Abstract
Aquaporins (AQPs) are a family of integral membrane proteins that selectively transport water and glycerol across the cell membrane. Because AQPs are involved in a wide range of physiological functions and pathophysiological conditions, AQP-based therapeutics may have the broad potential for clinical utility, including for disorders of water and energy balance. However, AQP modulators have not yet been developed as suitable candidates for clinical applications. In this study, to identify potential modulators of AQPs, we screened 31 natural products by measuring the water and glycerol permeability of mouse erythrocyte membranes using a stopped-flow light scattering method. None of the tested natural compounds substantially affected the osmotic water permeability. However, several compounds considerably affected the glycerol permeability. Stichoposide C increased the glycerol permeability of mouse erythrocyte membranes, whereas rhizochalin decreased it at nanomolar concentrations. Immunohistochemistry revealed that AQP7 was the main aquaglyceroporin in mouse erythrocyte membranes. We further verified the effects of stichoposide C and rhizochalin on aquaglyceroporins using human AQP3-expressing keratinocyte cells. Stichoposide C, but not stichoposide D, increased AQP3-mediated transepithelial glycerol transport, whereas the peracetyl aglycon of rhizochalin was the most potent inhibitor of glycerol transport among the tested rhizochalin derivatives. Collectively, stichoposide C and the peracetyl aglycon of rhizochalin might function as modulators of AQP3 and AQP7, and suggests the possibility of these natural products as potential drug candidates for aquaglyceroporin modulators.
1. Introduction
Marine natural products are a rich source of potential drugs because of their high biodiversity, low toxicity, suitability for oral applications, and a variety of biological actions [1,2,3]. According to the global marine pharmaceutical pipeline website (www.marinepharmacology.org) (accessed on 20 April 2024), 15 marine-derived drugs have been approved by the United States Food and Drug Administration (US FDA), and 32 marine-derived compounds are currently in various phases of clinical trials for drug development. Most have been developed or are under development as anticancer drugs, whereas a few are targeted to diseases other than cancer, such as pain, hypertriglyceridemia, Alzheimer’s disease, and viral infections. Considerable scientific and technological advances in analytical methods and functional assays in the past decade have opened up new opportunities for the exploration of marine natural products as novel potential therapeutic agents for more diverse disease entities [4,5,6].
Membrane transport proteins are important targets for drug discovery and delivery [7,8]. Transport proteins are essential for translocating solutes across the plasma or intracellular membranes, thus maintaining homeostasis. Transport proteins include channels and membrane transporters that are divided into three distinct classes: solute carriers (SLC), ATP-binding cassette (ABC) transporters, and ATPase ion pumps [9,10]. According to the data from The HUGO Gene Nomenclature Committee (https://genenames.org/) (accessed on 1 May 2024), transport proteins account for approximately 6% of all human protein-coding genes: 427 genes of the SLC superfamily, 312 genes of the ABC transporters, 125 genes of ATPase, and 291 genes of channels. The loss or alteration of the function of membrane transporters and channels causes a wide variety of human diseases, including channelopathies [11,12,13]. Membrane transporters are rapidly emerging as potential drug targets but have remained understudied until recently [14,15]. Moreover, since membrane transporters function as drug transporters, they play key roles in the absorption, distribution, and elimination of drugs and determine the therapeutic efficacy and adverse reactions of drugs in the process of drug discovery and development [16,17,18].
Aquaporins (AQPs) are a family of small integral membrane proteins that primarily transport water across cell membranes along osmotic gradients. To date, 13 AQP subtypes have been found in mammals (AQP0-12), some of which permit the transcellular passage of glycerol and urea as well as water (AQP3, 7, 9, and 10), and are thus called aquaglyceroporins [19]. AQPs are key players in maintaining a normal water balance and energy homeostasis in the body [20,21]. AQPs have been implicated in multiple disorders including diabetes insipidus, brain edema, cancer, obesity, cataracts, and neuromyelitis optica [22,23,24,25,26]. In addition to AQP-based diseases, AQP modulators have potential therapeutic utility in various pathological conditions for correcting abnormal transepithelial fluid and glycerol transport [20,21,27]. Notwithstanding the vigorous efforts to identify aquaporin modulators, limited progress has been made in the development of aquaporin-based therapeutics [28]. The relatively low hit rate, poor druggability due to toxicity, and lack of specificity makes the exploration of new AQP-targeted drugs challenging [29,30]. Because marine natural products not only possess greater structural and chemical diversity but also less toxicity than synthetic chemical libraries, the difficulty in finding promising lead compounds as AQP modulators using synthetic compound libraries encouraged us to conduct the present study to screen the marine natural products for novel drugs targeting AQPs.
2. Results
2.1. Effects of Marine Natural Compounds on the Osmotic Water Permeability of the Erythrocyte Membrane
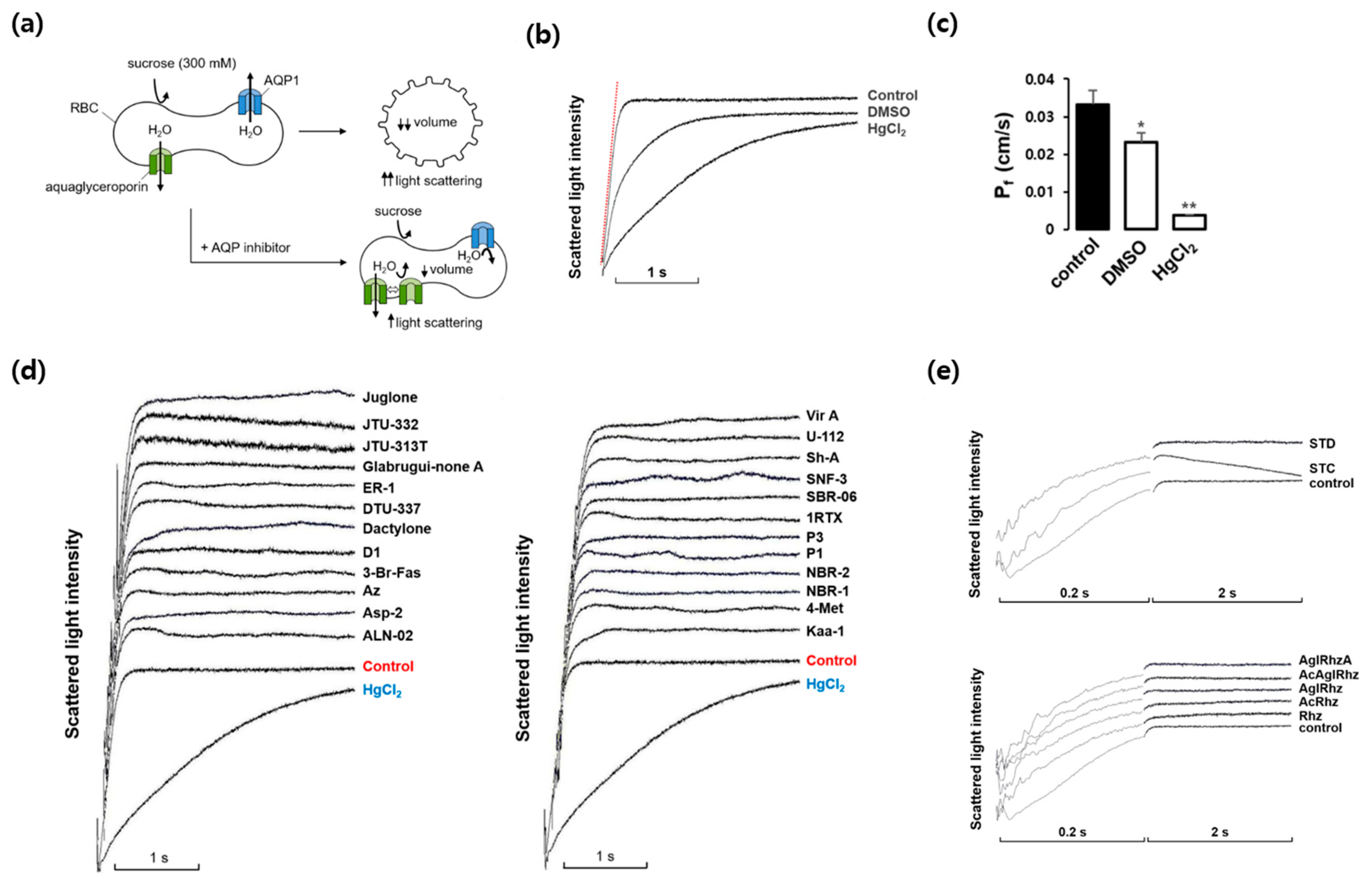
We first investigated whether marine natural compounds could alter the osmotic water permeability of mouse erythrocyte membranes. To measure the membrane water permeability, the kinetics of cell volume change were examined using light scattering (Figure 1a). In the presence of an inwardly directed gradient of an impermeable solute, water moves out of the cells according to the osmotic gradient through AQPs. Consequently, the cell volume decreases, which causes an increase in light scattering. The AQP inhibitors prevented water efflux and cell shrinkage, retarding the increase in light scattering. As shown in Figure 1b, in response to an inward gradient of 300 mM sucrose, the scattered light intensity rapidly increased and reached a steady state. HgCl2 is a well-known inhibitor of various AQPs and prevents cell shrinkage in response to osmotic gradients by inhibiting water efflux [31,32]. DMSO also slows osmotic water movement owing to an osmotic clamping effect rather than a direct inhibition of water efflux [33]. The value of the osmotic water permeability coefficient (Pf), which was calculated from the time course of the increase in light scattering upon exposure to 300 mM sucrose, was 0.033 ± 0.004 cm/s (Figure 1c). In the presence of DMSO or HgCl2, the Pf value decreased by 30% and 88%, respectively. Figure 1d,e show the representative light scattering traces for 24 marine natural compounds (100 nM) as well as those of stichoposide C, rhizochalin, and their derivatives in response to 300 mM sucrose. Though the late plateau phase of the traces after 0.2 sec was changed by several compounds, the initial slope of the rising phase was not affected and thus the calculated Pf values showed no statistical difference when compared with that of sucrose alone, suggesting no significant effects of these natural compounds on the osmotic water permeability.
