Marine Algae and Deriving Biomolecules for the Management of Inflammatory Bowel Diseases: Potential Clinical Therapeutics to Decrease Gut Inflammatory and Oxidative Stress Markers?
Abstract
:1. Introduction
2. Search Strategy
Methods
3. Pathophysiology of IBD
4. Inflammatory and Oxidative Stress Markers Related to IBD
4.1. Cytokines Related to the Inflammatory Response in IBD
4.2. NF-κB Pathway in IBD
4.3. Oxidative Stress Markers in IBD
4.4. Trefoil Factor 3 (TFF3)
4.5. Lactoferrin
4.6. SIRT1
5. Marine Ecosystem: A Useful Tool in the Management of IBD Symptomatology
5.1. Focusing on Marine Algae in the Treatment of IBD
5.1.1. Macroalgae
5.1.2. Microalgae
6. Pharmacokinetic Properties of Marine Algae-Derived Compounds
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Roda, G.; Ng, S.C.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Primers 2020, 6, 22. [Google Scholar] [CrossRef]
- Alemany-Cosme, E.; Sáez-González, E.; Moret, I.; Mateos, B.; Iborra, M.; Nos, P.; Sandoval, J.; Beltrán, B. Oxidative stress in the pathogenesis of Crohn’s disease and the interconnection with immunological response, microbiota, external environmental factors, and epigenetics. Antioxidants 2021, 10, 64. [Google Scholar] [CrossRef]
- Stroie, T.; Preda, C.; Meianu, C.; Croitoru, A.; Gheorghe, L.; Gheorghe, C.; Diculescu, M. Health-related quality of life in patients with inflammatory bowel disease in clinical remission: What should we look for? Medicina 2022, 58, 486. [Google Scholar] [CrossRef] [PubMed]
- Núñez, P.; Cleveland, N.K.; Quera, R.; Rubin, D.T. Evolving role of endoscopy in inflammatory bowel disease: Going beyond diagnosis. World J. Gastroenterol. 2021, 27, 2521. [Google Scholar] [CrossRef] [PubMed]
- de Chambrun, G.P.; Blanc, P.; Peyrin-Biroulet, L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cheon, J.H. Updates on conventional therapies for inflammatory bowel diseases: 5-aminosalicylates, corticosteroids, immunomodulators, and anti-TNF-α. Korean J. Intern. Med. 2022, 37, 895. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory bowel disease: Clinical diagnosis and surgical treatment-overview. Medicina 2022, 58, 567. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.C.; Guadagni, I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig. Liver Dis. 2021, 53, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Vebr, M.; Pomahačová, R.; Sýkora, J.; Schwarz, J. A Narrative Review of Cytokine Networks: Pathophysiological and Therapeutic Implications for Inflammatory Bowel Disease Pathogenesis. Biomedicines 2023, 11, 3229. [Google Scholar] [CrossRef]
- Ardizzone, A.; Capra, A.P.; Repici, A.; Lanza, M.; Bova, V.; Palermo, N.; Paterniti, I.; Esposito, E. Rebalancing NOX2/Nrf2 to limit inflammation and oxidative stress across gut-brain axis in migraine. Free. Radic. Biol. Med. 2024, 213, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Repici, A.; Capra, A.P.; De Gaetano, F.; Bova, V.; Casili, G.; Campolo, M.; Esposito, E. Efficacy of the Radical Scavenger, Tempol, to reduce inflammation and oxidative stress in a murine model of atopic dermatitis. Antioxidants 2023, 12, 1278. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics efficacy on oxidative stress values in inflammatory bowel disease: A randomized double-blinded placebo-controlled pilot study. Endocr. Metab. Immune Disord. Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2019, 19, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah-Bodaghi, M.; Maleki, I.; Agah, S.; Hekmatdoost, A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement. Ther. Med. 2019, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, G.; Zhao, H.; Dong, N.; Wu, B.; Chen, Y.; Lu, Q. Natural-derived polysaccharides from plants, mushrooms, and seaweeds for the treatment of inflammatory bowel disease. Front. Pharmacol. 2021, 12, 651813. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Ermakova, S.P. Extracts and marine algae polysaccharides in therapy and prevention of inflammatory diseases of the intestine. Mar. Drugs 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kamp, K.; Dudley-Brown, S.; Heitkemper, M.; Wyatt, G.; Given, B. Symptoms among emerging adults with inflammatory bowel disease: A descriptive study. Res. Nurs. Health 2020, 43, 48–55. [Google Scholar] [CrossRef]
- Parente, P.; Pastore, M.; Grillo, F.; Fassan, M.; Francalanci, P.; Dirodi, A.; Rossi, C.; Arpa, G.; De Angelis, P.; Gullo, I.; et al. Very Early Onset-IBD: Evidence for the need of a multidisciplinary approach. Pathologica 2022, 114, 3. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Garrone, A.; Bertolino, C.; Vanni, R.; Bretto, E.; Poshnjari, A.; Tribocco, E.; Frara, S.; Armandi, A.; Astegiano, M.; et al. Epidemiology of inflammatory bowel diseases: A population study in a healthcare district of North-West Italy. J. Clin. Med. 2023, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, S.M. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front. Pediatr. 2023, 10, 1103713. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The cost of inflammatory bowel disease: An initiative from the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar] [PubMed]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef]
