A Comparative Analysis of the Anti-Tumor Activity of Sixteen Polysaccharide Fractions from Three Large Brown Seaweed, Sargassum horneri, Scytosiphon lomentaria, and Undaria pinnatifida
Abstract
:1. Introduction
2. Results
2.1. Extraction, Purification, and Characterization of 16 Polysaccharide Fractions from Three Types of Brown Seaweed
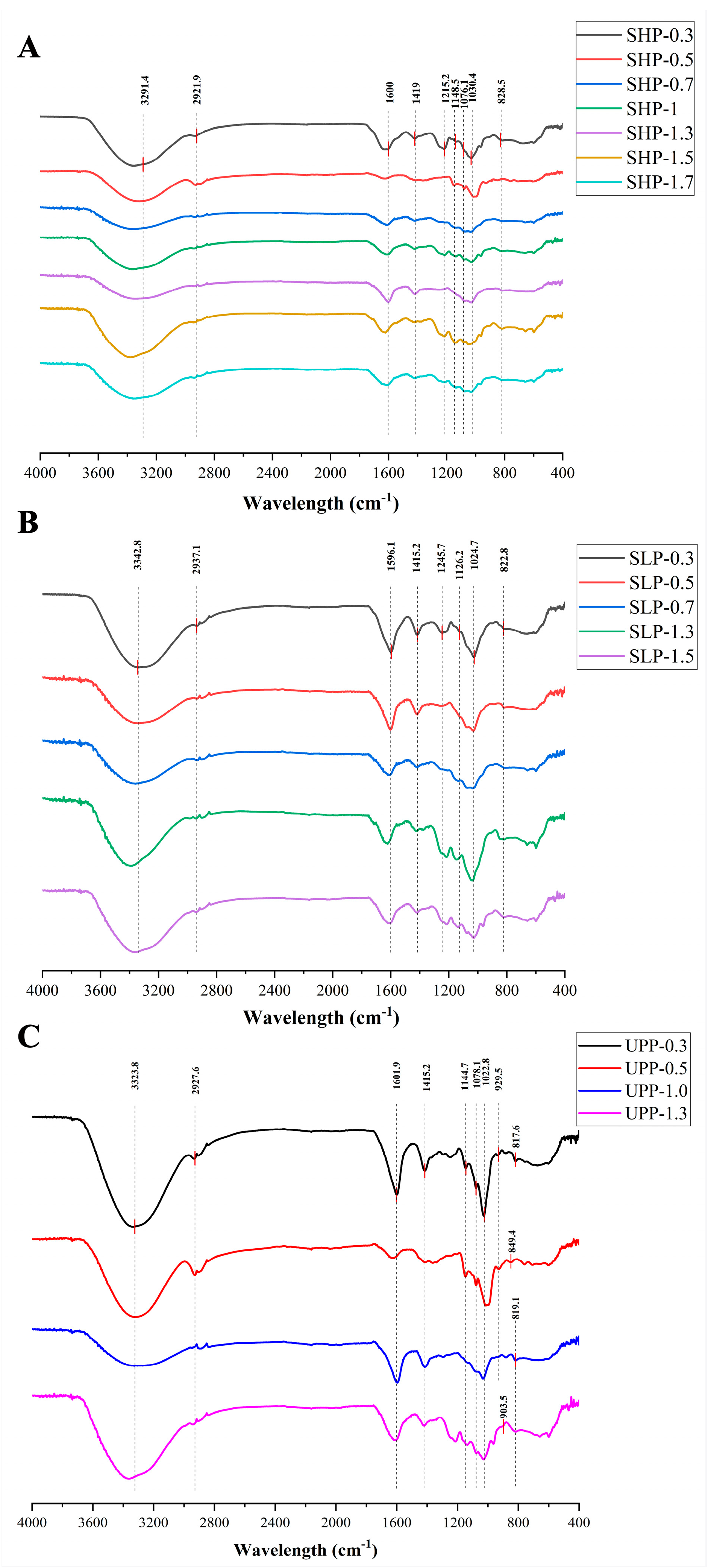
2.2. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis of 16 Polysaccharide Fractions
2.3. Triple-Helix Conformation
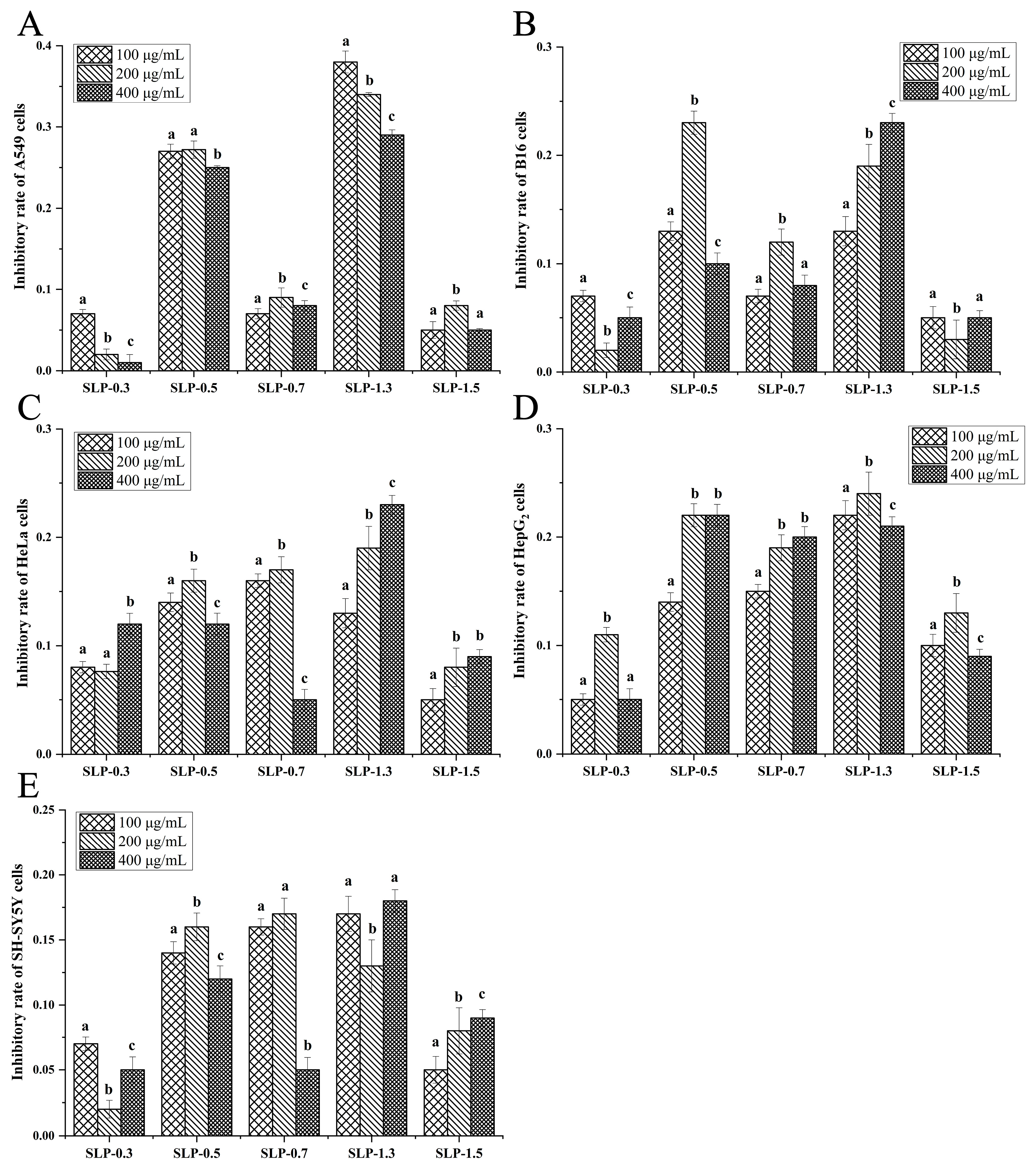
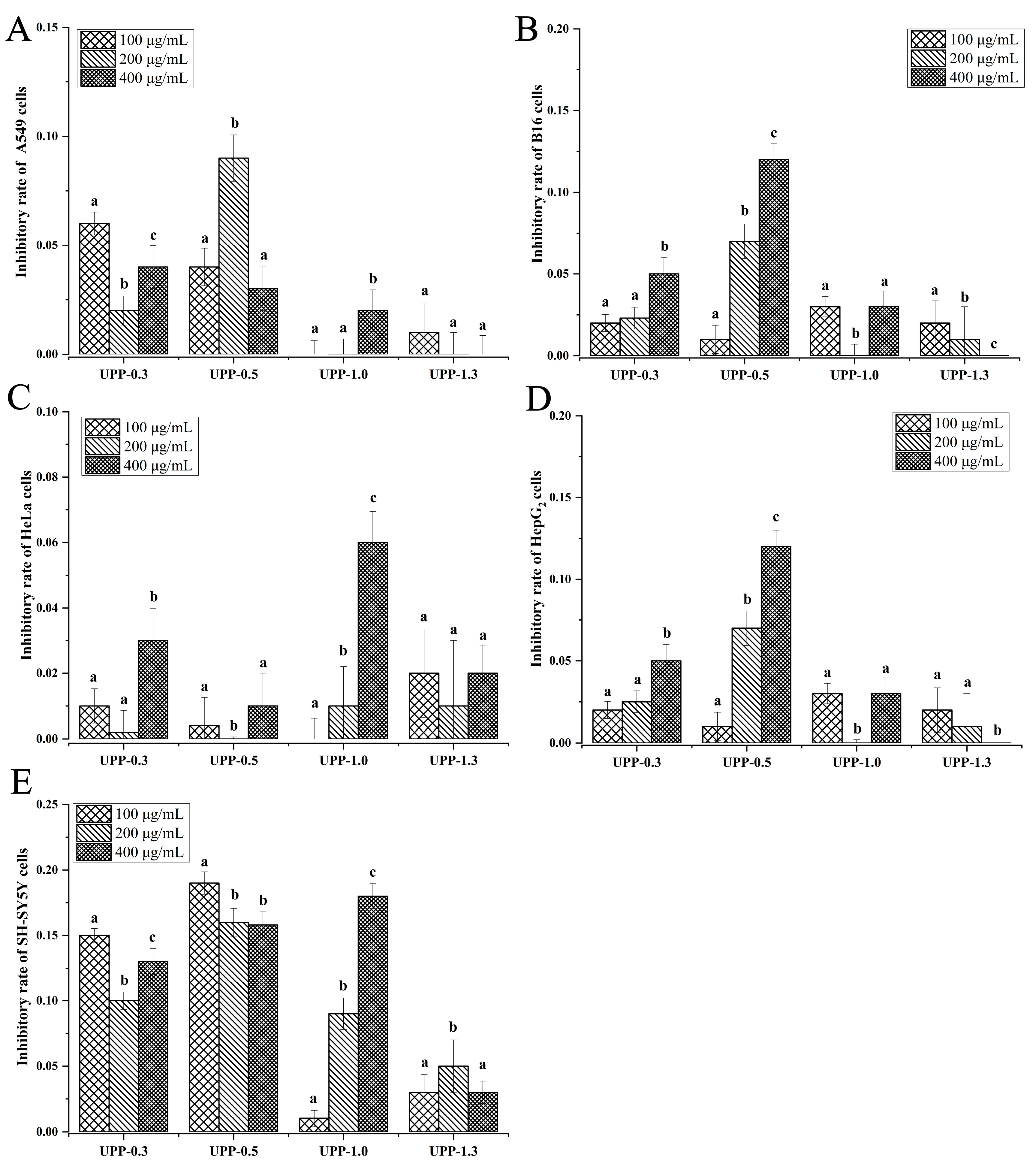
2.4. Comparison of Anti-Tumor Activities of 16 Polysaccharide Fractions
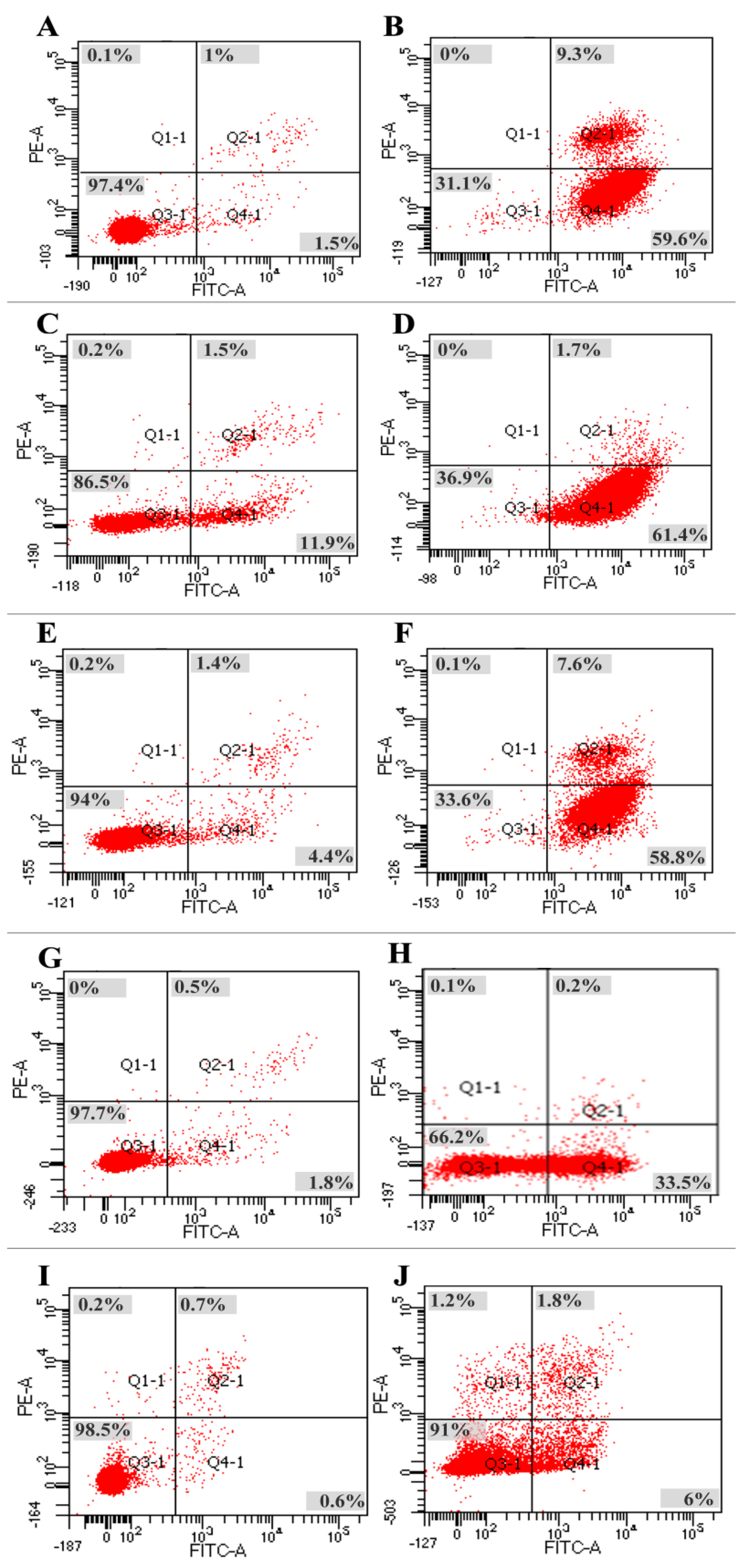
2.5. Apoptotic Effects of Polysaccharide Fractions on Tumor Cells
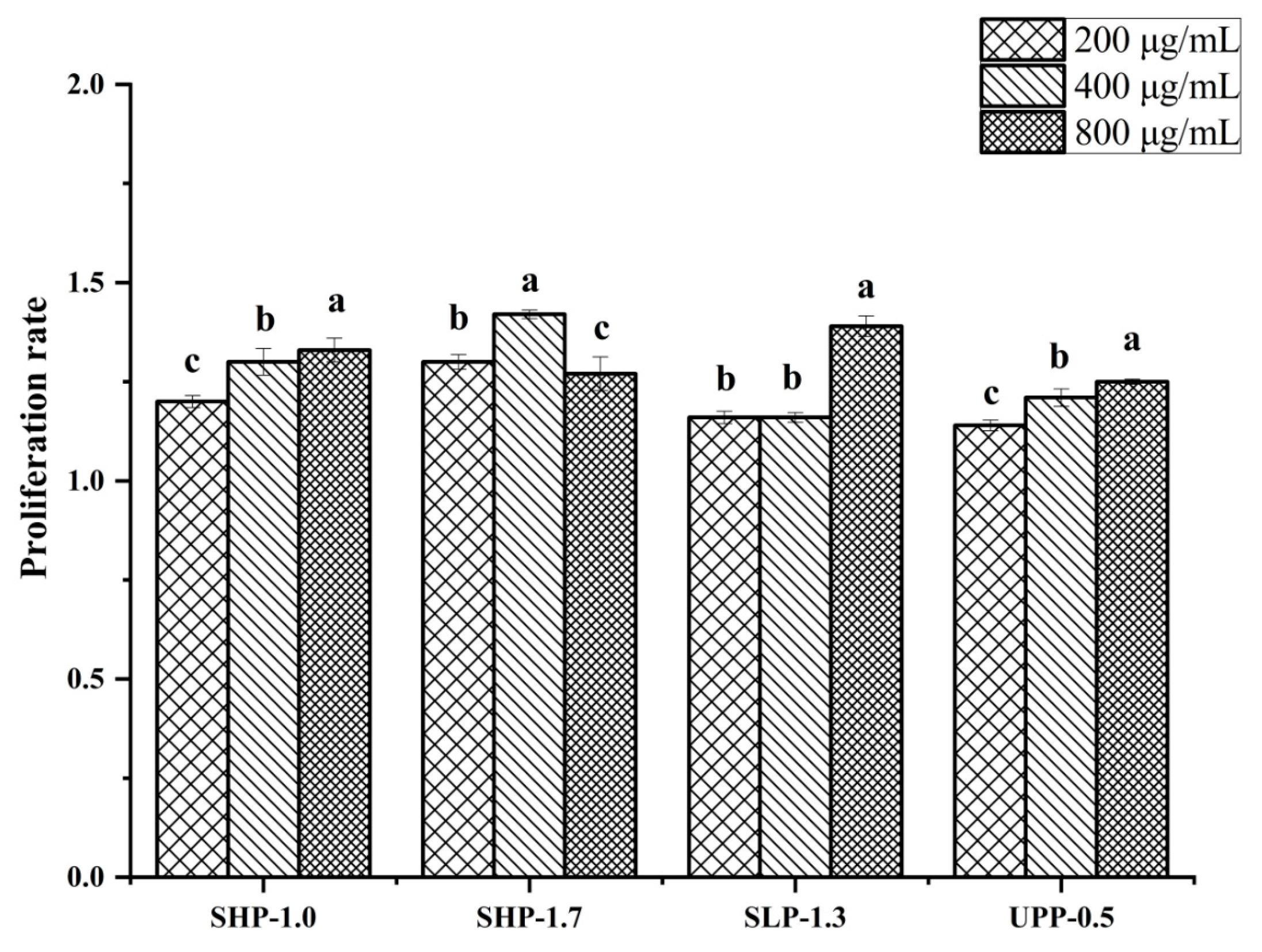
2.6. The Effect of Polysaccharide Fractions on Lymphocyte Proliferation
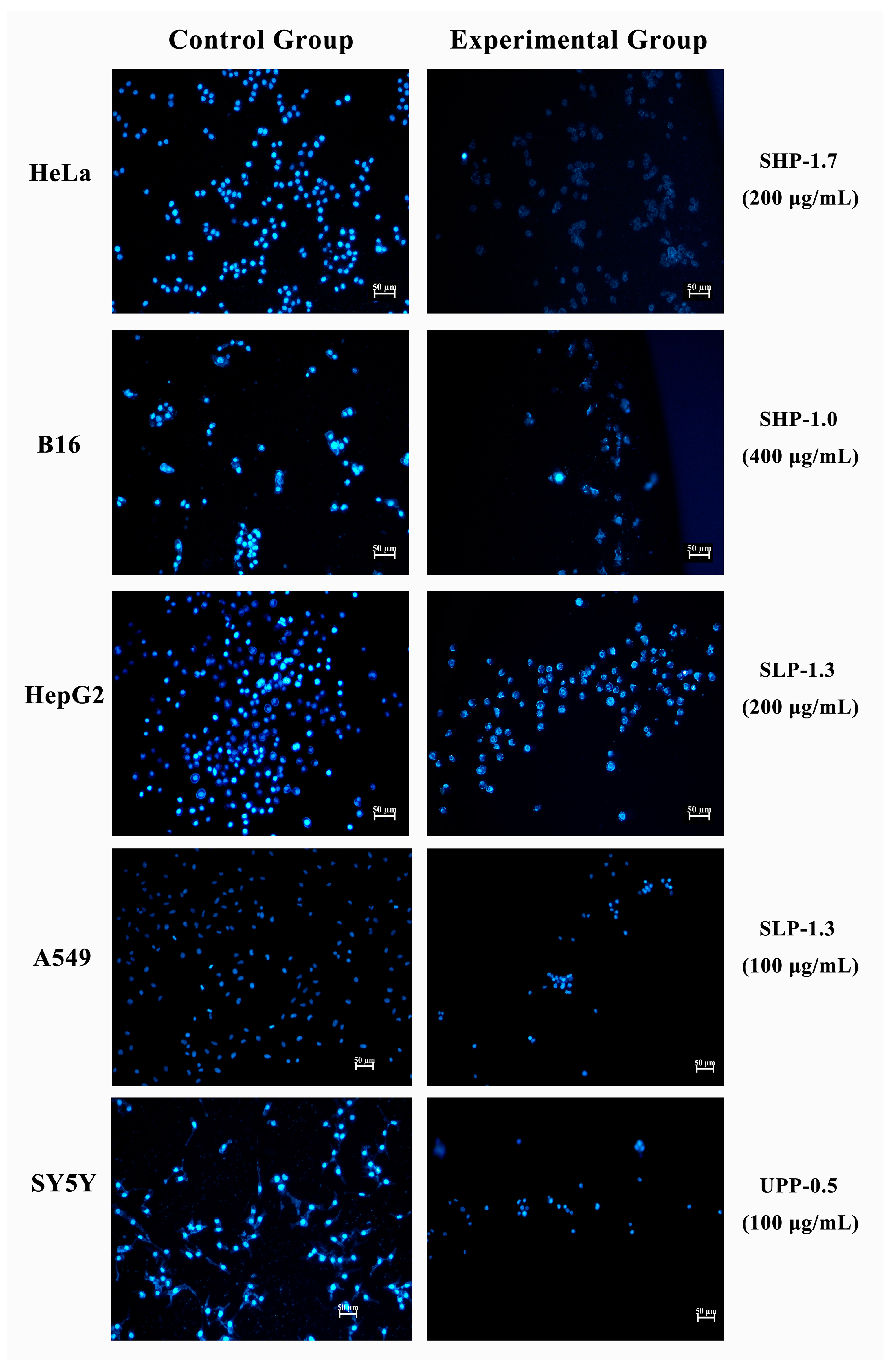
2.7. Morphological Analysis of Apoptosis by Hoechst 33342 Staining
2.8. Morphological Analysis of Apoptosis by Acridine Orange Staining
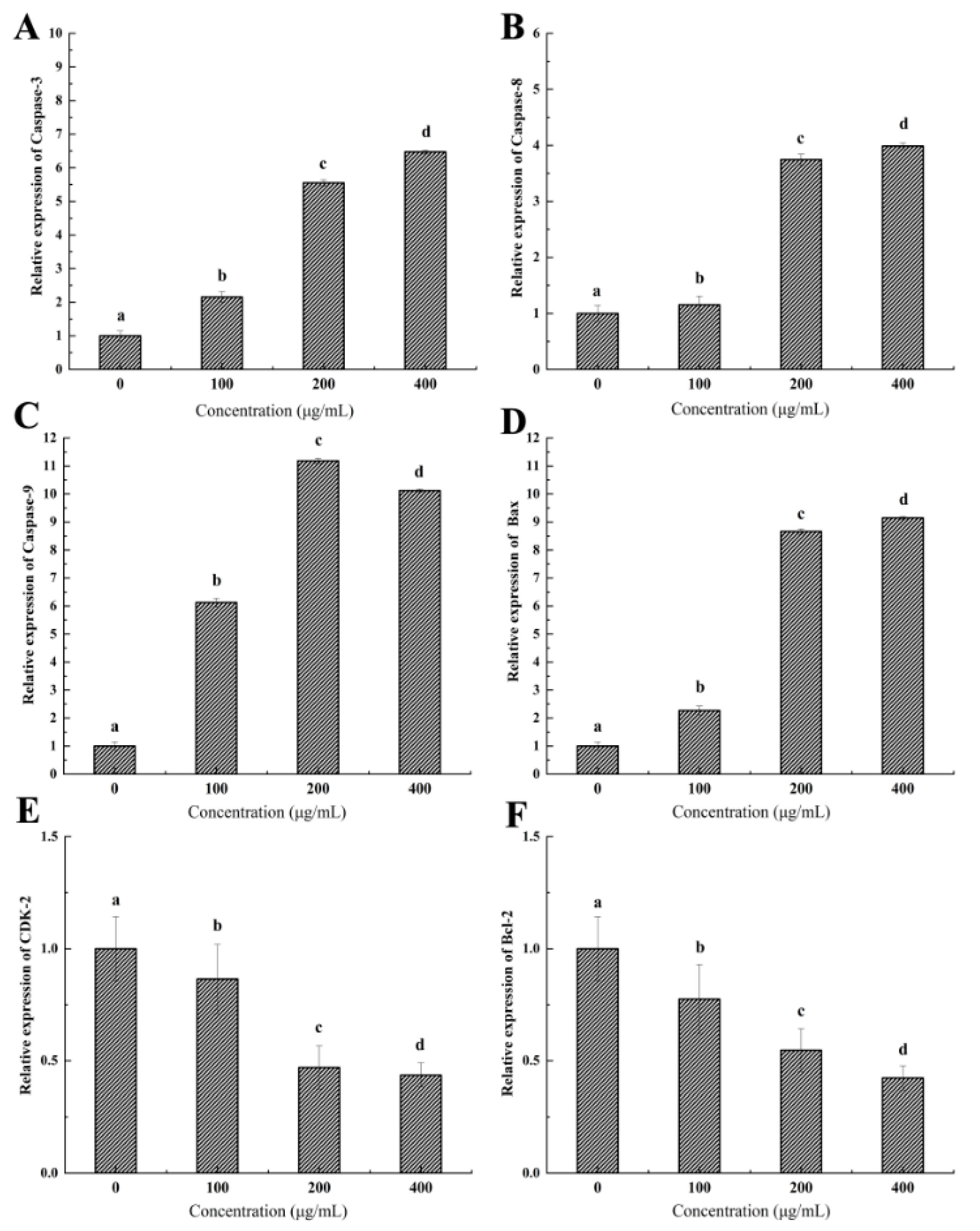
2.9. Expression of Genes Related to Tumor Apoptosis and Growth
2.10. Purification of SHP-1.0, SHP-1.7, SLP-1.3, and UPP-0.5
3. Discussion
3.1. Comparison of Different Anti-Tumor Activities of 16 Polysaccharide Fractions against Five Types of Tumor Cells
3.2. Relationship between Anti-Tumor Activity of Brown Seaweed Polysaccharides and Their Chemical Structures
3.3. Apoptosis Is the Primary Reason for the Anti-Tumor Effects of Brown Seaweed Polysaccharides
4. Materials and Methods
4.1. Seaweed Samples
4.2. Preparation of Crude Polysaccharides from Three Species of Brown Seaweed
4.3. Purification of Polysaccharides
4.4. Chemical Composition Analysis
4.5. FT-IR Characterization
4.6. Congo Red Analysis
4.7. Anti-Tumor Activity Assay
4.7.1. Cell Lines and Cell Culture
4.7.2. Cell Proliferation Assay
4.7.3. Flow Cytometry Analysis of Cell Apoptosis Rate
4.7.4. RAW264.7 Cell Proliferation/Cytotoxicity Assay
4.7.5. Hoechst 33342 Staining Assay
4.7.6. AO Staining Assay
4.7.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.8. Purification of the High Anti-Tumor Activity Fractions
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as Potential Anti-Tumor Biomacromolecules—A Review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.F.; Ye, H.; Zhu, Y.J.; Li, Y.P.; Wang, J.F.; Wang, P. Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative. Mar. Drugs 2019, 17, 21. [Google Scholar] [CrossRef]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural Characteristics and Anticancer Activity of Fucoidan from the Brown Alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef] [PubMed]
- Vanavil, B.; Selvaraj, K.; Aanandhalakshmi, R.; Usha, S.K.; Arumugam, M. Bioactive and thermostable sulphated polysaccharide from Sargassum swartzii with drug delivery applications. Int. J. Biol. Macromol. 2020, 153, 190–200. [Google Scholar] [CrossRef]
