Abstract
Four tunicamycin class compounds, tunicamycin VII (1), tunicamycin VIII (2), corynetoxin U17a (3), and tunicamycin IX (4), were isolated from the culture broth of the marine-derived actinomycete Streptomyces sp. MBTG32. The strain was identified using the 16S rDNA sequencing technique, and the isolated strain was closely related to Streptomyces bacillaris. The structures of the isolated compounds were elucidated based on spectroscopic data and comparisons with previously reported NMR data. Compounds 1–4 showed potent antibacterial activities against Gram-positive bacteria, especially Staphylococcus aureus, with MIC values of 0.13–0.25 µg/mL. Through a recombinant enzyme assay and overexpression analysis, we found that the isolated compounds exerted potent inhibitory effects on S. aureus MurNAc-pentapeptide translocase (MraY), with IC50 values of 0.08–0.21 µg/mL. The present results support that the underlying mechanism of action of tunicamycins isolated from marine-derived Streptomyces sp. is also associated with the inhibition of MraY enzyme activity in S. aureus.
1. Introduction
The prevalence of antibiotic-resistant bacteria has emerged as a major threat to human health, as infections from these bacteria are uncontrollable with commercial antibiotics [1]. Among the antibiotic-resistant bacteria, some are multidrug-resistant. Methicillin-resistant Staphylococcus aureus (MRSA) is a major pathogenic drug-resistant bacteria [2]. Drug resistance is caused by the selection of resistant organisms in antimicrobial conditions and the transfer of these resistance genes through mobile genetic elements, such as plasmids, bacteriophages, or transposons [3]. The acquisition of drug resistance is much faster than the rate of development of new antibacterial agents, but the investment in antibacterial drugs continues to decline [4]. As the number of newly discovered antibiotics is also decreasing, scientists have searched for new bioactive secondary metabolites from marine actinomycetes because their unique metabolisms might lead to structurally novel compounds [5,6]. As a result, various novel bioactive compounds have been discovered from rare marine actinomycetes [7].
Tunicamycins are a class of nucleoside sugar analog compounds that are characterized by the uracil moiety followed by an eleven-carbon dialdose sugar with an N-acetyl-D-glucosamine and an N-linked fatty acid residue [8]. Tunicamycins were first isolated from Streptomyces lysosuperificus as a mixture of homologs and later isolated as tunicamycin I, II, III, IV, and V [9,10]. Streptovirudins and corynetoxins, whose structures are similar to that of tunicamycins but differ in the N-linked acyl chains, were isolated from Streptomyces griseoflavus and Corynebacterium rathayi, respectively [11]. Tunicamycins are known to inhibit MurNAc-pentapeptide translocase (MraY), which is an essential membrane-associated enzyme in bacteria [12]. MraY transfers uridine diphosphate-MurNAc-pentapeptide to the lipid carrier bactoprenol phosphate (C55-P) to form lipid I, which is an intermediate of peptidoglycan synthesis [13,14]. MraY is a promising drug target for developing new antibacterial agents, though it is yet to be utilized as a target for commercial antibiotics [15]. Although tunicamycins have strong antibacterial activities against some Gram-positive bacteria—especially S. aureus—these compounds also inhibit dolichyl-phosphate N-acetylglucosamine-phosphotransferase 1 (DPAGT1) and show cytotoxicity in mammalian cells by damaging the membrane, leading to necroptosis [16,17]. While low specificity and cytotoxicity limited the use of tunicamycins as clinical drugs, recent attempts to achieve higher specificity toward DAPGT1 or antibacterial activity while lowering cytotoxicity has shown modified tunicamycins to be potential anticancer and antibacterial agents [17,18].
In the course of searching for bioactive secondary metabolites from marine-derived actinomycetes, we isolated the MBTG32 strain from marine sediment samples collected from Jeju Island, Republic of Korea. The strain was identified as Streptomyces bacillaris on the basis of its 16S rDNA sequence. This strain’s organic extract of a liquid culture exhibited potent antibacterial activity against S. aureus ATCC6538p with a minimum inhibitory concentration (MIC) of 0.5 μg/mL. Antibacterial-activity-guided separation of the extract using diverse chromatographic methods led to the isolation of four compounds: tunicamycin VII (1), tunicamycin VIII (2), corynetoxin U17a (3), and tunicamycin IX (4). We herein report the antibacterial activities of compounds 1–4 against several representative pathogenic bacteria, including S. aureus ATCC6538p and MRSA strains CCARM3090, ATCC43300, and ATCC700787. We also analyzed the mode of action of the compounds isolated through a MraY enzyme activity assay and overexpression assay.