- Loftus, E.; Silverstein, M.; Sandborn, W.; Tremaine, W.; Harmsen, W.; Zinsmeister, A.R. Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: Incidence, prevalence, and survival. Gut 2000, 46, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Mahid, S.S.; Minor, K.S.; Soto, R.E.; Hornung, C.A.; Galandiuk, S. Smoking and inflammatory bowel disease: A meta-analysis. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1462–1471. [Google Scholar]
- Tayyem, R.F.; Qalqili, T.R.; Ajeen, R.; Rayyan, Y.M. Dietary patterns and the risk of inflammatory bowel disease: Findings from a case-control study. Nutrients 2021, 13, 1889. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S. Dysbiosis in inflammatory bowel disease: Pathogenic role and potential therapeutic targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.-F.; Liang, Y.-J.; Xu, J.; Xu, H.-M.; Nie, Y.-Q.; Wang, L.-S.; Yao, J.; Li, D.-F. Immunology of inflammatory bowel disease: Molecular mechanisms and therapeutics. J. Inflamm. Res. 2022, 1825–1844. [Google Scholar] [CrossRef]
- Nanini, H.F.; Bernardazzi, C.; Castro, F.; de Souza, H.S.P. Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J. Gastroenterol. 2018, 24, 4622. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 1–28. [Google Scholar] [CrossRef]
- Neurath, M.F.; Leppkes, M. Resolution of ulcerative colitis. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 747–756. [Google Scholar]
- Brown, S.J.; Mayer, L. The immune response in inflammatory bowel disease. Off. J. Am. Coll. Gastroenterol. | ACG 2007, 102, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef]
- Tavakoli, P.P.; Vollmer-Conna, U.; Hadzi-Pavlovic, D.; Grimm, M.C. A review of inflammatory bowel disease: A model of microbial, immune and neuropsychological integration. Public Health Rev. 2021, 42, 1603990. [Google Scholar] [CrossRef] [PubMed]
- Niessner, M.; Volk, B. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin. Exp. Immunol. 1995, 101, 428–435. [Google Scholar] [CrossRef]
- Funderburg, N.T.; Park, S.R.S.; Sung, H.C.; Hardy, G.; Clagett, B.; Ignatz-Hoover, J.; Harding, C.V.; Fu, P.; Katz, J.A.; Lederman, M.M.; et al. Circulating CD 4+ and CD 8+ T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 2013, 140, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory bowel disease: Mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.A.; Schmidt, P.T.; Lang-Schwarz, C.; Vieth, M. Branching crypts in inflammatory bowel disease revisited. J. Gastroenterol. Hepatol. 2022, 37, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Johnson, K.; Yin, J.; Lee, S.; Lin, R.; Yu, H.; In, J.; Foulke-Abel, N.C.; Zachos, M.; Donowitz, M.; et al. Donowitz, Chronic inflammation in ulcerative colitis causes long-term changes in goblet cell function. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Shin-Ya, M.; Naito, Y.; Kishida, T.; Ito, R.; Suzuki, N.; Yasuda, H.; Sakagami, J.; Imanishi, J.; Kataoka, K.; et al. Early-stage blocking of Notch signaling inhibits the depletion of goblet cells in dextran sodium sulfate-induced colitis in mice. J. Gastroenterol. 2010, 45, 608–617. [Google Scholar] [CrossRef]
- Shanahan, F. Inflammatory bowel disease: Immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology 2001, 120, 622–635. [Google Scholar] [CrossRef]
- Tibble, J.; Bjarnason, I. Non-invasive investigation of inflammatory bowel disease. World J. Gastroenterol. 2001, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, D.; Bockemühl, M.; Ramadori, G. Quantitative measurement of cytokine mRNA in inflammatory bowel disease: Relation to clinical and endoscopic activity and outcome. Eur. J. Gastroenterol. Hepatol. 2005, 17, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Poullis, A.; Foster, R.; Northfield, T.; Mendall, M. Faecal markers in the assessment of activity in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2002, 16, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Umehara, Y.; Kudo, M.; Nakaoka, R.; Kawasaki, T.; Shiomi, M. Serum proinflammatory cytokines and adhesion molecules in ulcerative colitis. Hepato-Gastroenterol. 2006, 53, 879–882. [Google Scholar]
