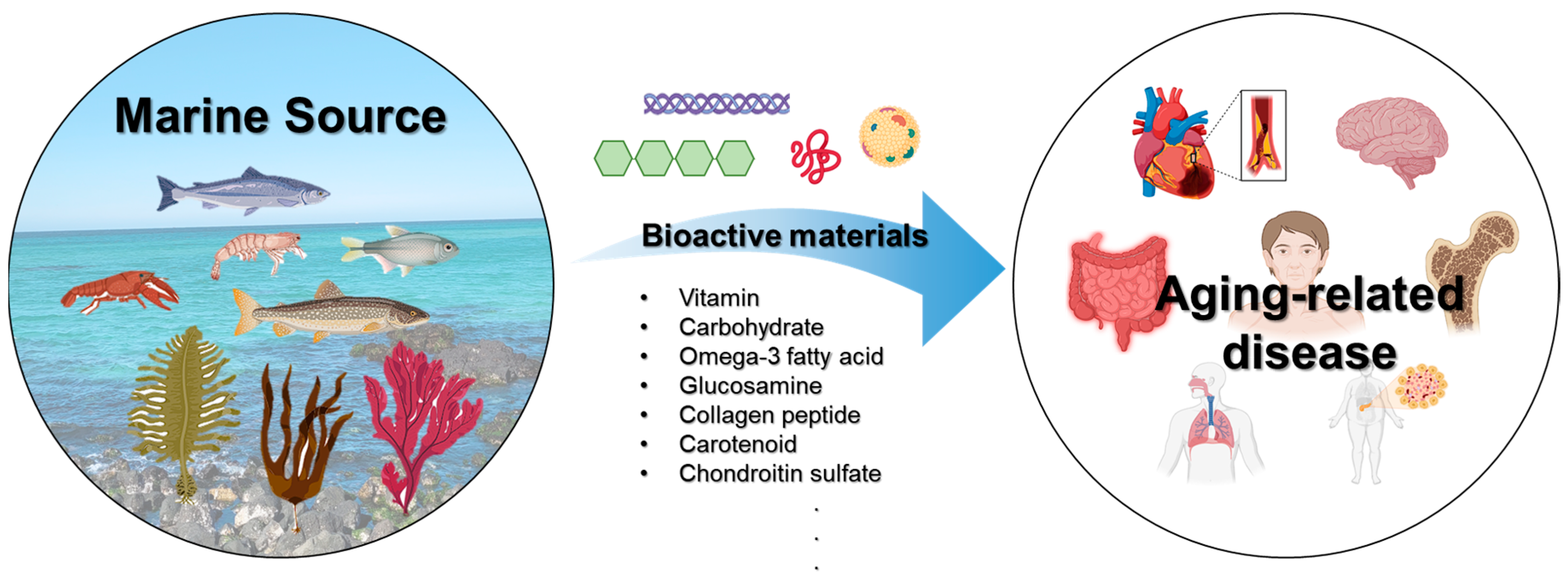
Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives
Abstract
:1. Introduction
2. Antioxidant Activity
2.1. Antioxidant Vitamins
2.2. Selenium
3. Marine Compounds for CVD
3.1. Omega-3 Fatty Acids
3.2. Zeaxanthin
3.3. Alginate Oligosaccharides
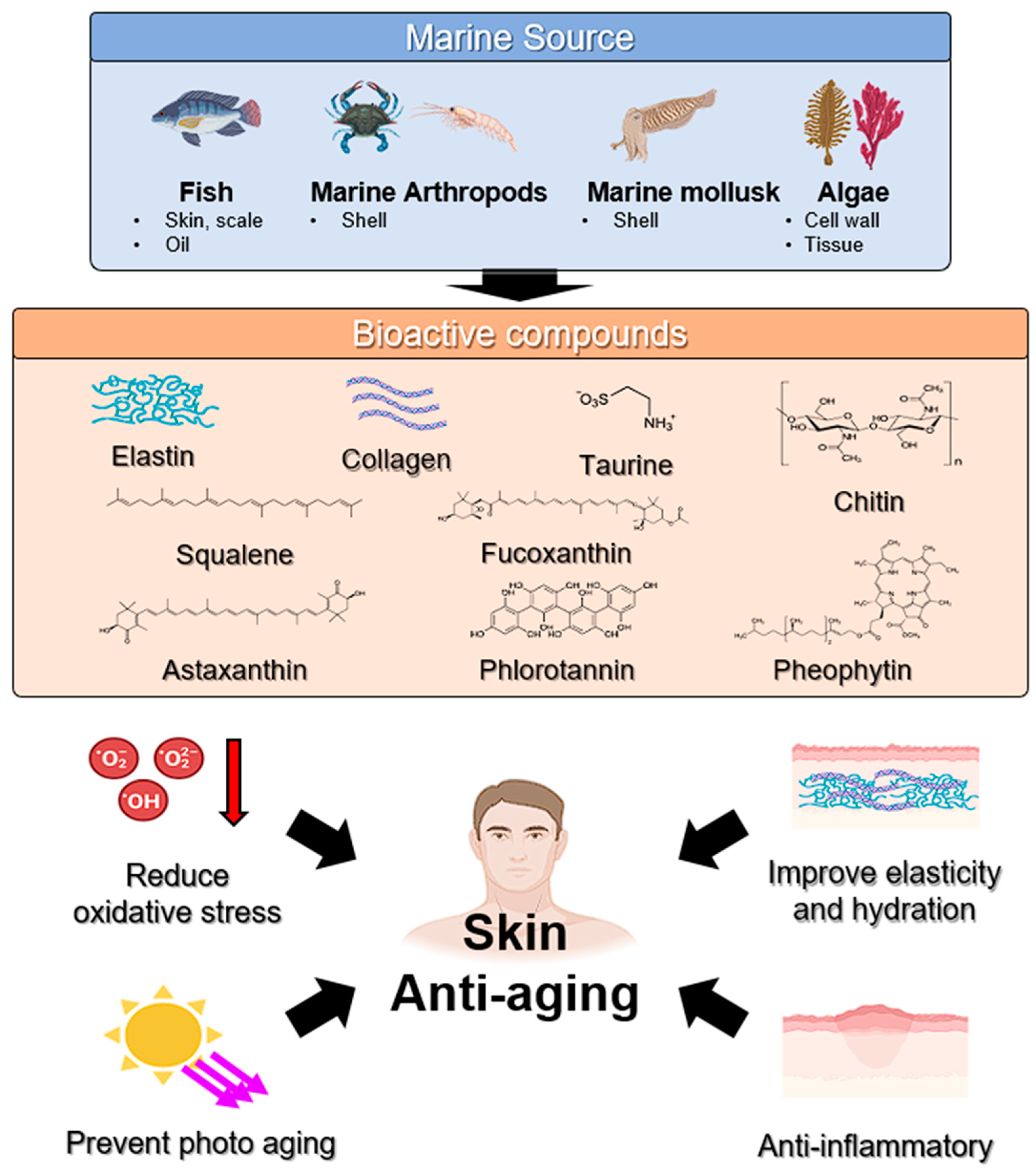
4. Marine Compounds for Skin Aging
Marine-Algae-Derived Carbohydrates
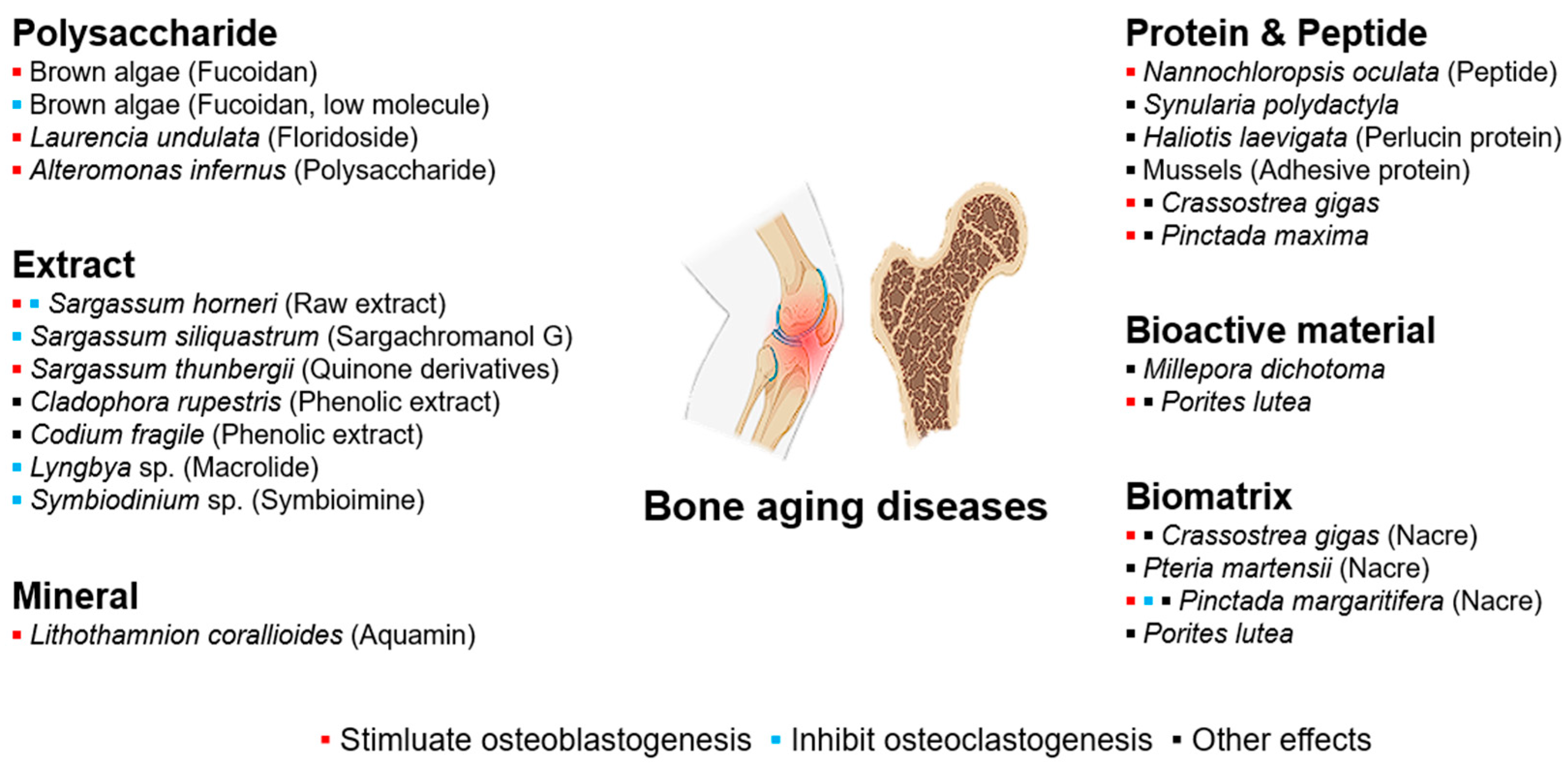
5. Marine Compounds for Bone and Joint Health
5.1. Osteoporosis
5.1.1. Marine-Algae-Derived Minerals
5.1.2. Aquamin®
5.2. Osteoarthritis
5.2.1. Glucosamine
5.2.2. Chondroitin Sulfate
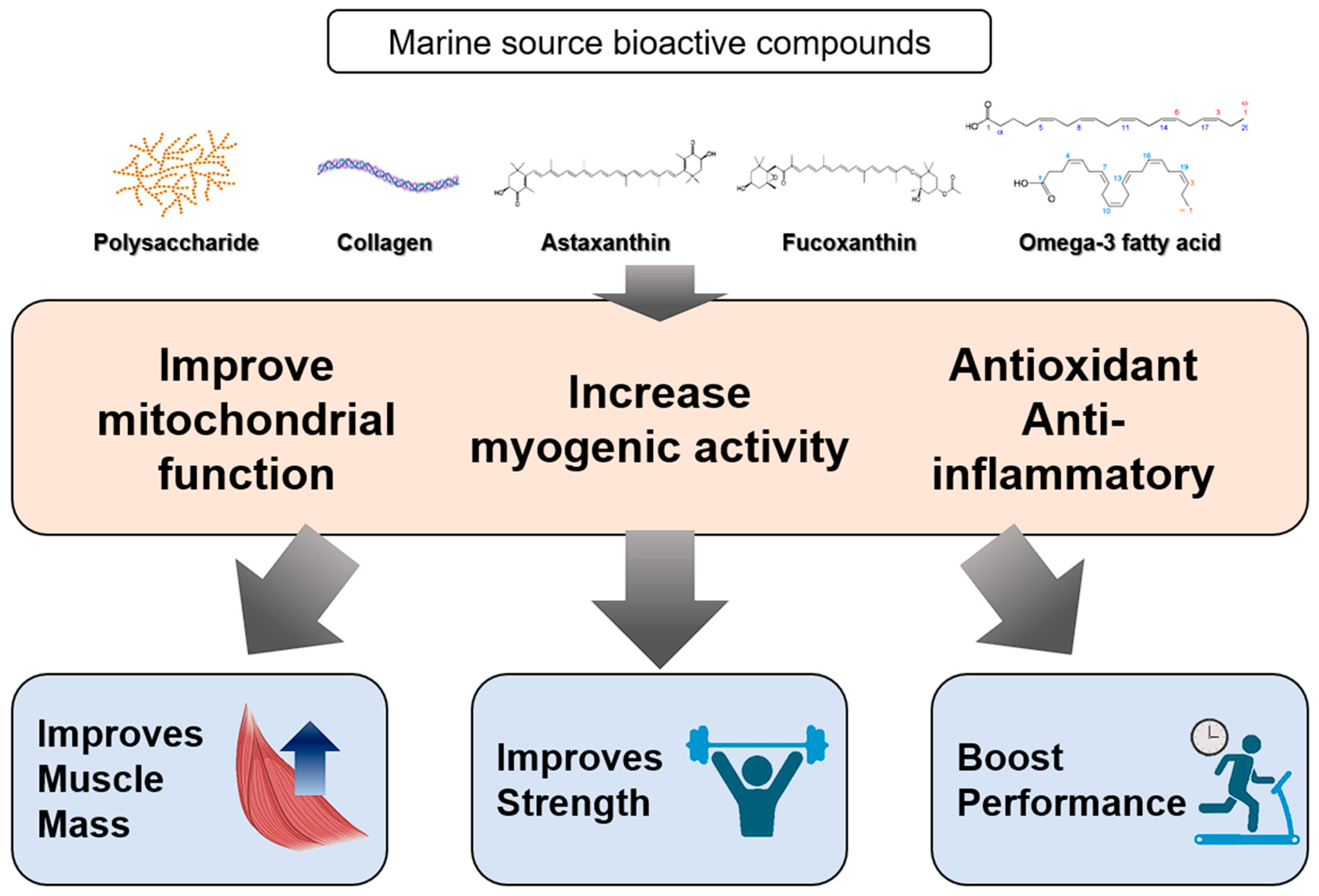
6. Marine Compounds for Sarcopenia
6.1. Collagen Peptide
6.2. Marine Carotenoids
7. Considerations for Practical Applications
8. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dharmarajan, T. Physiology of aging. In Geriatric Gastroenterology; Springer: Cham, Switzerland, 2021; pp. 101–153. [Google Scholar]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Antiaging Strategies and Remedies: A Landscape of Research Progress and Promise. ACS Chem. Neurosci. 2024, 15, 408–446. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Dryer, L.; Kalafsky, R. Approaches to the development of cosmetic products to counter the effects of skin aging. In Skin Aging Handbook; William Andrew: Norwich, NY, USA, 2009; pp. 265–290. [Google Scholar]
- Ki, M.-R.; Youn, S.; Kim, D.H.; Pack, S.P. Natural Compounds for Preventing Age-Related Diseases and Cancers. Int. J. Mol. Sci. 2024, 25, 7530. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; De Girolamo, M.; Cusenza, S.; Riccioni, G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 1242–1269. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Vellas, B.J.; Albarede, J.-L.; Garry, P.J. Diseases and aging: Patterns of morbidity with age; relationship between aging and age-associated diseases. Am. J. Clin. Nutr. 1992, 55, 1225S–1230S. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Grinin, L.; Grinin, A.; Korotayev, A. Anti-aging as a Key Challenge for the Medicine of the Future. In Cybernetic Revolution and Global Aging: Humankind on the Way to Cybernetic Society, or the Next Hundred Years; Springer: Cham, Switzerland, 2024; pp. 459–485. [Google Scholar]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.G.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Dashputre, N.L.; Sable, R.R.; Sawant, M.; Khairnar, S.J.; Ahire, E.D.; Patil, S.B.; Kadam, J.D. Marine-Derived Sources of Nutritional Vitamins. In Vitamins as Nutraceuticals: Recent Advances and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 129–166. [Google Scholar]
- Hajibabaei, K. Antioxidant properties of vitamin E. Ann. Res. Antioxid. 2016, 1, e22. [Google Scholar]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Dao, D.Q.; Ngo, T.C.; Thong, N.M.; Nam, P.C. Is vitamin A an antioxidant or a pro-oxidant? J. Phys. Chem. B 2017, 121, 9348–9357. [Google Scholar] [CrossRef]
- Fonseca, A.S.; Viitanen, A.K.; Kanerva, T.; Saamanen, A.; Aguerre-Chariol, O.; Fable, S.; Dermigny, A.; Karoski, N.; Fraboulet, I.; Koponen, I.K.; et al. Occupational Exposure and Environmental Release: The Case Study of Pouring TiO(2) and Filler Materials for Paint Production. Int. J. Environ. Res. Public Health 2021, 18, 418. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef]
