Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties
Abstract
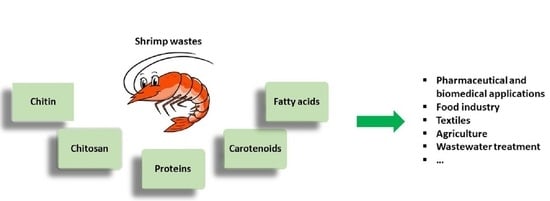
:1. Introduction
2. Composition of Shrimp Shell
3. Polysaccharides
3.1. Chitin
3.2. Chitosan
3.3. Extraction Processes
3.3.1. Chemical Extraction
3.3.2. Green Extraction of Polysaccharides
Biological Extraction (Microbial Fermentation)
Enzyme-Assisted Extraction (EAE)
Microwave-Assisted Extraction (MAE)
Subcritical Water Extraction (SWE)
Ultrasound-Assisted Extraction (UAE)
Chitosan Purification
Energy Consumption and Emission in Green Extraction
Future Directions
3.4. Applications of Polysaccharides
3.4.1. Anticancer Activity
3.4.2. Potential Drug Carrier: Microsphere
3.4.3. Blood-Compatible Membrane
3.4.4. Hemostatic Dressing
3.4.5. Bioimaging
3.4.6. Antioxidant Activity
3.4.7. Antimicrobial Treatment of Textiles
3.4.8. Antimicrobial and Stimulator Agents for Agriculture
3.4.9. Biomaterial for Industrial Packaging
4. Proteins
4.1. Extraction Methods
| Product | Shrimp Waste Sources | Enzyme | Conditions pH–Temperature–Time | DH or DP (%) | Composition of the Free Amino Acids (mg/mL) | Ref. |
|---|---|---|---|---|---|---|
| Flavor protein hydrolysate | Penaeus chinensis | Dispase | 6.5–57 °C–3 h | 57.7 (DH) | Threonine (0.62 mg/mL), valine (1.02 mg/mL), methionine (0.74 mg/mL), lysine (2.58 mg/mL), isoleucine (1.89 mg/mL), leucine (2.76 mg/mL), phenylalanine (1.69 mg/mL), serine (1.74 mg/mL), glutamic acid (2.24 mg/mL), glycine (3.19 mg/mL), histidine (0.39 mg/mL), arginine (4.48 mg/mL), alanine (3.08 mg/mL), proline (0.51 mg/mL), tyrosine (1.85 mg/mL), and aspartic acid (0.89 mg/mL) | [198] |
| Antioxidant protein hydrolysates | Metapenaeus dobsoni | Alcalase | 8.2–45.4 °C–3 h | 42.4 (DH) | - | [199] |
| Antioxidant protein hydrolysates | Shrimp byproducts | Alcalase | 7.2–NA–NA | NA | serine-valine-alanine-methionine-leucine-phenylalanine-histidine (804.4 Da) | [200] |
| Protein hydrolysates | Parapenaeus longirostris | Alcalase | 8–50 °C–30 min | 13 (DH) | Asparagine+aspartic acid (98/1000 PFCP), threonine (52/1000 PFCP), serine (56/1000 PFCP), glutamine+glutamic acid (113/1000 PFCP), glycine (128/1000 PFCP), alanine (96/1000 PFCP), cysteine (9/1000 PFCP), valine (56/1000 PFCP), methionine (27/1000 PFCP), isoleucine (48/1000 PFCP), leucine (34/1000 PFCP), tyrosine (34/1000 PFCP), phenylalanine (41/1000 PFCP), hydroxylysine (6/1000 PFCP), histidine (22/1000 PFCP), lysine (75/1000 PFCP), arginine (53/1000 PFCP), and proline (52/1000 PFCP) | [192] |
| Antioxidant protein hydrolysates | Farfantepenaeus subtilis | Alcalase | 8–55 °C–30–40 min | 3.6 (DH) | - | [117] |
| Antioxidant protein hydrolysates | Penaeus monodon and Penaeus indicus | Alcalase | 8.5–60 °C–90 min | 35 (DH) | - | [118] |
| Antioxidant protein hydrolysates | Shrimp waste | Digestive alkaline proteinases from Liza aurata | 8–45 °C–3 h | 76 (DP) | - | [197] |
| Protein hydrolysates | Litopenaeus vannamei 1 | Acid stable protease | 3.5–40 °C–6 h | 95 (DP) | - | [201] |
| Protein hydrolysate | Penaens vannamei | Endogenous enzyme | 9–50 °C–2 h | 92.1 (DP) 52.8 (DH) | - | [202] |
| Protein hydrolysate | Penaens vannamei | Endogenous enzyme | 8–50 °C–0.4 h | 87.5 (DP) 3 (DH) | Depending on the treatment applied, different proportions of histidine, isoleucine, leucine, lysine, phenylalanine, threonine, valine, alanine, arginine, aspartic acid, glutamic acid, glycine, tyrosine, and proline were found. | [203] |
| Protein hydrolysate | Litopenaeus vannamei 1 | Protease from Streptomyces griseus | 7–37 °C–3 h | 91.1 (DP) | - | [97] |
| Protein hydrolysate | Litopenaeus vannamei 1 | Trypsin or Ficin | 7.7 (trypsin) or 7.5 (Ficin)–RT–2 h | 92 (DP) | - | [113] |
| Carotenoprotein | Litopenaeus vannamei 1 | Albacore tuna spleen trypsin | 9.5–25 °C–45 min | NA | Alanine (38.58 mg/g sample), arginine (43.80 mg/g sample), aspartic acid/asparagine (71.78 mg/g sample), cysteine (0.21 mg/g sample), glutamic acid/glutamine (81.85 mg/g sample), glycine (54.10 mg/g sample), histidine (28.61 mg/g sample), hydroxylysine (0.66 mg/g sample), isoleucine (32.19 mg/g sample), leucine (52.60 mg/g sample), lysine (39.88 mg/g sample), methionine (16.47 mg/g sample), phenylalanine (35.04 mg/g sample), proline (28.32 mg/g sample), serine (32.97 mg/g sample), threonine (31.88 mg/g sample), tryptophan (8.57 mg/g sample), tyrosine (29.04 mg/g sample), and valine (36.18 mg/g sample) | [114] |
| Carotenoprotein | Litopenaeus vannamei 1 | Papain | 6.82–54.41 °C–48.91 min (shrimp shells) 7.22– 46.23 °C–51.17 min (shrimp heads) | 52.45 (shrimp shells; DH) 55.36 (shrimp heads; DH) | - | [115] |
| Protein hydrolysate | Litopenaeus vannamei 1 | Pepsin | 2–40 °C–16 h | 92 (DP) | - | [116] |
4.2. Application of Protein Hydrolysates
5. Lipidic Fraction
5.1. Carotenoids
5.1.1. Conventional Extraction of Astaxanthin
5.1.2. Green Extraction of Astaxanthin
Extraction with Vegetable Oils
Supercritical Fluid Extraction
High Pressure
Ultrasound-Assisted Extraction
Fermentation with Aerobic Bacteria
5.2. Fatty Acids
Extraction of Fatty Acids
5.3. Application of Carotenoids and Fatty Acids
5.3.1. Food Coloring
5.3.2. Antioxidant Activity
5.3.3. Anticancer Activity
5.3.4. Neuroprotection Activity
5.3.5. Anti-Inflammatory Activity
5.3.6. Wound-Healing Activity
5.3.7. Hepatoprotective Activity
5.3.8. Antidiabetic Activity and Cardiovascular Protection
6. Materials and Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jory, D. Annual Farmed Shrimp Production Survey: A Slight Decrease in Production Reduction in 2023 with Hopes for Renewed Growth in 2024; Global Seafood Alliance: Portsmouth, NH, USA, 2023. [Google Scholar]
- IMARC. Shrimp Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020–2025; International Mining and Resources Conference (IMARC): Sydney, Australia, 2020. [Google Scholar]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive Utilization of Shrimp Waste Based on Biotechnological Methods: A Review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.K.; Singh, S.P.; Awasthi, A. Immunostimulants for Shrimp Aquaculture: Paving Pathway towards Shrimp Sustainability. Environ. Sci. Pollut. Res. 2022, 30, 25325–25343. [Google Scholar] [CrossRef] [PubMed]
- Waldhorn, D.R.; Autric, E. Shrimp: The Animals Most Commonly Used and Killed for Food Production; Rethink Priorities: San Francisco, CA, USA, 2022. [Google Scholar]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in Shrimp Processing Waste Utilization: An Industrial Prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Sila, A.; Ghlissi, Z.; Kamoun, Z.; Makni, M.; Nasri, M.; Bougatef, A.; Sahnoun, Z. Astaxanthin from Shrimp By-Products Ameliorates Nephropathy in Diabetic Rats. Eur. J. Nutr. 2015, 54, 301–307. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Noriega, D.; Ramos, P.; Valcarcel, J.; Novoa-Carballal, R.; Pastrana, L.; Reis, R.L.; Pérez-Martín, R.I. Optimization of High Purity Chitin and Chitosan Production from Illex Argentinus Pens by a Combination of Enzymatic and Chemical Processes. Carbohydr. Polym. 2017, 174, 262–272. [Google Scholar] [CrossRef]
- Djellouli, M.; López-Caballero, M.E.; Arancibia, M.Y.; Karam, N.; Martínez-Alvarez, O. Antioxidant and Antimicrobial Enhancement by Reaction of Protein Hydrolysates Derived from Shrimp By-Products with Glucosamine. Waste Biomass Valorization 2020, 11, 2491–2505. [Google Scholar] [CrossRef]
- Leiva-Portilla, D.; Martínez, R.; Bernal, C. Valorization of Shrimp (Heterocarpus reedi) Processing Waste via Enzymatic Hydrolysis: Protein Extractions, Hydrolysates and Antioxidant Peptide Fractions. Biocatal. Agric. Biotechnol. 2023, 48, 102625. [Google Scholar] [CrossRef]
- Paul, T.; Halder, S.K.; Das, A.; Ghosh, K.; Mandal, A.; Payra, P.; Barman, P.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Production of Chitin and Bioactive Materials from Black Tiger Shrimp (Penaeus Monodon) Shell Waste by the Treatment of Bacterial Protease Cocktail. 3 Biotech 2015, 5, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Senphan, T.; Benjakul, S.; Kishimura, H. Characteristics and Antioxidative Activity of Carotenoprotein from Shells of Pacific White Shrimp Extracted Using Hepatopancreas Proteases. Food Biosci. 2014, 5, 54–63. [Google Scholar] [CrossRef]
