct maturations of N-propeptide domains in fibrillar procollagen molecules involved in the formation of heterotypic fibrils in adult sea urchin collagenous tissues. J. Biol. Chem. 2004, 279, 9811–9817. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Xue, C.; Li, Z.; Zhang, Y.; Dong, P.; Fu, X.; Gao, X. Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Tian, M.; Xue, C.; Chang, Y.; Shen, J.; Zhang, Y.; Li, Z.; Wang, Y. Collagen fibrils of sea cucumber (Apostichopus japonicus) are heterotypic. Food Chem. 2020, 316, 126272. [Google Scholar] [CrossRef] [PubMed]
- Hulmes, D.J.S. Collagen diversity, synthesis and assembly. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer US: Boston, MA, USA, 2008; pp. 15–47. ISBN 978-0-387-73906-9. [Google Scholar]
- Cluzel, C.; Lethias, C.; Humbert, F.; Garrone, R.; Exposito, J.Y. Characterization of fibrosurfin, an interfibrillar component of sea urchin catch connective tissues. J. Biol. Chem. 2001, 276, 18108–18114. [Google Scholar] [CrossRef] [PubMed]
- Sea Urchin Genome Sequencing Consortium; Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.A.; Bergeron, K.-F.; Whittle, J.; Brandhorst, B.P.; Burke, R.D.; Hynes, R.O. The echinoderm adhesome. Dev. Biol. 2006, 300, 252–266. [Google Scholar] [CrossRef]
- Wilkie, I.C.; McKew, M.; Candia Carnevali, M.D. Functional morphology of the compass-rotular ligament of Echinus esculentus (Echinodermata: Echinoida): A non-mutable collagenous component of Aristotle’s lantern. Zoomorphology 2005, 124, 9–26. [Google Scholar] [CrossRef]
- Wilkie, I.C. Mutable collagenous tissue: Overview and biotechnological perspective. In Echinodermata. Progress in Molecular and Subcellular Biology 39; Matranga, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 221–250. ISBN 978-3-540-24402-8. [Google Scholar] [CrossRef]
- Erlinger, R.; Welsch, U.; Scott, J.E. Ultrastructural and biochemical observations on proteoglycans and collagen in the mutable connective tissue of the feather star Antedon bifida (Echinodermata, Crinoidea). J. Anat. 1993, 183, 1–11. [Google Scholar]
- Wang, J.; Chang, Y.; Wu, F.; Xu, X.; Xue, C. Fucosylated chondroitin sulfate is covalently associated with collagen fibrils in sea cucumber Apostichopus japonicus body wall. Carbohyd. Polym. 2018, 186, 439–444. [Google Scholar] [CrossRef]
- Wilkie, I.C. The juxtaligamental jells of Ophiocomina nigra (Abildgaard) (Echinodermata: Ophiuroidea) and their possible role in mechano-effector function of collagenous tissue. Cell Tissue Res. 1979, 197, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Charlina, N.A.; Dolmatov, I.Y.; Wilkie, I.C. Juxtaligamental system of the disc and oral frame of the ophiuroid Amphipholis kochii (Echinodermata: Ophiuroidea) and its role in autotomy. Invertebr. Biol. 2009, 128, 145–156. [Google Scholar] [CrossRef]
- Hennebert, E.; Haesaerts, D.; Dubois, P.; Flammang, P. Evaluation of the different forces brought into play during tube foot activities in sea stars. J. Exp. Biol. 2010, 213, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, T. Mechanical mutability in connective tissue of starfish body wall. Biol. Bull. 2011, 221, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Demeuldre, M.; Hennebert, E.; Bonneel, M.; Lengerer, B.; Van Dyck, S.; Wattiez, R.; Ladurner, P.; Flammang, P. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J. Exp. Biol. 2017, 220, 2108–2119. [Google Scholar] [CrossRef] [PubMed]
