Cytotoxic Effects of ZnO and Ag Nanoparticles Synthesized in Microalgae Extracts on PC12 Cells
Abstract
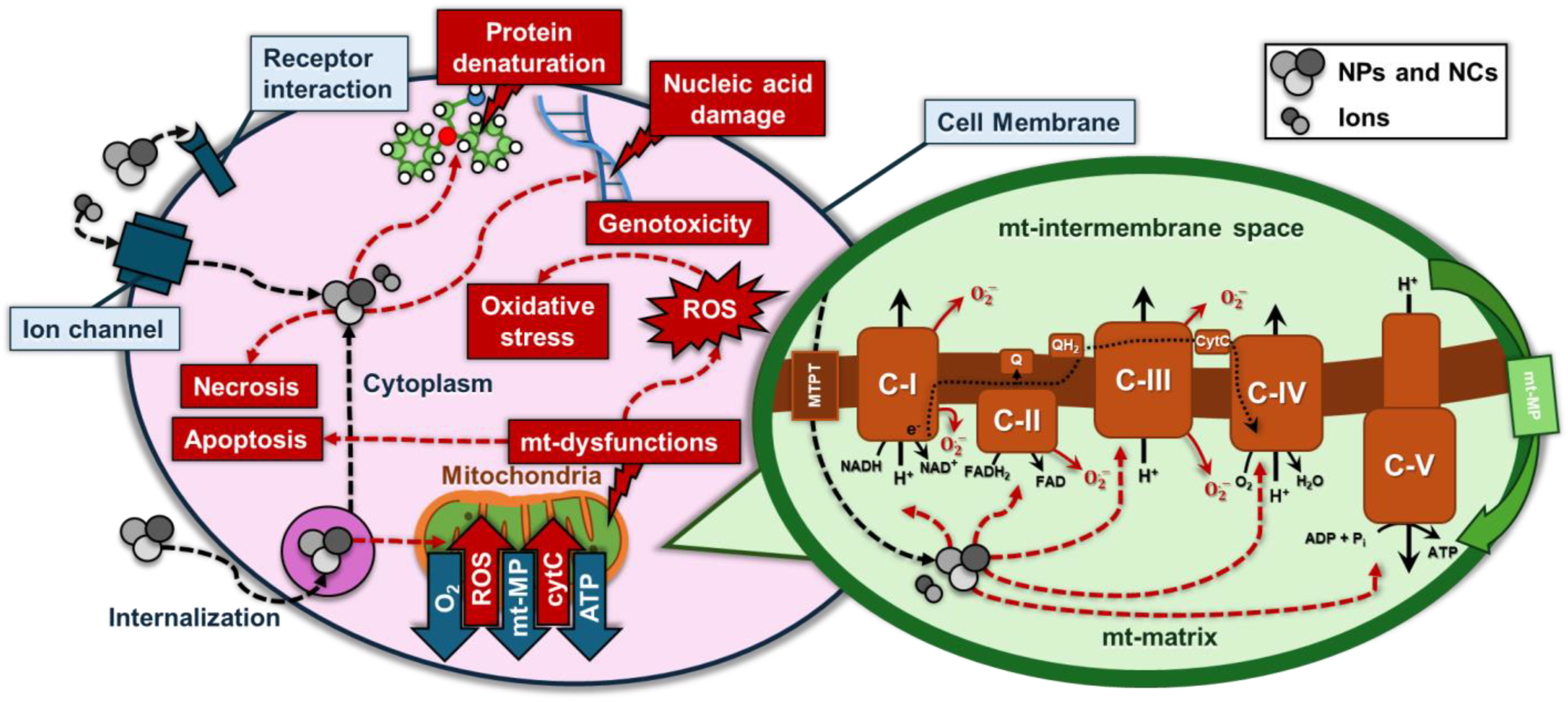
:1. Introduction
2. Results and Discussion
2.1. Phase Composition and Crystallinity of ZnO Nanoparticles Synthesized from Polar and Apolar Extracts
2.2. Cytotoxicity of the Produced Nanoparticles
2.2.1. MTT Assay
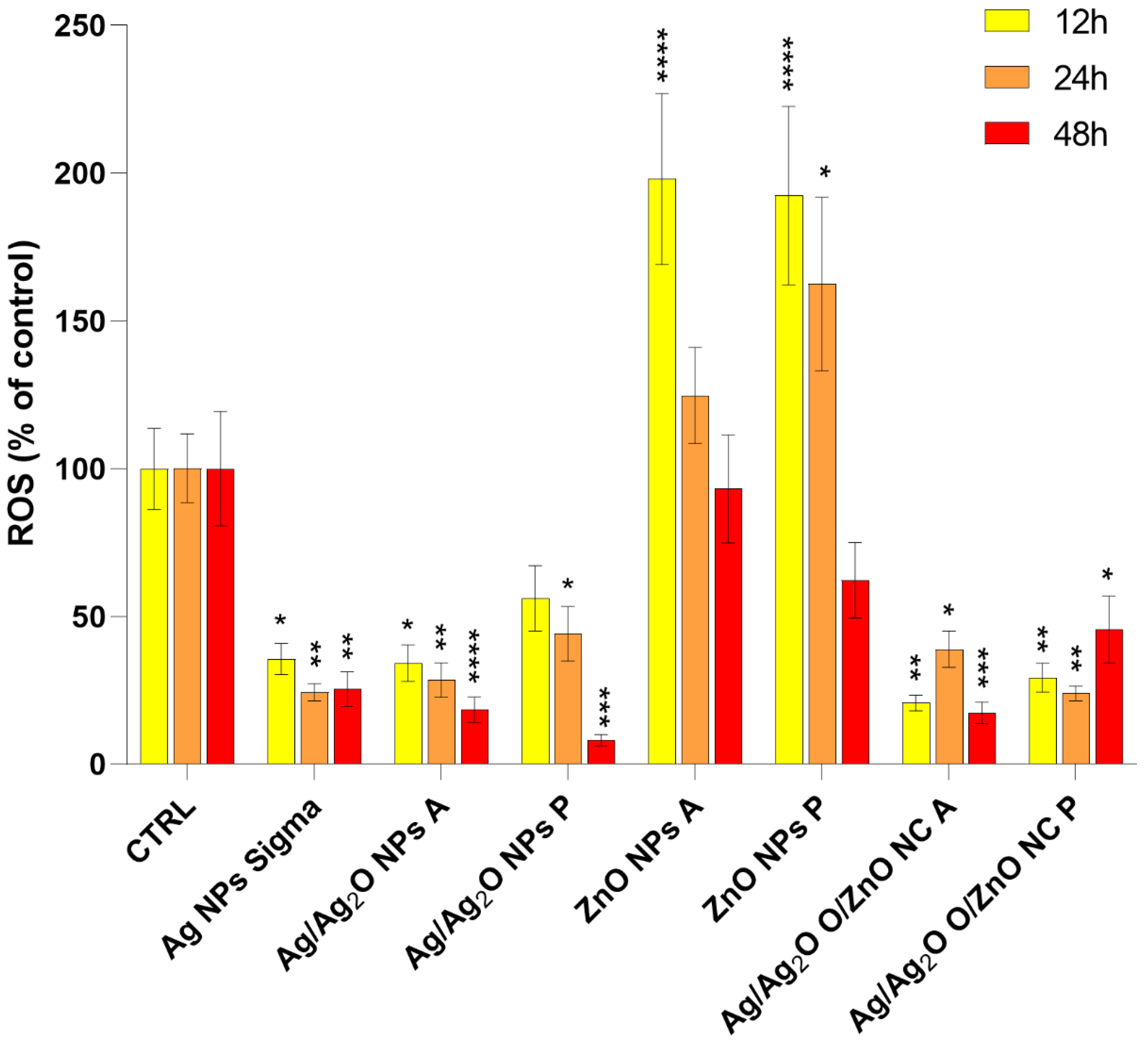
2.2.2. Role of ROS Generation in Nanoparticle-Induced Cytotoxicity
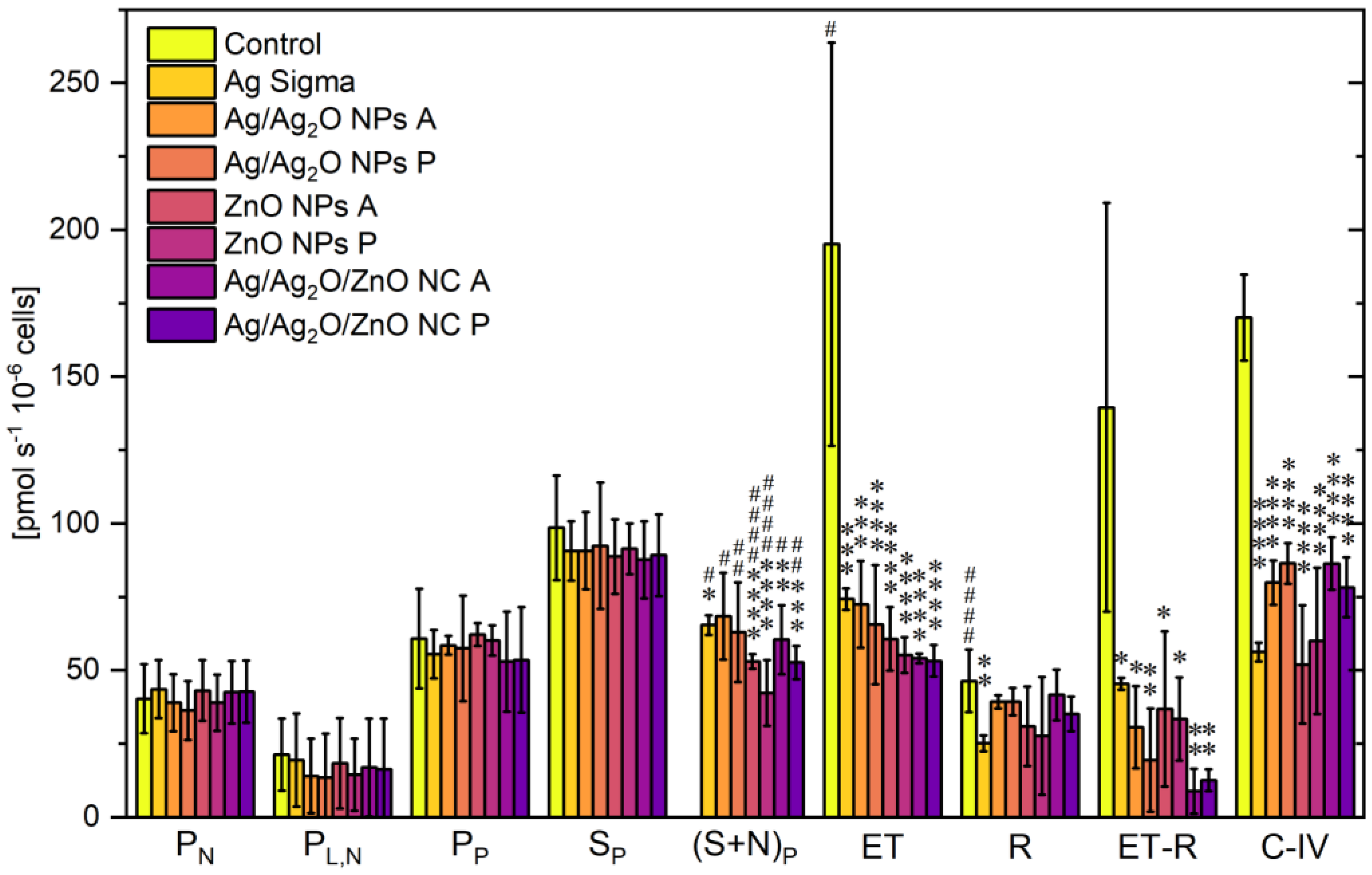
2.2.3. Bioenergetic Function
3. Materials and Methods
3.1. Synthesis of ZnO Nanoparticles Using Extracts from Chlorella vulgaris Biomass
3.2. Characterization of Synthesized Nanoparticles
3.3. Cell Culture Conditions
3.4. Nanoparticle Cytotoxicity in PC12 Cells via MTT Assay
3.5. Measurement of ROS Production in PC12 Cells
3.6. Measurement of Bioenergetic Function
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidorowicz, A.; Fais, G.; Casula, M.; Borselli, M.; Giannaccare, G.; Locci, A.M.; Lai, N.; Orrù, R.; Cao, G.; Concas, A. Nanoparticles from Microalgae and Their Biomedical Applications. Mar. Drugs 2023, 21, 352. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Kushwaha, P.; Ansari, T.M.; Kumar, A.; Rao, A. Recent Trends in Biologically Synthesized Metal Nanoparticles and Their Biomedical Applications: A Review. Biol. Trace Elem. Res. 2024, 202, 3383–3399. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in Cancer Therapy and Diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Fais, G.; Desogus, F.; Loy, F.; Licheri, R.; Lai, N.; Locci, A.M.; Cincotti, A.; Orrù, R.; Cao, G.; et al. Optimization of Brilliant Blue R Photocatalytic Degradation by Silver Nanoparticles Synthesized Using Chlorella Vulgaris. Environ. Sci. Pollut. Res. 2024, 31, 57765–57777. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Zhou, Q.; Chen, P.; Wang, Y.; Wang, Y.; Liu, T.; Xie, L. Inhibitory Effects of Polysaccharide Extract from Spirulina Platensis on Corneal Neovascularization. Mol. Vis. 2009, 15, 1951–1961. [Google Scholar]
- Zhou, X.; Hyyat, Z.; Zhang, D.; Li, M.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Khan, S.H. Green Nanotechnology for the Environment and Sustainable Development. Green Mater. Wastewater Treat. 2020, 38, 13–46. [Google Scholar]