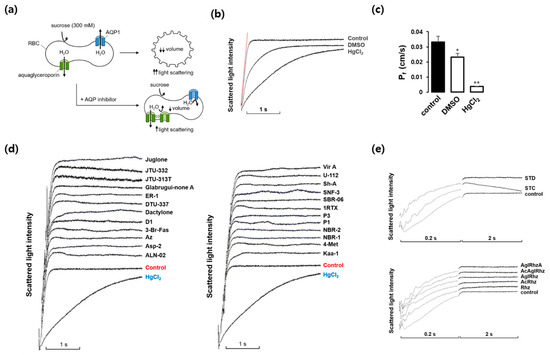
Figure 1.
Effects of various marine natural products on osmotic water permeability in mouse erythrocytes. (a) Assay method. Erythrocytes were subjected to a 300 mM sucrose gradient. A rapid decrease of the cell volume due to the water efflux through AQPs increased light scattering intensity. AQP inhibitors prevented water efflux and cell shrinkage. (b) Osmotic water permeability (Pf) was measured from the initial slope of the time course of scattered light intensity (a red line). Control (sucrose alone), DMSO (dimethylsulfoxide, 0.1%), and HgCl2 (0.5 mM). (c) The Pf values calculated from traces in (b). Values are the mean ± SD. * p < 0.05, ** p < 0.01. (d) The representative light scattering traces for 24 marine natural compounds (100 nM) in response to 300 mM sucrose. (e) The representative light scattering traces for stichoposide C (STC), stichoposide D (STD), rhizochalin (Rhz) and its derivatives in response to 300 mM sucrose. AglRhz, aglycon of rhizochalin; AcRhz, peracetyl rhizochalin; AcAglRhz, peracetyl aglycon of rhizochalin; AglRhzA, aglycon of rhizochalin A.
2.2. Effects of Marine Natural Compounds on Glycerol Permeability of Erythrocyte Membrane
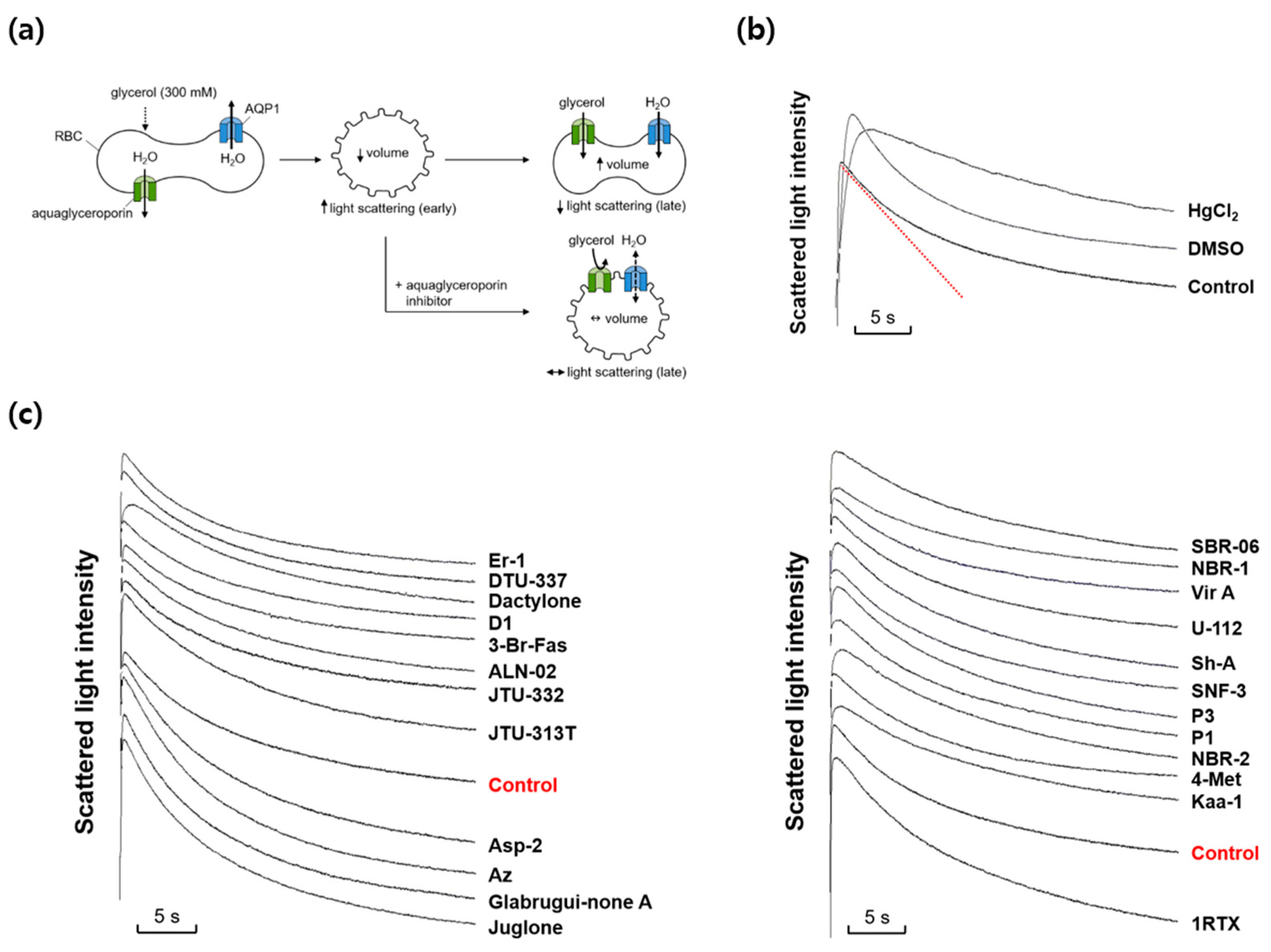
We next investigated whether any marine natural compounds might change the glycerol permeability of the mouse erythrocyte membrane. To measure the membrane glycerol permeability, changes in the cell volume in response to an imposed glycerol gradient were measured by light scattering (Figure 2a). In the presence of an inwardly directed gradient of glycerol, a permeable solute, a water efflux through AQPs occurs first according to the osmotic gradient, followed by a water influx accompanying a glycerol influx through aquaglyceroporins. The aquaglyceroporin inhibitors prevented the late re-swelling phase, retarding the decrease in light scattering. As shown in Figure 2b, scattered light intensity rapidly increased for the first ~0.3 s in response to an inwardly directed gradient of 300 mM glycerol in the same manner as that of sucrose, but slowly decreased afterwards as the cell volume was recovered by the entry of glycerol and water. DMSO slowed the initial increase in light scattering but did not affect the late decreasing phase. However, HgCl2, an inhibitor of AQPs including aquaglyceroporins, reduced not only the initial rise but also the late decreasing phase of light scattering. Figure 2c shows the representative light scattering traces for 24 marine natural compounds (100 nM) in response to 300 mM glycerol. Though several marine natural compounds increased or decreased the decrement rate of the late phase of light-scattering kinetics driven by a glycerol influx through aquaglyceroporins, the calculated glycerol permeability coefficient (Pglycerol) values showed no statistical difference when compared with that of sucrose alone.
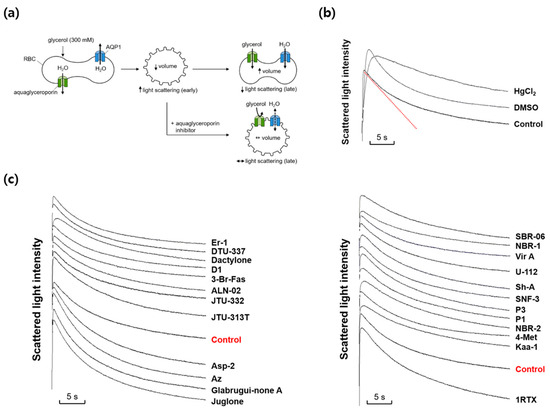
Figure 2.