2. Results and Discussion
2.1. Taxonomy and Phylogenetic Analysis of MBTG32
The phylogenetic analysis of the MBTG32 strain was conducted by amplifying the 16S rDNA sequence using polymerase chain reaction (PCR). The amplified sequence (1492 bp) was analyzed using the Basic Logic Alignment Search Tool (BLAST), and the sequence from the MBTG32 strain showed 100% identity with Streptomyces bacillaris strain NBRC13487 (GenBank accession number: NR041146). Therefore, this strain was designated as Streptomyces bacillaris strain MBTG32 (GenBank accession number: PP669811).
2.2. Isolation and Structural Elucidation of Compounds 1–4
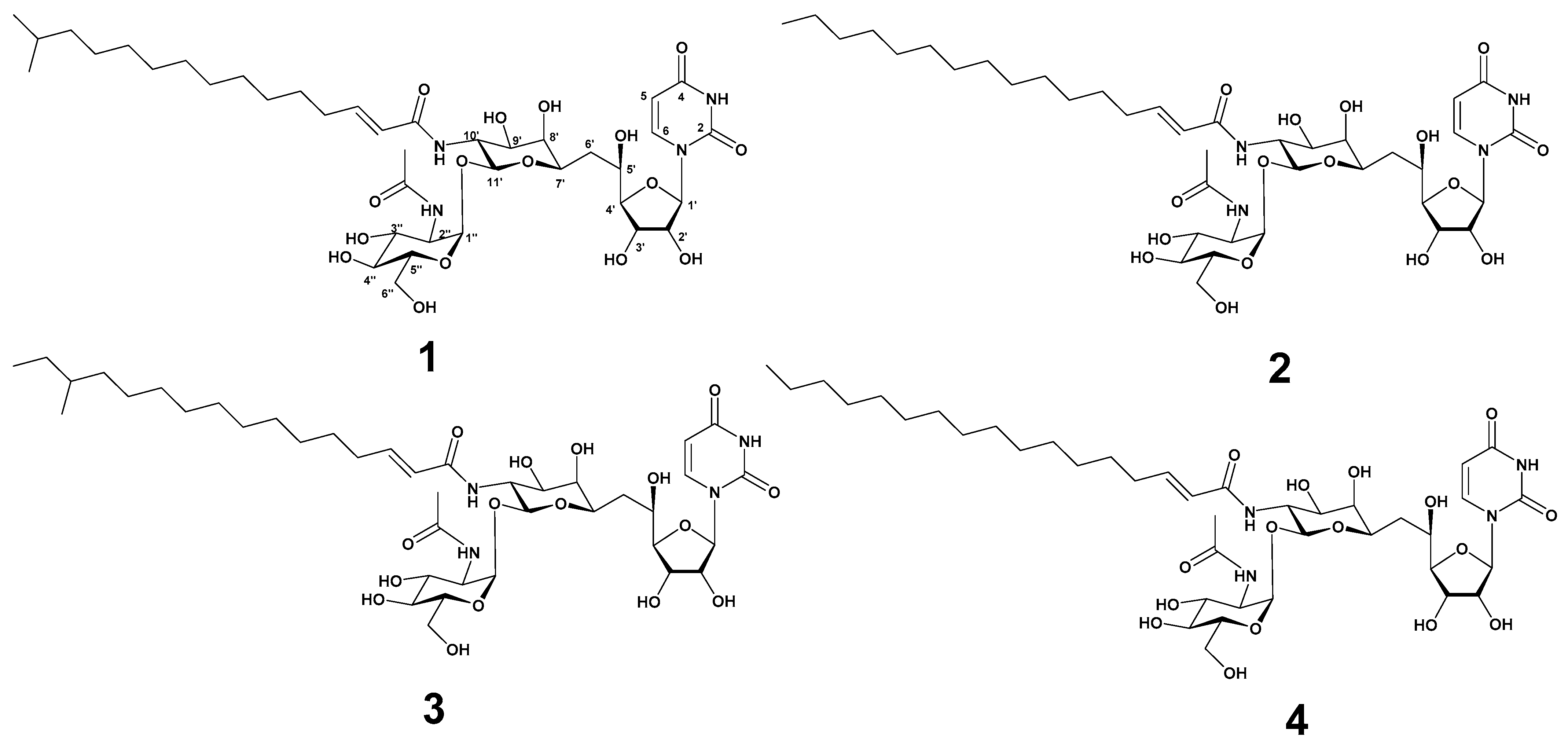
Strain MBTG32 was cultivated in GTYB broth and incubated for 7 days with shaking. The culture filtrate was extracted with n-hexane, and the aqueous fraction was subsequently extracted with ethyl acetate. The ethyl acetate fraction exhibited antibacterial activity against S. aureus, and further separation was conducted with C18 reversed-phase vacuum flash chromatography. After fractionation, the 100% methanol fraction was separated using semi-preparative high-pressure liquid chromatography (HPLC) to yield four compounds in the form of white powders. Based on spectroscopic analysis, including high-resolution electrospray ionization mass spectrometry (HR-ESI-MS), 1H, and 13C nuclear magnetic resonance (NMR), compounds 1–4 were identified as tunicamycin VII (1) [19,20], tunicamycin VIII (2) [20,21], corynetoxin U17a (3) [22], and tunicamycin IX (4) [20,21] (Figure 1). The acyl iso branching in compound 1 was determined from 1H NMR spectra, which showed a doublet (6H, J = 6.6 Hz, -CH(CH3)2) at δH 0.84. The presence of a linear acyl chain in compounds 2 and 4 was supported by 1H NMR spectrum at δH 0.80 (3H, t, J = 6.9 Hz) and δH 0.80 (3H, t, J = 7.0 Hz), respectively. In the 1H NMR spectrum of 3, the presence of the acyl anteiso branching was confirmed from one doublet and one triplet methyl proton at δH 0.82 (3H, d, J = 6.2 Hz) and δH 0.83 (3H, t, J = 7.0 Hz). These assignments were further supported by HR-ESI-MS data and notable fragmentation patterns. HR-ESI-MS analysis of compounds revealed a mass distribution and fragmentation pattern identical to those of tunicamycins [20]. Molecular ions were detected as [M + H]+, along with sodium adducts [M + Na]+, and two diagnostically useful fragment ions [M + H − 221]+ and [M + H − 203]+ (Figures S14–S17). The spectroscopic data of these compounds were in good agreement with those in the previous literature (Figures S1–S17, Tables S2 and S3).
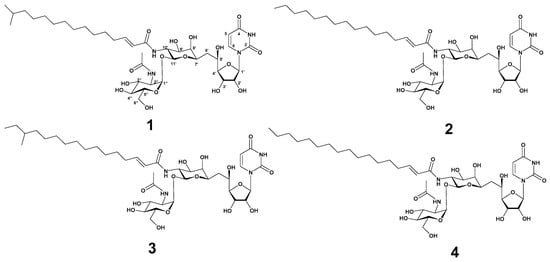
Figure 1.
The structure of tunicamycin VII (1), tunicamycin VIII (2), corynetoxin U17a (3), and tunicamycin IX (4).
2.3. Antibacterial Activities of Compounds 1–4
Tunicamycin from Streptomyces lysosuperficus was previously reported to show antibacterial activity against several Gram-positive bacteria (MIC = 0.1–20 µg/mL), particularly those in the Bacillus genus [9]. Therefore, the antibacterial activities of compounds 1–4 were evaluated against six representative pathogenic bacterial strains. These compounds showed significant antibacterial activities against Gram-positive bacteria, especially S. aureus ATCC6538p, with MICs in the range of 0.06–0.25 μg/mL (Table 1). However, these compounds showed weak or no activities against Gram-negative bacteria. Based on the reported data, compounds 1–4 were further evaluated against various methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA strains (Table 2). In the case of MSSA strains, compounds 1–4 exhibited potent antibacterial activities against CCARM0204 and CCARM0205 but substantially weak activities against CCARM0024 and CCARM3640. In the case of MRSA strains, compounds 1–4 did not show inhibitory activities against CCARM3089 and ATCC700788 and showed moderate to weak activities against CCARM3090, CCARM3634, CCARM3635, ATCC43300, and ATCC700787, with MIC values ranging from 2 to 64 μg/mL. These results indicate that the susceptibility of compounds 1–4 differs within S. aureus strains.

Table 1.
Results of the antimicrobial activities of compounds 1–4.

Table 2.
Antibacterial activities of compounds 1–4 against MSSA and MRSA strains.