- Cruz, D.M.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green Nanotechnology-Based Zinc Oxide (ZnO) Nanomaterials for Biomedical Applications: A Review. JPhys Mater. 2020, 3, 034005. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.-Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Jacob, J.M.; Ravindran, R.; Narayanan, M.; Samuel, S.M.; Pugazhendhi, A.; Kumar, G. Microalgae: A Prospective Low Cost Green Alternative for Nanoparticle Synthesis. Curr. Opin. Environ. Sci. Health 2021, 20, 100163. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Margarita, V.; Fais, G.; Pantaleo, A.; Manca, A.; Concas, A.; Rappelli, P.; Fiori, P.L.; Cao, G. Characterization of Nanomaterials Synthesized from Spirulina Platensis Extract and Their Potential Antifungal Activity. PLoS ONE 2022, 17, e0274753. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Alba-González, A.; Fernández-Bertólez, N.; Touzani, A.; Ramos-Pan, L.; Reis, A.T.; Moreda-Piñeiro, J.; Yáñez, J.; Laffon, B.; Folgueira, M. Effects of Zinc Oxide Nanoparticle Exposure on Human Glial Cells and Zebrafish Embryos. Int. J. Mol. Sci. 2023, 24, 12297. [Google Scholar] [CrossRef]
- Jha, S.; Rani, R.; Singh, S. Biogenic Zinc Oxide Nanoparticles and Their Biomedical Applications: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1437–1452. [Google Scholar] [CrossRef]
- Desai, A.S.; Ashok, A.; Edis, Z.; Bloukh, S.H.; Gaikwad, M.; Patil, R.; Pandey, B.; Bhagat, N. Meta-Analysis of Cytotoxicity Studies Using Machine Learning Models on Physical Properties of Plant Extract-Derived Silver Nanoparticles. Int. J. Mol. Sci. 2023, 24, 4220. [Google Scholar] [CrossRef]
- Sambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the Toxic Effect of Silver Nanoparticles on Mammalian Cell Lines. J. Nanomater. 2015, 2015, 136765. [Google Scholar] [CrossRef]
- Rudi, L.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Grozdov, D.; Kravtsova, A. The Impact of Silver Nanoparticles Functionalized with Spirulina Protein Extract on Rats. Pharmaceuticals 2024, 17, 1247. [Google Scholar] [CrossRef]