Effects of various marine natural products on glycerol permeability in mouse erythrocytes. (a) Assay method. Mouse erythrocytes were subjected to a 300 mM glycerol gradient. A rapid decrease of the cell volume (increased light scattering) due to the water efflux through AQPs was followed by cell re-swelling (decreased light scattering) due to glycerol and water influx. Aquaglyceroporin inhibitors prevented the late re-swelling phase. (b) Glycerol permeability was measured from the slope of the late decreasing phase of light scattering (a red line) in response to a 300 mM inwardly directed glycerol gradient (control). HgCl2 (0.5 mM); DMSO, dimethylsulfoxide (0.1%). (c) The representative light scattering traces for 24 marine natural compounds (100 nM) in response to 300 mM glycerol.
2.3. Effects of Stichoposide C, Rhizochalin, and Their Derivatives on Glycerol Permeability of Erythrocyte Membrane
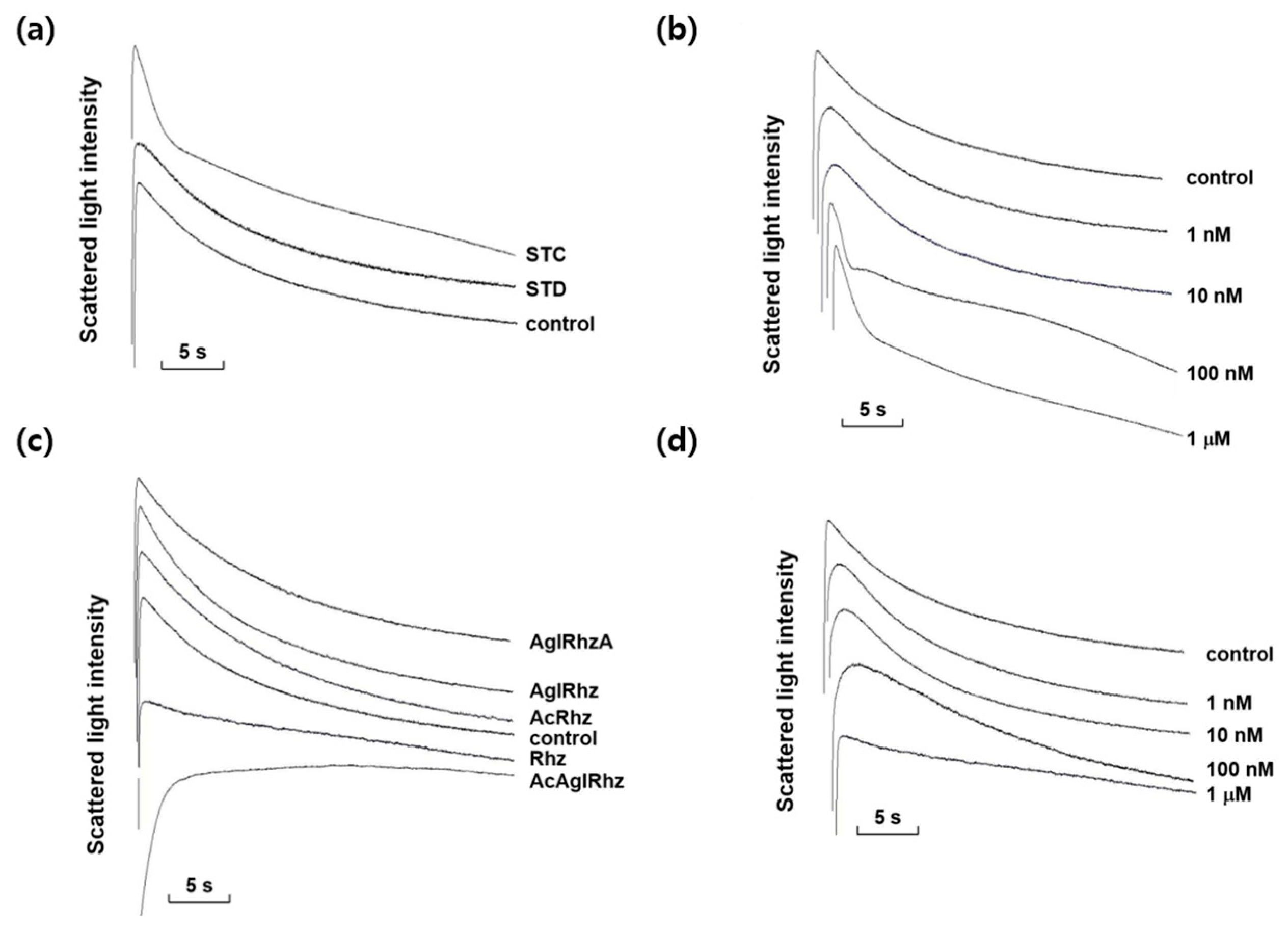
Among the marine natural products tested, stichoposide C, rhizochalin, and their derivatives most notably affected the decrement rate of the late phase of light-scattering kinetics driven by a glycerol influx through aquaglyceroporins. As shown in Figure 3, stichoposide C increased the glycerol permeability, whereas rhizochalin decreased it. The effect of stichoposide C on the glycerol permeability of erythrocyte membranes was further investigated. The promoting effect of stichoposide C on the glycerol permeability was concentration-dependent in the range from 100 nM to 1 μM (Figure 3b). In contrast to stichoposide C, stichoposide D did not exert any significant influence on the late phase of light-scattering kinetics up to a concentration of 1 μM (Figure 3a).
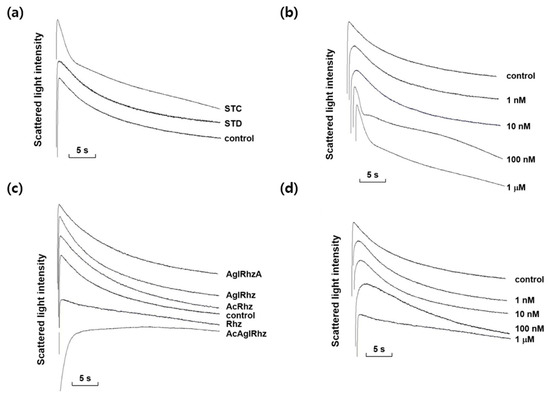
Figure 3.
Effects of stichoposide, rhizochalin, and their derivatives on glycerol permeability in mouse erythrocytes. (a) The representative light scattering traces for stichoposide C (STC, 1 μM) and stichoposide D (STD, 1 μM) in response to 300 mM glycerol. (b) The representative light scattering traces for the absence (control) or presence of the indicated concentrations of STC in response to 300 mM glycerol. (c) The representative light scattering traces for 1 μM of rhizochalin (Rhz) and its derivatives in response to 300 mM glycerol. AglRhz, aglycon of rhizochalin; AcRhz, peracetyl rhizochalin; AcAglRhz, peracetyl aglycon of rhizochalin; AglRhzA, aglycon of rhizochalin A. (d) The representative light scattering traces for the absence (control) or presence of the indicated concentrations of Rhz in response to 300 mM glycerol.
The effects of rhizochalin and its derivatives on the glycerol permeability of erythrocyte membranes were also investigated (Figure 3c). The peracetyl aglycon of rhizochalin decreased the glycerol permeability more profoundly than rhizochalin. However, the aglycon of rhizochalin, aglycon of Rhizochalin A, and rhizochalin peracetate did not have any significant effect on the glycerol permeability, in contrast to rhizochalin. Rhizochalin exhibited a concentration-dependent inhibition of glycerol permeability (Figure 3d).