2.4. Effects of Compounds 1–4 on MraY Expression and Preparation of Recombinant MraY
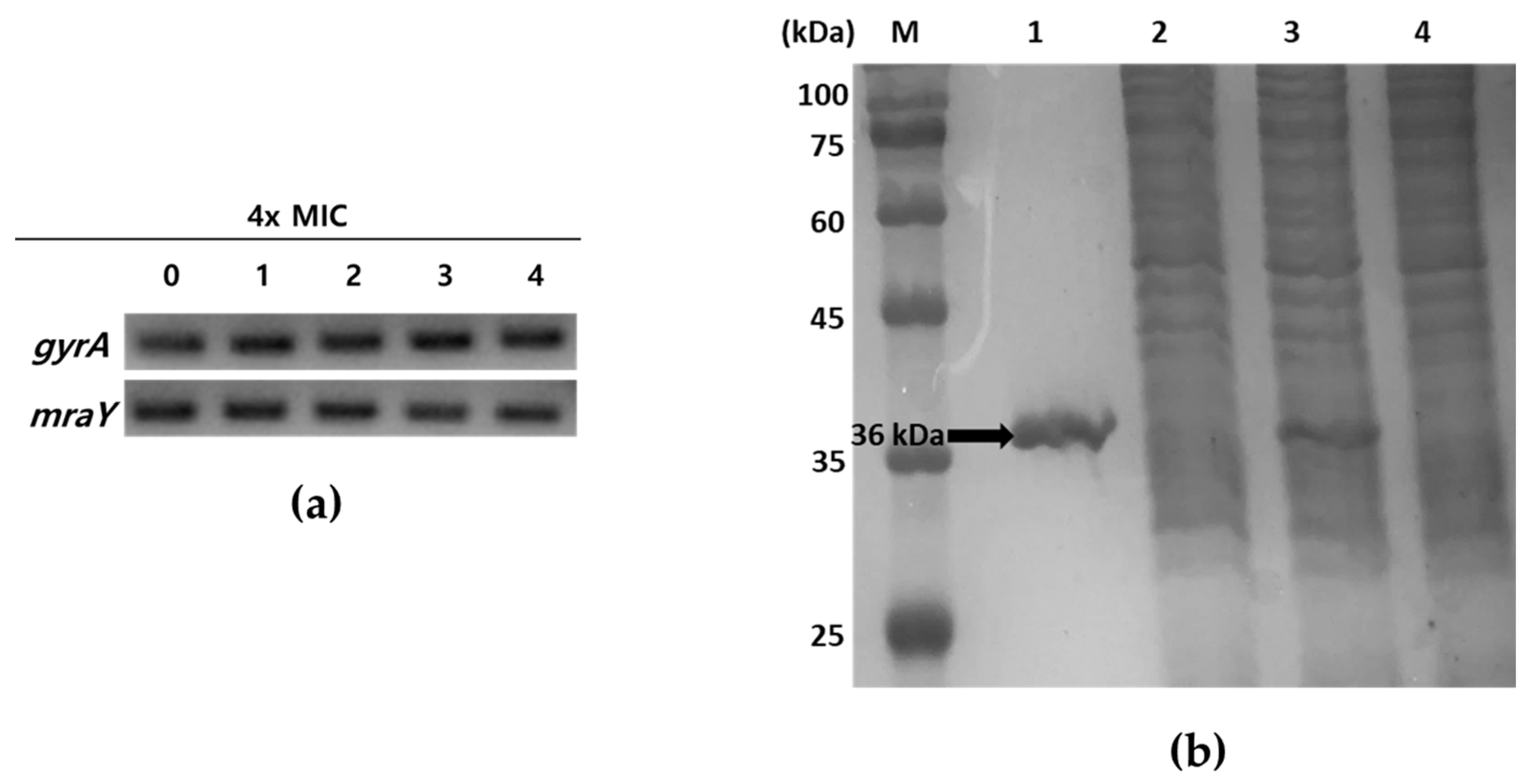
Tunicamycins, liposidomycins, capuramycins, mureidomycins, and muraymycins are known as inhibitors of MraY, the first membrane protein in peptidoglycan formation, which is essential for bacterial growth [13,23,24]. To ascertain whether tunicamycins inhibit the transcription of mraY or block the function of MraY at the enzyme level without affecting the expression of the mraY gene, the mRNA expression levels of mraY in response to tunicamycins were initially explored using semi-quantitative reverse-transcription PCR (RT-PCR). S. aureus ATCC6538p was grown to mid-log phase in MHB media and treated with four times the MIC of compounds 1–4 at 37 °C for 40 min. Total RNA isolated from S. aureus ATCC6538p was reverse-transcribed into cDNA, and the mraY gene was amplified using this cDNA as a template. As shown in Figure 2a, S. aureus ATCC6538p showed no inhibition of mraY gene transcription in response to treatment with compounds 1–4. Next, to determine the enzymatic activity inhibition of compounds 1–4, recombinant MraY derived from S. aureus ATCC6538p was prepared and purified (Figure 2b). S. aureus 6538p mraY was cloned into pET28a to obtain recombinant plasmid pET28a-mraY. The recombinant His6-tagged MraY enzyme was overexpressed in Escherichia coli BL21 with 1 mM IPTG and purified from the detergent-solubilized crude extract by binding with Ni2+-NTA-agarose resin and eluting with 250 mM imidazole. The elution contained the MraY enzyme, which showed a single band in lane 1 at the calculated size of 36 kDa in SDS-PAGE (Figure 2b).
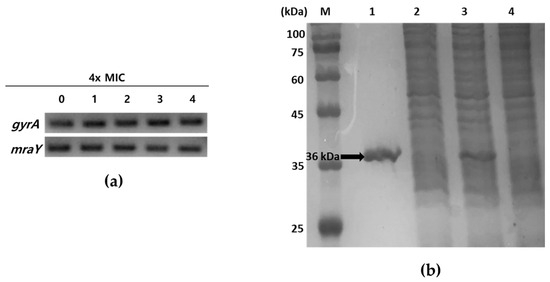
Figure 2.
Analysis of mraY expression in the presence of compounds 1–4 and SDS-PAGE of recombinant MraY. (a) Semi-quantitative RT-PCR of mraY in S. aureus ATCC6538p. A housekeeping gene gyrA was used as a loading control. 0: no compounds treated; 1–4: treatment with four times MIC of compounds 1–4, respectively. (b) SDS-PAGE analysis of purification of MraY. The samples were loaded on a 15% polyacrylamide gel. M: protein molecular weight standard; lane1: Ni2+-NTA bound proteins eluted with 250 mM imidazole; lane2: host with pET28a induced with 1 mM IPTG; lane3: host with pET28a-mraY induced with 1 mM IPTG; lane4: uninduced host with pET28a-mraY. The arrow indicates purified His6-tagged recombinant MraY (ca. 36 kDa).
2.5. In Vitro MraY Enzyme Inhibition Assay
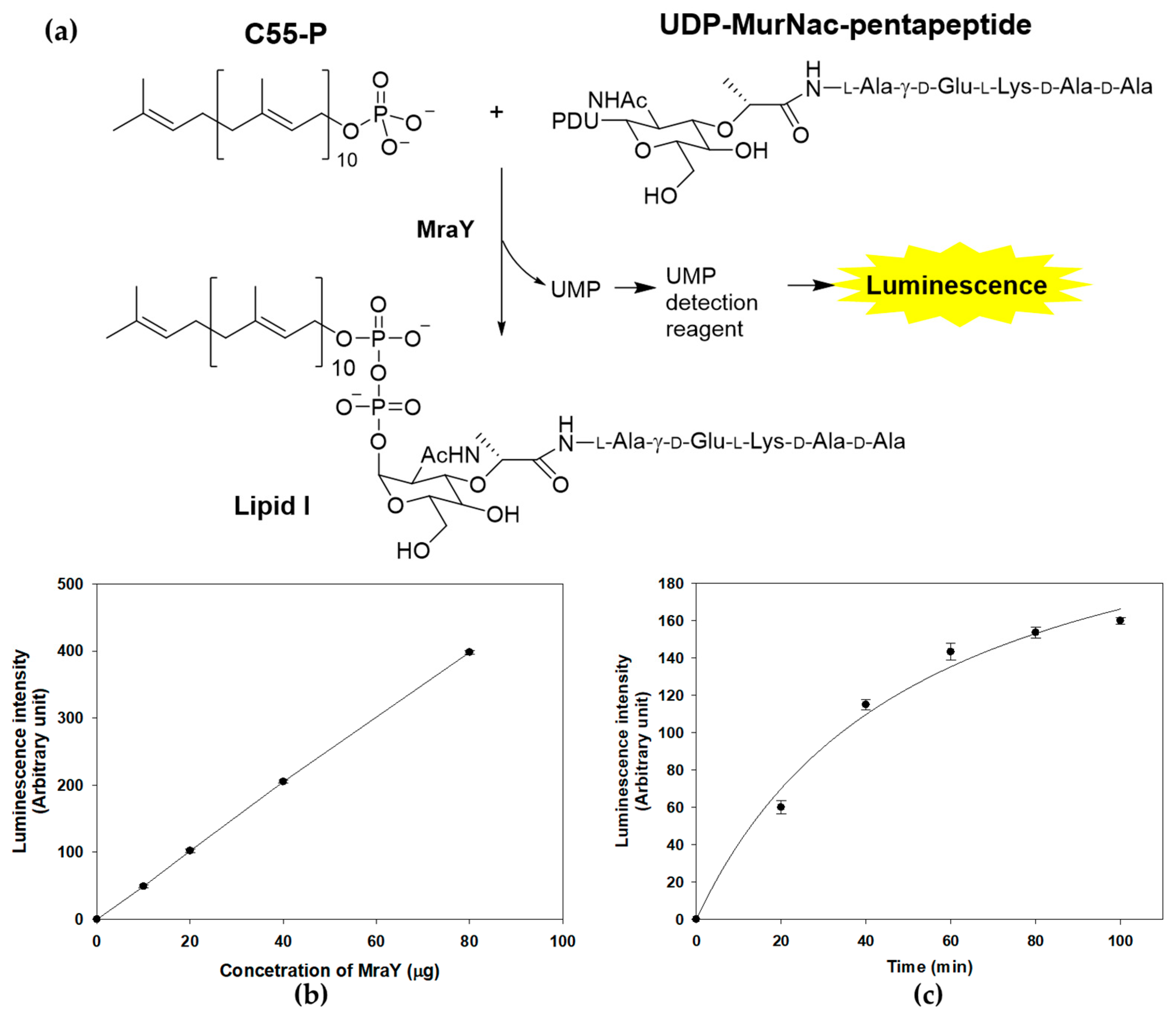
The enzymatic activity of MraY can be accurately and quantitatively assessed through a sensitive discontinuous coupled assay system, where the luminescence output serves as a reliable indicator of enzymatic activity. MraY transfers UDP-MurNAc-pentapeptide to C55-P, resulting in the formation of lipid I and the release of UMP, the byproduct of MraY reactions [15]. The enzyme activity of purified recombinant MraY was measured by detecting the generated UMP with a UMP-GloTM glycosyltransferase assay (Figure 3a) [25]. In the presence of both substrates and purified MraY, an increase in luminescence was observed, indicating that a UMP-generating reaction was detected (Figure 3b). The activity was dependent on the amount of enzyme and the incubation time, suggesting that the assay system can be used to measure MraY activity in vitro (Figure 3b,c).