- Zare Marzouni, H.; Tarkhan, F.; Aidun, A.; Shahzamani, K.; Jahan Tigh, H.R.; Malekshahian, S.; Esmaeil Lashgarian, H. Cytotoxic Effects of Coated Gold Nanoparticles on PC12 Cancer Cell. Galen. Med. J. 2018, 7, e1110. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Tischler, A.S. PC12 Pheochromocytoma Cultures in Neurobiological Research. Adv. Cell. Neurobiol. 1982, 3, 373–414. [Google Scholar]
- Larner, S.F.; Wang, J.; Goodman, J.; O’Donoghue Altman, M.B.; Xin, M.; Wang, K.K.W. In Vitro Neurotoxicity Resulting from Exposure of Cultured Neural Cells to Several Types of Nanoparticles. J. Cell Death 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Marongiu, M.E.; Piccardi, M.P.; Bernardi, F.; Corsini, G.U.; Del Zompo, M. Evaluation of the Toxicity of the Dopaminergic Neurotoxins MPTP and MPP+ in PC12 Pheochromocytoma Cells: Binding and Biological Studies. Neurosci. Lett. 1988, 94, 349–354. [Google Scholar] [CrossRef]
- Zhang, Z.; Meng, C.; Hou, K.; Wang, Z.; Huang, Y.; Lü, X. The Cytological and Electrophysiological Effects of Silver Nanoparticles on Neuron-like PC12 Cells. PLoS ONE 2022, 17, e0277942. [Google Scholar] [CrossRef]
- Powers, C.M.; Badireddy, A.R.; Ryde, I.T.; Seidler, F.J.; Slotkin, T.A. Silver Nanoparticles Compromise Neurodevelopment in PC12 Cells: Critical Contributions of Silver Ion, Particle Size, Coating, and Composition. Environ. Health Perspect. 2011, 119, 37–44. [Google Scholar] [CrossRef]
- Suthar, J.K.; Vaidya, A.; Ravindran, S. Size, Surface Properties, and Ion Release of Zinc Oxide Nanoparticles: Effects on Cytotoxicity, Dopaminergic Gene Expression, and Acetylcholinesterase Inhibition in Neuronal PC-12 Cells. Biol. Trace Elem. Res. 2024, 202, 2254–2271. [Google Scholar] [CrossRef]
- Fernández-Bertólez, N.; Alba-González, A.; Touzani, A.; Ramos-Pan, L.; Méndez, J.; Reis, A.T.; Quelle-Regaldie, A.; Sánchez, L.; Folgueira, M.; Laffon, B.; et al. Toxicity of Zinc Oxide Nanoparticles: Cellular and Behavioural Effects. Chemosphere 2024, 363, 142993. [Google Scholar] [CrossRef]
- Hasan, M.R.; Niebuur, B.J.; Siebrecht, M.; Kuttich, B.; Schweins, R.; Widmer-Cooper, A.; Kraus, T. The Colloidal Stability of Apolar Nanoparticles in Solvent Mixtures. ACS Nano 2023, 17, 9302–9312. [Google Scholar] [CrossRef]
