Jellyfish Venom Peptides Targeting Human Potassium Channels Identified through Ligand Screening: Morphometric and Molecular Identification of the Species and Antibiotic Potential
Abstract
:1. Introduction
2. Results
2.1. Morphological Identification
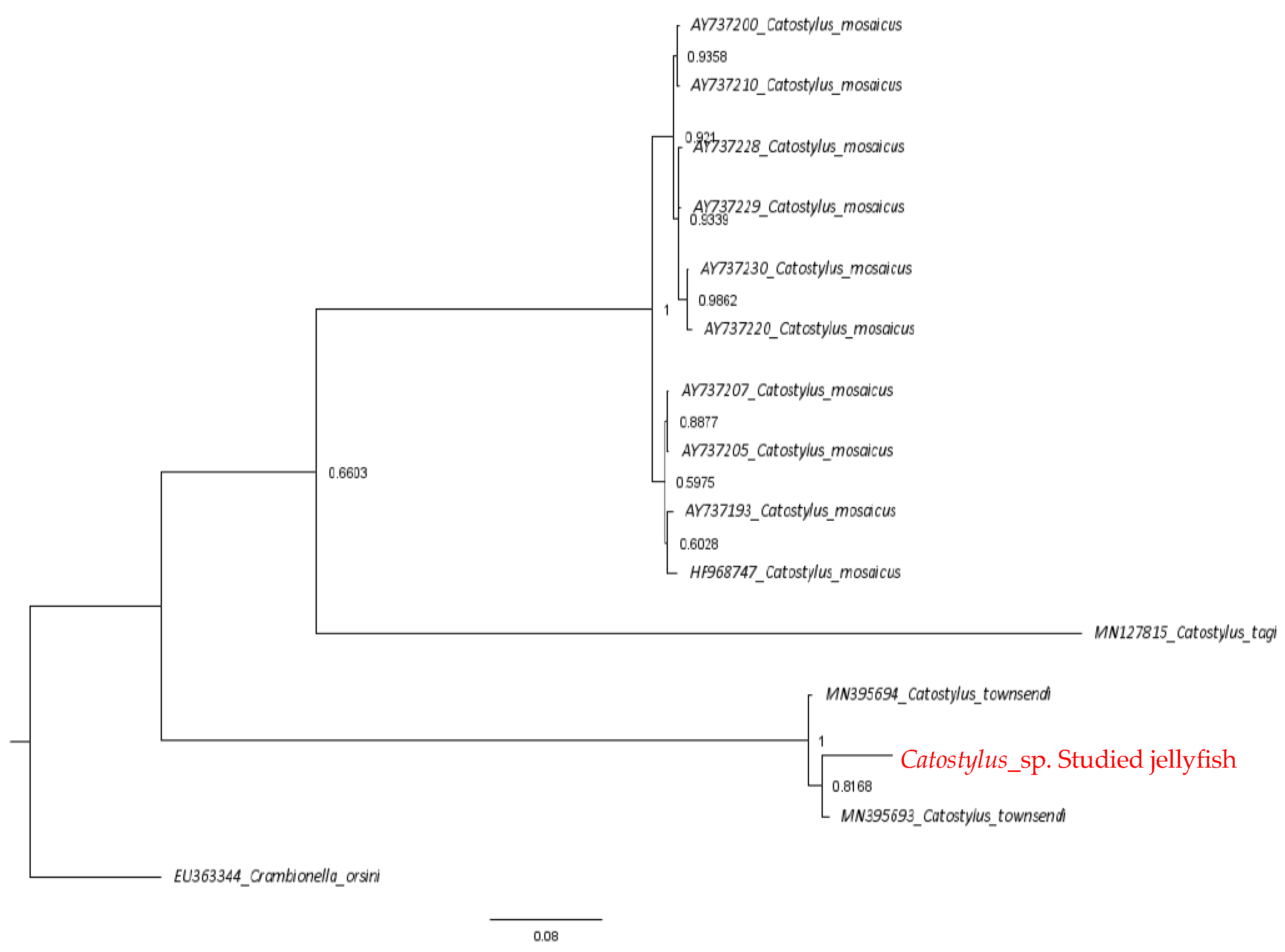
