Isolation and Purification of Chitosan Oligosaccharides (Mw ≤ 1000) and Their Protective Effect on Acute Liver Injury Caused by CCl4
Abstract
1. Introduction
2. Results
2.1. Isolation and Purification of COST
2.1.1. COST Component and DP4-6 COS Content
2.1.2. Gel Chromatographic Separation Yield
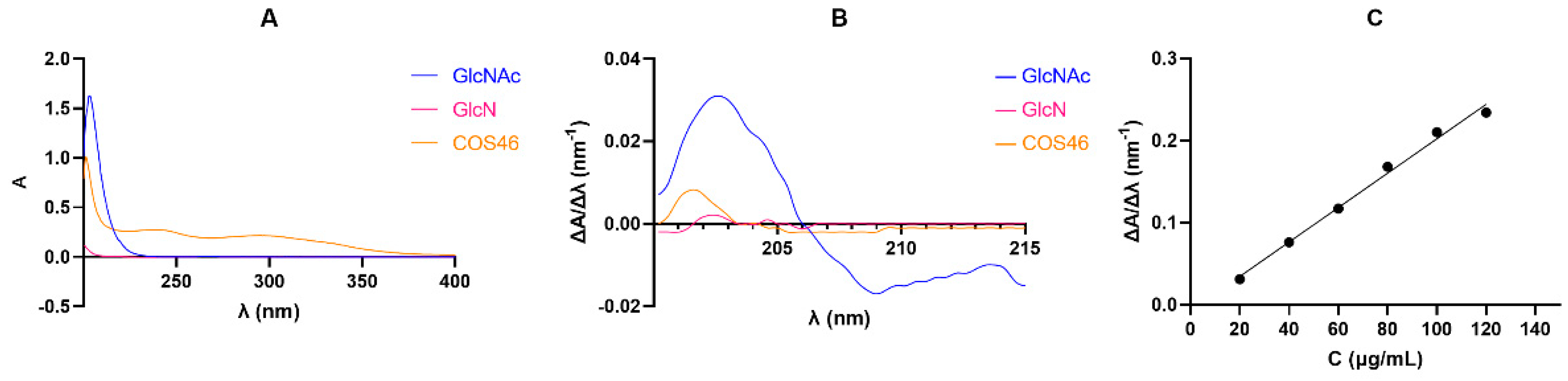
2.1.3. COS46 Purity and Infrared Spectrum
2.1.4. Determination of Deacetylation Degree
2.1.5. Molecular Weight of COS46 and COST
2.2. Oxidation Resistance of COS46 and COST
2.3. Effects of COS46 and COST on Hepatocyte Injury of L02
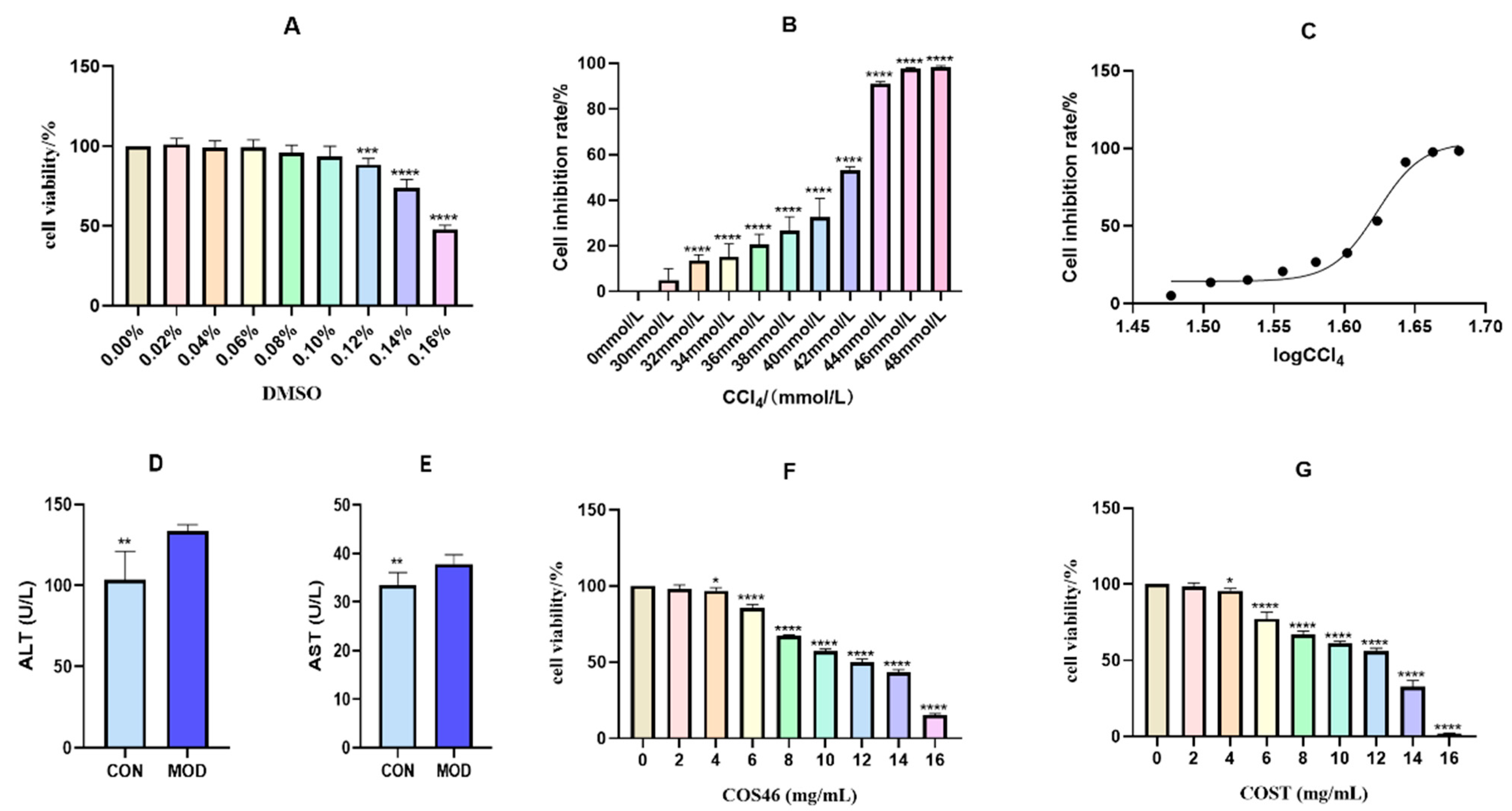
2.3.1. Modeling Conditions of Hepatocyte Injury and Dose Concentration
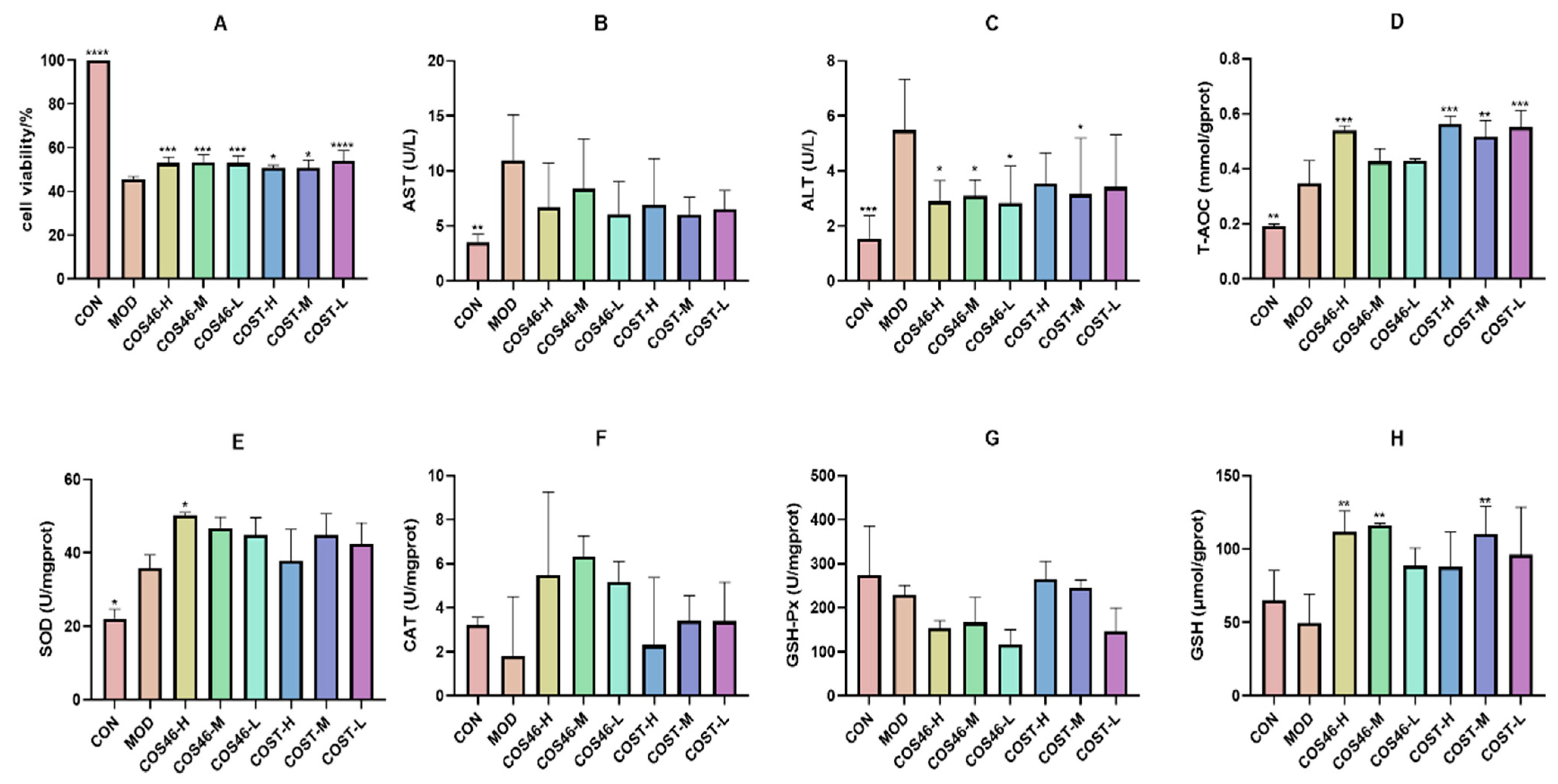
2.3.2. Cell Viability and Biochemical Indices
2.4. Effects of COS46 and COST on Mice with Liver Injury
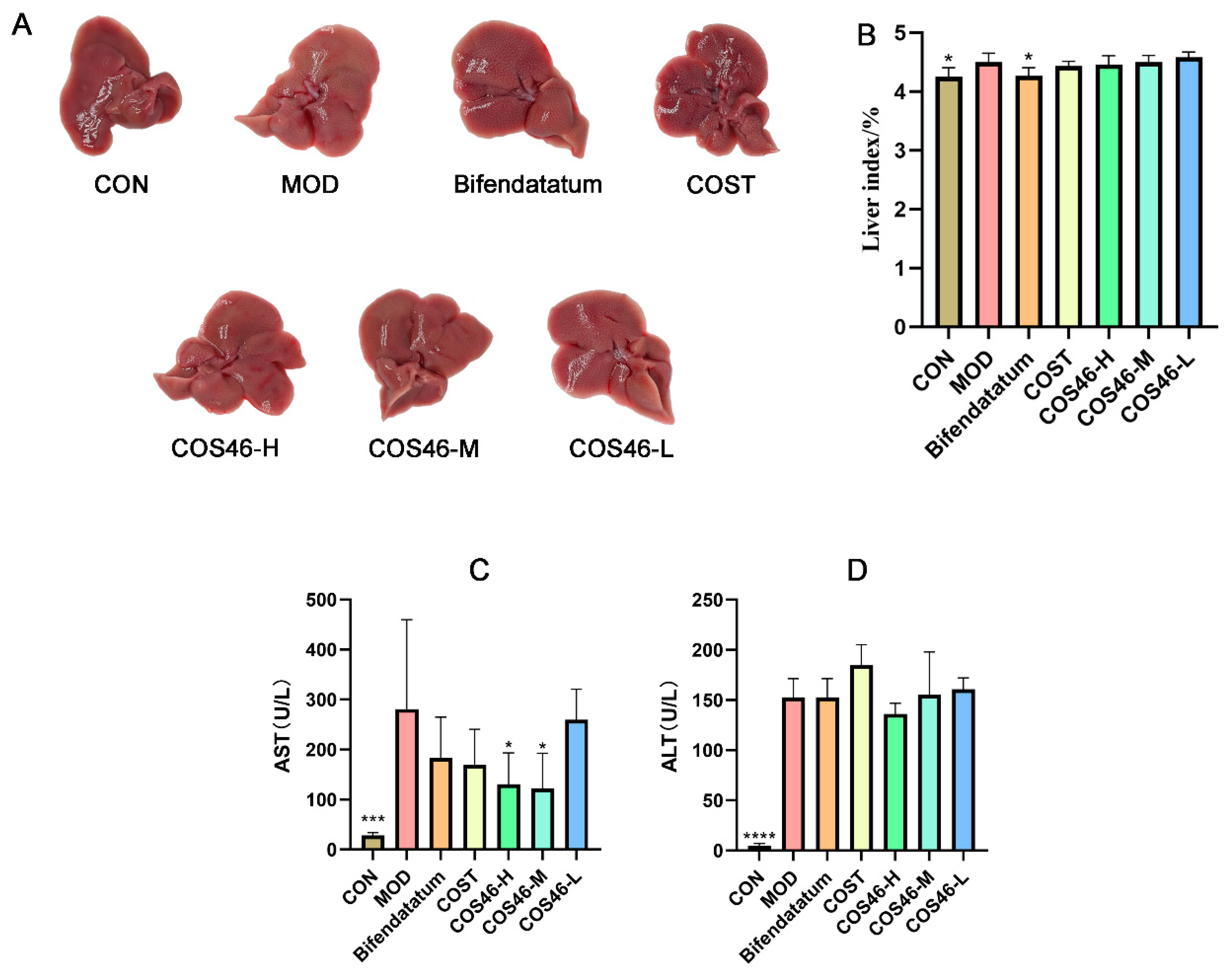
2.4.1. Effects of COS46 and COST on Liver Appearance, Liver Index, and Serum AST and ALT
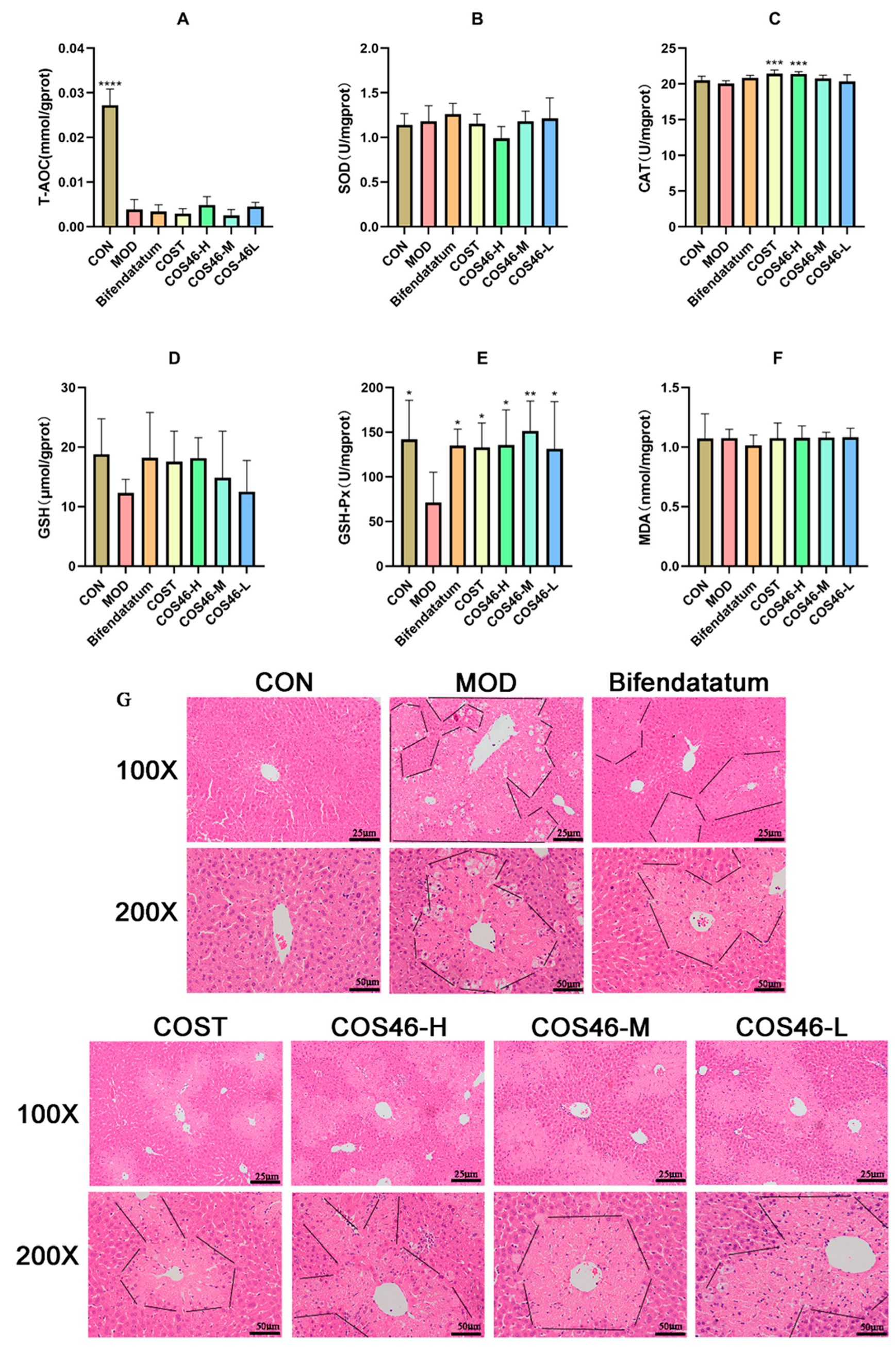
2.4.2. Mouse Liver Biochemical Indexes and HE Staining
3. Materials and Methods
3.1. Materials
3.2. COST Component Analysis and Purification of COS46
3.2.1. COST Component Analysis and DP4-6 Component Content Determination
3.2.2. Isolation and Identification of COS46
3.2.3. Determination of Deacetylation Degree
3.2.4. Determination of Number Average Molecular Weight of COST and COS46
3.3. Determination of Oxidation Resistance of COS46 and COST
3.3.1. Determination of Hydroxyl Radical Scavenging Ability
3.3.2. Determination of DPPH Free Radical Scavenging Ability
3.4. Cell Experiment
3.4.1. L02 Cell Culture
3.4.2. Establishment of Liver Injury Model Induced by CCl4 (Determination of DMSO and CCl4 Concentrations)
3.4.3. The Concentrations of COS46 and COST
3.4.4. Effects of COS46 and COST on Survival Rate of L02 Cells Treated with CCl4
3.4.5. Culture Medium and Antioxidant and Oxidation Indices Detection
3.5. Animal Experimental
3.5.1. Animal
