Phenolic Acid Functional Quaternized Chitooligosaccharide Derivatives: Preparation, Characterization, Antioxidant, Antibacterial, and Antifungal Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis and Characterization
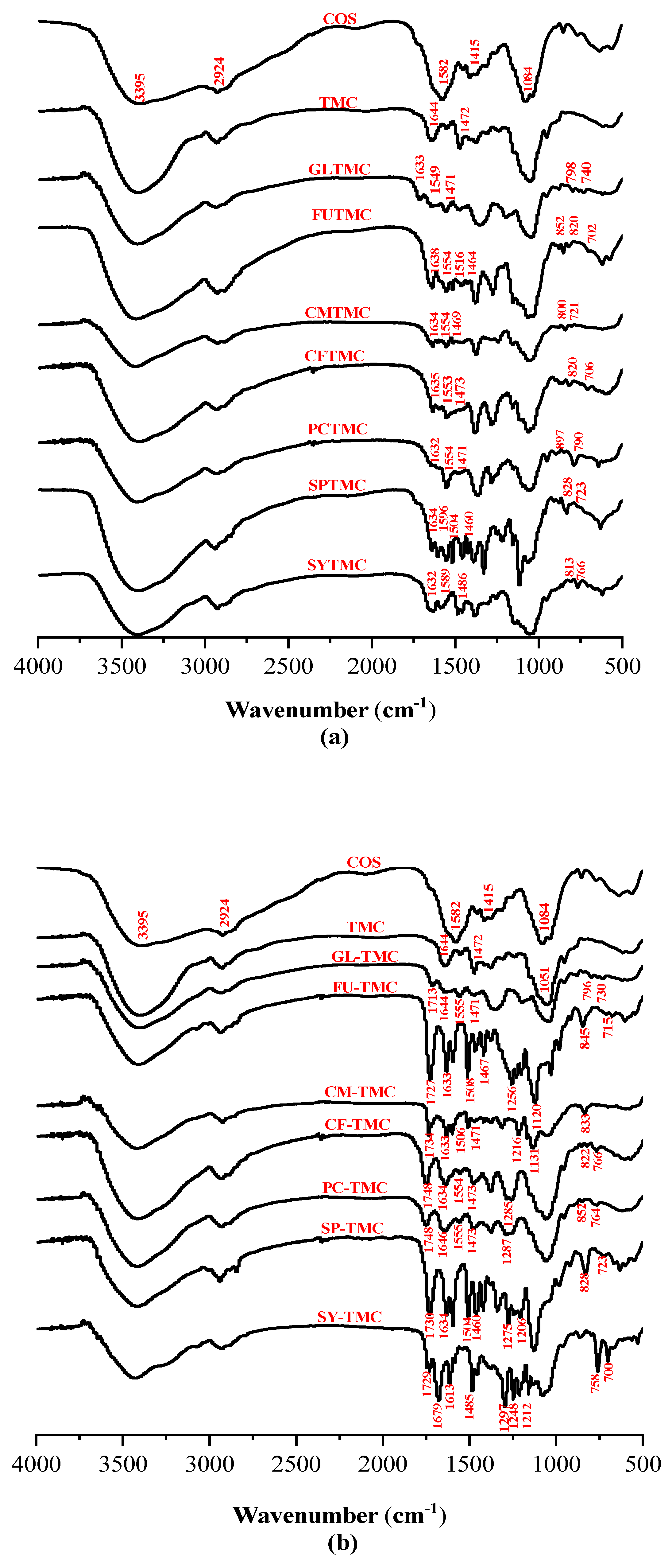
2.2. FT-IR Spectra
2.3. 1H NMR Spectra
2.4. Yields and the DS of Chitosan Derivatives
2.5. Thermal Gravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
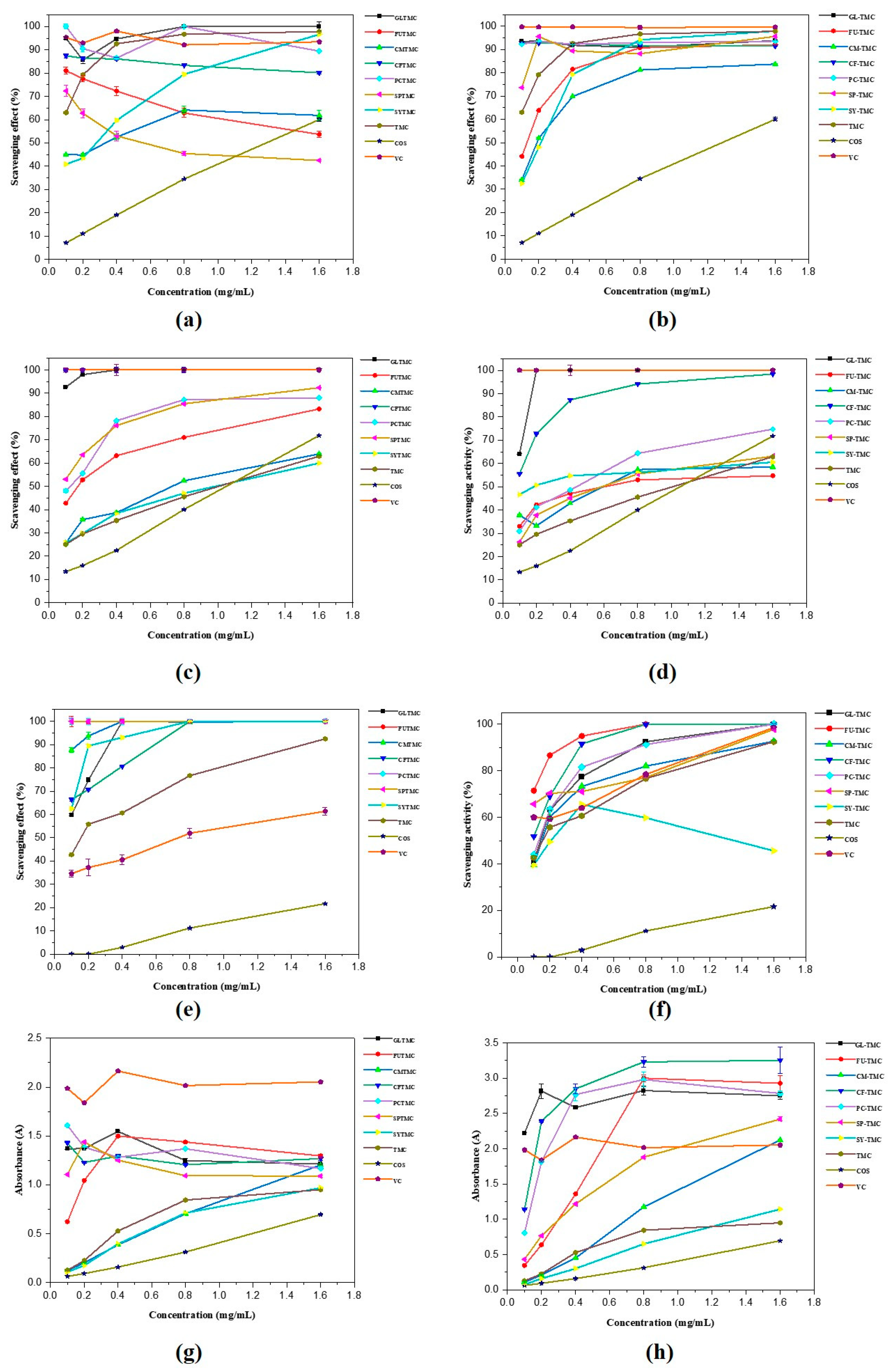
2.6. Antioxidant Activity
2.6.1. Scavenging Ability of the DPPH Radical
2.6.2. Scavenging Ability of Superoxide Radical
2.6.3. Scavenging Ability of Hydroxyl Radical
2.6.4. Reducing Power
2.7. Antibacterial Activity
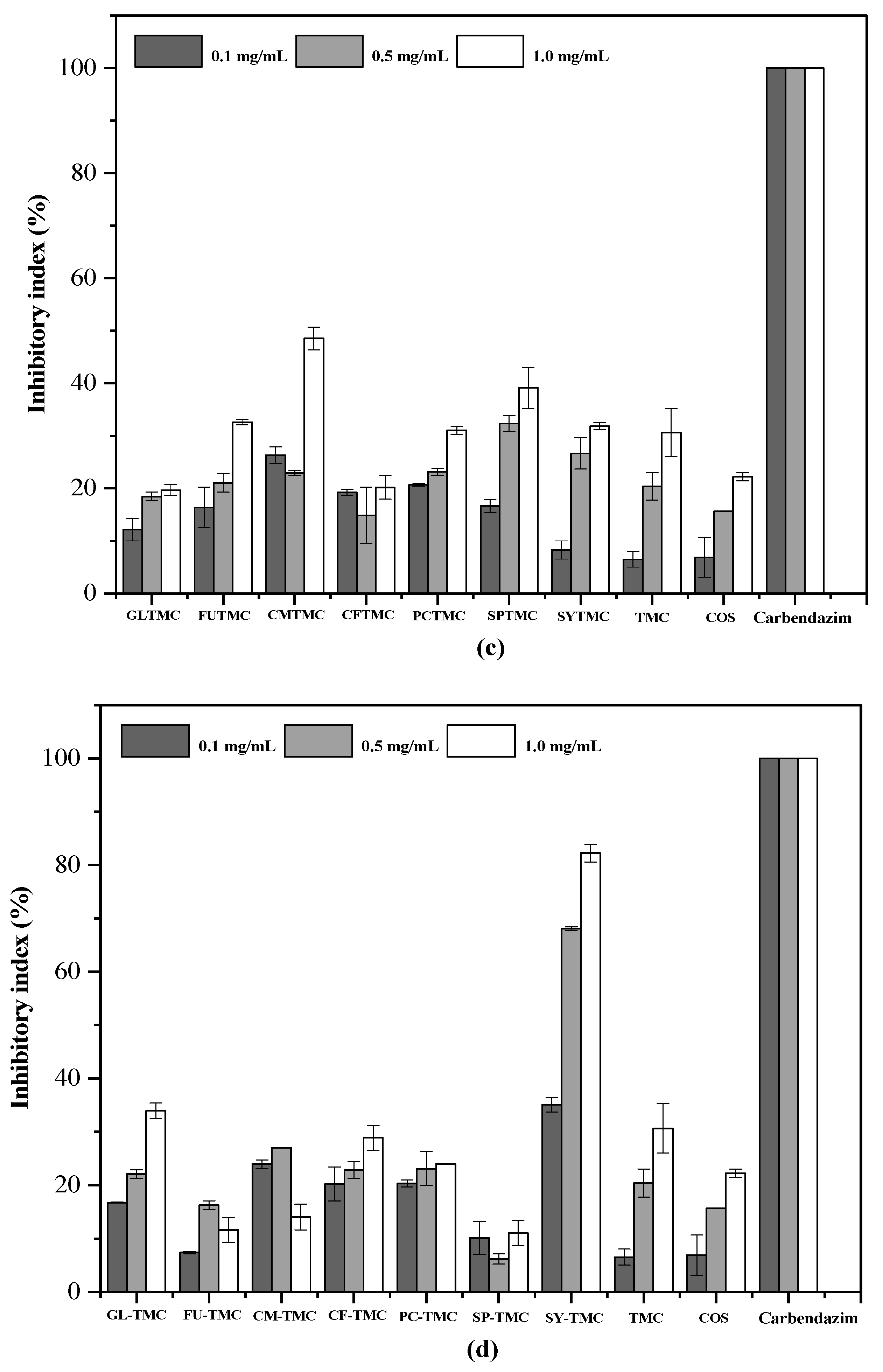
2.8. Antifungal Analysis
2.9. Cytotoxicity Analysis
3. Materials and Methods
3.1. Materials
3.2. Chemical Synthesis of COS Derivatives
3.2.1. Synthesis of N,N,N-Trimethylated Chitooligosaccharide
3.2.2. Synthesis of COS Derivatives—TMC Phenolic Acid Salts
3.2.3. Synthesis of COS Derivatives—Phenolic Acid Esterified TMC
3.3. Analytical Methods
3.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
3.3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.3.3. Yields and Degree of Substitution (DS) Analysis
3.3.4. Thermal Stability
3.4. Antioxidant Activity Assay
3.4.1. DPPH Radical Scavenging Activity Assay
3.4.2. Superoxide Radical Scavenging Activity Assay
3.4.3. Hydroxyl Radical Scavenging Activity Assay
3.4.4. Reducing Power Assay
3.5. Evaluation of Antibacterial Activity
3.5.1. Preparation of Solutions
3.5.2. Minimum Inhibitory Concentration and Minimum Biocidal Concentration
3.6. Antifungal Assay
3.6.1. Preparation of Solutions
3.6.2. Implementation of the Antifungal Experiment
3.7. Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, C.; Ren, D.; Wang, J.; Ma, C.; Abd El-Aty, A.M. The impact of chitooligosaccharides and their derivatives on the in vitro and in vivo antitumor activity: A comprehensive review. Carbohydr. Polym. 