Purification and Characterization of a Novel Fibrinolytic Enzyme from Marine Bacterium Bacillus sp. S-3685 Isolated from the South China Sea
Abstract
:1. Introduction
2. Results
2.1. Isolation and Identification of Strain S-3685
2.2. Purification of Fibrinolytic Enzyme
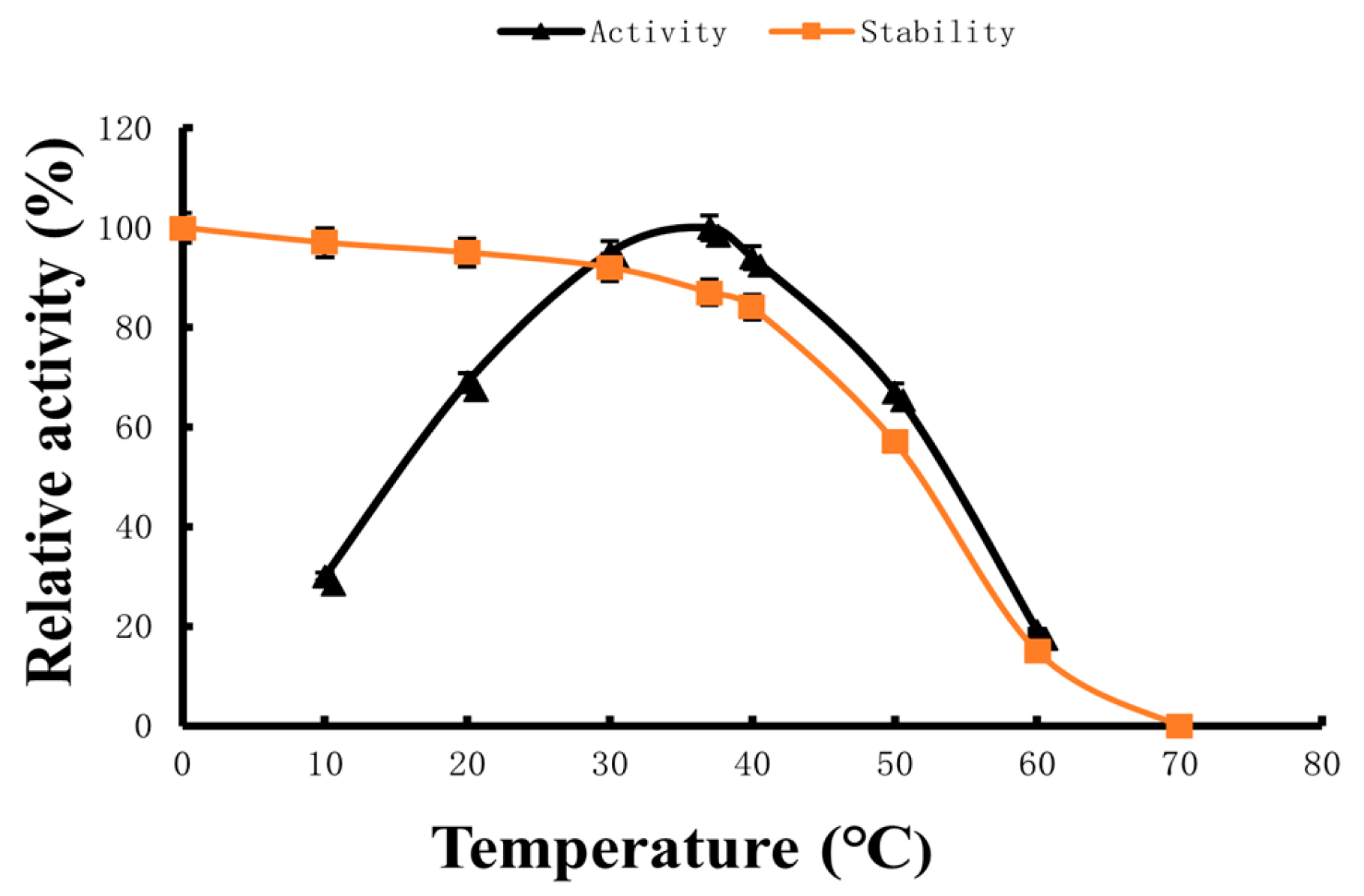
2.3. Optimum Reaction Temperature and Temperature Stability
2.4. Optimum Reaction pH and pH Stability
2.5. Kinetics Parameters of Fibrinolytic Enzyme
2.6. Effect of Different Ions on Fibrinolytic Enzyme Stability
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Separation and Purification
4.2.1. Isolation and Identification of Strain S-3685
4.2.2. Preparation of Sample
4.2.3. Purification by Ion Exchange Chromatography
4.2.4. Dextrin Gel Filtration
4.3. Optimum Reaction Temperature and Temperature Stability
4.4. Optimum Reaction pH and pH Stability
4.5. Effect of Different Ions on Fibrinolytic Enzyme Activity
4.6. Kinetic Parameters of Fibrinolytic Enzyme
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heit, J.A. Venous Thromboembolism: Disease Burden, Outcomes and Risk Factors. J. Thromb. Haemost. 2005, 3, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Næss, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrøm, J. Incidence and Mortality of Venous Thrombosis: A Population-based Study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef]
- Hu, S.-S. Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. J. Geriatr. Cardiol. 2023, 20, 399–430. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Targher, G.; Montagnana, M.; Lippi, G. The Metabolic Syndrome and the Risk of Arterial and Venous Thrombosis. Thromb. Res. 2008, 122, 727–735. [Google Scholar] [CrossRef]
- Björkman, J.-A.E.; Abrahamsson, T.I.; Nerme, V.K.; Mattsson, C.J. Inhibition of Carboxypeptidase U (TAFIa) Activity Improves Rt-PA Induced Thrombolysis in a Dog Model of Coronary Artery Thrombosis. Thromb. Res. 2005, 116, 519–524. [Google Scholar] [CrossRef]
- Tohgi, H.; Konno, S.; Takahashi, S.; Koizumi, D.; Kondo, R.; Takahashi, H. Activated Coagulation/Fibrinolysis System and Platelet Function in Acute Thrombotic Stroke Patients with Increased C-Reactive Protein Levels. Thromb. Res. 2000, 100, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.S. Potential Non-Glycoprotein IIb/IIIa Effects of Abciximab. Am. Heart J. 1999, 138, s1–s5. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Chen, W.-H.; Ng, W.; Cheng, X.; Kwok, J.Y.-Y.; Tse, H.-F.; Lau, C.-P. Low-Dose Aspirin Increases Aspirin Resistance in Patients with Coronary Artery Disease. Am. J. Med. 2005, 118, 723–727. [Google Scholar] [CrossRef]