- Sato, K.; Niki, E.; Shimasaki, H. Free radical-mediated chain oxidation of low density lipoprotein and its synergistic inhibition by vitamin E and vitamin C. Arch. Biochem. Biophys. 1990, 279, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Cárdenas, E.C.; Tuan, P.M.; Janssen, M.; Wijffels, R.H. Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol. Eng. 2003, 20, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xiang, J.; Yin, H.; He, H.; Hou, T. Progress in bioactive selenium-containing peptides. Food Sci. 2021, 42, 346–355. [Google Scholar]
- Mutanen, M.; Koivistoinen, P.; Morris, V.C.; Levander, O.A. Nutritional availability to rats of selenium in four seafoods: Crab (Callinectes sapidus), oyster (Crassostrea virginica), shrimp (Penaeus duorarum) and Baltic herring (Clupea harengus). Br. J. Nutr. 1986, 55, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Miao, J.; Chen, B.; Guo, J.; Ou, Y.; Liang, X.; Yin, Y.; Tong, X.; Cao, Y. Purification, identification, and antioxidative mechanism of three novel selenium-enriched oyster antioxidant peptides. Food Res. Int. 2022, 157, 111359. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Hussein, R.A.; Saleh, D.O.; Abdel Jaleel, G.A.R. Zeaxanthin Isolated from Dunaliella salina Microalgae Ameliorates Age Associated Cardiac Dysfunction in Rats through Stimulation of Retinoid Receptors. Mar. Drugs 2019, 17, 290. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Tsai, C.-H.; Chen, H.-Y.; Wang, K.-L.; Chang, H.-Y.; Huang, Y.-J.; Hong, Y.-H.; Ali, M.; Shieh, T.-M.; Huang, T.-C.; et al. Protective Effects of Fucoxanthin on Hydrogen Peroxide-Induced Calcification of Heart Valve Interstitial Cells. Mar. Drugs 2021, 19, 307. [Google Scholar] [CrossRef]
- Yan, Y.; Niu, Z.; Wang, B.; Zhao, S.; Sun, C.; Wu, Y.; Li, Y.; Ying, H.; Liu, H. Saringosterol from Sargassum fusiforme Modulates Cholesterol Metabolism and Alleviates Atherosclerosis in ApoE-Deficient Mice. Mar. Drugs 2021, 19, 485. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; Liu, D.; Wu, C.; Guo, P.; Lin, W. Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE−/− Mice. Mar. Drugs 2017, 15, 358. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujiwara, Y.; Hayashida, A.; Horlad, H.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Takeya, M.; et al. Manzamine A, a marine-derived alkaloid, inhibits accumulation of cholesterol ester in macrophages and suppresses hyperlipidemia and atherosclerosis in vivo. Bioorganic Med. Chem. 2013, 21, 3831–3838. [Google Scholar] [CrossRef]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-Rich Extract from the Green Alga Haematococcus pluvialis Lowers Plasma Lipid Concentrations and Enhances Antioxidant Defense in Apolipoprotein E Knockout Mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Li, Z.-C.; Li, Z.-H.; Chen, S.-H.; Jiang, M.-H.; Yang, H.-Y.; Liu, Y.-S.; Hu, R.; Zeng, Y.-W.; Dai, L.-H.; et al. Antiplatelet and Antithrombotic Effects of Isaridin E Isolated from the Marine-Derived Fungus via Downregulating the PI3K/Akt Signaling Pathway. Mar. Drugs 2022, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, Z.; Feng, W.; Hu, T.; Guan, H.; Mao, Y. AOS ameliorates monocrotaline-induced pulmonary hypertension by restraining the activation of P-selectin/p38MAPK/NF-κB pathway in rats. Biomed. Pharmacother. 2019, 109, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Lu, L.; Li, M.; Wang, G.; Sun, F.; Sun, H.; Wen, X.; Cheng, J.; Chen, J.; Pang, J.; et al. Xyloketal B alleviates cerebral infarction and neurologic deficits in a mouse stroke model by suppressing the ROS/TLR4/NF-κB inflammatory signaling pathway. Acta Pharmacol. Sin. 2017, 38, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Y.; Li, J.; Yuan, F.; Li, M.; Zhang, Q.; Huang, Y.-Y.; Pang, J.-Y.; Zhang, B.; Sun, F.-Y.; Sun, H.-S. Xyloketal B attenuates atherosclerotic plaque formation and endothelial dysfunction in apolipoprotein e deficient mice. Mar. Drugs 2015, 13, 2306–2326. [Google Scholar] [CrossRef]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Freeman, L.M. Beneficial effects of omega-3 fatty acids in cardiovascular disease. J. Small Anim. Pract. 2010, 51, 462–470. [Google Scholar] [CrossRef]
