Systematical Investigation on Anti-Fatigue Function and Underlying Mechanism of High Fischer Ratio Oligopeptides from Antarctic Krill on Exercise-Induced Fatigue in Mice
Abstract
:1. Introduction
2. Results
2.1. Effect of HFOs-AK on Body Weight and Organ Index of Fatigue Model of Mice
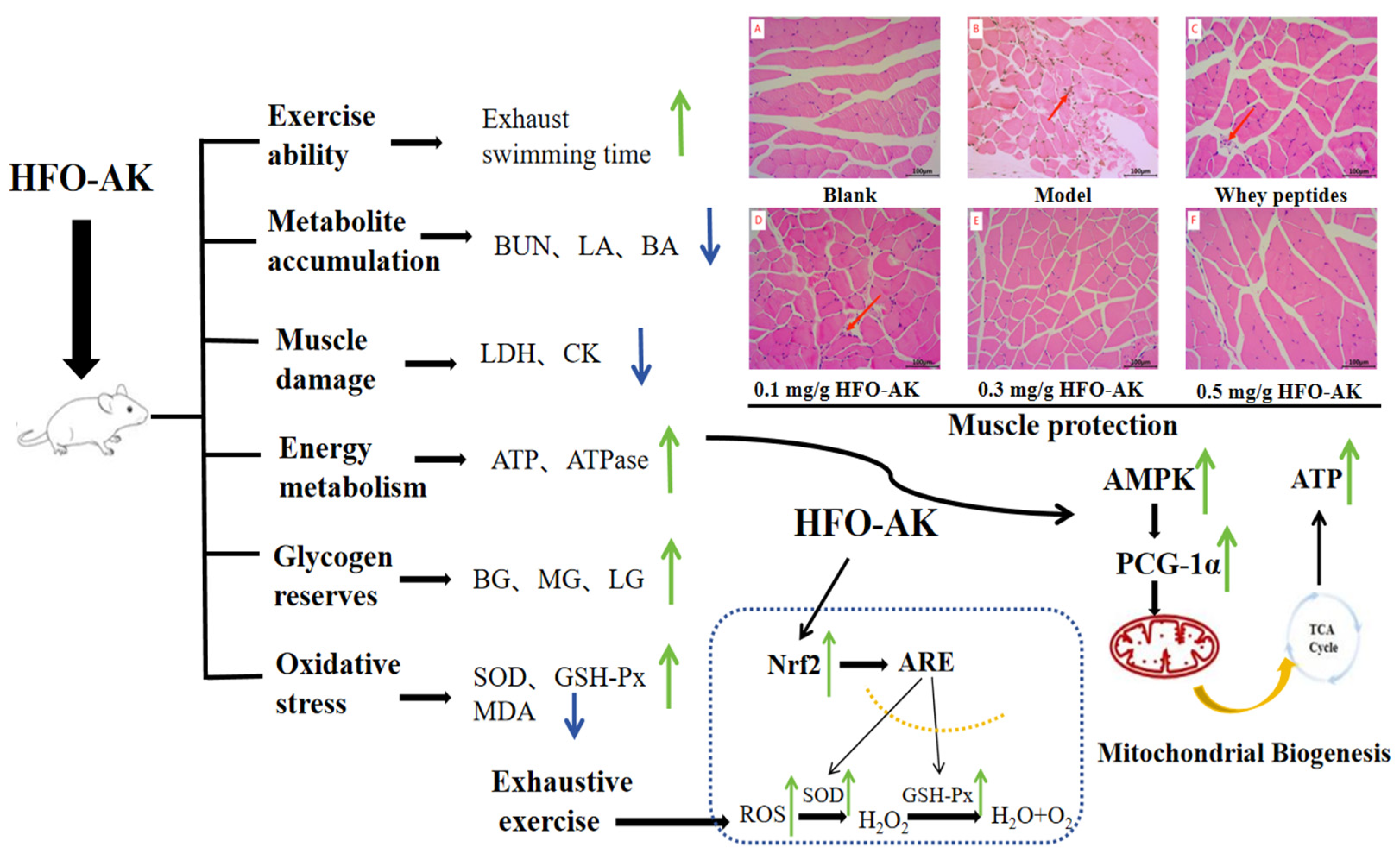
2.2. Effect of HFOs-AK on Tissue Morphology of Fatigue Model of Mice
2.3. Effect of HFOs-AK on Exercise Capacity and Metabolite Levels of BUN, Lactic Acid (LA), and Blood Ammonia (BA) in Fatigue Model of Mice
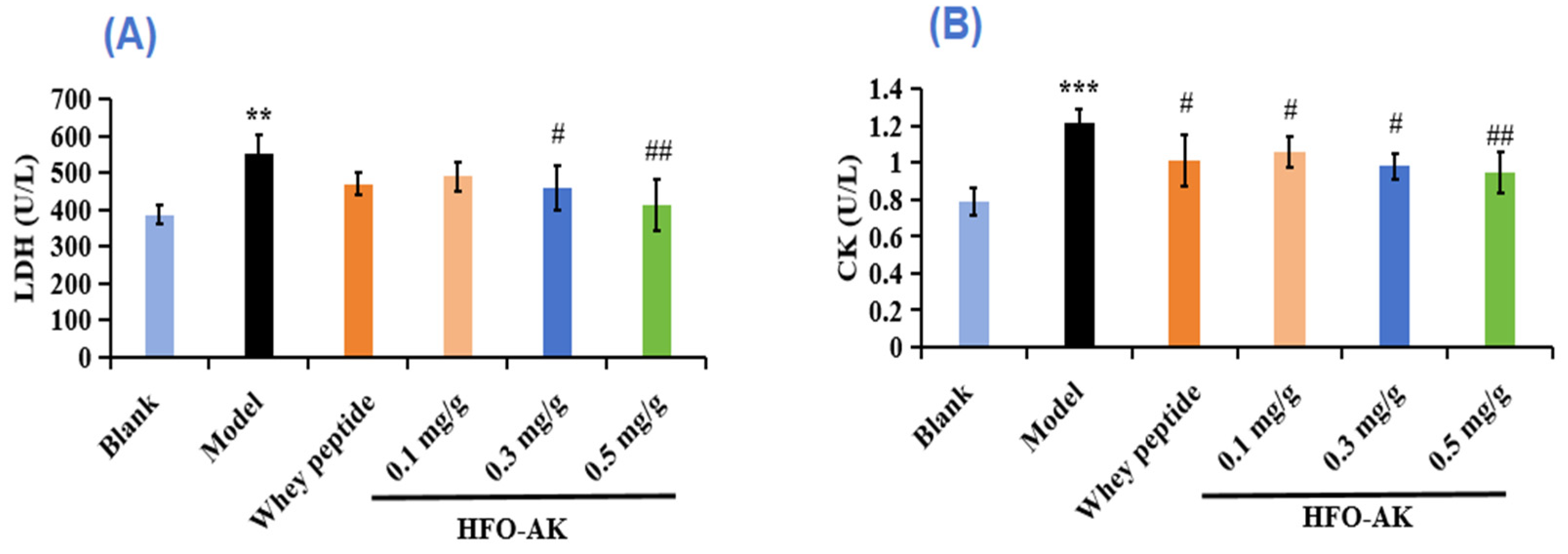
2.4. Effect of HFOs-AK on Lactate Dehydrogenase (LDH) and CK of Fatigue Model of Mice
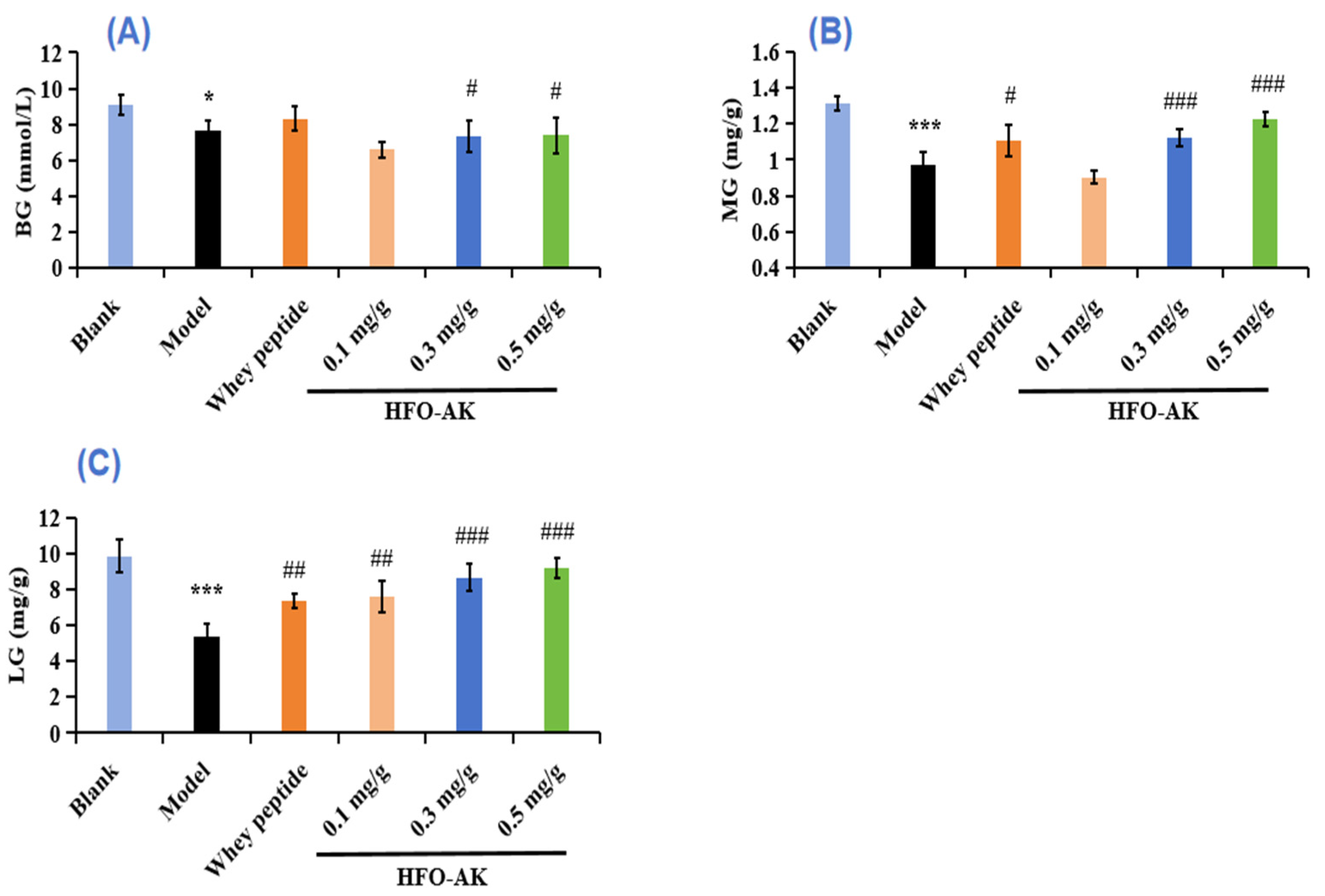
2.5. Effect of HFOs-AK on Blood Glucose (BG), Muscle Glycogen (MG), and Liver Glycogen (LG) of Fatigue Model of Mice
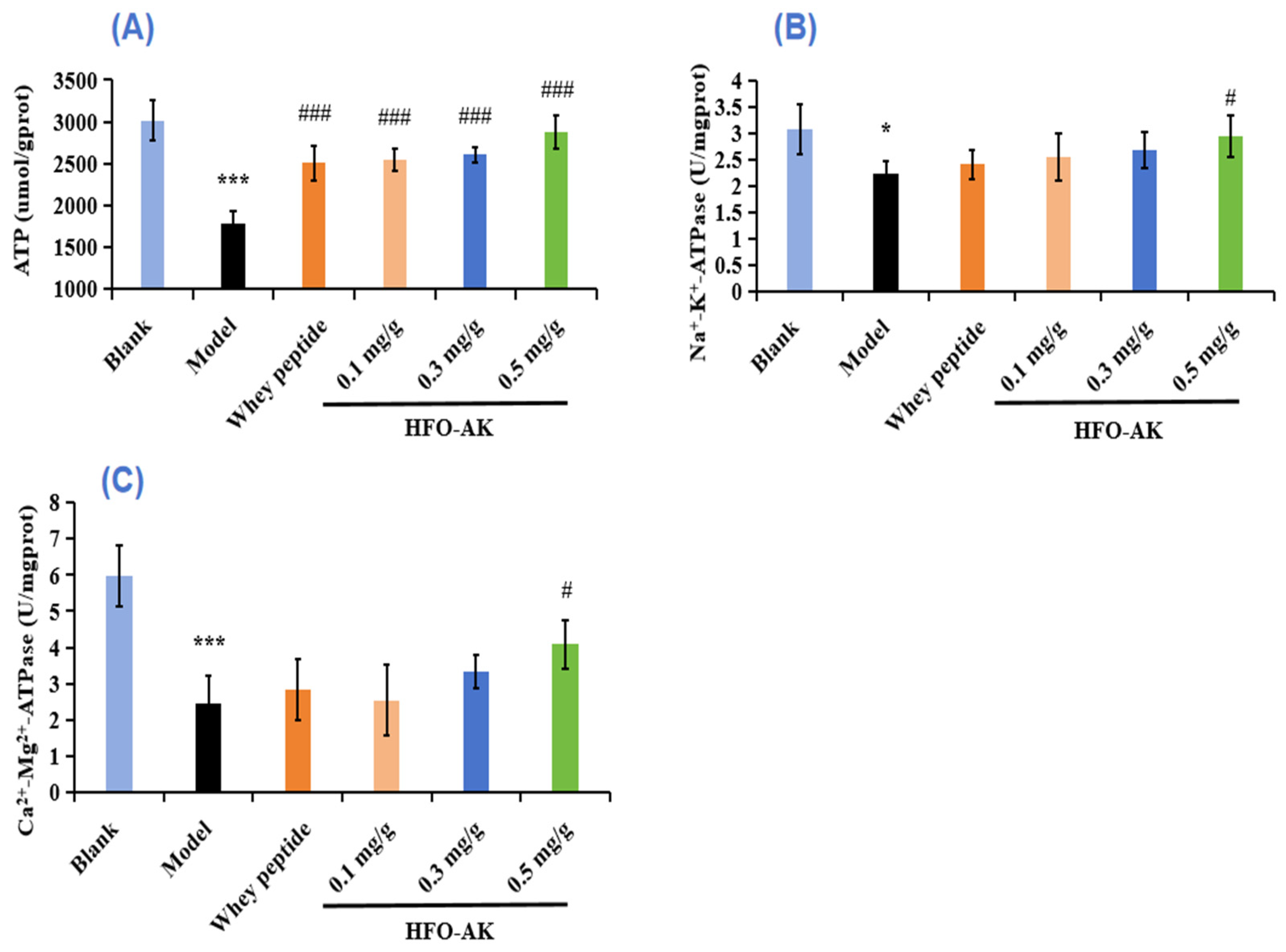
2.6. Effect of HFOs-AK on ATP Content and Activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase of Fatigue Model of Mice
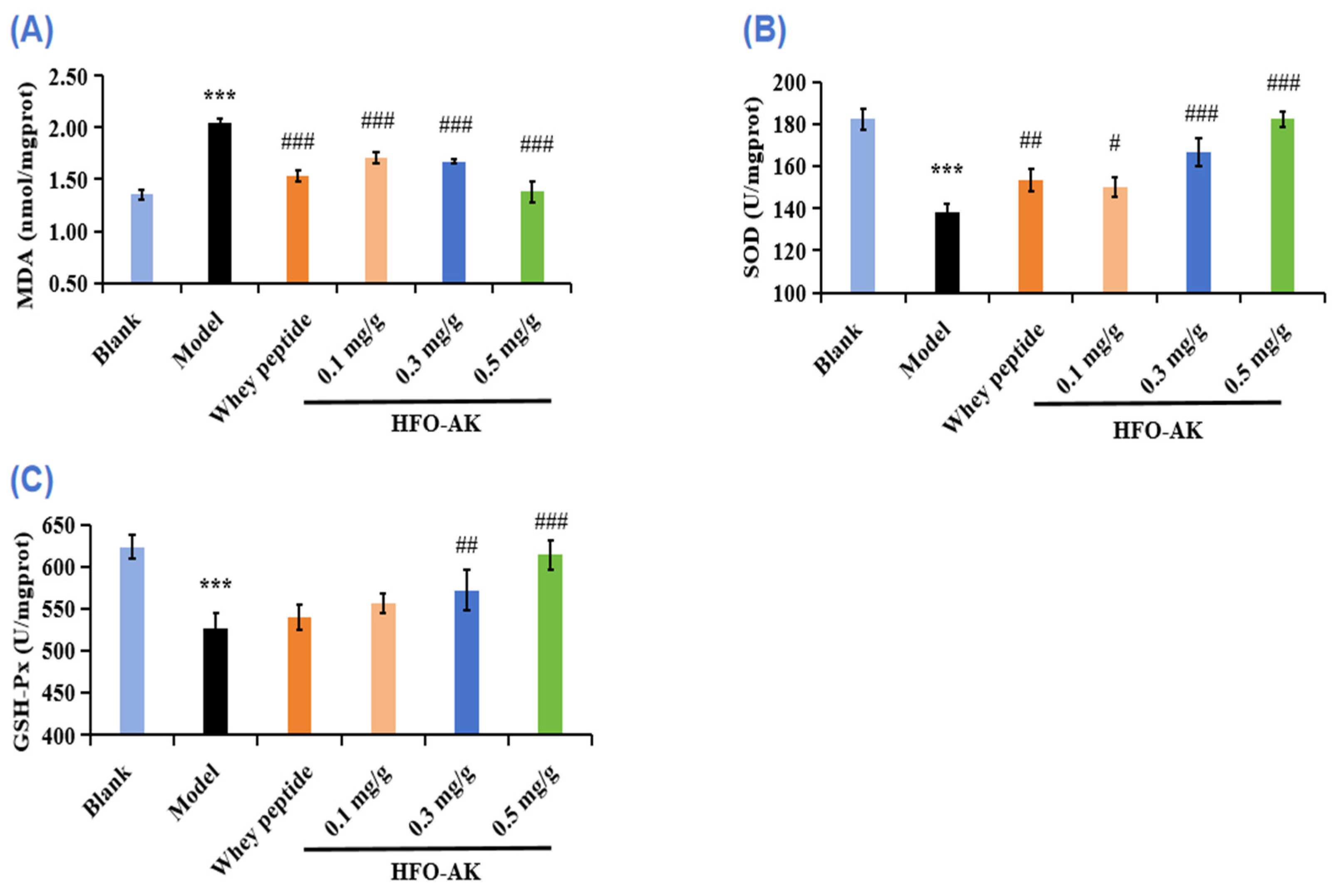
2.7. Effect of HFOs-AK on Antioxidant Capacity of Fatigue Model of Mice
2.8. Effect of HFOs-AK on Protein Expression of the AMPK/PGC-1α/Nrf2 Signaling Pathways in Fatigue Model of Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Chemical Reagents
4.2. Experimental Methods
4.2.1. Animal Breeding and Experimental Design
4.2.2. Establishment of a Fatigue Model of Endurance Swimming Mice
4.2.3. Measurement of Body Weight and Organ Index of Mice
4.2.4. Histopathological Changes Observed
4.2.5. Measurement of Biochemical Indexes among Mice
4.2.6. Detecting the Expression of Relevant Signaling Pathway Proteins
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Abachi, S.; Offret, C.; Fliss, I.; Marette, A.; Bazinet, L.; Beaulieu, L. Isolation of immunomodulatory biopeptides from Atlantic Mackerel (Scomber scombrus) protein hydrolysate based on molecular weight, charge, and hydrophobicity. Food Bioprocess Technol. 2022, 15, 852–874. [Google Scholar] [CrossRef]
- Hu, Y.D.; Xi, Q.H.; Kong, J.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from the collagens of monkfish (Lophius litulon) swim bladders: Isolation, characterization, molecular docking analysis and activity evaluation. Mar. Drugs 2023, 21, 516. [Google Scholar] [CrossRef] [PubMed]