- Mashanov, V.S.; Charlina, N.A.; Dolmatov, I.Y.; Wilkie, I.C. Juxtaligamental cells in the arm of the brittlestar Amphipholis kochii Lütken, 1872 (Echinodermata: Ophiuroidea). Russ. J. Mar. Biol. 2007, 33, 110–117. [Google Scholar] [CrossRef]
- Charlina, N.A.; Mashanov, V.S.; Dolmatov, I.Y.; Wilkie, I.C. Morphology of the juxtaligamental system in the ophiuroid Amphipholis kochii. In Proceedings of the 12th International Echinoderm Conference; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zueva, O.; Khoury, M.; Heinzeller, T.; Mashanova, D.; Mashanov, V. The complex simplicity of the brittle star nervous system. Front. Zool. 2018, 15, 1. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D. The juxtaligamental cells of echinoderms and their role in the mechano-effector function of connective tissue. In Frontiers in Invertebrate Physiology: A Collection of Reviews; Saleuddin, A.S., Leys, S., Roer, R., Wilkie, I.C., Eds.; Apple Academic Press: Cambridge, MA, USA, 2024; Volume 3, pp. 345–430. [Google Scholar]
- Bonneel, M.; Hennebert, E.; Aranko, A.S.; Hwang, D.S.; Lefevre, M.; Pommier, V.; Wattiez, R.; Delroisse, J.; Flammang, P. Molecular mechanisms mediating stiffening in the mechanically adaptable connective tissues of sea cucumbers. Matrix Biol. 2022, 108, 39–54. [Google Scholar] [CrossRef]
- Chen, T.; Ren, C.; Wong, N.-K.; Yan, A.; Sun, C.; Fan, D.; Luo, P.; Jiang, X.; Zhang, L.; Ruan, Y.; et al. The Holothuria leucospilota genome elucidates sacrificial organ expulsion and bioadhesive trap enriched with amyloid-patterned proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2213512120. [Google Scholar] [CrossRef]
- Bobrovskaya, N.V.; Dolmatov, I.Y. Autotomy of the visceral mass in the feather star Himerometra robustipinna (Crinoidea, Comatulida). Biol. Bull. 2014, 226, 81–91. [Google Scholar] [CrossRef]
- Wilkie, I.C. Is muscle involved in the mechanical adaptability of echinoderm mutable collagenous tissue? J. Exp. Biol. 2002, 205, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Hennebert, E.; Coelho, A.; Flammang, P. The echinoderm tube foot and its role in temporary underwater adhesion. In Functional Surfaces in Biology; Springer: Dordrecht, The Netherlands, 2009; Volume 2, pp. 9–41. ISBN 978-1-4020-6694-8. [Google Scholar]
- Bodnar, A.G. Cellular and molecular mechanisms of negligible senescence: Insight from the sea urchin. Invertebr. Reprod. Dev. 2015, 59, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Mashanov, V.; Whaley, L.; Davis, K.; Heinzeller, T.; Machado, D.J.; Reid, R.W.; Kofsky, J.; Janies, D. A subterminal growth zone at arm tip likely underlies life-long indeterminate growth in brittle stars. Front. Zool. 2022, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, S.; Barbaglio, A.; Burlini, N.; Giacco, L.D.; Ghilardi, A.; Sugni, M.; Benedetto, C.D.; Bonasoro, F.; Wilkie, I.C.; Candia Carnevali, M.D. New insights into the mutable collagenous tissue of Paracentrotus lividus: Preliminary results. Zoosymposia 2012, 7, 279–285. [Google Scholar] [CrossRef]