2.2. Phylogenetic Analysis
2.3. Analysis of Nematocysts
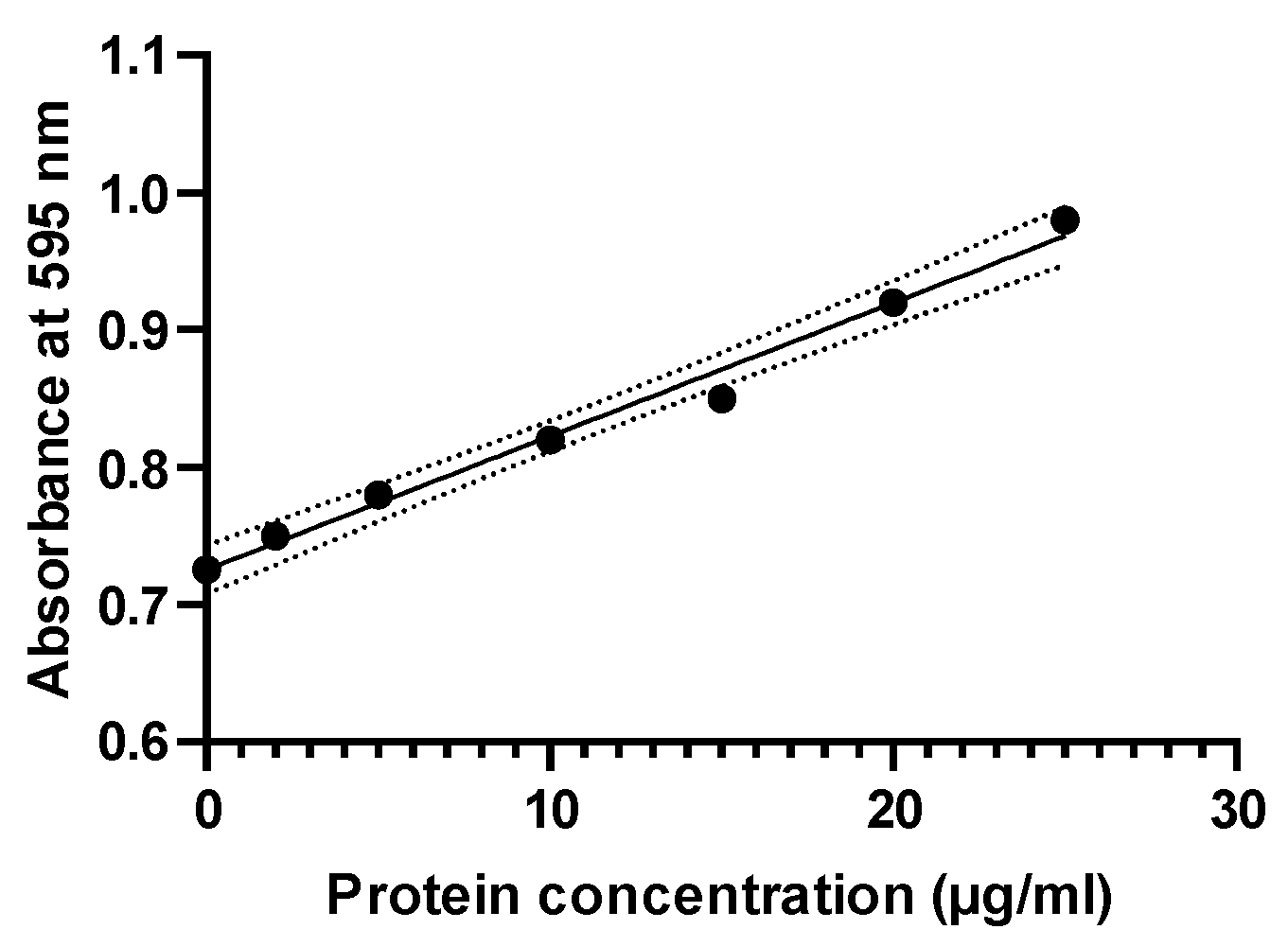
2.4. Determination of Protein Content
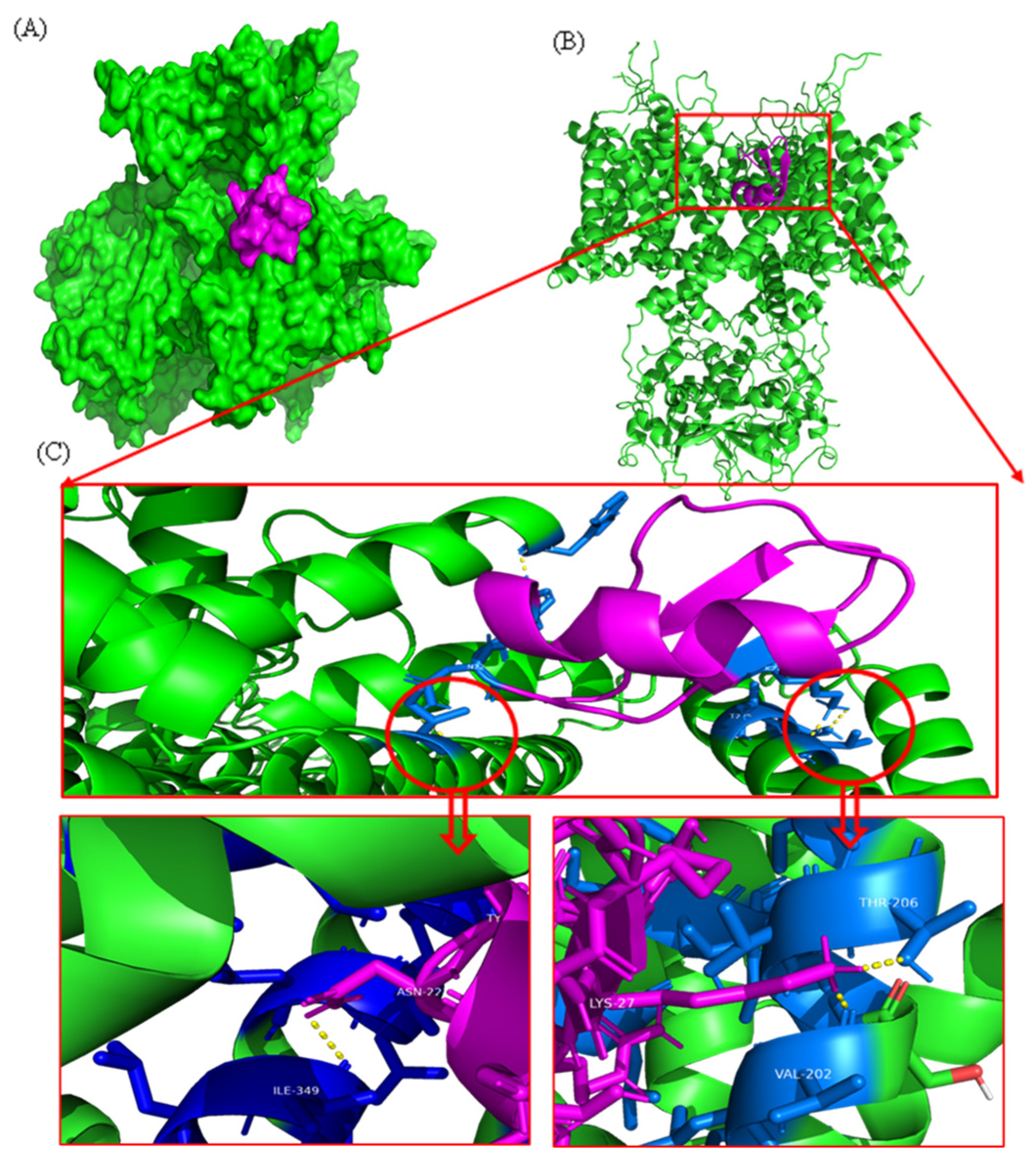
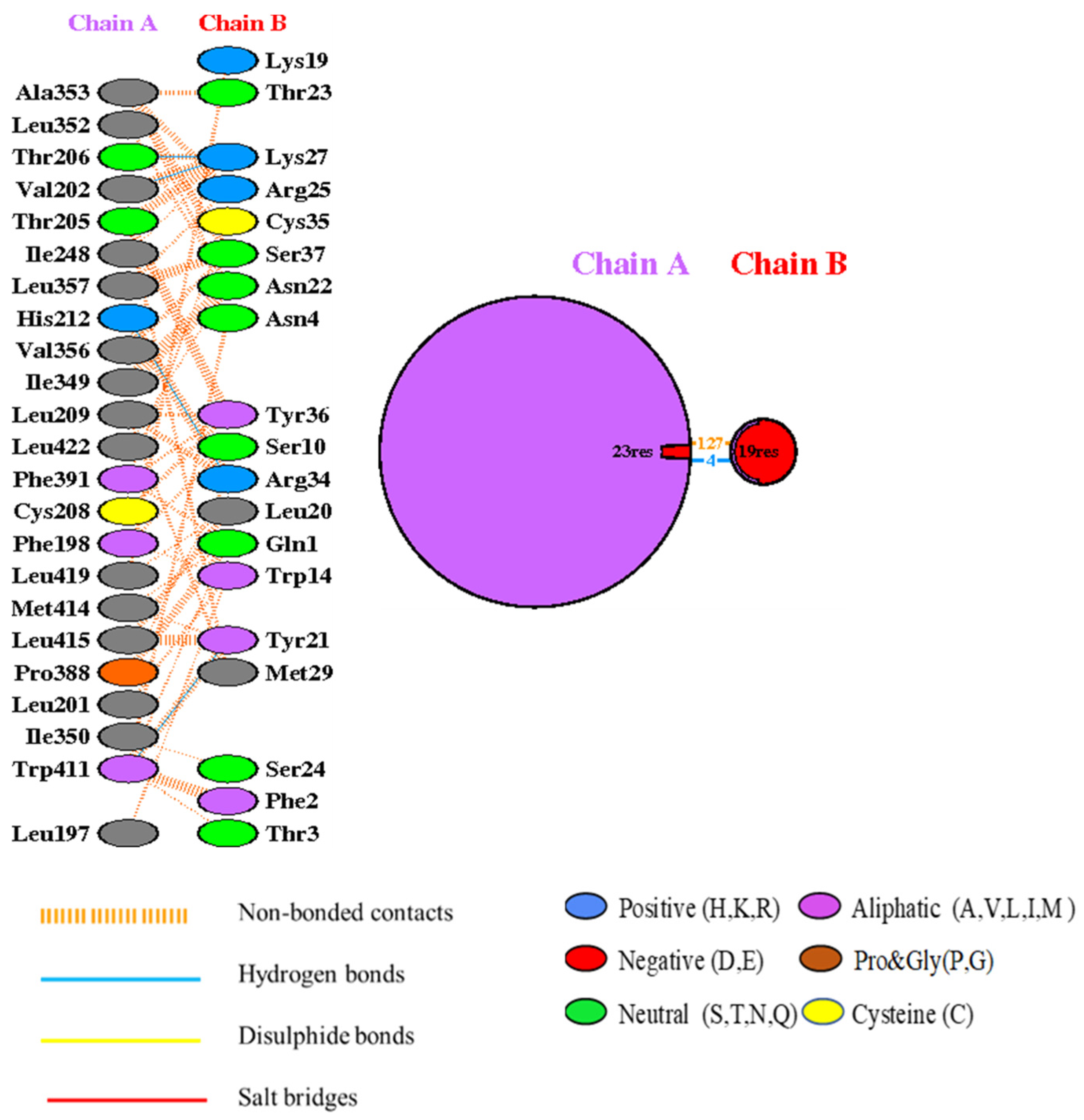
2.5. Docking Analysis
2.6. Antibacterial and Cytotoxicity Activities
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Collection and Identification
4.3. DNA Extraction and Sequencing
4.4. Phylogenetic Analysis
4.5. Nematocysts Isolation and Venom Extraction
4.6. Determination of Protein Concentration
4.7. SDS-PAGE Analysis and LC-Ms/MS Analysis
4.8. Antibacterial Activity
4.9. Cytotoxicity Studies
4.10. Functional Analysis of Neurotoxicity through Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
 ); (C) side view; (D) oral arms; (E) subumbrella region (arrow).
); (C) side view; (D) oral arms; (E) subumbrella region (arrow).
 ); (C) side view; (D) oral arms; (E) subumbrella region (arrow).