- Quintal-Bojórquez, N.; Segura-Campos, M.R. Bioactive peptides as therapeutic adjuvants for cancer. Nutr. Cancer 2021, 73, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Zhang, Z.; Sheng, Y.; Chi, C.F.; Wang, B. A systematic review on marine umami peptides: Biological sources, preparation methods, structure-umami relationship, mechanism of action and biological activities. Food Biosci. 2024, 57, 103637. [Google Scholar] [CrossRef]
- Lan, C.; Zhao, Y.Q.; Li, X.R.; Wang, B. High Fischer ratio oligopeptides determination from Antartic krill: Preparation, peptides profiles, and in vitro antioxidant activity. J. Food Biochem. 2019, 43, e12827. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Tanimoto, S.Y.; Watanabe, M.; Arai, S. Nutritional effects of an oligopeptide mixture with a very high Fischer ratio on the amino acid absorption and cerebral amine metabolism in rats suffering from galactosamine-induced liver injury. Agric. Biol. Chem. 1991, 55, 2585–2590. [Google Scholar]
- Wang, Z.G.; Ying, X.G.; Wang, Y.F.; Yu, X.W.; Luo, H.Y. Structural analysis and activity evaluation of high Fischer ratio oligopeptides from minced meat of skipjack (Katsuwonus pelamis). J. Aquat. Food Prod. Technol. 2019, 28, 1063–1075. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Ding, S.; Qi, B. Isolation and identification of novel antioxidant and antimicrobial oligopeptides from enzymatically hydrolyzed anchovy fish meal. Process Biochem. 2018, 74, 148–155. [Google Scholar] [CrossRef]
- Niu, R.; Feng, W. Research progress of phenylketonuria and its releveant treatment. Chin. New Drugs J. 2018, 27, 154–158. [Google Scholar]
- Liu, R.; Li, Z.; Yu, X.-C.; Hu, J.-N.; Zhu, N.; Liu, X.-R.; Hao, Y.-T.; Kang, J.-W.; Li, Y. The effects of peanut oligopeptides on exercise-induced fatigue in mice and its underlying mechanism. Nutrients 2023, 15, 1743. [Google Scholar] [CrossRef]
- Li, D.; Ren, J.-W.; Xu, T.; Li, L.; Liu, P.; Li, Y. Effect of bovine bone collagen oligopeptides on wound healing in mice. Aging 2021, 13, 9028. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Wang, Y.Z.; Zhao, Y.Q.; Chi, C.F.; Zhu, W.Y.; Wang, B. High Fischer ratio oligopeptides from hard-shelled mussel: Preparation and hepatoprotective effect against acetaminophen-induced liver injury in mice. Food Biosci. 2023, 53, 102638. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, M.; Hemar, Y. Study of the in vitro properties of oligopeptides from whey protein isolate with high Fisher’s ratio and their ability to prevent allergic response to β-lactoglobulin in vivo. Food Chem. 2023, 405, 134841. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Feng, Y.; Cui, Q. Changes in peptidomes and Fischer ratios of corn-derived oligopeptides depending on enzyme hydrolysis approaches. Food Chem. 2019, 297, 124931. [Google Scholar] [CrossRef]
- Ying, Z.; Yuyang, H.; Meiying, L.; Bingyu, S.; Linlin, L.; Mingshou, L.; Min, Q.; Huanan, G.; Xiuqing, Z. High Fischer ratio peptide of hemp seed: Preparation and anti-fatigue evaluation in vivo and in vitro. Food Res. Int. 2023, 165, 112534. [Google Scholar] [CrossRef] [PubMed]
- Salma, N.U.; Govindaraju, K.; Kumar, B.S.G.; Muthukumar, S.P.; Lakshmi, A.J. Ameliorative effect of enhanced Fischer ratio flaxseed protein hydrolysate in combination with antioxidant micronutrients on ethanol-induced hepatic damage in a rat model. Br. J. Nutr. 2022, 127, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Hou, Y.; Chen, X.; Zhang, M.; Hu, Z.; Chen, L.; Huang, J. High Fischer ratio oligopeptides of gluten alleviate alcohol-induced liver damage by regulating lipid metabolism and oxidative stress in rats. Foods 2024, 13, 436. [Google Scholar] [CrossRef]
- Qin, Y.; Cheng, M.; Fan, X.; Shao, X.; Wang, C.; Jiang, H.; Zhang, X. Preparation and antioxidant activities of high Fischer’s ratio oligopeptides from goat whey. Food Sci. Anim. Resour. 2022, 42, 800. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, C.; Cao, W.; Liu, S.; Ji, H. Preparation and characterisation of the pearl oyster (Pinctada martensii) meat protein hydrolysates with a high Fischer ratio. Int. J. Food Sci. Technol. 2009, 44, 1183–1191. [Google Scholar] [CrossRef]
- Xiong, K.; Liu, J.; Wang, X.; Sun, B.; Zhang, Y.; Zhao, Z.; Pei, P.; Li, X. Engineering a carboxypeptidase from Aspergillus niger M00988 by mutation to increase its ability in high Fischer ratio oligopeptide preparation. J. Biotechnol. 2021, 330, 1–8. [Google Scholar] [CrossRef]
- Feng, Z.; Wei, Y.; Xu, Y.; Zhang, R.; Li, M.; Qin, H.; Gu, R.; Cai, M. The anti-fatigue activity of corn peptides and their effect on gut bacteria. J. Sci. Food Agric. 2022, 102, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zeng, H.; Lin, S.; Zhang, Z.; Zhang, Y.; Hu, J. Anti-fatigue activities of hairtail (Trichiurus lepturus) hydrolysate in an endurance swimming mice model. J. Funct. Foods 2020, 74, 104207. [Google Scholar] [CrossRef]