- Yen, D.; Cheung, J.; Scheerens, H.; Poulet, F.; McClanahan, T.; McKenzie, B.; Kleinschek, M.A.; Owyang, A.; Mattson, J.; Blumenschein, W.; et al. IL-23 is essential for T cell–mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Investig. 2006, 116, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Pallone, F.; Monteleone, G. Interleukin-23 and Th17 cells in the control of gut inflammation. Mediat. Inflamm. 2009, 1, 297645. [Google Scholar] [CrossRef]
- Lucaciu, L.A.; Ilieș, M.; Vesa, Ș.C.; Seicean, R.; Din, S.; Iuga, C.A.; Seicean, A. Serum interleukin (IL)-23 and IL-17 profile in inflammatory bowel disease (IBD) patients could differentiate between severe and non-severe disease. J. Pers. Med. 2021, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Korta, A.; Kula, J.; Gomułka, K. The role of IL-23 in the pathogenesis and therapy of inflammatory bowel disease. Int. J. Mol. Sci. 2023, 24, 10172. [Google Scholar] [CrossRef]
- Ihara, S.; Hirata, Y.; Koike, K. TGF-β in inflammatory bowel disease: A key regulator of immune cells, epithelium, and the intestinal microbiota. J. Gastroenterol. 2017, 52, 777–787. [Google Scholar] [CrossRef]
- Ban, H.; Andoh, A.; Shioya, M.; Nishida, A.; Tsujikawa, T.; Fujiyama, Y. Increased number of FoxP3+ CD4+ regulatory T cells in inflammatory bowel disease. Mol. Med. Rep. 2008, 1, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, D.C.; Ma, J.; Vacharathit, V.; Shah, P.; Ramakrishnan, A.; Uprety, P.; Shen, Z.; Sheh, A.; Brayton, C.F.; Whary, M.T.; et al. The development of colitis in Il10−/−mice is dependent on IL-22. Mucosal Immunol. 2020, 13, 493–506. [Google Scholar] [CrossRef]
- Begue, B.; Verdier, J.; Rieux-Laucat, F.; Goulet, O.; Morali, A.; Canioni, D.; Hugot, J.-P.; Daussy, C.; Verkarre, V.; Pigneur, B.; et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Off. J. Am. Coll. Gastroenterol. | ACG 2011, 106, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan extracts ameliorate acute colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, Y.; Xing, Y.; Zhu, H.; Shen, J.; Tian, J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia–reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011, 49, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro anti-inflammatory activities of fucoidans from five species of brown seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Yoon, S.Y.; Oh, S.J.; Kim, S.K.; Kang, K.W. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 2006, 346, 345–350. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.; Nagahawatta, D.; Jayawardena, T.U.; Jeon, Y.-J. The role of seaweed polysaccharides in gastrointestinal health: Protective effect against inflammatory bowel disease. Life 2023, 13, 1026. [Google Scholar] [CrossRef]
- Phull, A.-R.; Majid, M.; Haq, I.-u.; Khan, M.R.; Kim, S.J. In Vitro and In Vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Park, J.; Cha, J.-D.; Choi, K.-M.; Lee, K.-Y.; Han, K.M.; Jang, Y.-S. Fucoidan inhibits LPS-induced inflammation In Vitro and during the acute response In Vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Herath, K.H.; Yang, H.-W.; Choi, C.S.; Jeon, Y.-J. Anti-inflammatory mechanisms of fucoidans to treat inflammatory diseases: A review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-H.; Shiue, S.-J.; Chen, C.-N.; Cheng, S.-W.; Lin, H.-Y.; Wu, L.-W.; Wu, M.-S. Fucoidan and fucoxanthin attenuate hepatic steatosis and inflammation of NAFLD through modulation of leptin/adiponectin axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- McFadden, B.A.; Vincenty, C.S.; Chandler, A.J.; Cintineo, H.P.; Lints, B.S.; Mastrofini, G.F.; Arent, S.M. Effects of fucoidan supplementation on inflammatory and immune response after high-intensity exercise. J. Int. Soc. Sports Nutr. 2023, 20, 2224751. [Google Scholar] [CrossRef]
- Yeh, C.-W.; Shih, C.-J.; Liu, T.-C.; Chiou, Y.-L. Effects of oligo-fucoidan on the immune response, inflammatory status and pulmonary function in patients with asthma: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2022, 12, 18150. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Cheng, J.; Xu, L.; Hood, M.; Zhong, D.; Cheng, M.; Liu, Y.; Chen, L.; Du, J. The combination of wheat peptides and fucoidan protects against chronic superficial gastritis and alters gut microbiota: A double-blinded, placebo-controlled study. Eur. J. Nutr. 2020, 59, 1655–1666. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Zhang, Y.; Zhang, Y.; Jiang, Z.-P.; Cui, Y.-L.; Wang, Q.-S. ROS-responsive thioketal-linked alginate/chitosan carriers for irritable bowel syndrome with diarrhea therapy. Int. J. Biol. Macromol. 2022, 209, 70–82. [Google Scholar] [CrossRef]