- Barbaglio, A.; Tricarico, S.; Ribeiro, A.R.; Di Benedetto, C.; Barbato, M.; Dessì, D.; Fugnanesi, V.; Magni, S.; Mosca, F.; Sugni, M.; et al. Ultrastructural and biochemical characterization of mechanically adaptable collagenous structures in the edible sea urchin Paracentrotus lividus. Zoology 2015, 118, 147–160. [Google Scholar] [CrossRef]
- Birk, D.E.; Nurminskaya, M.V.; Zycband, E.I. Collagen fibrillogenesis in situ: Fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995, 202, 229–243. [Google Scholar] [CrossRef] [PubMed]
- deVente, J.E.; Lester, G.E.; Trotter, J.A.; Dahners, L.E. Isolation of intact collagen fibrils from healing ligament. J. Electron Microsc. 1997, 46, 353–356. [Google Scholar] [CrossRef]
- Dahners, L.E.; Lester, G.E.; Caprise, P. The pentapeptide NKISK affects collagen fibril interactions in a vertebrate tissue. J. Orthopaed. Res. 2000, 18, 532–536. [Google Scholar] [CrossRef]
- Motokawa, T.; Tsuchi, A. Dynamic mechanical properties of body-wall dermis in various mechanical states and their implications for the behavior of sea cucumbers. Biol. Bull. 2003, 205, 261–275. [Google Scholar] [CrossRef]
- Tamori, M.; Yamada, A.; Nishida, N.; Motobayashi, Y.; Oiwa, K.; Motokawa, T. Tensilin-like stiffening protein from Holothuria leucospilota does not induce the stiffest state of catch connective tissue. J. Exp. Biol. 2006, 209, 1594–1602. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Fassini, D.; Cullorà, E.; Barbaglio, A.; Tricarico, S.; Sugni, M.; Del Giacco, L.; Candia Carnevali, M.D. Mechanical properties of the compass depressors of the sea-urchin Paracentrotus lividus (Echinodermata, Echinoidea) and the effects of enzymes, neurotransmitters and synthetic tensilin-like protein. PLoS ONE 2015, 10, e0120339. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Tamori, M.; Iketani, T.; Oiwa, K.; Motokawa, T. A novel stiffening factor inducing the stiffest state of holothurian catch connective tissue. J. Exp. Biol. 2010, 213, 3416–3422. [Google Scholar] [CrossRef]
- Takehana, Y.; Yamada, A.; Tamori, M.; Motokawa, T. Softenin, A novel protein that softens the connective tissue of sea cucumbers through inhibiting interaction between collagen fibrils. PLoS ONE 2014, 9, e85644. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, E.P.; Wilt, F.H. Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo. Dev. Biol. 1998, 196, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, C.; Robinson, J.J. Characterization of matrix metalloprotease activities induced in the sea urchin extraembryonic matrix, the hyaline layer. Biochem. Cell Biol. 2001, 79, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, J.L.; Rosa, R.; Ruiz, D.L.; García-Arrarás, J.E. Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev. Biol. 2002, 250, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Barbaglio, A.; Oliveira, M.J.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. Matrix metalloproteinases in a sea urchin ligament with adaptable mechanical properties. PLoS ONE 2012, 7, e49016. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Zhou, D.-Y.; Liu, Y.-X.; Yu, M.-M.; Liu, B.; Song, L.; Dong, X.-P.; Qi, H.; Shahidi, F. Inhibitory effect of natural metal ion chelators on the autolysis of sea cucumber (Stichopus japonicus) and its mechanism. Food Res. Int. 2020, 133, 109205. [Google Scholar] [CrossRef]
