Improvement of Alginate Extraction from Brown Seaweed (Laminaria digitata L.) and Valorization of Its Remaining Ethanolic Fraction
Abstract
1. Introduction
2. Results and Discussion
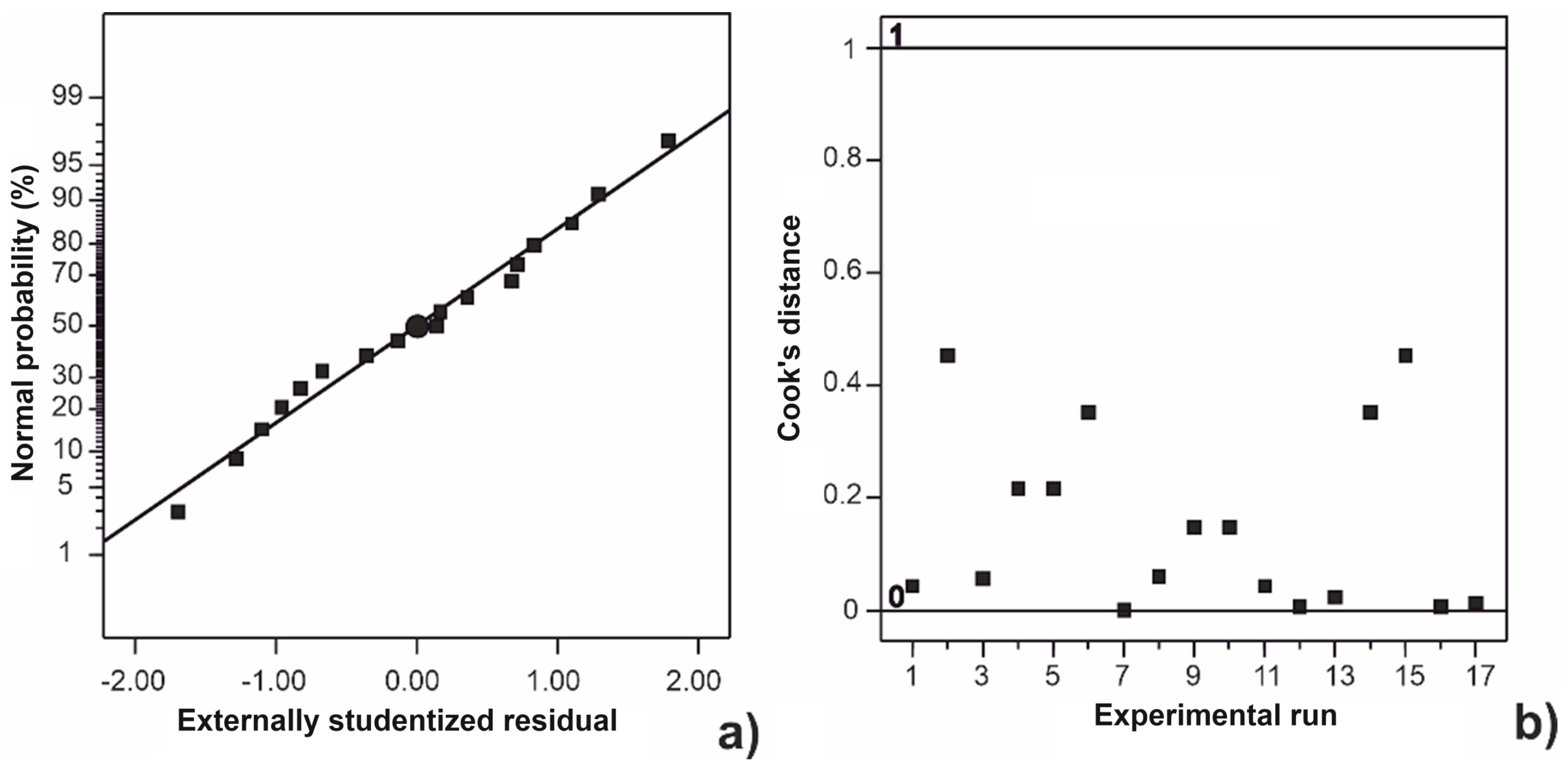
2.1. Modeling of the Acid Treatment Process
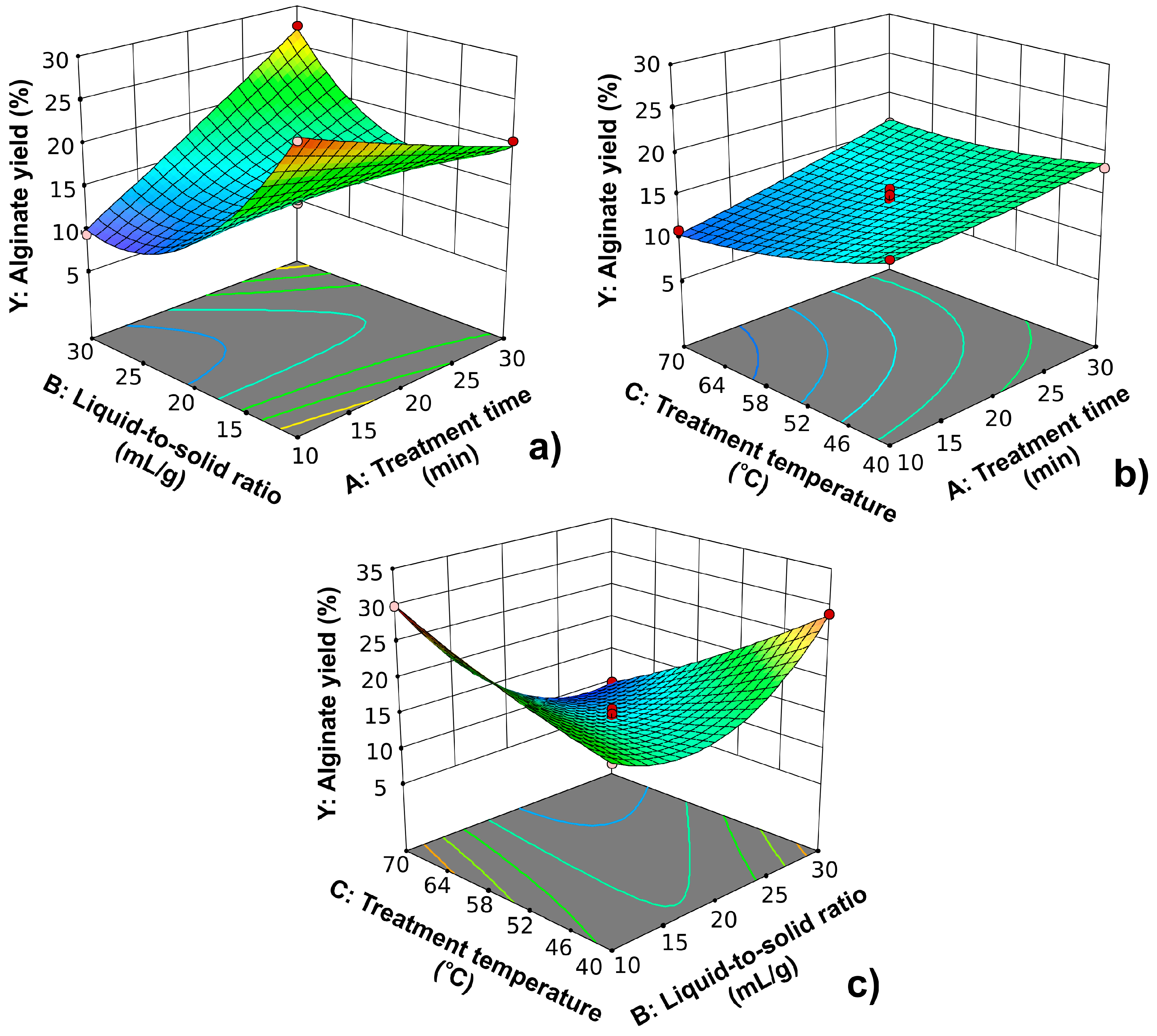
2.2. Numerical Optimization of Acid Treatment of Brown Seaweed Biomass
2.3. Fourier-Transform Infrared Analysis
2.4. Chemical Characterization of the Ethanolic Fraction of Brown Seaweed Biomass
2.4.1. Total Antioxidant Content and Total Flavonoid Content
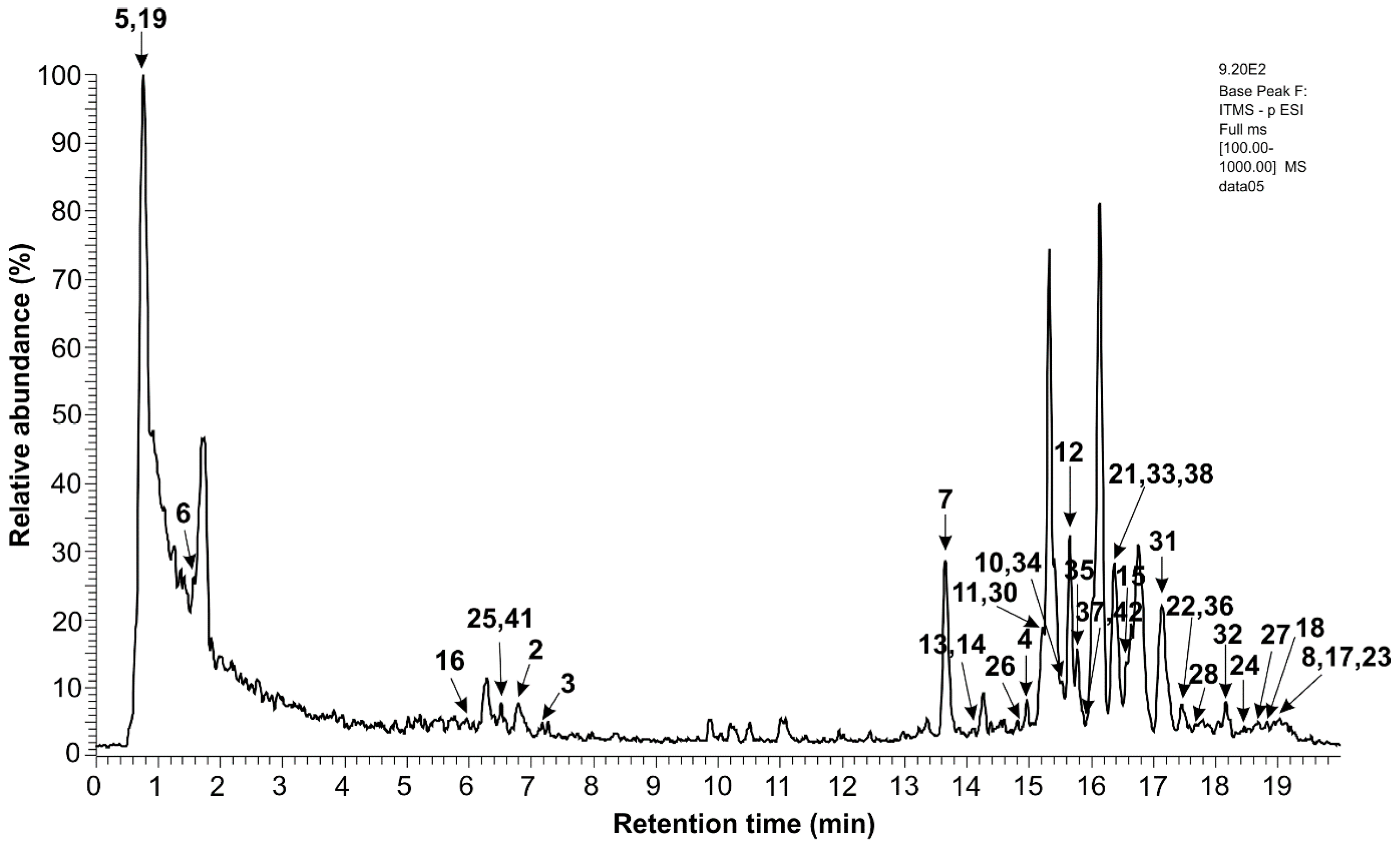
2.4.2. Identification of Extracts’ Chemical Constituents via the Chromatographic Method
2.4.3. Determination of the Concentrations of Macroelements and Microelements within the Studied Fraction
2.5. Biological Activities of the Ethanolic Fraction of Brown Seaweed Biomass
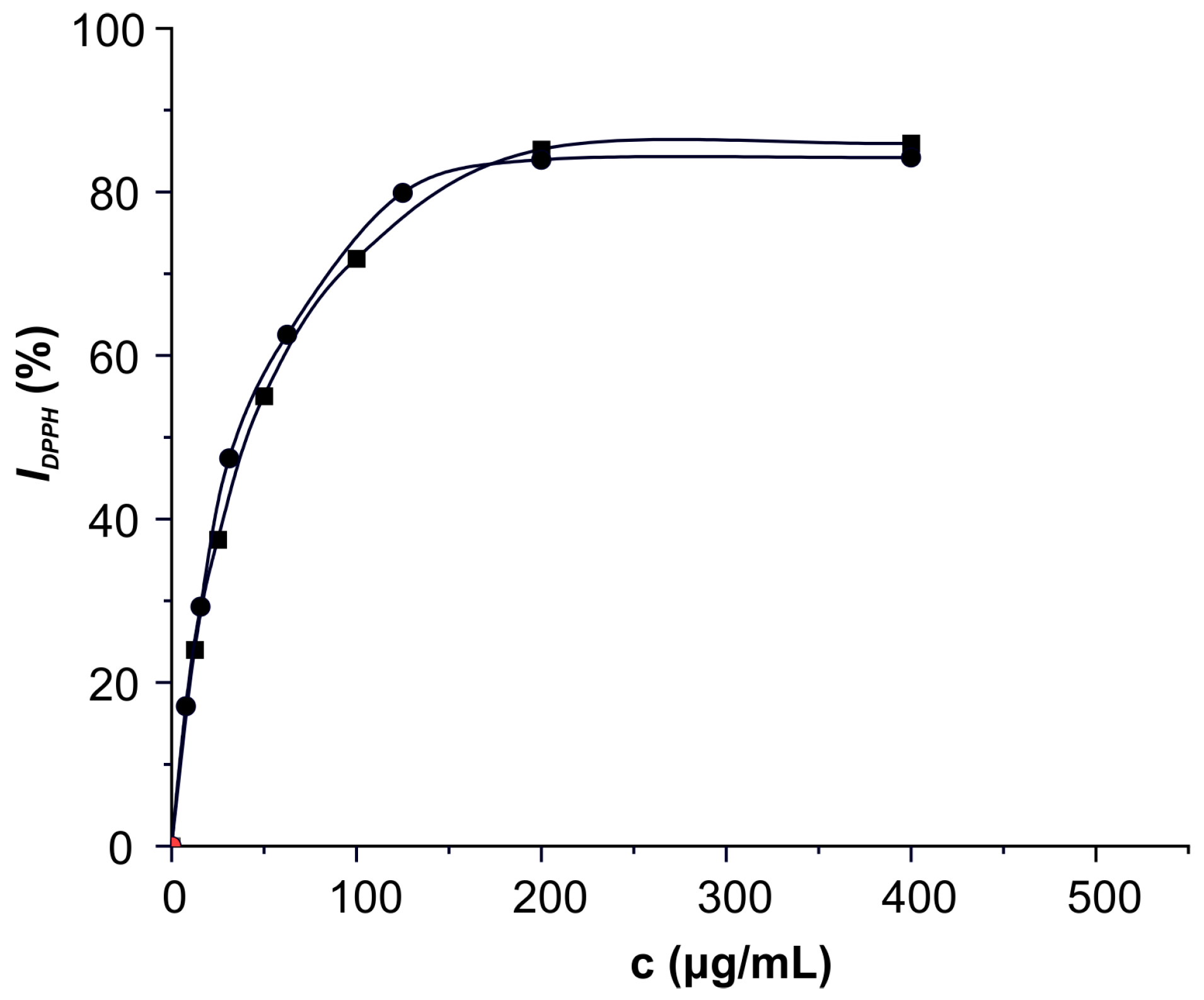
2.5.1. Radical Scavenging Activity
2.5.2. Antimicrobial Activity of Ethanolic Fraction of Brown Seaweed Biomass
3. Materials and Methods
3.1. Chemicals and Plant Material
3.2. Alginate Extraction from Brown Seaweed
3.3. Optimization of Acid Treatment of Biomass
3.4. Determination of Isolated Alginate Surface Chemistry
3.5. Chemical Characterization of the Ethanolic Fraction of Brown Seaweed Biomass
3.5.1. Determination of the Total Antioxidant Content
3.5.2. Determination of the Total Flavonoid Content
3.5.3. UHPLC-ESI-MS/MS Analysis
3.5.4. ICP-OES Analysis of Ethanolic Fraction
3.6. Biological Activities of the Ethanolic Fraction of Brown Seaweed Biomass
3.6.1. Radical Scavenging Activity
3.6.2. Antimicrobial Activity of the Ethanolic Fraction of Brown Seaweed Biomass
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A brief review on the development of alginate extraction process and its sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Rashedy, S.H.; Abd El Hafez, M.S.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and characterization of alginate extracted from brown seaweed collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A green approach for alginate extraction from Sargassum muticum brown seaweed using ultrasound-assisted technique. Int. J. Biol. Macromol. 2019, 124, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Torabi, P.; Hamdami, N.; Keramat, J. Microwave-assisted extraction of sodium alginate from brown macroalgae Nizimuddinia zanardini, optimization and physicochemical properties. Sep. Sci. Technol. 2022, 57, 872–885. [Google Scholar] [CrossRef]