2021, 266, 118132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, W.; Jiang, Q.; Yu, P.; Yue, L. Chitosan oligosaccharide-N-chlorokojic acid mannich base polymer as a potential antibacterial material. Carbohydr. Polym. 2018, 182, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Ko, S.-C.; Oh, G.-W.; Jang, Y.-M.; Kim, Y.-M.; Park, W.S.; Choi, I.-W.; Jung, W.-K. Characterization and biological activity of PVA hydrogel containing chitooligosaccharides conjugated with gallic acid. Carbohydr. Polym. 2018, 198, 197–205. [Google Scholar] [CrossRef]
- Oh, G.-W.; Kim, S.-C.; Kim, T.-H.; Jung, W.-K. Characterization of an oxidized alginate-gelatin hydrogel incorporating a COS-salicylic acid conjugate for wound healing. Carbohydr. Polym. 2021, 252, 117145. [Google Scholar] [CrossRef] [PubMed]
- Karagozlu, M.Z.; Karadeniz, F.; Kim, S.-K. Anti-HIV activities of novel synthetic peptide conjugated chitosan oligomers. Int. J. Biol. Macromol. 2014, 66, 260–266. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, X.; Jiang, Z. Research Progress in Functions of Bioactive Chitooligosaccharides in Prevention and Treatment of Alzheimers Disease. Food Sci. 2013, 34, 316–320. [Google Scholar]
- Liang, S.; Sun, Y.; Dai, X. A Review of the Preparation, Analysis and Biological Functions of Chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197. [Google Scholar] [CrossRef]
- Je, J.-Y.; Kim, S.-K. Chitooligosaccharides as potential nutraceuticals: Production and bioactivities. Adv. Food Nutr. Res. 2012, 65, 321–336. [Google Scholar]
- Boz, H. Ferulic Acid in Cereals—A Review. Czech J. Food Sci. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Bialecka-Florjanczyk, E.; Fabiszewska, A.; Zieniuk, B. Phenolic Acids Derivatives—Biotechnological Methods of Synthesis and Bioactivity. Curr. Pharm. Biotechnol. 2018, 19, 1098–1113. [Google Scholar] [CrossRef]