- Al-luhaibi, A.A.; Sendi, R.K. Synthesis, Potential of Hydrogen Activity, Biological and Chemical Stability of Zinc Oxide Nanoparticle Preparation by Sol–Gel: A Review. J. Radiat. Res. Appl. Sci. 2022, 15, 238–254. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Harun, Z.; Ismail, A.F.; Aziz, F. Constructing a Compact Heterojunction Structure of Ag2CO3/Ag2O in-Situ Intermediate Phase Transformation Decorated on ZnO with Superior Photocatalytic Degradation of Ibuprofen. Sep. Purif. Technol. 2020, 251, 117391. [Google Scholar] [CrossRef]
- Vinitha, V.; Preeyanghaa, M.; Anbarasu, M.; Jeya, G.; Neppolian, B.; Sivamurugan, V. Aminolytic Depolymerization of Polyethylene Terephthalate Wastes Using Sn-Doped ZnO Nanoparticles. J. Polym. Environ. 2022, 30, 3566–3581. [Google Scholar] [CrossRef]
- Singh, S.; Perween, S.; Ranjan, A. Dramatic Enhancement in Adsorption of Congo Red Dye in Polymer-Nanoparticle Composite of Polyaniline-Zinc Titanate. J. Environ. Chem. Eng. 2021, 9, 105149. [Google Scholar] [CrossRef]
- Kondratenko, T.; Ovchinnikov, O.; Grevtseva, I.; Smirnov, M.; Erina, O.; Khokhlov, V.; Darinsky, B.; Tatianina, E. Thioglycolic Acid Ftir Spectra on Ag2S Quantum Dots Interfaces. Materials 2020, 13, 909. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Charan, C.P. ZnO Nanofiller Concentrations Modified P(VDF-HFP)/PEO Polymer Matrix-Based Nanocomposites of Enhanced Optical and Dielectric Properties for Emerging Optoelectronic and Energy Storage Devices. Surf. Interfaces 2024, 46, 103945. [Google Scholar] [CrossRef]
- Muniyappan, M.; Iyandurai, N. Structural Analysis Of Aa2024 Interaction Reinforced With Carbon Nanotubes And Silicon Nanocomposites Studied By Fourier Transform Infrared Spectroscopy. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1219, 012046. [Google Scholar] [CrossRef]
- Vivek, C.; Balraj, B.; Thangavel, S. Structural, Optical and Electrical Behavior of ZnO@Ag Core–Shell Nanocomposite Synthesized via Novel Plasmon-Green Mediated Approach. J. Mater. Sci. Mater. Electron. 2019, 30, 11220–11230. [Google Scholar] [CrossRef]
- Shaban, A.S.; Owda, M.E.; Basuoni, M.M.; Mousa, M.A.; Radwan, A.A.; Saleh, A.K. Punica Granatum Peel Extract Mediated Green Synthesis of Zinc Oxide Nanoparticles: Structure and Evaluation of Their Biological Applications. Biomass Convers. Biorefin 2024, 14, 12265–12281. [Google Scholar] [CrossRef]