- Hanjabam, M.D.; Kumar, A.; Tejpal, C.S.; Krishnamoorthy, E.; Kishore, P.; Kumar, K.A. Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100200. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.-Y.; Ward, K. Multistage extraction and purification of waste Sargassum natans to produce sodium alginate: An optimization approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.T.; Chica, L.R.; Yamashita, C.; Nunes, N.S.S.; Moraes, I.C.F.; Branco, C.C.Z.; Branco, I.G. Optimal conditions for alkaline treatment of alginate extraction from the brown seaweed Sargassum cymosum C. Agardh by response surface methodology. App. Food Res. 2022, 2, 100141. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- García-Ríos, V.; Ríos-Leal, E.; Robledo, D.; Freile-Pelegrin, Y. Polysaccharides composition from tropical brown seaweeds. Phycol. Res. 2012, 60, 305–315. [Google Scholar] [CrossRef]
- Trica, B.; Delattre, C.; Gros, F.; Ursu, A.V.; Dobre, T.; Djelveh, G.; Michaud, P.; Oancea, F. Extraction and characterization of alginate from an edible brown seaweed (Cystoseira barbata) harvested in the Romanian Black Sea. Mar. Drug 2019, 17, 405. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Metz, M.; Martínez-Abad, A.; Knutsen, S.H.; Ballance, S.; López-Rubio, A.; Martínez-Sanz, M. Valorization of alginate-extracted seaweed biomass for the development of cellulose-based packaging films. Algal Res. 2022, 61, 102576. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Pauli, G.; Kamp, J. Plan A: The transformation of Argentina’s Economy; JJK Books: Santa Barbara, CA, USA, 2017; pp. 161–177. [Google Scholar]
- Purcell, D.; Packer, M.A.; Hayes, M. Angiotensin-I-converting enzyme inhibitory activity of protein hydrolysates generated from the macroalga Laminaria digitata (Hudson) JV Lamouroux 1813. Foods 2022, 11, 1792. [Google Scholar] [CrossRef]
- Strain, C.R.; Collins, K.C.; Naughton, V.; McSorley, E.M.; Stanton, C.; Smyth, T.J.; Soler-Vila, A.; Rea, M.C.; Ross, P.R.; Cherry, P.; et al. Effects of a polysaccharide-rich extract derived from Irish-sourced Laminaria digitata on the composition and metabolic activity of the human gut microbiota using an in vitro colonic model. Eur. J. Nutr. 2020, 59, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Feki, F.; Klisurova, D.; Masmoudi, M.A.; Choura, S.; Denev, P.; Trendafilova, A.; Chamkha, M.; Sayadi, S. Optimization of microwave assisted extraction of simmondsins and polyphenols from Jojoba (Simmondsia chinensis) seed cake using Box-Behnken statistical design. Food Chem. 2021, 356, 129670. [Google Scholar] [CrossRef]
