Recent Advances of the Zebrafish Model in the Discovery of Marine Bioactive Molecules
Abstract
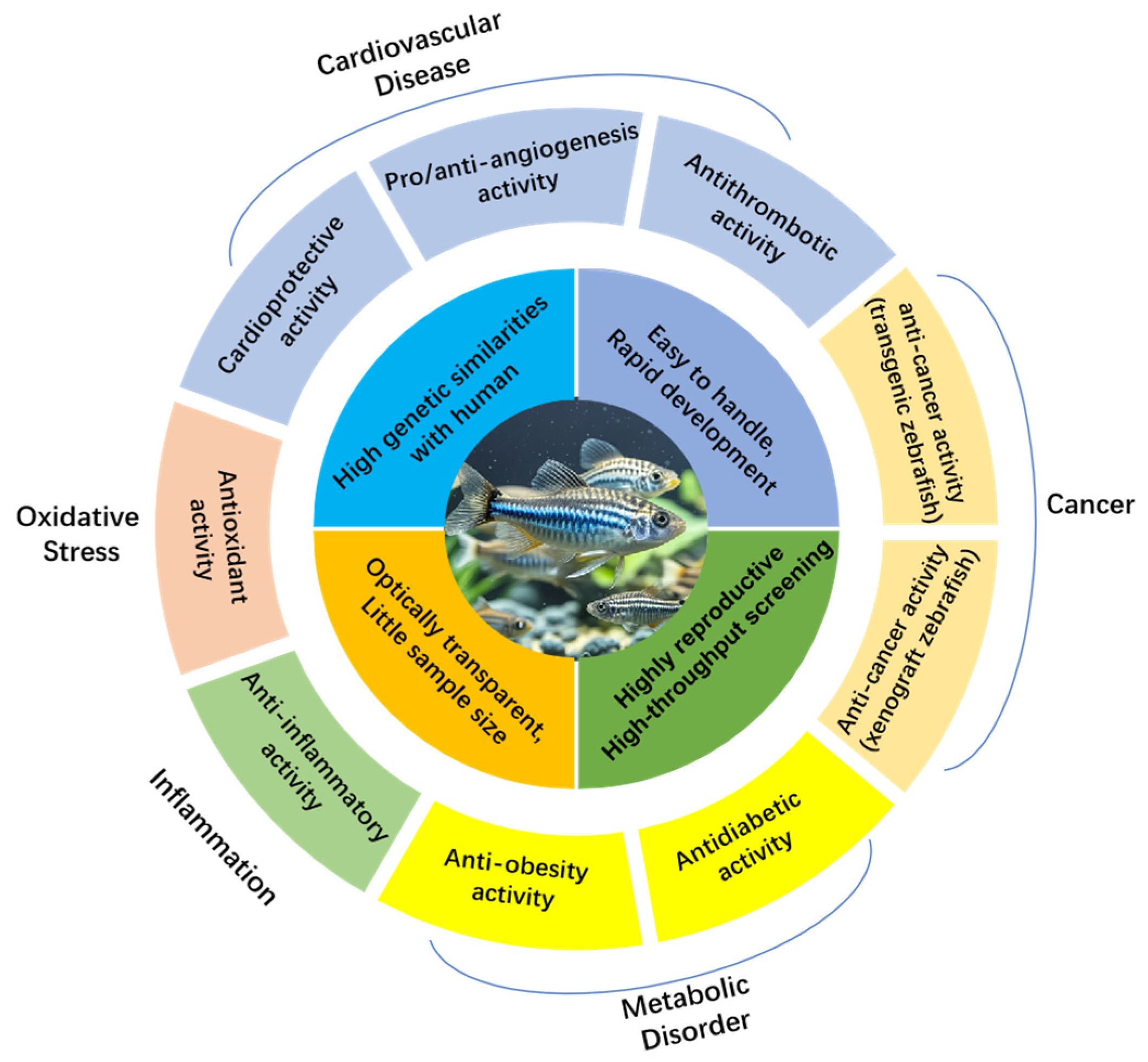
:1. Introduction
2. Zebrafish as a Model Species
3. Zebrafish Models for Evaluating Marine Bioactive Natural Products
3.1. Zebrafish Models Related to Cardiovascular Diseases
3.1.1. Evaluation of Cardioprotective Activity by Zebrafish Models
3.1.2. Evaluation of Angiogenesis Regulatory Activity by Zebrafish Models
3.1.3. Evaluation of Antithrombotic Activity by Zebrafish Models
3.2. Zebrafish Models Related to Cancer
3.2.1. Evaluation of Anticancer Activity by Transgenic Zebrafish Models
3.2.2. Evaluation of Anticancer Activity by Xenograft Zebrafish Models
3.3. Zebrafish Models Related to Metabolic Disorder
3.3.1. Evaluation of Antidiabetic Activity by Zebrafish Models
3.3.2. Evaluation of Anti-Obesity Activity by Zebrafish Models
3.4. Zebrafish Models Related to Inflammation
3.5. Zebrafish Models Related to Oxidative Stress
| Marine Nature Products | Source | Models | Main Effects (Concentrations) | References |
|---|---|---|---|---|
| Phospholipids | Shrimp heads, squid gonads, and viscera | AB or Tg(cmlc2: EGFP) zebrafish strain | Anti-heart failure and anti-arrhythmic effects (25, 50, 100 μg/mL and 20, 40, 80 μg/mL) | [49,50,51,52] |
| Peptide PcShK3 | zoantharian Palythoa caribaeorum | Tg(cmlc2: GFP) zebrafish strain | Cardio-protective activity (20 μM) | [53] |
| Calyculin A and okadaic acid | sponges | ILK-deficient msq mutant zebrafish embryos | Anti-heart failure activity (100 and 0.15 μM) | [54] |
| Dinotoamide J | Marine-derived fungus Aspergillus austroafricanus Y32-2 | Tg (vegfr2: GFP) zebrafish model | Proangiogenic activity (70, 120 μg/mL) | [60] |
| Communesin I, Fumiquinazoline Q and Protuboxepin E | Marine-derived fungus Penicillium expansum Y32 | Tg (vegfr2: GFP) zebrafish model | Proangiogenic activity (20 and 50 and 100 μg/mL) | [61] |
| N-(2-hydroxyphenyl)-acetamide, ethyl formyltyrosinate | Marine-derived fungus Penicillium chrysogenum Y20-2 | Tg (FLI1: EGFP) zebrafish model | Proangiogenic activity (25, 50, 100 μg/mL) | [62] |
| Chaetofanixins A-E | Hadal trench-derived fungus Chaetomium globosum YP-106 | Tg (flk1: EGFP) zebrafish model | Proangiogenic activity (20, 40, 80 μg/mL) | [63] |
| Bialorastin C | Deep-sea cold-seep-derived fungus Penicillium bialowiezense CS-283 | Tg(vegfr2: GFP) zebrafish model | Proangiogenic activity (20, 40 μM) | [64] |
| Sterigmatocystin A | Sponge-derived fungus Aspergillus versicolor (15XS43ZD-1) | Tg (vegfr2: GFP) zebrafish model | promoting angiogenesis activity (1.25 μM) | [65] |
| Agelanemoechine | South China sea sponge Agelas nemoechinata | Tg (vegfr2: GFP) zebrafish model | Proangiogenic activity (5 μM) | [66] |
| Lemnardosinanes A | Soft coral Lemnalia sp. | Tg (vegfr2: GFP) zebrafish model | Proangiogenic activity (20 μM) | [67] |
| Marchaetoglobin B and C | Marine-sponge-associated fungus Chaetomium globosum 162105 | Tg (vegfr2: GFP) zebrafish model | Proangiogenic activity (80 μM) | [68] |
| Chevalinulins A and B | Deep-sea cold-seep-derived fungus Aspergillus chevalieri CS-122 | Tg (vegfr2: GFP) zebrafish model | Proangiogenic activity (40 and 80 μg/mL) | [69] |
| Clavukoellian K | Marine soft coral Lemnalia sp | Tg (vegfr2: GFP) zebrafish model | Proangiogenic activity (2.5 μM) | [70] |
| Chaetoviridin L, chaetomugilin A, and chaephilone D | Hadal trench-derived fungus Chaetomium globosum YP-106 | Tg (flk1: EGFP) zebrafish model | Proangiogenic activity (20, 40, 80 μg/mL) | [71] |
| Fucoidan LMWF | Brown algae Saccharina japonica | High-glucose-induced zebrafish with blood vessel growth inhibition | Promotes subintestinal vessel formation in angiogenesis (100 μg/mL) | [72] |
| Polypeptide ZoaNPY | Zoanthus sociatus | Tg (Fli1a: EGFP) zebrafish model | Proangiogenic effects (100 pmol) | [73] |
| Cyclotripeptide X-13 | Mangrove fungus Xylaria sp. (no. 2508) | Tg (fli1: EGFP) zebrafish model | Proangiogenic activity (10, 50, 100 μM) | [59] |
| Pestaphilone J | Sea-mud-derived fungus Neopestalotiopsis sp. HN-1-6 | Tg (flk1:EGFP) zebrafish model | Proangiogenic activity (40 μM) | [74] |
| Pyrrolidinedione AD0157 | Marine fungi | Tg (fli1:EGFP)y1 zebrafish model | Anti-angiogenic activity (10 µM) | [76] |
| Catunaregin | Stem bark of Catunaregam spinosa | Tg (fli1: EGFP) zebrafish model | Anti-angiogenic activity (10, 50, 100 μM) | [77] |
| Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether (BDDE) | Marine algae Leathesia nana and Rhodomela confervoides | Zebrafish with Alkaline phosphatase staining | Anti-angiogenic activity (6.25, 12.5, 25 μM) | [78] |
| Stellettin B | Marine-sponge Stelletta sp. | Tg (fli1: EGFP)y1 transgenic zebrafish | Anti-angiogenic activity (≥50 nM) | [79] |
| Ishophloroglucin A | Ishige okamurae | Tg (flk: EGFP) zebrafish with high glucose-induced angiogenesis | Anti-angiogenic activity (0.015, 0.05, 0.15, 0.5 µM) | [80] |
| Polypeptide CS5931 | Ciona savignyi | Zebrafish model | Anti-angiogenic Activity (10, 20, 30 μg/mL) | [81] |
| Diphlorethohydroxycarmalol | brown alga Ishige okamurae | Tg (flk: EGFP) zebrafish with high glucose-induced angiogenesis | Anti-angiogenic Activity (0.06, 0.2, 0.6, 2 μM) | [82] |
| Protein ASP-3 | Arca subcrenata Lischke | Tg (fli1: GFP) zebrafish model | Anti-angiogenic Activity(18.8–150 μg/mL) | [83] |
| Polysaccharide SPS | Brown seaweed Sargassum integerrimum | Tg (fli1a:EGFP)y1 zebrafish model | Anti-angiogenic Activity (0, 1, 4 mg/mL) | [84] |
| Quinadoline B | marine-derived fungus Aspergillus clavutus LZD32-24 | Tg (fli1a: EGFP) zebrafish model | Anti-angiogenic Activity (2, 5, 10 μM) | [85] |
| Phloroglucinol and dieckol | Brown alga Ecklonia cava | Tg (flk: EGFP) zebrafish under high glucose conditions | Anti-angiogenic activity (0.24, 0.8, 2.4, 8 and 0.03, 0.1, 0.3, 1 μM) | [86] |
| Sinularin | Soft coral Sinularia flexibilis | The angiofluorescent zebrafish | Anti-angiogenic activity (5 μM) | [87] |
| Capnellene GB9 | Soft coral Capnella imbricata | Tg (fli: EGFP) zebrafish model | Anti-angiogenic activity (10 μM) | [88] |
| Toluquinol | Marine fungus Penicillium sp. HL-85-ALS5- R004 | Tg (fli1: EGFP)y1 zebrafish model | Anti-angiogenic activity (20 μM) | [89] |
| Solomonamide A | Marine algae Leathesia nana | Zebrafish model | Anti-angiogenic activity (5, 10 μM) | [90] |
| Somocystinamide A | Marine microorganisms Lyngbya majuscula | Tg(fli1: EGFP) zebrafish model | Anti-angiogenic activity (80, 160, 300, 1.6, 3 μM) | [91] |
| Fucoidan | Fucus vesiculosus | Tg(fli1: EGFP) zebrafish model | Anti-angiogenic activity (300 μg/mL) | [92] |
| Dihydroaustrasulfone alcohol WA-25 | Soft coral Cladiella australis | Tg(fli1: EGFP)y1 and Tg(kdrl: mCherryci5-fli1a: negfpy7) zebrafish model | Anti-angiogenic activity (50 μM) | [93] |
| Bis(2,3,6-tribromo-4,5-dihydroxybenzyl)ether (BTDE) | Marine red alga Symphyocladia latiuscula | Tg (flk1: EGFP) zebrafish model | Anti-angiogenic activity (2.5–10 μM) | [94] |
| Asperhiratide | Soft coral-derived fungus Aspergillus hiratsukae SCSIO 5Bn1003 | Tg (fli1: EGFP) zebrafish model | Anti-angiogenic activity | [95] |
| Pyrrole-pyridinimidazole derivative 8a | Marine sponge Agelas nakamurai | Tg (flk1: EGFP) zebrafish model | Anti-angiogenic activity(100, 150 μM) | [96] |
| Penisterine C and D | Marine brown alga derived fungus, Penicillium sumatraense SC29 | Tg (fli1: EGFP) zebrafish model | Anti-angiogenic activity (10.2, 20.4 and 8.6, 17.2 μg/mL) | [97] |
| Monacolin X | Fungi-NMK7 associated with marine sponge | Tg (Kdr: EGFP)/ko1 zebrafish model | Anti-angiogenic activity (0.5, 1 μM) | [98] |
| Dolastatin 15 | Marine cyanobacteria | vhl+/hu2117 heterozygous parents carrying the fli1a:egfpy1 transgene | Anti-vascularization effect (6 μM) | [99] |
| Murrangatin | Marine plant | Tg (fli1: EGFP) zebrafish model | Anti-angiogenic effects (10, 50, 100 μM) | [100] |
| 4-hydroxyphenylacetic acid | Marine-derived fungus Emericellopsis maritima Y39–2 | Arachidonic Acid induced AB zebrafish model | Antithrombotic activity(164.3, 328.6 μM) | [110] |
| Sarcoeleganolide I | Soft coral Sarcophyton elegans | Arachidonic Acid induced AB zebrafish model | Antithrombotic activity (20 μM) | [111] |
| Sarcoelegan C | Soft coral Sarcophyton elegans | Arachidonic Acid induced AB zebrafish model | Antithrombotic activity (20 μM) | [112] |
| sarcocinerenolides C and H | soft coral Sarcophyton cinereum | Arachidonic Acid induced AB zebrafish model | Antithrombotic activity (20 μM) | [113] |
| Pyruvylated and sulfated galactan (PSG) | Green alga Dictyosphaeria cavernosa | phenylhydrazine-induced thrombosis model of Tg(gata1: dSRed) and Tg(CD41: EGFP) zebrafish strains | Antithrombotic activity (100, 150 μg/mL) | [114] |
| Oligo-Fucoidan | Brown seaweed | AB, Tg (fabp10a: HBV-HBx-mCherry, myl7: EGFP), Tg (fabp10a: src, myl7: EGFP), Tg (fabp10a: HBV-HBx-mCherry, myl7: EGFP, p53−/+), Tg (fabp10a: src, myl7: EGFP, p53−/+) | Prevents radiation-induced fibrosis and secondary tumors (300 mg/kg) | [120] |
| Terphenyllin derivative CHNQD-00824 | Marine-derived compound library | Tg (fabp10: rtTA2s-M2; TRE2: EGFP krasG12V) | inhibit DOX-induced liver-specific enlargement (2.5, 5 μM) | [121] |