- Shen, Q.; Song, G.; Wang, H.; Zhang, Y.; Cui, Y.; Xie, H.; Xue, J.; Wang, H. Isolation and Lipidomics Characterization of Fatty Acids and Phospholipids in Shrimp Waste through GC/FID and HILIC-QTrap/MS. J. Food Compos. Anal. 2021, 95, 103668. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Alemán, A.; López-Caballero, M.E.; Baccan, G.C.; Montero, P.; Gómez-Guillén, M.C. Bioaccessibility and Antimicrobial Properties of a Shrimp Demineralization Extract Blended with Chitosan as Wrapping Material in Ready-to-Eat Raw Salmon. Food Chem. 2019, 276, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Gagnon, J.; Pelletier, C.; Tchoukanova, N.; Zhang, J.; Ewart, H.S.; Ewart, K.V.; Jiao, G.; Wang, Y. Shrimp Oil Extracted from the Shrimp Processing Waste Reduces the Development of Insulin Resistance and Metabolic Phenotypes in Diet-Induced Obese Rats. Appl. Physiol. Nutr. Metab. 2017, 42, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Nwanna, L.; Ajenifuja, A.M.; Enujiugha, V. Replacement of Fish Meal with Chemically Preserved Shrimp Head in the Diets of African Catfish, Clarias Gariepinus. J. Food Agric. Environ. 2004, 2, 79–83. [Google Scholar]
- Ritter, Á.M.; Borchardt, M.; Vaccaro, G.L.R.; Pereira, G.M.; Almeida, F. Motivations for Promoting the Consumption of Green Products in an Emerging Country: Exploring Attitudes of Brazilian Consumers. J. Clean. Prod. 2015, 106, 507–520. [Google Scholar] [CrossRef]
- Abuzar; Sharif, H.R.; Sharif, M.K.; Arshad, R.; Rehman, A.; Ashraf, W.; Karim, A.; Awan, K.A.; Raza, H.; Khalid, W.; et al. Potential Industrial and Nutritional Applications of Shrimp By-Products: A Review. Int. J. Food Prop. 2023, 26, 3407–3432. [Google Scholar] [CrossRef]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of Chitin/Chitosan and Their Derivatives in the Papermaking Industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, L.; Yang, G. Chitosan Application in Textile Processing. Curr. Trends Fash. Technol. Text. Eng. 2018, 4, 32–34. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; ul Gani Mir, T.; Rahayu, F.; Suhara, C.; Anjli, A.; Chopra, C.; Singh, R.; Prakash, A.; El Messaoudi, N.; et al. Eco-Friendly and Safe Alternatives for the Valorization of Shrimp Farming Waste. Environ. Sci. Pollut. Res. 2023, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Q.; Zhang, D.; Wei, S.; Sun, Q.; Xia, Q.; Shi, W.; Ji, H.; Liu, S. Comparison of the Proximate Composition and Nutritional Profile of Byproducts and Edible Parts of Five Species of Shrimp. Foods 2021, 10, 2603. [Google Scholar] [CrossRef] [PubMed]
- Iber, B.T.; Kasan, N.A. Recent Advances in Shrimp Aquaculture Wastewater Management. Heliyon 2021, 7, e08283. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.F.V. Arthropod Cuticle: A Natural Composite Shell System. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1311–1315. [Google Scholar] [CrossRef]
- Nakahara, H. Calcification of Gastropod Nacre. In Biomineralization and Biological Metal Accumulation; Westbroek, P., de Jong, E.W., Eds.; Springer: Dordrecht, The Netherlands, 1983; pp. 225–230. ISBN 978-94-009-7946-8. [Google Scholar]
- Kaur, S.; Dhillon, G.S. Recent Trends in Biological Extraction of Chitin from Marine Shell Wastes: A Review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Vani, R.; Stanley, S.A. Studies on the Extraction of Chitin and Chitosan from Different Aquatic Organisms. Adv. Biotech 2013, 12, 12–15. [Google Scholar]
- Sanjanwala, D.; Londhe, V.; Trivedi, R.; Bonde, S.; Sawarkar, S.; Kale, V.; Patravale, V. Polysaccharide-Based Hydrogels for Drug Delivery and Wound Management: A Review. Expert Opin. Drug Deliv. 2022, 19, 1664–1695. [Google Scholar] [CrossRef] [PubMed]
- Vieira, H.; Lestre, G.M.; Solstad, R.G.; Cabral, A.E.; Botelho, A.; Helbig, C.; Coppola, D.; de Pascale, D.; Robbens, J.; Raes, K.; et al. Current and Expected Trends for the Marine Chitin/Chitosan and Collagen Value Chains. Mar. Drugs 2023, 21, 605. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Das Saha, K. Chitosan-Decorated PLGA-NPs Loaded with Tannic Acid/Vitamin E Mitigate Colon Cancer via the NF-ΚB/β-Cat/EMT Pathway. ACS Omega 2021, 6, 28752–28769. [Google Scholar] [CrossRef] [PubMed]
- Dogan, N.O.; Bozuyuk, U.; Erkoc, P.; Karacakol, A.C.; Cingoz, A.; Seker-Polat, F.; Nazeer, M.A.; Sitti, M.; Bagci-Onder, T.; Kizilel, S. Parameters Influencing Gene Delivery Efficiency of PEGylated Chitosan Nanoparticles: Experimental and Modeling Approach. Adv. NanoBiomed Res. 2022, 2, 2100033. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Dave, U.; Somanader, E.; Baharlouei, P.; Pham, L.; Rahman, M.A. Applications of Chitin in Medical, Environmental, and Agricultural Industries. J. Mar. Sci. Eng. 2021, 9, 1173. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds Containing Chitosan, Gelatin and Graphene Oxide for Bone Tissue Regeneration In Vitro and In Vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.-K. In Vivo Study of Chitosan-Natural Nano Hydroxyapatite Scaffolds for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-H.; Chao, H.-M.; Chern, E.; Hsu, S. Chitosan 3D Cell Culture System Promotes Naïve-like Features of Human Induced Pluripotent Stem Cells: A Novel Tool to Sustain Pluripotency and Facilitate Differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef] [PubMed]
- Bagal-Kestwal, D.R.; Chiang, B.-H. Exploration of Chitinous Scaffold-Based Interfaces for Glucose Sensing Assemblies. Polymers 2019, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. Biomed Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.M.; Škalko-Basnet, N.; Jøraholmen, M.W. The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Mar. Drugs 2021, 19, 697. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; de Los Llanos Gandía, M.; Caballero, A.H. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Harkin, C.; Mehlmer, N.; Woortman, D.V.; Brück, T.B.; Brück, W.M. Nutritional and Additive Uses of Chitin and Chitosan in the Food Industry. In Sustainable Agriculture Reviews; Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Springer: Cham, Switzerland, 2019; pp. 1–43. [Google Scholar]
- Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications. Polymers 2023, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, X.; Hua, T.; Fu, J.; Koo, M.; Chan, W.; Poon, T. Chitosan Natural Polymer Material for Improving Antibacterial Properties of Textiles. ACS Appl. Bio Mater. 2021, 4, 4014–4038. [Google Scholar] [CrossRef] [PubMed]
- Sirajudheen, P.; Poovathumkuzhi, N.C.; Vigneshwaran, S.; Chelaveettil, B.M.; Meenakshi, S. Applications of Chitin and Chitosan Based Biomaterials for the Adsorptive Removal of Textile Dyes from Water—A Comprehensive Review. Carbohydr. Polym. 2021, 273, 118604. [Google Scholar] [CrossRef] [PubMed]
- Malayoglu, U. Removal of Heavy Metals by Biopolymer (Chitosan)/Nanoclay Composites. Sep. Sci. Technol. 2018, 53, 2741–2749. [Google Scholar] [CrossRef]
- Bose, I.; Singh, R.; Negi, P.; Singh, Y. Chitin as Bio-Based Nanomaterial in Packaging: A Review. Mater. Today Proc. 2021, 46, 11254–11257. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef] [PubMed]
- Rasweefali, M.K.; Sabu, S.; Muhammed Azad, K.S.; Raseel Rahman, M.K.; Sunooj, K.V.; Sasidharan, A.; Anoop, K.K. Influence of Deproteinization and Demineralization Process Sequences on the Physicochemical and Structural Characteristics of Chitin Isolated from Deep-Sea Mud Shrimp (Solenocera hextii). Adv. Biomark. Sci. Technol. 2022, 4, 12–27. [Google Scholar] [CrossRef]
- Berezina, N. Production and Application of Chitin. Phys. Sci. Rev. 2016, 1, 20160048. [Google Scholar] [CrossRef]
- Strnad, S.; Zemljič, L. Cellulose–Chitosan Functional Biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the Structural Diversity of Chitins as a Versatile Biomaterial. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200331. [Google Scholar] [CrossRef] [PubMed]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Du, J.; Tan, E.; Kim, H.J.; Zhang, A.; Bhattacharya, R.; Yarema, K.J. Comparative Evaluation of Chitosan, Cellulose Acetate, and Polyethersulfone Nanofiber Scaffolds for Neural Differentiation. Carbohydr. Polym. 2014, 99, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Medha; Kaith, B.S. A Review on Chitosan-Gelatin Nanocomposites: Synthesis, Characterization and Biomedical Applications. React. Funct. Polym. 2022, 179, 105362. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Sánchez, L.-F.; Cánepa, J.; Kim, S.; Nakamatsu, J. A Simple Approach to Produce Tailor-Made Chitosans with Specific Degrees of Acetylation and Molecular Weights. Polymers 2021, 13, 2415. [Google Scholar] [CrossRef] [PubMed]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and Their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef] [PubMed]
- Nouj, N.; Hafid, N.; El Alem, N.; Cretescu, I. Novel Liquid Chitosan-Based Biocoagulant for Treatment Optimization of Fish Processing Wastewater from a Moroccan Plant. Materials 2021, 14, 7133. [Google Scholar] [CrossRef]