- Souadia, A.; Gourine, N.; Yousfi, M. Optimization total phenolic content and antioxidant activity of Saccocalyx satureioides extracts obtained by ultrasonic-assisted extraction. J. Chemometr. 2022, 36, e3428. [Google Scholar] [CrossRef]
- Setyani, W.; Murwanti, R.; Sulaiman, T.N.S.; Hertiani, T. Application of Response Surface Methodology (RSM) for the optimization of ultrasound-assisted extraction (UAE) of Moringa oleifera: Extraction yield, content of bioactive compounds, and biological effects in vitro. Plants 2023, 12, 2455. [Google Scholar] [CrossRef]
- Ozioko, F.C.; Iweka, S.C.; Nwanze, N.E.; Adepoju, T.F. Effect of thermally extracted carrot oil using biomass solvent to boost consumer goods: Box-Behnken design approach. J. Eng. Res. 2024, 26, 113–124. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.M.; Mourtzinos, I.; Goula, A.M. Recovery of phenolic compounds from spent coffee grounds through optimized extraction processes. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- Beratto, A.; Agurto, C.; Freer, J.; Peña-Farfal, C.; Troncoso, N.; Agurto, A.; Castillo, R.D.P. Chemical characterization and determination of the anti-oxidant capacity of two brown algae with respect to sampling season and morphological structures using infrared spectroscopy and multivariate analyses. Appl. Spectrosc. 2017, 71, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Wekre, M.E.; Hellesen Brunvoll, S.; Jordheim, M. Advancing quantification methods for polyphenols in brown seaweeds-applying a selective qNMR method compared with the TPC assay. Phytochem. Anal. 2022, 33, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- D’Este, M.; Alvarado-Morales, M.; Ciofalo, A.; Angelidaki, I. Macroalgae Laminaria digitata and Saccharina latissima as potential biomasses for biogas and total phenolics production: Focusing on seasonal and spatial variations of the algae. Energy Fuels 2017, 31, 7166–7175. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid–liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drug 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, B.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Identification of phenolic compounds in Australian grown dragon fruits by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Arab. J. Chem. 2021, 14, 103151. [Google Scholar] [CrossRef]