- Lu, J.; Shi, K.K.; Chen, S.; Wang, J.; Hassouna, A.; White, L.N.; Merien, F.; Xie, M.; Kong, Q.; Li, J.; et al. Fucoidan Extracted from the New Zealand Undaria Pinnatifida—Physicochemical Comparison against Five Other Fucoidans: Unique Low Molecular Weight Fraction Bioactivity in Breast Cancer Cell Lines. Mar. Drugs 2018, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Usoltseva, R.V.; Shevchenko, N.M.; Isakov, V.V.; Zvyagintseva, T.N.; Ermakova, S.P. In Vitro Anticancer Activity of the Laminarans from Far Eastern Brown Seaweeds and Their Sulfated Derivatives. J. Appl. Phycol. 2016, 29, 543–553. [Google Scholar] [CrossRef]
- Liu, J.; Luthuli, S.; Yang, Y.; Cheng, Y.; Zhang, Y.; Wu, M.; Choi, J.; Tong, H. Therapeutic and nutraceutical potentials of a brown seaweed Sargassum fusiforme. Food Sci. Nutr. 2020, 8, 5195–5205. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process. Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A Review on Fucoidan Antitumor Strategies: From a Biological Active Agent to a Structural Component of Fucoidan-Based Systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Pinheiro, T.; Nascimento Santos, M.D.S.; Will Castro, L.S.E.; Paiva, A.A.D.O.; Alves, L.G.; Cruz, A.K.M.; Nobre, L.T.D.; Alves, M.G.D.C.; Leite, E.L. A Fucan of a Brown Seaweed and Its Antitumoral Property on Ht-29 and Immunomodulatory Activity in Murine Raw 264.7 Macrophage Cell Line. J. Appl. Phycol. 2017, 29, 2061–2075. [Google Scholar] [CrossRef]
- Lee, H.; Selvaraj, B.; Lee, J.W. Anticancer Effects of Seaweed-Derived Bioactive Compounds. Appl. Sci. 2021, 11, 11261. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.I.; et al. Different Extraction Methods Bring About Distinct Physicochemical Properties and Antioxidant Activities of Sargassum Fusiforme Fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Song, M.G.; Kim, Y.N.; Park, J.I.; Kwak, J.Y. The Mechanism of Fucoidan-Induced Apoptosis in Leukemic Cells: Involvement of Erk1/2, Jnk, Glutathione, and Nitric Oxide. Mol. Carcinog. 2010, 49, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y. Fucoidan as a Marine Anticancer Agent in Preclinical Development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from Brown Seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural Characteristics and Anticancer Activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; You, L.; Fu, X.; Liu, R.H. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr. Polym. 2017, 155, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Niu, Y.; Ju, H.; Chen, R.; Li, B.; Song, X.; Song, L. Chemical Characterization, Antitumor, and Immune-Enhancing Activities of Polysaccharide from Sargassum pallidum. Molecules 2021, 26, 7559. [Google Scholar] [CrossRef]
- Rasin, A.B.; Silchenko, A.S.; Kusaykin, M.I.; Malyarenko, O.S.; Zueva, A.O.; Kalinovsky, A.I.; Airong, J.; Surits, V.V.; Ermakova, S.P. Enzymatic Transformation and Anti-Tumor Activity of Sargassum Horneri Fucoidan. Carbohydr. Polym. 2020, 246, 116635. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Tran, H.T.Q.; Huang, H.T.; Nan, F.H.; Lee, M.C. Sargassum horneri extracts stimulate innate immunity, enhance growth performance, and upregulate immune genes in the white shrimp Litopenaeus vannamei. Fish. Shellfish. Immunol. 2020, 102, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, S.; Cheon, J.; Lee, J.; Kim, B.K.; Lee, S.H.; Kong, C.; Kim, Y.Y.; Kim, M. Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells. Nutr. Res. Pr. 2016, 10, 148–153. [Google Scholar] [CrossRef]