| Rakicidin B and B1 | Marine Micromonospora | Zebrafish xenotransplantation model with HCT-8 tumor cell | Antitumor (3, 10,30, 35, 40 ng/mL) | [127] |
| Rhopaloic acid A | Marine sponge Rhopaloeides sp. | Zebrafish oral and Bladder cancer xenotransplantation model | Antitumor effects against oral and Bladder cancer (0.03, 0.3 μg/mL) | [128,129] |
| Tilapia piscidin 4 | Nile tilapia | AB zebrafish bladder cancer model | Antitumor effects against bladder cancer (0.3, 1, 3 μg/mL) | [130] |
| Crambescidine-816 | Marine sponge Crambe crambe | Zebrafish xenotransplantation model with colorectal carcinoma cells | antitumor activity against colorectal carcinoma (1, 5, 10 μM) | [131] |
| Holothurian glycosaminoglycan | Sea cucumber Holothuria leucospilota | AB/Tubingen zebrafish xenotransplantation model with B16F10 tumor cell | Antitumor Effects (1 μM) | [132] |
| Saringosterol acetate | Brown alga Hizikia fusiforme | Tg(fli1: EGFP) zebrafish hepatocellular carcinoma xenograft model | Suppress hepatocellular carcinoma growth and metastasis (2 or 5 μg/g) | [133] |
| Cyclo (l-Pro-l-Leu), cyclo (l-Pro-l-Val), cyclo (l-Pro-l-Phe) and cyclo (l-Pro-l-Tyr) | Exiguobacterium acetylicum S01 | zebrafish xenogroft model with HT-29 tumor cells | Inhibit the tumor progression (50, 100, 150 μM) | [134] |
| Intestinal peptide (SCIP) | Sea cucumber | AB zebrafish xenogroft model with MCF-7 tumor cells | Inhibits the proliferation of MCF-7 tumor cells (27.8, 83.3, 250 μg/mL) | [135] |
| Peptides MP06 | Green sea algae Bryopsis plumosa | Tg(kdrl: GFP) zebrafish xenogroft model with A549 cells | Reduce metastatic dissemination (1, 2, 4, 10 μM) | [136] |
| Isofistularin-3 | Marine sponge Aplysina aerophoba | zebrafish xenogroft model with VampiroPC3 or Vampiro-SH-SY5Y cells | Antiproliferative activity (5, 10, 25, 50 μM) | [137] |
| Trabectedin | Marine derived Ecteinascidia turbinata | zebrafish xenogroft model with NCI-H295R, MUC-1, and TVBF-7 cells | Reduce ACC cell xenograft area and metastasis formation (15 nM) | [138] |
| Brasilterpenes A and C | Deep Sea-Derived Fungus Paraconiothyrium brasiliense HDN15-135 | Tg(-1.2ins:htBidTE–ON; LR) zebrafish model | Hypoglycemic activity (0, 1, 10, 50, 200 μM) | [154] |
| Penipyrol C | Mangrove derived fungus Penicillium sp. HDN-11-131 | Tg(-1.2ins: H2BmCherry) and Tg (-1.2ins: H2BmCherry) zebrafish model | Anti-diabetes (10 μM) | [155] |
| Extracts of Polysiphonia japonica and 5-Bromoprotocatechualdehyde | Polysiphonia japonica | Tg(ins: EGFP) zebrafish model | Protects against palmitate-induced β-cell dysfunction (10 and 50 μM) | [156,157] |
| Con-Ins G1, Con-Ins G3, Con-Ins T1A, Con-Ins T2, Con-Ins K1, Con-Ins K2 | Conus geographus, C.tulipa, C.kinoshitai | Streptozotocin-induced model of diabetes in zebrafish | Reduce blood glucose (65 ng/g) | [158] |
| Aspterric acid | Mangrove sediment-derived fungus Penicillium polonicum H175 | Tg (Ins: htBidTEON; LR) zebrafish model | Hypoglycaemic effect (10 μmol/L) | [159] |
| Antarctic krill enzymatic hydrolysates AKEH | Antarctic krill | Diet-induced diabetic zebrafish model | Hypoglycaemic effect (1.35,2.70, 5.40 g/L) | [160] |
| Dieckol | Ecklonia cava | Alloxan-induced diabetic zebrafish model | Anti-diabetes activity (1 µg/g body weight) | [161] |
| Palmaria mollis | The red seaweed Palmaria mollis | Diet-induced obese zebrafish model | Anti-obesity effects (PM dose of 2.5% (w/w)) | [178] |
| Citreorosein and questinol | Marine sponge-associated fungus Talaromyces stipitatus KUFA 0207. | Diet-induced obese zebrafish model | Anti-obesity effects (5 μM) | [176] |
| (1′Z)-2-(1′,5′-dimethylhexa-1′,4′-dieny1)-5-methylbenzene-1,4-diol, 6-(3-hydroxy-6-methyl-1,5-heptadien-2-yl)-3-methylbenzene-1,4-diol, 4-hydroxy-3,7-dimethyl-7-(3-methylbut-2-en-1-yl)benzofuran-15-one, 1,8-epoxy-1(6),2,4,7,10-bisaborapentaen-4-ol, 9-(3,3-dimethyloxiran-2-yl)-1,7-dimethyl-7-chromen-4-ol | Marine sponge Myrmekioderma sp. | Diet-induced obese zebrafish model | Lipid-reducing activity (10 µM) | [177] |
| Polysaccharide SS3-N1 | Suaeda salsa L. in coastal saline-alkali areas | Egg yolk powder-induced hyperlipidemic zebrafish model | Hypolipidemic activity (100 μg/mL) | [179] |
| Fucoxanthin | Marine brown algae | Egg yolk powder-induced hyperlipidemic zebrafish model | Inhibits lipid accumulation (3.125, 6.25, 12.5 μM) | [180] |
| Glycosaminoglycans | Ostrea rivularis | Hyperlipidemic zebrafish | Hypolipidemic effect (fed with 125, 250, 500 mg/(kg·day)) | [181] |
| Saringosterol acetate | Sargassum fusiforme | Diet-induced obese adult male zebrafish | Anti-obesity Activity (2.5% (w/w)) | [182] |
| Extracts of cyanobacteria | cyanobacteria | Diet-induced obese zebrafish | Lipid Reducing Activity (10 μg/mL) | [168,169] |
| The Extracts of seagrass Halophila stipulacea | Seagrass Halophila stipulacea | Diet-induced obese zebrafish | Lipid Reducing Activity (2, 6 μg/mL) | [170] |
| fractions of cyanobacteria | cyanobacterial library | Diet-induced obese zebrafish | Repress intestinal lipid absorption (10 μg/mL) | [171] |
| Exometabolome from Cyanobacteria | Cyanobacteria | Diet-induced obese zebrafish | Lipid-Reducing Activity (25 µg/mL) | [172] |
| Chlorophyll derivative 13-2-hydroxypheophytine | Cyanobacteria | Diet-induced obese zebrafish | Reduce neutral lipid reserves (7.5 μg/mL) | [173] |
| Vitamin K1-analog (OH-PhQ) | Cyanobacterium Tychonema sp. LEGE 07196 | Diet-induced obese zebrafish | Lipid reducing activity (10 μg/mL) | [174] |
| The Extracts of Microbacterium foliorum #91-29 and #91-40 | Microbacterium foliorum | Diet-induced obese zebrafish | Lipid reducing activity (10 μg/mL) | [175] |
| Peptide LLTRAGL | Rapana venosa | 2,4,6-trinitrobenzene sulfonic acid-induced Tg(zlyz: EGFP) zebrafish model | Protective effect against inflammatory bowel disease (20, 40, 80 μg/mL) | [190] |
| Septosone A | Marine Sponge Dysidea septosa | CuSO4-induced Tg(zlyz: EGFP) zebrafish model | Anti-inflammatory activity (2.5, 5, 10 μM) | [191] |
| Somalactam A | Streptomyces somaliensis 1107 | LPS-induced Tg(zlyz: EGFP) zebrafish model | Anti-inflammatory effect (10 μM) | [192] |
| Dysidinoid B | Sponge Dysidea septosa | CuSO4-induced Tg(zlyz: EGFP) zebrafish model | Anti-inflammatory activity (20, 40, 80 μM) | [193] |
| Sarcoelegan A, (±)-sarcoelegan D, sarcoelegan E, (+)-sarcoelegan F, and (+)-sarcoelegan H | Soft coral Sarcophyton elegans | CuSO4-induced Tg(zlyz: EGFP) zebrafish | Anti-inflammatory activity (20 μM) | [112] |
| Altechromone A | Marine-derived fungus Penicillium Chrysogenum (XY-14-0-4) | CuSO4-, tail-cutting-, and LPS-induced Tg(zlyz: EGFP) zebrafish and TNBS-induced zebrafish model | Anti-inflammatory activity (12.5, 25, 50 μg/mL) | [194] |
| Purpurols A and B | Sponge Pseudoceratina purpurea | CuSO4-induced Tg(zlyz: EGFP) zebrafish | Anti-inflammatory activity (40 µM) | [195] |
| Protein hydrolysate AJH-1 | sea cucumber Apostichopus japonicus | CuSO4-induced Tg(zlyz: EGFP) zebrafish | Anti-inflammatory activity (100, 250, 500 µg/mL) | [196] |
| Apo-9′-fucoxanthinone | Sargassum muticum | LPS-induced zebrafish model | Anti-inflammatory activity (25, 50, 100 μg/mL) | [197] |
| 5-Hydroxypalisadin B | Red seaweed Laurencia snackeyi | LPS-induced and tail-cutting-zebrafish model | Anti-inflammatory activity (0.25, 0.5, 1 μg/mL) | [198] |
| Fucoidan (SFF-PS-F5) | Fermented Sargassum fusiforme | LPS-induced zebrafish model | Anti-inflammatory activity (25, 50, 100 μg/mL) | [199] |
| Fucoidan LJSF4 | Saccharina japonica | LPS-induced zebrafish model | Anti-inflammatory effect (12.5–50 μg/mL) | [200] |
| Fucose containing sulfated polysaccharides (FCSP) | Turbinaria ornata from the Maldives | LPS-induced zebrafish model | Anti-inflammatory effect (12.5, 25, 50 μg/mL) | [201] |
| 24-Methylcholesta-5(6), 22-Diene-3β-ol | Marine Diatom Phaeodactylum tricornutum | LPS-induced zebrafish model | Anti-inflammatory effect (12.5, 25, 50 μg/mL) | [202] |
| Sulfated polysaccharides (CFCE-PS) | Green seaweed Codium fragile | LPS-induced zebrafish model | Anti-inflammatory effect (25, 50, 100 μg/mL) | [203] |
| Sulfated polysaccharides (SFPS) | Brown Seaweed Sargassum fulvellum | LPS-induced zebrafish model | Anti-inflammatory effect (25, 50, 100 μg/mL) | [204] |
| Sulfated Galactofucan LJNF3 | Brown seaweed Saccharina japonica | LPS-induced zebrafish model | Anti-inflammatory effect (12.5, 25, 50 μg/mL) | [205] |
| Eckmaxol and dieckol | Brown seaweed Ecklonia maxima | LPS-induced zebrafish model | Anti-inflammatory effect (12.5, 25, 50 μg/mL) | [206] |
| Enzymatic peptide SEP | Skipjack Katsuwonus pelamis | CuSO4-induced Tg(zlyz: EGFP) zebrafish | Anti-inflammatory activity (500 μg/mL) | [207] |
| Micrometam C | Micromelum falcatum | LPS-induced zebrafish model | Anti-inflammatory activity (10, 50, 200 μM) | [208] |
| Peptides YSQLENEFDR and YIAEDAER | Marine snail | metronidazole-treated Tg (krt4: NTR-hKikGR)cy17 zebrafish | Antioxidant activity (0.77, 7.70, 76.98 and 1.03, 10.36, 103.63 μM) | [213] |
| Frondoplysin A | Marine sponge Dysidea frondosa | metronidazole-treated Tg (krt4: NTR-hKikGR)cy17 zebrafish | Antioxidant activity (10, 20, 40 μM) | [214] |
