Abstract
Cyclic glycine-proline (cGP), a prevalent marine cyclic dipeptide, possesses a distinct pyrrolidine-2,5-dione scaffold, which contributes to the chemical diversity and broad bioactivities of cGP. The diverse sources from marine-related, endogenous biological, and synthetic pathways and the in vitro and in vivo activities of cGP are reviewed. The potential applications for cGP are also explored. In particular, the pivotal roles of cGP in regulating insulin-like growth factor-1 homeostasis, enhancing neuroprotective effects, and improving neurotrophic function in central nervous system diseases are described. The potential roles of this endogenous cyclic peptide in drug development and healthcare initiatives are also highlighted. This review underscores the significance of cGP as a fundamental building block in drug discovery with exceptional drug-like properties and safety. By elucidating the considerable value of cGP, this review aims to reignite interest in cGP-related research within marine medicinal chemistry and synthetic biology.
1. Introduction
Recently, the concept of ‘drugs from the sea’ has gained widespread acceptance, exemplified by marine-derived cyclic peptides like ziconotide and plitidepsin, which have been approved for the treatment of severe pain and multiple myeloma, respectively [1,2,3]. Interest in marine-derived cyclic peptides in drug discovery surged from 2010 to 2020; an average of 268 related publications per year described the broad biological activity and structural diversity of cyclic peptides [4]. Cyclic peptides lack the inherent disadvantages of linear peptides, including poor membrane permeability and enzymatic stability [5]. Their constrained conformation results in minimal entropic cost when binding to biological targets. Thus, cyclic peptides exhibit superior pharmacological properties compared to their linear counterparts [6]. The unique characteristics of cyclic peptides foster the development of novel drugs with extensive prospects, including antibiotics, immunosuppressants, and inhibitors of protein–protein interaction [7].
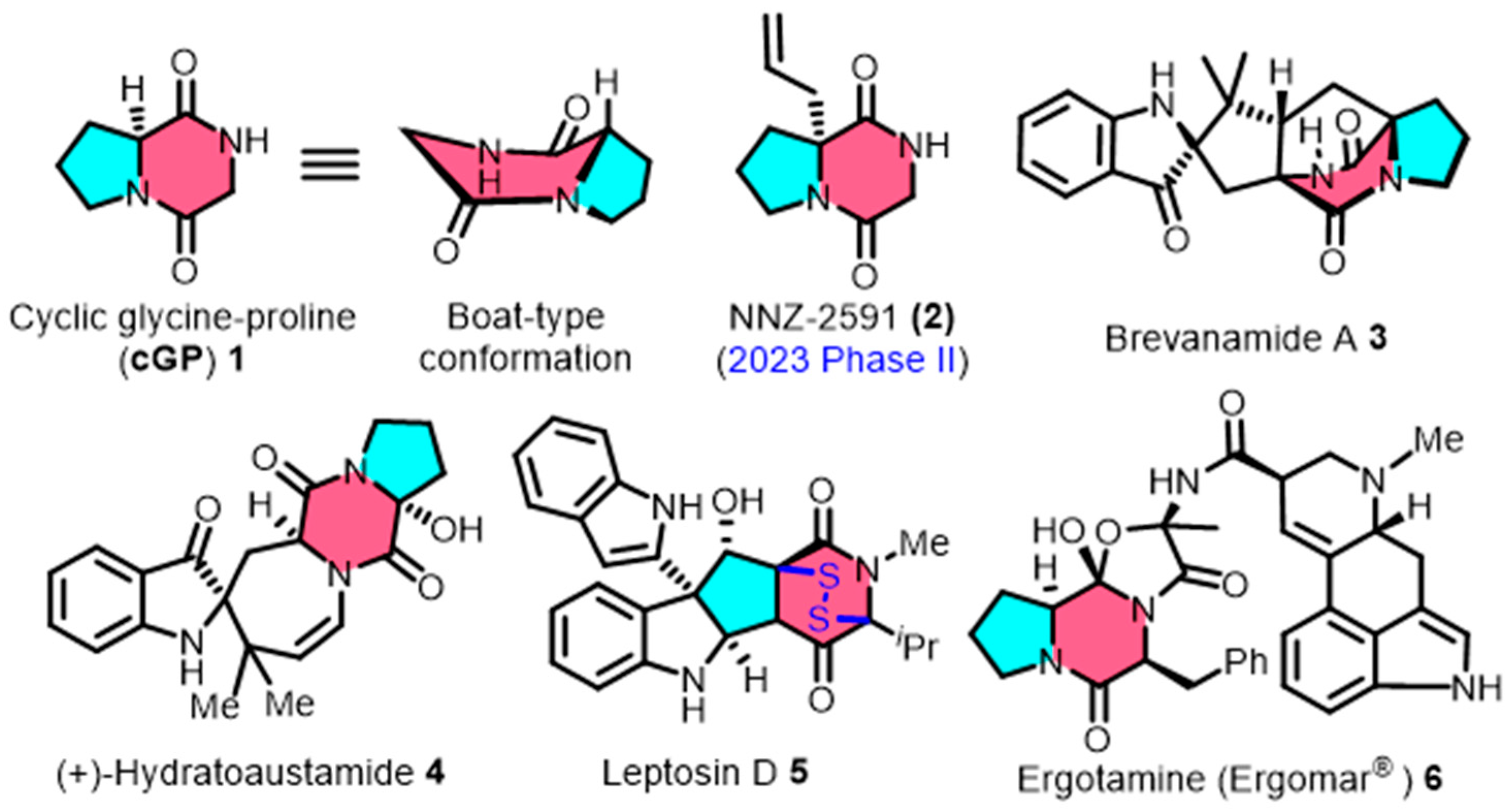
Cyclic glycine-proline (cGP, 1) is a small-molecule cyclic dipeptide, which is widely sourced from the secondary metabolism of marine microorganisms [8,9]. Cyclic GP consists of glycine and proline, which form a distinctive pyrrolidinedione framework and display a unique non-planar stereo structure (Figure 1). This molecule is found in the structural composition of clinical drugs and the fundamental skeletons of complex natural products. For example, the derivative NNZ-2591 (2) is currently undergoing Phase II clinical trials for the treatment of developmental neurological disorders [10]. The migraine medication ergotamine (Ergomar®) (6) also contains cGP [11]. Additionally, the cGP structure is present in natural products, including brevanamide A (3) [12], (+) hydratoaustamide (4) [13,14], and leptosin D (5) [15]. The cage-shaped bicyclo[2.2.2]diazaoctane (3) and the disulfide-bridged epipolythiodioxopiperazines (5) both incorporate cGP in their construction.
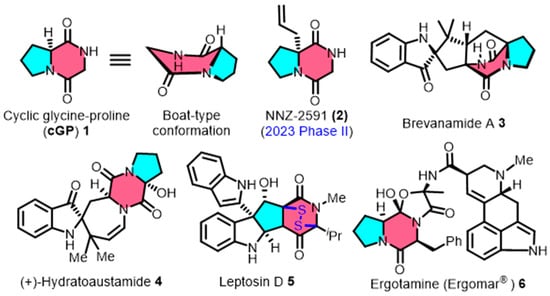
Figure 1.
The spatial conformation of cyclic glycine-proline (cGP), along with representative clinical drugs and complex natural products containing cGP scaffold.
Given the structural importance of cGP in drug discovery and its abundance in complex natural products, the potential of cGP has been extensively explored. Previous research focused on the optical chirality [16], diverse derivatives, and biological activities of cGP [8,9]. However, the potential of cGP-containing marine microbial organisms as a promising sustainable drug source has received less attention. This brief review covers various sources of cGP, including marine origins, biotransformation, and chemical synthesis, focusing on literature published between 2000 and 2022. Investigations into the in vitro and in vivo activity of cGP and the applications of cGP in preclinical drug discovery are highlighted. Additionally, future perspectives on engineered biosynthesis derived from marine microbial, diversity-building block libraries, and preclinical neurological drug candidates are discussed. This review provides valuable insights for further research and development of cGP.
2. Sources: Marine Origins, Endogenous Biological Transformation, and Chemical Synthesis
2.1. Marine Origins
The prevalent marine secondary metabolite of cGP was first isolated from Gulf of Mexico starfish, and its structure was later confirmed through X-ray analysis by Von Dreele in 1975 [17]. A decade later, the secondary metabolite of cGP was also found in Diodon novemacultus from the South China Sea [18]. Traces of the cyclic dipeptide have been discovered in various sources, including terrestrial fungi [19,20,21,22,23], lake cyanobacteria [24], animals [25], and even in food items such as coffee [26,27], beef [28], and blackcurrant [29]. However, marine organisms have emerged as a noteworthy natural source of cGP. This review primarily focuses on marine sources of cGP up to 2022, due to the prevalence of cGP in marine natural product chemistry (Table 1) [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. Interestingly, the majority of collection sites for marine sources of cGP are located in tropical and subtropical coastal areas [47], and several sites are closely associated with mangroves. In these environments, microorganisms, such as Penicillium, Aspergillus, and Streptomyces, can produce cGP and its diverse derivatives. Although extensive research has focused on the structural diversity of this cyclic peptide in marine natural product chemistry, biosynthesis of cGP has received less attention. Exploring the biosynthesis of cGP using genetically informative microbes may provide a sustainable drug source and offer a novel approach to achieving cyclic peptide green synthesis.

Table 1.
Overview of marine sources of cyclic glycine-proline (cGP) with their bioactivities from 2000 to 2022.