); (C) side view; (D) oral arms; (E) subumbrella region (arrow).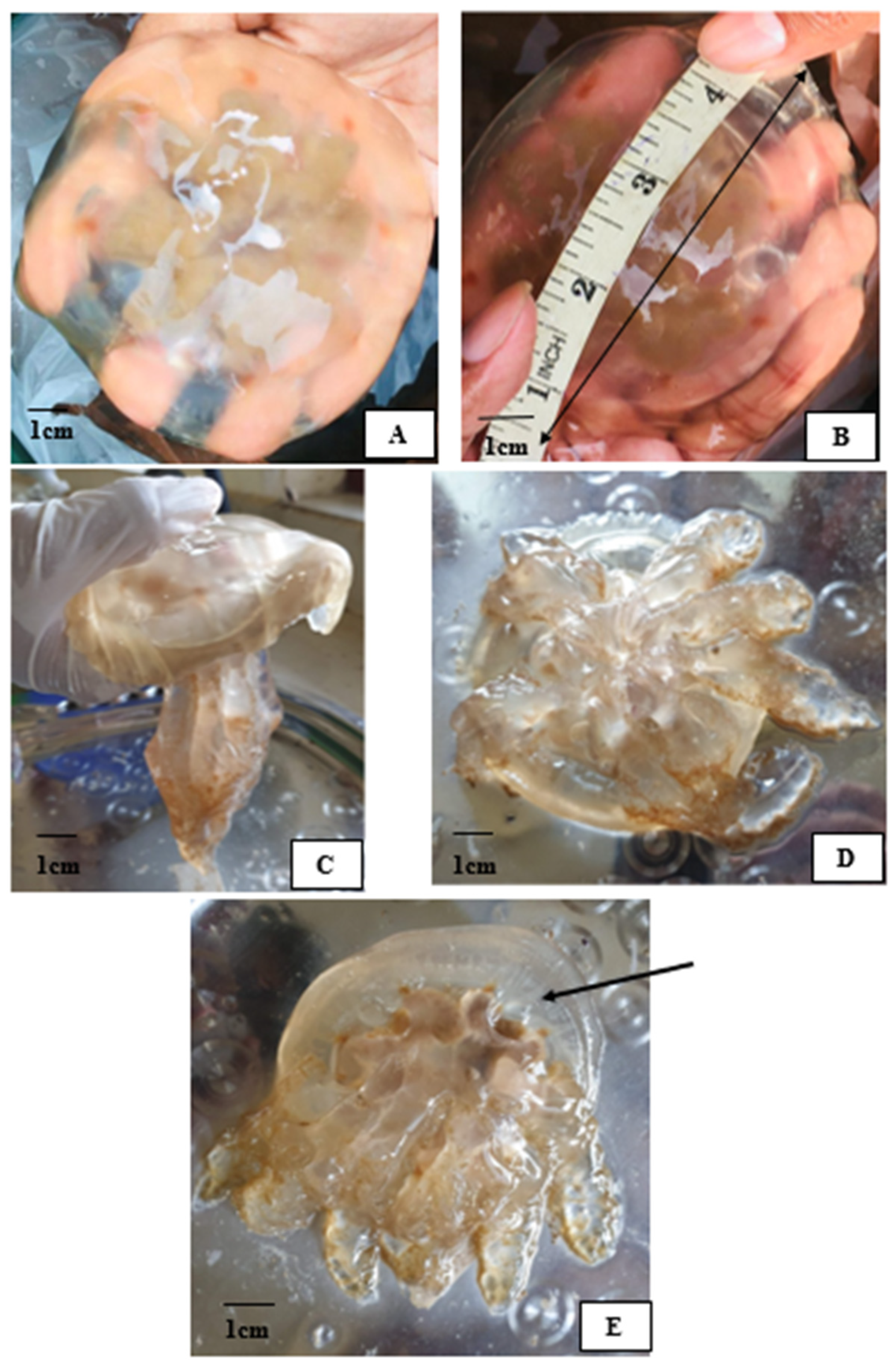
References
- Cheng, K. Learning in Cnidaria: A Systematic Review. Learn. Behav. 2021, 49, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.; McClain, M.; Rubinstein, B.; Sabin, K.Z.; McKinney, S.A.; Gibson, M.C. The Architecture and Operating Mechanism of a Cnidarian Stinging Organelle. Nat. Commun. 2022, 13, 3494. [Google Scholar] [CrossRef] [PubMed]
- Ozacmak, V.H.; Arrieta, A.R.; Thorington, G.U.; Hessinger, D.A. N-Acetyl Neuraminic Acid (NANA) Activates L-Type Calcium Channels on Isolated Tentacle Supporting Cells of the Sea Anemone (Aiptasia pallida). Biol. Bull. 2021, 241, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Oppong-Danquah, E.; Miranda, M.; Blümel, M.; Tasdemir, D. Bioactivity Profiling and Untargeted Metabolomics of Microbiota Associated with Mesopelagic Jellyfish Periphylla Periphylla. Mar. Drugs 2023, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Amreen Nisa, S.; Vinu, D.; Krupakar, P.; Govindaraju, K.; Sharma, D.; Vivek, R. Jellyfish Venom Proteins and Their Pharmacological Potentials: A Review. Int. J. Biol. Macromol. 2021, 176, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yu, H.; Li, R.; Yue, Y.; Yu, C.; Geng, H.; Liu, S.; Xing, R.; Li, P. Jellyfish Nemopilema Nomurai Causes Myotoxicity through the Metalloprotease Component of Venom. Biomed. Pharmacother. 2022, 151, 113192. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, C.; Li, R.; Xing, R.; Liu, S.; Li, P. Factors Influencing Hemolytic Activity of Venom from the Jellyfish Rhopilema Esculentum Kishinouye. Food Chem. Toxicol. 2007, 45, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, H.; Li, C.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Isolation and Characterization of Venom from Nematocysts of Jellyfish Rhopilema Esculentum Kishinouye. Chin. J. Oceanol. Limnol. 2009, 27, 869–874. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, Y.C.; Kim, E. Jellyfish Venom and Toxins: A Review. In Marine and Freshwater Toxins; Springer: Dordrecht, The Netherlands, 2015; pp. 1–14. [Google Scholar]
- Suganthi, K.; Bragadeeswaran, S. Antimicrobial and Immunomodulatory Activities of Jellyfish (Chrysaora quinquecirrha) Venom. In Prospects in Bioscience: Addressing the Issues; Springer: Delhi, India, 2012; pp. 283–292. [Google Scholar]
- Miyake, M.; Aungtonya, C.; Hoe, C.C.; Metillo, E.B.; Iesa, I.; Arsiranant, I.; Karunarathne, K.D.; Densing, L.A.F.; de Croos, M.D.S.T.; Yap, N.W.L.; et al. Field Guide to the Jellyfish in Western Pacific; Tan, A., Ed.; Centre for Marine and Coastal Studies, Universiti Sains Malaysia: Penang, Malaysia, 2021. [Google Scholar]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Belmar, M.; Milisenda, G.; Basso, L.; Doyle, T.K.; Leone, A.; Piraino, S. Jellyfish Impacts on Marine Aquaculture and Fisheries. Rev. Fish. Sci. Aquac. 2021, 29, 242–259. [Google Scholar] [CrossRef]
- Ruiz-Frau, A. Impacts of Jellyfish Presence on Tourists’ Holiday Destination Choices and Their Willingness to Pay for Mitigation Measures. J. Environ. Plan. Manag. 2023, 66, 2107–2125. [Google Scholar] [CrossRef]