- de Oliveira, D.N.; Ferreira, M.S.; Catharino, R.R. Rapid and simultaneous in situ assessment of aflatoxins and stilbenes using silica plate imprinting mass spectrometry imaging. PLoS ONE 2014, 9, e90901. [Google Scholar] [CrossRef]
- Fahmy, N.M.; El-Din, M.I.G.; Salem, M.M.; Rashedy, S.H.; Lee, G.S.; Jang, Y.S.; Kim, K.H.; Kim, C.S.; El-Shazly, M.; Fayez, S. Enhanced expression of p53 and suppression of PI3K/Akt/mTOR by three red sea algal extracts: Insights on their composition by LC-MS-Based metabolic profiling and molecular networking. Mar. Drug 2023, 21, 404. [Google Scholar] [CrossRef]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef]
- Ramabulana, A.T.; Steenkamp, P.; Madala, N.; Dubery, I.A. Profiling of chlorogenic acids from Bidens pilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, R.; Arafa, I.H.; Usam, A.M.; Abdullah Sulaiman, A.A.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Duan, J.A.; Tang, Y.P.; Guo, J.M.; Shang, E.X.; Zhu, Z.H. Identification of chemical constituents in SiWu decoction by UHPLC-DAD-TOF/MS. Acta Chromatogr. 2014, 26, 517–537. [Google Scholar] [CrossRef]
- Chandradevan, M.; Simoh, S.; Mediani, A.; Ismail, N.H.; Ismail, I.S.; Abas, F. UHPLC-ESI-Orbitrap-MS analysis of biologically active extracts from Gynura procumbens (Lour.) Merr. and Cleome gynandra L. leaves. Evid. Based Complement. Altern. Med. 2020, 2020, 3238561. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Z.; Li, P.; Ye, S.; Li, W.; Li, M.; Zhu, L.; Ding, Y. Investigation of the potential phlorotannins and mechanism of six brown algae in treating type II Diabetes mellitus based on biological activity, UPLC-QE-MS/MS, and Network Pharmacology. Foods 2023, 12, 3000. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sang, M.; Liu, E.; Banahene, P.O.; Zhang, Y.; Wang, T.; Han, L.; Gao, X. Rapid profiling and pharmacokinetic studies of major compounds in crude extract from Polygonum multiflorum by UHPLC-Q-TOF-MS and UPLC–MS/MS. J. Pharm. Biomed. Anal. 2017, 140, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and in vitro preliminary anti-inflammatory activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) burs, leaves, and chestnuts extracts and their metabolite profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Gašić, U.; Kečkeš, S.; Dabić, D.; Trifković, J.