- You, L.; Gong, Y.; Li, L.; Hu, X.; Brennan, C.; Kulikouskaya, V. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: An overview. Int. J. Food Sci. Technol. 2020, 55, 1199–1206. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 1–37. [Google Scholar] [CrossRef]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging roles for noncanonical NF-κB signaling in the modulation of inflammatory bowel disease pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Roessner, A.; Kuester, D.; Malfertheiner, P.; Schneider-Stock, R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol.-Res. Pract. 2008, 204, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Mishra, N.; Garg, A.; Sibuh, B.Z.; Taneja, P.; Rai, G.; Djearamane, S.; Wong, L.S.; Al-Dayan, N.; Roychoudhury, S.; et al. Recent updates on correlation between reactive oxygen species and synbiotics for effective management of ulcerative colitis. Front. Nutr. 2023, 10, 1126579. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Sies, H. On the history of oxidative stress: Concept and some aspects of current development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Branco, C.D.S.; de Lima, É.D.; Rodrigues, T.S.; Scheffel, T.B.; Scola, G.; Laurino, C.C.F.C.; Moura, S.; Salvador, M. Mitochondria and redox homoeostasis as chemotherapeutic targets of Araucaria angustifolia (Bert.) O. Kuntze in human larynx HEp-2 cancer cells. Chem.-Biol. Interact. 2015, 231, 108–118. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Langhorst, J.; Elsenbruch, S.; Mueller, T.; Rueffer, A.; Spahn, G.; Michalsen, A.; Dobos, G.J. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm. Bowel Dis. 2005, 11, 1085–1091. [Google Scholar] [CrossRef]
- Srivastava, S.; Kedia, S.; Kumar, S.; Mouli, V.P.; Dhingra, R.; Sachdev, V.; Tiwari, V.; Kurrey, L.; Pradhan, R.; Ahuja, V. Serum human trefoil factor 3 is a biomarker for mucosal healing in ulcerative colitis patients with minimal disease activity. J. Crohn’s Colitis 2015, 9, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, A.A.; El-Fayoumy, M.S.; El-Sherbiny, A.A.; El-shorbagy, M.S.; El-ghamry, F.A. Predictive value of trefoil factor 3 for identifying activity in ulcerative colitis patients: A comparison with fecal calprotectin and C-reactive protein. J. Recent Adv. Med. 2023, 4, 109–118. [Google Scholar] [CrossRef]
- Nakov, R.; Velikova, T.; Nakov, V.; Ianiro, G.; Gerova, V.; Tankova, L. Serum trefoil factor 3 predicts disease activity in patients with ulcerative colitis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 788–794. [Google Scholar] [PubMed]
- Kane, S.V.; Sandborn, W.J.; Rufo, P.A.; Zholudev, A.; Boone, J.; Lyerly, D.; Camilleri, M.; Hanauer, S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Off. J. Am. Coll. Gastroenterol. | ACG 2003, 98, 1309–1314. [Google Scholar] [CrossRef]
- Costa, F.; Mumolo, M.; Ceccarelli, L.; Bellini, M.; Romano, M.; Sterpi, C.; Ricchiuti, A.; Marchi, S.; Bottai, M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005, 54, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Land, M.; Cook, T.; Boone, J.; Lyerly, D.; Rufo, P. Serial fecal lactoferrin measurements are useful in the interval assessment of patients with active and inactive inflammatory bowel disease. In Gastroenterology; Elsevier: Amsterdam, The Netherlands, 2004; p. A215. [Google Scholar]
- Langhorst, J.; Elsenbruch, S.; Koelzer, J.; Rueffer, A.; Michalsen, A.; Dobos, G.J. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Off. J. Am. Coll. Gastroenterol. | ACG 2008, 103, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.; Singh, N.; Jaggi, A.S. Dual role of sirtuin 1 in inflammatory bowel disease. Immunopharmacol. Immunotoxicol. 2020, 42, 385–391. [Google Scholar] [CrossRef]
- Caruso, R.; Marafini, I.; Franzè, E.; Stolfi, C.; Zorzi, F.; Monteleone, I.; Caprioli, F.; Colantoni, A.; Sarra, M.; Sedda, S.; et al. Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol. 2014, 7, 1467–1479. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Son, S.-U.; Suh, H.J.; Shin, K.-S. Characterization of a novel sulfated-rhamnoglucuronan isolated from Korean seaweed Ulva pertusa and its efficacy for treatment of inflammatory bowel disease in mice. Carbohydr. Polym. 2024, 342, 122373. [Google Scholar] [CrossRef]
- Pereira, J.G.; Mesquita, J.X.; Aragão, K.S.; Franco, Á.X.; Souza, M.H.; Brito, T.V.; Dias, J.M.; Silva, R.O.; Medeiros, J.-V.R.; Oliveira, J.S.; et al. Polysaccharides isolated from Digenea simplex inhibit inflammatory and nociceptive responses. Carbohydr. Polym. 2014, 108, 17–25. [Google Scholar] [CrossRef]