2.2. Endogenous Biological Transformation
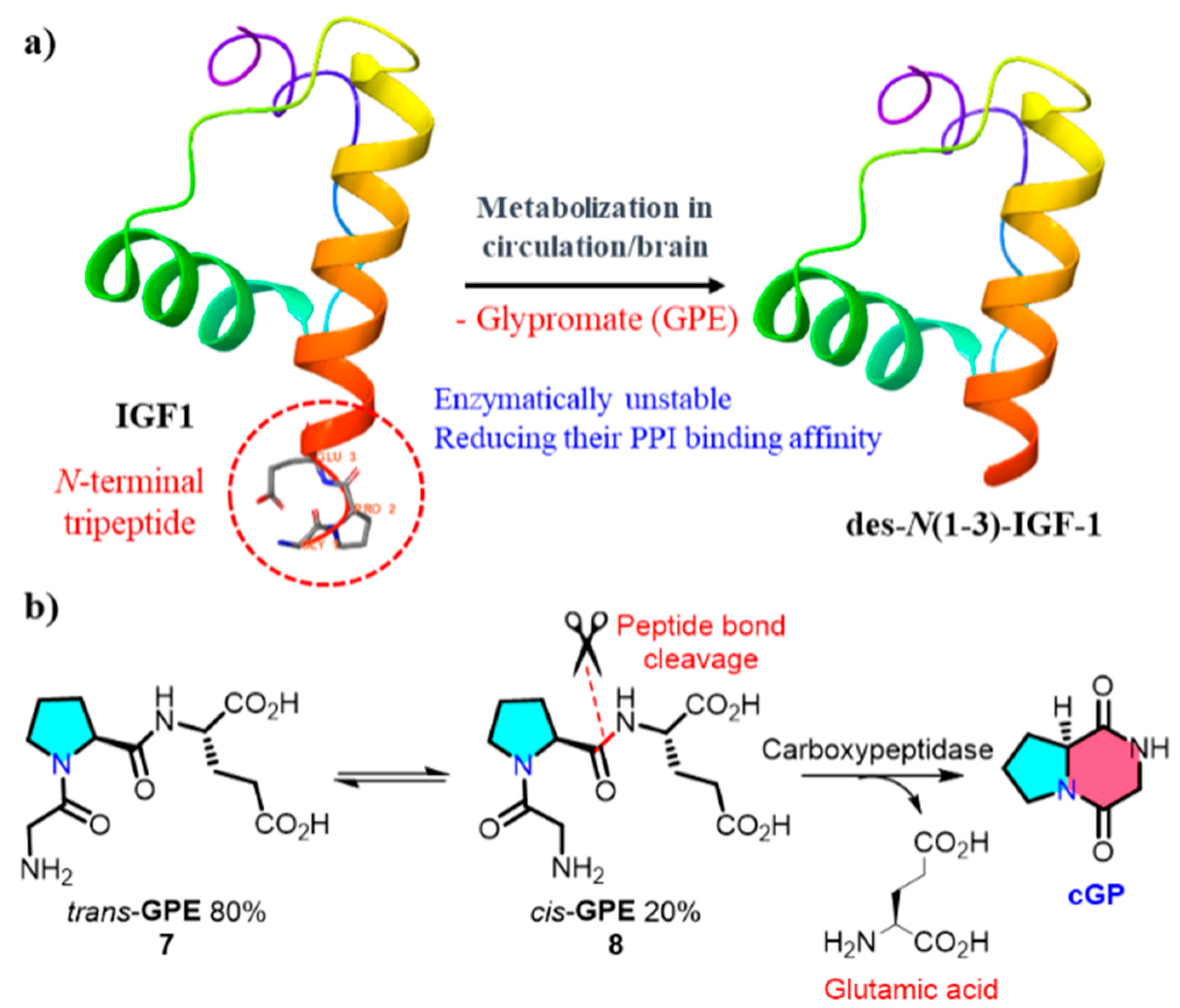
An endogenous biological source of cGP was identified by Gudasheva et al. in 1996; cGP was identified as an endogenous bioactive cyclic dipeptide in the rat brain at 2.8 nmol/g. The anti-amnesic activity of cGP, which is associated with memory regulation, was also demonstrated in this study [48]. Insulin-like growth factor-1 (IGF-1) is an insulin-like hormone that plays crucial regulatory roles in human growth, development, and metabolic processes [49]. Clinical trials demonstrated that low concentrations of cGP and decreased cGP/IGF-1 ratios in the plasma are associated with impaired IGF-1 function during the early stages of stroke. After ischemic brain injury, cGP exhibits neuroprotective effects and shares pharmacological similarities with IGF-1 [50,51]. IGF-1 undergoes enzymatic cleavage in the plasma and brain tissues, releasing des-N(1-3)-IGF-1 residues and linear glycine-proline glutamic acid (GPE, cis/trans = 1/4) (Figure 2). The reversible cis-GPE (8), metabolised by carboxypeptidase to release glutamic acid and cGP, dynamically regulates the bioavailability of cGP in the circulation to restore the normalisation of IGF-1 [52]. More details on the physiological functions of cGP will be elucidated in the next section.
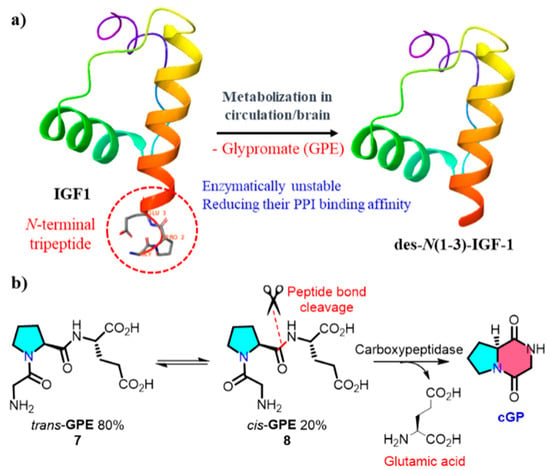
Figure 2.
(a) Metabolism of IGF-1 within the organism; (b) formation of endogenous cyclic glycine-proline (PPI, protein–protein interaction).
2.3. Chemical Synthesis
Hayasaka et al. developed a cost-effective strategy for generating tripeptide components rich in Gly-Pro-Y sequences, which were derived from the enzymatic hydrolysis of proteins. Subsequent heating converts the tripeptide into the cyclic glycine-proline dipeptide, with a conversion rate of 78%. This method represents an economically viable biotransformation protocol for achieving large-scale synthesis [53].
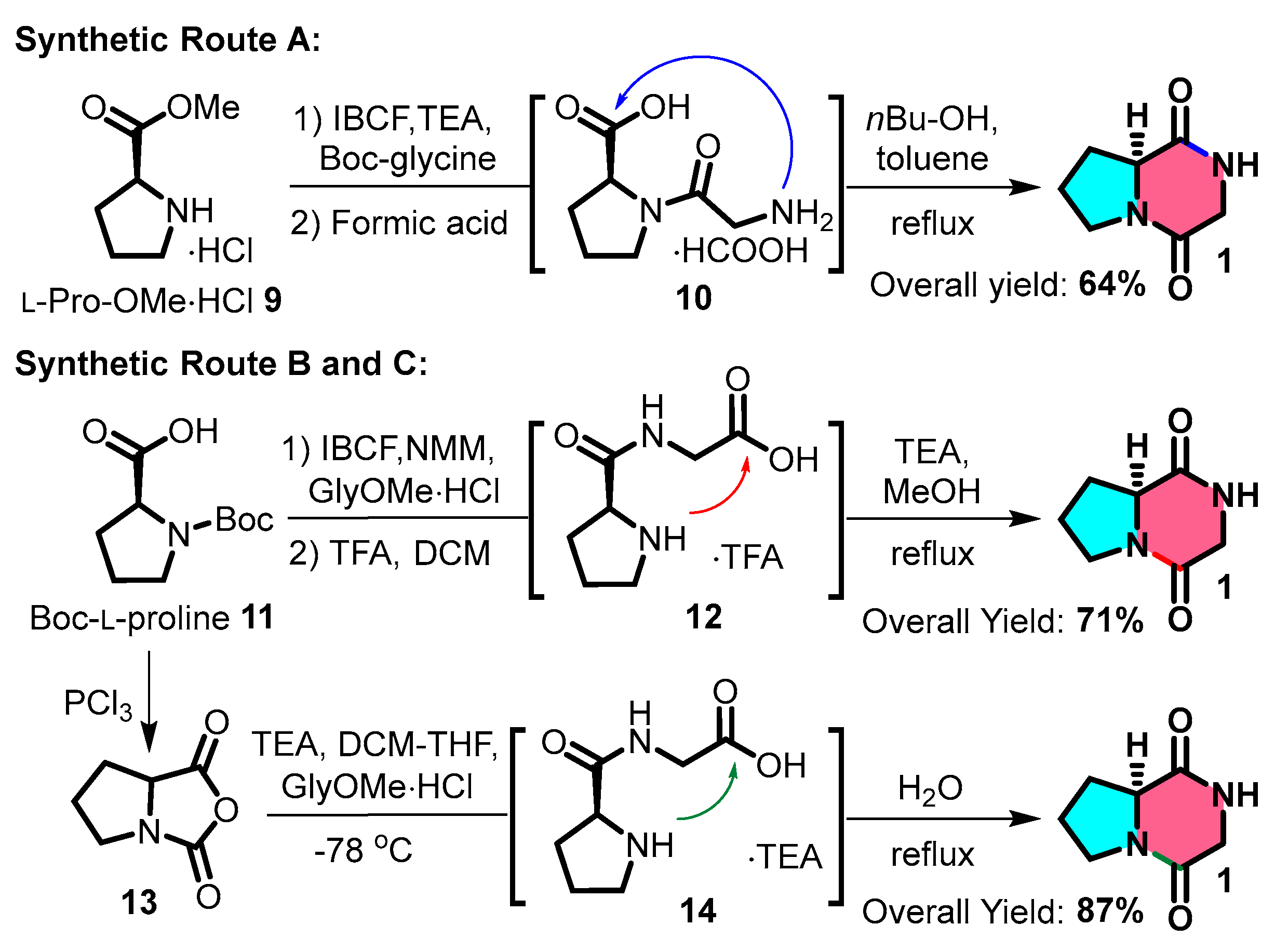
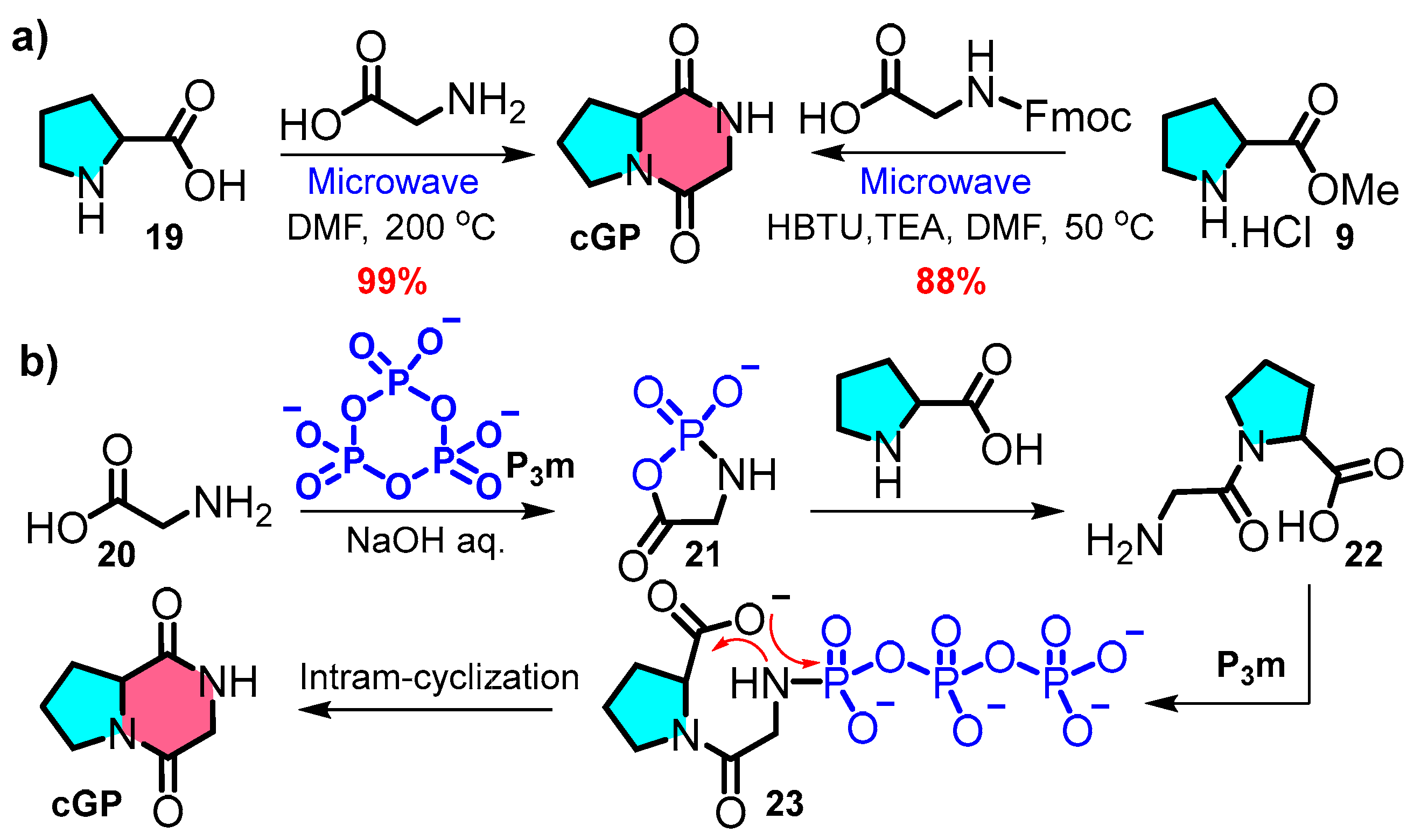
The routine synthesis of cGP typically involves several steps. N-Boc-protected glycine can be coupled with methylated L-proline 9 using isobutyl chloroformate and triethylamine (TEA). After removing the N-Boc protecting group, the intermediate undergoes intramolecular cyclisation upon heating, producing a cyclic dipeptide at a 64% yield (Figure 3: Route A). Another method involves a C-N bond-forming sequence using N-Boc-L-proline 11, followed by the steps listed above to produce a cyclic dipeptide at a 71% yield (Figure 3: Route B) [16,54,55]. A third approach includes acylating N-Boc-L-proline and coupling the resulting product with glycine methyl ester hydrochloride; refluxing with water produces a cyclic dipeptide at a yield of 87% (Figure 3: Route C) [56]. The third approach (synthetic pathway C) exhibits superior atom economy; peptide bond formation is achieved without condensing agents, providing a potential strategy for streamlined, multistep continuous flow synthesis in the future.