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. Phenolic profile and antioxidant activity of Serbian polyfloral honeys. Food Chem. 2014, 145, 599–607. [Google Scholar] [CrossRef]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae—Unraveling the polar lipid and fatty acid composition of Chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- Chouh, A.; Nouadri, T.; Catarino, M.D.; Silva, A.M.; Cardoso, S.M. Phlorotannins of the brown algae Sargassum vulgare from the Mediterranean Sea coast. Antioxidants 2022, 11, 1055. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Mannitol: Physiological functionalities, determination methods, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2020, 104, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, A.D.; Tuvikene, R.; Somasiri, M.N.R.; De Silva, M.L.W.P.; Adhikari, R.; Ranahewa, T.H.; Wijesundara, R.R.M.K.K.; Wijesekera, S.K.; Dissanayake, I.P.G.H.U.; Wangchuk, P.; et al. A novel therapeutic effect of mannitol-rich extract from the brown seaweed Sargassum ilicifolium using in vitro and in vivo models. BMC Complement. Med. Ther. 2023, 23, 26. [Google Scholar]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2007. [Google Scholar]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential. Mar. Drug 2020, 18, 331. [Google Scholar] [CrossRef]
- Hou, J.; Liang, L.; Su, M.; Yang, T.; Mao, X.; Wang, Y. Variations in phenolic acids and antioxidant activity of navel orange at different growth stages. Food Chem. 2021, 360, 129980. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, H.; Wang, Y.; Peng, Z.; Guo, Z.; Ma, Y.; Zhang, R.; Zhang, M.; Wu, Q.; Xiao, J.; et al. Effects of different extraction methods on contents, profiles, and antioxidant abilities of free and bound phenolics of Sargassum polycystum from the South China Sea. J. Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, C.; Guo, Z.; Li, S.; Zhu, Z.; Grimi, N.; Xiao, J. Fermentation of Betaphycus gelatinum using Lactobacillus brevis: Growth of probiotics, total polyphenol content, polyphenol profile, and antioxidant capacity. Foods 2023, 12, 3334. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar lipids composition, antioxidant and anti-inflammatory activities of the atlantic red seaweed Grateloupia turuturu. Mar. Drug 2021, 19, 414. [Google Scholar] [CrossRef]
- Arif, M.; Bai, Y.; Usman, M.; Jalalah, M.; Harraz, F.A.; Al-Assiri, M.S.; Li, X.; Salama, E.S.; Zhang, C. Highest accumulated microalgal lipids (polar and non-polar) for biodiesel production with advanced wastewater treatment: Role of lipidomics. Bioresour. Technol. 2020, 298, 122299. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news/item/31-01-2013-who-issues-new-guidance-on-dietary-salt-and-potassium (accessed on 9 May 2024).