| Peptide AFFP | Aquacultured flounder fish | 2,2-azobis-(2-amidinopropane) hydrochloride-induced oxidative damage in a zebrafish model | Antioxidative effects (25, 50, 100 μg/mL) | [215] |
| Seahorse peptide SHP | Hippocampus abdominalis | AAPH-induced oxidative stress in the zebrafish model | Antioxidative effects (50, 100, 200 μg/mL) | [216] |
| Phloroglucinol, eckol, dieckol, eckstolonol and triphloroethol A | Brown algae Ecklonia cava | AAPH-induced oxidative stress in the zebrafish model | Antioxidative effects (50 μM) | [217] |
| Agaro-oligosaccharides AO | Agar of Gracilaria lemaneiformis | H2O2-stimulated oxidative stress in zebrafish | Antioxidant activity (12.5, 25, 50 μg/mL) | [218] |
| Xyloketal B | Mangrove fungus Xylaria sp. (no. 2508) | Phorbol myristate acetate-induced ROS levels in zebrafish | Antioxidant activity (20 μM) | [219] |
| (−)-loliolide | Sargassum horneri | AAPH-induced oxidative damage in zebrafish models | Antioxidant activity (6.25, 12.5, 25 μg/mL) | [220] |
| Protein hydrolysate HPH | Seahorses Hippocampus abdominalis | AAPH-induced oxidative damage in zebrafish | Antioxidant activity (100, 400 μg/mL) | [221] |
| Fucoidan (HFPS-F4) | Hizikia fusiforme | H2O2-stimulated oxidative stress in zebrafish | Antioxidant activity (12.5, 25, 50 μg/mL) | [222] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current status and future prospects of marine natural products (MNPs) as antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.F.; Gan, C.F.; Cui, J.G. Advances in the research of bioactive steroids derived from marine microorganisms. Nat. Prod. Res. Dev. 2018, 30, 1266–1273. [Google Scholar]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.; Sousa, E. Targeting antimicrobial drug resistance with marine natural products. Int. J. Antimicrob. Agents 2020, 56, 106005. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, E.A.; Campitiello, R.; Caferri, R.; Pagliuca, V.F.; Li, J.; Agathos, S.N.; Cutolo, M. Immunomodulatory compounds from the sea: From the origins to a modern marine pharmacopoeia. Mar. Drugs 2024, 22, 304. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Vitiello, L.; Giannaccare, G. Marine natural products rescuing the eye: A narrative review. Mar. Drugs 2024, 22, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W. Efficient discovery and medicinal research of marine natural products. Chin. Tradit. Herb. Drugs 2019, 50, 5645–5652. [Google Scholar]
- Ha, Q.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine natural products in clinical use. Mar. Drugs 2022, 20, 528. [Google Scholar]
- Mandrekar, N.; Thakur, N.L. Significance of the zebrafish model in the discovery of bioactive molecules from nature. Biotechnol. Lett. 2009, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Jia, S.; Zhang, X. Recent advances in pharmaceutical screening using zebrafish as a disease model. Period. Ocean Univ. China 2019, 49, 59–65. [Google Scholar]
- West, K.H.; Crawford, A.D. Marine Biodiscovery Goes Deeper: Using In Vivo Bioassays Based on model organisms to identify biomedically relevant marine metabolites. Planta Med. 2016, 82, 754–760. [Google Scholar] [CrossRef]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A preclinical model for drug screening. Assay Drug Dev. Technol. 2002, 1 Pt 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.L.; Wall, E.S.; Sichel, S.; Watral, V.; Stagaman, K.; Sharpton, T.J.; Guillemin, K. Pseudocapillaria tomentosa, Mycoplasma spp., and intestinal lesions in experimentally infected zebrafish danio rerio. Zebrafish 2021, 18, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Hisaoka, K.K. The effects of 2-acetylaminofluorene on the embryonic development of the zebrafish. II. Histochemical studies. Cancer Res. 1958, 18, 664–667. [Google Scholar]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Streisinger, G.; Singer, F.; Walker, C.; Knauber, D.; Dower, N. Segregation analyses and genecentrome distances in zebrafish. Genetics 1986, 112, 311–319. [Google Scholar] [CrossRef]
- Peterson, R.T.; Link, B.A.; Dowling, J.E.; Schreiber, S.L. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl. Acad. Sci. USA 2000, 97, 12965–12969. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.J.; Li, H.; Wu, D.T.; Zhuang, Q.G.; Li, H.B.; Geng, F.; Gan, R.Y. Recent development in zebrafish model for bioactivity and safety evaluation of natural products. Crit. Rev. Food Sci. Nutr. 2022, 62, 8646–8674. [Google Scholar] [CrossRef] [PubMed]
- Kithcart, A.; MacRae, C.A. Using zebrafish for high-throughput screening of novel cardiovascular drugs. JACC Basic Transl. Sci. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Crawford, A.D.; Esguerra, C.V.; de Witte, P.A. Fishing for drugs from nature: Zebrafish as a technology platform for natural product discovery. Planta Med. 2008, 74, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.R. Zebrafish: Development of a vertebrate model organism. Curr. Protoc. Essent. Lab. Tech. 2018, 16, 19. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef]
- Cueto-Escobedo, J.; German-Ponciano, L.J.; Guillén-Ruiz, G.; Soria-Fregozo, C.; Herrera-Huerta, E.V. Zebrafish as a useful tool in the research of natural products with potential anxiolytic effects. Front. Behav. Neurosci. 2022, 15, 795285. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Wang, J.; Zhuang, K.; Jin, H.; Liu, K. Progress in using zebrafish as a toxicological model for traditional chinese medicine. J. Ethnopharmacol. 2022, 282, 114638. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Zhu, J.J.; Zhou, S.H.; Xin, Y.; Xuan, Y.; Li, C. Application of zebrafish model in drug toxicity and safety evaluation. J. Toxicol. 2012, 26, 224–228. [Google Scholar]
- Challal, S.; Bohni, N.; Buenafe, O.E.; Esguerra, C.V.; de Witte, P.A.; Wolfender, J.L.; Crawford, A.D. Zebrafish bioassay-guided microfractionation for the rapid in vivo identification of pharmacologically active natural products. Chimia 2012, 66, 229–232. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, Y.; Nel, A.E.; Lin, S. Zebrafish: An in vivo model for nano EHS studies. Small 2013, 9, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Bohni, N.; Cordero-Maldonado, M.L.; Maes, J.; Siverio-Mota, D.; Marcourt, L.; Munck, S.; Kamuhabwa, A.R.; Moshi, M.J.; Esguerra, C.V.; de Witte, P.A.; et al. Integration of microfractionation, qNMR and zebrafish screening for the in vivo bioassay-guided isolation and quantitative bioactivity analysis of natural products. PLoS ONE 2013, 8, e64006. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 7446, 496–505. [Google Scholar] [CrossRef]
- Giacomotto, J.; Ségalat, L. High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef]
- Liu, J.T.; Tong, K.; Cui, J.R. Application of zebrafish in tumor pharmacology. Chin. Pharm. J. 2010, 5, 1800–1804. [Google Scholar]
- Maximino, C.; da Silva, A.W.; Araújo, J.; Lima, M.G.; Miranda, V.; Puty, B.; Benzecry, R.; Picanço-Diniz, D.L.; Gouveia, A.; Oliveira, K.R., Jr.; et al. Fingerprinting of psychoactive drugs in zebrafish anxiety-like behaviors. PLoS ONE 2014, 9, e103943. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, W.; Wang, Z.; Zeng, Z.; Zhan, H.; Li, C.; Zhou, L.; Yan, C.; Spitsbergen, J.M.; Gong, Z. A transgenic zebrafish liver tumor model with inducible Myc expression reveals conserved Myc signatures with mammalian liver tumors. Dis. Models Mech. 2013, 6, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Kundap, U.P.; Kumari, Y.; Othman, I.; Shaikh, M.F. Zebrafish as a model for epilepsy-induced cognitive dysfunction: A pharmacological, biochemical and behavioral approach. Front. Pharmacol. 2017, 8, 515–851. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.L.; Moro, E. Using transgenic reporters to visualize bone and cartilage signaling during development in vivo. Front. Endocrinol. 2012, 3, 91. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, W.J.; Xue, Y. Research progress of zebrafish as a model for skeletal diseases. Acta Lab. Anim. Sci. Sin. 2019, 27, 248–253. [Google Scholar]
- Xin, S.C.; Zhao, Y.Q.; Li, S.; Lin, S.; Zhong, H.B. Application of zebrafish models in drug screening. Hereditas 2012, 34, 1144–1152. [Google Scholar] [PubMed]
- Bournele, D.; Beis, D. Zebrafish models of cardiovascular disease. Heart Fail. Rev. 2016, 21, 803–813. [Google Scholar] [CrossRef]
- Zhang, Y.P. Establishment of Thrombocyte Specific Transgenic Line and Expression Analysis and Generation of Gene Knockout Mutant with CRISPR/Cas9 System in Zebrafish. Master’s Thesis, Southern Medical University, Guangzhou, China, 2016. [Google Scholar]
- Qu, Y.G. Establishment and Preliminary Functional Study of Several Zebrafish Transgenic Lines. Master’s Thesis, Hunan Normal University, Changsha, China, 2014. [Google Scholar]
- Zhang, S.H.; Zhao, C.T.; Fen, X.Z. Research progress of zebrafish cancer model in tumor precision medicine. J. Clin. Pathol. Res. 2021, 41, 2193–2200. [Google Scholar]
- Lv, P.; Ma, D.; Gao, S.; Zhang, Y.; Bae, Y.K.; Liang, G.; Gao, S.; Choi, J.H.; Kim, C.H.; Wang, L.; et al. Generation of foxn1/casper mutant zebrafish for allograft and xenograft of normal and malignant cells. Stem Cell Rep. 2020, 15, 749–760. [Google Scholar] [CrossRef]