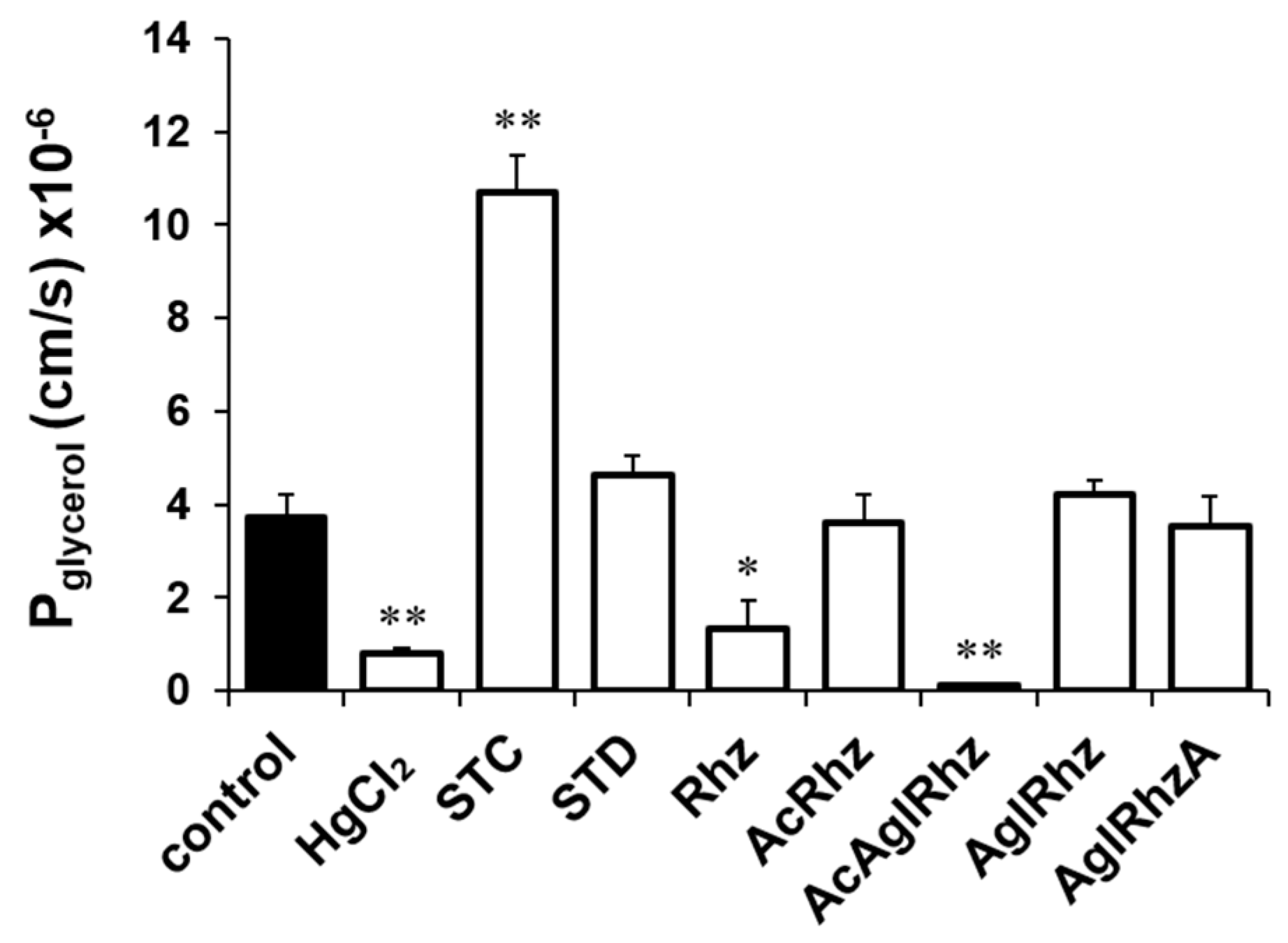
The glycerol permeability coefficients (Pglycerol) were calculated from the time course of the late decreasing phase of light scattering (Figure 4). The Pglycerol values in the presence of 300 mM glycerol alone or with HgCl2 were (3.71 ± 0.511) × 10−6 cm/s and (0.78 ± 0.134) ×10−6 cm/s, respectively. The Pglycerol values in the presence of stichoposide C or Stichoposide D were (10.71 ± 0.803) × 10−6 cm/s (2.88-fold increase, p < 0.01) and (4.64 ± 0.388) × 10−6 cm/s (1.25-fold increase), respectively. The Pglycerol values in the presence of rhizochalin or the peracetyl aglycon of rhizochalin were (1.33 ± 0.617) × 10−6 cm/s (2.79-fold decrease, p < 0.05) and (0.15 ± 0.013) × 10−6 cm/s (24.73-fold decrease, p < 0.01), respectively, indicating that the peracetyl aglycon of rhizochalin was a more potent inhibitor of glycerol permeability than that of rhizochalin.
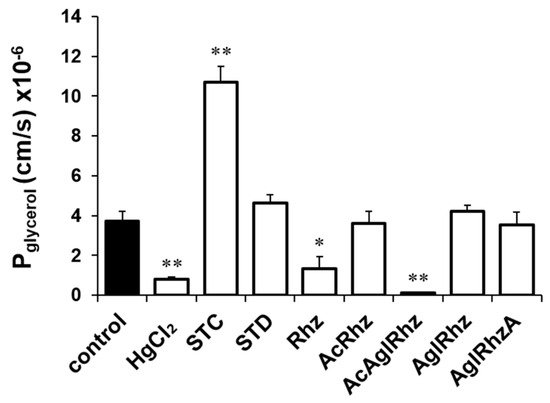
Figure 4.
Glycerol permeability coefficients (Pglycerol) of mouse erythrocytes. The Pglycerol value was calculated from the late decreasing phase of light scattering traces in the presence of 300 mM glycerol alone (control) or together with stichoposide (STC), rhizochalin (Rhz), and their derivatives (100 nM). STD, stichoposide D; AglRhz, aglycon of rhizochalin; AcRhz, peracetyl rhizochalin; AcAglRhz, peracetyl aglycon of rhizochalin; and AglRhzA, aglycon of rhizochalin A. Values are the mean ± SD (n = 20). * p < 0.05, ** p < 0.01 according to Student’s t-tests.
2.4. Expression of AQP Subtypes in Mouse Erythrocytes
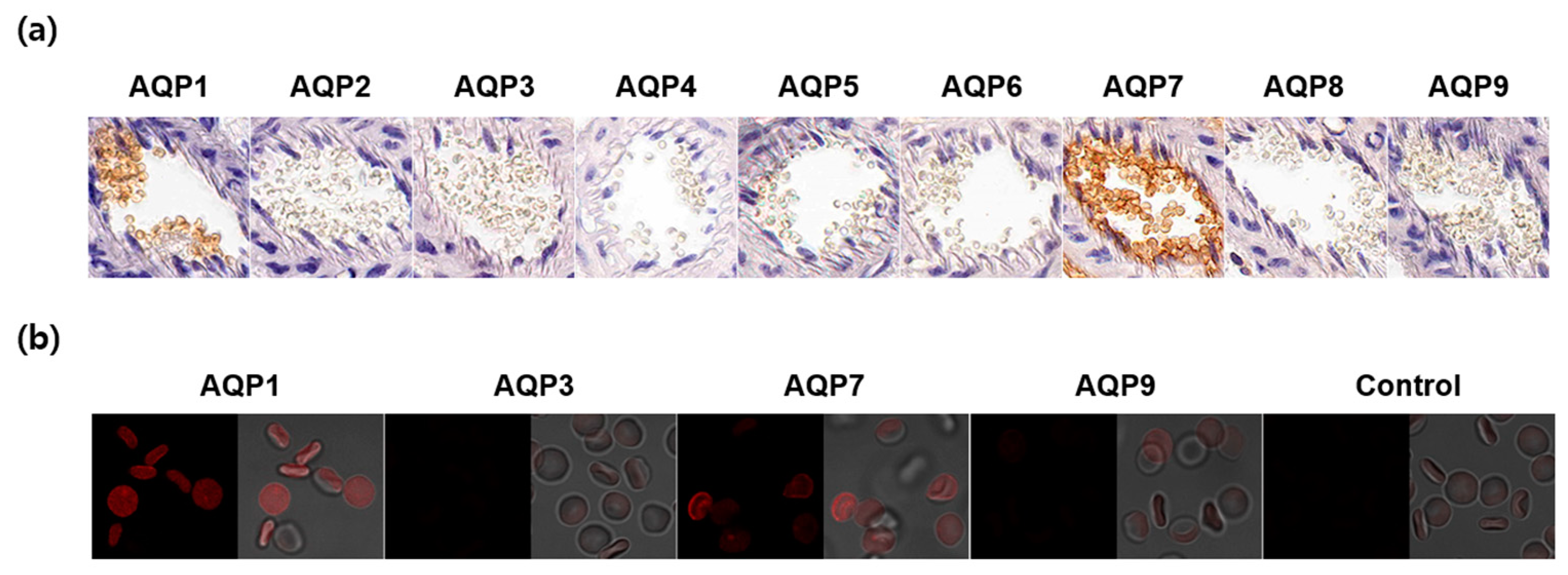
To elucidate which AQP subtypes are responsible for the water and glycerol permeability, we analyzed CD1 mouse erythrocytes in the blood vessels by immunohistochemistry using nine AQP subtypes. As shown in Figure 5a, AQP1 and AQP7 were strongly expressed in mouse erythrocyte membranes. We verified this finding with an immunofluorescence analysis using glutaraldehyde-fixed erythrocyte suspensions. Though a weak AQP9 signal was detected, AQP7 exhibited a strong expression in erythrocyte membranes comparable to AQP1 (Figure 5b). These results indicated that AQP7 was the main aquaglyceroporin that determined the glycerol permeability in CD1 mouse erythrocytes.
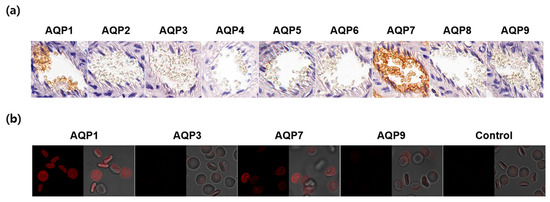
Figure 5.
Expression of AQP subtypes in CD1 mouse erythrocytes. (a) Immunohistochemistry of paraffin-embedded kidney sections using anti-AQP1-AQP9 antibodies. (b) Immunofluorescence analysis of glutaraldehyde-fixed erythrocyte suspensions using anti-AQP antibodies. Control, secondary antibody alone.