- Eom, S.J.; Kim, Y.E.; Kim, J.E.; Park, J.; Kim, Y.H.; Song, K.M.; Lee, N.H. Production of Undaria Pinnatifida Sporophyll Extract Using Pilot-Scale Ultrasound-Assisted Extraction: Extract Characteristics and Antioxidant and Anti-Inflammatory Activities. Algal Res. 2020, 51, 102039. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, J.; Jiang, P.; Zheng, W.; Li, X.; Song, S.; Ai, C. Polysaccharides from Edible Brown Seaweed Undaria Pinnatifida Are Effective against High-Fat Diet-Induced Obesity in Mice through the Modulation of Intestinal Microecology. Food Funct. 2022, 13, 2581–2593. [Google Scholar] [CrossRef]
- Shao, P.; Liu, J.; Chen, X.; Fang, Z.; Sun, P. Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int. J. Biol. Macromol. 2015, 73, 124–130. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Thakur, V.; Lu, J.; Roscilli, G.; Aurisicchio, L.; Cappelletti, M.; Pavoni, E.; White, W.L.; Bedogni, B. The natural compound fucoidan from New Zealand Undaria pinnatifida synergizes with the ERBB inhibitor lapatinib enhancing melanoma growth inhibition. Oncotarget 2017, 8, 17887–17896. [Google Scholar] [CrossRef]
- Wen, Z.S.; Liu, L.J.; OuYang, X.K.; Qu, Y.L.; Chen, Y.; Ding, G.F. Protective Effect of Polysaccharides from Sargassum Horneri against Oxidative Stress in Raw264.7 Cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef]
- Wen, Z.S.; Xiang, X.W.; Jin, H.X.; Guo, X.Y.; Liu, L.J.; Huang, Y.N.; OuYang, X.K.; Qu, Y.L. Composition and AntiInflammatory Effect of Polysaccharides from Sargassum horneri in Raw264.7 Macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Fernando, I.P.S.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Jee, Y.; Jeon, Y.J. In Vitro and in Vivo AntiInflammatory Activities of High Molecular Weight Sulfated Polysaccharide; Containing Fucose Separated from Sargassum horneri: Short Communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Kim, J.; Jeon, Y.J. Fucoidan Isolated from Invasive Sargassum Horneri Inhibit Lps-Induced Inflammation Via Blocking Nf-Κb and Mapk Pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Ponce, N.M.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Córdoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the Phaeophyta Scytosiphon lomentaria: Chemical Analysis and Antiviral Activity of the Galactofucan Component. Carbohydr. Res. 2019, 478, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jayawardhana, H.H.A.C.K.; Lee, H.G.; Liyanage, N.M.; Nagahawatta, D.P.; Ryu, B.; Jeon, Y.J. Structural Characterization and Anti-Inflammatory Potential of Sulfated Polysaccharides from Scytosiphon lomentaria; Attenuate Inflammatory Signaling Pathways. J. Funct. Foods 2023, 102, 105446. [Google Scholar] [CrossRef]
- Qi, H.; Yu, Y.; Sun, L.; Yang, J.; Li, D.; Xin, C.; Zhou, D.; Qin, L.; Murata, Y. Pu-rification and Partial Bioactivity in Vitro of Polysaccharides from Sporophyll of Undaria pinnatifida. J. Food Agric. Environ. 2012, 10, 197–201. [Google Scholar]