- Crupi, R.; Cuzzocrea, S. Role of EPA in inflammation: Mechanisms, effects, and clinical relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Calder, P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 2012, 56, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.; Yaqoob, P. Omega-3 (n-3) fatty acids, cardiovascular disease and stability of atherosclerotic plaques. Cell. Mol. Biol. 2010, 56, 28–37. [Google Scholar]
- Bhatt, D.L.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Steg, P.G.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C. REDUCE-IT USA: Results from the 3146 patients randomized in the United States. Circulation 2020, 141, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Pd, R.; Young, C.-C.; Hameed, A.; Lin, S.-Y.; Ab, A. Zeaxanthin Production by Novel Marine Isolates from Coastal sand of India and its Antioxidant Properties. Appl. Biochem. Biotechnol. 2013, 171, 817–831. [Google Scholar] [CrossRef]
- Tjandrawinata, R.R.; Nurkolis, F. A Comparative Analysis on Impact of Extraction Methods on Carotenoids Composition, Antioxidants, Antidiabetes, and Antiobesity Properties in Seagrass Enhalus acoroides: In Silico and In Vitro Study. Mar. Drugs 2024, 22, 365. [Google Scholar] [CrossRef]
- Zazirna, M.; Tischler, S.; Marko, D.; Varga, E.; Castejón, N. Ultrasound-based strategies for the recovery of microalgal carotenoids: Insights from green extraction methods to UV/MS-based identification. Food Res. Int. 2024, 187, 114354. [Google Scholar] [CrossRef]
- Ferraz, C.A.A.; Grougnet, R.; Nicolau, E.; Picot, L.; de Oliveira Junior, R.G. Carotenoids from Marine Microalgae as Antimelanoma Agents. Mar. Drugs 2022, 20, 618. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Worldwide burden of LDL cholesterol: Implications in cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 241–244. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T.; et al. Oxygenated Carotenoid Lutein and Progression of Early Atherosclerosis. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.H.; Paul-Labrador, M.J.; Fan, J.; Shircore, A.M.; Merz, C.N.B.; Dwyer, K.M. Progression of Carotid Intima-Media Thickness and Plasma Antioxidants: The Los Angeles Atherosclerosis Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.A. Zeaxanthin: Review of Toxicological Data and Acceptable Daily Intake. J. Ophthalmol. 2016, 2016, 3690140. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.; Rauf, A.; Khalil, A.A.; Shan, Z.; Chen, C.; Rengasamy, K.R.R.; Wan, C. Process and applications of alginate oligosaccharides with emphasis on health beneficial perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 303–329. [Google Scholar] [CrossRef]
- Ning, L.; Zhu, B.; Yao, Z. Separation, purification and structural characterization of marine oligosaccharides: A comprehensive and systematic review of chromatographic methods. J. Chromatogr. A 2024, 1719, 464755. [Google Scholar] [CrossRef]
- Yan, C.; Pan, M.; Geng, L.; Zhang, Q.; Hu, Y.; Wang, J.; Ye, S. A novel enzyme-assisted one-pot method for the extraction of fucoidan and alginate oligosaccharides from Lessonia trabeculata and their bioactivities. J. Oceanol. Limnol. 2024. [Google Scholar] [CrossRef]