- Mianzan, H.W.; Cornelius, P.F.S. Cubomedusae and Scyphomedusae, 1st ed.; Boltovskoy, D., Ed.; Packhuys Publishers: Leiden, The Netherlands, 1999; Volume 1, pp. 513–559. [Google Scholar]
- Silveira, F.L.; Cornelius, P.F.S. Novas Observações Sobre Medusas (Cnidaria, Scyphozoa, Rhizostomeae) No Nordeste e Sul Do. Brasil. Acta Biolog. Leopoldensia 2000, 22, 9–18. [Google Scholar]
- Morandini, A.C. Case 3485 Lychnorhiza Lucerna Haeckel, 1880 (Cnidaria, Scyphozoa, Rhizostomeae): Proposed Conservation of Generic and Specific Names. Bull. Zool. Nomencl. 2009, 66, 242–246. [Google Scholar] [CrossRef]
- Riyas, A.; Kumar, A.B.; Vakani, B. First Record of Rhizostome Jellyfish Catostylus Perezi Ranson 1945 (Cnidaria: Scyphozoa) from the Indian Coast. Thalass. Int. J. Mar. Sci. 2019, 35, 519–524. [Google Scholar] [CrossRef]
- Kramp, P.L. Synopsis of the Medusae of the World. J. Mar. Biol. Assoc. UK 1961, 40, 1–469. [Google Scholar] [CrossRef]
- Wiltshire, C.J.; Sutherland, S.K.; Fenner, P.J.; Young, A.R. Optimization and Preliminary Characterization of Venom Isolated from 3 Medically Important Jellyfish: The Box (Chironex fleckeri), Irukandji (Carukia barnesi), and Blubber (Catostylus mosaicus) Jellyfish. Wilderness Environ. Med. 2000, 11, 241–250. [Google Scholar] [CrossRef]
- Jouiaei, M. Evolution and Diversification of Cnidarian Venom System; The Unvrsity of Queensland: St Lucia, Australia, 2016. [Google Scholar]
- Yue, Y.; Yu, H.; Li, R.; Liu, S.; Xing, R.; Li, P. Insights into Individual Variations in Nematocyst Venoms from the Giant Jellyfish Nemopilema Nomurai in the Yellow Sea. Sci. Rep. 2019, 9, 3361. [Google Scholar] [CrossRef]
- Zare, A.; Afshar, A.; Khoradmehr, A.; Baghban, N.; Mohebbi, G.; Barmak, A.; Daneshi, A.; Bargahi, A.; Nabipour, I.; Almasi-Turk, S.; et al. Chemical Compositions and Experimental and Computational Modeling of the Anticancer Effects of Cnidocyte Venoms of Jellyfish Cassiopea andromeda and Catostylus mosaicus on Human Adenocarcinoma A549 Cells. Mar. Drugs 2023, 21, 168. [Google Scholar] [CrossRef]
- Dissanayake, I.H.; Bandaranayake, U.; Keerthirathna, L.R.; Manawadu, C.; Silva, R.M.; Mohamed, B.; Rizwan, A.; Peiris, D.C. Integration of in Vitro and In-Silico Analysis of Caulerpa racemosa against Antioxidant, Antidiabetic, and Anticancer Activities. Sci. Rep. 2022, 12, 20848. [Google Scholar] [CrossRef]
- Perera, D.D.B.D.; Perera, K.M.L.; Peiris, D.C. A Novel In Silico Benchmarked Pipeline Capable of Complete Protein Analysis: A Possible Tool for Potential Drug Discovery. Biology 2021, 10, 1113. [Google Scholar] [CrossRef]
- AlShammari, A.K.; Abd El-Aziz, T.M.; Al-Sabi, A. Snake Venom: A Promising Source of Neurotoxins Targeting Voltage-Gated Potassium Channels. Toxins 2023, 16, 12. [Google Scholar] [CrossRef]
- Liao, Q.; Feng, Y.; Yang, B.; Lee, S.M.-Y. Cnidarian Peptide Neurotoxins: A New Source of Various Ion Channel Modulators or Blockers against Central Nervous Systems Disease. Drug Discov. Today 2019, 24, 189–197. [Google Scholar] [CrossRef]