- Yan, L.-J.; Sun, L.; Cao, K.-Y.; Chen, Y.-L.; Zhang, L.-J.; Liu, G.-M.; Jin, T.; Cao, M.-J. Type I collagen from sea cucumber (Stichopus japonicus) and the role of matrix metalloproteinase-2 in autolysis. Food Biosci. 2021, 41, 100959. [Google Scholar] [CrossRef]
- Elphick, M.R. The protein precursors of peptides that affect the mechanics of connective tissue and/or muscle in the echinoderm Apostichopus japonicus. PLoS ONE 2012, 7, e44492. [Google Scholar] [CrossRef]
- Tamori, M.; Takemae, C.; Motokawa, T. Evidence that water exudes when holothurian connective tissue stiffens. J. Exp. Biol. 2010, 213, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Barbaglio, A.; Oliveira, M.J.; Santos, R.; Coelho, A.V.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. Correlations between the biochemistry and mechanical states of a sea-urchin ligament: A mutable collagenous structure. Biointerphases 2012, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Tamori, M.; Ishida, K.; Matsuura, E.; Ogasawara, K.; Hanasaka, T.; Takehana, Y.; Motokawa, T.; Osawa, T. Ultrastructural changes associated with reversible stiffening in catch connective tissue of sea cucumbers. PLoS ONE 2016, 11, e0155673. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Prévost, S.F.; Blowes, L.M.; Egertová, M.; Terrill, N.J.; Wang, W.; Elphick, M.R.; Gupta, H.S. Interfibrillar stiffening of echinoderm mutable collagenous tissue demonstrated at the nanoscale. Proc. Natl. Acad. Sci. USA 2016, 113, E6362–E6371. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.S.; Barbieri, E.; Inamdar, S.R.; Mo, J. Synchrotron X-ray imaging combined with multiscale modelling applied to biological soft tissues. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 34–60. [Google Scholar]
- Barbieri, E.; Mo, J.; Gupta, H.S. Chemoviscoelasticity of the interfibrillar matrix of the dermis of the black sea cucumber Holothuria atra. Mech. Mater. 2022, 168, 104252. [Google Scholar] [CrossRef]
- Sisican, K.M.D.; Torreno, V.P.M.; Yu, E.T.; Conato, M.T. Physicochemical and biochemical characterization of collagen from Stichopus cf. horrens tissues for use as stimuli-responsive thin films. ACS Omega 2023, 8, 35791–35799. [Google Scholar] [CrossRef]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From food waste to innovative biomaterial: Sea urchin-derived collagen for applications in skin regenerative medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Carolo, A.; Melotti, L.; Zivelonghi, G.; Sacchetto, R.; Akyürek, E.E.; Martinello, T.; Venerando, A.; Iacopetti, I.; Sugni, M.; Martinelli, G.; et al. Mutable collagenous tissue isolated from echinoderms leads to the production of a dermal template that is biocompatible and effective for wound healing in rats. Mar. Drugs 2023, 21, 506. [Google Scholar] [CrossRef]