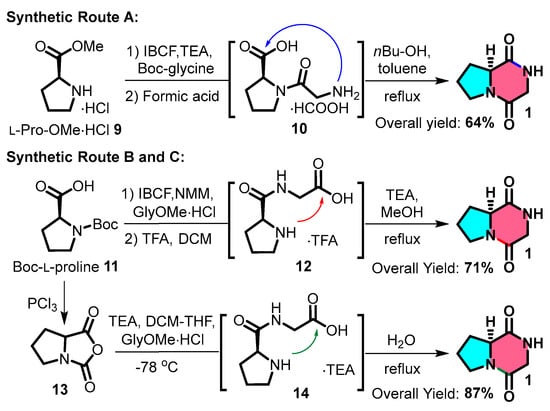
Figure 3.
General synthetic route for cyclic glycine-proline.
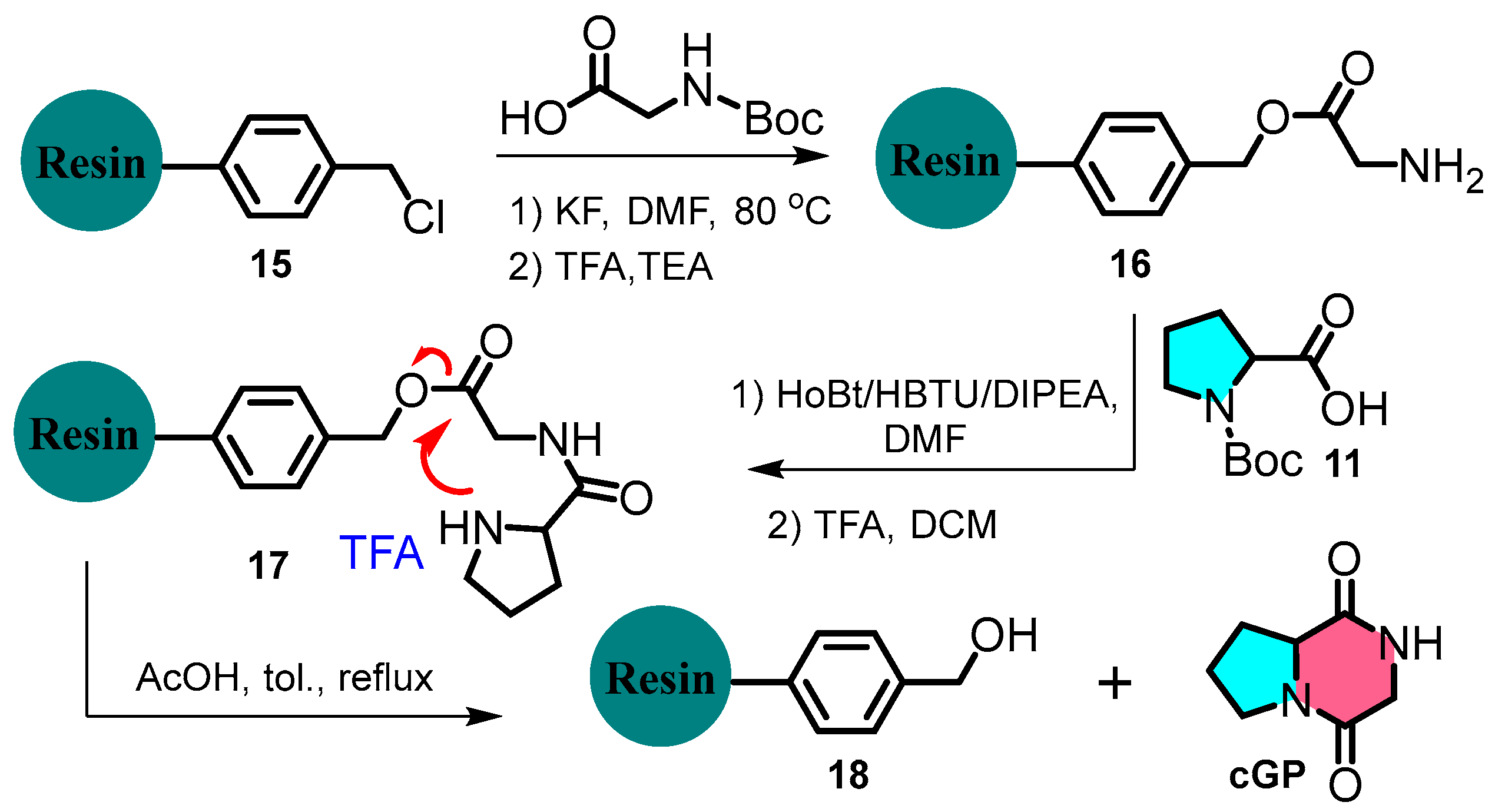
Chen et al. devised a rapid and flexible method to expand cyclic dipeptide diversity using immobilised Merrifield solid-phase peptide synthesis (Figure 4) [28]. For example, the N-Boc-glycine residue undergoes deprotection and condensation with N-Boc-L-proline, catalysed by HBTU/HOBt. The product is subsequently treated with TFA and TEA to form a cyclic dipeptide. This strategy contrasts with liquid-phase peptide synthesis and offers an efficient, versatile, and scalable approach to cyclic dipeptide synthesis.
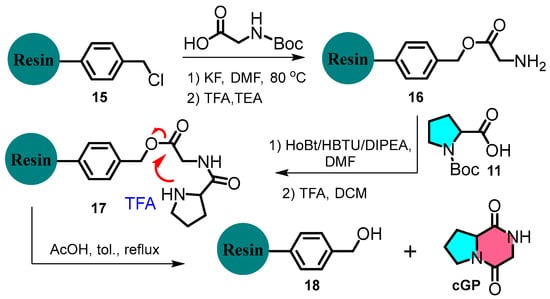
Figure 4.
Solid-phase peptide synthesis of cyclic glycine-proline.
Compared to the traditional multistep synthesis, a concise and efficient synthetic strategy offers a novel approach aligned with the principles of atom economy and environmentally friendly synthesis. Ahonen et al. introduced an effective green synthetic one-step synthesis protocol at a 100-milligram scale based on microwave reactions (Figure 5a left) [57]. The interaction of L-proline with glycine under high-temperature microwave conditions leads to rapid product formation through direct cooling and filtration and eliminates the need for chromatography purification. A similar ultrasound-assisted process, incorporating intramolecular cyclisation within a one-pot synthesis, was reported by Poonia et al. (Figure 5a right) [58]. The streamlined synthesis of cyclic peptides from free amino acids saves time and costs and reduces waste.
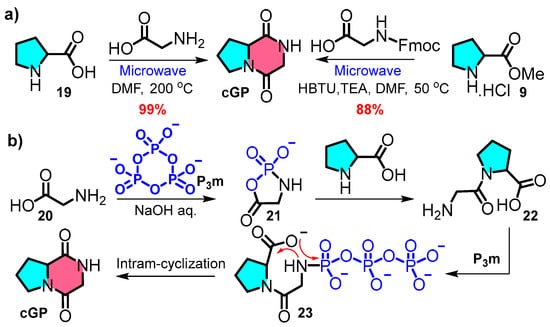
Figure 5.
(a) One-pot microwave protocol; (b) biomimetic synthetic route.
Zhao et al. demonstrated the prebiotic synthesis of cyclic peptides. In this reaction, the spontaneous formation of cyclic peptides from linear dipeptide 22 was facilitated by intramolecular cyclisation and had a calculated reaction barrier of 33.2 kcal·mol−1 [59,60]. Furthermore, the phosphorylation of glycine 20 by trimetaphosphate (P3m) generates an active five-membered intermediate 21, which interacts with L-proline and is further catalysed by P3m to yield cGP with a theoretical yield of 97% (Figure 5b).