2.5. Effects of Stichoposide C, Rhizochalin, and Their Derivatives on AQP3-Mediated Transepithelial Glycerol Transport
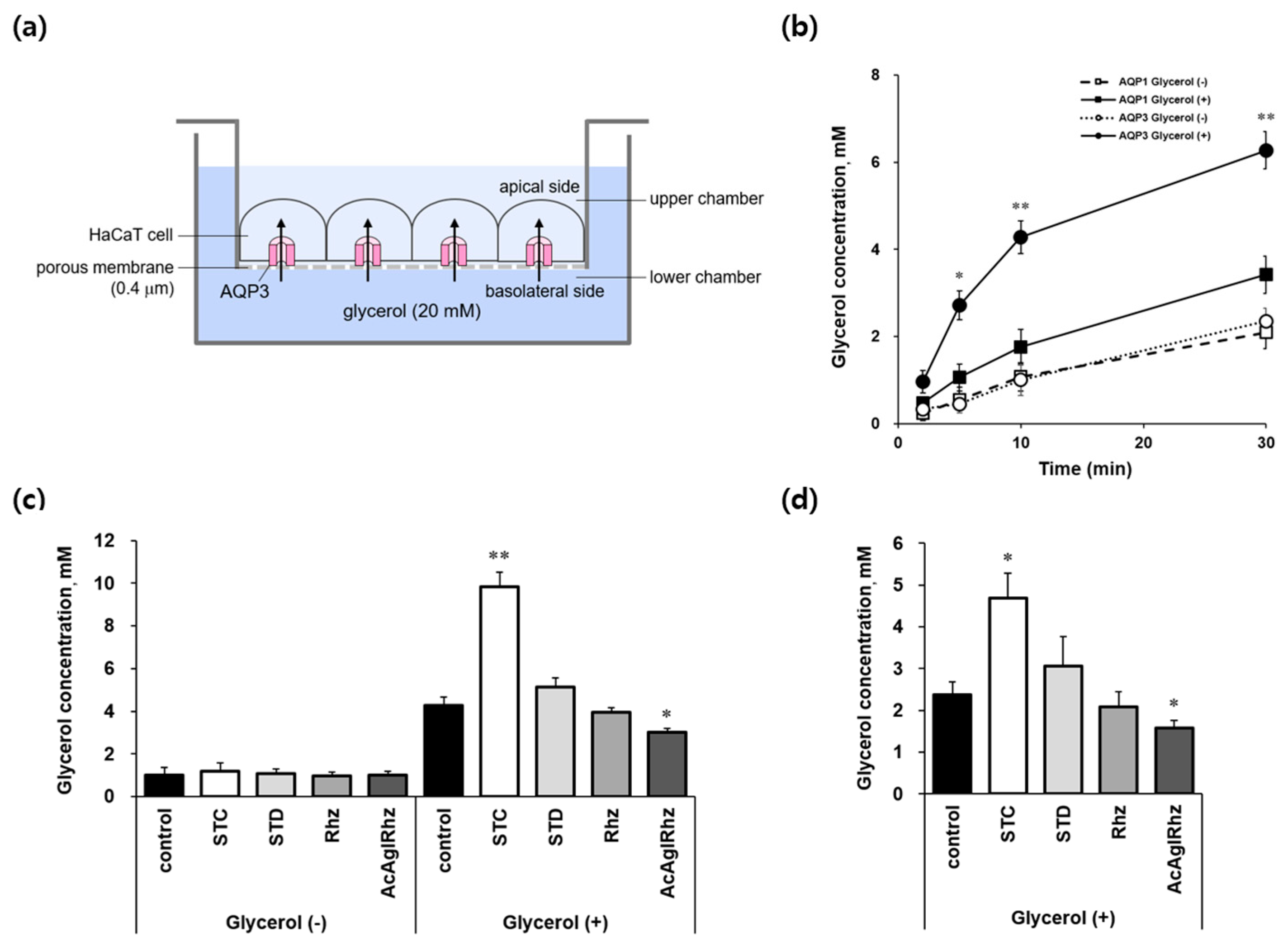
The effects of stichoposide C and rhizochalin on aquaglyceroporins were further explored using AQP3-expressing HaCaT cell monolayers to directly measure transepithelial glycerol transport (Figure 6a). When cells grown on permeable supports were exposed to 20 mM glycerol in the lower basolateral chamber, the glycerol concentrations in the upper apical chambers increased with time up to 30 min in both YFP-hAQP1 and YFP-hAQP3 HaCaT cells (Figure 6b). However, glycerol concentrations in YFP-hAQP3 HaCaT cells were over two-fold higher than those in YFP-hAQP1 HaCaT cells at every measured time point, which validated our method to measure AQP3-mediated transepithelial glycerol transport using YFP-hAQP3 HaCaT cells. Stichoposide C significantly stimulated AQP3-mediated basolateral glycerol transport in YFP-hAQP3 HaCaT cell monolayers (Figure 6c,d). The glycerol concentrations in the upper apical chambers at 10 min and inside the cells at 30 min were 2.30-fold (p < 0.01) and 1.97-fold (p < 0.05) increased, respectively, by Stichoposide C. The peracetyl aglycon of rhizochalin significantly decreased the glycerol concentrations in the upper apical chambers at 10 min (1.63-fold, p < 0.05) and inside the cells at 30 min (1.52-fold, p < 0.05). Stichoposide D increased, and rhizochalin decreased AQP3-mediated transepithelial glycerol transport to some extent, but there were no statistically significant differences.
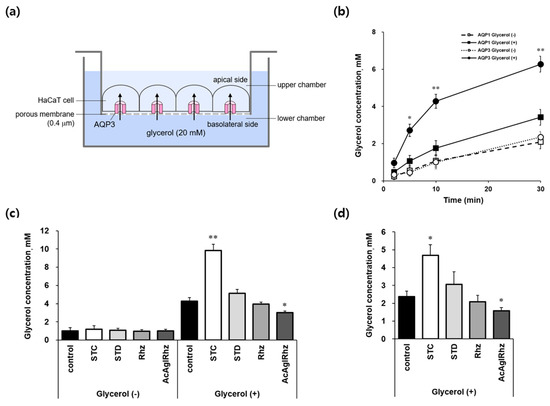
Figure 6.
AQP3-mediated transepithelial glycerol transport in HaCaT cell monolayers. (a) Assay method. YFP-hAQP3 HaCaT cells grown on permeable supports were exposed to 20 mM glycerol in the lower chamber of the Transwell. The basal-to-apical glycerol transport across the cell monolayer was assessed by measuring the glycerol concentration in the upper chamber as well as inside the cells. (b) The glycerol concentrations of the upper chamber bathed on the apical side of YFP-hAQP1 and YFP-hAQP3 HaCaT cells at the indicated time points. (c) The glycerol concentrations of the upper chamber bathed on the apical side of YFP-hAQP3 HaCaT cells at 10 min. 1 μM of stichoposide C (STC), stichoposide D (STD), rhizochalin (Rhz), or peracetyl aglycon of rhizochalin (AcAglRhz). (d) The glycerol concentrations inside YFP-hAQP3 HaCaT cells at 30 min. Values are the mean ± SD (n = 10). * p < 0.05, ** p < 0.01 according to one-way ANOVA followed by Duncan’s test.
3. Discussion
In this study, we aimed to identify potential AQP modulators in marine natural products. Although none of the marine natural compounds tested had significant effects on the osmotic water permeability, we found several compounds that significantly affected the glycerol permeability in mouse erythrocytes. We confirmed the modulatory effects of stichoposide C and rhizochalin on aquaglyceroporins through transepithelial glycerol transport assays using AQP3-expressing human keratinocytes.
Natural products, including plants, animals, and minerals, continue to be the best sources of novel drugs owing to their structural diversity and biodiversity [5,6]. In 2023, ten new natural products or their direct derivatives were drug approved by the US FDA, which corresponds to 18% of a total of 55 new drugs on the market [34]. Because the total number of species and biochemical diversity in oceans is higher than that on land, marine natural products are more invaluable sources for potential drug discovery than terrestrial products [3,4]. Moreover, natural products released into the water are rapidly diluted and, therefore, need to be highly potent to have any effect. The advantages of marine natural products, along with the growing appreciation for functional assays and phenotypic screening, may further contribute to the revival of interest in natural products for drug discovery and development [35,36].