- Singh, S.K.; Thakur, R.; Singh, M.K.; Singh, C.S.; Pal, S.K. Effect of fertilizer level and seaweed sap on productivity and profitability of rice (Oryza sativa). Indian J. Agric. Sci. 2015, 60, 420–425. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Mohabati, M.; Eslami, S.; Sohrabipour, J.; Miri, R. Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iran. J. Pharm. Res. 2013, 12, 339–348. [Google Scholar]
- Horincar, V.B.; Parfene, G.; Tyagi, A.K.; Gottardi, D.; Dinică, R.; Guerzoni, M.E.; Bahrim, G. Extraction and characterization of volatile compounds and fatty acids from red and green macroalgae from the Romanian Black Sea in order to obtain valuable bioadditives and biopreservatives. J. Appl. Phycol. 2014, 26, 551–559. [Google Scholar] [CrossRef]
- Padmakumar, K.; Ayyakkannu, K. Seasonal variation of antibacterial and antifungal activities of the extracts of marine algae from southern coasts of India. Bot. Mar. 1997, 40, 507–515. [Google Scholar] [CrossRef]
- Salem, W.M.; Galal, H.; Nasr El-deen, F. Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr. J. Microbiol. Res. 2011, 5, 2160–2167. [Google Scholar] [CrossRef]
- Hellio, C.; De La Broise, D.; Dufosse, L.; Le Gal, Y.; Bourgougnon, N. Inhibition of marine bacteria by extracts of macroalgae: Potential use for environmentally friendly antifouling paints. Mar. Environ. Res. 2001, 52, 231–247. [Google Scholar] [CrossRef]
- Boisvert, C.; Beaulieu, L.; Bonnet, C.; Pelletier, É. Assessment of the antioxidant and antibacterial activities of three species of edible seaweeds. J. Food Biochem. 2015, 39, 377–387. [Google Scholar] [CrossRef]
- Osman, M.E.; Aboshady, A.M.; Elshobary, M.E. Production and characterization of antimicrobial active substance from some macroalgae collected from Abu-Qir bay (Alexandria) Egypt. Afr. J. Biotechnol. 2013, 12, 6847–6858. [Google Scholar]
- Ismail, A.; Salah, K.B.H.; Ahmed, M.; Mastouri, M.; Bouraoui, A.; Aouni, M. Antibacterial and antifungal activities of brown alga Zonaria tournefortii (JV Lamouroux). Allelopathy J. 2014, 34, 143–154. [Google Scholar]
- Ina, K.; Takasawa, R.; Yagi, A.; Yamashita, N.; Etoh, H.H.; Sakata, K. An improved assay method for antifouling substances using the blue mussel, Mytilus edulis. Agric. Biol. Chem. 1989, 53, 3319–3321. [Google Scholar]
- Bhattacharya, S. Central composite design for response surface methodology and its application in pharmacy. In Response Surface Methodology in Engineering Science; Kayaroganam, P., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Savić Gajić, I.; Savić, I.; Boškov, I.; Žerajić, S.; Marković, I.; Gajić, D. Optimization of ultrasound-assisted extraction of phenolic compounds from black locust (Robiniae pseudoacaciae) flowers and comparison with conventional methods. Antioxidants 2019, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Savić, I.M.; Nikolić, V.D.; Savić, I.M.; Nikolić, L.J.B.; Stanković, M.Z.; Moder, K. Optimization of total flavonoid compound extraction from Camellia sinensis using the artificial neural network and response surface methodology. Hem. Ind. 2013, 67, 249–259. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Vasiljevic, Z.; Vunduk, J.; Bartolic, D.; Miskovic, G.; Ognjanovic, M.; Tadic, N.B.; Nikolic, M.V. An Eco-friendly approach to ZnO NP synthesis using Citrus reticulata Blanco peel/extract: Characterization and antibacterial and photocatalytic activity. ACS Appl. Bio Mater. 2024, 7, 3014–3032. [Google Scholar] [CrossRef]

| Run | Factor 1 | Factor 2 | Factor 3 | Response (Y): Alginate Yield (%) |
|---|---|---|---|---|
| A: Treatment Time (min) | B: Liquid-to-Solid Ratio (mL/g) | C: Treatment Temperature (°C) | Experimental | |
| 1 | 20 | 10 | 70 | 29.91 ± 0.23 |
| 2 | 30 | 10 | 55 | 20.39 ± 0.11 |
| 3 * | 20 | 20 | 55 | 13.12 ± 0.09 |
| 4 | 30 | 20 | 70 | 16.28 ± 0.10 |
| 5 | 10 | 20 | 40 | 16.78 ± 0.05 |
| 6 | 30 | 20 | 40 | 18.23 ± 0.13 |
| 7 * | 20 | 20 | 55 | 14.61 ± 0.04 |
| 8 * | 20 | 20 | 55 | 15.80 ± 0.19 |
| 9 | 10 | 10 | 55 | 27.98 ± 0.24 |
| 10 | 30 | 30 | 55 | 27.63 ± 0.02 |
| 11 | 20 | 30 | 40 | 28.88 ± 0.15 |
| 12 | 20 | 30 | 70 | 9.91 ± 0.03 |
| 13 * | 20 | 20 | 55 | 13.59 ± 0.10 |
| 14 | 10 | 20 | 70 | 10.92 ± 0.05 |
| 15 | 10 | 30 | 55 | 9.40 ± 0.06 |
| 16 | 20 | 10 | 40 | 19.17 ± 0.10 |