- Vadakkan, K.; Rumjit, N.P.; Ngangbam, A.K.; Vijayanand, S.; Nedumpillil, N.K. Novel Advancements in the Sustainable Green Synthesis Approach of Silver Nanoparticles (AgNPs) for Antibacterial Therapeutic Applications. Coord. Chem. Rev. 2024, 499, 215528. [Google Scholar] [CrossRef]
- Aspoukeh, P.K.; Barzinjy, A.A.; Hamad, S.M. A Novel Approach to the Green Synthesis of Zinc Oxide Nanorods Using Thymus Kotschyanus Plant Extract: Effect of Ammonium Hydroxide and Precursor Concentration. Nano Express 2023, 4, 045001. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Concas, A.; Orrù, R.; Cao, G.; Rupprechter, G. Microalgae-Derived Co3O4 Nanomaterials for Catalytic CO Oxidation. RSC Adv. 2024, 14, 4575–4586. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Amin, S.; Kudaibergenova, B.M.; Rauf, A.; Siddique, M.; Ullah, K.; Bawazeer, S.; Farooq, U.; Mabkhot, Y.N.; Ramadan, M.F. Green Synthesis and Antimicrobial Potential of Silver Nanoparticles with Boerhavia Procumbens Extract. J. Pure Appl. Microbiol. 2020, 14, 1437–1451. [Google Scholar] [CrossRef]
- Anum, F.; Jabeen, K.; Javad, S.; Iqbal, S.; Tahir, A.; Javed, Z.; Cruz-Martins, N.; Ayatollahi, S.A.; Sharifi-Rad, J.; Alshehri, M.M.; et al. Green Synthesized Silver Nanoparticles as Potent Antifungal Agent against Aspergillus Terreus Thom. J. Nanomater. 2021, 2021, 2992335. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Szymański, T.; Rybka, J.D. Photodegradation of Biohazardous Dye Brilliant Blue r Using Organometallic Silver Nanoparticles Synthesized through a Green Chemistry Method. Biology 2021, 10, 784. [Google Scholar] [CrossRef]
- Song, T.; Gao, F.; Guo, S.; Zhang, Y.; Li, S.; You, H.; Du, Y. A Review of the Role and Mechanism of Surfactants in the Morphology Control of Metal Nanoparticles. Nanoscale 2021, 13, 3895–3910. [Google Scholar] [CrossRef]
- Bari, A.H.; Jundale, R.B.; Kulkarni, A.A. Understanding the Role of Solvent Properties on Reaction Kinetics for Synthesis of Silica Nanoparticles. Chem. Eng. J. 2020, 398, 125427. [Google Scholar] [CrossRef]