- Ye, J.; Shen, C.H.; Huang, Y.Y.; Zhang, X.Q.; Xiao, M.T. Anti-fatigue activity of sea cucumber peptides prepared from Stichopus japonicus in an endurance swimming rat model. J. Sci. Food Agric. 2017, 97, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, R.X.; Wei, Y.; Ling, K.; Lu, L.; Wang, J.; Pan, X.C.; Cai, M.Y. Anti-fatigue effects of fermented soybean protein peptides in mice. J. Sci. Food Agric. 2022, 102, 2693–2703. [Google Scholar] [CrossRef]
- Ge, M.-X.; Chen, R.-P.; Zhang, L.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Novel Ca-chelating peptides from protein hydrolysate of Antarctic krill (Euphausia superba): Preparation, characterization, and calcium absorption efficiency in Caco-2 cell monolayer model. Mar. Drugs 2023, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, L.; Liu, F.; Li, M.; Zeng, S.; Gui, Y.; Zhao, Y.; Wang, J.J. Effects of soluble Antarctic krill protein-curcumin complex combined with photodynamic inactivation on the storage quality of shrimp. Food Chem. 2023, 403, 134388. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhao, Y.Q.; Wang, Y.M.; Zhao, W.H.; Wang, P.; Chi, C.F.; Wang, B. Antioxidant peptides from Antarctic Krill (Euphausia superba) hydrolysate: Preparation, identification and cytoprotection on H2O2-induced oxidative stress. J. Funct. Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Yue, H.; Cai, W.; Yin, H.; Tian, Y.; Dong, P.; Wang, J. Peptides from Antarctic krill (Euphausia superba) ameliorate acute liver injury in mice induced by carbon tetrachloride via activating the Nrf2/HO-1 pathway. Food Funct. 2023, 14, 3526–3537. [Google Scholar] [CrossRef]
- Yue, H.; Li, Y.; Cai, W.; Bai, X.; Dong, P.; Wang, J. Antarctic krill peptide alleviates liver fibrosis via downregulating the secondary bile acid mediated NLRP3 signaling pathway. Food Funct. 2022, 13, 7740–7749. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, Y.; Ding, J.; Sun, N.; Lin, S. Antarctic krill peptides improve scopolamine-induced memory impairment in mice. Food Biosci. 2022, 49, 101987. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Hu, S.; Wang, D.; Yao, H.; Sun, N. Co-administration of Antarctic krill peptide EEEFDATR and calcium shows superior osteogenetic activity. Food Biosci. 2022, 48, 101728. [Google Scholar] [CrossRef]