- Azila, N.; Siao, F.K.; Othman, I. Haemolytic, Oedema and Haemorrhage Inducing Activities of Tentacular Extract of the Blubber Jellyfish (Catostylus mosaicus). Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 99, 153–156. [Google Scholar] [CrossRef]
- Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological Applications of Scyphomedusae. Mar. Drugs 2019, 17, 604. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (Box Jellyfish) Venom Proteins. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef]
- Morales-Landa, J.L.; Zapata-Pérez, O.; Cedillo-Rivera, R.; Segura-Puertas, L.; Simá-Alvarez, R.; Sánchez-Rodríguez, J. Antimicrobial, Antiprotozoal, and Toxic Activities of Cnidarian Extracts from the Mexican Caribbean Sea. Pharm. Biol. 2007, 45, 37–43. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Panteleev, P.V.; Balandin, S.V.; Gizatullina, A.K.; Altukhov, D.A.; Finkina, E.I.; Kokryakov, V.N.; Arseniev, A.S.; Ovchinnikova, T.V. Recombinant Expression and Solution Structure of Antimicrobial Peptide Aurelin from Jellyfish Aurelia aurita. Biochem. Biophys. Res. Commun. 2012, 429, 63–69. [Google Scholar] [CrossRef]
- Xia, W.; Li, H.; Cheng, W.; Li, H.; Mi, Y.; Gou, X.; Liu, Y. High-Quality Genome Assembly of Chrysaora quinquecirrha Provides Insights into the Adaptive Evolution of Jellyfish. Front. Genet. 2020, 11, 535. [Google Scholar] [CrossRef]
- Kitamura, M.; Omori, M. Synopsis of Edible Jellyfish Collected from Southeast Asia with Notes on Jellyfish Fisheries. Plankton Benthos Res. 2010, 5, 106–118. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Dimopoulos, E.A.; Carmagnini, A.; Velsko, I.M.; Warinner, C.; Larson, G.; Frantz, L.A.F.; Irving-Pease, E.K. HAYSTAC: A Bayesian Framework for Robust and Rapid Species Identification in High-Throughput Sequencing Data. PLoS Comput. Biol. 2022, 18, e1010493. [Google Scholar] [CrossRef]
- McHugh, A.; Yablonsky, C.; Binford, G.; Agnarsson, I. Molecular Phylogenetics of Caribbean Micrathena (Araneae: Araneidae) Suggests Multiple Colonisation Events and Single Island Endemism. Invertebr. Syst. 2014, 28, 337. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nagai, H.; Takuwa, K.; Nakao, M.; Sakamoto, B.; Crow, G.L.; Nakajima, T. Isolation and Characterization of a Novel Protein Toxin from the Hawaiian Box Jellyfish (Sea Wasp) Carybdea alata. Biochem. Biophys. Res. Commun. 2000, 275, 589–594. [Google Scholar] [CrossRef]
- Dissanayake, D.M.I.H.; Perera, D.D.B.D.; Keerthirathna, L.R.; Heendeniya, S.; Anderson, R.J.; Williams, D.E.; Peiris, L.D.C. Antimicrobial Activity of Plumbago indica and Ligand Screening of Plumbagin against Methicillin-Resistant Staphylococcus aureus. J. Biomol. Struct. Dyn. 2020, 40, 3273–3284. [Google Scholar] [CrossRef]
- Heendeniya, S.N.; Keerthirathna Lakshika, R.; Manawadu, C.K.; Dissanayake, I.H.; Ali, R.; Mashhour, A.; Alzahrani, H.; Godakumbura, P.; Boudjelal, M.; Peiris, D.C. Therapeutic Efficacy of Nyctanthes arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Meta. Biomolecules 2020, 10, 165. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A Web Server for Predicting the Binding Affinity of Protein–Protein Complexes. Bioinformatics 2016, 32, btw514. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Macarthur, M.W.; Thornton, J.M. PROCHECK: Validation of Protein-Structure Coordinates; Wiley: Hoboken, NJ, USA, 2012; Chapter 21.4; pp. 684–687. [Google Scholar]