- Albisetti, M. Thrombolytic Therapy in Children. Thromb. Res. 2006, 118, 95–105. [Google Scholar] [CrossRef]
- Zenych, A.; Fournier, L.; Chauvierre, C. Nanomedicine Progress in Thrombolytic Therapy. Biomaterials 2020, 258, 120297. [Google Scholar] [CrossRef]
- Sinnaeve, P.; Van De Werf, F. Thrombolytic Therapy. Thromb. Res. 2001, 103, S71–S79. [Google Scholar] [CrossRef] [PubMed]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Moula Ali, A.M.; Bavisetty, S.C.B. Purification, Physicochemical Properties, and Statistical Optimization of Fibrinolytic Enzymes Especially from Fermented Foods: A Comprehensive Review. Int. J. Biol. Macromol. 2020, 163, 1498–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, L.; Zhu, Z.; Chen, H.; Liu, C.; Dai, T.; Gong, E.S. Protective Effect of Ovalbumin-Flavonoid Hydrogel on Thrombolytic Activity and Stability of Nattokinase. Food Res. Int. 2022, 156, 111188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Jing, Y.; Zheng, X. Purification and Biochemical Characterization of a Novel Fibrinolytic Enzyme from Culture Supernatant of Coprinus Comatus. Foods 2024, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Takao, T.; Kitatani, F.; Watanabe, N.; Yagi, A.; Sakata, K. A Simple Screening Method for Antioxidants and Isolation of Several Antioxidants Produced by Marine Bacteria from Fish and Shellfish. Biosci. Biotechnol. Biochem. 1994, 58, 1780–1783. [Google Scholar] [CrossRef]
- Arunachalam, K.; Amirtham Jacob Appadorai, R. Antioxidant Potential and Biochemical Evaluation of Metabolites from the Marine Bacteria Virgibacillus sp. Associated with the Sponge Callyspongia Diffusa. Free Radic. Antioxid. 2013, 3, 47–51. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chen, Y.; Chan, W.Y. Marine Natural Products with Anti-Inflammatory Activity. Appl. Microbiol. Biotechnol. 2016, 100, 1645–1666. [Google Scholar] [CrossRef]
- Srilekha, V.; Krishna, G.; Seshasrinivas, V.; Charya, M.S. Antibacterial and Anti-Inflammatory Activities of Marine Brevibacterium sp. Res. Pharm. Sci. 2017, 12, 283. [Google Scholar] [CrossRef]
- Tohme, R.; Darwiche, N.; Gali-Muhtasib, H. A Journey Under the Sea: The Quest for Marine Anti-Cancer Alkaloids. Molecules 2011, 16, 9665–9696. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically Active Secondary Metabolites from Marine Cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mayer, L.M.; Weston, D.P.; Bock, M.J.; Jumars, P.A. Inhibition of Digestive Enzyme Activities by Copper in the Guts of Various Marine Benthic Invertebrates. Environ. Toxicol. Chem. 2002, 21, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Nakata, H.; Tanabe, S. In Vitro Inhibition of Hepatic Cytochrome P450 and Enzyme Activity by Butyltin Compounds in Marine Mammals. Environ. Poll. 1998, 99, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Gohel, V.; Chaudhary, T.; Vyas, P.; Chhatpar, H.S. Isolation and Identification of Marine Chitinolytic Bacteria and Their Potential in Antifungal Biocontrol. Indian. J. Exp. Biol. 2004, 42, 715–720. [Google Scholar] [PubMed]
- El Amraoui, B.; El Amraoui, M.; Cohen, N.; Fassouane, A. Antifungal and Antibacterial Activity of Marine Microorganisms. Ann. Pharm. Franç. 2014, 72, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Mini-Review: Inhibition of Biofouling by Marine Microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; He, L.; Sun, Z.; Cui, Z.; Du, Y.; Kong, Y. Purification and Biochemical Characterization of a Novel Fibrinolytic Enzyme from Streptomyces sp. P3. J. Microbiol. Biotechnol. 2015, 25, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Chitte, R.R.; Dey, S. Potent Fibrinolytic Enzyme from a Thermophilic Streptomyces megasporus Strain SD5. Lett. Appl. Microbiol. 2000, 31, 405–410. [Google Scholar] [CrossRef]
- Dhamodharan, D. Novel Fibrinolytic Protease Producing Streptomyces radiopugnans VITSD8 from Marine Sponges. Mar. Drugs 2019, 17, 164. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Q.; Zhang, Z.; Wang, W.; Wei, D.; Huang, Y.; Liu, Z.; Shi, Y.; Goodfellow, M. Streptomyces radiopugnans sp. Nov., a Radiation-Resistant Actinomycete Isolated from Radiation-Polluted Soil in China. Int. J. Syst. Evol. Microbiol. 2007, 57, 2578–2582. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, Y.; Chu, M.; Zhu, J.; Zhang, Y.; Tang, Q.; Osman, G.; Jiang, L.; Zhang, Z. Screening of a Novel Fibrinolytic Enzyme-Producing Streptomyces from a Hyper-Arid Area and Optimization of Its Fibrinolytic Enzyme Production. Fermentation 2023, 9, 410. [Google Scholar] [CrossRef]
- Sharma, C.; Osmolovskiy, A.; Singh, R. Microbial Fibrinolytic Enzymes as Anti-Thrombotics: Production, Characterisation and Prodigious Biopharmaceutical Applications. Pharmaceutics 2021, 13, 1880. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Belur, P.D.; Subramanya, S.B. Methods Available to Assess Therapeutic Potential of Fibrinolytic Enzymes of Microbial Origin: A Review. J. Anal. Sci. Technol. 2018, 9, 10. [Google Scholar] [CrossRef]