- Kettborough, R.N.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2023, 496, 494–497. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little Fish, Big Data: Zebrafish as a Model for Cardiovascular and Metabolic Disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef] [PubMed]
- Kithcart, A.P.; MacRae, C.A. Zebrafish assay development for cardiovascular disease mechanism and drug discovery. Prog. Biophys. Mol. Biol. 2018, 138, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.P.; Pieperhoff, S. Design and physiology of the heart I cardiac anatomy in fishes. In Encyclopedia of Fish Physiology; Academic Press: Cambridge, MA, USA, 2011; pp. 998–1005. [Google Scholar]
- Zakaria, Z.Z.; Benslimane, F.M.; Nasrallah, G.K.; Shurbaji, S.; Younes, N.N.; Mraiche, F.; Da’as, S.I.; Yalcin, H.C. Using zebrafish for investigating the molecular mechanisms of drug-induced cardiotoxici. BioMed Res. Int. 2018, 2018, 1642684. [Google Scholar] [CrossRef] [PubMed]
- Ran, K.K.; Zhen, R.F.; XIA, Q.; Zhang, C.Q.; Ma, R.J.; Liu, K.Y.; Zhang, Y.; Xing, J.G. Application of model organism-zebrafish in cardiac function evaluation. Drug Eval. Res. 2021, 44, 1581–1587. [Google Scholar]
- Li, P.H.; Zhang, M.Q.; Xie, D.X.; Zhang, X.M.; Zhang, S.S.; Gao, F.Y.; Wang, Y.L.; Hsiao, C.D.; Li, X.B.; Liu, K.C. Characterization and bioactivities of phospholipids from squid viscera and gonads using ultra-performance liquid chromatography-Q-exactive orbitrap/mass spectrometry-based lipidomics and zebrafish models. Food Funct. 2021, 12, 7986–7996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Li, P.H.; Meng, R.H.; Li, X.B.; Qiu, Y.Z.; Wang, L.Z.; Zhang, S.S.; Zhang, X.M.; Lin, H.W.; Zhai, H.B.; et al. Lipid profiles of the heads of four shrimp species by UPLC-Q-Exactive Orbitrap/MS and their cardiovascular activities. Molecules 2022, 27, 350. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.X.; Li, P.H.; Zhu, Y.Q.; Zhang, M.Q.; He, J.W.; Liu, K.C.; Lin, H.W.; Zhai, H.B.; Li, X.B.; Ma, Y.H. Comparative bioactivity profile of phospholipids from three marine byproducts based on the zebrafish model. J. Food Biochem. 2022, 46, e14229. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Li, P.H.; Wang, F.X.; Zhang, S.S.; Li, H.N.; Zhang, Y.; Wang, X.M.; Liu, K.C.; Li, X.B. Separation, identification and cardiovascular activities of phospholipid classes from the head of Penaeus vannamei by lipidomics and zebrafish models. Food Funct. 2021, 12, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Gong, G.; Siu, S.W.I.; Wong, C.T.T.; Yu, H.; Tse, Y.C.; Rádis-Baptista, G.; Lee, S.M. A novel shk-like toxic peptide from the transcriptome of the cnidarian Palythoa caribaeorum displays neuroprotection and cardioprotection in zebrafish. Toxins 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Pott, A.; Shahid, M.; Köhler, D.; Pylatiuk, C.; Weinmann, K.; Just, S.; Rottbauer, W. Therapeutic chemical screen identifies phosphatase inhibitors to reconstitute PKB phosphorylation and cardiac contractility in ILK-Deficient zebrafish. Biomolecules 2018, 8, 153. [Google Scholar] [CrossRef]
- Hu, X.Y.; You, H.M.; Ren, C.Z.; Zhang, Y.D.; Liang, C. Application of zebrafish in drug screening research for cardiovascular diseases. Int. J. Cardiovasc. Dis. 2021, 48, 156–159. [Google Scholar]
- Chen, Y.; Xu, H.; Wang, Y.J.; Shi, Q.; Liang, Q.Q. Application of zebrafish model in study of traditional chinese medicine regulating angiogenesis. Chin. Arch. Tradit. Chin. Med. 2023, 41, 46–50. [Google Scholar]
- Wilkinson, R.N.; van Eeden, F.J. The zebrafish as a model of vascular development and disease. Prog. Mol. Biol. Transl. Sci. 2014, 124, 93–122. [Google Scholar]
- Matsuoka, R.L.; Stainier, D.Y.R. Recent insights into vascular development from studies in zebrafish. Curr. Opin. Hematol. 2018, 25, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Xu, Z.L.; Yao, X.L.; Su, F.J.; Ye, C.H.; Li, J.; Lin, Y.C.; Wang, G.L.; Zeng, J.S.; Huang, R.X.; et al. Marine cyclotripeptide X-13 promotes angiogenesis in zebrafish and human endothelial cells via PI3K/Akt/eNOS signaling pathways. Mar. Drugs 2012, 10, 1307–1320. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Li, H.; Wang, R.; Hou, H.; Li, X.; Liu, K.; Chen, H. New prenylated indole homodimeric and pteridine alkaloids from the marine-derived fungus Aspergillus austroafricanus Y32-2. Mar. Drugs 2021, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Q.; Li, P.H.; Chao, Y.X.; Chen, H.; Du, N.; He, Q.X.; Liu, K.C. Alkaloids with cardiovascular effects from the marine-derived fungus Penicillium expansum Y32. Mar. Drugs 2015, 13, 6489–6504. [Google Scholar] [CrossRef]
- Qiu, Y.Z.; Zhu, Y.Q.; Lu, H.; Li, X.B.; Liu, K.C.; Li, P.H.; Wang, L.Z.; Zhang, X.M.; Chen, H.; Lin, H.W.; et al. Secondary metabolites from the marine-derived fungus Penicillium chrysogenum Y20-2, and their pro-angiogenic activity. Z. Naturforschung C J. Biosci. 2023, 78, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, C.; Zhang, Y.; Ma, Z.; Li, P.; Guo, L.; Feng, T.; Zhou, L.; Xu, L. Pro-angiogenic new chloro-azaphilone derivatives from the hadal trench-derived fungus Chaetomium globosum YP-106. Front. Microbiol. 2022, 13, 943452. [Google Scholar] [CrossRef]
- Yan, L.H.; Li, P.H.; Li, X.M.; Yang, S.Q.; Liu, K.C.; Zhang, Y.; Wang, B.G.; Li, X. Bialorastins A–F, highly oxygenated and polycyclic andrastin-type meroterpenoids with proangiogenic activity from the deep-sea cold-seep-derived fungus Penicillium bialowiezense CS-283. Bioorganic Chem. 2024, 143, 107073. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tang, X.; Luo, X.; Sun, C.; Liu, K.; Zhang, Y.; Li, P.; Li, G. Isolation and identification of three new sterigmatocystin derivatives from the fungus Aspergillus versicolor guided by molecular networking approach. Chem. Biodivers. 2020, 17, e2000208. [Google Scholar] [CrossRef]
- Li, T.; Tang, X.; Luo, X.; Wang, Q.; Liu, K.; Zhang, Y.; de Voogd, N.J.; Yang, J.; Li, P.; Li, G. Agelanemoechine, a dimeric bromopyrrole alkaloid with a Pro-Angiogenic effect from the south china sea sponge Agelas nemoechinata. Org. Lett. 2019, 21, 9483–9486. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, P.; Tang, X.; Luo, X.; Liu, K.; Zhang, Y.; Wang, Q.; Li, G. Lemnardosinanes A–I: New bioactive sesquiterpenoids from soft coral Lemnalia sp. J. Org. Chem. 2021, 86, 970–979. [Google Scholar] [CrossRef]
- Miao, X.; Hong, L.; Ju, Z.; Liu, H.; Shang, R.; Li, P.; Liu, K.; Cheng, B.; Jiao, W.; Xu, S.; et al. Marchaetoglobins A-D: Four cytochalasans with proangiogenic activity from the marine-sponge-associated fungus Chaetomium globosum 162105. ACS Omega 2024, 9, 22450–22458. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.H.; Li, P.H.; Li, X.M.; Yang, S.Q.; Liu, K.C.; Wang, B.G.; Li, X. Chevalinulins A and B, proangiogenic alkaloids with a spiro[bicyclo[2.2.2]octane-diketopiperazine] skeleton from deep-sea cold-seep-derived fungus Aspergillus chevalieri CS-122. Org. Lett. 2022, 24, 2684–2688. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.; Liu, H.; Luo, X.; Sung, P.J.; Li, P.L.; Li, G.Q. Clavukoellians G–K, new nardosinane and aristolane sesquiterpenoids with angiogenesis promoting activity from the marine soft coral Lemnalia sp. Mar. Drugs 2020, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Q.; Jiang, C.; Zhang, Y.J.; Ma, Z.H.; Li, P.H.; Guo, L.Z.; Feng, T.; Zhou, L.M.; Xu, L.L. A new chloro-azaphilone derivative with pro-angiogenesis activity from the hadal trench-derived fungus Chaetomium globosum YP-106. J. Oceanol. Limnol. 2023, 41, 1145–1151. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Wang, J.; Yue, Y.; Geng, L.; Zhang, Q. Low molecular weight fucoidan alleviates cerebrovascular damage by promoting angiogenesis in type 2 diabetes mice. Int. J. Biol. Macromol. 2022, 217, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, N.; Zhao, C.; He, Y.L.; Kam, S.H.T.; Wu, X.; Huang, P.; Yang, M.; Wong, C.T.T.; Radis-Baptista, G.; et al. A new invertebrate NPY-like polypeptide, ZoaNPY, from the zoanthus sociatus, as a novel ligand of human NPY Y2 receptor rescues vascular insufficiency via PLC/PKC and Src- FAK-dependent signaling pathways. Pharmacol. Res. 2024, 203, 107173. [Google Scholar] [CrossRef]
- Feng, T.; Wu, R.; Wang, Y.; Wang, P.; Zhou, L.; Wang, C.; Kong, F. Proangiogenic azaphilones from the marine-derived fungus neopestalotiopsis sp. HN-1-6. Mar. Drugs 2024, 22, 241. [Google Scholar] [CrossRef]
- Tulotta, C.; He, S.; van der Ent, W.; Chen, L.; Groenewoud, A.; Spaink, H.P.; Snaar-Jagalska, B.E. Imaging cancer angiogenesis and metastasis in a zebrafish embryo model. Adv. Exp. Med. Biol. 2016, 916, 239–263. [Google Scholar] [PubMed]
- García-Caballero, M.; Cañedo, L.; Fernández-Medarde, A.; Medina, M.Á.; Quesada, A.R. The marine fungal metabolite, AD0157, inhibits angiogenesis by targeting the akt signaling pathway. Mar. Drugs 2014, 12, 279–299. [Google Scholar] [CrossRef]