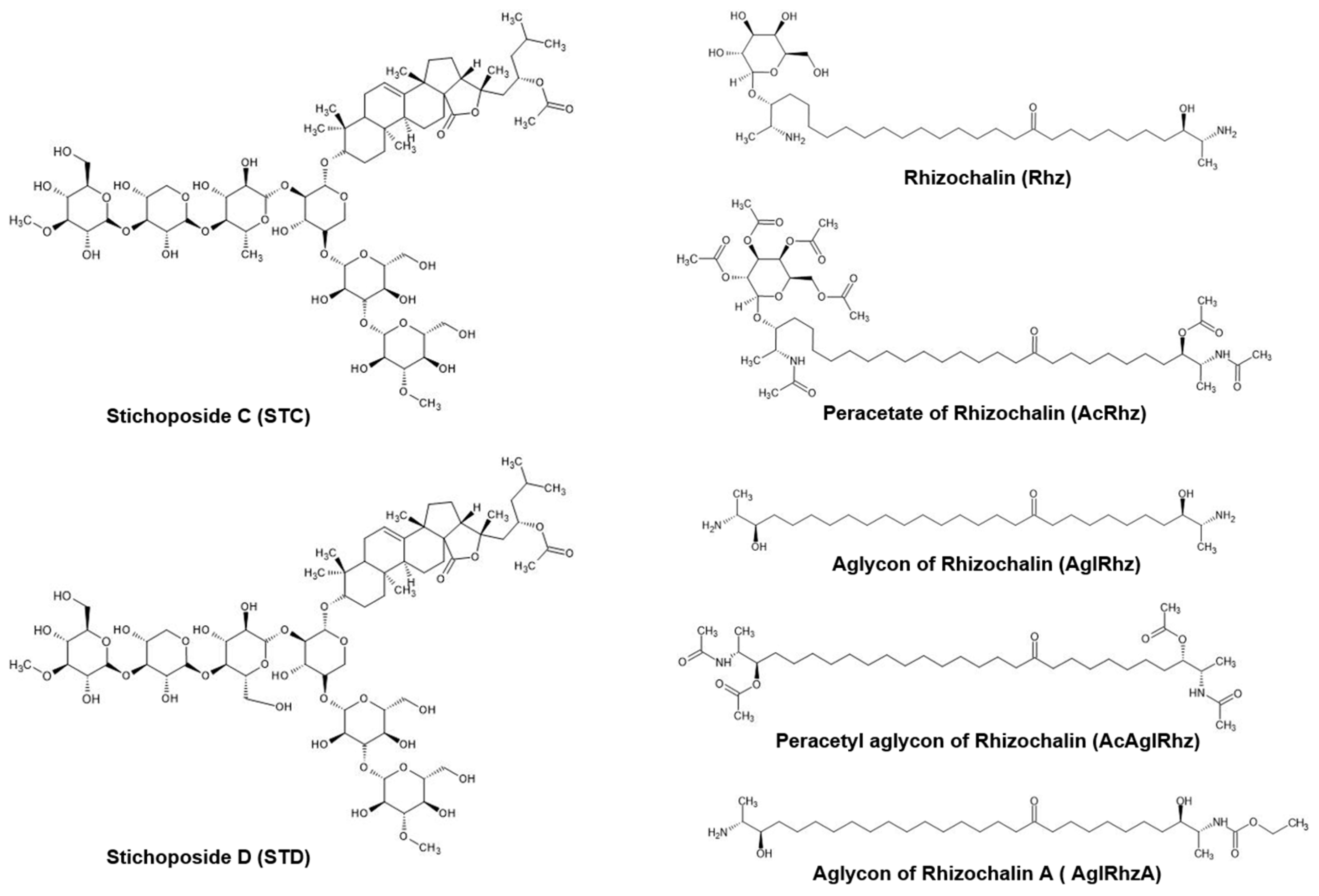
Triterpene glycosides are widely distributed not only in plants, but also in marine invertebrates, especially echinoderms, octocorals, and sponges [37,38]. Many investigators have attempted to develop marine triterpene glycosides as candidate anticancer agents based on in vitro and in vivo studies [39,40,41]. Stichoposides C and D are triterpene glycosides extracted from sea cucumbers of the family Stichopodidae [42,43]. Stichoposide C is a quinovose-containing hexoside, whereas stichoposide D is a glucose-containing hexoside (Figure 7). Stichoposide C is an active membrane-acting agent with anticancer activity, and is more potent than stichoposide D [44,45]. In the present study, stichoposide C strongly stimulated the glycerol permeability in murine erythrocytes and AQP3-expressing human keratinocytes, whereas stichoposide D did not. Based on the notion that a linear tetrasaccharide fragment in triterpene glycosides is essential for the actions leading to the modification of the cellular membrane [39,46,47], having quinovose, rather than glucose, as a second monosaccharide unit in glycosides seems to be critical for interaction with 5(6)-unsaturated sterols of cellular membranes in contact with aquaglyceroporins or for direct interaction with channel proteins. Otherwise, STC-specific activation of the sphingomyelinase-ceramide pathway might account for the different effects on aquaglyceroporins [44]. However, further studies on the structure–activity relationship of these molecules are needed to improve the efficacy and safety of these compounds in activating aquaglyceroporins.
Figure 7.
Structures of Stichoposide, Rhizochalin, and their derivatives.
Rhizochalin is a marine two-headed sphingolipid-like natural product isolated from the sponge Rhizochalina incrustata [48,49]. These compounds differ from classical sphingolipids in the presence of polar groups at α,ω-positions, which contain a terminal methyl group instead of a hydroxymethyl group unlike sphingoid bases, thus representing a unique class of bipolar lipids [50,51]. Rhizochalin and its analogs have been reported to have antibacterial, antifungal, and cytotoxic activities [52,53,54,55]. In the present study, the peracetyl aglycon of rhizochalin exhibited the most potent inhibitory effect on the glycerol permeability in murine erythrocytes and human AQP3-expressing epithelial cells. It might be assumed that the absence of a glucopyranosyl sugar moiety on one polar end, together with the presence of three acetyl moieties on the other polar end of two-headed bipolar lipids (Figure 7), favors an intimate interaction with aquaglyceroporin or neighboring membrane lipids and regulatory proteins. The molecular mechanism underlying the inhibitory effect of the peracetyl aglycon of rhizochalin on aquaglyceroporins and the structure–activity relationship of rhizochalin analogs require further investigation.
Aquaglyceroporins mediate various physiological and pathophysiological processes [56,57,58]. Water-selective AQPs function primarily in water and salt homeostasis, whereas water/glycerol-transporting aquaglyceroporins are primarily involved in energy metabolism and lubrication. Because aquaglyceroporin dysfunction is implicated in various human diseases and symptoms, including obesity, nonalcoholic fatty liver disease, psoriasis, cancer, polyuria, and glyceroluria, functional modulators of aquaglyceroporins are promising therapeutic targets for a wide range of clinical conditions [24,59,60,61]. A few dozen aquaglyceroporin modulators have been reported to date, but none have been developed or are under development. Metal compounds, such as HgCl2 and p-chloromercuribenzene sulfonate (pCMBS), were first identified as AQP inhibitors, including aquaglyceroporins, and are still the most commonly used inhibitors in functional assays [32]. The gold compound Auphen ([Au(phen)Cl2]Cl) and its copper analog Cuphen ([Cu(phen)Cl2]) inhibited AQP3 and AQP7 [62,63]. Recently, several aquaglyceroporin inhibitors were identified by screening a commercially available library of small molecules: DFP00173 for AQP3, Z433927330 for AQP7, and HTS13286 for AQP9 inhibitors [64,65]. Natural compounds were also reported to have modulating effects on aquaglyceroporins which are usually from plants, such as glycolic acid, 18β-glycyrrhetinic acid, curcumin, chrysin, and phloretin [61]. However, the application of aquaglyceroporin modulators in clinical trials is still far from being implemented, possibly because of the lack of selective and potent modulators that can be administered in vivo.
In this study, we searched for potential AQP modulators among marine natural products and found that stichoposide C and the peracetyl aglycon of rhizochalin were potential aquaglyceroporin modulators. Since mouse erythrocytes express AQP7 and transepithelial glycerol transport was measured using human AQP3-expressing cells, it can be stated that stichoposide C and the peracetyl aglycon of rhizochalin function as modulators of AQP3 and AQP7. AQP3 activators may exert therapeutic effects on defects in the hydration, lubrication, and proliferation of the skin, bladder, vagina, and respiratory system, whereas AQP7 activators may be applicable as useful adjuvants for obesity treatment. In addition, AQP3 and AQP7 inhibitors are worthy of development as anticancer drugs. Further studies are needed to verify the therapeutic effects of stichoposide C and the peracetyl aglycon of rhizochalin in animal models of human diseases or in 3D bioengineered disease models.
4. Materials and Methods
4.1. Marine Natural Products
The marine natural products were provided by Dr. V. A. Stonik at the Pacific Institute of Bioorganic Chemistry, Far Eastern Branch of the Russian Academy of Science, Vladivostok, Russian Federation. The thirty-one compounds tested were extracted from marine natural products. The compounds were dissolved in phosphate-buffered saline (PBS) or dimethyl sulfoxide (DMSO) as 1–2 mM stock solutions, and diluted to working concentrations ranging from 100 nM to 1 μM.
4.2. Erythrocyte Preparation
Thirty CD1 mice, 6–8 weeks old, were used in this experiment. The mice were anesthetized with avertin (2,2,2-tribromoethanol-tert-amyl alcohol, 250 mg/kg intraperitoneally). Blood was collected from the inferior vena cava in heparinized tubes. Freshly obtained whole blood was centrifuged at 120× g for 10 min to remove the plasma and buffy coat. The erythrocytes were washed three times and suspended in PBS to adjust the final hematocrit to 2%. All animal protocols were approved by the Dong-A University Medical School Institutional Animal Care and Use Committee (DIACUC-10-6-1).