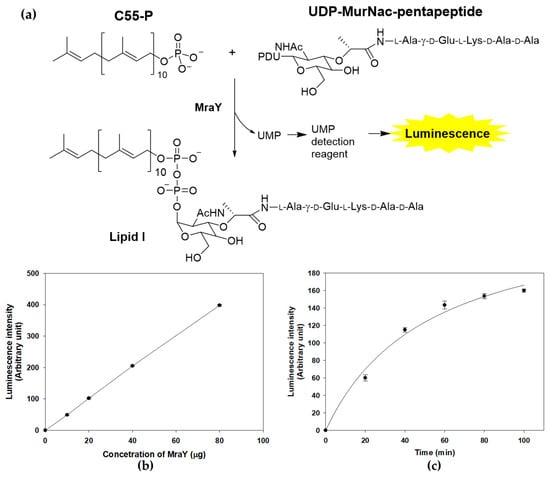
Figure 3.
MraY enzyme reaction and activity measurement using UMP detection. (a) MraY catalyzes the reaction of C55-P and UDP-MurNAc-pentapeptide to form Lipid I. The enzyme activity is measured by the luminescence of the luciferase reaction from the UMP detection reagent. (b) Concentration-dependent activity of the MraY enzyme. The enzyme reaction was carried out under standard assay conditions at 37 °C for 1 h. (c) Time course of the MraY enzyme reaction. Reactions were carried at 37 °C in 100 μL, containing 100 mM Tris-HCl, 500 mM NaCl, 10 mM MgCl2, 20 mM CHAPS, 150 μM UDP-MurNAc-pentapeptide, 250 μM C55-P, and 30 μg of purified MraY.
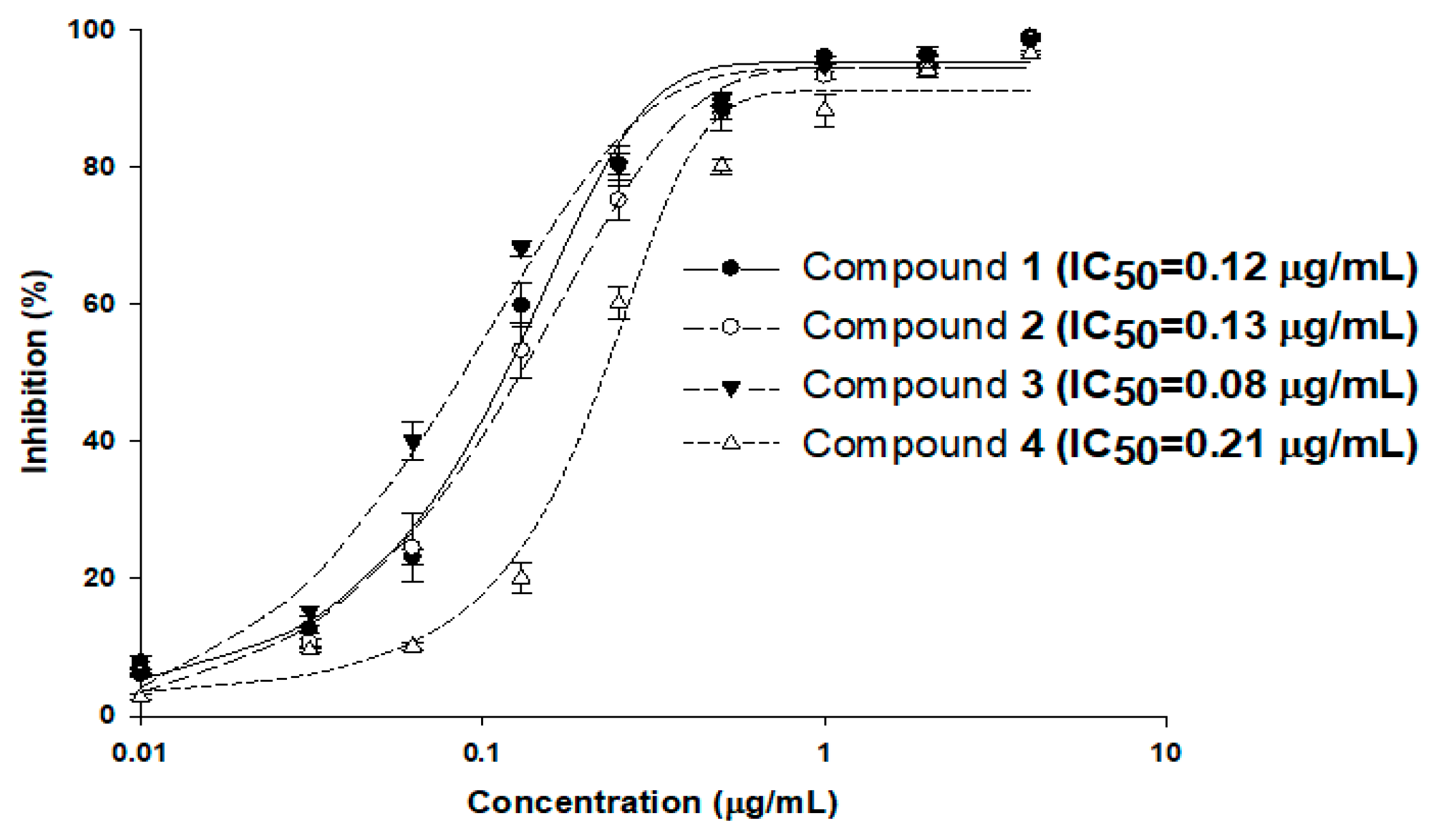
To evaluate the inhibitory activity of compounds 1–4 against MraY using the established luminescence assay system, MraY enzyme reactions were carried out with the addition of compounds in various concentrations in the range of 0.03 to 4 μg/mL, and the half-maximal inhibitory concentration (IC50) values were calculated (Figure 4). The reactions were carried out three times, and the calculated IC50 values of compounds 1–4 were 0.12, 0.13, 0.08, and 0.21 μg/mL, respectively. As the IC50 values of these compounds were in a similar range to that of the MIC values against S. aureus 6538p (0.13, 0.13, 0.06, and 0.25 μg/mL, respectively), MraY may be the primary target of the tunicamycins in S. aureus. The tendencies of the IC50 of MraY and antimicrobial activity were distinct among the MraY inhibitors. For instance, liposidomycins showed MraY inhibition with an IC50 at 0.03 μM and demonstrated antibacterial activity against Gram-positive bacteria. The MIC for Mycobacterium phlei was 1.6 μg/mL and did not show toxicity in mice [13,26]. Capuramycin strongly inhibited MraY (MurX) with an IC50 of 0.010 µg/mL and displayed selective antibacterial activity against mycobacteria. The MIC values were about 6.25 to 12.5 µg/mL for M. smegmatis [11]. Muraymycin A1 exhibited antibacterial activity against Gram-positive bacteria with MIC values of 2–16 µg/mL against Staphylococcus spp. It also showed therapeutic efficacy in S. aureus-infected mice at an effective dose of 1.1 mg/kg [24]. Further investigation is necessary to uncover the pharmacological properties observed among nucleoside inhibitors targeting MraY, as this aspect remains poorly understood.