- JCarneiro, G.; Rodrigues, J.A.G.; de Sousa Oliveira Vanderlei, E.; Souza, R.B.; Quinderé, A.L.G.; Coura, C.O.; de Araújo, I.W.F.; Chaves, H.V.; Bezerra, M.M.; Benevides, N.M.B. Peripheral antinociception and anti-inflammatory effects of sulphated polysaccharides from the alga Caulerpa mexicana. Basic Clin. Pharmacol. Toxicol. 2014, 115, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.G.; Holanda, T.D.B.L.; Quinderé, A.L.G.; Frota, A.F.; Soares, V.V.M.; Sousa, R.S.D.; Carneiro, M.A.; Martins, D.S.; Duarte, A.S.G.; Benevides, N.M.B. Gastroprotective effects of sulphated polysaccharides from the alga Caulerpa mexicana reducing ethanol-induced gastric damage. Pharmaceuticals 2018, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.-Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 9, e113509. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.F.; Pires, S.; Rupert, A.; Oguntunmibi, S.; Jin, W.-B.; Marderstein, A.; Funez-dePagnier, G.; Maldarelli, G.; Viladomiu, M.; Putzel, G. The gut microbiome regulates the clinical efficacy of sulfasalazine therapy for IBD-associated spondyloarthritis. Cell Rep. Med. 2024, 5, 101431. [Google Scholar] [CrossRef] [PubMed]
- Sudirman, S.; Hsu, Y.-H.; He, J.-L.; Kong, Z.-L. Dietary polysaccharide-rich extract from Eucheuma cottonii modulates the inflammatory response and suppresses colonic injury on dextran sulfate sodium-induced colitis in mice. PLoS ONE 2018, 13, e0205252. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.V.; Barros, F.C.; Silva, R.O.; Júnior, G.J.D.; Júnior, J.S.C.; Franco, Á.X.; Soares, P.M.; Chaves, L.S.; Abreu, C.M.; de Paula, R.C.; et al. Sulfated polysaccharide from the marine algae Hypnea musciformis inhibits TNBS-induced intestinal damage in rats. Carbohydr. Polym. 2016, 151, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; O’Shea, C.; Collins, C.; O’Doherty, J.; Sweeney, T. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon. J. Anim. Sci. 2012, 90, 263–265. [Google Scholar] [CrossRef]
- Ardizzone, A.; Filippone, A.; Mannino, D.; Scuderi, S.A.; Casili, G.; Lanza, M.; Cucinotta, L.; Campolo, M.; Esposito, E. Ulva pertusa, a marine green alga, attenuates DNBS-induced colitis damage via NF-κB/Nrf2/SIRT1 signaling pathways. J. Clin. Med. 2022, 11, 4301. [Google Scholar] [CrossRef]
- Li, Y.; Ye, H.; Wang, T.; Wang, P.; Liu, R.; Li, Y.; Tian, Y.; Zhang, J. Characterization of low molecular weight sulfate ulva polysaccharide and its protective effect against IBD in mice. Mar. Drugs 2020, 18, 499. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, I.W.F.; Rodrigues, J.A.G.; Quinderé, A.L.G.; Silva, J.D.F.T.; de Freitas Maciel, G.; Ribeiro, N.A.; Vanderlei, E.D.S.O.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.A. Analgesic and anti-inflammatory actions on bradykinin route of a polysulfated fraction from alga Ulva lactuca. Int. J. Biol. Macromol. 2016, 92, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Mannino, D.; Capra, A.P.; Repici, A.; Filippone, A.; Esposito, E.; Campolo, M. New insights into the mechanism of Ulva pertusa on colitis in mice: Modulation of the pain and immune system. Mar. Drugs 2023, 21, 298. [Google Scholar] [CrossRef]
- Ma, M.; Fu, T.; Wang, Y.; Zhang, A.; Gao, P.; Shang, Q.; Yu, G. Polysaccharide from edible alga Enteromorpha clathrata improves ulcerative colitis in association with increased abundance of Parabacteroides spp. in the gut microbiota of dextran sulfate sodium-fed mice. Mar. Drugs 2022, 20, 764. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-J.; Bu, Y.; Bae, J.; Bang, Y.-M.; Kim, J.; Lee, H.; Beom-Joon, L.; Hyun, Y.H.; Park, J.-W. Protective effect of Laminaria japonica with probiotics on murine colitis. Mediat. Inflamm. 2014, 2014, 417814. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.A.; Silva, H.M.; Abílio, G.M.; Miranda, G.E.; Moura, A.M.; Araújo-Júnior, J.X.D.; Silveira, E.J.; Santos, B.V.; Souto, J.T. Anti-inflammatory effects of methanolic extract of green algae Caulerpa mexicana in a murine model of ulcerative colitis. Rev. Bras. De Farmacogn. 2015, 25, 677–682. [Google Scholar] [CrossRef]
- Ribeiro, N.A.; Abreu, T.M.; Chaves, H.V.; Bezerra, M.M.; Monteiro, H.S.A.; Jorge, R.J.B.; Benevides, N.M.B. Sulfated polysaccharides isolated from the green seaweed Caulerpa racemosa plays antinociceptive and anti-inflammatory activities in a way dependent on HO-1 pathway activation. Inflamm. Res. 2014, 63, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Velankanni, P.; Go, S.-H.; Jin, J.B.; Park, J.-S.; Park, S.; Lee, S.-B.; Kwon, H.-K.; Pan, C.-H.; Cha, K.H.; Lee, C.-G. Chlorella vulgaris Modulates Gut Microbiota and Induces Regulatory T Cells to Alleviate Colitis in Mice. Nutrients 2023, 15, 3293. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef]