| 17 * | 20 | 20 | 55 | 15.14 ± 0.13 |
| Sum of Squares | df | Mean Squares | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 716.2 | 9 | 79.6 | 83.3 | <0.0001 * |
| A-A | 37.6 | 1 | 37.6 | 39.4 | 0.0004 * |
| B-B | 58.8 | 1 | 58.8 | 61.6 | 0.0001 * |
| C-C | 32.7 | 1 | 32.7 | 34.2 | 0.0006 * |
| AB | 165.4 | 1 | 165.4 | 173.2 | <0.0001 * |
| AC | 4.0 | 1 | 4.0 | 4.2 | 0.0795 |
| BC | 222.2 | 1 | 222.2 | 232.7 | <0.0001 * |
| A2 | 0.3 | 1 | 0.3 | 0.3 | 0.6190 |
| B2 | 187.2 | 1 | 187.2 | 196.0 | <0.0001 * |
| C2 | 3.3 | 1 | 3.3 | 3.4 | 0.1073 |
| Residual | 6.7 | 7 | 1.0 | ||
| Lack-of-fit | 1.9 | 3 | 0.6 | 0.5 | 0.677 |
| Pure error | 4.7 | 4 | 1.2 | ||
| Cor total | 722.8 | 16 | |||
| Standard deviation | 0.9772 | R2 | 0.9908 | ||
| Mean value | 18.09 | adjusted R2 | 0.9789 | ||
| CV % | 5.40 | predicted R2 | 0.9468 | ||
| Adequate precision | 27.1 | ||||
| No. | [M − H]− or [M + HCOOH]− m/z | tR, min | MS/MS Fragment Ions, m/z | Compound | The Literature |
|---|---|---|---|---|---|
| 1. | 180.90 | 0.86 | 163, 143, 131, 119, 101 (100%), 89, 83, 59 | d-(−)-mannitol * | |
| 2. | 236.93 | 6.89 | 193 (100%) | 7-hydroxyflavone * | |
| 3. | 260.95 | 7.20 | 189, 165 (100%) | 4-hydroxybenzoic acid-4-O-glucoside | Chen et al. [28] |
| 4. | 265.12 | 14.94 | 97 (100%) | oxidized fatty acid * | |
| 5. | 272.92 [M − H + HCOOH]− | 0.65 | 227, 217, 159 (100%), 115 | resveratrol | de Oliveira et al. [29] |
| 6. | 277.01 | 1.56 | 185 (100%), 141, 97 | octadecatrienoic acid | Fahmy et al. [30] |
| 7. | 293.04 | 13.59 | 249, 236 (100%), 221, 217, 193, 136 | hydroxy-octadecatrienoic acid | Fahmy et al. [30] |
| 8. | 304.87 | 19.00 | 175 (100%), 131 | 2-isopropylmalic acid derivative | Sobeh et al. [31] |
| 9. | 305.04 | 11.00 | 249, 231 (100%) | unknown | |
| 10. | 311.22 | 15.48 | 243, 211, 183, 149 (100%) | caftaric acid | Ramabulana et al. [32] |
| 11. | 317.03 | 15.11 | 299, 255, 249 (100%), 228, 215, 181, 163, 135 | p-coumaric acid derivative * | |
| 12. | 319.18 | 15.61 | 301 (100%), 275, 257, 203, 179, 167, 115 | 5-O-p-coumaroylshikimic acid | Ben Said et al. [33] |
| 13. | 351.03 | 14.14 | 315 (100%), 297, 222, 161, 111 | isorhamnetin derivative | Ben Said et al. [33] |
| 14. | 353.21 | 14.20 | 335, 317, 310, 256, 191 (100%), 173, 123 | 1-caffeoylquinic acid | Zhu et al. [34] |
| 15. | 355.30 | 16.54 | 337, 309 (100%), 205, 130 | coumaroylglucaric acid isomer | Chandradevan et al. [35] |
| 16. | 360.93 | 6.02 | 317 (100%), 293, 273, 231, 185 | phlorotannin derivative | Chen et al. [36] |
| 17. | 372.83 | 18.91 | 305 (100%) | fucophlorethol | Chen et al. [36] |
| 18. | 378.69 | 18.89 | 311 (100%), 249, 179 | caftaric acid derivative | Ramabulana et al. [32] |
| 19. | 379.12 | 0.70 | 343 (100%), 179 | quercetin derivative | Ben Said et al. [33] |
| 20. | 384.77 | 17.84 | 317, 249 (100%) | phlorotannin derivative | Chen et al. [36] |
| 21. | 385.20 | 16.29 | 317, 249, 227 (100%), 195, 175, 157 | phlorotannin derivative | Chen et al. [36] |
| 22. | 439.29 | 17.52 | 281 (100%), 227 | sterol (C:N 25:0;O6) | Fahmy et al. [30] |
| 23. | 440.87 | 18.94 | 425, 373, 305 (100%), 175 | epigallocatechin derivative | Chandradevan et al. [35] |
| 24. | 452.73 | 17.66 | 385, 317 (100%), 249 | phlorotannin derivative | Fahmy et al. [30] |
| 25. | 484.95 | 6.38 | 441, 397, 349, 315, 301 (100%), 297, 271, 257, 225 | quercetin derivative * | |
| 26. | 501.12 | 14.84 | 484, 458, 433, 380 (100%), 365, 285, 272, 212, 197 | kaempferol derivative | Chandradevan et al. [35] |
| 27. | 508.70 | 18.70 | 441 (100%), 373, 312, 305 | epigallocatechin derivative | Chandradevan et al. [35] |
| 28. | 520.65 | 17.69 | 452, 387 (100%), 317 | kaempferol-3-O-glucoside | Chandradevan et al. [35] |
| 29. | 527.09 | 7.15 | 499, 481, 409, 401 (100%), 387, 367, 341, 329, 313, 282, 271 | unknown | |
| 30. | 527.30 | 15.21 | 334, 299, 244, 225 (100%), 207, 165, 153 | orientin-sulphate | Ben Said et al. [33] |
| 31. | 547.40 | 17.07 | 479, 379, 285 (100%), 279, 267 | luteolin derivative | Chandradevan et al. [35] |
| 32. | 549.45 | 18.16 | 482, 405, 287, 279 (100%), 269 | liquiritigenin-hexose-xyl/ara | Wang et al. [37] |
| 33. | 555.32 | 16.27 | 508, 299 (100%), 225 | sulfoquinovosyl monoacylglycerol (C:N 16:0) | Fahmy et al. [30] |