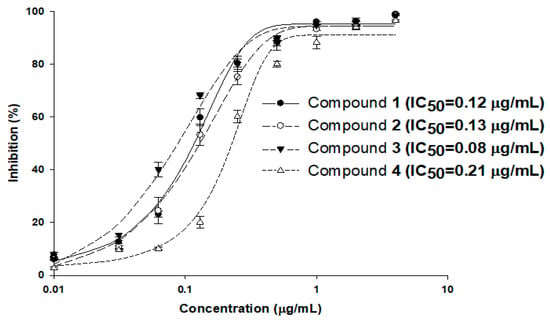
Figure 4.
MraY enzyme inhibition by compounds 1–4. The enzyme reactions were carried out three times under standard assay conditions, and the calculated IC50 values of compounds 1–4 were 0.12, 0.13, 0.08, and 0.21 μg/mL, respectively.
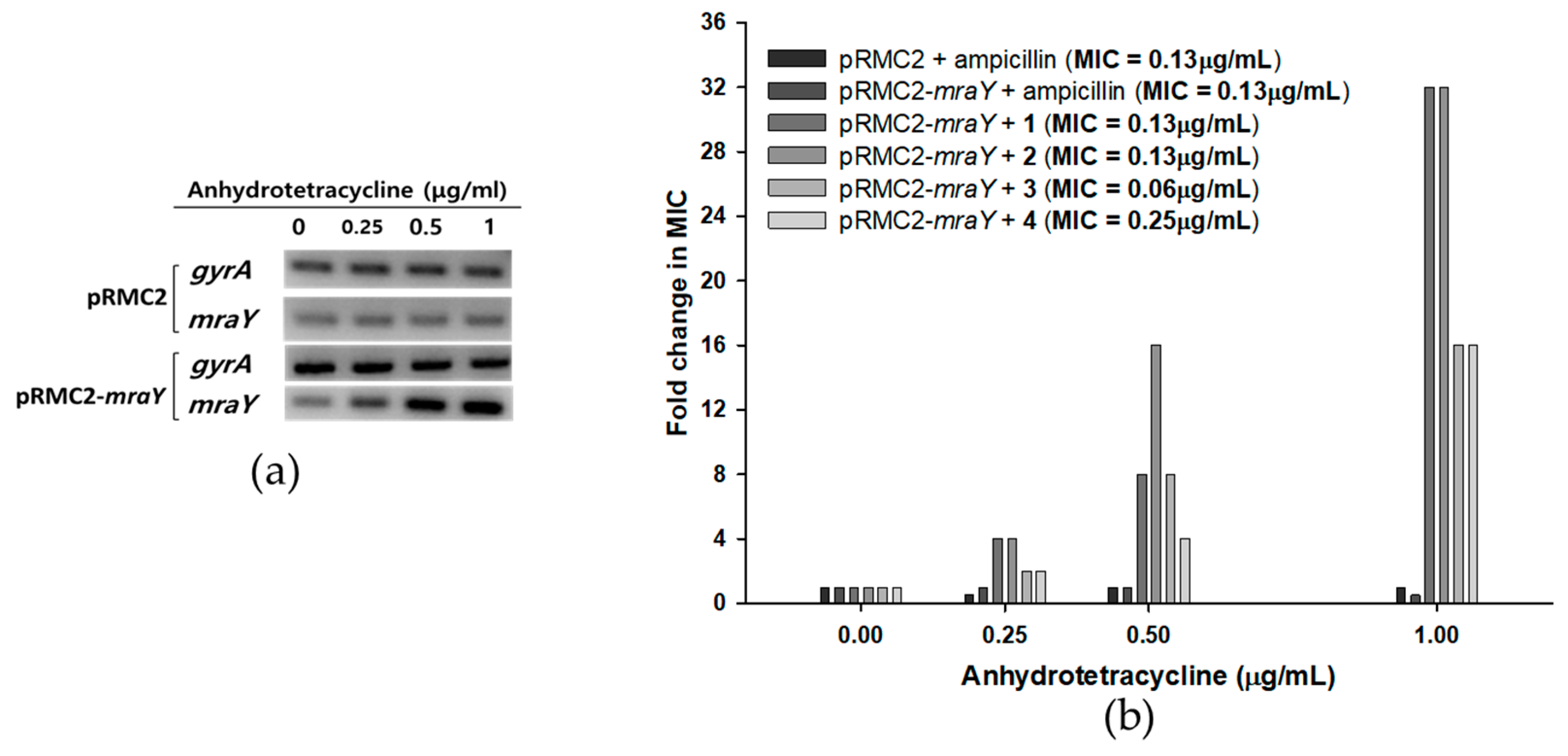
2.6. MraY Overexpression In Vivo
Since MraY is crucial for the survival of bacteria but absent in eukaryotes, it presents an excellent target for the development of novel antibacterial agents [13,17,27]. As MraY is an enzyme that is essential for the growth of S. aureus, deletion mutants could not survive without complementary copies of mraY [28]. In this case, overexpression studies have attempted to monitor the change in susceptibility to overexpressed conditions to validate the targets of inhibitors [29,30]. An overexpression study of mraY in S. aureus was recently conducted, showing that a synthetic compound, 8-octyl berberine, inhibits S. aureus peptidoglycan synthesis and that the overexpression of mraY resulted in a 16-fold increase in the MIC [31]. To further investigate the activity of tunicamycins with respect to MraY in vivo, an overexpression analysis of mraY was conducted by cloning the gene into a tetracycline-inducible plasmid pRMC2 to obtain a recombinant plasmid pRMC2-mraY [32]. The recombinant plasmid pRMC2-mraY was transformed into S. aureus 6538p, resulting in a tetracycline-inducible mraY overexpression system. Anhydrotetracycline, a less potent analog of tetracycline, was used for induction, and the overexpression of mraY was confirmed with semi-quantitative RT-PCR (Figure 5a). The MIC of anhydrotetracycline in S. aureus 6538p was 8 μg/mL, whereas the MIC of tetracycline was 0.13 μg/mL. S. aureus bearing the pRMC2-mraY plasmid was induced with anhydrotetracycline in the concentrations of 0.25, 0.5, and 1 μg/mL. The overexpressed S. aureus cells were collected to measure the MIC of compounds 1–4. In mraY-overexpressed conditions, the MIC of compounds 1–4 showed concentration dependency toward anhydrotetracycline (Figure 5b). At 0.25 μg/mL anhydrotetracycline, the MIC of compounds 1 and 2 increased four-fold, and the MIC of compounds 3 and 4 increased two-fold. As the concentrations of anhydrotetracycline were increased to 0.5 and 1 μg/mL, the MIC of compounds 1–4 further increased 32-fold in the cases of compounds 1 and 2 and 16-fold in the cases of compounds 3 and 4. The overexpression of mraY in S. aureus resulted in far less potency of compounds 1–4, with up to a 32-fold increase in the MIC with respect to that in uninduced conditions. The MIC of ampicillin did not increase in any overexpressed conditions in either pRMC2 or pRMC2-mraY, meaning that the MIC change was specifically observed in compounds 1–4. The MIC fold changes in MraY overexpression conditions show that S. aureus can overcome tunicamycin susceptibility when the MraY expression level is artificially increased; this result was similar to those of previously reported MraY overexpression tests of 8-octyl berberine [31], indicating that the antimicrobial activity of compounds 1–4 was related to inhibition of MraY in vivo. While extensive research is necessary to fully understand the effects of MraY overexpression in S. aureus, the overexpression system outlined in this study holds potential for evaluating the in vivo activity of MraY inhibitors.