- Han, Y.; Wu, J.; Liu, T.; Hu, Y.; Zheng, Q.; Wang, B.; Lin, H.; Li, X. Separation, characterization and anticancer activities of a sulfated polysaccharide from Undaria pinnatifida. Int. J. Biol. Macromol. 2015, 83, 42–49. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Wang, X.; Zhang, X.; Liu, W.; Wang, Y.; Zhang, Y.; Pan, H.; Wang, Q.; Han, Y. Effect of Polysaccharide from Undaria pinnatifida on Proliferation, Migration and Apoptosis of Breast Cancer Cell Mcf7. Int. J. Biol. Macromol. 2019, 121, 734–742. [Google Scholar] [CrossRef]
- Song, Z.; Li, H.; Liang, J.; Xu, Y.; Zhu, L.; Ye, X.; Wu, J.; Li, W.; Xiong, Q.; Li, S. Sulfated polysaccharide from Undaria pinnatifida stabilizes the atherosclerotic plaque via enhancing the dominance of the stabilizing components. Int. J. Biol. Macromol. 2019, 140, 621–630. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Xu, Y.; Chen, G.; Qiu, Y. Sulfated Polysaccharide from Undaria pinnatifida Induces Apoptosis and Inhibits Proliferation, Migration, and Invasion in Ovarian Cancer Via Suppressing the Hedgehog Signaling Pathway. Front. Mater. 2021, 8, 795061. [Google Scholar] [CrossRef]
- Wang, F.L.; Ji, Y.B.; Yang, B. Sulfated Modification, Characterization and Monosaccharide Composition Analysis of Undaria pinnatifida Polysaccharides and Anti-Tumor Activity. Exp. Ther. Med. 2020, 20, 630–636. [Google Scholar] [CrossRef]
- Zhong, Q.W.; Zhou, T.S.; Qiu, W.H.; Wang, Y.K.; Xu, Q.L.; Ke, S.Z.; Wang, S.J.; Jin, W.H.; Chen, J.W.; Zhang, H.W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Xia, X.; Wong, I.N.; Chung, S.K.; Xu, B.; El-Seedi, H.R.; Wang, B.; Huang, R. Sulfated Heteropolysaccharides from Undaria pinnatifida: Structural Characterization and Transcript-Metabolite Profiling of Immunostimulatory Effects on Raw264. 7 Cells Food Chem. X 2022, 13, 100251. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, J.H.; Kim, S.M.; Kim, J.Y.; Kim, J.H.; Eom, S.J.; Kang, M.C.; Song, K.M. The Antioxidant Activity of Undaria pinnatifida Sporophyll Extract Obtained Using Ultrasonication: A Focus on Crude Polysaccharide Extraction Using Ethanol Precipitation. Antioxidants 2023, 12, 1904. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.; Gao, Y.; Teng, X.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; et al. Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydr. Polym. 2013, 93, 478–483. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.; Fernando, I.P.; Kim, E.A.; Ahn, G.; Jee, Y.; Jeon, Y.J.; Noh, H.J.; Koh, H.B.; Kim, H.K.; Cho, H.H.; et al. Anti-Inflammatory Activity of a Sulfated Polysaccharide Isolated from an Enzymatic Digest of Brown Seaweed Sargassum horneri in Raw 264. 7 Cells Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Athukorala, Y.; Jeon, Y.J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Wei, X.L.; Sun, Z.L.; Wang, C.Y. Extraction, Fractionation, and Chemical Characterisation of fucoidans from the Brown Seaweed Sargassum pallidum. Czech J. Food Sci. 2016, 34, 406–413. [Google Scholar] [CrossRef]