- Liu, J.X.; Luo, M.Q.; Xia, M.; Wu, Q.; Long, S.M.; Hu, Y.; Gao, G.C.; Yao, X.L.; He, M.; Su, H.; et al. Marine compound catunaregin inhibits angiogenesis through the modulation of phosphorylation of akt and eNOS in vivo and in vitro. Mar. Drugs 2014, 12, 2790–2801. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, G.; Qiu, L.; Lin, X.; Liu, M. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, represses angiogenesis in HUVEC cells and in zebrafish embryos via inhibiting the VEGF signal systems. Biomed. Pharmacother. 2015, 75, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Chen, N.F.; Lin, P.Y.; Su, J.H.; Chen, B.H.; Kuo, H.M.; Sung, C.S.; Sung, P.J.; Wen, Z.H.; Chen, W.F. Anti-invasion and antiangiogenic effects of stellettin B through Inhibition of the Akt/Girdin signaling pathway and VEGF in glioblastoma cells. Cancers 2019, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Fernando, K.H.N.; Yang, H.W.; Jiang, Y.; Jeon, Y.J.; Ryu, B. Ishige okamurae extract and its constituent ishophloroglucin a attenuated in vitro and in vivo high glucose-induced angiogenesis. Int. J. Mol. Sci. 2019, 20, 5542. [Google Scholar] [CrossRef]
- Liu, G.; Liu, M.; Wei, J.; Huang, H.; Zhang, Y.; Zhao, J.; Xiao, L.; Wu, N.; Zheng, L.; Lin, X. CS5931, a novel polypeptide in ciona savignyi, represses angiogenesis via inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs). Mar. Drugs 2014, 12, 1530–1544. [Google Scholar] [CrossRef]
- Fernando, K.H.N.; Yang, H.W.; Jiang, Y.; Jeon, Y.J.; Ryu, B. Diphlorethohydroxycarmalol isolated from ishige okamurae represses high glucose-induced angiogenesis in vitro and in vivo. Mar. Drugs 2018, 16, 375. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Shi, H.; Li, C.; Luo, Y.; Bi, S.; Yu, R.; Wang, H.; Liu, W.; Zhu, J.; Huang, W.; et al. Identification and characterization of a novel protein ASP-3 purified from arca subcrenata and its antitumor mechanism. Mar. Drugs 2019, 17, 528. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kuang, S.; Wu, S.; Jin, W.; Sun, C. A novel polysaccharide from Sargassum integerrimum induces apoptosis in A549 cells and prevents angiogensis in vitro and in vivo. Sci. Rep. 2016, 6, 26722. [Google Scholar] [CrossRef]
- Guo, X.; Fan, A.; Qi, X.; Liu, D.; Huang, J.; Lin, W. Indoloquinazoline alkaloids suppress angiogenesis and inhibit metastasis of melanoma cells. Bioorganic Chem. 2023, 141, 106873. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yang, H.W.; Lu, Y.A.; Je, J.G.; Lee, H.G.; Fernando, K.H.N.; Jeon, Y.J.; Ryu, B. Phloroglucinol and dieckol isolated from ecklonia cava suppress impaired diabetic angiogenesis; a study of in-vitro and in-vivo. Biomed. Pharmacother. 2021, 138, 111431. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Wen, Z.H.; Shih, P.C.; Kuo, H.M.; Lin, S.C.; Liu, H.T.; Lee, Y.H.; Wang, Y.J.; Chen, W.F.; Chen, N.F. Sinularin induces oxidative stress-mediated apoptosis and mitochondrial dysfunction, and inhibits angiogenesis in glioblastoma cells. Antioxidants 2022, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Wu, B.J.; Chiu, C.C.; Chen, C.L.; Zhou, J.Q.; Liang, S.R.; Duh, C.Y.; Sung, P.J.; Wen, Z.H.; Wu, C.Y. Coral-derived natural marine compound GB9 impairs vascular development in zebrafish. Int. J. Mol. Sci. 2017, 18, 1696. [Google Scholar] [CrossRef] [PubMed]
- García-Caballero, M.; Marí-Beffa, M.; Cañedo, L.; Medina, M.Á.; Quesada, A.R. Toluquinol, a marine fungus metabolite, is a new angiosuppresor that interferes with the akt pathway. Biochem. Pharmacol. 2013, 85, 1727–1740. [Google Scholar] [CrossRef]
- Carrillo, P.; Martínez-Poveda, B.; Cheng-Sánchez, I.; Guerra, J.; Tobia, C.; López-Romero, J.M.; Sarabia, F.; Medina, M.Á.; Quesada, A.R. Exploring the antiangiogenic potential of solomonamide a bioactive precursors: In vitro and in vivo evidences of the inhibitory activity of Solo F-OH during angiogenesis. Mar. Drugs 2019, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Wrasidlo, W.; Mielgo, A.; Torres, V.A.; Barbero, S.; Stoletov, K.; Suyama, T.L.; Klemke, R.L.; Gerwick, W.H.; Carson, D.A.; Stupack, D.G. The marine lipopeptide somocystinamide A triggers apoptosis via caspase 8. Proc. Natl. Acad. Sci. USA 2008, 105, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Lee, J.Y.; Yang, C.; Song, G.; Lim, W. Fucoidan derived from Fucus vesiculosus inhibits the development of human ovarian cancer via the disturbance of calcium homeostasis, endoplasmic reticulum stress, and angiogenesis. Mar. Drugs 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Huang, S.C.; Kuo, H.M.; Chen, C.H.; Ma, Y.L.; Chu, T.H.; Bee, Y.S.; Wang, E.M.; Wu, C.Y.; Sung, P.J.; et al. Coral-derived compound WA-25 inhibits angiogenesis by attenuating the VEGF/VEGFR2 signaling pathway. Mar. Drugs 2015, 13, 861–878. [Google Scholar] [CrossRef]
- Dong, S.T.; Chen, Z.Y.; Wang, L.; Liu, Y.K.; Stagos, D.; Lin, X.K.; Liu, M. Marine bromophenol bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether inhibits angiogenesis in human umbilical vein endothelial cells and reduces vasculogenic mimicry in human lung cancer A549 cells. Mar. Drugs 2021, 19, 641. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Y.C.; Wang, J.F.; Shi, X.F.; Che, Y.H.; Chen, X.Y.; Zhong, W.M.; Zhang, W.M.; Wei, X.Y.; Wang, F.Z.; et al. Diverse secondary metabolites from the coral-derived fungus Aspergillus hiratsukae SCSIO 5Bn1003. Mar. Drugs 2022, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Tang, Y.; Wang, Y.; Guan, X.; Yu, W.; Jiang, T.; Lu, L.; Gu, Y. A sirt6 inhibitor, marine-derived pyrrole-pyridinimidazole derivative 8a, suppresses angiogenesis. Mar. Drugs 2023, 21, 517. [Google Scholar] [CrossRef] [PubMed]
- Hsi, H.Y.; Wang, S.W.; Cheng, C.H.; Pang, K.L.; Leu, J.Y.; Chang, S.H.; Lee, Y.T.; Kuo, Y.H.; Huang, C.Y.; Lee, T.H. Chemical Constituents and Anti-Angiogenic Principles from a Marine Algicolous Penicillium sumatraense SC29. Molecules 2022, 27, 8940. [Google Scholar] [CrossRef] [PubMed]
- Nagabhishek, S.N.; Madan Kumar, A.; B, S.; Balakrishnan, A.; Katakia, Y.T.; Chatterjee, S.; Nagasundaram, N. A marine sponge associated fungal metabolite monacolin X suppresses angiogenesis by down regulating VEGFR2 signaling. RSC Adv. 2019, 9, 26646–26667. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, R.; Gunasekera, S.P.; Ma, J.J.; Dang, L.H.; Carney, T.J.; Paul, V.J.; Luesch, H. Dolastatin 15 from a marine cyanobacterium suppresses HIF-1α mediated cancer cell viability and vascularization. ChemBioChem 2020, 21, 2356–2366. [Google Scholar] [CrossRef]
- Long, W.; Wang, M.; Luo, X.; Huang, G.; Chen, J. Murrangatin suppresses angiogenesis induced by tumor cell-derived media and inhibits AKT activation in zebrafish and endothelial cells. Drug Des. Dev. Ther. 2018, 12, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.R.; Gihr, G.; Gawaz, M.P.; Müller, I.I. Hemostasis in Danio rerio: Is the zebrafish a useful model for platelet research? J. Thromb. Haemost. 2010, 8, 1159–1169. [Google Scholar] [CrossRef]
- Thijs, T.; Deckmyn, H.; Broos, K. Model systems of genetically modified platelets. Blood 2012, 119, 1634–1642. [Google Scholar] [CrossRef]
- Weyand, A.C.; Shavit, J.A. Zebrafish as a model system for the study of hemostasis and thrombosis. Curr. Opin. Hematol. 2014, 21, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Liu, H.C.; Guo, S.Y.; Xia, B.; Song, R.S.; Lao, Q.C.; Xuan, Y.X.; Li, C.Q. A zebrafish thrombosis model for assessing antithrombotic drugs. Zebrafish 2016, 13, 335–344. [Google Scholar] [CrossRef]
- Rajagopal, R.E.; Balasubramanian, M.; Kalyanaraman, S. Phenylhydrazine hydrochloride induced dosedependent embryo cytotoxicity in zebrafish. Bioinformation 2019, 15, 255–260. [Google Scholar] [CrossRef]
- Gao, P.Y.; Li, L.Z.; Liu, K.C.; Sun, C.; Sun, X.; Wu, Y.N.; Song, S.J. Natural terpenoid glycosides with in vitro/vivo antithrombotic profiles from the leaves of crataegus pinnatifida. RSC Adv. 2019, 7, 48466–48474. [Google Scholar] [CrossRef]
- Mo, C.L.; Li, J.; Wang, J.Z.; Liu, X.; Lin, S.H. Applicability of a zebrafish thrombosis model in screening active ingredients of traditional Chinese medicine. Shandong Sci. 2021, 34, 52–59. [Google Scholar]
- Shi, Y.P.; Zhang, Y.G.; Li, H.N.; Kong, H.T.; Zhang, S.S.; Zhang, X.M.; Li, X.B.; Liu, K.C.; Han, L.W.; Tian, Q.P. Discovery and identification of antithrombotic chemical markers in gardenia fructus by herbal metabolomics and zebrafish model. J. Ethnopharmacol. 2020, 253, 112679. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Yang, Z.; Yu, Q.; Zhao, L.; Wang, Y. Synergistic effects of cryptotanshinone and senkyunolide I in guanxinning tablet against endogenous thrombus formation in zebrafish. Front. Pharmacol. 2021, 11, 622787. [Google Scholar] [CrossRef]
- Xin, S.S.; Zhang, M.Q.; Li, P.H.; Wang, L.Z.; Zhang, X.M.; Zhang, S.S.; Mu, Z.Q.; Lin, H.W.; Li, X.B.; Liu, K.C. Marine-fungus-derived natural compound 4-Hydroxyphenylacetic acid induces autophagy to exert antithrombotic effects in zebrafish. Mar. Drugs 2024, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Zhang, J.R.; Jin, T.Y.; Shi, X.; Wang, M.F.; Zhang, W.J.; Zong, Y.; Li, G.Q.; Li, P.L. Sarcoeleganolides H-K, new anti-thrombotic cembranes from soft coral Sarcophyton elegans. Nat. Prod. Res. 2024, 38, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Gan, Y.; Wang, M.F.; Li, X.L.; Liu, X.H.; Shi, X.; Mi, Y.; Liu, K.C.; Zhang, Y.; et al. Sarcoelegans A-H, eight undescribed cembranes with anti-inflammatory and anti-thrombotic activities from the south china sea soft coral sarcophyton elegans. Phytochemistry 2023, 207, 113578. [Google Scholar] [CrossRef]