3.5.2. Administration and Establishment of Acute Liver Injury Model
3.6. Statistical Analysis
4. Discussions and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COS | chitosan oligosaccharides |
| COST | chitosan oligosaccharides (Mw ≤ 1000) |
| COS46 | chitosan oligosaccharides with degree of 4–6 polymerization |
| COS4 | chitotetraose |
| COS5 | chitopentaose |
| COS6 | chitohexaose |
| DD | degree of deacetylation |
| DP | degree of polymerization |
| GlcNAc | acetylglucosamine |
| GlcN | aminoglucose |
References
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-P.; Liu, J.; Sui, Z.-J.; Xu, M.-J.; Zhu, Z.-Y. Preparation and antibacterial effect of chitooligosaccharides monomers with different polymerization degrees from crab shell chitosan by enzymatic hydrolysis. Biotechnol. Appl. Biochem. 2023, 70, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wei, X.Y.; Xie, Y.Y.; Zhou, T. A novel chitosan oligosaccharide derivative: Synthesis, antioxidant and antibacterial properties. Carbohydr. Polym. 2022, 291, 119608. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yuan, S.; Yang, X.; Guo, Y.; Li, L.; Xu, C.; Zhai, X.; Wang, J. Structural characterization and antitumor effects of chitosan oligosaccharides against orthotopic liver tumor via NF-κB signaling pathway. J. Funct. Foods 2019, 57, 157–165. [Google Scholar] [CrossRef]
- Zhai, X.; Li, C.; Ren, D.; Wang, J.; Ma, C.; Abd El-Aty, A.M. The impact of chitooligosaccharides and their derivatives on the in vitro and in vivo antitumor activity: A comprehensive review. Carbohydr. Polym. 2021, 266, 118132. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Tan, W.; Li, Q.; Guo, Z.; Zhang, J. Preparation and antioxidant activity of novel chitosan oligosaccharide quinolinyl urea derivatives. Carbohydr. Res. 2022, 521, 108678. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Liu, Y.; Gong, T.; Hou, M. The Anti-inflammatory Effect of Chitosan Oligosaccharide on Heart Failure in Mice. Biomed. Res. Int. 2022, 2022, 8746530. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Zhuravleva, I.L.; Bezrodnykh, E.A.; Berezin, B.B.; Kulikov, S.N.; Tikhonov, V.E. Complexation of oligochitosan with sodium caseinate in alkalescent and weakly acidic media. Carbohydr. Polym. 2023, 302, 120391. [Google Scholar] [CrossRef]