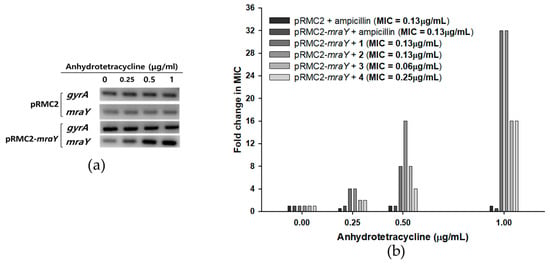
Figure 5.
Effects of mraY overexpression in S. aureus 6538p cells on the MICs of compounds 1–4. (a) Semi-quantitative RT-PCR of mraY in S. aureus bearing pRMC2 or pRMC2-mraY plasmids induced with anhydrotetracycline. Anhydrotetracycline was treated for 1 h, and overexpression of mraY was observed in S. aureus with pRMC2-mraY, while mraY in S. aureus with pRMC2 was not overexpressed. A housekeeping gene, gyrA, was used as a loading control. (b) S. aureus MIC fold change with mraY overexpression. Anhydrotetracycline was added in concentrations of 0.25, 0.5, and 1 μg/mL, and the MICs of compounds 1–4 were tested.
3. Materials and Methods
3.1. General Experimental Procedures
1H and 13C NMR spectra were recorded using a Bruker Avance 600 MHz instrument (Billerica, MA, USA) in both MeOH-d4 and DMSO-d6 solutions. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) data were acquired at the National Instrumentation Center for Environmental Management (Seoul, Republic of Korea) using an AB Sciex 5600 QTOF HR-MS instrument (Sciex, MA, USA). HPLC separations were conducted on a SpectraSYSTEM p2000 equipped with a UV-Vis detector (Gilson, Middleton, WI, USA). All solvents used were either of spectroscopic grade or were distilled prior to use. Substrates and antibiotics were from Sigma-Aldrich (St Louis, MO, USA) if not otherwise mentioned.
3.2. Taxonomic Identification of the Tunicamycin-Producing Microorganism
The bacterial strain MBTG32 was isolated from a marine sediment sample obtained from a seashore on Jeju Island, Republic of Korea. The strain identification was confirmed by sequencing the internal transcribed spacer region after PCR amplification of extracted DNA. Genomic DNA was extracted from MBTG32 mycelium using the i-Genomic BYF DNA Extraction Mini Kit (Intron Biotechnology, Seoul, Republic of Korea) following the manufacturer’s protocols. PCR amplification of the 16S rDNA sequence was accomplished under optimized conditions using primers 27F and 1429R (Table S1). The obtained nucleotide sequence was deposited in GenBank under the accession number PP669811.
3.3. Cultivation, Extraction, and Isolation
The MBTG32 strain was streaked to sporulate on GTYB agar plates composed of 10 g of glucose, 2 g of tryptone, 1 g of yeast extract, 1 g of beef extract, and 20 g of agar in 1 L of artificial seawater and incubated for 5 days at 28 °C. Mature spores were then transferred to 25 mL of GTYB broth and incubated for 24 h at 28 °C on a rotary shaker. Subsequently, each seed culture was scaled up to 500 mL of GTYB broth and incubated for 7 days at 28 °C on a rotary shaking incubator at 230 rpm. The resulting culture filtrate (40 L) was partitioned using equal volumes of n-hexane (0.6 g) and ethyl acetate (3.17 g).
The ethyl acetate fraction was separated by C18 reversed-phase vacuum flash chromatography using a sequential mixture of methanol and water as eluents (seven fractions in the gradient, water–methanol, from 100:0 to 0:100). The fraction eluted with 100% methanol which exhibited inhibitory activity against S. aureus (MIC = 0.26 μg/mL) was further separated with semi-preparative reversed-phase HPLC (Agilent C18 column, 10 × 250 mm; flow rate: 2.0 mL/min; water–acetonitrile, 50:50), yielding compounds 1 (tR = 13.2 min), 2 (tR = 14.1 min), 3 (tR = 18.9 min), and 4 (tR = 22.0 min). Additional purification of compounds 1 and 2 was conducted using analytical HPLC (Agilent C18 column, 4.6 × 250 mm; flow rate: 0.7 mL/min; water–methanol, 20:80), while compounds 3 and 4 were purified using analytical HPLC (Agilent C18 column, 4.6 × 250 mm; flow rate: 0.7 mL/min; gradient water–acetonitrile, 58:42 to 53:47 for 70 min). The isolated metabolites were obtained in quantities of 8.3, 4.5, 6.4, and 3.2 mg for compounds 1–4, respectively.
3.4. Antibacterial Activity Assays
Antibacterial activity assays were conducted by following the guidelines outlined by the Clinical and Laboratory Standards Institute [33]. The bacterial strains used in these assays were from the Culture Collection of Antimicrobial Resistant Microorganisms (CCARM) at Seoul Women’s University (Seoul, Republic of Korea) and the American Type Culture Collection (ATCC) (see Table 1 and Table 2). The cells were cultured in Mueller–Hinton broth (MHB) at 37 °C for 16 h, collected via centrifugation, and washed with sterile distilled water. The test compounds were dissolved in DMSO and diluted two-fold with MHB from 0.016 to 128 μg/mL. DMSO was added to each well to give a final 1% DMSO concentration. Then, 90 μL of MHB containing the test compound was mixed with 10 μL of broth of the test bacterium containing 5 × 106 colony-forming units (cfu)/mL in each well of a 96-well plate (final concentration, 5 × 105 cfu/mL). The plates were then incubated for 16 h at 37 °C. The MIC was determined to be the lowest concentration of the test compound, and it completely inhibited cell growth. Ampicillin and tetracycline were used as positive controls.
3.5. Gene Expression Analysis
For the analysis of mraY expression, overnight-cultured S. aureus 6538p was diluted with MHB medium and allowed to reach the mid-log phase through further incubation. Fourfold concentrations of the MIC of compounds 1–4 were introduced and incubated for 40 min at 37 °C. Lysostaphin was employed to enzymatically degrade the cell wall of S. aureus, followed by total RNA extraction using the easy-BLUE™ reagent (Intron Biotechnology, Seoul, Republic of Korea). Subsequently, cDNA was synthesized using the SuperScript III cDNA synthesis kit (Enzynomics, Seoul, Republic of Korea), and semi-quantitative RT–PCR was conducted with mraY-specific primers (mraY For and mraY Rev) (Table S1) under standard conditions. The DNA gyrase subunit A (gyrA) gene was utilized as a loading control.