- Agarwal, M.; Agarwal, M.K.; Shrivastav, N.; Pandey, S.; Gaur, P. A Simple and Effective Method for Preparation of Chitosan from Chitin. Int. J. Life-Sciences Sci. Res. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A Review on Properties, Biological Activities and Recent Progress in Biomedical Applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Scherließ, R.; Buske, S.; Young, K.; Weber, B.; Rades, T.; Hook, S. In Vivo Evaluation of Chitosan as an Adjuvant in Subcutaneous Vaccine Formulations. Vaccine 2013, 31, 4812–4819. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Roman, M. Effects of Chitosan Molecular Weight and Degree of Deacetylation on Chitosan–Cellulose Nanocrystal Complexes and Their Formation. Molecules 2023, 28, 1361. [Google Scholar] [CrossRef] [PubMed]
- Lagat, M.K.; Were, S.; Ndwigah, F.; Kemboi, V.J.; Kipkoech, C.; Tanga, C.M. Antimicrobial Activity of Chemically and Biologically Treated Chitosan Prepared from Black Soldier Fly (Hermetia illucens) Pupal Shell Waste. Microorganisms 2021, 9, 2417. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.C., Jr.; Ribeaux, D.R.; da Silva, C.A.; De Campos-Takaki, G.M. Physicochemical and Antibacterial Properties of Chitosan Extracted from Waste Shrimp Shells. Int. J. Microbiol. 2016, 2016, 5127515. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin Extraction from Shrimp Shell Using Enzymatic Treatment. Antitumor, Antioxidant and Antimicrobial Activities of Chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Samar, M.M.; El-Kalyoubi, M.; Khalaf, M.; El-Razik, M.A. Physicochemical, Functional, Antioxidant and Antibacterial Properties of Chitosan Extracted from Shrimp Wastes by Microwave Technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Devi, C.S. Studies on Heavy Metal Removal Efficiency and Antibacterial Activity of Chitosan Prepared from Shrimp Shell Waste. 3 Biotech 2014, 4, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, M.Y.; Alemán, A.; Calvo, M.M.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Antimicrobial and Antioxidant Chitosan Solutions Enriched with Active Shrimp (Litopenaeus vannamei) Waste Materials. Food Hydrocoll. 2014, 35, 710–717. [Google Scholar] [CrossRef]
- Saravanan, A.; Parthasarathy, V.; Kumar, P.S. Antimicrobial, Antifungal, and Antioxidant Activities of the Extracted Chitosan from Shrimp Shell Waste and Its Bioadsorption Study of Heavy Metals from the Aqueous Solution. Biomass Convers. Biorefinery 2023, 1–11. [Google Scholar] [CrossRef]
- Resmi, R.; Yoonus, J.; Beena, B. Anticancer and Antibacterial Activity of Chitosan Extracted from Shrimp Shell Waste. Mater. Today Proc. 2021, 41, 570–576. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory Effect of Dietary Chitosan Nanoparticles on the Performance of Nile Tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol. 2019, 88, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Prasetyaningsih, A.; Adi, Y.K.; Wicaksono, A.A.; Prakasita, V.C. The Effect of Shrimp Shell (Litopenaeus vannamei) Extract on Testicular Parameters of Streptozotocin-Induced Diabetic Rats. World Vet. J. 2023, 13, 144–151. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and Characterization of Chitin and Chitosan from Fishery Waste by Chemical Method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and Chitosan Preparation from Shrimp Shells Penaeus Monodon and Its Human Ovarian Cancer Cell Line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Buntong, B.; Sophal, L.; Vann, S.; Acedo, A., Jr. Effects of Chemical Extraction Methods on Physicochemical Properties of Shrimp Chitosan. Int. J. Environ. Rural Dev. 2021, 12, 170–175. [Google Scholar]
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of Chitosan and Its Oligomers from Shrimp Shell Waste, Their Characterization and Antimicrobial Effect. Vet. World 2017, 10, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kandile, N.G.; Zaky, H.T.; Mohamed, M.I.; Nasr, A.S.; Ali, Y.G. Extraction and Characterization of Chitosan from Shrimp Shells. Open J. Org. Polym. Mater. 2018, 08, 33–42. [Google Scholar] [CrossRef]
- Wang, D.; Lee, S.H.; Kim, J.; Park, C.B. “Waste to Wealth”: Lignin as a Renewable Building Block for Energy Harvesting/Storage and Environmental Remediation. ChemSusChem 2020, 13, 2807–2827. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-L.; Zhang, M.-S.; Deng, J.-J.; Lu, W.-J.; Yang, Z.-D.; Li, Z.-W.; Chen, Y.-C.; Luo, X.-C. Highly Efficient Shrimp Shell Recovery by Solid-State Fermentation with Streptomyces Sp. SCUT-3. Chem. Eng. J. 2023, 458, 141256. [Google Scholar] [CrossRef]
- Hamdi, M.; Hammami, A.; Hajji, S.; Jridi, M.; Nasri, M.; Nasri, R. Chitin Extraction from Blue Crab (Portunus segnis) and Shrimp (Penaeus kerathurus) Shells Using Digestive Alkaline Proteases from P. segnis Viscera. Int. J. Biol. Macromol. 2017, 101, 455–463. [Google Scholar] [CrossRef] [PubMed]
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and Efficient Extraction of Chitin and Chitosan for Scale-up Production: Effect of Process Parameters on Deacetylation Degree and Molecular Weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-Assisted Extraction of Chitosan from Squid Pen: Molecular Characterization and Fat Binding Capacity. J. Food Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Effendy, M.; Clausse, D.; Saleh, K.; Guénin, E. Heterogeneous Deacetylation Reaction of Chitin under Low-Frequency Ultrasonic Irradiation. Carbohydr. Polym. 2021, 267, 118180. [Google Scholar] [CrossRef] [PubMed]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and Chitosan Production from Shrimp Shells Using Ammonium-Based Ionic Liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Espíndola-Cortés, A.; Moreno-Tovar, R.; Bucio, L.; Gimeno, M.; Ruvalcaba-Sil, J.L.; Shirai, K. Hydroxyapatite Crystallization in Shrimp Cephalothorax Wastes during Subcritical Water Treatment for Chitin Extraction. Carbohydr. Polym. 2017, 172, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, K.; Stępniak, I.; Langer, E.; Tsurkan, M.; Wysokowski, M.; Petrenko, I.; Khrunyk, Y.; Fursov, A.; Bo, M.; Bavestrello, G.; et al. Electrochemical Approach for Isolation of Chitin from the Skeleton of the Black Coral Cirrhipathes sp. (Antipatharia). Mar. Drugs 2020, 18, 297. [Google Scholar] [CrossRef]
- Dan, G.; Zhang, Z.H.; Zeng, X.A.; Han, Z.; Luo, W.B.; Tang, C.; Quek, S.Y. Synergetic Effects of Pulsed Electric Field and Ozone Treatments on the Degradation of High Molecular Weight Chitosan. Int. J. Food Eng. 2014, 10, 775–784. [Google Scholar] [CrossRef]
- Saravana, P.S.; Ho, T.C.; Chae, S.-J.; Cho, Y.-J.; Park, J.-S.; Lee, H.-J.; Chun, B.-S. Deep Eutectic Solvent-Based Extraction and Fabrication of Chitin Films from Crustacean Waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Hongkulsup, C.; Khutoryanskiy, V.V.; Niranjan, K. Enzyme Assisted Extraction of Chitin from Shrimp Shells (Litopenaeus vannamei). J. Chem. Technol. Biotechnol. 2016, 91, 1250–1256. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Wang, C.-L.; Wang, S.-L. Microbial Conversion of Shrimp Heads to Proteases and Chitin as an Effective Dye Adsorbent. Polymers 2020, 12, 2228. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, C.-L.; Wang, S.-L. Proteases Production and Chitin Preparation from the Liquid Fermentation of Chitinous Fishery By-Products by Paenibacillus Elgii. Mar. Drugs 2021, 19, 477. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.-L. Chitin Extraction from Shrimp Waste by Liquid Fermentation Using an Alkaline Protease-Producing Strain, Brevibacillus Parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Abun, A.; Rusmana, D.; Widjastuti, T.; Haetami, K. Prebiotics BLS from Encapsulated of Extract of Shrimp Waste Bioconversion on Feed Supplement Quality and Its Implication of Metabolizable Energy and Digestibility at Indonesian Local Chicken. J. Appl. Anim. Res. 2021, 49, 295–303. [Google Scholar] [CrossRef]
- Cabanillas-Bojórquez, L.A.; Gutiérrez-Grijalva, E.P.; Castillo-López, R.I.; Contreras-Angulo, L.A.; Angulo-Escalante, M.A.; López-Martínez, L.X.; Ríos-Iribe, E.Y.; Heredia, J.B. Bioprocessing of Shrimp Waste Using Novel Industrial By-Products: Effects on Nutrients and Lipophilic Antioxidants. Fermentation 2021, 7, 312. [Google Scholar] [CrossRef]
- Neves, A.C.; Zanette, C.; Grade, S.T.; Schaffer, J.V.; Alves, H.J.; Arantes, M.K. Optimization of Lactic Fermentation for Extraction of Chitin from Freshwater Shrimp Waste. Acta Sci. Technol. 2017, 39, 125–133. [Google Scholar] [CrossRef]