- Min, Y.; Suminda, G.G.D.; Heo, Y.; Kim, M.; Ghosh, M.; Son, Y.O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants 2023, 12, 703. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Mech, F.; Zitzmann, C.; Hasenberg, M.; Gunzer, M.; Figge, M.T. Automated Characterization and Parameter-Free Classification of Cell Tracks Based on Local Migration Behavior. PLoS ONE 2013, 8, e80808. [Google Scholar] [CrossRef]
- Sambale, F.; Stahl, F.; Rüdinger, F.; Seliktar, D.; Kasper, C.; Bahnemann, D.; Scheper, T. Iterative Cellular Screening System for Nanoparticle Safety Testing. J. Nanomater. 2015, 2015, 691069. [Google Scholar] [CrossRef]
- Issler, T.; Turner, R.J.; Prenner, E.J. Membrane-Nanoparticle Interactions: The Impact of Membrane Lipids. Small 2024, 20, e2404152. [Google Scholar] [CrossRef] [PubMed]
- Labena, A.; Hegazy, M.A.; Kamel, W.M.; Elkelish, A.; Hozzein, W.N. Enhancement of a Cationic Surfactant by Capping Nanoparticles: Synthesis, Characterization and Multiple Applications. Molecules 2020, 25, 2007. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Meng, L.; Gao, J.; Lu, M.; Guo, C.; Li, Y.; Rong, Z.; Ye, Y. PHB2 Promotes Colorectal Cancer Cell Proliferation and Tumorigenesis through NDUFS1-Mediated Oxidative Phosphorylation. Cell Death Dis. 2023, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Keskin-Erdogan, Z.; Sawadkar, P.; Nik Sharifulden, N.S.A.; Shannon, M.R.; Patel, M.; Silva, L.B.; Patel, R.; Chau, D.Y.S.; Knowles, J.C.; et al. Oxidative Stress Modulating Nanomaterials and Their Biochemical Roles in Nanomedicine. Nanoscale Horiz. 2024, 9, 1630–1682. [Google Scholar] [CrossRef]
- Naseer, F.; Ahmed, M.; Majid, A.; Kamal, W.; Phull, A.R. Green Nanoparticles as Multifunctional Nanomedicines: Insights into Anti-Inflammatory Effects, Growth Signaling and Apoptosis Mechanism in Cancer. Semin. Cancer Biol. 2022, 86, 310–324. [Google Scholar] [CrossRef]
- Maurer, L.L.; Meyer, J.N. A Systematic Review of Evidence for Silver Nanoparticle-Induced Mitochondrial Toxicity. Environ. Sci. Nano 2016, 3, 311–322. [Google Scholar] [CrossRef]
- Pashootan, P.; Saadati, F.; Fahimi, H.; Rahmati, M.; Strippoli, R.; Zarrabi, A.; Cordani, M.; Moosavi, M.A. Metal-Based Nanoparticles in Cancer Therapy: Exploring Photodynamic Therapy and Its Interplay with Regulated Cell Death Pathways. Int. J. Pharm. 2024, 649, 123622. [Google Scholar] [CrossRef]