3.6. MraY Molecular Cloning
The DNA sequence of the S. aureus 6538p mraY gene was amplified via PCR using specific primers (mraY For-NcoI and mraY Rev-XhoI for pET28a plasmid inserts; mraY For-SacI and mraY Rev-EcoRI for pRMC2 plasmid inserts) (Table S1), incorporating appropriate restriction enzyme sites for recombinant plasmid construction. The resulting recombinant plasmids containing S. aureus ATCC6538p mraY, namely, pET28a-mraY and pRMC2-mraY, were sequenced to confirm the inserts using the Sanger sequencing method. Plasmid pET28a-mraY was transformed into E. coli strain BL21(DE3), while plasmid pRMC2-mraY was transformed into S. aureus ATCC6538p using the S. aureus RN4220 strain as an intermediate to methylate DNA prior to electroporation [34].
3.7. MraY Enzyme Purification and Activity Assay
Recombinant MraY was prepared and purified by following previously described protocols [35]. Initially, E. coli strain BL21(DE3) containing the recombinant plasmid pET28a-mraY was cultured in LB media at 37 °C until reaching an absorbance of approximately 0.7 at 600 nm. Subsequently, 1 mM of IPTG (isopropyl β-D-1-thiogalactopyranoside) was added to induce protein expression and incubated for 16 h at 25 °C with agitation. Cells were harvested via centrifugation, and the membrane fraction was solubilized using a sodium phosphate–glycerol detergent buffer [35]. The solubilized crude extract was subjected to affinity chromatography using Ni2+-NTA-agarose resin, eluted with 250 mM imidazole to obtain purified MraY, and analyzed using SDS-PAGE.
Enzymatic activity assays for MraY were conducted according to previously described methods with slight modifications [36]. The UMP-GloTM glycosyltransferase assay (Promega Corporation, Madison, WI, USA) was performed according to the manufacturer’s instructions [25]. Then, 150 μM UDP-MurNAc-pentapeptide (Evitachem, Beijing, China) and 250 μM C55-P (Larodan AB, Solna, Sweden) were added to the Tris-HCl buffer system [36] and incubated with 300 μg/mL of purified MraY for 60 min at 37 °C. The luminescence resulting from the reaction was measured using a SpectraMax M3 multi-mode microplate reader (Molecular Devices, San Jose, CA, USA).
3.8. MraY Overexpression In Vivo
To induce the overexpression of tetracycline-inducible plasmids, a previously established method was employed [32]. S. aureus 6538p containing the recombinant plasmid pRMC2-mraY was cultured at 37 °C in MHB until reaching an absorbance of approximately 0.7 at 600 nm. Anhydrotetracycline was added to the culture at concentrations of 0.25, 0.5, and 1 μg/mL and incubated for 1 h. Following incubation, cells were harvested via centrifugation and diluted with MHB medium to achieve a concentration of approximately 5 × 106 cfu/mL. Subsequently, the antibacterial activity of compounds 1–4 was evaluated as described above.
4. Conclusions
A marine-derived actinomycete (Streptomyces sp. MBTG32) exhibiting antibacterial activity against Staphylococcus aureus was investigated. The strain was identified using the 16S rDNA sequencing technique, and the isolated strain was closely related to Streptomyces bacillaris. Four tunicamycins, tunicamycin VII (1), tunicamycin VIII (2), corynetoxin U17a (3), and tunicamycin IX (4), were isolated from the ethyl acetate extract of the culture broth. The structures of the isolated compounds were elucidated based on comparisons of spectroscopic data with previously reported NMR data. Compounds 1–4 showed potent antibacterial activities against Gram-positive bacteria—especially S. aureus—with MIC values of 0.13–0.25 µg/mL. The inhibitory activities of these compounds against recombinant MraY, an essential enzyme that catalyzes the membrane-associated initial step of peptidoglycan synthesis, were assayed by using a discontinuous coupled assay system. The IC50 values of compounds 1–4 against MraY were 0.12, 0.13, 0.08, and 0.21 μg/mL, respectively. As MraY is an enzyme essential for the growth of S. aureus, the construction of deletion mutants of this gene could not be achieved. To overcome this limitation, the effects of mraY overexpression in S. aureus cells on the MICs of compounds 1–4 were evaluated. Overexpression studies showed that the MICs of compounds 1–4 increased up to 32-fold, indicating that the antibacterial activity of these compounds is associated with the inhibition of MraY. Therefore, we confirmed that the marine-derived tunicamycins reported here are strong MraY inhibitors, effectively inhibiting the growth of the selected MRSA strains. As MraY inhibitors are a promising novel target for drug-resistant Gram-positive bacteria, the overexpression system used in this study could be an additional tool for evaluating in vivo activity of MraY inhibitors.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/md22070293/s1, Figure S1: 1H NMR (600 MHz, MeOH-d4) spectrum of 1; Figure S2: 13C NMR (150 MHz, MeOH-d4) spectrum of 1; Figure S3: 13C NMR (150 MHz, DMSO-d6) spectrum of 1; Figure S4: 1H NMR (600 MHz, DMSO-d6) spectrum of 2; Figure S5: 13C NMR (600 MHz, DMSO-d6) spectrum of 2; Figure S6: 1H NMR (600 MHz, DMSO-d6) spectrum of 3; Figure S7: 13C NMR (600 MHz, DMSO-d6) spectrum of 3; Figure S8: 1H NMR (600 MHz, DMSO-d6) spectrum of 4; Figure S9: 13C NMR (600 MHz, DMSO-d6) spectrum of 4; Figure S10: HR-ESI-MS data of 1; Figure S11: HR-ESI-MS data of 2; Figure S12: HR-ESI-MS data of 3; Figure S13: HR-ESI-MS data of 4; Figure S14: HR-ESI-MS fragmentation analysis of 1; Figure S15: HR-ESI-MS fragmentation analysis of 2; Figure S16: HR-ESI-MS fragmentation analysis of 3; Figure S17: HR-ESI-MS fragmentation analysis of 4; Table S1: List of oligonucleotides used in this study; Table S2: 13C NMR comparison of 1 with tunicamycin V in MeOH-d4; Table S3: 13C NMR comparison of 3 with corynetoxin U17a in DMSO-d6.
Author Contributions
Conceptualization, K.-B.O. and J.S.; methodology, J.L., J.-Y.H. and D.O.; validation, J.L., J.-Y.H. and D.O.; formal analysis, J.L., J.-Y.H. and D.O.; investigation, J.L., J.-Y.H. and K.-B.O.; resources, J.L. and K.-B.O.; data curation, J.L., J.-Y.H., D.O., D.-C.O., H.-g.P., K.-B.O. and J.S.; writing—original draft preparation, J.L. and K.-B.O.; writing—review and editing, D.-C.O., H.-g.P., K.-B.O. and J.S.; supervision, K.-B.O. and J.S.; project administration, K.-B.O. and J.S.; funding acquisition, K.-B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF-2021R1F1A1048154) of Korea and funded by the Ministry of Education, Science, and Technology.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are contained within this article and the Supplementary Materials.