- Speck, O.; Speck, D.; Horn, R.; Gantner, J.; Sedlbauer, K.P. Biomimetic bio-inspired biomorph sustainable? An attempt to classify and clarify biology-derived technical developments. Bioinspir. Biomim. 2017, 12, 011004. [Google Scholar] [CrossRef]
- Nepal, D.; Kang, S.; Adstedt, K.M.; Kanhaiya, K.; Bockstaller, M.R.; Brinson, L.C.; Buehler, M.J.; Coveney, P.V.; Dayal, K.; El-Awady, J.A.; et al. Hierarchically structured bioinspired nanocomposites. Nat. Mater. 2023, 22, 18–35. [Google Scholar] [CrossRef]
- Drack, M.; Limpinsel, M.; De Bruyn, G.; Nebelsick, J.H.; Betz, O. Towards a theoretical clarification of biomimetics using conceptual tools from engineering design. Bioinspir. Biomim. 2017, 13, 016007. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Yuan, C.; Liang, X.; Dong, T.; Liu, T.; Zhang, J.; Zou, J.; Yang, H.; Bowen, C. Soft actuators and robotic devices for rehabilitation and assistance. Adv. Intell. Syst. 2022, 4, 2100140. [Google Scholar] [CrossRef]
- Huang, L.; Chen, L.; Chen, H.; Wang, M.; Jin, L.; Zhou, S.; Gao, L.; Li, R.; Li, Q.; Wang, H.; et al. Biomimetic scaffolds for tendon tissue regeneration. Biomimetics 2023, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Military Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, H.; Ma, T.; Yang, Y.; Luo, J.; Wang, H.; Jiang, P. A review of soft actuator motion: Actuation, design, manufacturing and applications. Actuators 2022, 11, 331. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Wang, Y.; Mu, X.; Ling, S.; Yu, H.; Chen, W.; Guo, C.; Watson, M.C.; Yu, Y.; et al. Stimuli-responsive composite biopolymer actuators with selective spatial deformation behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 14602–14608. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z. Adaptive and self-shaping materials. In Biomimetic Principles and Design of Advanced Engineering Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 79–100. ISBN 978-1-118-92625-3. [Google Scholar]
- Li, M.; Pal, A.; Aghakhani, A.; Pena-Francesch, A.; Sitti, M. Soft actuators for real-world applications. Nat. Rev. Mater. 2022, 7, 235–249. [Google Scholar] [CrossRef]
- Capadona, J.R.; Shanmuganathan, K.; Tyler, D.J.; Rowan, S.J.; Weder, C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 2008, 319, 1370–1374. [Google Scholar] [CrossRef]
- Jorfi, M.; Skousen, J.L.; Weder, C.; Capadona, J.R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 2015, 12, 011001. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Park, D.J.; Skousen, J.L.; Hess-Dunning, A.E.; Tyler, D.J.; Rowan, S.J.; Weder, C.; Capadona, J.R. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng. 2014, 11, 056014. [Google Scholar] [CrossRef]
- Mendez, J.; Annamalai, P.K.; Eichhorn, S.J.; Rusli, R.; Rowan, S.J.; Foster, E.J.; Weder, C. Bioinspired mechanically adaptive polymer nanocomposites with water-activated shape-memory effect. Macromolecules 2011, 44, 6827–6835. [Google Scholar] [CrossRef]
- Potter-Baker, K.A.; Capadona, J.R. Reducing the “stress”: Antioxidative therapeutic and material approaches may prevent intracortical microelectrode failure. ACS Macro Lett. 2015, 4, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.K.; Capadona, J.R. Understanding the role of innate immunity in the response to intracortical microelectrodes. Crit. Rev. Biomed. Eng. 2018, 46, 341–367. [Google Scholar] [CrossRef]