- Zhang, H.; Yun, S.; Song, L.; Zhang, Y.; Zhao, Y. The Preparation and Characterization of Chitin and Chitosan under Large-Scale Submerged Fermentation Level Using Shrimp by-Products as Substrate. Int. J. Biol. Macromol. 2017, 96, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Abbasiliasi, S.; Lee, C.K.; Phapugrangkul, P. Chitin Extraction from Shrimp Wastes by Single Step Fermentation with Lactobacillus Acidophilus FTDC3871 Using Response Surface Methodology. J. Food Process. Preserv. 2020, 44, e14895. [Google Scholar] [CrossRef]
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Chitin from Penaeus Merguiensis via Microbial Fermentation Processing and Antioxidant Activity. Int. J. Biol. Macromol. 2016, 82, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Mondal, S.; Pal, K.; Jana, A.; Soren, J.P.; Barman, P.; Mondal, K.C.; Halder, S.K. Extraction of Chitin from Litopenaeus Vannamei Shell and Its Subsequent Characterization: An Approach of Waste Valorization through Microbial Bioprocessing. Bioprocess Biosyst. Eng. 2021, 44, 1943–1956. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Bioconversion of Shrimp Waste Penaeus Merguiensis Using Lactic Acid Fermentation: An Alternative Procedure for Chemical Extraction of Chitin and Chitosan. Int. J. Biol. Macromol. 2017, 104, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Verardi, A.; Sangiorgio, P.; Moliterni, S.; Errico, S.; Spagnoletta, A.; Dimatteo, S. Advanced Technologies for Chitin Recovery from Crustacean Waste. Clean Technol. Recycl. 2023, 3, 4–43. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Nielsen, P.H. Environmental Assessment of Enzyme Use in Industrial Production—A Literature Review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Marzieh, M.-N.; Zahra, F.; Tahereh, E.; Sara, K.-N. Comparison of the Physicochemical and Structural Characteristics of Enzymatic Produced Chitin and Commercial Chitin. Int. J. Biol. Macromol. 2019, 139, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Poonsin, T.; Simpson, B.K.; Benjakul, S.; Visessanguan, W.; Yoshida, A.; Klomklao, S. Albacore Tuna Spleen Trypsin: Potential Application as Laundry Detergent Additive and in Carotenoprotein Extraction from Pacific White Shrimp Shells. Biocatal. Agric. Biotechnol. 2019, 17, 638–646. [Google Scholar] [CrossRef]
- Dayakar, B.; Xavier, K.A.M.; Ngasotter, S.; Layana, P.; Balange, A.K.; Priyadarshini, B.; Nayak, B.B. Characterization of Spray-Dried Carotenoprotein Powder from Pacific White Shrimp (Litopenaeus vannamei) Shells and Head Waste Extracted Using Papain: Antioxidant, Spectroscopic, and Microstructural Properties. LWT 2022, 159, 113188. [Google Scholar] [CrossRef]
- Duong, N.T.H.; Nghia, N.D. Kinetics and Optimization of the Deproteinization by Pepsin in Chitin Extraction from White Shrimp Shell. J. Chitin Chitosan Sci. 2014, 2, 21–28. [Google Scholar] [CrossRef]
- Vieira, M.A.; Oliveira, D.D.; Kurozawa, L.E. Production of Peptides with Radical Scavenging Activity and Recovery of Total Carotenoids Using Enzymatic Protein Hydrolysis of Shrimp Waste. J. Food Biochem. 2016, 40, 517–525. [Google Scholar] [CrossRef]
- Dey, S.S.; Dora, K.C. Antioxidative Activity of Protein Hydrolysate Produced by Alcalase Hydrolysis from Shrimp Waste (Penaeus monodon and Penaeus indicus). J. Food Sci. Technol. 2014, 51, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Adhiksana, A.; Wahyudi, W.; Arifin, Z.; Irwan, M. Effect of microwave irradiation time to deacetylation process of chitin from shrimp shells. J. Kim. Ris. 2023, 8, 1–7. [Google Scholar] [CrossRef]
- Prajapat, A.L.; Gogate, P.R. Depolymerization of Guar Gum Solution Using Different Approaches Based on Ultrasound and Microwave Irradiations. Chem. Eng. Process. Process Intensif. 2015, 88, 1–9. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Yeates, S.G. “Green” Molecular Weight Degradation of Chitosan Using Microwave Irradiation. Polym. Degrad. Stab. 2013, 98, 863–867. [Google Scholar] [CrossRef]
- Tsubaki, S.; Azuma, J. Total Fractionation of Green Tea Residue by Microwave-Assisted Alkaline Pretreatment and Enzymatic Hydrolysis. Bioresour. Technol. 2013, 131, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Reynaud, S.; Desbrieres, J. Amphiphilic Derivatives of Chitosan Using Microwave Irradiation. Toward an Eco-Friendly Process to Chitosan Derivatives. Carbohydr. Polym. 2015, 116, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Kim, S.; Selling, G.W.; Cheng, H.N. Conversion of Agricultural Residues to Carboxymethylcellulose and Carboxymethylcellulose Acetate. Ind. Crops Prod. 2014, 60, 259–265. [Google Scholar] [CrossRef]
- dos Santos, D.M.; Bukzem, A.d.L.; Ascheri, D.P.R.; Signini, R.; de Aquino, G.L.B. Microwave-Assisted Carboxymethylation of Cellulose Extracted from Brewer’s Spent Grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Khodaiyan, F.; Razavi, S.H.; Mousavi, M. Improvement of Chitosan Production from Persian Gulf Shrimp Waste by Response Surface Methodology. Food Hydrocoll. 2016, 59, 50–58. [Google Scholar] [CrossRef]
- Pratiwi, R.D.; El Muttaqien, S.; Gustini, N.; Difa, N.S.; Syahputra, G.; Rosyidah, A. Eco-Friendly Synthesis of Chitosan and Its Medical Application: From Chitin Extraction to Nanoparticle Preparation. ADMET DMPK 2023, 11, 435–455. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; El Khalfaouy, R.; Laajeb, A.; Addaou, A.; Lahsini, A. Eco-Friendly Extraction and Characterization of Chitin and Chitosan from the Shrimp Shell Waste via Microwave Irradiation. Process Saf. Environ. Prot. 2016, 104, 395–405. [Google Scholar] [CrossRef]
- Zaeni, A.; Safitri, E.; Fuadah, B.; Sudiana, I.N. Microwave-Assisted Hydrolysis of Chitosan from Shrimp Shell Waste for Glucosammine Hydrochlorid Production. J. Phys. Conf. Ser. 2017, 846, 012011. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Chen, S.; Fu, H.; Zhao, Y. Microwave-assisted Degradation of Chitosan with Hydrogen Peroxide Treatment Using Box-Behnken Design for Enhanced Antibacterial Activity. Int. J. Food Sci. Technol. 2018, 53, 156–165. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and Eco-Friendly Approaches for the Extraction of Chitin and Chitosan: A Review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Radoiu, M.; Fabiano-Tixier, A.-S.; Chemat, F. From Laboratory to Industry: Scale-Up, Quality, and Safety Consideration for Microwave-Assisted Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 207–229. [Google Scholar]
- Chan, C.; Yusoff, R.; Ngoh, G. Assessment of Scale-Up Parameters of Microwave-Assisted Extraction via the Extraction of Flavonoids from Cocoa Leaves. Chem. Eng. Technol. 2015, 38, 489–496. [Google Scholar] [CrossRef]
- Suryawanshi, N.; Ayothiraman, S.; Eswari, J.S. Ultrasonication Mode for the Expedition of Extraction Process of Chitin from the Maritime Shrimp Shell Waste. Indian J. Biochem. Biophys. 2020, 57, 431–438. [Google Scholar]
- Villa-Lerma, G.; González-Márquez, H.; Gimeno, M.; López-Luna, A.; Bárzana, E.; Shirai, K. Ultrasonication and Steam-Explosion as Chitin Pretreatments for Chitin Oligosaccharide Production by Chitinases of Lecanicillium Lecanii. Bioresour. Technol. 2013, 146, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.G.; de Moura Delezuk, J.A.; Campana-Filho, S.P. Ultrasound-Assisted Conversion of Alpha-Chitin into Chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, T.; Prince; Kumar, V.; Sharma, S.; Rani, J. A Review on Conversion of Food Wastes and By-Products into Value Added Products. Int. J. Chem. Stud. 2019, 7, 2068–2073. [Google Scholar]
- Paul, S.; Jayan, A.; Sasikumar, C.S.; Cherian, S.M. Extraction and Purification of Chitosan from Chitin Isolated from Sea Prawn Fenneropenaeus Indicus. Asian J. Pharm. Clin. Res. 2014, 7, 201–204. [Google Scholar]
- Tissera, W.M.J.C.; Rathnayake, S.I.; Abeyrathne, E.D.N.S.; Nam, K.-C. An Improved Extraction and Purification Method for Obtaining High-Quality Chitin and Chitosan from Blue Swimmer (Portunus pelagicus) Crab Shell Waste. Food Sci. Biotechnol. 2021, 30, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, A.P.; Pătru, A.; Gubler, L. Review—CO2 Separation and Transport via Electrochemical Methods. J. Electrochem. Soc. 2020, 167, 133504. [Google Scholar] [CrossRef]
- Ražić, S.; Arsenijević, J.; Đogo Mračević, S.; Mušović, J.; Trtić-Petrović, T. Greener Chemistry in Analytical Sciences: From Green Solvents to Applications in Complex Matrices. Current Challenges and Future Perspectives: A Critical Review. Analyst 2023, 148, 3130–3152. [Google Scholar] [CrossRef] [PubMed]