- Wu, D.; Ma, Y.; Cao, Y.; Zhang, T. Mitochondrial Toxicity of Nanomaterials. Sci. Total Environ. 2020, 702, 134994. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, G.; Lu, L.; Yin, H.; Wang, Y.; Wang, B.; Pan, R.; Zheng, C.; Jin, X. Crucial Physicochemical Factors Mediating Mitochondrial Toxicity of Nanoparticles at Noncytotoxic Concentration. Sci. Total Environ. 2024, 908, 168211. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A.S. Establishment of a Noradrenergic Clonal Line of Rat Adrenal Pheochromocytoma Cells Which Respond to Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Tharushi Perera, P.G.; Linklater, D.P.; Kosyer, E.; Croft, R.; Ivanova, E.P. Localization of Nanospheres in Pheochromocytoma-like Cells Following Exposure to High-Frequency Electromagnetic Fields at 18 GHz. R. Soc. Open Sci. 2022, 9, 220520. [Google Scholar] [CrossRef]
- Fasching, M.; Fontana-Ayoub, M.; Gnaiger, E. O2k-Procedures Chemicals Mitochondrial Physiology Network Mitochondrial Respiration Medium-MiR06; Oroboros: Innsbruck, Austria, 2016; Volume 13, pp. 1–4. [Google Scholar]
- Gnaiger, E.; Cecatto, C.; Gradl, L. O2k-Manual: DatLab 7.4 DatLab 7.4 Guide; Oroboros: Innsbruck, Austria, 2021; Volume 26. [Google Scholar]
- Lai, N.; Fealy, C.E.; Kummitha, C.M.; Cabras, S.; Kirwan, J.P.; Hoppel, C.L. Mitochondrial Utilization of Competing Fuels Is Altered in Insulin Resistant Skeletal Muscle of Non-Obese Rats (Goto-Kakizaki). Front. Physiol. 2020, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Hoppel, C.L. Measuring Oxidative Phosphorylation in Human Skin Fibroblasts. Anal. Biochem. 2013, 437, 52–58. [Google Scholar] [CrossRef] [PubMed]

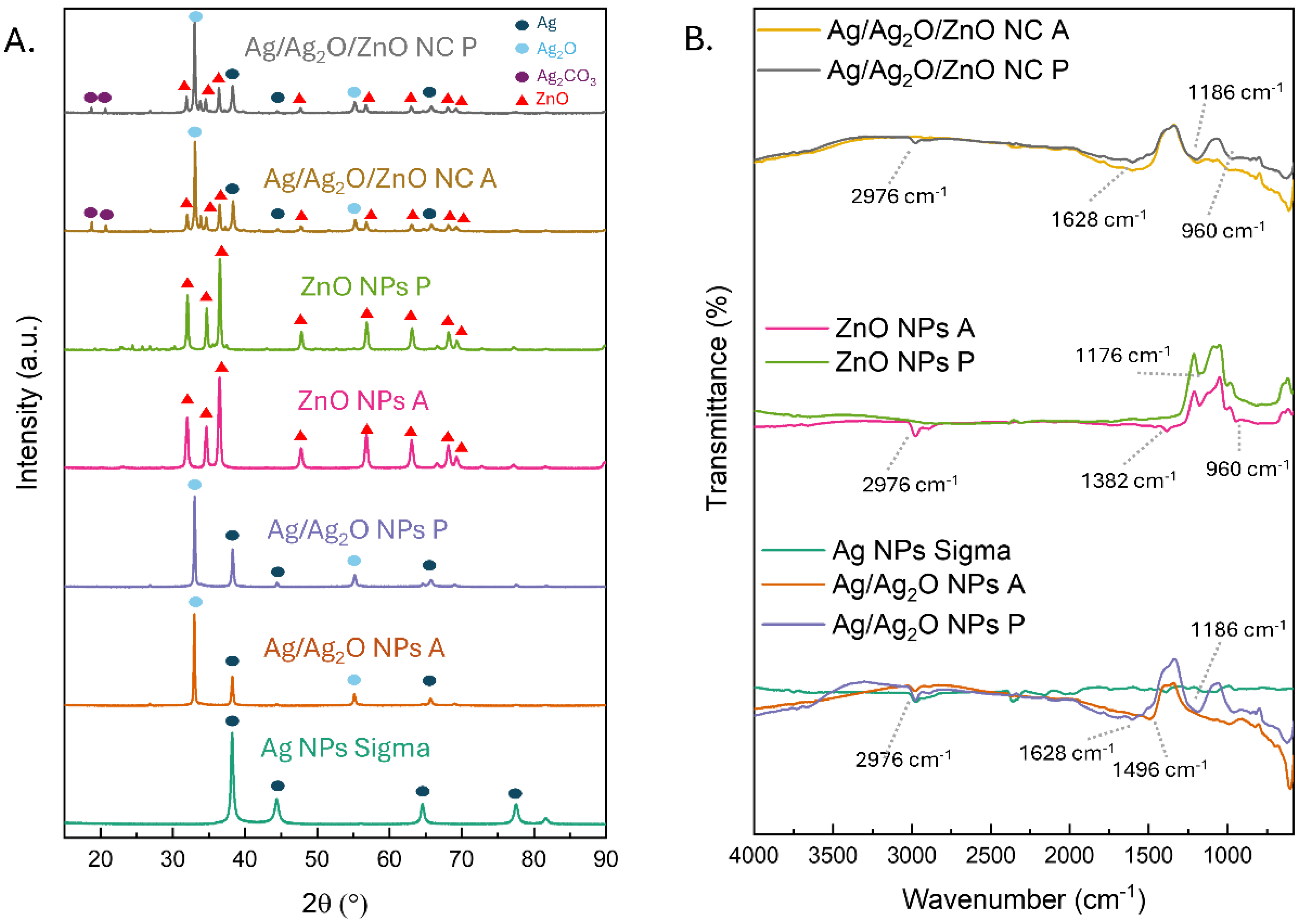
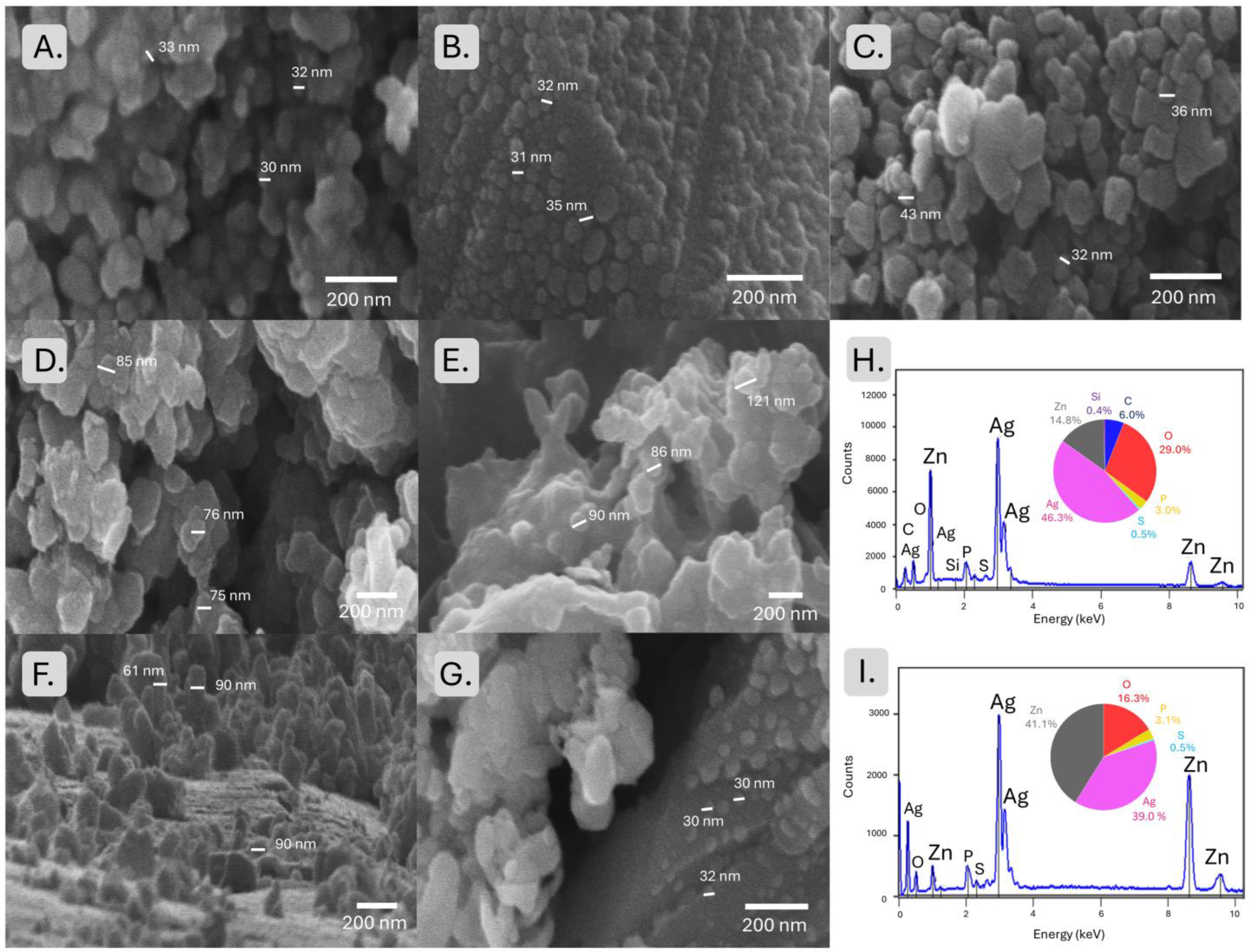
| Nanoparticle Type | Crystallite Size (nm) | Phase Composition | Morphology (SEM) |
|---|---|---|---|
| Ag NPs Sigma | 14.4 | Cubic Fm-3m (Ag) | Uniformly dispersed, spherical shape |
| Ag/Ag2O NPs A | 30.7 | Cubic Pn-3m (Ag) and Cubic Pn-3m (Ag2O) | Spherical particles, tend to form clusters |
| Ag/Ag2O NPs P | 24.8 | Cubic Pn-3m (Ag) and Cubic Pn-3m (Ag2O) | Relatively uniform, spherical particles with minimal agglomeration |
| ZnO NPs A | 22.4 | Hexagonal P63mc Wurtzite (ZnO) | Mixture of spherical and rod-like particles, diverse shapes |