- Han, Z.-L.; Chen, M.; Fu, X.-D.; Yang, M.; Hrmova, M.; Zhao, Y.-H.; Mou, H.-J. Potassium Alginate Oligosaccharides Alter Gut Microbiota, and Have Potential to Prevent the Development of Hypertension and Heart Failure in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2021, 22, 9823. [Google Scholar] [CrossRef]
- Xu, Z.; Lam, M.T. Alginate Application for Heart and Cardiovascular Diseases. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 185–212. [Google Scholar]
- Mizuno, H.; Bamba, S.; Abe, N.; Sasaki, M. Effects of an alginate-containing variable-viscosity enteral nutrition formula on defecation, intestinal microbiota, and short-chain fatty acid production. J. Funct. Foods 2020, 67, 103852. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; He, J. Effects of alginate oligosaccharide on the growth performance, antioxidant capacity and intestinal digestion-absorption function in weaned pigs. Anim. Feed Sci. Technol. 2017, 234, 118–127. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Liu, J.; Hu, S.; Shi, S.; Feng, W.; Mao, Y. Alginate oligosaccharide alleviates vascular aging by upregulating glutathione peroxidase 7. J. Nutr. Biochem. 2024, 126, 109578. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; D′Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ki, M.-R.; Min, K.H.; Pack, S.P. Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy. Antioxidants 2023, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.C.; Hu, H.-C.; Yu, S.-Y.; Tsai, Y.-H.; Korinek, M.; Wu, Y.-C.; Chang, F.-R.; Chen, Y.-J. Development on potential skin anti-aging agents of Cosmos caudatus Kunth via inhibition of collagenase, MMP-1 and MMP-3 activities. Phytomedicine 2023, 110, 154643. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata Fucoidan on Expression of Matrix Metalloproteinase-1 Promoter, mRNA, and Protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef]
- Chen, J.; Rashid, A.; Wang, S.; Liu, X.; Gao, G. Metabolisms and multiple functions of laminaran in marine algae: A critical review. Carbohydr. Polym. 2024, 327, 121652. [Google Scholar] [CrossRef]
- Cui, L.-B.; Zhou, X.-Y.; Zhao, Z.-J.; Li, Q.; Huang, X.-Y.; Sun, F.-Z. The Kunming mouse: As a model for age-related decline in female fertility in human. Zygote 2013, 21, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Biological activities of carrageenan. Adv. Food Nutr. Res. 2014, 72, 113–124. [Google Scholar] [PubMed]
- Ren, S.-W.; Li, J.; Wang, W.; Guan, H.-S. Protective effects of κ-ca3000+ CP against ultraviolet-induced damage in HaCaT and MEF cells. J. Photochem. Photobiol. B Biol. 2010, 101, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Lee, J.W.; Papaccio, F.; Bellei, B.; Picardo, M. Alterations of the pigmentation system in the aging process. Pigment Cell Melanoma Res. 2021, 34, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Balcos, M.C.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Kim, M.-K.; Kim, D.-S. ERK activation by fucoidan leads to inhibition of melanogenesis in Mel-Ab cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2015, 19, 29–34. [Google Scholar] [CrossRef]
- Quah, C.C.; Kim, K.H.; Lau, M.S.; Kim, W.R.; Cheah, S.H.; Gundamaraju, R. Pigmentation and dermal conservative effects of the astonishing algae Sargassum polycystum and Padina tenuis on guinea pigs, human epidermal melanocytes (HEM) and Chang cells. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 77–83. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, C.; Lu, C.; Mo, C.; Zeng, J.; Yao, M.; Jia, B.; Liu, Z.; Yuan, P.; Xu, S. Age-related bone diseases: Role of inflammaging. J. Autoimmun. 2024, 143, 103169. [Google Scholar] [CrossRef]