- Faisal, S.N.; Iacopi, F. Thin-film electrodes based on two-dimensional nanomaterials for neural interfaces. ACS Appl. Nano Mater. 2022, 5, 10137–10150. [Google Scholar] [CrossRef]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent fundamental advances and emerging biological and biomimicking applications. Adv. Mater. 2021, 33, 2004349. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Luo, Q.; Zhou, G.; Xu, X. Recent advances on cellulose nanocrystals and their derivatives. Polymers 2021, 13, 3247. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J.; et al. Understanding nanocellulose–water interactions: Turning a detriment into an asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Li, K.; Yang, H.; Chen, P.-Y. Recent advances in integration of 2D materials with soft matter for multifunctional robotic materials. Mater. Horiz. 2020, 7, 54–70. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Lee, Y.-J.; Yang, S. Responsive and foldable soft materials. Trends Chem. 2020, 2, 107–122. [Google Scholar] [CrossRef]
- Khadivi, P.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Lotfi, R. Fabrication of microphase-separated polyurethane/cellulose nanocrystal nanocomposites with irregular mechanical and shape memory properties. Appl. Phys. A 2019, 125, 779. [Google Scholar] [CrossRef]
- Lim, H.L.; Hwang, Y.; Kar, M.; Varghese, S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, R. Biomimetic Sea-Cucumber: Stiffness Fast-Reversible, Turbidity Switchable, Shape-Memorable and Self-Healing Hydrogel. M.Sc. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2019. [Google Scholar]
- Beaumont, M.; Potthast, A.; Rosenau, T. Cellulose nanofibrils: From hydrogels to aerogels. In Cellulose Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 277–339. ISBN 978-1-119-21761-9. [Google Scholar]
- Eyley, S.; Schütz, C.; Thielemans, W. Surface chemistry and characterization of cellulose nanocrystals. In Cellulose Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 223–252. ISBN 978-1-119-21761-9. [Google Scholar]
- Rosenau, T.; Potthast, A.; Hofinger, A.; Bacher, M.; Yoneda, Y.; Mereiter, K.; Nakatsubo, F.; Jaeger, C.; French, A.; Kajiwara, K. Toward a better understanding of cellulose swelling, dissolution, and regeneration on the molecular level. In Cellulose Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 99–125. ISBN 978-1-119-21758-9. [Google Scholar]
- Studart, A.R.; Erb, R.M. Bioinspired materials that self-shape through programmed microstructures. Soft Matter 2014, 10, 1284–1294. [Google Scholar] [CrossRef]
- Montero De Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired polymer systems with stimuli-responsive mechanical properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Wang, F.; Yang, Z.; Niu, X.; Wu, Q.; Sun, P. High-performance polyurethane nanocomposites based on UPy-modified cellulose nanocrystals. Carbohyd. Polym. 2019, 219, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, M.; Grunin, L.; Morris, R.H.; Imamutdinova, A. Nanocellulose-based thermoplastic polyurethane biocomposites with shape memory effect. J. Compos. Sci. 2023, 7, 168. [Google Scholar] [CrossRef]