- Insaward, A.; Duangmal, K.; Mahawanich, T. Mechanical, Optical, and Barrier Properties of Soy Protein Film As Affected by Phenolic Acid Addition. J. Agric. Food Chem. 2015, 63, 9421–9426. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Lluis Torres, J. Analysis of Nonextractable Phenolic Compounds in Foods: The Current State of the Art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.; Fernando, W.M.A.D.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health—A review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef]
- Eom, T.-K.; Senevirathne, M.; Kim, S.-K. Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ. Toxicol. Pharmacol. 2012, 34, 519–527. [Google Scholar] [CrossRef]
- Vo, T.-S.; Ngo, D.-H.; Kim, S.-K. Gallic acid-grafted chitooligosaccharides suppress antigen-induced allergic reactions in RBL-2H3 mast cells. Eur. J. Pharm. Sci. 2012, 47, 527–533. [Google Scholar] [CrossRef]
- Chen, D.; Bai, R.; Yong, H.; Zong, S.; Jin, C.; Liu, J. Improving the digestive stability and prebiotic effect of carboxymethyl chitosan by grafting with gallic acid: In vitro gastrointestinal digestion and colonic fermentation evaluation. Int. J. Biol. Macromol. 2022, 214, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, X.; Cui, J.; Mi, Y.; Zhang, J.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives. Mar. Drugs 2022, 20, 489. [Google Scholar] [CrossRef]
- Dai-Nghiep, N.; Kim, M.-M.; Kim, S.-K. Protective effects of aminoethyl-chitooligosaccharides against oxidative stress in mouse macrophage RAW 264.7 cells. Int. J. Biol. Macromol. 2012, 50, 624–631. [Google Scholar]
- Mi, Y.; Tan, W.; Zhang, J.; Guo, Z. Modification of Hydroxypropyltrimethyl Ammonium Chitosan with Organic Acid: Synthesis, Characterization, and Antioxidant Activity. Polymers 2020, 12, 2460. [Google Scholar] [CrossRef]
- Xing, R.; Xu, C.; Gao, K.; Yang, H.; Liu, Y.; Fan, Z.; Liu, S.; Qin, Y.; Yu, H.; Li, P. Characterization of Different Salt Forms of Chitooligosaccharides and Their Effects on Nitric Oxide Secretion by Macrophages. Molecules 2021, 26, 2563. [Google Scholar] [CrossRef]
- Belalia, R.; Grelier, S.; Benaissa, M.; Coma, V. New bioactive biomaterials based on quaternized chitosan. J. Agric. Food Chem. 2008, 56, 1582–1588. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Luan, F.; Yin, X.; Dong, F.; Li, Q.; Guo, Z. Synthesis of Quaternary Ammonium Salts of Chitosan Bearing Halogenated Acetate for Antifungal and Antibacterial Activities. Polymers 2018, 10, 530. [Google Scholar] [CrossRef]