- Gao, Y.; Patil, S.; Jia, J. The Development of Molecular Biology of Osteoporosis. Int. J. Mol. Sci. 2021, 22, 8182. [Google Scholar] [CrossRef]
- Vun, J.; Iqbal, N.; Jones, E.; Ganguly, P. Anti-Aging Potential of Platelet Rich Plasma (PRP): Evidence from Osteoarthritis (OA) and Applications in Senescence and Inflammaging. Bioengineering 2023, 10, 987. [Google Scholar] [CrossRef]
- Dan, Y.L.; Yang, Y.Q.; Zhu, D.C.; Bo, L.; Lei, S.F. Accelerated biological aging as a potential risk factor for rheumatoid arthritis. Int. J. Rheum. Dis. 2024, 27, e15156. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Gonzalez, O.A. Mucosal circadian rhythm pathway genes altered by aging and periodontitis. PLoS ONE 2022, 17, e0275199. [Google Scholar] [CrossRef] [PubMed]
- Ringe, J.; Kaps, C.; Burmester, G.-R.; Sittinger, M. Stem cells for regenerative medicine: Advances in the engineering of tissues and organs. Naturwissenschaften 2002, 89, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Nguyen, T.K.M.; Park, T.-I.; Park, H.-M.; Pack, S.P. Biomimetic Silica Particles with Self-Loading BMP-2 Knuckle Epitope Peptide and Its Delivery for Bone Regeneration. Pharmaceutics 2023, 15, 1061. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.A.; Clarke, S.A. Bioactive Compounds from Marine Organisms: Potential for Bone Growth and Healing. Mar. Drugs 2018, 16, 340. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.A.; Walsh, P.; Maggs, C.A.; Buchanan, F. Designs from the deep: Marine organisms for bone tissue engineering. Biotechnol. Adv. 2011, 29, 610–617. [Google Scholar] [CrossRef]
- Granito, R.N.; Custódio, M.R.; Rennó, A.C.M. Natural marine sponges for bone tissue engineering: The state of art and future perspectives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1717–1727. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Bouvard, B.; Annweiler, C.; Legrand, E. Osteoporosis in older adults. Jt. Bone Spine 2021, 88, 105135. [Google Scholar] [CrossRef]
- Pu’Ad, N.M.; Koshy, P.; Abdullah, H.; Idris, M.; Lee, T. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Kim, D.H.; Min, K.H.; Pack, S.P. Efficient Bioactive Surface Coatings with Calcium Minerals: Step-Wise Biomimetic Transformation of Vaterite to Carbonated Apatite. Biomimetics 2024, 9, 402. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Kim, K.H.; Seo, J.-H.; Pack, S.P. Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration. Biomimetics 2024, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, 9–19. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Xuan, M.; Yang, R.; Zhang, J.; Chang, J. Marine biomaterials for sustainable bone regeneration. Giant 2024, 19, 100298. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Aenglong, C.; Tang, Q.-J.; Tanasawet, S.; Klaypradit, W.; Sukketsiri, W. Osteogenic properties and anti-osteoporosis activity of calcium hydroxyapatite from Katsuwonus pelamis bone and its water-soluble forms. Fish. Sci. 2023, 89, 837–852. [Google Scholar] [CrossRef]
- O’Gorman, D.M.; Tierney, C.M.; Brennan, O.; O’Brien, F.J. The Marine-derived, Multi-mineral formula, Aquamin, Enhances Mineralisation of Osteoblast Cells In Vitro. Phytother. Res. 2012, 26, 375–380. [Google Scholar] [CrossRef]
- Brennan, O.; Sweeney, J.; O’meara, B.; Widaa, A.; Bonnier, F.; Byrne, H.J.; O’Gorman, D.M.; O’Brien, F.J. A natural, calcium-rich marine multi-mineral complex preserves bone structure, composition and strength in an ovariectomised rat model of osteoporosis. Calcif. Tissue Int. 2017, 101, 445–455. [Google Scholar] [CrossRef]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6. [Google Scholar]