- Sharma, C.; Salem, G.E.M.; Sharma, N.; Gautam, P.; Singh, R. Thrombolytic Potential of Novel Thiol-Dependent Fibrinolytic Protease from Bacillus cereus RSA1. Biomolecules 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, P.; Rengaswamy, D. Fibrinolytic Enzyme—An Overview. Curr. Pharm. Biotechnol. 2022, 23, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Pathom-aree, W. Actinobacteria From Desert: Diversity and Biotechnological Applications. Front. Microbiol. 2021, 12, 765531. [Google Scholar] [CrossRef] [PubMed]
- Bay, S.K.; Waite, D.W.; Dong, X.; Gillor, O.; Chown, S.L.; Hugenholtz, P.; Greening, C. Chemosynthetic and Photosynthetic Bacteria Contribute Differentially to Primary Production across a Steep Desert Aridity Gradient. ISME J. 2021, 15, 3339–3356. [Google Scholar] [CrossRef] [PubMed]
- Molina-Menor, E.; Gimeno-Valero, H.; Pascual, J.; Peretó, J.; Porcar, M. High Culturable Bacterial Diversity From a European Desert: The Tabernas Desert. Front. Microbiol. 2021, 11, 583120. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Yu, B.; Chen, G.; Liang, Z. Purification and Characterization of a Fibrinolytic Enzyme from Marine Bacillus velezensis Z01 and Assessment of Its Therapeutic Efficacy In Vivo. Microorganisms 2022, 10, 843. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Kumar, T.S.J.; Vijayaraghavan, P.; Elshikh, M.S.; Alkufeidy, R.M.; Alkubaisi, N.A.; Alshammari, M.K. Enhanced Production, Purification and Biochemical Characterization of Therapeutic Potential Fibrinolytic Enzyme from a New Bacillus flexus from Marine Environment. J. King Saud Univ. Sci. 2020, 32, 3174–3180. [Google Scholar] [CrossRef]
- Kumar, S.S.; Haridas, M.; Abdulhameed, S. A Novel Fibrinolytic Enzyme from Marine Pseudomonas aeruginosa KU1 and Its Rapid in Vivo Thrombolysis with Little Haemolysis. Int. J. Biol. Macromol. 2020, 162, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lin, X.; Fu, J.; Zhang, J.; Tang, W.; He, Z. A Novel Bi-Functional Fibrinolytic Enzyme with Anticoagulant and Thrombolytic Activities from a Marine-Derived Fungus Aspergillus Versicolor ZLH-1. Mar. Drugs 2022, 20, 356. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Khan, Z.; Tamadoni Jahromi, S.; Pourmozaffar, S.; Gozari, M.; Nahavandi, R. A Critical Review on Marine Serine Protease and Its Inhibitors: A New Wave of Drugs? Int. J. Biol. Macromol. 2021, 170, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-J.; Choi, K.-H.; Kim, M.-J.; Park, C.-S.; Cha, J. Purification and Characterization of a New Fibrinolytic Enzyme of Bacillus licheniformis KJ-31, Isolated from Korean Traditional Jeot-Gal. J. Microbiol. Biotechnol. 2007, 17, 1469–1476. [Google Scholar] [PubMed]