- Li, Y.; Qin, G.; Cheng, C.; Yuan, B.; Huang, D.; Cheng, S.; Cao, C.; Chen, G. Purification, characterization and anti-tumor activities of polysaccharides from Ecklonia kurome obtained by three different extraction methods. Int. J. Biol. Macromol. 2019, 150, 1000–1010. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.Y.; Liu, C.; Liu, A.J. The structural characteristics of an acid-soluble polysaccharide from Grifola frondosa and its antitumor effects on H22-bearing mice. Int. J. Biol. Macromol. 2020, 158, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides. Mar. Drugs 2016, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begun, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor potential of carrageenans from marine red algae. Carbohydr. Polym. 2020, 246, 116568. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. The Derivatization and Antitumor Mechanisms of Polysaccharides. Futur. Med. Chem. 2017, 9, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–Function Relationships of Immunostimulatory Polysaccharides: A Review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, X. Structure elucidation and immunological activity of a novel pectic polysaccharide from the stems of Avicennia marina. Eur. Food Res. Technol. 2013, 236, 243–248. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; González-Muñoz, M.J.; Domínguez, H. Influence of molecular weight on the properties of Sargassum muticum fucoidan. Algal Res. 2019, 38, 101393. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, W.; Liu, G.; Yao, J.; Shan, T.; Sun, C.; Zhang, Q. The Structure-Activity Relationship between Polysaccharides from Sargassum thunbergii and Anti-Tumor Activity. Int. J. Biol. Macromol. 2017, 105, 686–692. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.L.; Jin, C.; Chen, H.J.; Li, S.H.; Li, S.Y.; Dou, X.F.; Jia, J.Q.; Gui, Z.Z. Cordyceps Militaris Polysaccharide Triggers Apoptosis and G0/G1 Cell Arrest in Cancer Cells. J. Asia-Pac. Entomol. 2015, 18, 433–438. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Barabanova, A.O.B.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’Eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ghlissi, Z.; Krichen, F.; Kallel, R.; Amor, I.B.; Boudawara, T.; Gargouri, J.; Zeghal, K.; Hakim, A.; Bougatef, A.; Sahnoun, Z. Sulfated Polysaccharide Isolated from Globularia alypum L.: Structural Characterization, in Vivo and in Vitro Anticoagulant Activity, and Toxicological Profile. Int. J. Biol. Macromol. 2019, 123, 335–342. [Google Scholar] [CrossRef]
- Brunetti, J.; Riolo, G.; Depau, L.; Mandarini, E.; Bernini, A.; Karousou, E.; Passi, A.; Pini, A.; Bracci, L.; Falciani, C. Unraveling Heparan Sulfate Proteoglycan Binding Motif for Cancer Cell Selectivity. Front. Oncol. 2019, 9, 843. [Google Scholar] [CrossRef]
- Suresh, V.; Senthilkumar, N.; Thangam, R.; Rajkumar, M.; Anbazhagan, C.; Rengasamy, R.; Gunasekaran, P.; Kannan, S.; Palani, P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process. Biochem. 2013, 48, 364–373. [Google Scholar] [CrossRef]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Tarbeeva, D.V.; Ermakova, S.P.; Zvyagintseva, T.N. Structural Characteristics and Biological Activity of Fucoidans from the Brown Algae alaria sp. and Saccharina japonica of Different Reproductive Status. Chem. Biodivers. 2012, 9, 817–828. [Google Scholar] [CrossRef]