- Hatanaka, A.; Miyahara, H.; Suzuki, K.I.; Sato, S. Isolation and identification of antihypertensive peptides from Antarctic krill tail meat hydrolysate. J. Food Sci. 2009, 74, H116–H120. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Xu, H. Analysis of chemical components of shiitake polysaccharides and its anti-fatigue effect under vibration. Int. J. Biol. Macromol. 2009, 45, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wu, R.; Jin, J.; Ning, K.; Wang, Z.; Yi, X.; Kapilevich, L.; Liu, J. Signaling pathways regulated by natural active ingredients in the fight against exercise fatigue—A review. Front. Pharmacol. 2023, 14, 1269878. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.W.; Chen, Q.H.; Li, D.; Mao, R.X.; Liu, X.R.; Li, Y. Small molecule oligopeptides isolated from walnut (Juglans regia L.) and their anti-fatigue effects in mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef]
- Zhong, L.; Zhao, L.; Yang, F.; Yang, W.; Sun, Y.; Hu, Q. Evaluation of anti-fatigue property of the extruded product of cereal grains mixed with Cordyceps militaris on mice. J. Int. Soc. Sports Nutr. 2017, 14, 15. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Huang, L.; Ou, Y.; Wu, J.; Guo, Z.; Zheng, B. The anti-fatigue effect of glycoprotein from Hairtail fish (Trichiurus lepturus) on BALB/c mice. Foods 2023, 12, 1245. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, G.; Jiang, Y.; Li, B.; Feng, C.; Ge, Y.; Le, H.; Jiang, L.; Liu, H.; Shi, Y.; et al. Sea cucumber peptides improved the mitochondrial capacity of mice: A potential mechanism to enhance gluconeogenesis and fat catabolism during exercise for improved antifatigue property. Oxidative Med. Cell. Longev. 2020, 2020, 4604387. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef]
- Suo, S.K.; Zheng, S.L.; Chi, C.F.; Luo, H.Y.; Wang, B. Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts-milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells. Front. Nutr. 2022, 9, 957778. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, M.Y.; Xing, Y.B.; Wang, X.R.; Cui, C.B. Anti-oxidation and anti-fatigue effects of the total flavonoids of Sedum aizoon L. J. Agric. Food Res. 2023, 12, 100560. [Google Scholar] [CrossRef]
- Zou, D.; Liu, P.; Chen, K.; Xie, Q.; Liang, X.; Bai, Q.; Zhou, Q.; Liu, K.; Zhang, T.; Zhu, J.; et al. Protective effects of myricetin on acute hypoxia-induced exercise intolerance and mitochondrial impairments in rats. PLoS ONE 2015, 10, e0124727. [Google Scholar]