3. In Vitro and In Vivo Effects
In vitro screening in tumour cells revealed the anti-tumour effects of cGP, ranging from modest to weak. For example, cGP exhibited an IC50 value of 101.8 µM against HepG2 cells [42] and an IC50 value of 206 µM against A549 cells [23], indicating limited anti-tumour activity. The effects of cGP on cytokine release have also been investigated. Jia et al. observed that cGP, at a concentration of 5.0 µg/mL, substantially upregulated IFN-c in the murine macrophage-like cell line J774A.1 but had only moderate effects in MCP-1 and IL-10 cells and had only a minor impact on TNF-α secretion [43]. In contrast, Khan et al. detected the inhibition of TNF-α release by cGP in LPS-induced RAW 264.7 macrophage, with an IC50 value of 4.5 µg/mL [61]. This was accompanied by the downregulation of IL-1β and IL-6 expression. The notable decrease in cGP levels during LPS-induced NO production, resulting in minimal cytotoxicity, underscores the promising anti-inflammatory properties of cGP. These results emphasise the need for further development of this small cyclic dipeptide as a cytokine modulator.
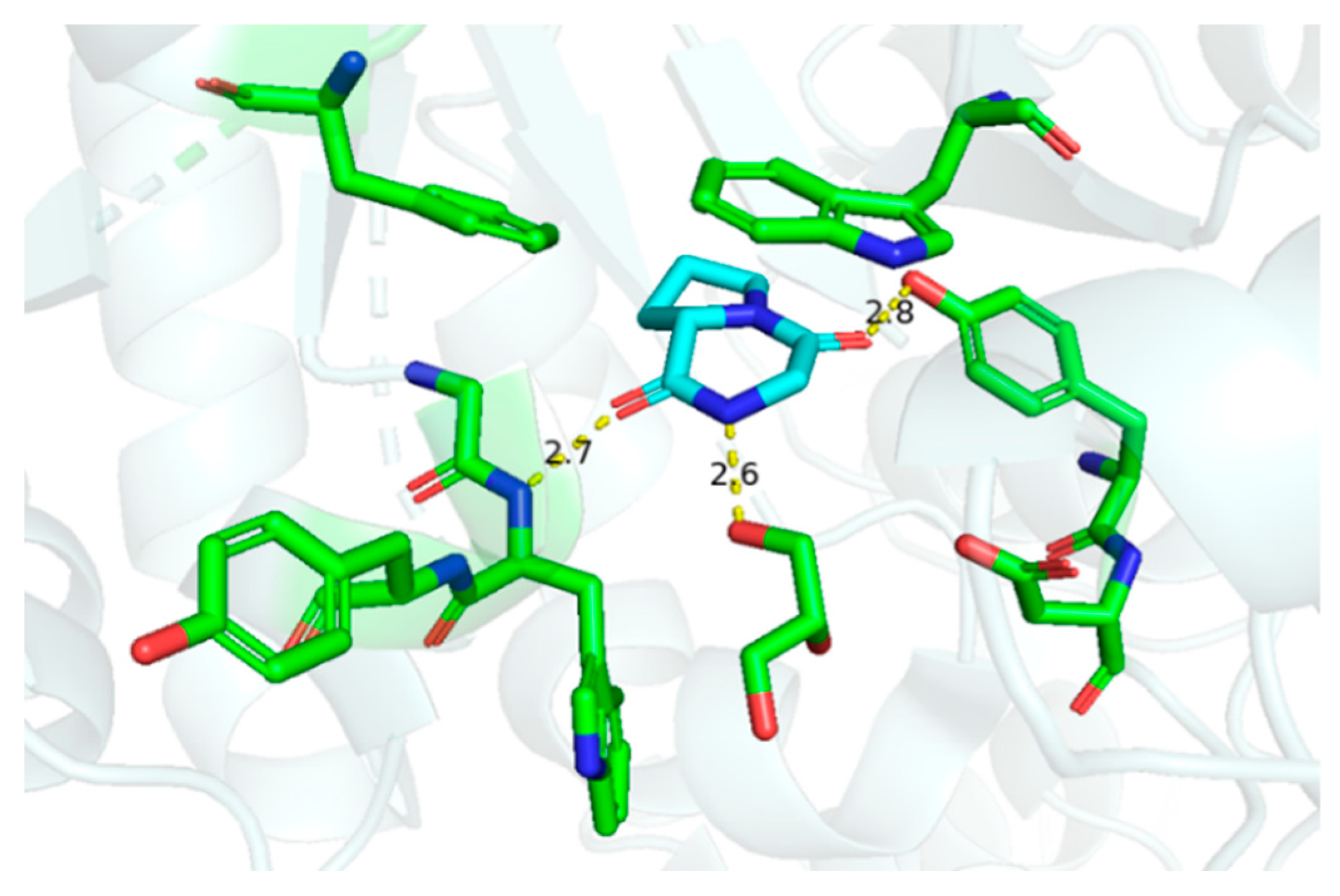
Although cGP exhibited negligible antimicrobial and antifungal activities against microbes such as Bacillus subtilis, Escherichia coli, and Saccharomyces cerevisiae [34], cGP selectively inhibits chitinase enzymes at a concentration of 5.0 mM [62]. Thus, cGP is a promising candidate for crop protection and the prevention of nosocomial cross-infection. The crystal structure discloses hydrogen bonding interactions, such as carbonyl and N-H, in the cyclic dipeptide scaffold and amino acid residues, providing a foundation for designing novel inhibitors targeting Serratia marcescens chitinase (Figure 6). Moreover, cGP markedly enhances antibiotic production in microorganisms, likely due to the stimulation of bacterial quorum-sensing signals [32].
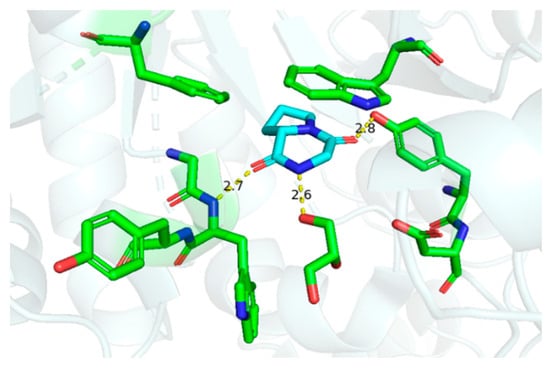
Figure 6.
Interactions between cyclic glycine-proline and chitinase (hydrogen bonding distance unit: Å) [62].
In puncture spray trials, cGP exhibited efficient and selective phytotoxicity against the crop weed A. euphorbiicola at a concentration of 1.0 mM [63]. Additionally, investigations into its acaricidal activity against T. urticae revealed an LC50 value of 96 µM [64,65]. Although these screenings yielded moderate results in terms of phytotoxicity and anti-pest activity, cGP is a promising candidate for use as a green agricultural product for biological control due to its availability from natural sources and selective inhibitory properties [66].
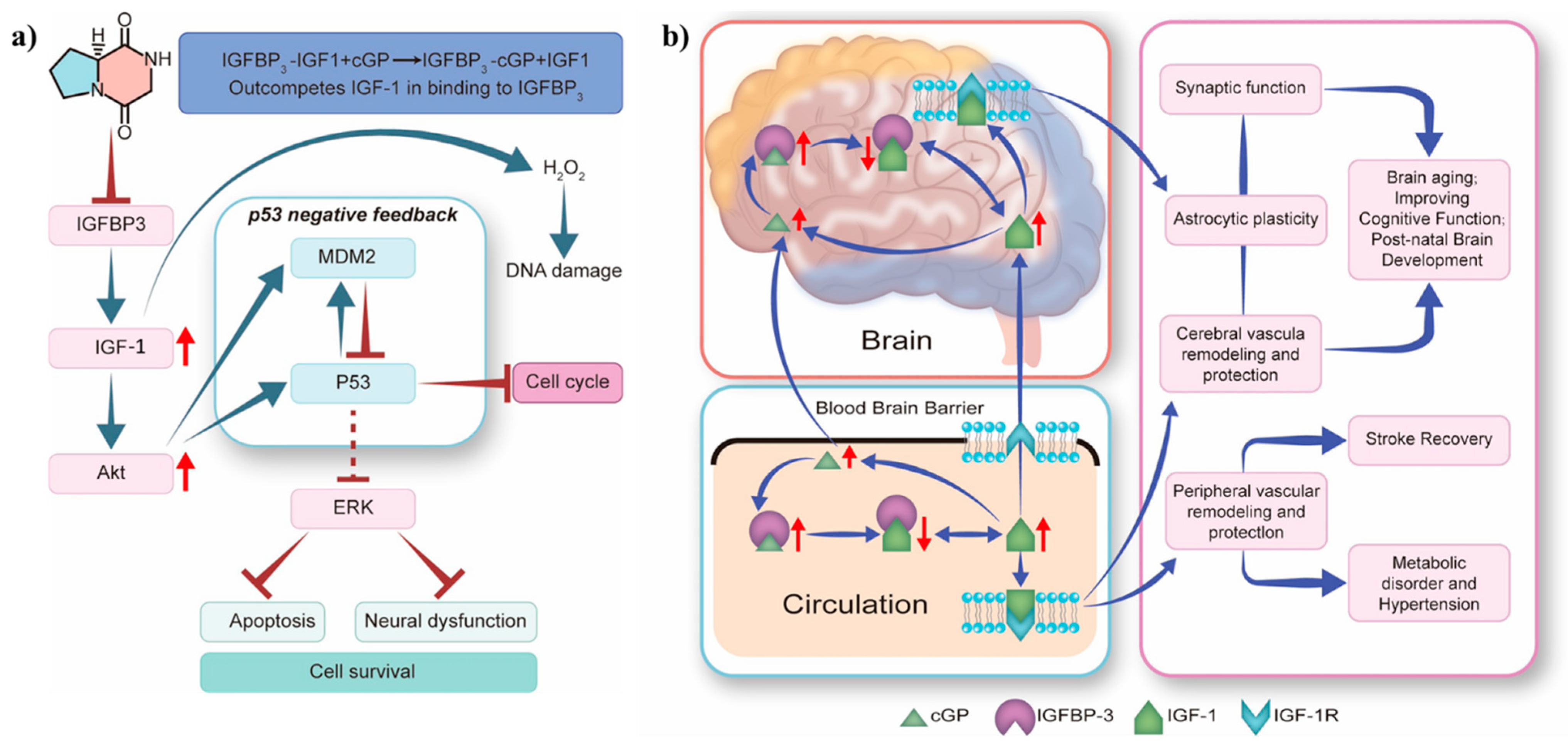
In vitro studies revealed that cyclic dipeptide can alleviate oxidative stress in human foetal neural stem cells (hfNSC). The cyclic dipeptide maintains IGF-1 homeostasis, activates Akt signalling, and enhances MDM2 E3 ubiquitin ligase expression. This regulation influences the interaction between IGF-1 signalling and MDM2-p53 pathways, resulting in a dose-dependent reduction in hfNSC cell death and apoptosis (Figure 7a) [67]. Importantly, cGP mimics the pharmacological effects of pramiracetam, including enhanced cognition [48,68], reduced anxiety [69], neuroprotection [51], analgesia [70], and antidepressant properties [71]. Mechanistic studies demonstrate that cGP modulates AMPA receptor-mediated currents in Purkinje neuronal cells [72,73]. Furthermore, cGP upregulates and activates the AMPA receptor-mediated brain-derived neurotrophic factor-tropomyosin receptor kinase B signalling pathway. These studies highlight the positive allosteric modulator ampakine-like pharmacological characteristics that contribute to the neuropsychotropic effects of cGP.