- Masilamani, R.; Natarajan, S. Molecular Cloning, Overexpression and Characterization of a New Thiol-Dependent, Alkaline Serine Protease with Destaining Function and Fibrinolytic Potential from Marinobacter aquaeolei MS2-1. Biologia 2015, 70, 1143–1149. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Belur, P.D. A Novel Fibrinolytic Serine Metalloprotease from the Marine Serratia marcescens subsp. sakuensis: Purification and Characterization. Int. J. Biol. Macromol. 2018, 112, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.E.D.C.E.; Barros, R.C.D.; Albuquerque, W.W.C.; Brandão, R.M.P.; Bezerra, R.P.; Porto, A.L.F. In Vitro Thrombolytic Activity of a Purified Fibrinolytic Enzyme from Chlorella vulgaris. J. Chromatogr. B 2018, 1092, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, P.; Prakash Vincent, S.G. A Low Cost Fermentation Medium for Potential Fibrinolytic Enzyme Production by a Newly Isolated Marine Bacterium, Shewanella sp. IND20. Biotechnol. Rep. 2015, 7, 135–142. [Google Scholar] [CrossRef]
- Barros, P.D.S.D.; Silva, P.E.C.E.; Nascimento, T.P.; Costa, R.M.P.B.; Bezerra, R.P.; Porto, A.L.F. Fibrinolytic Enzyme from Arthrospira platensis Cultivated in Medium Culture Supplemented with Corn Steep Liquor. Int. J. Biol. Macromol. 2020, 164, 3446–3453. [Google Scholar] [CrossRef]
- Yao, Z.; Kim, J.A.; Kim, J.H. Properties of a Fibrinolytic Enzyme Secreted by Bacillus subtilis JS2 Isolated from Saeu (Small Shrimp) Jeotgal. Food Sci. Biotechnol. 2018, 27, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Pan, S.; Chen, G.; Huang, S.; Zhang, Z.; Li, Y.; Liang, Z. Biochemical Characteristics of a Fibrinolytic Enzyme Purified from a Marine Bacterium, Bacillus subtilis HQS-3. Int. J. Biol. Macromol. 2013, 62, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Kim, J.A.; Kim, J.H. Gene Cloning, Expression, and Properties of a Fibrinolytic Enzyme Secreted by Bacillus pumilus BS15 Isolated from Gul (Oyster) Jeotgal. Biotechnol. Bioproc. E 2018, 23, 293–301. [Google Scholar] [CrossRef]
- Mahajan, P.M.; Nayak, S.; Lele, S.S. Fibrinolytic Enzyme from Newly Isolated Marine Bacterium Bacillus subtilis ICTF-1: Media Optimization, Purification and Characterization. J. Biosci. Bioeng. 2012, 113, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T. Purification and Characterization of Fibrinolytic Enzyme from Bacillus velezensis and Evaluation of Its Thrombolytic Effect In Vitro/Vivo. Ph.D. Thesis, Guangxi University, Nanning, China, 2022. [Google Scholar]
- Hu, H.L. Screening and Inhibitory Activity of Polypeptide Toxin Acting on Type IItransmembrane Serine Protease Matriptase. Master’s Thesis, Hunan Normal University, Changsha, China, 2021. [Google Scholar]