- Bannuru, R.R.; Vaysbrot, E.E.; Sullivan, M.C.; McAlindon, T.E. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 43, 593–599. [Google Scholar] [CrossRef]
- Ding, C. Do NSAIDs affect the progression of osteoarthritis? Inflammation 2002, 26, 139–142. [Google Scholar] [CrossRef]
- Brief, A.A.; Maurer, S.G.; Di Cesare, P.E. Use of Glucosamine and Chondroitin Sulfatein the Management of Osteoarthritis. JAAOS J. Am. Acad. Orthop. Surg. 2001, 9, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Novel functional food ingredients from marine sources. Curr. Opin. Food Sci. 2015, 2, 123–129. [Google Scholar] [CrossRef]
- Chen, J.; Huo, L.-N.; Gao, Y.; Zhang, Y.-L.; Chen, Y. Two new N-acetyl-ᴅ-glucosamine derivatives from the medical algae-derived endophytic fungus Penicillium chrysogenum. Nat. Prod. Res. 2022, 36, 3988–3991. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Nishioka, K. Effects of glucosamine/chondroitin supplement on osteoarthritis: Involvement of PGE2 and YKL-40. Jpn. J. Rheum. Jt. Surg. 2002, 21, 175–184. [Google Scholar]
- Piperno, M.; Reboul, P.; Le Graverand, M.H.; Peschard, M.; Annefeld, M.; Richard, M.; Vignon, E. Glucosamine sulfate modulates dysregulated activities of human osteoarthritic chondrocytes in vitro. Osteoarthr. Cartil. 2000, 8, 207–212. [Google Scholar] [CrossRef]
- Meulyzer, M.; Vachon, P.; Beaudry, F.; Vinardell, T.; Richard, H.; Beauchamp, G.; Laverty, S. Comparison of pharmacokinetics of glucosamine and synovial fluid levels following administration of glucosamine sulphate or glucosamine hydrochloride. Osteoarthr. Cartil. 2008, 16, 973–979. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; do Rosário Bronze, M. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Roughley, P.J. The structure and function of cartilage proteoglycans. Eur. Cell. Mater. 2006, 12, 92–101. [Google Scholar] [CrossRef]
- Ronca, F.; Palmieri, L.; Panicucci, P.; Ronca, G. Anti-inflammatory activity of chondroitin sulfate. Osteoarthr. Cartil. 1998, 6, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Iovu, M.; Dumais, G.d.; Du Souich, P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthr. Cartil. 2008, 16, S14–S18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, J.; Sun, Y.; Han, S. Chondroitin sulfate from sturgeon bone protects chondrocytes via inhibiting apoptosis in osteoarthritis. Int. J. Biol. Macromol. 2019, 134, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of Collagen Derived From Tropical Freshwater Carp Fish Scale Wastes and Its Amino Acid Sequence. Nat. Prod. Commun. 2019, 14, 1934578X19866288. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kwon, E.-Y.; Han, Y. A Collagen Hydrolysate Containing Tripeptides Ameliorates Sarcopenia in Middle-Aged Mice. Molecules 2022, 27, 2718. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Zhang, L.; Zhang, T.; Wang, P.; Wang, J. Type II collagen from squid cartilage mediated myogenic IGF-I and irisin to activate the Ihh/PThrp and Wnt/β-catenin pathways to promote fracture healing in mice. Food Funct. 2021, 12, 6502–6512. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef]