4.3. Stopped-Flow Light Scattering Measurements
The water and glycerol permeabilities were measured via light scattering using a stopped-flow apparatus (SFM-3, Biologic, Seyssinet-Pariset, France). The erythrocyte suspension was subjected to a 300 mM inwardly directed gradient of either sucrose or glycerol. The kinetics of decreasing cell volume were measured from the time course of 90° scattered light intensity at 530 nm [66]. The osmotic water and glycerol permeability coefficients were calculated from the time course of light scattering using the following equation:
where V(t) is the relative red cell volume as a function of time, SAV is the red cell surface area-to-initial volume ratio, MVW is the molar volume of water (18 cm3/mole), and Cin and Cout (mole/cm3) are the initial concentrations of the intracellular and extracellular solutes, respectively. The average erythrocyte surface area and volume were 7.2 × 10−7 cm2 and 3.1 × 10−11 cm3, respectively [67].
dV(t)/dt = Pf × SAV × MVW × (Cin − Cout)
4.4. Tissues Preparation and Immunohistochemistry
CD1 mouse kidneys were fixed in 10% neutral buffered formalin for 48 h, paraffin-embedded, and sectioned at 5 μm using a microtome (RM 2125RT, Leica, Wetzlar, Germany). Paraffin sections were deparaffinized with xylene, serially rehydrated in 100%, 95%, and 70% ethanol, and boiled for 15 min in citrate buffer (10 mM sodium citrate, 0.05% Tween 20, and pH 6.0) for antigen retrieval. After blocking with 5% bovine serum albumin for 2 h, the sections were incubated with the following primary antibodies overnight at 4 °C at a dilution of 1:300: anti-AQP1, AQP2, AQP3, and AQP4 (Merck Millipore, Billerica, MA, USA); anti-AQP5, AQP6, and AQP7 (Alomone Labs, Jerusalem, Israel); anti-AQP8 (Mybiosource, San Diego, CA, USA); and anti-AQP9 (Bioss, Woburn, MA, USA). Subsequently, the sections were incubated with secondary anti-rabbit horseradish peroxidase (HRP)-linked IgG (Dako, Glostrup, Denmark) for 40 min at 25 °C, and HRP activity was determined by adding DAB+ chromogen (Dako). The sections were mounted using Marinol Mount (Muto Pure Chemicals, Tokyo, Japan) solution and xylene (Muto Pure Chemicals) (1:1), and then visualized using a digital slide scanner (Pannoramic Midi, 3DHistech, Budapest, Hungary) at the Neuroscience Translational Research Solution Center (Busan, Republic of Korea).
4.5. Cell Cultures
HaCaT cells, an immortalized human keratinocyte line, were stably transfected with a plasmid encoding the yellow fluorescent protein (YFP) and either human AQP1 (YFP-hAQP1) or human AQP3 (YFP-hAQP3) (gifts from Dr. Alan Verkman at the University of California, San Francisco, CA, USA) and selected using G418 (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Cells were grown in a DMEM medium (Gibco, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (HycloneTM, Cytiva, Logan, UT, USA), penicillin G (100 U/mL), and streptomycin (100 μg/mL) and maintained at 37 °C in a humidified 5% CO2 incubator.
4.6. Measurement of Transepithelial Glycerol Transport
YFP-hAQP1-HaCaT cells and YFP-hAQP3-HaCaT cells were seeded at a density of 2 × 104 cells/well onto 6.5 mm, 0.4 μm pore size Transwell permeable supports (Corning Life Sciences, Tewksbury, MA, USA), cultured for 3–5 days to a confluent state, and serum-deprived for 2–3 days before the experiments. Once the cells reached a confluent state, the integrity of the cellular barriers was assessed by transepithelial electrical resistance (TEER) using an epithelial voltohmmeter. When the TEER values were above 200 Ω·cm2, transepithelial flux experiments were performed [68].
Glycerol transport across HaCaT cell monolayers was determined by measuring the glycerol concentrations of the culture medium in the upper and lower compartments, as well as in the Transwell inserts. The cells were pretreated with marine natural products or HgCl2 for 10 min, and 20 mM glycerol was added to the bath solution on the basal side of the cells. Aliquots (10 µL) of samples were collected from the medium of the opposite side from the glycerol addition at the indicated time points. The glycerol concentrations were measured using a glycerol assay kit (Sigma-Aldrich, Saint Louis, MO, USA) according to the manufacturer’s instructions.
4.7. Statistical Analysis
All the data were expressed as the mean ± standard deviation (SD). For data analysis of the Pf and Pglycerol values, Student’s t-test was used to compare the mean values between the two groups. For data analysis of transepithelial glycerol transport, one-way analysis of variance (ANOVA), Duncan’s multiple range test was employed to determine the statistical significance of differences between the groups. We used the Statistical Package for Social Science (SPSS) ver. 22.0 (SPSS Inc., Chicago, IL, USA) with a significance level set at p < 0.05.
Author Contributions
Conceptualization, J.-Y.K. and H.-R.B.; methodology, J.W.I., J.H.L. and H.-R.B.; software, S.J. and M.S.; validation, J.W.I., S.J. and H.-R.B.; formal analysis, J.W.I. and H.-R.B.; investigation, J.W.I. and J.H.L.; resources, J.-Y.K. and V.A.S.; data curation, J.W.I. and H.-R.B.; writing—original draft preparation, J.W.I., J.H.L. and H.-R.B.; writing—review and editing, M.S. and H.-R.B.; visualization, J.W.I. and J.H.L.; supervision, J.-Y.K. and H.-R.B.; project administration, H.-R.B.; funding acquisition, H.-R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2021R111A3057381).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stonik, V.A. Marine natural products: A way to new drugs. Acta Nat. 2009, 1, 15–25. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef]
- Jiménez, C. Marine natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- The International Transporter Consortium; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef]
- Alam, S.; Doherty, E.; Ortega-Prieto, P.; Arizanova, J.; Fets, L. Membrane transporters in cell physiology, cancer metabolism and drug response. Dis. Models Mech. 2023, 16, dmm050404. [Google Scholar] [CrossRef]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A Guide to Plasma Membrane Solute Carrier Proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef]
- Thomas, C.; Tampé, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Kass, R.S. The Channelopathies: Novel Insights into Molecular and Genetic Mechanisms of Human Disease. J. Clin. Investig. 2005, 115, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.A.; Clémençon, B.; Burrier, R.E.; Bruford, E.A. The ABCs of Membrane Transporters in Health and Disease (SLC Series): Introduction. Mol. Asp. Med. 2013, 34, 95–107. [Google Scholar] [CrossRef]
- Harraz, O.F.; Delpire, E. Recent Insights into Channelopathies. Physiol. Rev. 2024, 104, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC Transporters as Therapeutic Targets: Emerging Opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef] [PubMed]
- César-Razquin, A.; Snijder, B.; Frappier-Brinton, T.; Isserlin, R.; Gyimesi, G.; Bai, X.; Reithmeier, R.A.; Hepworth, D.; Hediger, M.A.; Edwards, A.M.; et al. A Call for Systematic Research on Solute Carriers. Cell 2015, 162, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Niwa, T.; Yotsumoto, Y.; Sugiyama, Y. Impact of Drug Transporter Studies on Drug Discovery and Development. Pharmacol. Rev. 2003, 55, 425–461. [Google Scholar] [CrossRef]
- Shugarts, S.; Benet, L.Z. The Role of Transporters in the Pharmacokinetics of Orally Administered Drugs. Pharm. Res. 2009, 26, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Galetin, A.; Brouwer, K.L.R.; Tweedie, D.; Yoshida, K.; Sjöstedt, N.; Aleksunes, L.; Chu, X.; Evers, R.; Hafey, M.J.; Lai, Y.; et al. Membrane Transporters in Drug Development and as Determinants of Precision Medicine. Nat. Rev. Drug Discov. 2024, 23, 255–280. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Morishita, Y.; Tanaka, Y. The Evolutionary Aspects of Aquaporin Family. Adv. Exp. Med. Biol. 2017, 969, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins at a Glance. J. Cell Sci. 2011, 124, 2107–2112. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.B.; Rittig, S.; Fenton, R.A. Nephrogenic Diabetes Insipidus: Essential Insights into the Molecular Background and Potential Therapies for Treatment. Endocr. Rev. 2013, 34, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 Deletion in Mice Reduces Brain Edema after Acute Water Intoxication and Ischemic Stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Hibuse, T.; Funahashi, T. Role of Aquaporin-7 and Aquaporin-9 in Glycerol Metabolism; Involvement in Obesity. In Handbook of Experimental Pharmacology; Beitz, E., Ed.; Springer Naure: Berlin, Germany, 2009; Volume 190, pp. 233–249. [Google Scholar] [CrossRef]