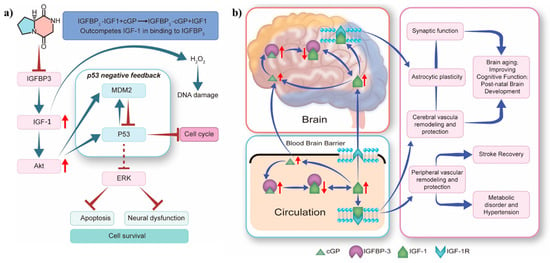
Figure 7.
Proposed mechanisms for the effects of cyclic glycine-proline: (a) induction of apoptosis in human foetal neural stem cells; (b) regulation of IGF-1 homeostasis in the circulatory system and brain tissue. Akt, protein kinase B; cGP, cyclic glycine-proline; ERK, extracellular signal-regulated kinase; IGF-1 or IGF1, insulin-like growth factor 1; IGFBP-3 or IGFBP3, insulin-like growth factor-binding protein 3; IGF-1R, insulin-like growth factor 1 receptor; MDM2, mouse double minute 2 homologous protein (Note: Redrawn graphics are based on original artwork by Murotomi [67] and Guan [74]. The red arrows on the left indicate the up-regulated levels of related proteins, while the red arrows on the right indicate changes in their relative amounts).
The neuroactive peptide cGP, derived from the brain neurotrophic factor IGF-1, regulates IGF-1 homeostasis by modulating the binding of IGF-binding protein 3 (IGFBP-3) to IGF-1. This modulation normalises IGF-1 bioavailability and function, especially in the context of brain ageing and age-related neurological conditions. Additionally, cGP maintains IGF-1 function within the optimal physiological range, enhancing function when IGF-1 function is deficient and inhibiting function when IGF-1 is excessive. IGF-1 function is optimised by the inherent affinity for cGP, which originates from the binding domain of IGF-1, enabling cGP to compete with IGF-1 for IGFBP-3 binding. Thus, IGF-1 function is regulated by the circulating cGP/IGF-1 molar ratio. Reduced IGF-1 bioavailability in patients often indicates age-related conditions such as hypertension, stroke, and neurological disorders with cognitive impairment. An increase in the cGP/IGF-1 ratio is associated with more favourable clinical outcomes, including improved memory retention and stroke recovery. Moreover, the absorption of cGP is enhanced in human tissues, and cGP efficiently and directly crosses the blood–brain barrier into the cerebrospinal fluid. Activation of IGF-1 receptors in capillaries promotes astrocyte plasticity and vascular formation to enhance memory (Figure 7b) [74]. According to Fan et al., the intake of exogenous cyclic dipeptide is associated with reduced cardiovascular systolic pressure and improved stroke recovery [29]. For instance, the cognitive health supplement cGPMAX™ Brain Health enhances mental clarity, improves sleep, and balances stress and emotions in older individuals. The outstanding safety profile and beneficial effects of cGP on the nervous system emphasise the potential commercial use of cGP in the pharmaceutical and healthcare sectors, particularly for the treatment of neurological disorders.
4. Exploring Applications for Building Blocks
Structurally, cGP consists of lipophilic and hydrophobic pyrrolidine rings with hydrogen bond donors and acceptors. According to Swiss ADME predictions [75,76], cGP adheres well to the “Rule of Two” [77,78,79] for drug-likeness building blocks (Table 2). Additionally, cGP exhibits a high-saturation carbon atom distribution rate (Fsp3) [80] of 0.73, representing remarkable three-dimensional features. Based on the empirical rules from Lipinski [81] and Veber [82], the topological polar surface area (TPSA) [83] of 49.4 Å2 for cGP exceeds 30.8 Å2 (0.2 × MW) [84] and is less than 60–70 Å2. Thus, the TPSA falls within the range conducive [85,86] to crossing the blood–brain and intestinal barriers. These predictions are consistent with the exceptional membrane permeability, oral availability, and metabolic stability of cGP, which facilitate the optimisation of lead drug-likeness.

Table 2.
Comparative predictive drug-likeness parameters of cyclic glycine-proline using empirical rule parameters.
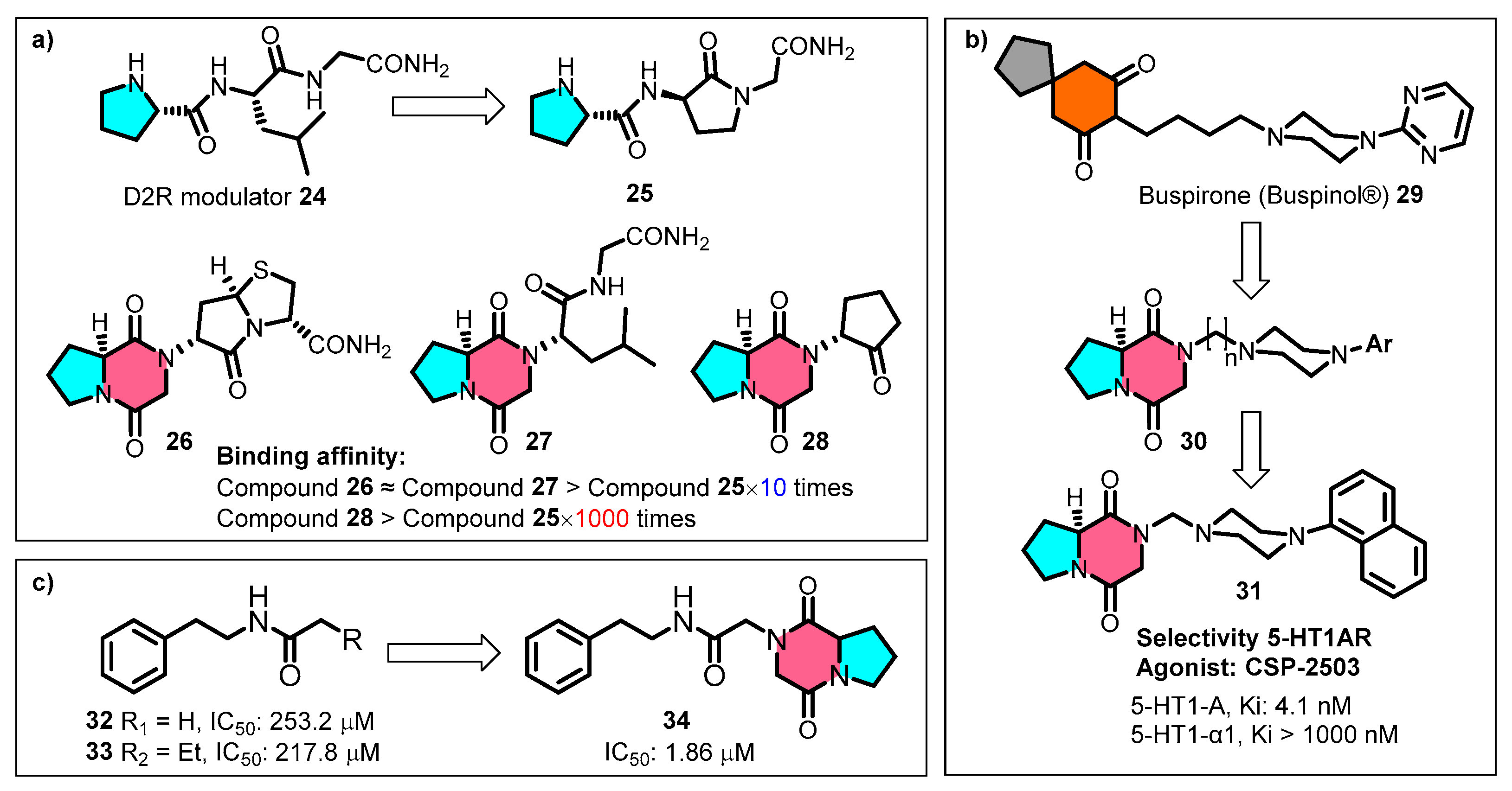
Capitalising on the specific topological molecular structure of cGP, Baures et al. replaced the proline portions of the dopamine receptor-regulating peptide 25 with cGP, resulting in conformationally restricted analogues 26–28 with exponentially improved binding affinities (Figure 8a) [87]. The N-positioned pharmacophore cyclopentanone 28 exhibits significantly enhanced receptor affinity by 1000-fold compared to the prototype compound 25. Lopez-Rodriguez et al. incorporated the cyclic dipeptide as a conformational mimic into the phenylpiperazine pharmacophore scaffold of the anxiolytic agent butorphanol 29, producing a series of selective 5-HT1A receptor agonists 30 (Figure 8b) [88,89]. The clinical candidate CSP-2503 (31) was validated pharmacologically as a 5-HT1AR agonist, acting on both the somatodendritic and postsynaptic sites. CSP-2503 also exhibits high-affinity antagonistic effects on 5-HT2A and 5-HT3 receptors [90,91].
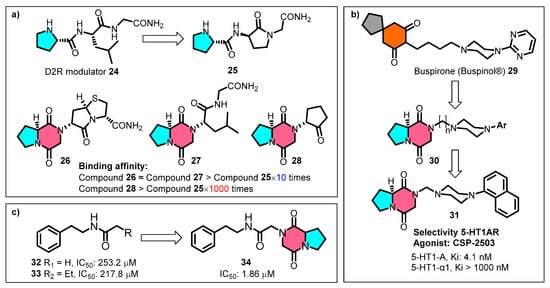
Figure 8.
Application of cyclic glycine-proline for building blocks: (a) dopamine receptor-regulating peptide (D2R) analogues; (b) selective 5-HT1A receptor agonists; (c) serotonin-specific receptor (Se-5HTR) inhibitors.