- Li, F.; Li, Z.P. Expression and Activity of Tissue-type Plasminogen Activator Mutant Reteplase with Deletion of PAI-1 Binding Sites. Prog. Modern Biomed. 2014, 14, 3029–3032. [Google Scholar]
- Li, Y. Purification, Characterization, Gene Cloning and Exression of the Cocoonase from the Chinese Oak Silkworm, Antheraea pernyi. Master’s Thesis, Dalian University of Technology, Dalian, China, 2013. [Google Scholar]
- Chen, T.C.; Wang, P.W. Purification and biochemical properties of a fibrinolytic enzyme from Bacillus subtitles fermented red bean. Food Chem. 2012, 133, 1611–1617. [Google Scholar]
- Poonam, M.; Seung, S.C. A low molecular weight chymotrypsin-like novel fibrinolytic enzyme from Streptomyces sp. CS624. Proc. Biochem. 2011, 46, 1449–1455. [Google Scholar]
- Ju, X.Y.; Cao, X.Y.; Sun, Y. Purification and characterization of a fibrinolytic enzyme from Streptomyces sp. XZNUM 00004. World J. Microbiol. Biotechnol. 2012, 28, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Hoe, C.K.; Bong, S.C. Purification and characterization of a novel, highly potent fibrinolytic enzyme from Paecilomyces tenuipes. Proc. Biochem. 2011, 46, 1545–1553. [Google Scholar]
- Yang, H.; Liu, Y.; Ning, Y.; Wang, C.; Zhang, X.; Weng, P.; Wu, Z. Characterization of an Intracellular Alkaline Serine Protease from Bacillus velezensis SW5 with Fibrinolytic Activity. Curr. Microbiol. 2020, 77, 1610–1621. [Google Scholar] [CrossRef]
- Lu, M.; Gao, Z.; Xing, S.; Long, J.; Li, C.; He, L.; Wang, X. Purification, Characterization, and Chemical Modification of Bacillus velezensis SN-14 Fibrinolytic Enzyme. Int. J. Biol. Macromol. 2021, 177, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Taneja, K.; Bajaj, B.K.; Kumar, S.; Dilbaghi, N. Production, Purification and Characterization of Fibrinolytic Enzyme from Serratia sp. KG-2-1 Using Optimized Media. 3 Biotech 2017, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.E.; De Souza, F.A.S.D.; Teixeira, J.A.; Porto, T.S.; Porto, A.L.F. Integrated Process Production and Extraction of the Fibrinolytic Protease from Bacillus sp. UFPEDA 485. Appl. Biochem. Biotechnol. 2013, 170, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, W.; Han, B.; Xu, R. Biochemical and Enzymatic Properties of a Novel Marine Fibrinolytic Enzyme from Urechis unicinctus. Appl. Biochem. Biotechnol. 2007, 136, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Ambati, R.R.; Cai, Z.; Lei, B. Purification and Characterization of Fibrinolytic Enzyme from a Bacterium Isolated from Soil. 3 Biotech 2018, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Purification and Characterization of a Novel Alkaline Serine Protease Secreted by Vibrio metschnikovii. Int. J. Mol. Med. 2011, 29, 263–268. [Google Scholar] [CrossRef]