- Karagozlu, M.Z.; Kim, S.-K. Anticancer Effects of Chitin and Chitosan Derivatives. Adv. Food Nutr. Res. 2014, 72, 215–225. [Google Scholar] [PubMed]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Virmani, T.; Kumar, G.; Sharma, A.; Pathak, K.; Akhtar, M.S.; Afzal, O.; Altamimi, A.S.A. Amelioration of Cancer Employing Chitosan, Its Derivatives, and Chitosan-Based Nanoparticles: Recent Updates. Polymers 2023, 15, 2928. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiao, Y.; Zhou, C. Impacts of Chitosan Oligosaccharide (COS) on Angiogenic Activities. Microvasc. Res. 2021, 134, 104114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl Chitosan Represses Tumor Angiogenesis in Vitro and in Vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mu, M.; Fan, R.; Zou, B.; Guo, G. Functionalized Chitosan as a Promising Platform for Cancer Immunotherapy: A Review. Carbohydr. Polym. 2022, 290, 119452. [Google Scholar] [CrossRef]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.-A. Chitosan Exerts Anticancer Activity through Induction of Apoptosis and Cell Cycle Arrest in Oral Cancer Cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef]
- Mary Lazer, L.; Sadhasivam, B.; Palaniyandi, K.; Muthuswamy, T.; Ramachandran, I.; Balakrishnan, A.; Pathak, S.; Narayan, S.; Ramalingam, S. Chitosan-Based Nano-Formulation Enhances the Anticancer Efficacy of Hesperetin. Int. J. Biol. Macromol. 2018, 107, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Aljaeid, B. Preparation, Characterization, and Potential Application of Chitosan, Chitosan Derivatives, and Chitosan Metal Nanoparticles in Pharmaceutical Drug Delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Goyal, J.; Behera, P.K.; Devi, S.; Singh, S.K.; Garg, V.; Mittal, N. Unraveling the Role of Chitosan for Nasal Drug Delivery Systems: A Review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100316. [Google Scholar] [CrossRef]
- Hussain, S.A.; Abdelkader, H.; Abdullah, N.; Kmaruddin, S. Review on Micro-Encapsulation with Chitosan for Pharmaceuticals Applications. MOJ Curr. Res. Rev. 2018, 1, 77–84. [Google Scholar] [CrossRef]
- He, T.; Wang, W.; Chen, B.; Wang, J.; Liang, Q.; Chen, B. 5-Fluorouracil Monodispersed Chitosan Microspheres: Microfluidic Chip Fabrication with Crosslinking, Characterization, Drug Release and Anticancer Activity. Carbohydr. Polym. 2020, 236, 116094. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Fresta, M.; Paolino, D. Rutin-Loaded Chitosan Microspheres: Characterization and Evaluation of the Anti-Inflammatory Activity. Carbohydr. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.R.; Moura, A.; Leiro, V.; Silva-Carvalho, R.; Estevinho, B.N.; Seabra, C.L.; Henriques, P.C.; Lucena, M.; Teixeira, C.; Gomes, P.; et al. Grafting MSI-78A onto Chitosan Microspheres Enhances Its Antimicrobial Activity. Acta Biomater. 2022, 137, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Thaya, R.; Vaseeharan, B.; Sivakamavalli, J.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Synthesis of Chitosan-Alginate Microspheres with High Antimicrobial and Antibiofilm Activity against Multi-Drug Resistant Microbial Pathogens. Microb. Pathog. 2018, 114, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Cai, H.-Y.; Chen, J.-P.; Li, R.; Zhong, S.-Y.; Jia, X.-J.; Liu, X.-F.; Song, B.-B. Chitosan/Alginate Nanoparticles for the Enhanced Oral Antithrombotic Activity of Clam Heparinoid from the Clam Coelomactra Antiquata. Mar. Drugs 2022, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Pk, C.; Dev, S.K. Formulation and In-Vitro-In-Vivo Evaluation of Alginate-Chitosan Microspheres of Glipizide by Ionic Gelation Method. Asian J. Pharm. Clin. Res. 2017, 10, 385. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Li, R.; Yin, X.; Dong, W.; Pan, C. Fabrication of a Blood Compatible Composite Membrane from Chitosan Nanoparticles, Ethyl Cellulose and Bacterial Cellulose Sulfate. RSC Adv. 2018, 8, 31322–31330. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A Review on Recent Advances in Chitosan Based Composite for Hemostatic Dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, X.; Huang, P. Graphene-Based Nanomaterials for Bioimaging. Adv. Drug Deliv. Rev. 2016, 105, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yin, M. Design and Development of Fluorescent Nanostructures for Bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Cai, H.; Yao, P. In Situ Preparation of Gold Nanoparticle-Loaded Lysozyme–Dextran Nanogels and Applications for Cell Imaging and Drug Delivery. Nanoscale 2013, 5, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xia, X.; Huang, Y.; Chen, X.; Han, J.-D.J. Bioimaging for Quantitative Phenotype Analysis. Methods 2016, 102, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ke, F.; Mararenko, A.; Wei, Z.; Banerjee, P.; Zhou, S. Responsive Polymer–Fluorescent Carbon Nanoparticle Hybrid Nanogels for Optical Temperature Sensing, near-Infrared Light-Responsive Drug Release, and Tumor Cell Imaging. Nanoscale 2014, 6, 7443–7452. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, D.; Li, P.; Wu, Q.; Bo, X.; Liu, B.; Bao, S.; Su, T.; Xu, H.; Wang, Q. Dual-Enzyme-Loaded Multifunctional Hybrid Nanogel System for Pathological Responsive Ultrasound Imaging and T 2 -Weighted Magnetic Resonance Imaging. ACS Nano 2015, 9, 5646–5656. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.J.; Kwag, D.S.; Lee, U.Y.; Youn, Y.S.; Lee, E.S. Acid PH-Activated Glycol Chitosan/Fullerene Nanogels for Efficient Tumor Therapy. Carbohydr. Polym. 2014, 101, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Kerch, G. The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases. Mar. Drugs 2015, 13, 2158–2182. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean Waste-Derived Chitosan: Antioxidant Properties and Future Perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and Antimicrobial Proprieties of Chitin and Chitosan Extracted from Parapenaeus Longirostris Shrimp Shell Waste. Ann. Pharm. Françaises 2016, 74, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Li, H.; Du, Y.; Shi, X. Recent Advances in Chitosan-Based Self-Healing Materials. Res. Chem. Intermed. 2018, 44, 4827–4840. [Google Scholar] [CrossRef]
- Moattari, M.; Kouchesfehani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M. Evaluation of Nerve Growth Factor (NGF) Treated Mesenchymal Stem Cells for Recovery in Neurotmesis Model of Peripheral Nerve Injury. J. Cranio-Maxillofacial Surg. 2018, 46, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Huh, B.K.; Kim, S.N.; Lee, J.Y.; Park, C.G.; Mikos, A.G.; Choy, Y. Bin Application of Materials as Medical Devices with Localized Drug Delivery Capabilities for Enhanced Wound Repair. Prog. Mater. Sci. 2017, 89, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Kelly, A.; Oldham, T.; Rogers, R.D. Agricultural Uses of Chitin Polymers. Environ. Chem. Lett. 2020, 18, 53–60. [Google Scholar] [CrossRef]
- Saharan, V.; Pal, A. Current and Future Prospects of Chitosan-Based Nanomaterials in Plant Protection and Growth. In Chitosan Based Nanomaterials in Plant Growth and Protection; SpringerBriefs in Plant Science; Springer: New Delhi, India, 2016; pp. 43–48. [Google Scholar]
- Amine, R.; Abla, E.H.; Mohammed, B.I.; Khadija, O. The Amendment with Chitin and/or Chitosan Improves the Germination and Growth of Lycopersicon esculentum L., Capsicum annuum L. and Solanum melongena L. Indian J. Agric. Res. 2020, 54, 420–428. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36; Crini, G., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–123. [Google Scholar]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Elhussieny, A.; Faisal, M.; D’Angelo, G.; Aboulkhair, N.T.; Everitt, N.M.; Fahim, I.S. Valorisation of Shrimp and Rice Straw Waste into Food Packaging Applications. Ain Shams Eng. J. 2020, 11, 1219–1226. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 11, 1599. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Cheng, A.; Wang, R.; Zhang, R. Marine Biofilms: Diversity, Interactions and Biofouling. Nat. Rev. Microbiol. 2022, 20, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, R.M.; Al-Hilifi, S.A.; Rashed, M.M.A. Fabrication, Characterization, and Anti-free Radical Performance of Edible Packaging-chitosan Film Synthesized from Shrimp Shell Incorporated with Ginger Essential Oil. J. Food Meas. Charact. 2021, 15, 2951–2962. [Google Scholar] [CrossRef]
- Tamer, T.; Aacute, K.; Mohyeldin, M.; Soltes, L. Free Radical Scavenger Activity of Chitosan and Its Aminated Derivative. J. Appl. Pharm. Sci. 2016, 6, 195–201. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Dasumiati; Saridewi, N.; Malik, M. Food Packaging Development of Bioplastic from Basic Waste of Cassava Peel (Manihot uttilisima) and Shrimp Shell. IOP Conf. Ser. Mater. Sci. Eng. 2019, 602, 012053. [Google Scholar] [CrossRef]