- Mi, Y.; Han, X.; Yu, X.; Li, L.; Tang, X.; Li, G. Sarcocinerenolides A, an open-loop decarbonizing cembranolide, and sarcocinerenolides B-I, eight polyoxygenated cembranolides with anti-thrombotic activity from the south china sea soft coral sarcophyton cinereum. Phytochemistry 2024, 223, 114109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, L.; Lu, X.; Lu, L.; Mao, W. A pyruvylated and sulfated galactan from the green alga dictyosphaeria cavernosa: Structure, anticoagulant property and inhibitory effect on zebrafish thrombosis. Carbohydr. Polym. 2024, 324, 121492. [Google Scholar] [CrossRef]
- Li, L.; Qiu, R.L.; Wei, K.F.; Sun, D.D. Advances in zebrafish models of tumor. Chin. Pharmacol. Bull. 2022, 38, 168–172. [Google Scholar]
- Wu, Z.B.; Zhang, H.H.; Li, R.X.; Sun, N.Y.; Liu, C.X. Progress in the application of zebrafish model in cancer research. China Med. Her. Vol. 2023, 20, 45–48. [Google Scholar]
- Raby, L.; Völkel, P.; Le Bourhis, X.; Angrand, P.O. Genetic engineering of zebrafish in cancer research. Cancers 2020, 12, 2168. [Google Scholar] [CrossRef] [PubMed]
- Le, X.N.; Zon, L.I. Potential of zebrafish for anticancer drug screening. Expert Opin. Drug Discov. 2008, 3, 1451–1460. [Google Scholar]
- Callahan, S.J.; Tepan, S.; Zhang, Y.M.; Lindsay, H.; Burger, A.; Campbell, N.R.; Kim, I.S.; Hollmann, T.J.; Studer, L.; Mosimann, C.; et al. Cancer modeling by transgene electroporation in adult zebrafish (TEAZ). Dis. Model Mech. 2018, 11, 034561. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Yang, W.Y.; Cheng, C.C.; Hsiao, M.C.; Tsai, S.L.; Lin, H.K.; Lin, K.H.; Yuh, C.H. Low molecular weight fucoidan prevents radiation-induced fibrosis and secondary tumors in a zebrafish model. Cancers 2020, 12, 1608. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Z.; Zhang, B.Q.; Wang, C.F.; Yin, J.N.; Haider, W.; Said, G.; Wei, M.Y.; Lu, L. A terphenyllin derivative CHNQD-00824 from the marine compound library induced DNA damage as a potential anticancer agent. Mar. Drugs 2023, 21, 512. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sheng, R.; Guo, R. Application of zebrafish as a model for anti-cancer activity evaluation and toxicity testing of natural products. Pharmaceuticals 2023, 16, 827. [Google Scholar] [CrossRef] [PubMed]
- Fazio, M.; Ablain, J.; Chuan, Y.; Langenau, D.M.; Zon, L.I. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat. Rev. Cancer 2020, 20, 263–273. [Google Scholar] [CrossRef]
- Letrado, P.; de Miguel, I.; Lamberto, I.; Díez-Martínez, R.; Oyarzabal, J. Zebrafish: Speeding up the cancer drug discovery process. Cancer Res. 2018, 78, 6048–6058. [Google Scholar] [CrossRef]
- Osmani, N.; Goetz, J.G. Multiscale imaging of metastasis in zebrafish. Trends Cancer 2019, 5, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Lovera, C.; Vázquez-Ríos, A.J.; Guerra-Varela, J.; Sánchez, L.; de la Fuente, M. The potential of zebrafish as a model organism for improving the translation of genetic anticancer nanomedicines. Genes 2017, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, W.Q.; Kuang, Y.M.; Xu, W.F.; Jin, P.F. Research advances on zebrafish models in the application of drug screening for anti-tumorconstituents. Northwest Pharm. J. 2018, 33, 422–425. [Google Scholar]
- Hung, S.Y.; Chen, W.F.; Lee, Y.C.; Su, J.H.; Juan, Y.S.; Lin, I.P.; Zhang, Y.H.; Chang, M.K.; Lin, M.Y.; Chen, C.Y.; et al. Rhopaloic acid a induces apoptosis, autophagy and MAPK activation through ROS-mediated signaling in bladder cancer. Phytomedicine 2021, 92, 153720. [Google Scholar] [CrossRef]
- Chen, W.F.; Tsai, S.C.; Zhang, Y.H.; Chang, H.M.; Wu, W.J.; Su, J.H.; Wu, B.N.; Chen, C.Y.; Lin, M.Y.; Chen, H.L.; et al. Rhopaloic acid a triggers mitochondria damage-induced apoptosis in oral cancer by JNK/BNIP3/Nix-mediated mitophagy. Phytomedicine 2024, 132, 155855. [Google Scholar] [CrossRef]
- Chang, C.F.; Chang, P.C.; Lee, Y.C.; Pan, C.Y.; Chang, H.M.; Wu, W.J.; Lin, M.Y.; Chen, C.Y.; Wen, Z.H.; Lee, C.H. The antimicrobial peptide tilapia piscidin 4 induced the apoptosis of bladder cancer through ERK/SIRT1/PGC-1alpha signaling pathway. Probiotics Antimicrob. Proteins, 2024; online ahead of print. [Google Scholar]
- Roel, M.; Rubiolo, J.A.; Guerra-Varela, J.; Silva, S.B.; Thomas, O.P.; Cabezas-Sainz, P.; Sánchez, L.; López, R.; Botana, L.M. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget 2016, 7, 83071–83087. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.Q.; Wang, A.Y.; Zhu, Z.J.; Tao, L.; Li, Y.; Zhou, L.; Chen, W.X.; Lu, Y. Holothurian glycosaminoglycan inhibits metastasis via inhibition of P-selectin in B16F10 melanoma cells. Mol. Cell. Biochem. 2015, 410, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Lee, J.H.; Heo, S.J.; Jeon, Y.J. Saringosterol acetate isolated from Hizikia fusiforme, an edible brown alga, suppressed hepatocellular carcinoma growth and metastasis in a zebrafish xenograft model. Chem. Biol. Interact. 2021, 335, 109362. [Google Scholar] [CrossRef] [PubMed]
- Jinendiran, S.; Teng, W.; Dahms, H.U.; Liu, W.; Ponnusamy, V.K.; Chiu, C.C.; Kumar, B.S.D.; Sivakumar, N. Induction of mitochondria-mediated apoptosis and suppression of tumor growth in zebrafish xenograft model by cyclic dipeptides identified from Exiguobacterium acetylicum. Sci. Rep. 2020, 10, 13721. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Fan, X.M.; Jia, S.H.; Zhang, X.P.; Zhang, Z.; Zhang, X.J.; Zhang, J.X.; Zhang, Y.W. Sea cucumber intestinal peptide induces the apoptosis of MCF-7 cells by inhibiting PI3K/AKT pathway. Front. Nutr. 2021, 8, 763692. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.T.; Jung, S.H.; Han, J.W.; Jo, S.; Kim, I.-G.; Kim, R.-K.; Kahm, Y.-J.; Choi, T.I.; Kim, C.H.; et al. A novel anticancerPeptide derived from bryopsisplumosa regulates proliferation and invasion in non-small cell lung cancer cells. Mar. Drugs 2023, 21, 607. [Google Scholar] [CrossRef] [PubMed]
- Florean, C.; Schnekenburger, M.; Lee, J.Y.; Kim, K.R.; Mazumder, A.; Song, S.; Kim, J.M.; Grandjenette, C.; Kim, J.G.; Yoon, A.Y.; et al. Discovery and characterization of isofistularin-3, a marine brominated alkaloid, as a new DNA demethylating agent inducing cell cycle arrest and sensitization to TRAIL in cancer cells. Oncotarget 2016, 7, 24027–24049. [Google Scholar] [CrossRef]
- Abate, A.; Tamburello, M.; Rossini, E.; Basnet, R.M.; Ribaudo, G.; Gianoncelli, A.; Hantel, C.; Cosentini, D.; Laganà, M.; Grisanti, S.; et al. Trabectedin impairs invasiveness and metastasis in adrenocortical carcinoma preclinical models. Endocr. Relat. Cancer 2022, 30, e220273. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Li, B.Y.; Xu, X.Y.; Gan, R.Y.; Sun, Q.C.; Meng, J.M.; Shang, A.; Mao, Q.Q.; Li, H.B. Targeting gut microbiota for the prevention and management of diabetes mellitus by dietary natural products. Foods 2019, 8, 440. [Google Scholar] [CrossRef]
- Shang, A.; Gan, R.Y.; Xu, X.Y.; Mao, Q.Q.; Zhang, P.Z.; Li, H.B. Effects and mechanisms of edible and medicinal plants on obesity: An updated review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2061–2077. [Google Scholar] [CrossRef]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a model for obesity and diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Tai, H.; Jung, D.W.; Williams, D.R. Fishing for nature’s hits: Establishment of the zebrafish as a model for screening antidiabetic natural products. Evid. Based Complement. Altern. Med. 2015, 2015, 287847. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Maddison, L.A.; Chen, W. Modeling pancreatic endocrine cell adaptation and diabetes in the zebrafish. Front. Endocrinol. 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, L.W.; He, Q.X.; Han, J.; Wang, R.C. Application of zebrafish models in research of diabetes. Chin. J. Comp. Med. 2017, 27, 1–5. [Google Scholar]
- Lai, A.K.; Lo, A.C. Animal models of diabetic retinopathy: Summary and comparison. Diabetes Res. 2013, 2013, 106594. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. Repeated 14 blood collection for blood tests in adult zebrafish. J. Vis. Exp. 2015, 2015, e53272. [Google Scholar]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Diogo, P.; Martins, G.; Simão, M.; Marreiros, A.; Eufrásio, A.C.; Cabrita, E.; Gavaia, P.J. Type I diabetes in zebrafish reduces sperm quality and increases insulin and glucose transporter transcripts. Int. J. Mol. Sci. 2023, 24, 7035. [Google Scholar] [CrossRef]
- Jiang, X.; Qian, H.J.; Wei, X.; Zheng, Y.X.; Zhou, Z.Y. Development and application of zebrafish diabetes model. Lab. Anim. Comp. Med. 2020, 40, 547–552. [Google Scholar]