- Jing, W.; Chen, C.; Wang, G.; Han, M.; Chen, S.; Jiang, X.; Shi, C.; Sun, P.; Yang, Z.; Shi, B.; et al. Metabolic Modulation of Intracellular Ammonia via Intravesical Instillation of Nanoporter-Encased Hydrogel Eradicates Bladder Carcinoma. Adv. Sci. 2023, 10, e2206893. [Google Scholar] [CrossRef]
- Na, K.; Wei, J.; Zhang, L.; Fang, Y.; Li, X.; Lu, S.; Guo, X. Effects of chitosan oligosaccharides (COS) and FMT from COS-dosed mice on intestinal barrier function and cell apoptosis. Carbohydr. Polym. 2022, 297, 120043. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhu, L.; Chang, K.; Zhang, R.; Chen, Y.; Yin, H.; Jin, J.; Zhao, L. Chitooligosaccahrides: Digestion characterization and effect of the degree of polymerization on gut microorganisms to manage the metabolome functional diversity in vitro. Carbohydr. Polym. 2022, 275, 118716. [Google Scholar] [CrossRef]
- Tzeng, H.P.; Liu, S.H.; Chiang, M.T. Antidiabetic Properties of Chitosan and Its Derivatives. Mar. Drugs 2022, 20, 784. [Google Scholar] [CrossRef]
- He, N.; Wang, S.; Lv, Z.; Zhao, W.; Li, S. Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct. 2020, 11, 9947–9959. [Google Scholar] [CrossRef]
- Karadeniz, F. In Vitro Anti-HIV-1 Activity of Chitosan Oligomers N-Conjugated with Asparagine and Glutamine. BioTech 2023, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.Q.; Zhao, S.; Li, S.D.; Song, C. Application of Chitosan, Chitooligosaccharide, and Their Derivatives in the Treatment of Alzheimer’s Disease. Mar. Drugs 2017, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar] [CrossRef]