- Xu, J.; Ye, S.; Fu, J. Novel sea cucumber-inspired material based on stiff, strong yet tough elastomer with unique self-healing and recyclable functionalities. J. Mater. Chem. A 2018, 6, 24291–24297. [Google Scholar] [CrossRef]
- Yuan, D.; Delpierre, S.; Ke, K.; Raquez, J.-M.; Dubois, P.; Manas-Zloczower, I. Biomimetic water-responsive self-healing epoxy with tunable properties. ACS Appl. Mater. Interfaces 2019, 11, 17853–17862. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Toyota, T. Photo-triggered solvent-free metamorphosis of polymeric materials. Nat. Commun. 2017, 8, 502. [Google Scholar] [CrossRef]
- Lancia, F.; Ryabchun, A.; Nguindjel, A.-D.; Kwangmettatam, S.; Katsonis, N. Mechanical adaptability of artificial muscles from nanoscale molecular action. Nat. Commun. 2019, 10, 4819. [Google Scholar] [CrossRef]
- Jiao, D.; Lossada, F.; Yu, W.; Guo, J.; Hoenders, D.; Walther, A. Non-equilibrium, light-adaptive, steady-state reconfiguration of mechanical patterns in bioinspired nanocomposites. Adv. Funct. Mater. 2020, 30, 1905309. [Google Scholar] [CrossRef]
- Jiao, D.; Lossada, F.; Guo, J.; Skarsetz, O.; Hoenders, D.; Liu, J.; Walther, A. Electrical switching of high-performance bioinspired nanocellulose nanocomposites. Nat. Commun. 2021, 12, 1312. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Akama, S.; Ohori, S.; Kawai, M.; Mitsumata, T. Magnetic elastomers with smart variable elasticity mimetic to sea cucumber. Biomimetics 2019, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Tsai, Y.-L.; Lin, S.-H.; Hsu, S. Smart polymers for cell therapy and precision medicine. J. Biomedic. Sci. 2019, 26, 73. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- De France, K.; Cranston, E.; Hoare, T. Mechanically reinforced injectable hydrogels. ACS Appl. Polym. Mat. 2020, 2, 1016–1030. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Li, Y.; Xu, B.; Cao, Z.; Liu, W. Sea cucumber-inspired autolytic hydrogels exhibiting tunable high mechanical performances, repairability, and reusability. ACS Appl. Mater. Interfaces 2016, 8, 8956–8966. [Google Scholar] [CrossRef]
- Li, W.; Guan, Q.; Li, M.; Saiz, E.; Hou, X. Nature-inspired strategies for the synthesis of hydrogel actuators and their applications. Prog. Polym. Sci. 2023, 140, 101665. [Google Scholar] [CrossRef]
- Mao, S.; Dong, E.; Jin, H.; Xu, M.; Zhang, S.; Yang, J.; Low, K.H. Gait study and pattern generation of a starfish-like soft robot with flexible rays actuated by SMAs. J. Bionic Eng. 2014, 11, 400–411. [Google Scholar] [CrossRef]
- Bell, M.A. Manufacturing and Design of Echinoderm-Inspired Underwater Soft Robots. Doctoral Dissertation, Harvard University Graduate School of Arts and Sciences, Cambridge, MA, USA, 2021. [Google Scholar]
- Picardi, G.; Astolfi, A.; Chatzievangelou, D.; Aguzzi, J.; Calisti, M. Underwater legged robotics: Review and perspectives. Bioinspir. Biomim. 2023, 18, 031001. [Google Scholar] [CrossRef]