- Ewais, A.; Saber, R.A.; Ghany, A.A.; Sharaf, A.; Sitohy, M. High Quality, Low Molecular Weight Shrimp and Crab Chitosans Obtained by Short-Time Holistic High-Power Microwave Technology. SN Appl. Sci. 2023, 5, 365. [Google Scholar] [CrossRef]
- Sila, A.; Sayari, N.; Balti, R.; Martinez-Alvarez, O.; Nedjar-Arroume, N.; Moncef, N.; Bougatef, A. Biochemical and Antioxidant Properties of Peptidic Fraction of Carotenoproteins Generated from Shrimp By-Products by Enzymatic Hydrolysis. Food Chem. 2014, 148, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.D.S.; De Souza, M.M.; Da Rocha, M.; Wasielesky, W.F.; Prentice, C. Functional Properties of White Shrimp (Litopenaeus vannamei) By-Products Protein Recovered by Isoelectric Solubilization/Precipitation. J. Aquat. Food Prod. Technol. 2019, 28, 649–657. [Google Scholar] [CrossRef]
- De Los Ángeles Pereira, N.; Fangio, M.F.; Rodriguez, Y.E.; Bonadero, M.C.; Harán, N.S.; Fernández-Gimenez, A.V. Characterization of Liquid Protein Hydrolysates Shrimp Industry Waste: Analysis of Antioxidant and Microbiological Activity, and Shelf Life of Final Product. J. Food Process. Preserv. 2022, 46, e15526. [Google Scholar] [CrossRef]
- Krichen, F.; Sila, A.; Caron, J.; Kobbi, S.; Nedjar, N.; Miled, N.; Blecker, C.; Besbes, S.; Bougatef, A. Identification and Molecular Docking of Novel ACE Inhibitory Peptides from Protein Hydrolysates of Shrimp Waste. Eng. Life Sci. 2018, 18, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, K.; Tokur, B. Optimization of Hydrolysis Conditions for the Production of Protein Hydrolysates from Fish Wastes Using Response Surface Methodology. Food Biosci. 2022, 45, 101312. [Google Scholar] [CrossRef]
- Bkhairia, I.; Ktari, N.; Younes, I.; Kammoun, M.; Nasri, M.; Ghorbel, S. Golden Grey Mullet (Liza aurata) Alkaline Proteases: Biochemical Characterization, Application in the Shrimp Wastes Deproteinization, Laundry Commercial Detergents, and Preparation of Antioxidant Protein Hydrolysate. J. Aquat. Food Prod. Technol. 2015, 24, 597–613. [Google Scholar] [CrossRef]
- Guo, X.; Han, X.; He, Y.; Du, H.; Tan, Z. Optimization of Enzymatic Hydrolysis for Preparation of Shrimp Flavor Precursor Using Response Surface Methodology. J. Food Qual. 2014, 37, 229–236. [Google Scholar] [CrossRef]
- Gunasekaran, J.; Kannuchamy, N.; Kannaiyan, S.; Chakraborti, R.; Gudipati, V. Protein Hydrolysates from Shrimp (Metapenaeus dobsoni) Head Waste: Optimization of Extraction Conditions by Response Surface Methodology. J. Aquat. Food Prod. Technol. 2015, 24, 429–442. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, G.; Jiang, J. Purification and Characterization of a New DPPH Radical Scavenging Peptide from Shrimp Processing By-Products Hydrolysate. J. Aquat. Food Prod. Technol. 2013, 22, 281–289. [Google Scholar] [CrossRef]
- Baron, R.; Socol, M.; Arhaliass, A.; Bruzac, S.; Le Roux, K.; del Pino, J.R.; Bergé, J.P.; Kaas, R. Kinetic Study of Solid Phase Demineralization by Weak Acids in One-Step Enzymatic Bio-Refinery of Shrimp Cuticles. Process Biochem. 2015, 50, 2215–2223. [Google Scholar] [CrossRef]
- Cao, W.; Tan, C.; Zhan, X.; Li, H.; Zhang, C. Ultraviolet Irradiation and Gradient Temperature Assisted Autolysis for Protein Recovery from Shrimp Head Waste. Food Chem. 2014, 164, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Murueta, J.H.; Garcia-Carreno, F.L.; Navarrete-del-Toro, M.d.l.A. PH-Solubilzation Process as an Alternative to Enzymatic Hydrolysis Applied to Shrimp Waste. Turk. J. Fish. Aquat. Sci. 2013, 13, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, R.-D. Progress in Bioextraction Processes of Chitin from Crustacean Biowastes. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 545–554. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, J.; Kan, F.; Gao, Y.; Lan, J.; Zhang, X.; Hu, Z.; Li, Y.; Lin, H. Antioxidant Production and Chitin Recovery from Shrimp Head Fermentation with Streptococcus Thermophilus. Food Sci. Biotechnol. 2013, 22, 1023–1032. [Google Scholar] [CrossRef]
- Kan, K. Therapeutic Effect of a Chitin Oligosaccharide Mixture by per Os Administration on Human Cancer. J. Chitin Chitosan Sci. 2014, 2, 205–208. [Google Scholar] [CrossRef]
- Sun, R.; Liu, C.; Zhang, H.; Wang, Q. Benzoylurea Chitin Synthesis Inhibitors. J. Agric. Food Chem. 2015, 63, 6847–6865. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mao, X. An Environmental Friendly Process for Antarctic Krill (Euphausia superba) Utilization Using Fermentation Technology. J. Clean. Prod. 2016, 127, 618–623. [Google Scholar] [CrossRef]
- Ghorbel-Bellaaj, O.; Jellouli, K.; Maalej, H. Shrimp Processing By-Products Protein Hydrolysates: Evaluation of Antioxidant Activity and Application in Biomass and Proteases Production. Biocatal. Biotransformation 2017, 35, 287–297. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein Hydrolysates from Animal Processing By-Products as a Source of Bioactive Molecules with Interest in Animal Feeding: A Review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Dort, J.; Leblanc, N.; Bryl, P.; Fortin, M.-G.; Carbonneau, M.-E.; Lavigne, C.; Jacques, H. Shrimp Protein Hydrolysate Modulates the Timing of Proinflammatory Macrophages in Bupivacaine-Injured Skeletal Muscles in Rats. Biomed Res. Int. 2016, 2016, 5214561. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; Shahidi, F. Bioactive Peptides from Shrimp Shell Processing Discards: Antioxidant and Biological Activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Yuan, G.; Li, W.; Pan, Y.; Wang, C.; Chen, H. Shrimp Shell Wastes: Optimization of Peptide Hydrolysis and Peptide Inhibition of α-Amylase. Food Biosci. 2018, 25, 52–60. [Google Scholar] [CrossRef]
- da Silva, C.P.; Bezerra, R.S.; dos Santos, A.C.O.; Messias, J.B.; de Castro, C.R.O.B.; Carvalho, L.B., Jr. Biological Value of Shrimp Protein Hydrolysate By-Product Produced by Autolysis. LWT 2017, 80, 456–461. [Google Scholar] [CrossRef]
- Guilherme-Fernandes, J.; Aires, T.; Fonseca, A.J.M.; Yergaliyev, T.; Camarinha-Silva, A.; Lima, S.A.C.; Maia, M.R.G.; Cabrita, A.R.J. Squid Meal and Shrimp Hydrolysate as Novel Protein Sources for Dog Food. Front. Vet. Sci. 2024, 11, 1360939. [Google Scholar] [CrossRef]
- Su, F.; Huang, B.; Liu, J. The Carotenoids of Shrimps (Decapoda: Caridea and Dendrobranchiata) Cultured in China. J. Crustac. Biol. 2018, 38, 523–530. [Google Scholar] [CrossRef]
- López, A.M.Q.; dos Santos, F.A.R.; Martins, E.S.; dos Silva, A.L.S.; dos Santos, E.C.L. Pink and White Shrimps from the Brazilian Coast: Pigment Identification, Antioxidant Activity and Microbial Quality under Different Freezing-Times. Food Sci. Technol. 2021, 41, 447–457. [Google Scholar] [CrossRef]
- Parjikolaei, B.R.; El-Houri, R.B.; Fretté, X.C.; Christensen, K.V. Influence of Green Solvent Extraction on Carotenoid Yield from Shrimp (Pandalus borealis) Processing Waste. J. Food Eng. 2015, 155, 22–28. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Characterization and Storage Stability of Astaxanthin Esters, Fatty Acid Profile and α-Tocopherol of Lipid Extract from Shrimp (L. vannamei) Waste with Potential Applications as Food Ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, X.; Zhang, L.; Shao, P.; Wu, W.; Chen, Z.; Li, J.; Renard, C.M.G.C. An Overview of Carotenoid Extractions Using Green Solvents Assisted by Z-Isomerization. Trends Food Sci. Technol. 2022, 123, 145–160. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef]
- Taheri, F.; Sattari, E.; Hormozi, M.; Ahmadvand, H.; Bigdeli, M.R.; Kordestani-Moghadam, P.; Anbari, K.; Milanizadeh, S.; Moghaddasi, M. Dose-Dependent Effects of Astaxanthin on Ischemia/Reperfusion Induced Brain Injury in MCAO Model Rat. Neurochem. Res. 2022, 47, 1736–1750. [Google Scholar] [CrossRef]
- Sayuti, N.H.; Nawawi, K.N.M.; Goon, J.A.; Mokhtar, N.M.; Makpol, S.; Tan, J.K. Preventative and Therapeutic Effects of Astaxanthin on NAFLD. Antioxidants 2023, 12, 1552. [Google Scholar] [CrossRef]
- Patil, A.D.; Kasabe, P.J.; Dandge, P.B. Pharmaceutical and Nutraceutical Potential of Natural Bioactive Pigment: Astaxanthin. Nat. Products Bioprospect. 2022, 12, 25. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Muskhazli, M.; Nor Azwady, A.A.; Ahmad, S.A.; Adli, A.A. Three Dimensional Optimisation for the Enhancement of Astaxanthin Recovery from Shrimp Shell Wastes by Aeromonas Hydrophila. Biocatal. Agric. Biotechnol. 2020, 27, 101649. [Google Scholar] [CrossRef]