- Chikkulapalli, A.; Aavula, S.K.; Mona, R.N.P.; Karthikeyan, C.; Kumar, V.C.H.; Sulur, M.G.; Sumathi, S. Convenient N-acetylation of amines in N,N-dimethylacetamide with N,N-carbonyldiimidazole. Tetrahedron Lett. 2015, 56, 3799–3803. [Google Scholar] [CrossRef]
- Engstrom, K.M. Practical Considerations for the Formation of Acyl Imidazolides from Carboxylic Acids and N,N′-Carbonyldiimidazole: The Role of Acid Catalysis. Org. Process Res. Dev. 2018, 22, 1294–1297. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Gao, Y.; Gao, W.; Hou, Z.; Zhu, Y. Facile Method for Surface-Grafted Chitooligosaccharide on Medical Segmented Poly(ester-urethane) Film to Improve Surface Biocompatibility. Membranes 2021, 11, 37. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wang, G.; Yin, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of N,N,N-trimethyl chitosan salts. Int. J. Biol. Macromol. 2018, 118, 9–14. [Google Scholar] [CrossRef]
- Witt, J.D.; Gabriel, M.K.; Julian, R.L. Gc-Ft-Ir Analysis Of Commercial Divinyl Benzene. J. Chromatogr. Sci. 1979, 17, 445–448. [Google Scholar] [CrossRef]
- Sedman, J.; Butler, I.S. Polarized Ft-Ir Spectra of Benzene and Anthracene Oriented in A Nematic Liquid-Crystal Solvent. Appl. Spectrosc. 1985, 39, 621–625. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Zhang, J.; Gu, G.; Guo, Z. Significantly enhanced antioxidant activity of chitosan through chemical modification with coumarins. Polym. Chem. 2019, 10, 1480–1488. [Google Scholar] [CrossRef]
- Wang, Y.; Cen, C.; Chen, J.; Fu, L. MgO/carboxymethyl chitosan nanocomposite improves thermal stability, waterproof and antibacterial performance for food packaging. Carbohydr. Polym. 2020, 236, 116078. [Google Scholar] [CrossRef]