- Tao, W.; Wang, G.; Pei, X.; Sun, W.; Wang, M. Chitosan Oligosaccharide Attenuates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction through Suppressing the Inflammatory Response and Oxidative Stress in Mice. Antioxidants 2022, 11, 1384. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, X.; Cui, J.; Mi, Y.; Zhang, J.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives. Mar. Drugs 2022, 20, 489. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Amiri, H.; Moosavi Basri, S.M.; Rastegari, H.; Lam, S.S.; Pan, J.; Gupta, V.K.; Tabatabaei, M. Tuning chitosan’s chemical structure for enhanced biological functions. Trends Biotechnol. 2022, 41, 785–797. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Xing, R.; Qin, Y.; Li, P. Preparation, characterization and antioxidant activity of two partially N-acetylated chitotrioses. Carbohydr. Polym. 2013, 92, 1730–1736. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and its derivatives: Preparation and biological a pplications. BioMed. Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- Bonin, M.; Sreekumar, S.; Cord-Landwehr, S.; Moerschbacher, B.M. Preparation of Defined Chitosan Oligosaccharides Using Chitin Deacetyl ases. Int. J. Mol. Sci. 2020, 21, 7835. [Google Scholar] [CrossRef]
- Xiong, C.; Wu, H.; Wei, P.; Pan, M.; Tuo, Y.; Kusakabe, I.; Du, Y. Potent angiogenic inhibition effects of deacetylated chitohexaose sepa rated from chitooligosaccharides and its mechanism of action in vitro. Carbohydr. Res. 2009, 344, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Sørbotten, A.; Horn, S.J.; Eijsink, V.G.H.; Vårum, K.M. Degradation of chitosans with chitinase B from Serratia marcescens. Pr oduction of chito-oligosaccharides and insight into enzyme processivit y. FEBS J. 2005, 272, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, Q.L.; Chen, Z.X.; Wu, W.; Xiao, H.X. Preparation of chitosan oligomers COS and their effect on the retrogra dation of intermediate amylose rice starch. J. Food Sci. Technol. 2012, 49, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, Y.; Zhao, L.; Zhou, J.; Xia, Q.; Jiang, L.; Fan, L. Purification of DP 6 to 8 chitooligosaccharides by nanofiltration from the prepared chitooligosaccharides syrup. Bioresour. Bioprocess. 2014, 1, 20. [Google Scholar] [CrossRef][Green Version]