- Zou, D.; Chen, K.; Liu, P.; Chang, H.; Zhu, J.; Mi, M. Dihydromyricetin improves physical performance under simulated high altitude. Med. Sci. Sports Exerc. 2014, 46, 2077–2084. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Y.; Zhang, Y.; Li, S.; Sun, Y.; Chen, Z.; Teng, L.; Wang, D. Antifatigue potential activity of Sarcodon imbricatus in acute excise-treated and chronic fatigue syndrome in mice via regulation of Nrf2-mediated oxidative stress. Oxidative Med. Cell. Longev. 2018, 2018, 9140896. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ji, R.; Dam, J. Antifatigue effect of coenzyme Q10 in mice. J. Med. Food 2010, 13, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Osman, W.N.W.; Mohamed, S. Standardized Morinda citrifolia L. and Morinda elliptica L. leaf extracts alleviated fatigue by improving glycogen storage and lipid/carbohydrate metabolism. Phytother. Res. 2018, 32, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Lin, Y.Q.; Chen, L.N.; Chen, M.T.; Huang, C.C. Effects of perch essence supplementation on improving exercise performance and anti-fatigue in mice. Int. J. Environ. Res. Public Health 2022, 19, 1155. [Google Scholar] [CrossRef]
- Falavigna, G.; Junior, J.A.D.A.; Rogero, M.M.; Pires, I.S.D.O.; Pedrosa, R.G.; Junior, E.M.; de Castro, I.A.; Tirapegui, J. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients 2012, 4, 1767–1780. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, D.; Guo, J.; Zhang, Y.; Zheng, B. In vitro antioxidant activity and in vivo anti-fatigue effect of sea horse (Hippocampus) peptides. Molecules 2017, 22, 482. [Google Scholar] [CrossRef]
- Lv, J.J.; Liu, Y.; Zeng, X.Y.; Yu, J.; Li, Y.; Du, X.Q.; Wu, Z.B.; Hao, S.L.; Wang, B.C. Anti-fatigue peptides from the enzymatic hydrolysates of Cervus elaphus blood. Molecules 2021, 26, 7614. [Google Scholar] [CrossRef]
- Cai, B.; Yi, X.; Wang, Z.; Zhao, X.; Duan, A.; Chen, H.; Wan, P.; Chen, D.; Huang, J.; Pan, J. Anti-fatigue effects and mechanism of Syngnathus schlegeli peptides supplementation on exercise-fatigued mice. J. Funct. Foods 2023, 110, 105846. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, H.; Chi, Y.; Deng, R.; He, Q. Structural characterization, erythrocyte protection, and antifatigue effect of antioxidant collagen peptides from tilapia (Oreochromis nilotica L.) skin. Food Funct. 2020, 11, 10149–10160. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zou, X.; Wang, K.; Liu, H.; Hong, H.; Luo, Y.; Tan, Y. Antifatigue and microbiome reshaping effects of yak bone collagen peptides on Balb/c mice. Food Biosci. 2023, 52, 102447. [Google Scholar] [CrossRef]