Based on a structure–activity relationship study of Spodoptera exigua serotonin-specific receptor (Se-5HTR) inhibitors, Hasan et al. linked the cGP fragment to the end of the carbon chain, which enhanced the specificity and competitive inhibitory activity of compound 34 against Se-5HTR (Figure 8c) [92]. This modification resulted in a two-order-of-magnitude improvement in the in vitro immunosuppressive activity compared to the prototype compounds (32–33). Thus, leveraging the outstanding drug-likeness and bonding affinity advantages of cGP provides efficient pharmaceutical building blocks for lead optimisation in the early stages of drug discovery.
5. Perspectives and Conclusions
Although the biosynthesis mechanism of cGP is largely unexplored, cGP may originate from glycine and proline, which are abundant in nature. Peptide biosynthesis involves nonribosomal peptide synthetase (NRPS), encoded by biosynthetic gene clusters (BGCs). NRPS is a core biosynthetic enzyme responsible for the key metabolic step in natural peptide biosynthesis [93,94]. Maiya et al. demonstrated that the biosynthesis pathway of cGP compounds involves the BGC ftmA, encoding enzymes for peptide condensation via NRPS to produce the analogue, brevianamide F [95,96]. Further research by Dubois et al. explored the feasibility of constructing the cGP framework in natural product biosynthesis using Escherichia coli; co-expressing NRPS with a new ribosome-independent enzyme, cyclodipeptide synthase [97,98], results in an increase in the derivative tryprostatin B, yielding up to 26 mg/L [99]. Exploring efficient chemo- and enantio-selective peptidyl transferases from diverse mangrove microbial strains may lay the foundation for the biosynthesis of cGP directly from glycine and L-proline. Alternatively, integrating BGC profiles from marine microbes with advanced techniques such as CRISPR/Cas9 gene editing, metabolic flux analysis, atmospheric and room temperature plasma mutagenesis, and adaptive laboratory evolution technology may provide insights into the technical challenges associated with extremely low biosynthetic productivity.
Increasing the prevalence of quaternary carbon centres, rather than relying solely on flat aromatic rings, represents a crucial strategy in contemporary drug discovery aimed at transcending two-dimensional molecular structures. This paradigm, often referred to as the ‘escaping the flatland’ [100,101] approach, has been consistently associated with enhanced drug-like properties and the increased success rate of clinical drug candidates. Augmenting the topological polar surface area and introducing three-dimensional spatial characteristics facilitates the optimisation of pharmacological profiles. Consequently, the unique topological scaffold, optical chirality, and chemical diversity inherent to cGP render it particularly valuable for early-stage drug discovery applications, including bioisostere and scaffold-hopping endeavours. Nonetheless, the development of precise chiral chemical synthesis methodologies, specifically targeting particular sites to expand building block libraries, remains a challenge. This is particularly true for incorporating new quaternary carbon centres and introducing functional substituents containing fluorine or boron.
NNZ-2591, an allylic analogue situated at the quaternary bridgehead carbon centre, has undergone clinical trials for the treatment of developmental neurological disorders, including Rett syndrome and fragile X syndrome [102,103]. Additionally, a phase II study targeting Prader–Willi syndrome received FDA accelerated approval in 2023 [104]. Given the role of cGP in regulating IGF-1 across various neurological and metabolic disorders, exploring the structure–activity relationship of cGP-derived compounds could offer valuable insights for the development of preventive healthcare and treatment strategies.
In summary, this review explores the diverse sources of cGP from marine-related, endogenous biological, and synthetic pathways, along with its in vitro and in vivo activities. The presence of cGP sourced sustainably from marine mangrove microbial origins, coupled with cutting-edge synthetic biological methodologies, has ignited significant interest in its biosynthesis. Moreover, the potential roles of this endogenous cyclic peptide in drug development and healthcare initiatives are emphasised. For example, the pivotal role of cGP in regulating insulin-like growth factor-1 homeostasis positively impacts the treatment of central nervous system diseases. Finally, the precise binding affinity, pharmacological attributes, exceptional drug-like characteristics, and safety profile of cGP collectively expand its applications as a fundamental building block, paving the way for novel drug discoveries in the early stages.
Author Contributions
L.H. wrote the paper; J.L., F.Q. and L.X. checked the paper; and L.L. verified the content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fujian Provincial Natural Science Foundation (No. 2023D022), the Youth Science Fund of Xiamen (No. 3502Z202372059), and the Xiamen Medical College education teaching reform project (No. XBYX2023017).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data in this article is openly available without any restrictions.
Acknowledgments
We thank Raphaël Robiette (Université Catholique de Louvain, Belgium) and Guillaume Berionni (University of Namur, Belgium) for the stimulating discussions on this manuscript. We acknowledge Zhong-Hang Wen (WuXi STA, China), Yung-Husan Chen (Xiamen Medical College, China), and Gang Zhang (Xiamen Medical College, China) for helpful discussions related to the preparation of the manuscript and for proofreading the manuscript. We thank LetPub (www.letpub.com, accessed on 22 April 2024) for linguistic assistance and pre-submission expert review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruggieri, G.D. Drugs from the sea: Marine organisms with novel chemical constituents are excellent sources of new drugs. Science 1976, 194, 491–497. [Google Scholar] [CrossRef]
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martín, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Devel. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef]
- Costa, L.; Sousa, E.; Fernandes, C. Cyclic peptides in pipeline: What future for these great molecules? Pharmaceuticals 2023, 16, 996. [Google Scholar] [CrossRef]
- Sindhikara, D.; Borrelli, K. High throughput evaluation of macrocyclization strategies for conformer stabilization. Sci. Rep. 2018, 8, 6585. [Google Scholar] [CrossRef]
- Buckton, L.K.; Rahimi, M.N.; McAlpine, S.R. Cyclic peptides as drugs for intracellular targets: The next frontier in peptide therapeutic development. Chem. Eur. J. 2021, 27, 1487–1513. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S. Cyclic peptide drugs approved in the last two decades (2001–2021). RSC Chem. Biol. 2022, 3, 18–31. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and biological activities of diketopiperazines from marine organisms: A review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef] [PubMed]
- Gizzo, L.; Bliss, G.; Palaty, C.; Kolevzon, A. Caregiver perspectives on patient-focused drug development for Phelan-McDermid syndrome. Orphanet J. Rare Dis. 2024, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Bednarski, M.; Sapa, J.; Nowak, G. Ergotamine and nicergoline-facts and myths. Pharmacol. Rep. 2015, 67, 360–363. [Google Scholar] [CrossRef]
- Williams, R.M.; Cox, R.J. Paraherquamides, brevianamides, and asperparalines: Laboratory synthesis and biosynthesis. An interim report. Acc. Chem. Res. 2003, 36, 127–139. [Google Scholar] [CrossRef]
- Baran, P.S.; Corey, E.J. A short synthetic route to (+)-austamide,(+)-deoxyisoaustamide, and (+)-hydratoaustamide from a common precursor by a novel palladium-mediated indole → dihydroindoloazocine cyclization. J. Am. Chem. Soc. 2002, 124, 7904–7905. [Google Scholar] [CrossRef]
- Steyn, P.S. The structures of five diketopiperazines from Aspergillus ustus. Tetrahedron 1973, 29, 107–120. [Google Scholar] [CrossRef]
- Takahashi, C.; Minoura, K.; Yamada, T.; Numata, A.; Kushida, K.; Shingu, T.; Hagishita, S.; Nakai, H.; Sato, T.; Harada, H. Potent cytotoxic metabolites from a leptosphaeria species. Structure determination and conformational analysis. Tetrahedron 1995, 51, 3483–3498. [Google Scholar] [CrossRef]
- Ordóñez, M.; Torres-Hernández, F.; Viveros-Ceballos, J.L. Highly diastereoselective synthesis of cyclic α-aminophosphonic and α-aminophosphinic acids from glycyl-L-Proline 2,5-diketopiperazine. Eur. J. Org. Chem. 2019, 2019, 7378–7383. [Google Scholar] [CrossRef]
- Von Dreele, R.B. The crystal structure of cyclo-L-prolylglycyl: A refinement of high-angle diffraction data. Acta Crystallogr. B 1975, 31, 966–970. [Google Scholar] [CrossRef]
- Li, R.S.; Shi, K.L.; Long, K.H.; Mak, T.C.W. Studies on chemical constituents of Chinese marine folk remedies-isolation and X-ray structural characterization of cyclo-L-prolyl-glycyl from Diodon Novemacultus (Bleeker). J. Struct. Chem. 1985, 4, 210–213. [Google Scholar] [CrossRef]
- Trigos, A.; Reyna, S.; Gutierrez, M.L.; Sanchez, M. Diketopiperazines from cultures of the fungus Colletotrichum gloesporoides. Nat. Prod. Lett. 1997, 11, 13–16. [Google Scholar] [CrossRef]
- Trigos, A.; Reyna, S.; Cervantes, L. Three diketopiperazines from the cultivated fungus Fusarium oxysporum. Nat. Prod. Lett. 1995, 6, 241–246. [Google Scholar] [CrossRef]