- Miao, J.; Regenstein, J.M.; Qiu, J.; Zhang, J.; Zhang, X.; Li, H.; Zhang, H.; Wang, Z. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia—A review. Int. J. Biol. Macromol. 2020, 150, 102–113. [Google Scholar] [CrossRef]
- Hu, J.; Jia, X.; Fang, X.; Li, P.; He, C.; Chen, M. Ultrasonic Extraction, Antioxidant and Anti-cancer Activities of Novel Polysaccharides from Chuanxiong Rhizome. Int. J. Biol. Macromol. 2016, 85, 277–284. [Google Scholar] [CrossRef]
- Hashemifesharaki, R.; Xanthakis, E.; Altintas, Z.; Guo, Y.; Gharibzahedi, S.M.T. Microwave-Assisted Extraction of Polysaccharides from the Marshmallow Roots: Optimization, Purification, Structure, and Bioactivity. Carbohydr. Polym. 2020, 240, 116301. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wu, W.; Tang, H.; Wei, B.; Wang, H.; Sun, J.; Zhang, W.; Zhong, W. Structure Analysis and Anti-Tumor and Anti-Angiogenic Activities of Sulfated Galactofucan Extracted from Sargassum thunbergii. Mar. Drugs 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Kimmelman, A.C.; White, E. Autophagy and Tumor Metabolism. Cell Metab. 2017, 25, 1037–1043. [Google Scholar] [CrossRef]
- Xie, M.Y.; Nie, S.P. A Review About the Research of Structure and Function of Polysaccharides from Natural Products. J. Chin. Inst. Food Sci. Technol. 2010, 10, 1–11. [Google Scholar]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between Antitumor Activity, Molecular Weight, and Conformation of Lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The Structures and Biological Functions of Polysaccharides from Traditional Chinese Herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical Modifications of Polysaccharides and Their Anti-Tumor Activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H.Y. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kleiboeker, S.B. Porcine Reproductive and Respiratory Syndrome Virus Induces Apoptosis through a Mitochondria-Mediated Pathway. Virology 2007, 365, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, H.; Hou, Y.; Jin, Y.; Zhang, L. Combined Administration of Poly-ADP-Ribose Polymerase-1 and Caspase-3 Inhibitors Alleviates Neuronal Apoptosis After Spinal Cord Injury in Rats. World Neurosurg. 2019, 127, e346–e352. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Wang, J.; Li, Y.; Li, Y.; Wei, J.; Zheng, T.; Jin, M.; Sun, Z. Silica Nanoparticles Induced Intrinsic Apoptosis in Neuroblastoma Sh-Sy5y Cells Via Cytc/Apaf-1 Pathway. Environ. Toxicol. Pharmacol. 2017, 52, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, Z.C.; Shen, Z.Y.; Shen, X.J.; Tang, S.M. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol. 2021, 148, 111971. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Strasser, A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011, 18, 1414–1424. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Maller, J.L. A Centrosomal Localization Signal in Cyclin E Required for Cdk2-Independent S Phase Entry. Science 2004, 306, 885–888. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Xiong, Q.; Song, Z.; Hu, W.; Liang, J.; Jing, Y.; He, L.; Huang, S.; Wang, X.; Hou, S.; Xu, T.; et al. Methods of Extraction, Separation, Purification, Structural Characterization for Polysaccharides from Aquatic Animals and Their Major Pharmacological Activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 48–63. [Google Scholar] [CrossRef]