- Tanoue, T.; Nishitani, Y.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. In Vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264. 7 cells. Biochem. Biophys. Res. Commun. 2008, 374, 565–569. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of marine-derived drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, D.; Zhao, X.; Jin, W.; Wang, J.; Zhang, Q. Microanalysis and preliminary pharmacokinetic studies of a sulfated polysaccharide from Laminaria japonica. Chin. J. Oceanol. Limnol. 2016, 34, 177–185. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, E.; Hu, B.; Liang, H.; Song, S.; Ji, A. Study on absorption mechanism and tissue distribution of fucoidan. Molecules 2020, 25, 1087. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The pharmacokinetics of fucoidan after topical application to rats. Mar. Drugs 2019, 17, 687. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar. Drugs 2014, 13, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Zhan, E.; Chu, F.; Zhao, T.; Chai, Y.; Liang, H.; Song, S.; Ji, A. Determination of fucoidan in rat plasma by HPLC and its application in pharmacokinetics. Pak. J. Pharm. Sci. 2020, 33, 1–9. [Google Scholar] [PubMed]
- Irhimeh, M.R.; Fitton, J.; Lowenthal, R.; Kongtawelert, P. A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 705–710. [Google Scholar] [CrossRef]
- Tokita, Y.; Hirayama, M.; Nakajima, K.; Tamaki, K.; Iha, M.; Nagamine, T. Detection of fucoidan in urine after oral intake of traditional Japanese seaweed, Okinawa mozuku (Cladosiphon okamuranus Tokida). J. Nutr. Sci. Vitaminol. 2017, 63, 419–421. [Google Scholar] [CrossRef]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption study of mozuku fucoidan in Japanese volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.; Kouokam, J.C.; Hurst, H.; Palmer, K.E. Pharmacokinetics of the antiviral lectin griffithsin administered by different routes indicates multiple potential uses. Viruses 2016, 8, 331. [Google Scholar] [CrossRef]
- Barton, C.; Kouokam, J.C.; Lasnik, A.B.; Foreman, O.; Cambon, A.; Brock, G.; Montefiori, D.C.; Vojdani, F.; McCormick, A.A.; O’Keefe, B.R.; et al. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Yokose, T.; Yamamoto, Y.; Yamaguchi, K.; Oda, T. Detection and pharmacokinetics of alginate oligosaccharides in mouse plasma and urine after oral administration by a liquid chromatography/tandem mass spectrometry (LC-MS/MS) method. Biosci. Biotechnol. Biochem. 2008, 72, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Hagen, A.; Skjak-Braek, G.; Dornish, M. Pharmacokinetics of sodium alginate in mice. Eur. J. Pharm. Sci. 1996, 4, S100. [Google Scholar] [CrossRef]
- Egorin, M.J.; Sentz, D.L.; Rosen, D.M.; Ballesteros, M.F.; Kearns, C.M.; Callery, P.S.; Eiseman, J.L. Plasma pharmacokinetics, bioavailability, and tissue distribution in CD2F1 mice of halomon, an antitumor halogenated monoterpene isolated from the red algae Portieria hornemannii. Cancer Chemother. Pharmacol. 1996, 39, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing eckol as a therapeutic aid: A systematic review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Petri, D.; Lundebye, A.-K. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in Haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef]
- Obluchinskaya, E.; Pozharitskaya, O.; Flisyuk, E.; Shikov, A. Optimization of the composition and production technology of fucoidan tablets and their biopharmaceutical evaluation. Pharm. Chem. J. 2020, 54, 509–513. [Google Scholar] [CrossRef]

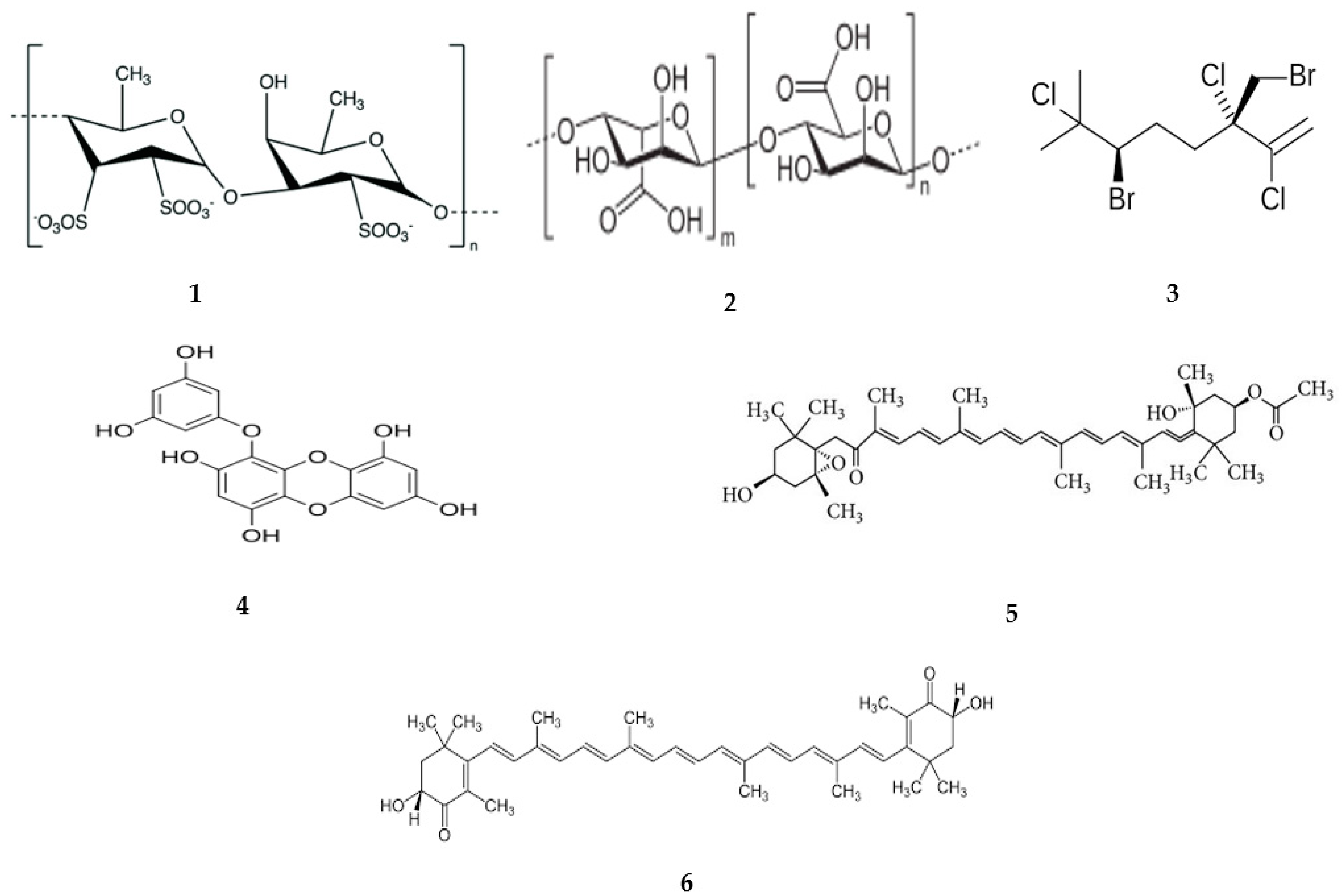
| Type of Algae | Study Design and Concentrations | In Vitro Cell Type | Control | Main Finding | Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Korean seaweeds, Ulva pertusa polysaccharide (UPP) | 1.5 × 106 cells/well of LS174T and Caco-2 was incubated for 24 h (5 % CO2, 37 °C). UUP (1, 10, and 100 μg/mL) were added to the cells and incubated for 24 h. Total mRNA was obtained for further analysis. Culture media was used as a negative control (NC), and propionic acid (5 mM) as a positive control (PC). | LS174T and Caco-2 cells | Culture media, propionic acid 5 mM | ↑ mRNA expression of MUC2, ZO-1, occludin, and TFF3 | Strength Tight junction (TJ) and proliferation and survival of the epithelial cells. | [94] |
| Fucoidan, a sulfated polysaccharide from brown algae | Caco-2 cells were seeded at 3.75 × 105 cells/well, the culture media was changed every 3 days until the cells fully differentiated, and the cells were used in passage number 48–62. RAW264.7 cells were seeded at 8.5 × 106 cells/well and were used in passage number 10–30, 1.5 fucoidan (500 µg/mL) was applied to the apical side for 3 h and LPS 100 ng·mL was added to the basolateral side and after additional 3 h, the basolateral supernatant was collected to be used for marker analysis. | co-cultured Caco-2 and RAW264.7 | Budesonide 1 µM and TNF- α 50 µg/mL | ↓ mRNA expression of IL-8, TNF-α | Inhibition of TNF-α secretion resulted from LPS-stimulated RAW246.7. | [113] |
| Zonarol extracted from the brown algae, Dictyopteris undulata | RAW246.7 cells were seeded at 1 × 105 cell/cm2, and after 24 h LPS 10µg/mL was added with or without zonarol 2 µM, 24 h later the cells were subjected to the required assay. | RAW264.7 | The cells not treated by LPS or zonarol were used as a normal control | ↓ NO, IL-1b, IL-6 and iNOS | Inhibit the inflammatory reaction in the macrophage cell line | [98] |