- Zang, L.; Shimada, Y.; Nishimura, N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci. Rep. 2017, 7, 1461. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Liu, Y.; Zhang, Y.; Wu, J.; Zhang, G.; Che, Q.; Zhu, T.; Li, M.; Li, D. Brasilterpenes A–E, bergamotane sesquiterpenoid derivatives with hypoglycemic activity from the deep sea-derived fungus paraconiothyrium brasiliense HDN15-135. Mar. Drugs 2022, 20, 338. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.Q.; Che, Q.; Zhu, T.J.; Zhang, G.J.; Zhang, X.K.; Li, M.Y.; Li, D.H. Penipyrols C-G and methyl-penipyrol A, α-pyrone polyketides from the mangrove derived fungus Penicillium sp. HDN-11-131. Bioorganic Chem. 2021, 113, 104975. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Kim, H.S.; Hwang, Y.; Jeon, Y.J.; Jun, H.S. Polysiphonia japonica extract attenuates palmitate-induced toxicity and enhances insulin secretion in pancreatic beta-cells. Oxidative Med. Cell. Longev. 2018, 2018, 4973851. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Zhang, C.; Heo, S.J.; Jun, H.S. 5-Bromoprotocatechualdehyde combats against palmitate toxicity by inhibiting parkin degradation and reducing ros-induced mitochondrial damage in pancreatic β-cells. Antioxidants 2021, 10, 264. [Google Scholar] [CrossRef]
- Ahorukomeye, P.; Disotuar, M.M.; Gajewiak, J.; Karanth, S.; Watkins, M.; Robinson, S.D.; Flórez Salcedo, P.; Smith, N.A.; Smith, B.J.; Schlegel, A.; et al. Fish-hunting cone snail venoms are a rich source of minimized ligands of the vertebrate insulin receptor. eLife 2019, 8, e41574. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Tang, X.X.; He, F.M.; Jia, J.X.; Hu, H.; Xie, B.Y.; Li, M.Y.; Qiu, Y.K. Two new compounds from a mangrove sediment-derived fungus Penicillium polonicum H175. Nat. Prod. Res. 2022, 36, 2370–2378. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.H.; Allan, V.K.; Song, C. Establishment of diabetic zebrafish model and evaluation of hypoglycemic activity of antarctic krill enzymatic hydrolysates. Food Mach. 2019, 35, 24–29. [Google Scholar]
- Kim, E.A.; Lee, S.H.; Lee, J.H.; Kang, N.; Oh, J.Y.; Cha, S.H.; Ahn, G.; Ko, S.C.; Fernando, I.P.S.F.; Kim, S.Y. A marine algal polyphenol, dieckol, attenuates blood glucose levels by Akt pathway in alloxan induced hyperglycemia zebrafish model. RSC Adv. 2016, 6, 78570–78575. [Google Scholar] [CrossRef]
- Zhang, C.; Forlano, P.M.; Cone, R.D. AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost. Cell Metab. 2012, 15, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Models Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Gibert, Y.; Berekelya, L.; Bernard, L.; Brunet, F.; Guillot, E.; Le Bail, J.C.; Sánchez, J.A.; Galzin, A.M.; Triqueneaux, G.; et al. Fasting induces CART down-regulation in the zebrafish nervous system in a cannabinoid receptor 1-dependent manner. Mol. Endocrinol. 2012, 26, 1316–1326. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Y.Q.; Guo, S.Y.; Li, C.Q. Rapid analysis of hypolipidemic drugs in a live zebrafish assay. Pharmacol. Toxicol. Methods 2015, 72, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, Z.K.; Yang, X.T.; Yang, T.X.; Zhang, T. Construction of zebrafish obesity model and its application in lipidlowering substances. Mod. Prev. Med. 2021, 48, 2622–2625. [Google Scholar]
- Costa, M.; Rosa, F.; Ribeiro, T.; Hernandez-Bautista, R.; Bonaldo, M.; Gonçalves Silva, N.; Eiríksson, F.; Thorsteinsdóttir, M.; Ussar, S.; Urbatzka, R. Identification of cyanobacterial strains with potential for the treatment of obesity-related co-morbidities by bioactivity, toxicity evaluation and metabolite profiling. Mar. Drugs 2019, 17, 280. [Google Scholar] [CrossRef]
- Ribeiro, T.; Jónsdóttir, K.; Hernandez-Bautista, R.; Silva, N.G.; Sánchez-Astráin, B.; Samadi, A.; Eiriksson, F.F.; Thorsteinsdóttir, M.; Ussar, S.; Urbatzka, R. Metabolite profile characterization of cyanobacterial strains with bioactivity on lipid metabolism using in vivo and in vitro approaches. Mar. Drugs 2023, 21, 498. [Google Scholar] [CrossRef]
- Bel Mabrouk, S.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L.; et al. The marine seagrass Halophila stipulacea as a source of bioactive metabolites against obesity and biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Bellver, M.; Costa, S.L.d.; Sanchez, B.A.; Vasconcelos, V.; Urbatzka, R. Inhibition of intestinal lipid absorption by cyanobacterial strains in zebrafish larvae. Mar. Drugs 2021, 19, 161. [Google Scholar] [CrossRef]
- Luz, R.; Cordeiro, R.; Gonçalves, V.; Vasconcelos, V.; Urbatzka, R. Screening of lipid-reducing activity and cytotoxicity of the exometabolome from cyanobacteria. Mar. Drugs 2024, 22, 412. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Del Amor, A.; Bautista, R.H.; Ussar, S.; Cristobal, S.; Urbatzka, R. Insights into the mechanism of action of the chlorophyll derivative 13-2-hydroxypheophytine a on reducing neutral lipid reserves in zebrafish larvae and mice adipocytes. Eur. J. Pharmacol. 2023, 960, 176158. [Google Scholar] [CrossRef]
- Silva, N.G.; Preto, M.; Vasconcelos, V.; Urbatzka, R. Reduction of neutral lipid reservoirs, bioconversion and untargeted metabolomics reveal distinct roles for vitamin K isoforms on lipid metabolism. Food Funct. 2024, 15, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.; Vitorino, I.; De la Cruz, M.; Díaz, C.; Cautain, B.; Annang, F.; Pérez-Moreno, G.; Gonzalez Martinez, I.; Tormo, J.R.; Martín, J.M.; et al. Bioactivities and extract dereplication of Actinomycetales isolated from marine sponges. Front. Microbiol. 2019, 10, 727. [Google Scholar] [CrossRef]
- Noinart, J.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.A.; Urbatzka, R.; Freitas, S.; Lee, M.; Silva, A.M.S.; Pinto, M.M.M.; et al. A new ergosterol nalog, a new bis-anthraquinone and anti-obesity activity of anthraquinones froam the marine sponge-associated fungus Talaromyces stipitatus KUFA 0207. Mar. Drugs 2017, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Coello, L.; Urbatzka, R.; Pérez, M.; Thorsteinsdottir, M. New aromatic bisabolane derivatives with lipid-reducing activity from the marine sponge Myrmekioderma sp. Mar. Drugs 2019, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Shimada, Y.; Zang, L.Q.; Terasawa, M.; Nishiura, K.; Matsuda, K.; Toombs, C.; Langdon, C.; Nishimura, N. Novel anti-obesity properties of palmaria mollis in zebrafish and mouse models. Nutrients 2018, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, Y.; Tong, X.; Xu, Z.; Xia, Q.; Xu, K. Structural characterization and hypolipidemic activity of a natural polysaccharide from Suaeda salsa L. Int. J. Biol. Macromol. 2024, 279 Pt 4, 135480. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Seo, Y.J.; Pan, C.H.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Fucoxanthin suppresses lipid accumulation and ROS production during differentiation in 3T3-L1 Adipocytes. Phytother. Res. 2016, 30, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, Y.; Dai, Z.R.; Qin, M.; Fan, H.L.; Hao, J.G.; Zhang, C.X.; Zhong, Q.P.; Qi, C.; Wang, P. Glycosaminoglycan from Ostrea rivularis attenuates hyperlipidemia and regulates gut microbiota in high-cholesterol diet-fed zebrafish. Food Sci. Nutr. 2021, 9, 5198–5210. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Fu, Y.H.; Li, F.T.; Yang, D.; Wei, L.N.; Yue, H.; Dai, Y.L.; Jeon, Y.J. Treatment of obese zebrafish with saringosterol acetate through AMP activated protein kinase pathway. Chem. Biodivers. 2022, 19, e202200495. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Lan, Z.; Xin, Z.; He, C.; Guo, Z.; Xia, X.; Hu, T. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J. Cell. Physiol. 2020, 235, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Zhang, Y.; Li, P.H.; Li, H.; Yang, G.W.; Liu, K.C. Anti-inflammatory activity of viridicatol based on zebrafish model. Chin. J. Antibiot. 2023, 48, 172–178. [Google Scholar]
- Duan, X.Y.; Ma, R.J.; Zhang, Y.; Liu, K.C. Zebrafish inflammation model and its application in anti-inflammatory field of traditional chinese medicine. Drug Eval. Res. 2021, 44, 1573–1580. [Google Scholar]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutiérrez-de Frías, C.; Cortés, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef] [PubMed]
- LeBert, D.C.; Huttenlocher, A. Inflammation and wound repair. Semin. Immunol. 2014, 26, 315–320. [Google Scholar] [CrossRef]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, F.; Xia, Q.; Liu, K.; Lin, H.; Zhang, S.; Zhang, Y. The peptide LLTRAGL derived from rapana venosa exerts protective effect against inflammatory bowel disease in zebrafish model by regulating multi-pathways. Mar. Drugs 2024, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.H.; Jiao, W.H.; Zhou, M.; Zhang, Y.; Zeng, D.Q.; Zhu, H.R.; Liu, K.C.; Sun, F.; Chen, H.F.; Lin, H.W. Septosones A–C, in vivo anti-inflammatory meroterpenoids with rearranged carbon skeletons from the marine sponge dysidea septosa. Org. Lett. 2019, 21, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sang, M.; Lu, J.R.; Zhao, H.M.; Zou, Y.; Wu, W.; Yu, Y.; Liu, Y.W.; Ma, W.; Zhang, Y.; et al. Somalactams A–D: Anti-inflammatory macrolide lactams with unique ring systems from an arctic actinomycete strain. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218085. [Google Scholar] [CrossRef]