- Yan, W.; Niu, Y.; Lv, J.; Xie, Z.; Jin, L.; Yao, W.; Gao, X.; Yu, L.L. Characterization of a Heteropolysaccharide Isolated from Diploid Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2013, 92, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.C.; Yin, J.; Wu, J.Y. Effect of polysaccharide chain conformation on ultrasonic degradation of curdlan in alkaline solution. Carbohydr. Polym. 2018, 195, 298–302. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Wei, J.; Sun, X.; Yuan, J.; Li, F.; Xiang, J. Whole Transcriptome Analysis Provides Insights into Molecular Mechanisms for Molting in Litopenaeus vannamei. PLoS ONE 2015, 10, e0144350. [Google Scholar] [CrossRef] [PubMed]



| Species | Polysaccharide Fractions | Molecular Weight | Studied Activity | References |
|---|---|---|---|---|
| Sargassum horneri | SHS1 and SHS0.5 | -- | Antioxidant activity | [31] |
| SHP30, SHP60, and SHP80 | 1.58 × 103 kDa, 1.92 × 103 kDa, and 11.2 kDa | Antioxidant and anti-tumor (gastric cancer and DLD intestinal cancer) activity | [29] | |
| SHPSA | 5.78 × 105 kDa | Anti-tumor (DLD intestinal cancer) activity | [28] | |
| SHPH1 and SHPH2 | 78.31 kDa and 21.42 kDa | Antioxidant and moisture-preserving activities | [32] | |
| F1 and F2 | -- | Anti-inflammatory activity | [33] | |
| F1, F2, F3, and F4 | <5 kDa, 5–10 kDa, 10–30 kDa, and >30 kDa | Anti-inflammatory activity | [34] | |
| SHCF1 and SHCF2 | >50 kDa and <50 kDa | Anti-inflammatory activity | [35] | |
| F1, F2, and F3 | 63 kDa, 114 kDa, and 138 kDa | Anti-tumor (human colon carcinoma cancer) and radiosensitizing activities | [23] | |
| Scytosiphon lomentaria | A5, A10, A20, A30, and A40 | 11.2 kDa,10.6 kDa,13.3 kDa, 8.5 kDa, and 12.1 kDa | Antiviral activity | [36] |
| SLCF1, SLCF2, SLCF3, SLCF4, and SLCF5 | -- | Anti-inflammatory activity | [37] | |
| Undaria pinnatifida | PSU1-4 | -- | Anti-inflammatory and antioxidant activities | [38] |
| SPUP | 97.9 kDa | Anti-tumor (breast cancer and ovarian cancer) activity Anti-atherosclerotic effects | [39,40,41,42] | |
| UPPS-B1 and S-UPPS-B1 | 37 kDa and 110 kDa | Anti-tumor (ascites tumor) activity | [43] | |
| Up-3, Up-4, and Up-5 | 84.8 kDa, 41.4 kDa, and 330.7 kDa | Hypoglycemic activity | [44] | |
| UPP 1, UPP 2, and UPP 3 | 7.212 kDa, 13.924 kDa, and 55.875 kDa | Immunostimulatory activity | [45] | |
| UPE_8, UPE_8S, and UPE_8P | 1062kDa, 447 kDa, and 745 kDa | Antioxidant activity | [46] |
| Fractions | Total Saccharides | Sulfate Contents | Molecular Weight (kDa) | Monosaccharide Composition (Molar Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | Glu | Xyl | Fuc | Gal | ||||
| SHP-0.3 | 32% | 7.62% | 10.493 | 0.468 | 1.1 | Nd | 0.126 | 9.18 | Nd | Nd |
| SHP-0.5 | 26% | 6.49% | 30.945 | 0.725 | Nd | 2.29 | Nd | 0.771 | Nd | Nd |
| SHP-0.7 | 19% | 6.91% | 82.341 | 2.2 | 1.44 | 1.26 | 1.65 | 1.76 | 0.328 | Nd |
| SHP-1.0 | 36% | 2.2% | 614.40 | 5.22 | 3.377 | Nd | 0.497 | Nd | 2.256 | 0.07 |
| SHP-1.3 | 18% | 2.91% | 620.64 | 2.46 | Nd | 19.9 | 0.37 | 1.82 | 3 | 0.17 |