- Cui, J.; Sun, Y.; Wang, L.; Miao, Q.; Tan, W.; Guo, Z. Preparation of l-Arginine Schiff Bases Modified Chitosan Derivatives and Their Antimicrobial and Antioxidant Properties. Mar. Drugs 2022, 20, 688. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. -Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Masumizu, T.; Maitani, Y.; Nagai, T. Evaluation of superoxide anion radical scavenging activity of shikonin by electron spin resonance. Int. J. Pharm. 1998, 174, 133–139. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yao, Q.; Zhou, D.; Mao, F. Antioxidant activity of N-carboxymethyl chitosan oligosaccharides. Bioorganic Med. Chem. Lett. 2008, 18, 5774–5776. [Google Scholar] [CrossRef]
- Singhal, M.; Paul, A.; Singh, H.P. Synthesis and reducing power assay of methyl semicarbazone derivatives. J. Saudi Chem. Soc. 2014, 18, 121–127. [Google Scholar] [CrossRef]
- Yang, X.; Lan, W.; Sun, X. Antibacterial and antioxidant properties of phenolic acid grafted chitosan and its application in food preservation: A review. Food Chem. 2023, 428, 136788. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Yao, Y.; Wang, D.; Kitts, D.D. Prooxidant capacity of phenolic acids defines antioxidant potential. Biochim. Et Biophys. Acta-Gen. Subj. 2023, 1867, 130371. [Google Scholar] [CrossRef]
- Mansouri, M.; Mobarez, A.M.; Nojoomi, F. Comprehensive study of common Enterogenic E. coli in Iran during 2010–2020: A systematic review. Gene Rep. 2021, 25, 101316. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Jin, J.; Li, X.; Zhang, H.; Shi, X.; Zhao, C. Prevalence, antibiotic resistance, and enterotoxin genes of Staphylococcus aureus isolated from milk and dairy products worldwide: A systematic review and meta-analysis. Food Res. Int. 2022, 162, 111969. [Google Scholar] [CrossRef] [PubMed]
- Munhuweyi, K.; Lennox, C.L.; Meitz-Hopkins, J.C.; Caleb, O.J.; Opara, U.L. Major diseases of pomegranate (Punica granatum L.), their causes and management-A review. Sci. Hortic. 2016, 211, 126–139. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wei, L.; Chen, Y.; Mi, Y.; Sun, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis of urea-functionalized chitosan derivatives for potential antifungal and antioxidant applications. Carbohydr. Polym. 