- Zhiyin, L.; Jinliang, C.; Qiunan, C.; Yunfei, Y.; Qian, X. Fucoxanthin rescues dexamethasone induced C2C12 myotubes atrophy. Biomed. Pharmacother. 2021, 139, 111590. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Nishino, H.; Hashimoto, T. Effects of fucoxanthin on the inhibition of dexamethasone-induced skeletal muscle loss in mice. Nutrients 2021, 13, 1079. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Fujita, T.; Nishino, H.; Hashimoto, T. Fucoxanthinol attenuates oxidative stress-induced atrophy and loss in myotubes and reduces the triacylglycerol content in mature adipocytes. Mol. Biol. Rep. 2020, 47, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miyaji, N.; Yang, M.; Mills, E.M.; Taniyama, S.; Uchida, T.; Nikawa, T.; Li, J.; Shi, J.; Tachibana, K.; et al. Astaxanthin Prevents Atrophy in Slow Muscle Fibers by Inhibiting Mitochondrial Reactive Oxygen Species via a Mitochondria-Mediated Apoptosis Pathway. Nutrients 2021, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ren, P.; Yang, R.; Yue, H.; Tang, Q.; Xue, C. Astaxanthin Ameliorates Skeletal Muscle Atrophy in Mice With Cancer Cachexia. Nutr. Cancer 2024, 76, 529–542. [Google Scholar] [CrossRef]
- Dai, W.; He, S.; Huang, L.; Lin, S.; Zhang, M.; Chi, C.; Chen, H. Strategies to reduce fishy odor in aquatic products: Focusing on formation mechanism and mitigation means. Food Chem. 2024, 444, 138625. [Google Scholar] [CrossRef]
- Zaky, A.; Abomohra, A. Marine-Based Biorefinery: A Path Forward to a Sustainable Future. Fermentation 2023, 9, 554. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ejeromedoghene, O.; Okoye, C.O.; Ezeorba, T.P.C.; Nyaruaba, R.; Ikechukwu, C.K.; Oladipo, A.; Orege, J.I. Microalgae biorefinery: An integrated route for the sustainable production of high-value-added products. Energy Convers. Manag. X 2022, 16, 100323. [Google Scholar] [CrossRef]
- Musa, M.; Ayoko, G.A.; Ward, A.; Rösch, C.; Brown, R.J.; Rainey, T.J. Factors Affecting Microalgae Production for Biofuels and the Potentials of Chemometric Methods in Assessing and Optimizing Productivity. Cells 2019, 8, 851. [Google Scholar] [CrossRef]
- Takahashi, T. Routine Management of Microalgae Using Autofluorescence from Chlorophyll. Molecules 2019, 24, 4441. [Google Scholar] [CrossRef]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained Photobiological Hydrogen Gas Production upon Reversible Inactivation of Oxygen Evolution in the Green Alga Chlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gujjala, L.K.S.; Varjani, S.; Kumar, S. Emerging microalgae-based technologies in biorefinery and risk assessment issues: Bioeconomy for sustainable development. Sci. Total Environ. 2022, 813, 152417. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, S.; Javeed, A.; Jian, C.; Liu, Y.; Sun, J.; Wu, S.; Fu, P.; Han, B. Structures and Anti-Allergic Activities of Natural Products from Marine Organisms. Mar. Drugs 2023, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Unar, A.; Sarfraz, M.; Ajarem, J.S.; Allam, A.A.; Bhatti, U.; Chanihoon, G.Q.; Afridi, H.I. Mitigating marine hazardous contaminants: A sustainable management perspective. Chemosphere 2023, 338, 139292. [Google Scholar] [CrossRef] [PubMed]
- Perez-Palacios, T.; Ruiz-Carrascal, J.; Solomando, J.C.; de-la-Haba, F.; Pajuelo, A.; Antequera, T. Recent Developments in the Microencapsulation of Fish Oil and Natural Extracts: Procedure, Quality Evaluation and Food Enrichment. Foods 2022, 11, 3291. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent advances in fishy odour in aquatic fish products, from formation to control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- Prester, L. Seafood Allergy, Toxicity, and Intolerance: A Review. J. Am. Coll. Nutr. 2016, 35, 271–283. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Song, W.-J.; Cho, S.-H.; Chang, Y.-S. Time trends of the prevalence of allergic diseases in Korea: A systematic literature review. Asia Pac. Allergy 2018, 8, e8. [Google Scholar] [CrossRef]