- Hooshmand, H.; Shabanpour, B.; Moosavi-Nasab, M.; Golmakani, M.T. Optimization of Carotenoids Extraction from Blue Crab (Portunus pelagicus) and Shrimp (Penaeus semisulcatus) Wastes Using Organic Solvents and Vegetable Oils. J. Food Process. Preserv. 2017, 41, e13171. [Google Scholar] [CrossRef]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and Purification of Astaxanthin from Shrimp Shells and the Effects of Different Treatments on Its Content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Dave, D.; Liu, Y.; Pohling, J.; Trenholm, S.; Murphy, W. Astaxanthin Recovery from Atlantic Shrimp (Pandalus Borealis) Processing Materials. Bioresour. Technol. Rep. 2020, 11, 100535. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In Vitro Antioxidant, Antityrosinase, and Cytotoxic Activities of Astaxanthin from Shrimp Waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Irna, C.; Jaswir, I.; Othman, R.; Jimat, D.N. Comparison Between High-Pressure Processing and Chemical Extraction: Astaxanthin Yield From Six Species of Shrimp Carapace. J. Diet. Suppl. 2018, 15, 805–813. [Google Scholar] [CrossRef]
- Darachai, P.; Limpawattana, M.; Hawangjoo, M.; Klaypradit, W. Effects of Shrimp Waste Types and Their Cooking on Properties of Extracted Astaxanthin and Its Characteristics in Liposomes. J. Food Nutr. Res. 2019, 7, 530–536. [Google Scholar] [CrossRef]
- Maia, M.L.; Grosso, C.; Barroso, M.F.; Silva, A.; Delerue-Matos, C.; Domingues, V.F. Bioactive Compounds of Shrimp Shell Waste from Palaemon Serratus and Palaemon Varians from Portuguese Coast. Antioxidants 2023, 12, 435. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Arena, R.; Renda, G.; Ficano, G.; Randazzo, M.; Fricano, S.; Sadok, S.; Santulli, A. In Vitro Bioactivity of Astaxanthin and Peptides from Hydrolisates of Shrimp (Parapenaeus longirostris) By-Products: From the Extraction Process to Biological Effect Evaluation, as Pilot Actions for the Strategy “From Waste to Profit”. Mar. Drugs 2021, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.K.N.; Rodrigues, B.D.; da Silva, L.H.M.; da Rodrigues, A.M.C. Drying and Extraction of Astaxanthin from Pink Shrimp Waste (Farfantepenaeus subtilis): The Applicability of Spouted Beds. Food Sci. Technol. 2018, 38, 454–461. [Google Scholar] [CrossRef]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Rahman, R.A. Co-Solvent Selection for Supercritical Fluid Extraction of Astaxanthin and Other Carotenoids from Penaeus Monodon Waste. J. Oleo Sci. 2014, 63, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, N.; Martínez, J.; Maraschin, M.; Ferreira, S.R.S. Pink Shrimp (P. brasiliensis and P. paulensis) Residue: Supercritical Fluid Extraction of Carotenoid Fraction. J. Supercrit. Fluids 2013, 74, 22–33. [Google Scholar] [CrossRef]
- Cabanillas-Bojórquez, L.A.; Gutiérrez-Grijalva, E.P.; González-Aguilar, G.A.; López-Martinez, L.X.; Castillo-López, R.I.; de Jesús Bastidas-Bastidas, P.; Heredia, J.B. Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity. Molecules 2021, 26, 4465. [Google Scholar] [CrossRef] [PubMed]
- Shazana, A.R.; Masturah, M.; Badlishah, S.B.; Rashidi, O.; Russly, A.R. Optimisation of Supercritical Fluid Extraction of Astaxanthin from Penaeus Monodon Waste Using Ethanol-Modified Carbon Dioxide. J. Eng. Sci. Technol. 2016, 11, 722–736. [Google Scholar]
- Li, J.; Sun, W.; Ramaswamy, H.S.; Yu, Y.; Zhu, S.; Wang, J.; Li, H. High Pressure Extraction of Astaxanthin from Shrimp Waste (Penaeus vannamei Boone): Effect on Yield and Antioxidant Activity. J. Food Process Eng. 2017, 40, e12353. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Clinical Significance of Polyunsaturated Fatty Acids in the Prevention of Cardiovascular Diseases. Front. Nutr. 2022, 9, 998291. [Google Scholar] [CrossRef] [PubMed]
- Balzano, M.; Pacetti, D.; Lucci, P.; Fiorini, D.; Frega, N.G. Bioactive Fatty Acids in Mantis Shrimp, Crab and Caramote Prawn: Their Content and Distribution among the Main Lipid Classes. J. Food Compos. Anal. 2017, 59, 88–94. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M.M.; Gómez-Guillén, M.C.; Gómez-Estaca, J. Microcapsules Containing Astaxanthin from Shrimp Waste as Potential Food Coloring and Functional Ingredient: Characterization, Stability, and Bioaccessibility. LWT 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin Inhibits NF-ΚB and Wnt/β-Catenin Signaling Pathways via Inactivation of Erk/MAPK and PI3K/Akt to Induce Intrinsic Apoptosis in a Hamster Model of Oral Cancer. Biochim. Biophys. Acta 2013, 1830, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-C.; Chen, J.-C.; Wang, T.-J.; Zheng, H.-Y.; Chen, W.-C.; Chang, P.-Y.; Lin, Y.-W. Astaxanthin Down-Regulates Rad51 Expression via Inactivation of AKT Kinase to Enhance Mitomycin C-Induced Cytotoxicity in Human Non-Small Cell Lung Cancer Cells. Biochem. Pharmacol. 2016, 105, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, P.R.-R.; Arathi, B.P.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Astaxanthin from Shrimp Efficiently Modulates Oxidative Stress and Allied Cell Death Progression in MCF-7 Cells Treated Synergistically with β-Carotene and Lutein from Greens. Food Chem. Toxicol. 2017, 106, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Aneesh, P.A.; Ajeeshkumar, K.K.; Lekshmi, R.G.K.; Anandan, R.; Ravishankar, C.N.; Mathew, S. Bioactivities of Astaxanthin from Natural Sources, Augmenting Its Biomedical Potential: A Review. Trends Food Sci. Technol. 2022, 125, 81–90. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Saravana, P.S.; Chun, B.-S.; Kang, H.W. Astaxanthin-Alpha Tocopherol Nanoemulsion Formulation by Emulsification Methods: Investigation on Anticancer, Wound Healing, and Antibacterial Effects. Colloids Surf. B Biointerfaces 2018, 172, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. In Vitro Antitumor Potential of Astaxanthin Nanoemulsion against Cancer Cells via Mitochondrial Mediated Apoptosis. Int. J. Pharm. 2019, 560, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Hao, C.; Mao, X.; Zhang, L. Astaxanthin Protects PC12 Cells from Glutamate-Induced Neurotoxicity through Multiple Signaling Pathways. J. Funct. Foods 2015, 16, 137–151. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidve, P.; Prajapati, N.; Kalia, K.; Tiwari, V. Astaxanthin Ameliorates Behavioral and Biochemical Alterations in In-Vitro and in-Vivo Model of Neuropathic Pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, F.; Ying, C.J.; Chen, J.; Chen, L.; Dong, J.; Shi, Y.; Tang, M.; Hu, X.T.; Pan, Z.H.; et al. Inhibition of INOS Alleviates Cognitive Deficits and Depression in Diabetic Mice through Downregulating the NO/SGC/CGMP/PKG Signal Pathway. Behav. Brain Res. 2017, 322, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In Vitro Studies of the Neuroprotective Activities of Astaxanthin and Fucoxanthin against Amyloid Beta (Aβ 1-42) Toxicity and Aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef]
- Wen, X.; Huang, A.; Hu, J.; Zhong, Z.; Liu, Y.; Li, Z.; Pan, X.; Liu, Z. Neuroprotective Effect of Astaxanthin against Glutamate-Induced Cytotoxicity in HT22 Cells: Involvement of the Akt/GSK-3β Pathway. Neuroscience 2015, 303, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, X.; Feng, J.; Xie, T.; Si, P.; Wang, W. Neuroprotective Effect of Astaxanthin on Newborn Rats Exposed to Prenatal Maternal Seizures. Brain Res. Bull. 2019, 148, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory Effect of Astaxanthin in Phthalic Anhydride-induced Atopic Dermatitis Animal Model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef]
- Bi, J.; Cui, R.; Li, Z.; Liu, C.; Zhang, J. Astaxanthin Alleviated Acute Lung Injury by Inhibiting Oxidative/Nitrative Stress and the Inflammatory Response in Mice. Biomed. Pharmacother. 2017, 95, 974–982. [Google Scholar] [CrossRef]