2019, 215, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, C.; Tan, W.; Gu, G.; Guo, Z. Novel Amino-Pyridine Functionalized Chitosan Quaternary Ammonium Derivatives: Design, Synthesis, and Antioxidant Activity. Molecules 2017, 22, 156. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Q.; Tan, W.; Dong, F.; Luan, F.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Double Quaternized Chitosan Derivatives. Molecules 2017, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J.; Mi, Y.; Miao, Q.; Tan, W.; Li, Q.; Guo, Z. Synthesis, characterization, and antioxidant activity of carboxymethyl chitosan derivatives containing sulfonium salt. J. Oceanol. Limnol. 2022, 40, 284–295. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Li, W.; Dong, F.; Guo, Z. Synthesis and antioxidant property of novel 1,2,3-triazole-linked starch derivatives via ‘click chemistry’. Int. J. Biol. Macromol. 2016, 82, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Masson, M.; Meyer, R.L. Quaternary Ammoniumyl Chitosan Derivatives for Eradication of Staphylococcus aureus Biofilms. Biomacromolecules 2018, 19, 3649–3658. [Google Scholar] [CrossRef]
- Mi, Y.; Li, Q.; Miao, Q.; Tan, W.; Zhang, J.; Guo, Z. Enhanced antifungal and antioxidant activities of new chitosan derivatives modified with Schiff base bearing benzenoid/heterocyclic moieties. Int. J. Biol. Macromol. 2022, 208, 586–595. [Google Scholar] [CrossRef]






| Compounds | Yields (%) | DS (%) | Compounds | Yields (%) | DS (%) |
|---|---|---|---|---|---|
| COS | - | - | TMC | 78.57 | 94.88 |
| GLTMC | 86.47 | 80.00 | GL-TMC | 58.96 | 32.00 |
| FUTMC | 24.20 | 66.00 | FU-TMC | 39.42 | 43.00 |
| CMTMC | 20.40 | 51.00 | CM-TMC | 32.40 | 41.00 |
| CFTMC | 68.97 | 54.00 | CF-TMC | 59.88 | 15.00 |
| PCTMC | 44.49 | 36.00 | PC-TMC | 53.16 | 12.00 |
| SPTMC | 65.42 | 46.00 | SP-TMC | 64.34 | 47.00 |
| SYTMC | 73.60 | 41.00 | SY-TMC | 73.40 | 43.00 |