| Type of Algae | Study Design and Dose | Model | Inducer/Control | Main Findings | Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Korean seaweeds, Ulva pertusa polysaccharide (UPP) | Oral prophylactic Administration of UPP at doses (50, 100, and 200 mg/kg) once daily for three weeks After UPP administration, a 5% dextran sulfate sodium (DSS) was administered for the next eight days to induce UC | Mouse model | Dextran sulfate sodium, mesalazine (100 mg/kg) | ↑ IL-4, IL-10, and IgA, occludin, and claudin-1 ↓ IL-1β, TNF-α, iNOS, IL-6, MPO, ERK, and p38 phosphorylation | Interfere with MAPK and NF-κB signaling pathway leading to inhibition of the inflammatory response-associated signaling pathways. | [94] |
| Depyrogenated fucoidan (DPF) and fucoidan-polyphenol complex (Maritech Synergy) extracted from Fucus vesiculosus | UC was induced by using DSS in the drinking water from day 1 to day 8. then the treatment group received two fucoidan extracts for 7 days, either by oral Synergy or DPF) or intraperitoneal (DPF). | Male C57BL/6 mice | Dextran sulfate sodium/no positive control, the mice that didn’t receive SSD were a healthy control | IP DPF, ↓ −36.4% IL-1α, −28.6% IL-1β, −31.1% IL-10, −52.8% MIP-1α, −43.7% G-GSF, MCP-1 (%NA) and ↑ 58.6% IFN- γ, 115.8% RANTES. Oral DPF, ↓ −55.9% IL-1α, −51.2% IL-1β, −62.2% IL-10, −46.5% MIP-1α, −60.0% MIP-1β, −80.6% G-CSF and −51.0% GM-GSF. Oral Synergy, ↓ −71.3% IL-1α, −55.7% IL-1β, −50.7% IL-3, −69.2% IL-10, −26.5% IL-12(P40), −29.4% IL-12 (P70), −30.8% IL-13, −60.9% MIP-1α, −72.7% MIP-1β, −85.2% G-CSF and −51.9% GM-GSF, −36.6% Exotaxin, and −68.0% TNF- α. | Affect pro-inflammatory signaling and macrophage pathways such as p38, Erk, JNK, HMGB1, and NF-κB. | [56] |
| Hydroquinone zonarol from Dictyopteris undulata (brown algae) | 2% of DSS was used in drinking water for 14 days and at the same time 5-aminosalicylic acid (5-ASA at a dose of 50 mg/kg, and/or zonarol at doses of 10 and 20 mg/kg were used orally once a day for 14 days. | Male Slc:ICR mice | Dextran sulfate sodium/5-ASA 50 mg/kg as a positive control | ↓ 44.4% TNF-α, 15.2% IL-1β, 21.5% iNOS, 28.1%, TUNEL+ | Inhibit the TNF-α signaling pathway. | [98] |
| polysaccharide-rich extract from Eucheuma cottonii (EC) | 2.5% (w/v) DSS was used in drinking water for 7 days, at the same time, EC at the dose 0.35; 0.70; or 1.75 g/kg, and curcumin 0.10 g/kg was orally delivered once per day of DSS treatment for 7 days. | male BALB/c mice | dextran sulfate sodium/curcumin 0.10 g/kg as a positive control | ↓ TNF-α, IL-1β, and IL-6 in serum | Block TNF-α signaling pathway. | [100] |
| sulfated polysaccharide (PLS) from Hypnea musciformis | The animals were pretreated orally with PLS (10, 30, and 60 mg/kg, for three days, or dexamethasone (1 mg/kg, s.c.) then trinitrobenzene sulfonic acid (TNBS) was administered as a single intracolonic | male Wistar rats | trinitrobenzene sulfonic acid (TNBS) in a 50% ethanol/Dexamethasone (1 mg/kg, s.c.) as a positive control | ↑ 246.3% GSH ↓ 46.8% MDA, 220% NO2/NO3, 22.7% IL-1β, and 27.4% TNF-α | Inhibit the synthesis and release of the product of the Inflammation. | [101] |
| β-glucans derivedfrom seaweeds Laminaria hyperborea and Laminariadigitata | The animals were divided into 4 dietary groups (i) basal diet (BD), (ii) BD + β-glucans from Laminaria hyperborea, (iii) BD + β-glucans from Laminaria digitata, and (iv) BD + β-glucans from Saccharomyces cerevisiae. | Pigs | NA | ↓ IL-17a, IL-17F, IL-22, IL-23R, IL-5, IL-6 in S. cerevisiae group. ↓ IL-17a, IL-22, IL-23R, IL-10, IL-6 in L. digitata group. ↓ IL-17a, IL-17F, IL-22, IL-23R, IL-6 in L. hyperborea group. | Modulate the Th17 pathways without affecting TREG pathways. | [102] |
| Ulva pertusa (green alga) | 2,4,6-dinitrobenzene sulphonic acid (DNBS in 50% ethanol was administered intrarectal as a single dose to induce colitis. Then Ulva Pertusa extract was administered orally for 4 days. | male CD1 mice | 2,4,6-dinitrobenzene sulphonic acid (DNBS)/the control group treated with saline | ↓ nitrotyrosine, Bax/Bcl-2 ratio, mast cell infiltration, NF-κB, IL-5, IL-9, IL13, iNOS, COX2, P53, Bax, Caspase-3, Caspase-8, Caspase-9, MDA, ↑ IκBα, IL-4, Bcl-2, Nrf2, GSH, CAT, SOD, SIRT1, Mn-SOD, HO-1 | Modulate Nrf2/SIRT1 and NF-κB pathway. | [103] |
| polysaccharide extracted from Enteromorpha clathrate (EPC) | dextran sulfate sodium (DSS) in 2.0% (w/v) in drinking water for 8 days, at the same time EPC at a dosage of 100 mg/kg/day was used orally. | male c57bl/6j mice | dextran sulfate sodium (DSS)/normal control group which serves as the baseline for comparison. | No markers evaluated in this study | N.A | [107] |