- Dong, X.; Shen, A.; Gou, Z.; Chen, D.; Liang, X. Hydrophilic interaction/weak cation-exchange mixed-mode chromatography for chitooligosaccharides separation. Carbohydr. Res. 2012, 361, 195–199. [Google Scholar] [CrossRef]
- Le Dévédec, F.; Bazinet, L.; Furtos, A.; Venne, K.; Brunet, S.; Mateescu, M.A. Separation of chitosan oligomers by immobilized metal affinity chromat ography. J. Chromatogr. A 2008, 1194, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, L.; Qu, Y.; Lu, J.; Xia, W. Chitosan oligosaccharides exert neuroprotective effects via mod ulating the PI3K/Akt/Bcl-2 pathway in a Parkinsonian model. Food Funct. 2022, 13, 5838–5853. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wu, J.; Li, X.; Zheng, L.; Wu, M.; Liu, P.; Huang, Q. Validated HPAEC-PAD Method for the Determination of Fully Deacetylated Chitooligosaccharides. Int. J. Mol. Sci. 2016, 17, 1699. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Guo, Z.; Wang, C.; Yang, Y.; Liang, X.; Ding, F. Neural activity analysis of pure chito-oligomer components separated f rom a mixture of chitooligosaccharides. Neurosci. Lett. 2014, 581, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Y.; Zhao, Y.; Zhang, H.-D.; Wang, W.-X.; Cong, H.-H.; Yin, H. Characterization of the Specific Mode of Action of a Chitin Deacetylas e and Separation of the Partially Acetylated Chitosan Oligosaccharides. Mar. Drugs 2019, 17, 74. [Google Scholar] [CrossRef]
- Hattori, T.; Anraku, N.; Kato, R. Capillary electrophoresis of chitooligosaccharides in acidic solution: Simple determination using a quaternary-ammonium-modified column and indirect photometric detection with crystal violet. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 878, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.-E.; Gauthier, J.; Boucher, I.; Waldron, K.C. Capillary electrophoresis separation of a mixture of chitin and chitos an oligosaccharides derivatized using a modified fluorophore conjugati on procedure. J. Sep. Sci. 2005, 28, 1390–1398. [Google Scholar] [CrossRef]