- Gui, Y.H.; Liu, L.; Wu, W.; Zhang, Y.; Jia, Z.L.; Shi, Y.P.; Kong, H.T.; Liu, K.C.; Jiao, W.H.; Lin, H.W. Discovery of nitrogenous sesquiterpene quinone derivatives from sponge dysidea septosa with anti-inflammatory activity in vivo zebrafish model. Bioorganic Chem. 2020, 94, 103435. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Feng, L.; Xu, L.; Lin, H.; Liu, K.; Li, X.; Wang, R. Altechromone A Ameliorates Inflammatory Bowel Disease by Inhibiting NF-κB and NLRP3 Pathways. Mar. Drugs 2024, 22, 410. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Han, X.; Mi, Y.; Cui, Y.; Fu, A.; Liu, K.; Li, X.; Tang, X.; Li, G. Anti-inflammatory and cytotoxicity nitrogenous merosesquiterpenoids from the sponge Pseudoceratina purpurea. Phytochemistry 2024, 226, 114220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Li, H.N.; Wang, L.Z.; Zhang, S.S.; Wang, F.X.; Lin, H.W.; Gao, S.; Li, X.B.; Liu, K.C. Anti-inflammatory peptides and metabolomics-driven biomarkers discovery from sea cucumber protein hydrolysates. J. Food Sci. 2021, 86, 3540–3549. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.; Kim, E.A.; Kang, M.C.; Lee, W.W.; Lee, H.S.; Vairappan, C.S.; Jeon, Y.J. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed laurencia snackeyi in zebrafish embryo in vivo model. Environ. Toxicol. Pharmacol. 2014, 37, 110–117. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Y.R.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.J. Anti-inflammatory effect of fucoidan isolated from fermented sargassum fusiforme in in vitro and in vivo models. Int. J. Biol. Macromol. 2022, 222 Pt B, 2065–2071. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in turbinaria ornata from the maldives; inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Kuruppu, A.I.; Ko, J.Y.; Lee, J.H.; Jeon, Y.J. Structural characterizationand anti-Inflammatory effects of 24-Methylcholesta-5(6), 22-Diene-3β-ol from the cultured marine diatom phaeodactylum tricornutum; attenuate inflammatory signaling pathways. Mar. Drugs 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Je, J.G.; Huang, C.; Oh, J.Y.; Fu, X.; Wang, K.; Ahn, G.; Xu, J.; Gao, X.; Jeon, Y.J. AntiInflammatory effect of sulfated polysaccharides isolated from codium fragile In vitro in RAW 264.7 macrophages and in vivo in zebrafish. Mar. Drugs 2022, 20, 391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.-W.; Ahn, G.; Fu, X.; Xu, J.; Gao, X.; Jeon, Y.J. In vitro and in vivo anti-inflammatory effects of sulfated polysaccharides isolated from the edible brown seaweed, sargassum fulvellum. Mar. Drugs 2021, 19, 277. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ni, L.; Fu, X.; Wang, L.; Duan, D.; Huang, L.; Xu, J.; Gao, X. Molecular mechanism of anti-inflammatory activities of a novel sulfated galactofucan from saccharina japonica. Mar. Drugs 2021, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Je, J.G.; An, H.; Baek, K.; Lee, J.M.; Yim, M.J.; Ko, S.C.; Kim, J.Y.; Oh, G.W.; Kang, M.C.; et al. Isolation and characterization of efficient active compounds using high-performance centrifugal partition chromatography (CPC) from anti-inflammatory activity fraction of ecklonia maxima in south africa. Mar. Drugs 2022, 20, 471. [Google Scholar] [CrossRef]
- Wang, Z.G.; Ying, X.G.; Gao, P.; Wang, C.L.; Wang, Y.F.; Yu, X.W.; Chen, J.; Wang, B.; Luo, H.Y. Anti-inflammatory activity of a peptide from skipjack (Katsuwonus pelamis). Mar. Drugs 2019, 17, 582. [Google Scholar] [CrossRef]
- Tang, H.; Ge, H.; Chen, Z.B.; Luo, X.M.; Su, F.J.; Liang, Y.B.; Li, Z.Y.; Wu, J.G.; Yang, Q.; Zeng, L.J.; et al. Micrometam C protects against oxidative stress in inflammation models in zebrafish and RAW264.7 macrophages. Mar. Drugs 2015, 13, 5593–5605. [Google Scholar] [CrossRef]
- Fei, T.T.; Liang, T.Y.; Cao, P.X.; Meng, X.; Wu, H. Research progress on the role of oxidative stress in cardiovascular disease in zebrafish. Chin. J. Comp. Med. 2024, 34, 172–178. [Google Scholar]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kim, H.S.; Oh, J.Y.; Lee, D.S.; Yang, H.W.; Kang, M.C.; Kim, E.A.; Kang, N.; Kim, J.; Heo, S.J.; et al. Potential antioxidant properties of enzymatic hydrolysates from stichopus japonicus against hydrogen peroxide-induced oxidative stress. Antioxidants 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Miller, Y.I. Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids. Free Radic. Biol. Med. 2012, 53, 1411–1420. [Google Scholar] [CrossRef]
- Zhang, S.S.; Han, L.W.; Shi, Y.P.; Li, X.B.; Zhang, X.M.; Hou, H.R.; Lin, H.W.; Liu, K.C. Two novel multi-functional peptides from meat and visceral mass of marine snail Neptunea arthritica cumingii and their activities in vitro and in vivo. Mar. Drugs 2018, 16, 473. [Google Scholar] [CrossRef]
- Jiao, W.H.; Li, J.; Zhang, M.M.; Cui, J.; Gui, Y.H.; Zhang, Y.; Li, J.Y.; Liu, K.C.; Lin, H.W. Frondoplysins A and B, unprecedented terpene-alkaloid bioconjugates from Dysidea frondosa. Org. Lett. 2019, 21, 6190–6193. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Y.; Kim, E.A.; Lee, J.H.; Kang, M.C.; Lee, J.S.; Kim, J.S.; Jung, W.K.; Jeon, Y.J. Protective effect of aquacultured flounder fish-derived peptide against oxidative stress in zebrafish. Fish Shellfish Immunol. 2014, 36, 320–323. [Google Scholar] [CrossRef]
- Lee, H.G.; Nagahawatta, D.P.; Yang, F.; Jayawardhana, H.H.A.C.K.; Liyanage, N.M.; Lee, D.S.; Lee, J.M.; Yim, M.J.; Ko, S.C.; Kim, J.Y.; et al. Antioxidant potential of hydrolysate-derived seahorse (Hippocampus abdominalis) peptide: Protective effects against AAPH-induced oxidative damage in vitro and in vivo. Food Chem. 2023, 407, 135130. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Cha, S.H.; Wijesinghe, W.A.; Kang, S.M.; Lee, S.H.; Kim, E.A.; Song, C.B.; Jeon, Y.J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef]
- Wang, L.; Fu, X.; Hyun, J.; Xu, J.; Gao, X.; Jeon, Y.-J. In vitro and in vivo protective effects of agaro-oligosaccharides against hydrogen peroxide-stimulated oxidative stress. Polymers 2023, 15, 1612. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Chen, J.W.; Yuan, F.; Huang, Y.Y.; Zhao, L.Y.; Li, J.; Su, H.X.; Liu, J.; Pang, J.Y.; Lin, Y.C.; et al. Xyloketal B exhibits its antioxidant activity through induction of HO-1 in vascular endothelial cells and zebrafish. Mar. Drugs 2013, 11, 504–522. [Google Scholar] [CrossRef]
- Kim, H.S.; Wang, L.; Fernando, I.P.S.; Je, J.G.; Ko, S.C.; Kang, M.C.; Lee, J.M.; Yim, M.J.; Jeon, Y.J.; Lee, D.S. Antioxidant efficacy of (−)-loliolide isolated from sargassum horneri against AAPH-induced oxidative damage in vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.Y.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Wang, L.; Lee, S.H.; Ko, S.C.; Kang, M.C.; Jayawardena, T.U.; Jeon, Y.J. Free radical scavenging activity of the peptide from the alcalase hydrolysate of the edible aquacultural seahorse (Hippocampus abdominalis). J. Food Biochem. 2019, 43, e12833. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.J. Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, H.; Han, L.; Mervin, L.H.; Hosseini-Gerami, L.; Li, P.; Wright, P.; Trapotsi, M.-A.; Liu, K.; Fan, T.-P.; et al. In silico prediction and biological assessment of novel angiogenesis modulators from traditional Chinese medicine. Front. Pharmacol. 2023, 14, 1116081. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhao, X.; Liu, H.; Wang, Y.; Zhao, C.; Zhao, T.; Zhao, B.; Wang, Y. Identification of a Quality Marker (Q-Marker) of Danhong Injection by the Zebrafish Thrombosis Model. Molecules 2017, 22, 1443. [Google Scholar] [CrossRef]
| Organism | Nematode | Fruit fly | Zebrafish | Mouse |
|---|---|---|---|---|
| Generation time | 3–4 days | 11–12 days | 3 months | 2 months |
| Adult size | 1–1.3 mm | 3–4 mm | 3–5 cm | 6–10 cm |
| Embryos size | 50 μm | 100 μm | 1–5 mm | N/A |
| Brood size | ~140 eggs/day | ~120 eggs/day | 200–300 eggs/week | 6–12 pups/month |
| Growth conditions | Solid or liquid medium | Solid medium | Liquid medium | Cages |
| Genome size | ~97 Mb | ~180 Mb | ~1500 Mb | ~3000 Mb |
| Homology to human (genome) | >50% | >60% | >80% | >90% |
| Transgenic organism Generation | weeks | weeks | months | months |
| Culture in microtiter plate | Eggs to adults | Eggs to larvae | Eggs to larvae | N/A |
| High-throughput drug screening | +++ | ++ | ++ | N/A |
| Whole biological complexity | + | + | ++ | +++ |
| Current use in drug discovery | + | + | ++ | +++ |
| Ease of experimental operation | +++ | ++ | ++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, J.; Wang, D.; Liu, J.; Liu, K.; Li, P.; Zhang, Y. Recent Advances of the Zebrafish Model in the Discovery of Marine Bioactive Molecules. Mar. Drugs 2024, 22, 540. https://doi.org/10.3390/md22120540
Liu C, Li J, Wang D, Liu J, Liu K, Li P, Zhang Y. Recent Advances of the Zebrafish Model in the Discovery of Marine Bioactive Molecules. Marine Drugs. 2024; 22(12):540. https://doi.org/10.3390/md22120540
Chicago/Turabian StyleLiu, Changyu, Jiaxun Li, Dexu Wang, Jibin Liu, Kechun Liu, Peihai Li, and Yun Zhang. 2024. "Recent Advances of the Zebrafish Model in the Discovery of Marine Bioactive Molecules" Marine Drugs 22, no. 12: 540. https://doi.org/10.3390/md22120540
APA StyleLiu, C., Li, J., Wang, D., Liu, J., Liu, K., Li, P., & Zhang, Y. (2024). Recent Advances of the Zebrafish Model in the Discovery of Marine Bioactive Molecules. Marine Drugs, 22(12), 540. https://doi.org/10.3390/md22120540