| 34. | 557.14 | 15.46 | 525, 511 (100%), 421, 308, 287, 275, 253, 231, 161 | caffeic acid derivative | Chandradevan et al. [35] |
| 35. | 559.15 | 15.75 | 513 (100%), 491, 423, 289, 277, 253 | caffeic acid derivative | Chandradevan et al. [35] |
| 36. | 571.31 | 17.40 | 521, 449, 311, 293 (100%), 277, 231 | caffeic acid derivative | Chandradevan et al. [35] |
| 37. | 579.25 | 15.90 | 307, 225 (100%) | sulfoquinovosyl monoacylglycerol (C:N 18:2) | Cerulli et al. [38] |
| 38. | 581.30 | 16.32 | 535, 377, 308, 299 (100%), 282, 225, 208 | 3-O-methylorobol derivative | Ben Said et al. [33] |
| 39. | 588.70 | 17.64 | 521 (100%), 453, 317 | unknown | |
| 40. | 599.06 | 10.29 | 555, 534, 507, 473 (100%), 459, 439, 411, 399, 383, 355, 343, 312, 275, 254 | unknown | |
| 41. | 609.02 | 6.60 | 565 (100%), 439, 395, 301, 297 | Rutin | Gašić et al. [39] |
| 42. | 665.46 | 17.88 | 634, 619, 605, 561, 545, 510, 445, 354 (100%), 337, 310, 293, 282, 266, 251, 239, 228 | posphatidic acid (C:N 34:4) | Melo et al. [40] |
| 43. | 719.09 | 15.18 | 673, 583, 447 (100%), 397, 243 | posphatidylglycerol (C:N 32:1) | Melo et al. [40] |
| 44. | 721.15 [M + HCOO]− | 15.50 | 675 (100%), 653, 397 | digalactosyl monoacylglycerol (C:N 18:3) | Fahmy et al. [30] |
| 45. | 723.10 | 15.92 | 677, 452 | phlorotannin sulfate | Chouh et al. [41] |
| 46. | 725.14 | 16.52 | 689, 679, 589, 453, 429 (100%), 397, 379 | unknown | |
| 47. | 774.00 | 17.02 | 737 (100%), 688, 455, 443 | unknown | |
| 48. | 805.43 | 16.94 | 761, 391 (100%), 347, 305 | unknown |
| Macroelements | Macroelement Content (mg/g d.w.) | Microelements | Microelement Content (μg/g d.w.) |
|---|---|---|---|
| Ca | 0.11 ± 0.006 | Al | 1.92 ± 0.19 |
| K | 36.77 ± 0.92 | B | 198.84 ± 2.51 |
| Mg | 11.90 ± 0.43 | Ba | 0.07 ± 0.01 |
| Na | 106.59 ± 1.23 | Cd | 0.05 ± 0.004 |
| P | 4.57 ± 0.16 | Co | 0.03 ± 0.003 |
| S | 18.88 ± 0.25 | Cr | 0.09 ± 0.005 |
| Si | 0.03 ± 0.002 | Cu | 1.50 ± 0.01 |
| Fe | 17.26 ± 0.48 | ||
| Li | 1.24 ± 0.02 | ||
| Mn | 8.80 ± 0.11 | ||
| Ni | 0.62 ± 0.05 | ||
| Pb | 0.48 ± 0.03 | ||
| Sr | 1.49 ± 0.13 | ||
| Zn | 7.06 ± 0.21 | ||
| Total macroelements | 178.84 | Total microelements | 239.45 |
| Microorganism | Sample | Gentamicin | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| Staphylococcus aureus ATCC 6538 | 5 | 10 | 0.125 | / |
| Enterococcus faecalis ATCC 29219 | 10 | 20 | 0.062 | 0.062 |
| Bacillus cereus ATCC 10876 | 5 | 10 | 0.015 | 0.125 |
| Escherichia coli ATCC 25922 | 10 | 10 | 0.015 | 0.015 |
| Pseudomonas aeruginosa ATCC 27853 | 10 | 20 | 0.500 | 1.000 |
| Shigella sonnei ATCC 29930 | 10 | 20 | 0.078 | 0.031 |
| Nystatin | ||||
| MIC (mg/mL) | MFC (mg/mL) | MIC (mg/mL) | MFC (mg/mL) | |
| Candida albicans ATCC 1231 | 20 | / | 0.0077 | 0.0625 |
| Factors | Coded Values | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Actual Values | |||
| Treatment time [min] | 10 | 20 | 30 |
| Liquid-to-solid ratio [mL/g] | 10 | 20 | 30 |
| Treatment temperature [°C] | 40 | 55 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savić Gajić, I.M.; Savić, I.M.; Ivanovska, A.M.; Vunduk, J.D.; Mihalj, I.S.; Svirčev, Z.B. Improvement of Alginate Extraction from Brown Seaweed (Laminaria digitata L.) and Valorization of Its Remaining Ethanolic Fraction. Mar. Drugs 2024, 22, 280. https://doi.org/10.3390/md22060280
Savić Gajić IM, Savić IM, Ivanovska AM, Vunduk JD, Mihalj IS, Svirčev ZB. Improvement of Alginate Extraction from Brown Seaweed (Laminaria digitata L.) and Valorization of Its Remaining Ethanolic Fraction. Marine Drugs. 2024; 22(6):280. https://doi.org/10.3390/md22060280
Chicago/Turabian StyleSavić Gajić, Ivana M., Ivan M. Savić, Aleksandra M. Ivanovska, Jovana D. Vunduk, Ivana S. Mihalj, and Zorica B. Svirčev. 2024. "Improvement of Alginate Extraction from Brown Seaweed (Laminaria digitata L.) and Valorization of Its Remaining Ethanolic Fraction" Marine Drugs 22, no. 6: 280. https://doi.org/10.3390/md22060280
APA StyleSavić Gajić, I. M., Savić, I. M., Ivanovska, A. M., Vunduk, J. D., Mihalj, I. S., & Svirčev, Z. B. (2024). Improvement of Alginate Extraction from Brown Seaweed (Laminaria digitata L.) and Valorization of Its Remaining Ethanolic Fraction. Marine Drugs, 22(6), 280. https://doi.org/10.3390/md22060280