| Ulva pertusa (green alga) | 2,4,6-dinitrobenzene sulphonic acid (DNBS in 50% ethanol was administered intrarectal as a single dose to induce colitis. Then Ulva Pertusa extract was administered orally for 4 days. | Male CD1 mice | 2,4,6-dinitrobenzene sulphonic acid (DNBS)/the control group treated with saline | ↓ ICAM)-1 and p-Selectin, CD68, CD4, CD8, TLR4, MYD88, TRAF6, NLRP3, ASC, Caspase-1 ↓ IL-6, IL-17, and IL-23 in the serum ↑ serum IL-10 | Modulate TLR4/Myd88/TRAF6 Pathway and NLPR3 inflammasome. | [106] |
| Low Molecular Weight Sulfate Ulva Polysaccharide (LMW-ulvan) from Ulva pertusa | 2% (w/v) DSS drinking water for 5 days, then LMW-ulvan at doses 50 and 100 mg/kg was administered orally for 7 days | Male C57BL/6 SPF mice | dextran sulfate sodium (DSS)/5-ASA (50 mg/kg) | ↑ IL-4, CAT, GPx enzyme activity, occludin, ZO-1, Claudin-1↓ IL-1β, IFN-γ, and MDA in serum and colon | Suppress NLRP3 inflammasome activation, inhibit Th1 cell response, and enhance antioxidant defense system. | [104] |
| Laminaria japonica extract (LJE) | 5% DSS in drinking water for 7 days, during the same time LJE was administered orally twice a day at doses of 100 and 300 mg/kg with probiotics at a dose of 300 mg/kg. | Male Balb/c mice | dextran sulfate sodium/normal animals were used as a control group | ↓ IL-1β, IL-6, TNF-α, IL-17, IL-12 (P40) | Modulate Th17 and Th1/Th17 dependent pathway | [108] |
| Chlorella vulgaris (C.V) (green algae) | 2.5% (w/v) DSS in drinking water for 7 days. Then C. vulgaris extract was orally administered 2 g/kg for 3 weeks | female C57BL/6 mice | dextran sulfate sodium/normal animals without treatment used as a control group | ↑ Treg, short-chain fatty acid (SCFAs), absolute CD4 + Foxp3+, Rort + Foxp3+ regulatory cells, and CD4+ T cells in the spleen and mouse lymph node | Improve immune tolerance by immune regulatory mechanisms mediated by Tregs and modulate the microbiome. | [111] |
| Caulerpa mexicana extract (green algae) | 3% DSS in drinking water for 14 days. During this period, the Caulerpa mexicana methanolic extract (2 mg/kg/day) was administered intravenously on alternate days | Male BALB/c mice | dextran sodium sulfate/control group received only saline without the DSS or the algae extract, serving as a baseline for comparison | ↓ Th1, Th17, IL-6, IL-12, TNF-α, IFN-γ, and IL-17 | Modulate Th1 and Th17 pathway. | [109] |
| Spirulina platensis (SP), Dunaliella salina (DS) | After sedating the animals by IP injection of pentobarbitone (35 mg/kg), acetic acid (4%, v/v) in saline was infused for 30 s into the colon to a distance of 8c cm using a polyethylene tube (2 mm) at the day 16, SP and DS was administered orally for 15 consecutive days with dose 500 mg/kg, in another group sulfasalazine was administered orally with dose 500 mg/kg at 13th, 14th, and 15th days. | Male Wistar albino rats | 4% v/v acetic acid (AA)/normal control group received only saline, the group with a single dose of AA without treatment served as a positive control group, another group received sulfasalazine | ↑ GSH, CAT, SOD ↓ MDA, PCO, MPO, TNF-α, IL-1β, IL-6, PEG2 | Scavenge free radicals and decrease Infiltration of Inflammatory cells. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repici, A.; Hasan, A.; Capra, A.P.; Scuderi, S.A.; Paterniti, I.; Campolo, M.; Ardizzone, A.; Esposito, E. Marine Algae and Deriving Biomolecules for the Management of Inflammatory Bowel Diseases: Potential Clinical Therapeutics to Decrease Gut Inflammatory and Oxidative Stress Markers? Mar. Drugs 2024, 22, 336. https://doi.org/10.3390/md22080336
Repici A, Hasan A, Capra AP, Scuderi SA, Paterniti I, Campolo M, Ardizzone A, Esposito E. Marine Algae and Deriving Biomolecules for the Management of Inflammatory Bowel Diseases: Potential Clinical Therapeutics to Decrease Gut Inflammatory and Oxidative Stress Markers? Marine Drugs. 2024; 22(8):336. https://doi.org/10.3390/md22080336
Chicago/Turabian StyleRepici, Alberto, Ahmed Hasan, Anna Paola Capra, Sarah Adriana Scuderi, Irene Paterniti, Michela Campolo, Alessio Ardizzone, and Emanuela Esposito. 2024. "Marine Algae and Deriving Biomolecules for the Management of Inflammatory Bowel Diseases: Potential Clinical Therapeutics to Decrease Gut Inflammatory and Oxidative Stress Markers?" Marine Drugs 22, no. 8: 336. https://doi.org/10.3390/md22080336
APA StyleRepici, A., Hasan, A., Capra, A. P., Scuderi, S. A., Paterniti, I., Campolo, M., Ardizzone, A., & Esposito, E. (2024). Marine Algae and Deriving Biomolecules for the Management of Inflammatory Bowel Diseases: Potential Clinical Therapeutics to Decrease Gut Inflammatory and Oxidative Stress Markers? Marine Drugs, 22(8), 336. https://doi.org/10.3390/md22080336