- Boll, M.; Lutz, W.D.; Becker, E.; Stampfl, A. Mechanism of Carbon Tetrachloride-Induced Hepatotoxicity. Hepatocellular Damage by Reactive Carbon Tetrachloride Metabolites. Z. Für Naturforschung C 2001, 56, 649–659. [Google Scholar] [CrossRef]
- Sun, J.; Wen, X.; Liu, J.; Kan, J.; Qian, C.; Wu, C.; Jin, C. Protective effect of an arabinogalactan from black soybean against carbon tetrachloride-induced acute liver injury in mice. Int. J. Biol. Macromol. 2018, 117, 659–664. [Google Scholar] [CrossRef]
- Li, S.; Zhou, J.; Xu, S.; Li, J.; Liu, J.; Lu, Y.; Shi, J.; Zhou, S.; Wu, Q. Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury. Biomed. Pharmacother. 2019, 117, 109073. [Google Scholar] [CrossRef]
- Unsal, V.; Cicek, M.; Sabancilar, I. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev. Environ. Health 2021, 36, 279–295. [Google Scholar] [CrossRef]
- He, S.; Zhao, W.; Chen, X.; Li, J.; Zhang, L.; Jin, H. Ameliorative Effects of Peptide Phe-Leu-Ala-Pro on Acute Liver and Kidney Injury Caused by CCl4 via Attenuation of Oxidative Stress and Inflammation. ACS Omega 2022, 7, 44796–44803. [Google Scholar] [CrossRef]
- Ismail, A.F.; Salem, A.A.; Eassawy, M.M. Modulation of gamma-irradiation and carbon tetrachloride induced oxidative stress in the brain of female rats by flaxseed oil. J. Photochem. Photobiol. B 2016, 161, 91–99. [Google Scholar] [CrossRef]
- Mazani, M.; Ojarudi, M.; Banaei, S.; Salimnejad, R.; Latifi, M.; Azizi, H.; Rezagholizadeh, L. The protective effect of cinnamon and ginger hydro-alcoholic extract on carbon tetrachloride-induced testicular damage in rats. Andrologia 2020, 52, e13651. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, C.; Wu, S.; Liu, G.; Guo, J.; Su, Z. Determination of the Deacetylation Degree of Chitooligosaccharides. Mar. Drugs 2017, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, K.; Li, P. Review: Advances in preparation of chitooligosaccharides with heteroge neous sequences and their bioactivity. Carbohydr. Polym. 2021, 252, 117206. [Google Scholar] [CrossRef]
- Hao, W.; Li, K.; Ma, Y.; Li, R.; Xing, R.; Yu, H.; Li, P. Preparation and Antioxidant Activity of Chitosan Dimers with Different Sequences. Mar. Drugs 2021, 19, 366. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Z.; Bao, L.; Wen, F.; Yang, H. The preparation and antioxidant activities of four 2-aminoacyl-chitooligosaccharides. Carbohydr. Res. 2022, 521, 108667. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, J.; Xie, H.; Liu, Y.; Bai, Y.; Che, Q.; Cao, H.; Huang, G.; Guo, J.; Su, Z. Protective effect and mechanism of chitooligosaccharides on acetaminop hen-induced liver injury. Food Funct. 2021, 12, 9979–9993. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, H.; Xu, H.; Gong, J.; Jiang, M.; Qian, J.; Xu, Z.; Shi, J. Chitooligosaccharides Attenuated Hepatic Encephalopathy in Mice throug h Stabilizing Gut-Liver-Brain Disturbance. Mol. Nutr. Food Res. 2023, 67, e2200158. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, H.; Gong, J.; Geng, Y.; Jiang, M.; Xu, H.; Xu, Z.; Shi, J. Chitooligosaccharides alleviate hepatic fibrosis by regulating the pol arization of M1 and M2 macrophages. Food Funct. 2022, 13, 753–768. [Google Scholar] [CrossRef]


| MassCOST (g) | MassCOS46 (g) | Yield (%) | Average Yield (%) |
|---|---|---|---|
| 0.3059 | 0.0891 | 29.13 | 30.39 ± 1.57 |
| 0.3343 | 0.1002 | 29.97 | |
| 0.3290 | 0.1055 | 28.72 | |
| 0.3289 | 0.1055 | 32.08 | |
| 0.3228 | 0.1035 | 32.06 |
| Sample | Concentration cos46 (μg/mL) | Deacetylation Degree (DD)% | Average DD (%) |
|---|---|---|---|
| COS46-1 | 705.3 | 97.23 | 97.71 |
| COS46-2 | 705.3 | 97.33 | |
| COS46-3 | 705.3 | 98.58 |