- Farruggia, C.; Kim, M.-B.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Han, M.J.; Park, Y.-K.; Lee, J.-Y. Astaxanthin Exerts Anti-Inflammatory and Antioxidant Effects in Macrophages in NRF2-Dependent and Independent Manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Yaghooti, H.; Mohammadtaghvaei, N.; Mahboobnia, K. Effects of Palmitate and Astaxanthin on Cell Viability and Proinflammatory Characteristics of Mesenchymal Stem Cells. Int. Immunopharmacol. 2019, 68, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Meephansan, J.; Rungjang, A.; Yingmema, W.; Deenonpoe, R.; Ponnikorn, S. Effect of Astaxanthin on Cutaneous Wound Healing. Clin. Cosmet. Investig. Dermatol. 2017, 10, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of Astaxanthin Incorporated Collagen Film Developed from the Outer Skin Waste of Squid Doryteuthis Singhalensis for Wound Healing and Tissue Regenerative Applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin Protects against Early Burn-Wound Progression in Rats by Attenuating Oxidative Stress-Induced Inflammation and Mitochondria-Related Apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Bi, J.; Gu, J.; Deng, Y.; Liu, C. Astaxanthin Pretreatment Attenuates Acetaminophen-Induced Liver Injury in Mice. Int. Immunopharmacol. 2017, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Park, Y.-K.; Koo, S.I.; Lee, J.-Y. Astaxanthin Prevents TGFβ1-Induced pro-Fibrogenic Gene Expression by Inhibiting Smad3 Activation in Hepatic Stellate Cells. Biochim. Biophys. Acta 2015, 1850, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S.-J. Astaxanthin Reduces Hepatic Lipid Accumulations in High-Fat-Fed C57BL/6J Mice via Activation of Peroxisome Proliferator-Activated Receptor (PPAR) Alpha and Inhibition of PPAR Gamma and Akt. J. Nutr. Biochem. 2016, 28, 9–18. [Google Scholar] [CrossRef]
- Chan, K.; Chen, S.; Chen, P. Astaxanthin Attenuated Thrombotic Risk Factors in Type 2 Diabetic Patients. J. Funct. Foods 2019, 53, 22–27. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin Improves Glucose Metabolism and Reduces Blood Pressure in Patients with Type 2 Diabetes Mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Song, M.-H.; Rengasamy, K.R.R.; Ko, E.-Y.; Keum, Y.-S. Red Shrimp Are a Rich Source of Nutritionally Vital Lipophilic Compounds: A Comparative Study among Edible Flesh and Processing Waste. Foods 2020, 9, 1179. [Google Scholar] [CrossRef]




| Field | Example of Application | Ref. |
|---|---|---|
| Pharmaceutical | - Excipients and drug carriers - Drug/gene delivery - Cancer diagnosis and treatment | [30,31,32,33,34] |
| Biomedical and biotechnology | - Scaffolds fabrication for tissue regeneration - Three-dimensional cell culture systems - Barrier of chitin-based dressings for microbial infections - Surgical stitches development - Hemostatic dressing - Enzyme immobilization - Biosensors manufacturing | [35,36,37,38,39,40,41,42,43] |
| Cosmetic and cosmeceutical | - Vehicle for active ingredients in skin care | [44,45] |
| Food and nutrition | - Emulsifying, fining, thickening, and stabilizing agents, antioxidants, and low-calorie food mimetics - Composite films/coatings - Food preservation | [46,47] |
| Textile | - Antimicrobial and non-allergenic fibers - Agents for dyes and chemicals removal from textile wastewaters | [48,49] |
| Industrial | - Adsorbents for removing heavy metals from water - Biomaterial for industrial packaging | [50,51] |
| Agriculture | - Plant root growth enhancer - Plant protection (antimicrobial, antifungal, and antiviral activities) | [52] |
| Papermaking industry | - Retention and drainage agents, paper strength agents - Coating agents | [19] |
| Extraction Methods | Treatment | Advantages | Disadvantages | Quality of Chitin |
|---|---|---|---|---|
| Traditional chemical extraction | Acidic treatment: Demineralization with decalcifying agents (HCl, HNO3, H2SO4, CH3COOH, and HCOOH). Alkali treatment: Deproteinization with NaOH. | - Used at the industrial scale for chitin preparation. - Short processing time. | Environmentally unfriendly; high cost because of the effluent treatment generated after acid and alkaline reagents. Removed proteins and minerals cannot be used as human and animal food supplements after being in contact with the acidic and alkali treatment. | Despite the complete removal of organic salts, there can be deacetylation and depolymerization reactions. |
| Biological extraction | Lactic acid treatment: Demineralization with lactic-acid-producing bacteria. Proteases treatment: Deproteinization with proteases-producing bacteria. | - Environmentally safe and low-cost because of the absence of effluent. - Solubilized proteins and minerals may be used as human and animal nutrients. | Limited to laboratory scale studies only; excessive time of process; need for more complex experimental devices. | Homogeneous and high-quality final products. |
| Mechanism of Anticancer Activity of Chitosan and Derivatives | Description | Ref. |
|---|---|---|
| Permeation enhancer | Amino group in chitosan leads to protonation in acidic–neutral medium. The positive charge developed makes chitosan water soluble and bioadhesive to bind with and enhance permeation through negatively charged epithelial surfaces. Therefore, chitosan can enhance the passage of polar drugs through epithelial surfaces. | [145,146,147] |
| Antiangiogenic effect | Chitosan oligosaccharides have an inhibitory effect on angiogenic activities of HUVECs. Chitosan oligosaccharide inhibited cell proliferation activity, cell migration, and vascular endothelial cell growth factor (VEGF) expression of human umbilical vein endothelial cells. VEGF is a potent angiogenic factor. | [145,148,149] |
| Immune enhancer | Chitosan demonstrates immune enhancement through two distinct mechanisms. Firstly, acting as an immune adjuvant, it triggers immune system activation, leading to the generation of proinflammatory factors. Secondly, it serves as a carrier for immunotherapeutic drugs, facilitating targeted delivery and enhancing bioavailability while mitigating systemic toxicity. | [145,150] |
| Apoptotic effect | Low-molecular-weight chitosan can induce G1/S cell cycle arrest and caspase activation in cell lines. | [145,151,152] |
| Shrimp Waste Sources | Type of Extraction | Extraction Conditions | Observations | Ref. |
|---|---|---|---|---|
| Pandalus borealis | Extraction with hexane/isopropanol, (60:40 v/v) | 5 g sample:25 mL solvent; repeated 4 times | 41.1 mg astaxanthin/Kg wet waste material | [218] |
| Litopenaeus vannamei | Stirring with ethyl acetate | 10 g sample: 50 mL solvent; stirred for 30 min at room temperature in darkness | 7 mg astaxanthin/g lipid extract | [219] |
| Penaeus semisulcatus | Stirring with different solvents (acetone, hexane, acetone/hexane (50:50), isopropyl alcohol, isopropyl alcohol/hexane (50:50)) | 10 g sample: 20 mL solvent; stirring (2 min) at room temperature | Best extraction yield with acetone (61.3 µg carotenoids/g waste) | [227] |
| Litopenaeus vannamei, Macrobrachium rosenbergii, Penaeus monodon, Fenneropenaeus chinensis, Penaeus japonicus | Agitation with an oscillator using dichloromethane/methanol (1:3, v/v) as a solvent | 200 mg: 5 mL solvent, agitation for 3 h | From 2.91 μg astaxanthin/g (Penaeus monodon) to 19.20 μg astaxanthin/g (Litopenaeus vannamei) | [22] |
| Litopenaeus vannamei | Blending with ethanol | 500 g sample: 1000 mL solvent. | 28.9 mg astaxanthin/g shrimp shells | [230] |
| Parapenaeopsis sculptili, Metapenaeus lysianassa, Macrobrachium rosenbergii, Metapenaeopsis hardwickii, Penaeus merguiensis, and Penaeus monodon | Mixing with acetone/methanol (7:3, v/v) | 1 g sample: 5 mL solvent at room temperature | P. monodon contained the highest total carotenoid (46.95 µg/mL) and astaxanthin (29.44 µg/g dw) content | [231] |
| Litopenaeus vannamei | Mixing with ethanol | Raw materials were mixed with ethanol (raw material/ethanol, 1:2 w/v); the extraction was repeated in triplicate | The yields of astaxanthin from fresh shrimp heads, cooked shrimp heads, fresh shrimp shells, and cooked shrimp shells were 3.64, 2.38, 14.65, and 11.76 mg/g crude extract | [232] |
| Palaemon serratus and Palaemon varians | Stirring macerations with different solvents (ethanol, 50% ethanol and 70% acetone), different solid/solvent ratios (5 g:50 mL and 5 g:100 mL), and different temperatures (room temperature and 40 °C) were tested | The optimal condition was 5 g of shrimp shell and 50 mL of absolute ethanol solution under agitation for 2 h at room temperature | The mean values ranged between 1.1 and 26.1 μg/g dw | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, N.; Grosso, C.; Delerue-Matos, C. Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties. Mar. Drugs 2024, 22, 153. https://doi.org/10.3390/md22040153
Rossi N, Grosso C, Delerue-Matos C. Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties. Marine Drugs. 2024; 22(4):153. https://doi.org/10.3390/md22040153
Chicago/Turabian StyleRossi, Nicola, Clara Grosso, and Cristina Delerue-Matos. 2024. "Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties" Marine Drugs 22, no. 4: 153. https://doi.org/10.3390/md22040153
APA StyleRossi, N., Grosso, C., & Delerue-Matos, C. (2024). Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties. Marine Drugs, 22(4), 153. https://doi.org/10.3390/md22040153