| Bacterial Species | Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COS | TMC | GLTMC | FUTMC | CMTMC | CFTMC | PCTMC | SPTMC | SYTMC | ||
| S. aureus | MIC (mg/mL) | >8 | >8 | 0.5 | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 | 2 |
| MBC (mg/mL) | >8 | >8 | 1 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 4 | |
| E. coli | MIC (mg/mL) | >8 | >8 | 0.25 | 1 | 0.0625 | 0.5 | 1 | 0.5 | >8 |
| MBC (mg/mL) | >8 | >8 | 0.5 | 2 | 0.125 | 1 | 2 | 1 | >8 | |
| Bacterial Species | Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COS | TMC | GL-TMC | FU-TMC | CM-TMC | CF-TMC | PC-TMC | SP-TMC | SY-TMC | ||
| S. aureus | MIC (mg/mL) | >8 | >8 | 4 | 0.25 | 0.125 | 0.25 | 2 | 1 | 0.25 |
| MBC (mg/mL) | >8 | >8 | 8 | 0.5 | 0.25 | 0.5 | 4 | 2 | 0.5 | |
| E. coli | MIC (mg/mL) | >8 | >8 | 0.125 | 0.125 | 0.03125 | 0.5 | 1 | 0.5 | 0.25 |
| MBC (mg/mL) | >8 | >8 | 0.25 | 0.25 | 0.0625 | 1 | 2 | 1 | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Cui, J.; Tian, L.; Mi, Y.; Guo, Z. Phenolic Acid Functional Quaternized Chitooligosaccharide Derivatives: Preparation, Characterization, Antioxidant, Antibacterial, and Antifungal Activity. Mar. Drugs 2023, 21, 535. https://doi.org/10.3390/md21100535
Sun Y, Cui J, Tian L, Mi Y, Guo Z. Phenolic Acid Functional Quaternized Chitooligosaccharide Derivatives: Preparation, Characterization, Antioxidant, Antibacterial, and Antifungal Activity. Marine Drugs. 2023; 21(10):535. https://doi.org/10.3390/md21100535
Chicago/Turabian StyleSun, Yan, Jingmin Cui, Liguang Tian, Yingqi Mi, and Zhanyong Guo. 2023. "Phenolic Acid Functional Quaternized Chitooligosaccharide Derivatives: Preparation, Characterization, Antioxidant, Antibacterial, and Antifungal Activity" Marine Drugs 21, no. 10: 535. https://doi.org/10.3390/md21100535
APA StyleSun, Y., Cui, J., Tian, L., Mi, Y., & Guo, Z. (2023). Phenolic Acid Functional Quaternized Chitooligosaccharide Derivatives: Preparation, Characterization, Antioxidant, Antibacterial, and Antifungal Activity. Marine Drugs, 21(10), 535. https://doi.org/10.3390/md21100535