| ZnO NPs P | 28.2 | Hexagonal P63mc Wurtzite (ZnO) | Predominantly hexagonal, well dispersed |
| Ag/Ag2O/ZnO NC A | 30.0 | Cubic Pn-3m (Ag), Cubic Pn-3m (Ag2O), and Hexagonal P63mc Wurtzite (ZnO) | Aggregated, heterogeneous structures, larger clusters |
| Ag/Ag2O/ZnO NC P | 31.4 | Cubic Pn-3m (Ag), Cubic Pn-3m (Ag2O), and Hexagonal P63mc Wurtzite (ZnO) | Smaller spherical particles anchored on larger ZnO surfaces |
| NPs and NCs | 12 h | 24 h | 48 h |
|---|---|---|---|
| Ag NPs Sigma | >120 | >120 | >120 |
| Ag/Ag2O NPs A | 0.59 | 0.89 | 0.72 |
| Ag/Ag2O NPs P | 0.75 | 0.88 | 0.92 |
| ZnO NPs A | 21.36 | 11.27 | 8.78 |
| ZnO NPs P | 39.21 | 21.32 | 10.53 |
| Ag/Ag2O/ZnO NC A | 1.72 | 1.27 | 1.49 |
| Ag/Ag2O/ZnO NC P | 1.30 | 1.30 | 1.92 |
| LD50 (μg/mL) of Nanoparticles and Nanocomposites | ||
|---|---|---|
| NPs and NCs | ND | D |
| Ag NPs Sigma | >120 * | >120 * |
| Ag/Ag2O NPs A | 0.89 | 0.81 |
| Ag/Ag2O NPs P | 0.88 | 1.42 |
| ZnO NPs A | 11.27 | >120 * |
| ZnO NPs P | 21.32 | >120 * |
| Ag/Ag2O O/ZnO NC A | 1.27 | 1.37 |
| Ag/Ag2O O/ZnO NC P | 1.30 | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fais, G.; Sidorowicz, A.; Perra, G.; Dessì, D.; Loy, F.; Lai, N.; Follesa, P.; Orrù, R.; Cao, G.; Concas, A. Cytotoxic Effects of ZnO and Ag Nanoparticles Synthesized in Microalgae Extracts on PC12 Cells. Mar. Drugs 2024, 22, 549. https://doi.org/10.3390/md22120549
Fais G, Sidorowicz A, Perra G, Dessì D, Loy F, Lai N, Follesa P, Orrù R, Cao G, Concas A. Cytotoxic Effects of ZnO and Ag Nanoparticles Synthesized in Microalgae Extracts on PC12 Cells. Marine Drugs. 2024; 22(12):549. https://doi.org/10.3390/md22120549
Chicago/Turabian StyleFais, Giacomo, Agnieszka Sidorowicz, Giovanni Perra, Debora Dessì, Francesco Loy, Nicola Lai, Paolo Follesa, Roberto Orrù, Giacomo Cao, and Alessandro Concas. 2024. "Cytotoxic Effects of ZnO and Ag Nanoparticles Synthesized in Microalgae Extracts on PC12 Cells" Marine Drugs 22, no. 12: 549. https://doi.org/10.3390/md22120549
APA StyleFais, G., Sidorowicz, A., Perra, G., Dessì, D., Loy, F., Lai, N., Follesa, P., Orrù, R., Cao, G., & Concas, A. (2024). Cytotoxic Effects of ZnO and Ag Nanoparticles Synthesized in Microalgae Extracts on PC12 Cells. Marine Drugs, 22(12), 549. https://doi.org/10.3390/md22120549