Acknowledgments
We are grateful to the National Center for Inter-University Research Facilities, Seoul National University, for providing the mass and NMR data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, R.; Eggleston, K.; Rotimi, V.; Zeckhauser, R.J. Antibiotic resistance as a global threat: Evidence from China, Kuwait and the United States. Glob. Health 2006, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Hawley, L.A.; Fridkin, S.K.; Whitney, C.G. Drug-resistant Streptococcus pneumoniae and methicillin-resistant Staphylococcus aureus surveillance. Emerg. Infect. Dis. 2003, 9, 1358–1359. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Aalbersberg, W. Marine actinomycetes: An ongoing source of novel bioactive metabolites. Microbiol. Res. 2012, 167, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Mincer, T.J.; Williams, P.G.; Fenical, W. Marine actinomycete diversity and natural product discovery. Antonie Van Leeuwenhoek 2005, 87, 43–48. [Google Scholar] [CrossRef]
- Yang, C.; Qian, R.; Xu, Y.; Yi, J.; Gu, Y.; Liu, X.; Yu, H.; Jiao, B.; Lu, X.; Zhang, W. Marine actinomycetes-derived natural products. Curr. Top. Med. Chem. 2019, 19, 2868–2918. [Google Scholar] [CrossRef]
- Price, N.P.; Tsvetanova, B. Biosynthesis of the tunicamycins: A review. J. Antibiot. 2007, 60, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, A.; Arima, K.; Tamura, G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J. Antibiot. 1971, 24, 215–223. [Google Scholar] [CrossRef]
- Takatsuki, A.; Kawamura, K.; Okina, M.; Kodama, Y.; Ito, T.; Tamura, G. The Structure of Tunicamycin. Agric. Biol. Chem. 1977, 41, 2307–2309. [Google Scholar] [CrossRef]
- Eckardt, K. Tunicamycins, streptovirudins, and corynetoxins, a special subclass of nucleoside antibiotics. J. Nat. Prod. 1983, 46, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.K.; Boon, D.Y.; Crum, F.C. N-Acetylglucosamine-1-phosphate transferase from hen oviduct: Solubilization, characterization, and inhibition by tunicamycin. Biochemistry 1979, 18, 3946–3952. [Google Scholar] [CrossRef]
- Brandish, P.E.; Kimura, K.I.; Inukai, M.; Southgate, R.; Lonsdale, J.T.; Bugg, T.D. Modes of action of tunicamycin, liposidomycin B, and mureidomycin A: Inhibition of phospho-N-acetylmuramyl-pentapeptide translocase from Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Hering, J.; Dunevall, E.; Ek, M.; Branden, G. Structural basis for selective inhibition of antibacterial target MraY, a membrane-bound enzyme involved in peptidoglycan synthesis. Drug Discov. Today 2018, 23, 1426–1435. [Google Scholar] [CrossRef]
- Lovering, A.L.; Safadi, S.S.; Strynadka, N.C. Structural perspective of peptidoglycan biosynthesis and assembly. Annu. Rev. Biochem. 2012, 81, 451–478. [Google Scholar] [CrossRef]
- Hakulinen, J.K.; Hering, J.; Branden, G.; Chen, H.; Snijder, A.; Ek, M.; Johansson, P. MraY-antibiotic complex reveals details of tunicamycin mode of action. Nat. Chem. Biol. 2017, 13, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Mitachi, K.; Mingle, D.; Effah, W.; Sánchez-Ruiz, A.; Hevener, K.E.; Narayanan, R.; Clemons, W.M.; Sarabia, F.; Kurosu, M. Concise synthesis of tunicamycin V and discovery of a cytostatic DPAGT1 Inhibitor. Angew. Chem. 2022, 134, e202203225. [Google Scholar] [CrossRef]
- Price, N.P.; Hartman, T.M.; Li, J.; Velpula, K.K.; Naumann, T.A.; Guda, M.R.; Yu, B.; Bischoff, K.M. Modified tunicamycins with reduced eukaryotic toxicity that enhance the antibacterial activity of β-lactams. J. Antibiot. 2017, 70, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, B. A modular approach to the total synthesis of tunicamycins. Angew. Chem. Int. Ed. Engl. 2015, 54, 6618–6621. [Google Scholar] [CrossRef]
- Tsvetanova, B.C.; Price, N.P. Liquid chromatography-electrospray mass spectrometry of tunicamycin-type antibiotics. Anal. Biochem. 2001, 15, 147–156. [Google Scholar] [CrossRef]
- Mitachi, K.; Mingle, D.; Kurosu, M. An improved total synthesis of tunicamycin V. MethodsX 2023, 10, 102095. [Google Scholar] [CrossRef]
- Frahn, J.L.; Edgar, J.A.; Jones, A.J.; Cockrum, P.A.; Anderton, N.A.; Culvenor, C.C.J. Structure of the corynetoxins, metabolites of Corynebacterium rathayi responsible for toxicity of annual ryegrass (Lolium rigidum) pastures. Aust. J. Chem. 1984, 37, 165–182. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Arai, M.; Sakaida, Y.; Takamatsu, Y.; Miyakoshi, S.; Inukai, M. Studies on novel bacterial translocase I inhibitors, A-500359s. V. Enhanced production of capuramycin and A-500359 A in Streptomyces griseus SANK 60196. J. Antibiot. 2006, 59, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, D.; Koppermann, S.; Wirth, M.; Niro, G.; Leyerer, K.; Ducho, C. Muraymycin nucleoside-peptide antibiotics: Uridine-derived natural products as lead structures for the development of novel antibacterial agents. Beilstein J. Org. Chem. 2016, 12, 769–795. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Walvoort, M.T.; Lukose, V.; Imperiali, B. A rapid and efficient luminescence-based method for assaying phosphoglycosyltransferase enzymes. Sci. Rep. 2016, 6, 33412. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ikeda, Y.; Kagami, S.; Yoshihama, M.; Suzuki, K.; Osada, H.; Isono, K. Selective inhibition of the bacterial peptidoglycan biosynthesis by the new types of liposidomycins. J. Antibiot. 1998, 51, 1099–1104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, J.; Singh, A.K.; Santa Maria, J.P., Jr.; Kim, Y.; Brown, S.; Swoboda, J.G.; Mylonakis, E.; Wilkinson, B.J.; Walker, S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 2011, 6, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Kurokawa, K.; Lee, B.L.; Sekimizu, K. Shuttle vectors derived from pN315 for study of essential genes in Staphylococcus aureus. Biol. Pharm. Bull. 2010, 33, 198–203. [Google Scholar] [CrossRef]
- Prelich, G. Gene overexpression: Uses, mechanisms, and interpretation. Genetics 2012, 190, 841–854. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Young, C.L.; Stone, K.C.; Dean, F.B.; Janjic, N.; Critchley, I.A. Mode of action and biochemical characterization of REP8839, a novel inhibitor of methionyl-tRNA synthetase. Antimicrob. Agents Chemother. 2005, 49, 4253–4262. [Google Scholar] [CrossRef]
- Li, X.; Ma, Z.; Tang, Q.; Gui, Z.; Zhang, B.; Sun, G.; Li, J.; Li, J.; Li, M.; Li, X.; et al. 8-Octyl berberine combats Staphylococcus aureus by preventing peptidoglycan synthesis. Eur. J. Pharm. Sci. 2023, 191, 106602. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.M.; Foster, T.J. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid 2009, 61, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Wikler, M.A.; Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard, 8th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Monk, I.R.; Shah, I.M.; Xu, M.; Tan, M.W.; Foster, T.J. Transforming the untransformable: Application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 2012, 3, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Bouhss, A.; Crouvoisier, M.; Blanot, D.; Mengin-Lecreulx, D. Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J. Biol. Chem. 2004, 279, 29974–29980. [Google Scholar] [CrossRef]
- Mashalidis, E.H.; Kaeser, B.; Terasawa, Y.; Katsuyama, A.; Kwon, D.Y.; Lee, K.; Hong, J.; Ichikawa, S.; Lee, S.Y. Chemical logic of MraY inhibition by antibacterial nucleoside natural products. Nat. Commun. 2019, 10, 2917. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).