- Wang, X.Q.; Yu, H.H.; Xing, R.G.; Liu, S.; Chen, X.L.; Li, P.C. Structural properties, anti-fatigue and immunological effect of low molecular weight peptide from monkfish. J. Funct. Foods 2023, 105, 105546. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Shrikanth, C.B.; Nandini, C.D. AMPK in microvascular complications of diabetes and the beneficial effects of AMPK activators from plants. Phytomedicine 2020, 73, 152808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Wu, W.; Hou, L.; Chen, H.; Zuo, B.O.; Xiong, Y.; Yang, J. Skeletal muscle-specific overexpression of PGC-1α induces fiber-type conversion through enhanced mitochondrial respiration and fatty acid oxidation in mice and pigs. Int. J. Biol. Sci. 2017, 13, 1152. [Google Scholar] [CrossRef]
- Cai, W.-W.; Hu, X.-M.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive peptides from Skipjack tuna cardiac arterial bulbs: Preparation, identification, antioxidant activity, and stability against thermal, pH, and simulated gastrointestinal digestion treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef]
- Duan, F.F.; Guo, Y.; Li, J.W.; Yuan, K. Antifatigue effect of luteolin-6-C-neohesperidoside on oxidative stress injury induced by forced swimming of rats through modulation of Nrf2/ARE signaling pathways. Oxidative Med. Cell. Longev. 2017, 3159358. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, M.; Han, W. Anti-fatigue activity of parsley (Petroselinum crispum) flavonoids via regulation of oxidative stress and gut microbiota in mice. J. Funct. Foods 2022, 89, 104963. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lu, L.; Zhu, D.J.; Wang, M.; Yin, Y.; Chen, D.X.; Wei, L.B. Effects of astragalus polysaccharides on dysfunction of mitochondrial dynamics induced by oxidative stress. Oxidative Med. Cell. Longev. 2016, 9573291. [Google Scholar]
- Zhao, C.; Gong, Y.; Zheng, L.; Zhao, M. Whey protein hydrolysate enhances exercise endurance, regulates energy metabolism, and attenuates muscle damage in exercise mice. Food Biosci. 2023, 52, 102453. [Google Scholar] [CrossRef]