- Furtado, N.A.; Pupo, M.T.; Carvalho, I.; Campo, V.L.; Duarte, M.C.T.; Bastos, J.K. Diketopiperazines produced by an Aspergillus fumigatus Brazilian strain. J. Braz. Chem. Soc. 2005, 16, 1448–1453. [Google Scholar] [CrossRef]
- Tan, Q.W.; Gao, F.; Wang, F.R.; Chen, Q.J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus tubingensis FJBJ11. Int. J. Mol. Sci. 2015, 16, 5750–5761. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, H.; Yang, J. 2,5-Diketopiperazines from Aspergillus sp., the endophytic fungus of Astragalus membranaceus and their anticancer assay. Chem. Nat. Compd. 2020, 56, 583–585. [Google Scholar] [CrossRef]
- Lin, S.; Geng, M.; Liu, X.; Tan, J.; Yang, H. On the control of Microcystis aeruginosa and Synechococccus species using an algicidal bacterium, Stenotrophomonas F6, and its algicidal compounds cyclo-(Gly-Pro) and hydroquinone. J. Appl. Phycol. 2016, 28, 345–355. [Google Scholar] [CrossRef]
- Cao, X.T.; Wang, D.; Wang, N.; Cui, Z. Water-soluble constitutions from the skin of Bufo bufo gargarizans Cantor. Chin. J. Nat. Med. 2009, 7, 181–183. [Google Scholar] [CrossRef]
- Guigoz, Y.; Solms, J. Bitter peptides, occurrence and structure. Chem. Senses 1976, 2, 71–84. [Google Scholar] [CrossRef]
- Ginz, M.; Engelhardt, U.H. Identification of new diketopiperazines in roasted coffee. Eur. Food Res. Technol. 2001, 213, 8–11. [Google Scholar] [CrossRef]
- Chen, M.Z.; Dewis, M.L.; Kraut, K.; Merritt, D.; Reiber, L.; Trinnaman, L.; Da Costa, N.C. 2,5-Diketopiperazines (cyclic dipeptides) in beef: Identification, synthesis, and sensory evaluation. J. Food Sci. 2009, 74, C100–C105. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of blackcurrant anthocyanins increased cyclic glycine-proline in the cerebrospinal fluid of Parkinson patients: Potential treatment to improve insulin-like growth factor-1 function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Q.; Ma, J.; Kang, W. Chemical constituents of Bacillus coagulans LL1103. Chem. Nat. Compd. 2018, 54, 419–420. [Google Scholar] [CrossRef]
- Dao, P.T.; Huong, D.T.M.; Murphy, B.; Van Minh, C.; Van Cuong, P. Compounds from culture broth of marine bacterium Oceanisphaera sp. Vietnam J. Chem. 2015, 53, 120–123. [Google Scholar]
- Jiang, Z.; Boyd, K.G.; Mearns-Spragg, A.; Adams, D.R.; Wright, P.C.; Burgess, J.G. Two diketopiperazines and one halogenated phenol from cultures of the marine bacterium, Pseudoalteromonas luteoviolacea. Nat. Prod. Lett. 2000, 14, 435–440. [Google Scholar] [CrossRef]
- Rosandy, A.R.; Ying, Y.C.; Kqueen, C.Y.; Lim, S.J.; Latip, J.; Murad, A.; Bakar, M.A.; Khalid, R.M. (-)-Glaciantarcin, a new dipeptide and some secondary metabolites from the psychrophilic yeast Glaciozyma antarctica PI12. Sains Malays. 2018, 47, 2693–2698. [Google Scholar] [CrossRef]
- Mitova, M.; Popov, S.; De Rosa, S. Cyclic peptides from a Ruegeria strain of bacteria associated with the sponge Suberites domuncula. J. Nat. Prod. 2004, 67, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.N.; Chen, G.; Bai, J.; Hua, H.M.; Xu, W.F.; Pei, Y.H. Isolation and identification of secondary metabolites from Penicillium oxalicum HSY-P-1. J. Shenyang Pharm. Univ. 2014, 31, 360–362. (In Chinese) [Google Scholar]
- Xie, L.R.; Li, D.Y.; Wang, P.L.; Hua, H.M.; Wu, X.; Li, Z.L. A new 3,4-seco-lanostane triterpenoid from a marine-derived fungus Ascotricha sp. ZJ-M-5. Acta Pharm. Sin. B 2013, 48, 89–93. [Google Scholar]
- Liu, H.B.; Gao, H.; Wang, N.L.; Lin, H.P.; Hong, K.; Yao, X.S. Cyclic dipeptides from the mangrove fungus Penicillium oxalicum (092007). J. Shenyang Pharm. Univ. 2007, 24, 474–478. (In Chinese) [Google Scholar]
- Wang, Y.; Zhang, Y.Q.; Xu, J.F.; Bai, Q.Q.; Chen, W.H.; Chen, G.Y. Study on diketopiperazine secondary metabolites of endophytic fungus Aspergillus terreus HT-1. Chin. J. Mar. Drugs 2022, 41, 10–16. (In Chinese) [Google Scholar]
- Li, H.J.; Lin, Y.C.; Liu, X.H.; Zhou, S.N. Peptide components of mangrove endogenous fungus No. 2524 (I). Acta Sci. Nat. Univ. Sunyatseni 2002, 41, 110–112. (In Chinese) [Google Scholar]
- Danh, C.D.; Dao, P.T.; Huong, D.T.M.; Thach, T.D.; Anh, N.M.; Minh, L.T.H.; Anh, T.T.; Van Cuong, P. Cyclopeptides from marine actinomycete Streptomyces sp. G261. Vietnam J. Chem. 2018, 56, 570–573. [Google Scholar] [CrossRef]
- Hue, N.T.; Linh, N.T.; Minh, L.T.H.; Minh, L.T.H.; Huyen, V.T.T.; Huong, D.T.M.; Cuong, P.V. Chemical constituents of marine-derived cctinomycete Streptomyces fradiae G650. Vietnam J. Sci. Technol. 2022, 60, 974–981. [Google Scholar]
- Chen, C.; Ye, Y.; Wang, R.; Zhang, Y.; Wu, C.; Debnath, S.C.; Ma, Z.; Wang, J.; Wu, M. Streptomyces nigra sp. nov. is a novel actinobacterium isolated from mangrove soil and exerts a potent antitumor activity in vitro. Front Microbiol. 2018, 9, 1587. [Google Scholar] [CrossRef]
- Chen, J.H.; Lan, X.P.; Liu, Y.; Jia, A.Q. The effects of diketopiperazines from Callyspongia sp. on release of cytokines and chemokines in cultured J774A.1 macrophages. Bioorg. Med. Chem. Lett. 2012, 22, 3177–3180. [Google Scholar]
- Chen, Y.; Peng, Y.; Gao, C.; Huang, R. A new diketopiperazine from South China Sea marine sponge Callyspongia sp. Nat. Prod. Res. 2014, 28, 1010–1014. [Google Scholar] [CrossRef]
- Huang, R.; Yan, T.; Peng, Y.; Zhou, X.; Yang, X.; Liu, Y. Diketopiperazines from the marine sponge Axinella sp. Chem. Nat. Compd. 2014, 50, 191–193. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, W.; Guan, Z.B.; Liao, X.J.; Zhao, B.X.; Xu, S.H. A new thiodiketopiperzaine from the marine sponge Tedania sp. Nat. Prod. Res. 2020, 34, 1113–1117. [Google Scholar]
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The state of the world’s mangrove forests: Past, present, and future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Boyko, S.S.; Akparov, V.K.; Ostrovskaya, R.U.; Skoldinov, S.P.; Rozantsev, G.G.; Voronina, T.A.; Zherdev, V.P.; Seredenin, S.B. Identification of a novel endogenous memory facilitating cyclic dipeptide cyclo-prolylglycine in rat brain. FEBS Lett. 1996, 391, 149–152. [Google Scholar] [CrossRef]
- Keating, G.M. Mecasermin. BioDrugs 2008, 22, 177–188. [Google Scholar] [CrossRef]
- Fan, D.; Krishnamurthi, R.; Harris, P.; Barber, P.A.; Guan, J. Plasma cyclic glycine proline/IGF-1 ratio predicts clinical outcome and recovery in stroke patients. Ann. Clin. Transl. Neurol. 2019, 6, 669–677. [Google Scholar] [CrossRef]
- Guan, J.; Gluckman, P.D. IGF-1 derived small neuropeptides and analogues: A novel strategy for the development of pharmaceuticals for neurological conditions. Br. J. Pharmacol. 2009, 157, 881–891. [Google Scholar] [CrossRef]
- Guan, J.; Harris, P.; Brimble, M.; Lei, Y.; Lu, J.; Yang, Y.; Gunn, A.J. The role for IGF-1-derived small neuropeptides as a therapeutic target for neurological disorders. Expert Opin. Ther. Targets 2015, 19, 785–793. [Google Scholar] [CrossRef]
- Hayasaka, F.; Yamamoto, S.; Sakai, Y. Production method for cyclic dipeptide derived from native collagen. Food Sci. Technol. 2016, 22, 477–483. [Google Scholar] [CrossRef]
- Ishizu, T.; Tokunaga, M.; Fukuda, M.; Matsumoto, M.; Goromaru, T.; Takemoto, S. Molecular capture and conformational change of diketopiperazines containing proline residues by epigallocatechin-3-O-gallate in water. Chem. Pharm. Bull. 2021, 69, 585–589. [Google Scholar] [CrossRef]
- de Costa, B.R.; He, X.; Linders, J.T.M.; Dominguez, C.; Gu, Z.Q.; Williams, W.; Bowen, W. Synthesis and evaluation of conformationally restricted N-[2-(3, 4-dichlorophenyl) ethyl]-N-methyl-2-(1-pyrrolidinyl) ethylamines at. sigma. receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds. J. Med. Chem. 1993, 36, 2311–2320. [Google Scholar] [CrossRef]
- Hendea, D.; Laschat, S.; Baro, A.; Frey, W. Diastereoselective alkylation of a proline-derived bicyclic lactim ether. Helv. Chim. Acta 2006, 89, 1894–1909. [Google Scholar] [CrossRef]
- Ahonen, K.; Lahtinen, M.; Kolehmainen, E. Cyclic dipeptides: Catalyst/promoter-free, rapid and environmentally benign cyclization of free amino acids. Green Chem. 2011, 13, 1203–1209. [Google Scholar]
- Poonia, B.K.; Sidhu, A.; Sharma, A.B. Cyclo (L-proline-L-serine) dipeptide suppresses seed borne fungal pathogens of rice: Altered cellular membrane integrity of fungal hyphae and seed quality benefits. J. Agric. Food Chem. 2022, 70, 2160–2168. [Google Scholar] [CrossRef]
- Ying, J.; Lin, R.; Xu, P.; Wu, Y.; Liu, Y.; Zhao, Y. Prebiotic formation of cyclic dipeptides under potentially early earth conditions. Sci. Rep. 2018, 8, 936. [Google Scholar] [CrossRef]
- Guo, Y.; Ying, J.; Sun, D.; Zhang, Y.; Zheng, M.; Ding, R.; Liu, Y.; Zhao, Y. Cyclic Dipeptides formation from linear dipeptides under potentially prebiotic earth conditions. Front. Chem. 2021, 9, 675821. [Google Scholar] [CrossRef]
- Khan, R.; Basha, A.; Goverdhanam, R.; Rao, P.C.; Tanemura, Y.; Fujimoto, Y.; Begum, A.S. Attenuation of TNF-α secretion by L-proline-based cyclic dipeptides produced by culture broth of Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2015, 25, 5756–5761. [Google Scholar] [CrossRef]
- Houston, D.R.; Synstad, B.; Eijsink, V.G.H.; Stark, M.J.R.; Eggleston, I.M.; Van Aalten, D.M.F. Structure-based exploration of cyclic dipeptide chitinase inhibitors. J. Med. Chem. 2004, 47, 5713–5720. [Google Scholar] [CrossRef]