| Peptide | Accession Number | Molecular wt. (Da) | Organism | Mode of Action |
|---|---|---|---|---|
| Toxin KTx 12.5 | P0CH12 | 6720 | Lychas mucronatus | Potassium ion channel inhibitor |
| Toxin BmKaTx10 | Q9NJC5 | 9374 | Mesobuthus martensii | Sodium ion channel inhibitor |
| Turripeptide VIII-01 | D5KXH3 | 16,124 | Gemmula speciosa | Neurotoxic |
| Basic phospholipase A2 sistruxin B | Q6EER2 | 15,844 | Sistrurus tergeminus | Phospholipase A2 |
| Phospholipase A2 | Q45Z47 | 16,104 | Oxyuranus scutellatus | Phospholipase A2 |
| Phospholipase A1 | Q9U6W0 | 33,484 | Polistes annularis | Phospholipase A1 |
| Snake venom serine protease pictobin | U5YCR8 | 27,783 | Bothrops pictus | Serine protease |
| Thrombin-like enzyme elegaxobin1 | P84788 | 25,440 | Protobothrops elegans | Serine protease |
| Venom factor | J3S836 | 184,923 | Crotalus adamanteus | Proteinase inhibitor |
| Toxin CrTX-A | Q9GV72 | 49,392 | Carybdea rastonii | Pore-forming toxin |
| Small cysteine-rich protein 1 2 | C0H694 | 8892 | Montipora capitata | Venom allergen |
| Putative antimicrobial peptide 7848 | L0GCJ6 | 8854 | Urodacus yaschenkoi | Antimicrobial |
| Strain | Inhibitory Diameter (mm) |
|---|---|
| Bacillus cereus | - |
| Staphylococcus aureus | - |
| Klebsiella pneumonia | 12 ± 0.06 |
| Pseudomonas aeruginosa | - |
| Escherichia coli | - |
| Ampicillin (standard drug) | 19 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edirisinghe, E.A.H.W.; Athukorala, B.N.; Perera, M.; Abeywardana, B.A.S.D.; Sigera, P.S.T.; Eranga, P.; Theekshana, K.D.; Boudjelal, M.; Ali, R.; Peiris, D.C. Jellyfish Venom Peptides Targeting Human Potassium Channels Identified through Ligand Screening: Morphometric and Molecular Identification of the Species and Antibiotic Potential. Mar. Drugs 2024, 22, 333. https://doi.org/10.3390/md22080333
Edirisinghe EAHW, Athukorala BN, Perera M, Abeywardana BASD, Sigera PST, Eranga P, Theekshana KD, Boudjelal M, Ali R, Peiris DC. Jellyfish Venom Peptides Targeting Human Potassium Channels Identified through Ligand Screening: Morphometric and Molecular Identification of the Species and Antibiotic Potential. Marine Drugs. 2024; 22(8):333. https://doi.org/10.3390/md22080333
Chicago/Turabian StyleEdirisinghe, Edirisinghe Arachchige Hashini Wasthala, Buddhima Nirmani Athukorala, Minoli Perera, Bothunga Arachchige Shamali Dilhara Abeywardana, Polgahawattage Sachini Tarushika Sigera, Pasindu Eranga, Kavindu Dinuhara Theekshana, Mohamad Boudjelal, Rizwan Ali, and Dinithi Champika Peiris. 2024. "Jellyfish Venom Peptides Targeting Human Potassium Channels Identified through Ligand Screening: Morphometric and Molecular Identification of the Species and Antibiotic Potential" Marine Drugs 22, no. 8: 333. https://doi.org/10.3390/md22080333