| OD1-COS46 | OD2-COS46 | DP | Average Molecular Weight | ||
|---|---|---|---|---|---|
| Absorbance | Average | Absorbance | Average | ||
| 0.2706 | 0.2711 | 0.1021 | 0.1028 | 3.79 | 628.50 |
| 0.2676 | 0.1040 | ||||
| 0.2752 | 0.1023 | ||||
| OD1-COST | OD2-COST | DP | Average Molecular Weight | ||
|---|---|---|---|---|---|
| Absorbance | Average | Absorbance | Average | ||
| 0.2342 | 0.2108 | 0.1131 | 0.1135 | 5.38 | 906.12 |
| 0.2536 | 0.1175 | ||||
| 0.1445 | 0.1098 | ||||
| Item | Condition | |
|---|---|---|
| LC | Column | GL Sciences Inertsil NH2 3.0 mm × 150 mm, 5 µm |
| T | 35 °C | |
| μ | 0.6 mL/min | |
| Mobile phase | CH₃CN:0.3%NH3∙H2O = 65:35 | |
| MS | Spray Voltage | 3200 V |
| Capillary Temperature | 270.00 °C | |
| Sheath Gas | 40.00 Arb | |
| Aux Gas | 8.00 Arb | |
| Max Spray Current | 100.00 µA | |
| Probe Heater Temp. | 250.00 °C | |
| Ion Source | ESI + ms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Yu, D.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. Isolation and Purification of Chitosan Oligosaccharides (Mw ≤ 1000) and Their Protective Effect on Acute Liver Injury Caused by CCl4. Mar. Drugs 2024, 22, 128. https://doi.org/10.3390/md22030128
Wang K, Yu D, Bai Y, Cao H, Guo J, Su Z. Isolation and Purification of Chitosan Oligosaccharides (Mw ≤ 1000) and Their Protective Effect on Acute Liver Injury Caused by CCl4. Marine Drugs. 2024; 22(3):128. https://doi.org/10.3390/md22030128
Chicago/Turabian StyleWang, Kai, Dawei Yu, Yan Bai, Hua Cao, Jiao Guo, and Zhengquan Su. 2024. "Isolation and Purification of Chitosan Oligosaccharides (Mw ≤ 1000) and Their Protective Effect on Acute Liver Injury Caused by CCl4" Marine Drugs 22, no. 3: 128. https://doi.org/10.3390/md22030128
APA StyleWang, K., Yu, D., Bai, Y., Cao, H., Guo, J., & Su, Z. (2024). Isolation and Purification of Chitosan Oligosaccharides (Mw ≤ 1000) and Their Protective Effect on Acute Liver Injury Caused by CCl4. Marine Drugs, 22(3), 128. https://doi.org/10.3390/md22030128