- Zheng, S.L.; Wang, Y.M.; Chi, C.F.; Wang, B. Chemical characterization of honeysuckle polyphenols and their alleviating function on ultraviolet B-damaged HaCaT cells by modulating the Nrf2/NF-κB signaling pathways. Antioxidants 2024, 13, 294. [Google Scholar] [CrossRef] [PubMed]




| Groups | Body Weight (g) | ||
|---|---|---|---|
| Starting Weight | Final Weight | Weight Change | |
| Blank | 22.85 ± 1.00 | 37.81 ± 2.45 | 13.33 ± 1.73 b |
| Model | 22.96 ± 0.82 | 37.97 ± 3.17 | 15.01 ± 2.35 a,b |
| Whey peptides | 23.31 ± 0.72 | 37.71 ± 1.86 | 14.40 ± 1.14 a,b |
| Low-dose HFOs-AK | 22.91 ± 0.97 | 37.92 ± 1.22 | 15.01 ± 0.25 a,b |
| Mid-dose HFOs-AK | 21.53 ± 0.94 | 37.57 ± 0.66 | 16.03 ± 0.28 a,b |
| High-dose HFOs-AK | 22.38 ± 0.99 | 38.67 ± 2.60 | 16.29 ± 1.61 a |
| Groups | Liver (%) | Spleen (%) | Gallbladder (%) | Thymus Gland (%) |
|---|---|---|---|---|
| Blank | 5.95 ± 0.41 a | 0.32 ± 0.05 a | 1.59 ± 0.14 a | 0.15 ± 0.01 a |
| Model | 5.85 ± 0.12 a,b | 0.32 ± 0.01 a | 1.56 ± 0.04 a | 0.14 ± 0.01 a |
| Whey peptides | 5.59 ± 0.05 a,b | 0.31 ± 0.02 a | 1.55 ± 0.04 a | 0.13 ± 0.04 a |
| Low-dose HFOs-AK | 5.88 ± 0.13 a | 0.29 ± 0.02 a | 1.56 ± 0.09 a | 0.15 ± 0.06 a |
| Mid-dose HFOs-AK | 5.59 ± 0.23 b | 0.29 ± 0.01 a | 1.51 ± 0.05 a | 0.14 ± 0.04 a |
| High-dose HFOs-AK | 5.69 ± 0.03 a,b | 0.30 ± 0.03 a | 1.55 ± 0.03 a | 0.14 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, S.-Y.; Suo, S.-K.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Systematical Investigation on Anti-Fatigue Function and Underlying Mechanism of High Fischer Ratio Oligopeptides from Antarctic Krill on Exercise-Induced Fatigue in Mice. Mar. Drugs 2024, 22, 322. https://doi.org/10.3390/md22070322
Mao S-Y, Suo S-K, Wang Y-M, Chi C-F, Wang B. Systematical Investigation on Anti-Fatigue Function and Underlying Mechanism of High Fischer Ratio Oligopeptides from Antarctic Krill on Exercise-Induced Fatigue in Mice. Marine Drugs. 2024; 22(7):322. https://doi.org/10.3390/md22070322
Chicago/Turabian StyleMao, Sha-Yi, Shi-Kun Suo, Yu-Mei Wang, Chang-Feng Chi, and Bin Wang. 2024. "Systematical Investigation on Anti-Fatigue Function and Underlying Mechanism of High Fischer Ratio Oligopeptides from Antarctic Krill on Exercise-Induced Fatigue in Mice" Marine Drugs 22, no. 7: 322. https://doi.org/10.3390/md22070322