- Liu, K.; Cheng, L.; Zhang, N.; Pan, H.; Fan, X.; Li, G.; Zhang, Z.; Zhao, D.; Zhao, J.; Yang, X.; et al. Biomimetic impact protective supramolecular polymeric materials enabled by quadruple H-bonding. J. Am. Chem. Soc. 2021, 143, 1162–1170. [Google Scholar] [CrossRef]
- Li, L.; Lin, Q.; Tang, M.; Tsai, E.H.R.; Ke, C. An integrated design of a polypseudorotaxane-based sea cucumber mimic. Angew. Chem. Int. Ed. 2021, 60, 10186–10193. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, R.; Bouzari, N.; Huang, J.; Golzar, H.; Jankhani, S.; Tang, X.; Mekonnen, T.; Aghakhani, A.; Shahsavan, H. Programmable nanocomposites of cellulose nanocrystals and zwitterionic hydrogels for soft robotics. Nat. Commun. 2023, 14, 6108. [Google Scholar] [CrossRef] [PubMed]
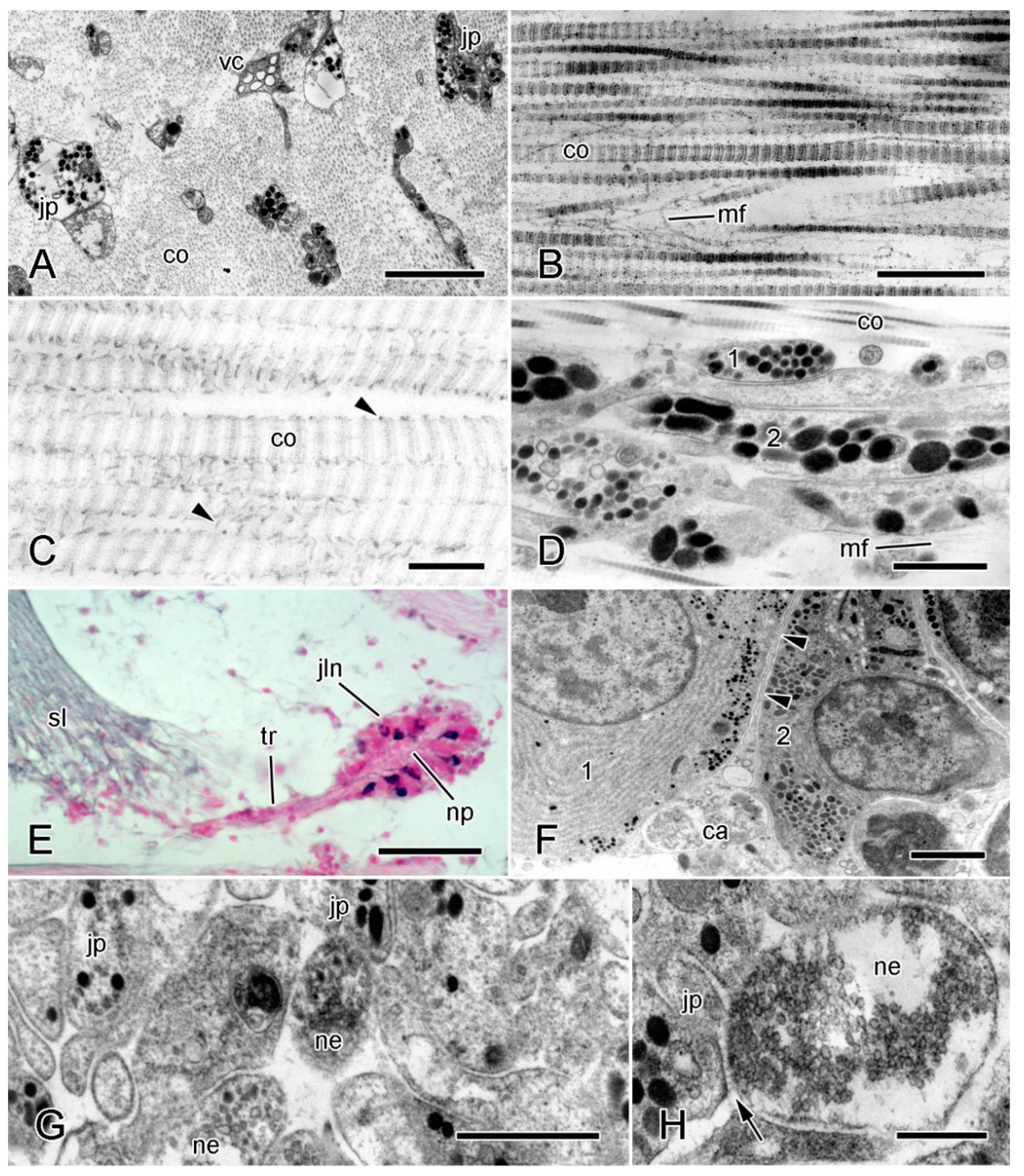
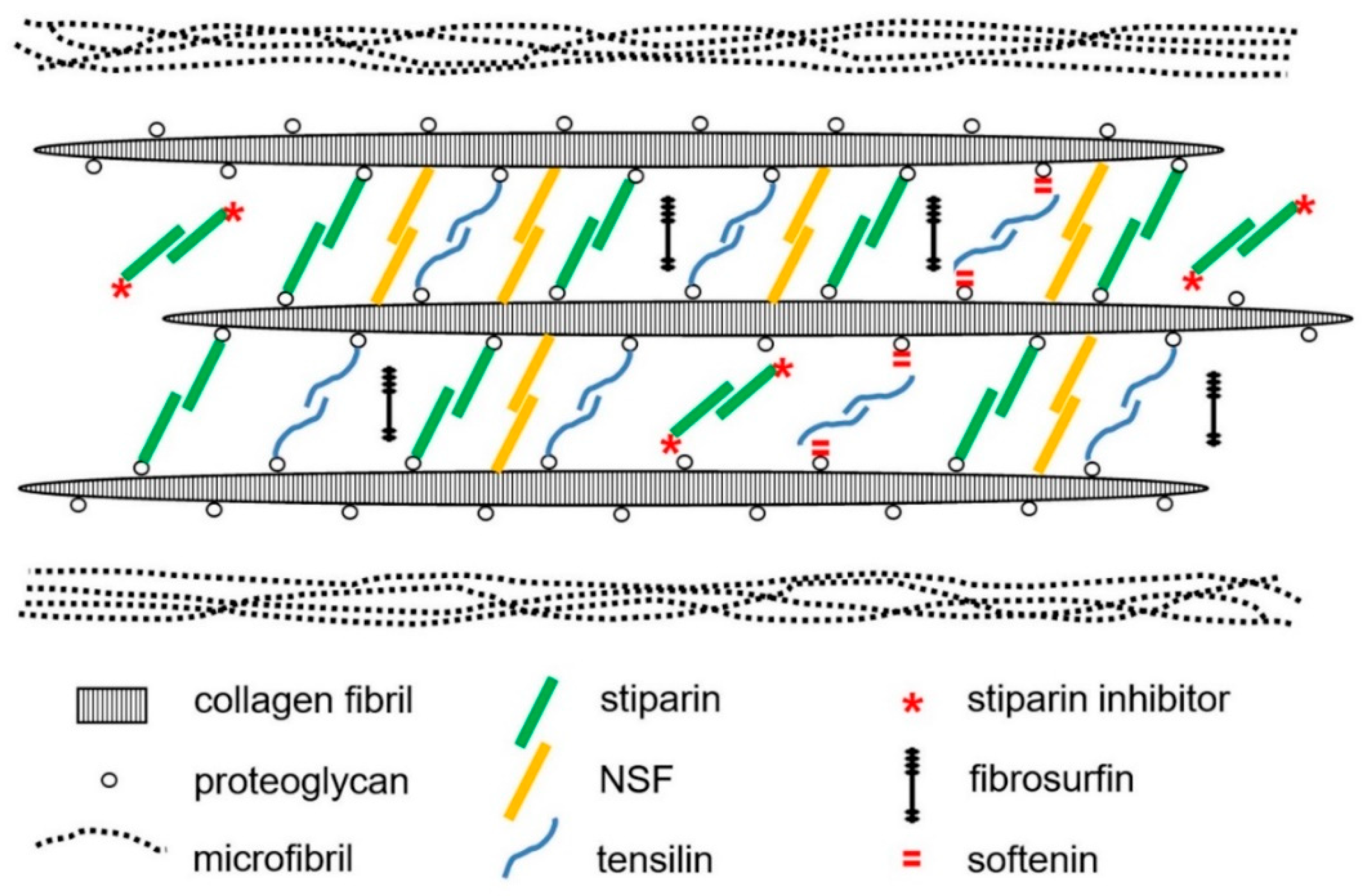
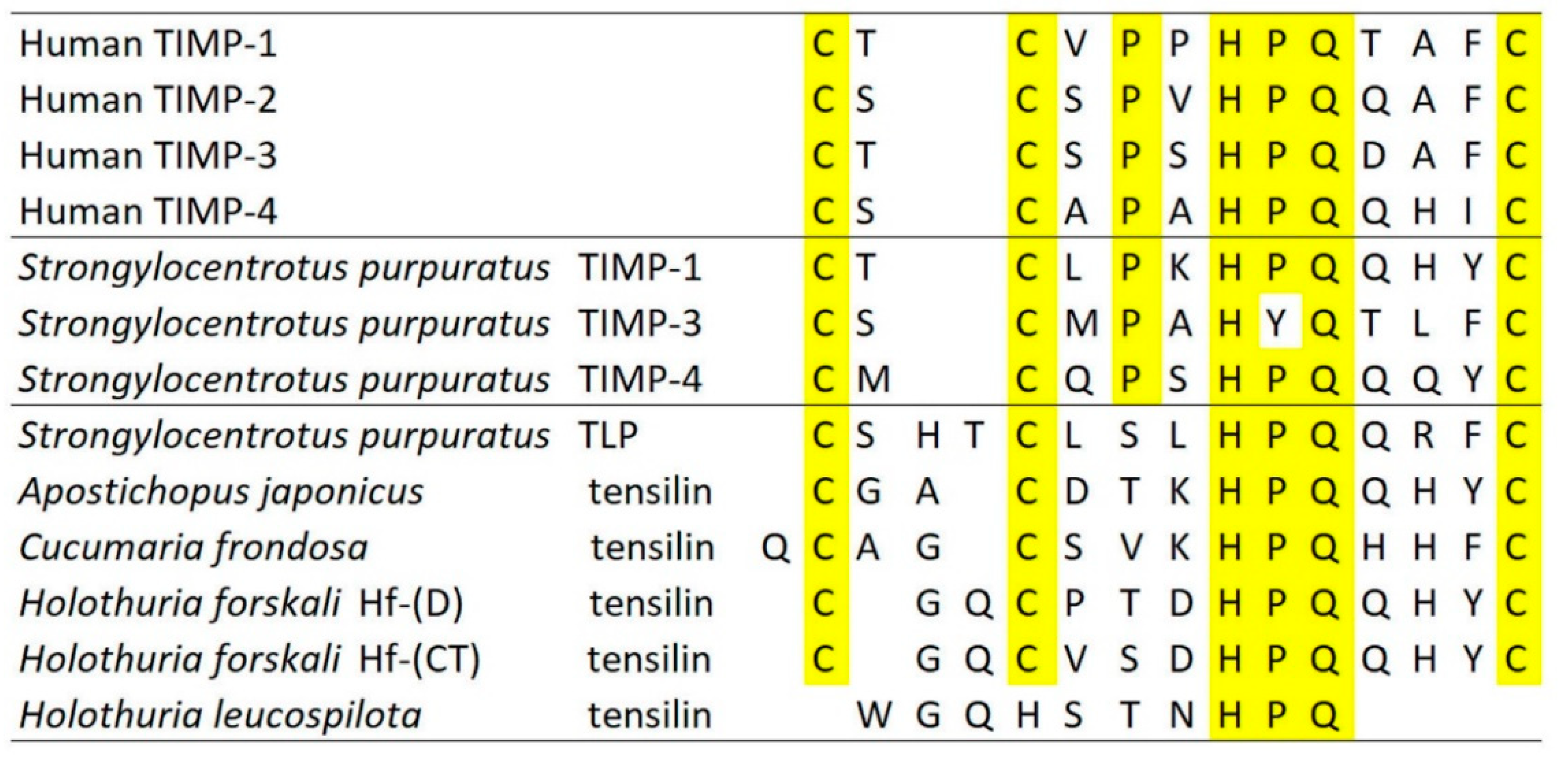

| Composition | Characteristics | Employability | Main Applications |
|---|---|---|---|
| Nanocellulose components: CNCs/CNFs (functionalized or chemically modified or not); CNC/CNF derivatives Polymeric matrix: PVA, UE, PU, and many others Possible additional components (bioderived or artificial): carbon nanotubes, etc. | Advantages: ease of production; sustainability; high commercial potential Structural characteristics: hierarchical organization; self-shaping, self-assembling; molecular interactions Functional properties: shape-memory; self-healing; gluing and adhesiveness; foldability; multi-stimuli responsiveness; modulable mechanical behavior; actuation abilities Specific biological functionalities: biocompatibility; biodegradability; low cytotoxicity; low invasivity; injectability; reusability | Biomedical area Tissue engineering Regenerative medicine Cosmetics Biotechnological area Agriculture Laminate industry | Multifunctional and diverse hybrid hydrogels; 2D and 3D scaffolding Cell culture adaptable substrata Injectable biocompatible fluids and substrata Tendon, ligament and skin repair/regeneration Ophthalmic applications Fingerprint detection; 3D printing inks; electric circuits Self-healable conductors/sensors Multifunctional robotic materials Magnetic elastomers Protective layers for non-biological materials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candia Carnevali, M.D.; Sugni, M.; Bonasoro, F.; Wilkie, I.C. Mutable Collagenous Tissue: A Concept Generator for Biomimetic Materials and Devices. Mar. Drugs 2024, 22, 37. https://doi.org/10.3390/md22010037
Candia Carnevali MD, Sugni M, Bonasoro F, Wilkie IC. Mutable Collagenous Tissue: A Concept Generator for Biomimetic Materials and Devices. Marine Drugs. 2024; 22(1):37. https://doi.org/10.3390/md22010037
Chicago/Turabian StyleCandia Carnevali, M. Daniela, Michela Sugni, Francesco Bonasoro, and Iain C. Wilkie. 2024. "Mutable Collagenous Tissue: A Concept Generator for Biomimetic Materials and Devices" Marine Drugs 22, no. 1: 37. https://doi.org/10.3390/md22010037
APA StyleCandia Carnevali, M. D., Sugni, M., Bonasoro, F., & Wilkie, I. C. (2024). Mutable Collagenous Tissue: A Concept Generator for Biomimetic Materials and Devices. Marine Drugs, 22(1), 37. https://doi.org/10.3390/md22010037