- Mir, S.A.; Farooq, S.; Shah, M.A.; Mir, M.B. Decontamination of Fish and Fish Products. In Microbial Decontamination of Food; Shah, M.A., Mir, S.A., Eds.; Springer Nature: Singapore, 2022; pp. 251–257. [Google Scholar]
- Martelli, F.; Cirlini, M.; Dellafiora, L.; Neviani, E.; Dall’Asta, C.; Bernini, V. Mitigation of marine toxins by interactions with bacteria: The case of okadaic acid and tetrodotoxin. Food Control 2022, 131, 108428. [Google Scholar] [CrossRef]
- Ali, A.; Wei, S.; Ali, A.; Khan, I.; Sun, Q.; Xia, Q.; Wang, Z.; Han, Z.; Liu, Y.; Liu, S. Research Progress on Nutritional Value, Preservation and Processing of Fish—A Review. Foods 2022, 11, 3669. [Google Scholar] [CrossRef]
- Zawistowski, J.; Kopeć, A. Chapter 13—Effect of functional food ingredients on nutrient absorption and digestion. In Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health; Bagchi, D., Ohia, S.E., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 173–189. [Google Scholar]
- Motohashi, N.; Gallagher, R.; Anuradha, V.; Gollapudi, R. Functional foods and their importance in geriatric nutrition. J. Clin. Nutr. Metab. 2017, 1, 2. [Google Scholar]

| Marine Source | Bioactive Compound | Target CVDs | Biological Effects | Ref. |
|---|---|---|---|---|
| Microalgae (Dunaliella Salina) | Zeaxanthin | Cardiac dysfunction | Elevates serum levels of homocysteine, creatinine kinase isoenzymes, and lactate dehydrogenase | [30] |
| Seaweed | Fucoxanthin | Heart valve disease | Decreases oxidative-stress-induced apoptosis and modulates Akt/ERK-related protein expression | [31] |
| Algae (Sargassum fusiforme) | Saringosterol | Atherosclerosis | Activates liver X receptors α and β to regulate cholesterol levels | [32] |
| Fungi (Aspergillus sp.) | Asperlin | Atherosclerosis | Reduces pro-inflammatory factors and decreases levels of iNOS, IL-1β, and TNFα expression | [33] |
| Marine sponge (Acanthostrongylophora ingens) | Manzamine A | Atherosclerosis | Decreases the total levels of free and LDL cholesterol and triglycerides | [34] |
| Algae (Haematococcus pluvialis) | Astaxanthin | Atherosclerosis | Decreases the total levels of triglyceride and cholesterol | [35] |
| Fungi (Amphichorda feline) | Isaridin E | Atherosclerosis | Downregulates the PI3K/Akt signaling pathway and has anti-inflammatory and anti-thrombotic effects | [36] |
| Brown algae | Alginate oligosaccharides | Hypertension | Decrease the expression of P-selectin and inhibit the p38MAPK/NF-κB pathway | [37] |
| Mangrove fungi | Xyloketal B | Atherosclerosis, hypertension, cardiac stroke | Promotes endothelial NO release, regulation of the Akt/eNOS pathway, and reductions in oxidative stress and has an antihypertensive effect | [38,39] |
| Fish oil | Omega-3 fatty acids (EPA and DHA) | Atherosclerosis, myocardial infarction, cardiac arrhythmia | Reduce inflammation, lower blood pressure, and improve lipid profiles | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Kim, D.H.; Pack, S.P. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Mar. Drugs 2024, 22, 496. https://doi.org/10.3390/md22110496
Han Y, Kim DH, Pack SP. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Marine Drugs. 2024; 22(11):496. https://doi.org/10.3390/md22110496
Chicago/Turabian StyleHan, Youngji, Dong Hyun Kim, and Seung Pil Pack. 2024. "Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives" Marine Drugs 22, no. 11: 496. https://doi.org/10.3390/md22110496
APA StyleHan, Y., Kim, D. H., & Pack, S. P. (2024). Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Marine Drugs, 22(11), 496. https://doi.org/10.3390/md22110496