- Berry, V.; Francis, P.; Kaushal, S.; Moore, A.; Bhattacharya, S. Missense Mutations in MIP Underlie Autosomal Dominant ‘polymorphic’ and Lamellar Cataracts Linked to 12q. Nat. Genet. 2000, 25, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin 4 and Neuromyelitis Optica. Lancet Neurol. 2012, 11, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in Clinical Medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Tradtrantip, L.; Jin, B.J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. Adv. Exp. Med. Biol. 2017, 969, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but Elusive Drug Targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Yool, A.J.; Bill, R.M. Recent Breakthroughs and Future Directions in Drugging Aquaporins. Trends Pharmacol. Sci. 2022, 43, 30–42. [Google Scholar] [CrossRef]
- Preston, G.M.; Jung, J.S.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [CrossRef]
- Yang, B.; Kim, J.K.; Verkman, A.S. Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 2006, 580, 6679–6684. [Google Scholar] [CrossRef] [PubMed]
- van Hoek, A.N.; de Jong, M.D.; van Os, C.H. Effects of dimethylsulfoxide and mercurial sulfhydryl reagents on water and solute permeability of rat kidney brush border membranes. Biochim. Biophys. Acta 1990, 1030, 203–210. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2023: An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2024, 29, 585. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine Natural Products: A New Wave of Drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from Sea Cucumbers (Holothuroids): Chemical Structures, Properties, Taxonomic Distribution, Biosynthesis and Evolution. J. Nat. Toxins 1999, 8, 235–248. [Google Scholar] [PubMed]
- Kalinin, V.I.; Ivanchina, N.V.; Krasokhin, V.B.; Makarieva, T.N.; Stonik, V.A. Glycosides from Marine Sponges (Porifera, Demospongiae): Structures, Taxonomical Distribution, Biological Activities and Biological Roles. Mar. Drugs 2012, 10, 1671–1710. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer Activity of Sea Cucumber Triterpene Glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Sim, E.H.; Han, S.H.; Kim, T.R.; Ju, M.H.; Han, J.Y.; Jeong, J.S.; Kim, S.H.; Silchenko, A.S.; Stonik, V.A.; et al. In Vitro and In Vivo Anti-Leukemic Effects of Cladoloside C2 Are Mediated by Activation of Fas/Ceramide Synthase 6/P38 Kinase/c-Jun NH2-Terminal Kinase/Caspase-8. Oncotarget 2017, 9, 495–511. [Google Scholar] [CrossRef]
- Menchinskaya, E.S.; Dyshlovoy, S.A.; Venz, S.; Jacobsen, C.; Hauschild, J.; Rohlfing, T.; Silchenko, A.S.; Avilov, S.A.; Balabanov, S.; Bokemeyer, C.; et al. Anticancer Activity of the Marine Triterpene Glycoside Cucumarioside A2-2 in Human Prostate Cancer Cells. Mar. Drugs 2024, 22, 20. [Google Scholar] [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Inamoto, T.; Yasuzawa, T.; Kyogoku, Y. The Structures of Six Antifungal Oligoglycosides, Stichlorosides A1, A2, B1, B2, C1, and C2, from the Sea Cucumber Stichopus Chloronotus (BRANDT). Chem. Pharm. Bull. 1981, 29, 2387–2391. [Google Scholar] [CrossRef]
- Stonik, V.A.; Mal’tsev, I.I.; Kalinovskii, A.I.; Conde, C.; Elyakov, G.B. Glycosides of Marine Invertegrates. XI. Two New Triterpene Glycosides from Holothurians of the family Stichopadidae. Chem. Nat. Compd. 1982, 18, 177–182. [Google Scholar] [CrossRef]
- Yun, S.H.; Park, E.S.; Shin, S.W.; Na, Y.W.; Han, J.Y.; Jeong, J.S.; Shastina, V.V.; Stonik, V.A.; Park, J.I.; Kwak, J.Y. Stichoposide C Induces Apoptosis through the Generation of Ceramide in Leukemia and Colorectal Cancer Cells and Shows In Vivo Antitumor Activity. Clin. Cancer Res. 2012, 18, 5934–5948. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Dyshlovoy, S.A.; Kuzmich, A.S.; Shubina, L.; Avilov, S.A.; Silchenko, A.S.; Bode, A.M.; Dong, Z.; Stonik, V.A. In Vitro Anticancer Activities of Some Triterpene Glycosides from Holothurians of Cucumariidae, Stichopodidae, Psolidae, Holothuriidae and Synaptidae Families. Nat. Prod. Commun. 2016, 11, 1239–1242. [Google Scholar] [PubMed]
- Park, J.I.; Bae, H.R.; Kim, C.G.; Stonik, V.A.; Kwak, J.Y. Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front. Chem. 2014, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Y.; Franco, C.M.M. Acetylated Triterpene Glycosides and Their Biological Activity from Holothuroidea Reported in the Past Six Decades. Mar. Drugs 2016, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Denisenko, V.A.; Stonik, V.A.; Milgrom, Y.M.; Rashkes, Y.V. Rhizochalin, a Novel Secondary Metabolite of Mixed Biosynthesis from the Sponge Rhizochalina incrustata. Tetrahedron Lett. 1989, 30, 6581–6584. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Santalova, E.A.; Pokanevich, E.V.; Molinski, T.F.; Stonik, V.A. Rhizochalin A, a Novel Two-Headed Sphingolipid from the Sponge Rhizochalina incrustata. J. Nat. Prod. 2005, 68, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Makarieva, T.N.; Stonik, V.A. (-)-Rhizochalin Is a Dimeric Enantiomorphic (2R)-Sphingolipid: Absolute Configuration of Pseudo-C(2v)-Symmetric Bis-2-Amino-3-Alkanols by CD. Angew. Chem. Int. Ed. Engl. 2000, 39, 4076–4079. [Google Scholar] [CrossRef]
- Pruett, S.T.; Bushnev, A.; Hagedorn, K.; Adiga, M.; Haynes, C.A.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H. Biodiversity of Sphingoid Bases (“sphingosines”) and Related Amino Alcohols. J. Lipid Res. 2008, 49, 1621–1639. [Google Scholar] [CrossRef]
- Nicholas, G.M.; Hong, T.W.; Molinski, T.F.; Lerch, M.L.; Cancilla, M.T.; Lebrilla, C.B. Oceanapiside, an Antifungal Bis-Alpha,Omega-Amino Alcohol Glycoside from the Marine Sponge Oceanapia Phillipensis. J. Nat. Prod. 1999, 62, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Shastina, V.; Park, J.I.; Han, J.Y.; Makarieva, T.; Fedorov, S.; Rasskazov, V.; Stonik, V.A.; Kwak, J.Y. Differential Induction of Apoptosis of Leukemic Cells by Rhizochalin, Two Headed Sphingolipids from Sponge and Its Derivatives. Biol. Pharm. Bull. 2009, 32, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Makarieva, T.N.; Guzii, A.G.; Shubina, L.K.; Kwak, J.Y.; Stonik, V.A. Marine Two-Headed Sphingolipid-like Compound Rhizochalin Inhibits EGF-Induced Transformation of JB6 P+ Cl41 Cells. Lipids 2009, 44, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Hauschild, J.; Venz, S.; Krisp, C.; Kolbe, K.; Zapf, S.; Heinemann, S.; Fita, K.D.; Shubina, L.K.; Makarieva, T.N.; et al. Rhizochalinin Exhibits Anticancer Activity and Synergizes with EGFR Inhibitors in Glioblastoma In Vitro Models. Mol. Pharm. 2023, 20, 4994–5005. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Physiological Roles of Glycerol-Transporting Aquaporins: The Aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef]
- Rojek, A.; Praetorius, J.; Frøkiaer, J.; Nielsen, S.; Fenton, R.A. A Current View of the Mammalian Aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Delporte, C. Involvement of Aquaglyceroporins in Energy Metabolism in Health and Disease. Biochimie 2021, 188, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Sohara, E.; Rai, T.; Miyazaki, J.; Verkman, A.S.; Sasaki, S.; Uchida, S. Defective Water and Glycerol Transport in the Proximal Tubules of AQP7 Knockout Mice. Am. J. Physiol. Ren. Physiol. 2005, 289, F1195–F1200. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Perret, J.; Delporte, C. Aquaglyceroporins: Drug Targets for Metabolic Diseases? Front. Physiol. 2018, 9, 851. [Google Scholar] [CrossRef]
- Pimpão, C.; Wragg, D.; da Silva, I.V.; Casini, A.; Soveral, G. Aquaglyceroporin Modulators as Emergent Pharmacological Molecules for Human Diseases. Front. Mol. Biosci. 2022, 9, 845237. [Google Scholar] [CrossRef]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Galán Cobo, A.; Echevarría, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting Aquaporin Function: Potent Inhibition of Aquaglyceroporin-3 by a Gold-Based Compound. PLoS ONE 2012, 7, e37435. [Google Scholar] [CrossRef] [PubMed]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a Potent Copper-Based Aquaporin Inhibitor with Cytotoxic Effect against Cancer Cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Jelen, S.; Wacker, S.; Aponte-Santamaría, C.; Skott, M.; Rojek, A.; Johanson, U.; Kjellbom, P.; Nielsen, S.; de Groot, B.L.; Rützler, M. Aquaporin-9 Protein Is the Primary Route of Hepatocyte Glycerol Uptake for Glycerol Gluconeogenesis in Mice. J. Biol. Chem. 2011, 286, 44319–44325. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and Characterization of Potent and Selective Aquaporin-3 and Aquaporin-7 Inhibitors. J. Biol. Chem. 2019, 294, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- van Hoek, A.N.; Verkman, A.S. Functional reconstitution of the isolated erythrocyte water channel CHIP28. J. Biol. Chem. 1992, 267, 18267–18269. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fukuda, N.; van Hoek, A.; Matthay, M.A.; Ma, T.; Verkman, A.S. Carbon Dioxide Permeability of Aquaporin-1 Measured in Erythrocytes and Lung of Aquaporin-1 Null Mice and in Reconstituted Proteoliposomes. J. Biol. Chem. 2000, 275, 2686–2692. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).