- Jespersen, J.; Astrup, T. A study of the fibrin plate assay of fibrinolytic agents. Optimal conditions, reproducibility and precision. Haemostasis 1983, 13, 301–315. [Google Scholar]



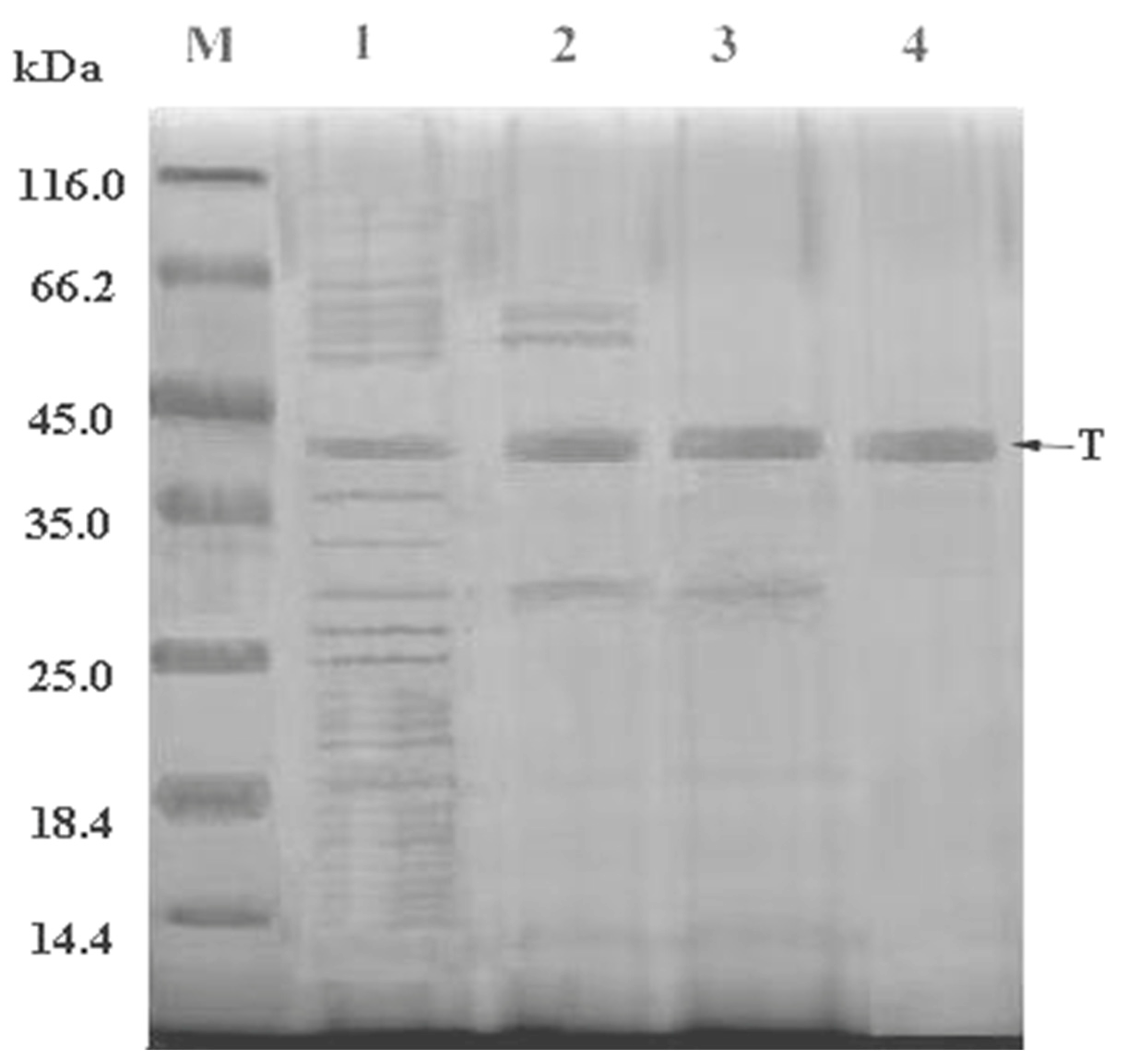

| Purification Steps | Total Activity A (×103 U) | Total Protein B (mg) | Specific Activity (U/mg) | Yield (%) | Purification Fold |
|---|---|---|---|---|---|
| Culture broth | 1956.23 ± 10.12 | 2871.67 ± 9.37 | 681.30 ± 0.53 | 100 | 1 |
| Ammonium sulfate precipitation | 1406.38 ± 7.75 | 2002.53 ± 8.13 | 702.29 ± 0.78 | 69.73 ± 2.12 | 1.03 |
| Q-Sepharose FF anion exchange | 832.65 ± 5.06 | 1153.64 ± 6.31 | 721.20 ± 1.03 | 40.18 ± 1.45 | 1.05 |
| Sephacryl®S-100 gel filtration | 533.37 ± 1.93 | 723.75 ± 3.57 | 736.44 ± 1.51 | 25.21 ± 1.02 | 1.08 |
| Metal Ions | Relative Activity (%) | Metal Ions | Relative Activity (%) |
|---|---|---|---|
| None | 100 | ||
| Na+ (NaCl) | 109 | Mn2+ (MnCl2) | 103 |
| Ba2+ (BaCl2) | 107 | Mg2+ (MgCl2) | 98.6 |
| K+ (KCl) | 103 | Al3+ (AlCl3) | 105 |
| Fe3+ (FeCl3) | 96.9 | Zn2+ (ZnCl2) | 95.3 |
| Co2+ (CoCl2) | 102 | Cu2+ (CuCl2) | 102 |
| Ca2+ (CaCl2) | 92.0 | Fe2+ (FeCl2) | 92.6 |
| Enzyme | Km | Vmax |
|---|---|---|
| BSFE1 | 2.1 μM | 49.0 μmol min−1 mg−1 |
| Velefibrinase [55] | 18.2 μM | 59.5 μmol min−1 mg−1 |
| Matriptase [56] | 30.9 μM | |
| Cocoonase [57] | 2.58 μM | 40.9 μmol min−1 mg−1 |
| Li [58] | 0.33 μM | |
| Chen [59] | 59.0 μM | |
| CS624 [60] | 21.8 μM | 84.0 μmol min−1 mg−1 |
| XZNUM 00004 [61] | 0.96 mg/mL | |
| Paecilomyces [62] | 17.0 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Elango, J.; Hao, J.; Wu, W. Purification and Characterization of a Novel Fibrinolytic Enzyme from Marine Bacterium Bacillus sp. S-3685 Isolated from the South China Sea. Mar. Drugs 2024, 22, 267. https://doi.org/10.3390/md22060267
Ma Z, Elango J, Hao J, Wu W. Purification and Characterization of a Novel Fibrinolytic Enzyme from Marine Bacterium Bacillus sp. S-3685 Isolated from the South China Sea. Marine Drugs. 2024; 22(6):267. https://doi.org/10.3390/md22060267
Chicago/Turabian StyleMa, Zibin, Jeevithan Elango, Jianhua Hao, and Wenhui Wu. 2024. "Purification and Characterization of a Novel Fibrinolytic Enzyme from Marine Bacterium Bacillus sp. S-3685 Isolated from the South China Sea" Marine Drugs 22, no. 6: 267. https://doi.org/10.3390/md22060267