- Varejão, E.V.V.; Demuner, A.J.; de Almeida Barbosa, L.C.; Barreto, R.W. Phytotoxic effects of metabolites from Alternaria euphorbiicola against its host plant Euphorbia heterophylla. Quim. Nova 2013, 36, 1004–1007. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, Y.H.; Yang, J.; Cui, W.Y.; He, P.J.; Munir, S.; He, P.F.; Wu, Y.X.; He, Y.Q. Acaricidal activity of cyclodipeptides from Bacillus amyloliquefaciens W1 against Tetranychus urticae. J. Agric. Food Chem. 2018, 66, 10163–10168. [Google Scholar] [CrossRef]
- Li, X.; Munir, S.; Xu, Y.; Wang, Y.; He, Y. Combined mass spectrometry-guided genome mining and virtual screening for acaricidal activity in secondary metabolites of Bacillus velezensis W1. RSC Adv. 2021, 11, 25441–25449. [Google Scholar] [CrossRef]
- Mendes, L.L.; Varejão, J.O.S.; de Souza, J.A.; de M. Carneiro, J.W.; Valdo, A.K.S.M.; Martins, F.T.; Ferreira, B.W.; Barreto, R.W.; da Silva, T.I.; Kohlhoff, M.; et al. 2,5-Diketopiperazines via intramolecular N-alkylation of Ugi adducts: A contribution to the synthesis, density functional theory study, X-ray characterization, and potential herbicide application. J. Agric. Food Chem. 2022, 70, 1799–1809. [Google Scholar]
- Murotomi, K.; Kagiwada, H.; Hirano, K.; Yamamoto, S.; Numata, N.; Matsumoto, Y.; Kaneko, H.; Namihira, M. Cyclo-glycylproline attenuates hydrogen peroxide-induced cellular damage mediated by the MDM2-p53 pathway in human neural stem cells. J. Cell. Physiol. 2023, 238, 434–446. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Ostrovskaya, R.U.; Maksimova, F.V.; Chuppin, A.V.; Trofimov, S.S.; Lezina, V.P.; Voronina, T.A.; Skoldinov, A.P. Proline-based topologic pyracetam analogs and their nootropic activity. Pharm. Chem. J. 1989, 23, 203–208. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Konstantinopol’skii, M.A.; Ostrovskaya, R.U.; Seredenin, S.B. Anxiolytic activity of endogenous nootropic dipeptide cycloprolylglycine in elevated plus-maze test. Bull. Exp. Biol. Med. 2001, 131, 464–466. [Google Scholar] [CrossRef]
- Ferro, J.N.; de Aquino, F.L.; de Brito, R.G.; dos Santos, P.L.; Quintans, J.S.S.; de Souza, L.C.; de Araújo, A.F.; Diaz, B.L.; Lucca--Júnior, W.; Quintans-Júnior, L.J.; et al. Cyclo-Gly-Pro, a cyclic dipeptide, attenuates nociceptive behaviour and inflammatory response in mice. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1287–1295. [Google Scholar] [CrossRef]
- Garibova, T.L.; Gudasheva, T.A.; Seredenin, S.B. A new component in the mechanism of regulation of endogenous depressive-like states. Dokl. Biochem. Biophys. 2019, 488, 324–326. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Grigoriev, V.V.; Koliasnikova, K.N.; Zamoyski, V.L.; Seredenin, S.B. Neuropeptide cycloprolylglycine is an endogenous positive modulator of AMPA receptors. Dokl. Biochem. Biophys. 2016, 471, 387–389. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Povarnina, P.Y.; Koliasnikova, K.N.; Alyaeva, A.G.; Vorontsova, O.N.; Seredenin, S.B. The anxiolytic effect of the neuropeptide cycloprolylglycine is mediated by AMPA and TrkB receptors. Dokl. Biochem. Biophys. 2020, 493, 190–192. [Google Scholar] [CrossRef]
- Guan, J.; Li, F.; Kang, D.; Pitcher, T.; Dalrymple-Alford, J.; Shorten, P.; Singh-Mallah, G. Cyclic glycine-proline (cGP) normalises insulin-like growth factor-1 (IGF-1) function: Clinical significance in the ageing brain and in age-related neurological conditions. Molecules 2023, 28, 1021. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics Free Web Services. Slovensky Grob, Slovakia. Available online: https://www.molinspiration.com (accessed on 14 February 2024).
- Goldberg, W.; Kettle, J.G.; Kogej, T. Designing novel building blocks is an overlooked strategy to improve compound quality. Drug Discov. Today 2015, 20, 11–17. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Ryabukhin, S.V.; Judd, D.B. The symbiotic relationship between drug discovery and organic chemistry. Chem. Eur. J. 2020, 26, 1196–1237. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Vashchenko, B.V. Emerging building blocks for medicinal chemistry: Recent synthetic advances. Eur. J. Org. Chem. 2021, 2021, 6478–6510. [Google Scholar] [CrossRef]
- Wei, W.; Cherukupalli, S.; Jing, L.; Liu, X.; Zhan, P. Fsp3: A new parameter for drug-likeness. Drug Discov. Today 2020, 25, 1839–1845. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Whitty, A.; Zhong, M.; Viarengo, L.; Beglov, D.; Hall, D.R.; Vajda, S. Quantifying the chameleonic properties of macrocycles and other high-molecular-weight drugs. Drug Discov. Today 2016, 21, 712–717. [Google Scholar] [CrossRef]
- Clark, D.E.; Grootenhuis, P.D. Predicting passive transport in silico: History, hype, hope. Curr. Top. Med. Chem. 2003, 3, 1193–1203. [Google Scholar] [CrossRef]
- Lobell, M.; Molnar, L.; Keseru, G.M. Recent advances in the prediction of blood-brain partitioning from molecular structure. J. Pharm. Sci. 2003, 92, 360–370. [Google Scholar] [CrossRef]
- Baures, P.W.; Ojala, W.H.; Costain, W.J.; Ott, M.C.; Pradhan, A.; Gleason, W.B.; Mishra, R.K.; Johnson, R.L. Design, synthesis, and dopamine receptor modulating activity of diketopiperazine peptidomimetics of L-prolyl-L-leucylglycinamide. J. Med. Chem. 1997, 40, 3594–3600. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, M.L.; Morcillo, M.J.; Fernandez, E.; Benhamú, B.; Tejada, I.; Ayala, D.; Viso, A.; Campillo, M.; Pardo, L.; Delgado, M.; et al. Synthesis and structure-activity relationships of a new model of arylpiperazines. 8. computational simulation of ligand-receptor interaction of 5-HT1AR agonists with selectivity over α1-adrenoceptors. J. Med. Chem. 2005, 48, 2548–2558. [Google Scholar] [CrossRef]
- Sato, H.; Skelin, L.; Debonnel, G.; Diksic, M. Chronic buspirone treatment normalizes open field behavior in olfactory bulbectomized rats: Assessment with a quantitative autoradiographic evaluation of the 5-HT1A binding sites. Brain Res. Bull. 2008, 75, 545–555. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, M.L.; Morcillo, M.J.; Fernandez, E.; Benhamú, B.; Tejada, I.; Ayala, D.; Viso, A.; Olivella, M.; Pardo, L.; Delgado, M.; et al. Design and synthesis of S-(−)-2-[[4-(napht-1-yl) piperazin-1-yl] methyl]-1,4-dioxoperhydropyrrolo [1,2-a] pyrazine (CSP-2503) using computational simulation. A 5-HT1A receptor agonist. Bioorg. Med. Chem. Lett. 2003, 13, 1429–1432. [Google Scholar] [CrossRef]
- Delgado, M.; Caicoya, A.G.; Greciano, V.; Benhamú, B.; López-Rodríguez, M.L.; Fernández-Alfonso, M.S.; Pozo, M.A.; Manzanares, J.; Fuentes, J.A. Anxiolytic-like effect of a serotonergic ligand with high affinity for 5-HT1A, 5-HT2A and 5-HT3 receptors. Eur. J. Pharmacol. 2005, 511, 9–19. [Google Scholar] [CrossRef]
- Hasan, A.; Yeom, H.S.; Ryu, J.; Bode, H.B.; Kim, Y. Phenylethylamides derived from bacterial secondary metabolites specifically inhibit an insect serotonin receptor. Sci. Rep. 2019, 9, 20358. [Google Scholar] [CrossRef]
- Kwon, M.J.; Steiniger, C.; Cairns, T.C.; Wisecaver, J.H.; Lind, A.L.; Pohl, C.; Regner, C.; Rokas, A.; Meyer, V. Beyond the biosynthetic gene cluster paradigm: Genome-wide coexpression networks connect clustered and unclustered transcription factors to secondary metabolic pathways. Microbiol. Spectr. 2021, 9, e00898-21. [Google Scholar] [CrossRef]
- Khaldi, N.; Seifuddin, F.T.; Turner, G.; Haft, D.; Nierman, W.C.; Wolfe, K.H.; Fedorova, N.D. SMURF: Genomic mapping of fungal secondary metabolite clusters. Fungal Genet. Biol. 2010, 47, 736–741. [Google Scholar] [CrossRef]
- Maiya, S.; Grundmann, A.; Li, S.M.; Turner, G. The fumitremorgin gene cluster of Aspergillus fumigatus: Identification of a gene encoding brevianamide F synthetase. ChemBioChem 2006, 7, 1062–1069. [Google Scholar] [CrossRef]
- Maiya, S.; Grundmann, A.; Li, S.M.; Turner, G. Improved tryprostatin B production by heterologous gene expression in Aspergillus nidulans. Fungal Genet. Biol. 2009, 46, 436–440. [Google Scholar] [CrossRef]
- Canu, N.; Moutiez, M.; Belin, P.; Gondry, M. Cyclodipeptide synthases: A promising biotechnological tool for the synthesis of diverse 2,5-diketopiperazines. Nat. Prod. Rep. 2020, 37, 312–321. [Google Scholar] [CrossRef]
- Gondry, M.; Sauguet, L.; Belin, P.; Thai, R.; Amouroux, R.; Tellier, C.; Tuphile, K.; Jacquet, M.; Braud, S.; Courçon, M.; et al. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes. Nat. Chem. Biol. 2009, 5, 414–420. [Google Scholar] [CrossRef]
- Dubois, P.; Correia, I.; Le Chevalier, F.; Dubois, S.; Jacques, I.; Canu, N.; Moutiez, M.; Thai, R.; Gondry, M.; Lequin, O.; et al. Reprogramming Escherichia coli for the production of prenylated indole diketopiperazine alkaloids. Sci. Rep. 2019, 9, 9208. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Lovering, F. Escape from flatland 2: Complexity and promiscuity. Med. Chem. Comm. 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Horrigan, J.P.; Tartaglia, N.; Hagerman, R.; Kolevzon, A.; Erickson, C.A.; Hatti, S.; Snape, M.; Yaroshinsky, A.; Stoms, G. A double-blind, randomized, placebo-controlled clinical study of Trofinetide in the treatment of Fragile X Syndrome. Pediatr. Neurol. 2020, 110, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Glaze, D.G.; Neul, J.L.; Percy, A.; Feyma, T.; Beisang, A.; Yaroshinsky, A.; Stoms, G.; Zuchero, D.; Horrigan, J.; Glass, L.; et al. A double-blind, randomized, placebo-controlled clinical study of Trofinetide in the treatment of rett syndrome. Pediatr. Neurol. 2017, 76, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).