| SHP-1.5 | 23% | 8.63% | 627.85 | 7.18 | Nd | 1.67 | 0.21 | 1.19 | 2.32 | 1.38 |
| SHP-1.7 | 37% | 10.77% | 729.67 | Nd | Nd | 2.46 | 1.44 | 0.85 | 4.43 | Nd |
| Fractions | Total Saccharides | Sulfate Contents | Molecular Weight (kDa) | Monosaccharide Composition (Molar Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | Glu | Xyl | Fuc | Gal | ||||
| SLP-0.3 | 24% | 2.34% | 301.31 | 4.347 | 0.076 | 0.05 | 0.842 | 0.021 | 0.333 | 0.01 |
| SLP-0.5 | 30% | 2.06% | 375.76 | 0.0538 | 0.215 | Nd | 0.0323 | 0.2 | 0.016 | 0.027 |
| SLP-0.7 | 75% | 6.49% | 448.64 | 0.154 | 0.832 | 3.921 | 1.218 | 0.068 | 0.069 | 0.086 |
| SLP-1.3 | 60% | 2.48% | 518.36 | 0.96 | 0.182 | 0.813 | 0.424 | Nd | 0.317 | 0.087 |
| SLP-1.5 | 36% | 1.49% | 646.83 | 0.091 | 0.0935 | Nd | 0.836 | Nd | 0.0347 | 0.075 |
| Fractions | Total Saccharides | Sulfate Contents | Molecular Weight (kDa) | Monosaccharide Composition (Molar Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | Glu | Xyl | Fuc | Gal | ||||
| UPP-0.3 | 90% | 0.50% | 8.889 | 4.302 | Nd | Nd | 1.032 | Nd | 0.418 | Nd |
| UPP-0.5 | 13% | 1.05% | 341.01 | 0.596 | Nd | Nd | 1.002 | Nd | 0.011 | Nd |
| UPP-1.0 | 42% | 1.34% | 402.35 | Nd | 6.29 | Nd | 0.105 | Nd | 0.942 | 0.533 |
| UPP-1.3 | 25% | 1.06% | 444.96 | 0.974 | Nd | 1.22 | 0.966 | 7.05 | 0.441 | Nd |
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| Caspase 3 | F R | TGACATCTCGGTCTGGTA AACATCACGCATCAATTCC |
| Caspase 8 | F R | TTCCTGAGCCTGGACTACATT GAAGTTCCCTTTCCATCTCCT |
| Caspase 9 | F R | ACTAACAGGCAAGCAGCAAA CCAAATCCTCCAGAACCAAT |
| CDK 2 | F R | AACACAGAGGGGGCCATCAAGC CAGGAGCTCGGTACCACAGGGTC |
| Bax | F R | AGGTCTTTTTCCGAGTGGCAGC CCCGGAGGAAGTCCAATGTCC |
| Bcl-2 | F R | ATGTGTGTGGAGAGCGTCAA GAGACAGCCAGGAGAAATCA |
| β-actin | F R | CGTGGACATCCGCAAAG GCACTCGTCATACTCCTGCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Niu, Y.; Chen, R.; Ju, H.; Liu, Z.; Zhang, B.; Xie, W.; Gao, Y. A Comparative Analysis of the Anti-Tumor Activity of Sixteen Polysaccharide Fractions from Three Large Brown Seaweed, Sargassum horneri, Scytosiphon lomentaria, and Undaria pinnatifida. Mar. Drugs 2024, 22, 316. https://doi.org/10.3390/md22070316
Song L, Niu Y, Chen R, Ju H, Liu Z, Zhang B, Xie W, Gao Y. A Comparative Analysis of the Anti-Tumor Activity of Sixteen Polysaccharide Fractions from Three Large Brown Seaweed, Sargassum horneri, Scytosiphon lomentaria, and Undaria pinnatifida. Marine Drugs. 2024; 22(7):316. https://doi.org/10.3390/md22070316
Chicago/Turabian StyleSong, Lin, Yunze Niu, Ran Chen, Hao Ju, Zijian Liu, Bida Zhang, Wancui Xie, and Yi Gao. 2024. "A Comparative Analysis of the Anti-Tumor Activity of Sixteen Polysaccharide Fractions from Three Large Brown Seaweed, Sargassum horneri, Scytosiphon lomentaria, and Undaria pinnatifida" Marine Drugs 22, no. 7: 316. https://doi.org/10.3390/md22070316
APA StyleSong, L., Niu, Y., Chen, R., Ju, H., Liu, Z., Zhang, B., Xie, W., & Gao, Y. (2024). A Comparative Analysis of the Anti-Tumor Activity of Sixteen Polysaccharide Fractions from Three Large Brown Seaweed, Sargassum horneri, Scytosiphon lomentaria, and Undaria pinnatifida. Marine Drugs, 22(7), 316. https://doi.org/10.3390/md22070316




