Protection of Tight Junctional Complexes between hCMEC/D3 Cells by Deep-Sea Fibrinolytic Compound FGFC1
Abstract
:1. Introduction
2. Results
2.1. The Construction of Barrier In Vitro Model in hCMEC/D3 Cells
2.2. The H2O2-Induced Barrier Dysfunction of hCMEC/D3 Cells
2.3. The Protection of TJCs in hCMEC/D3 Cells from Barrier Dysfunction by FGFC1
2.4. The Alleviation by FGFC1 of TJC Depression Related to Barrier Dysfunction in hCMEC/D3 Cells
2.5. The Inhibitory Effects of FGFC1 on the Down-Regulation of TJCs’ mRNA Expression in hCMEC/D3 Cells
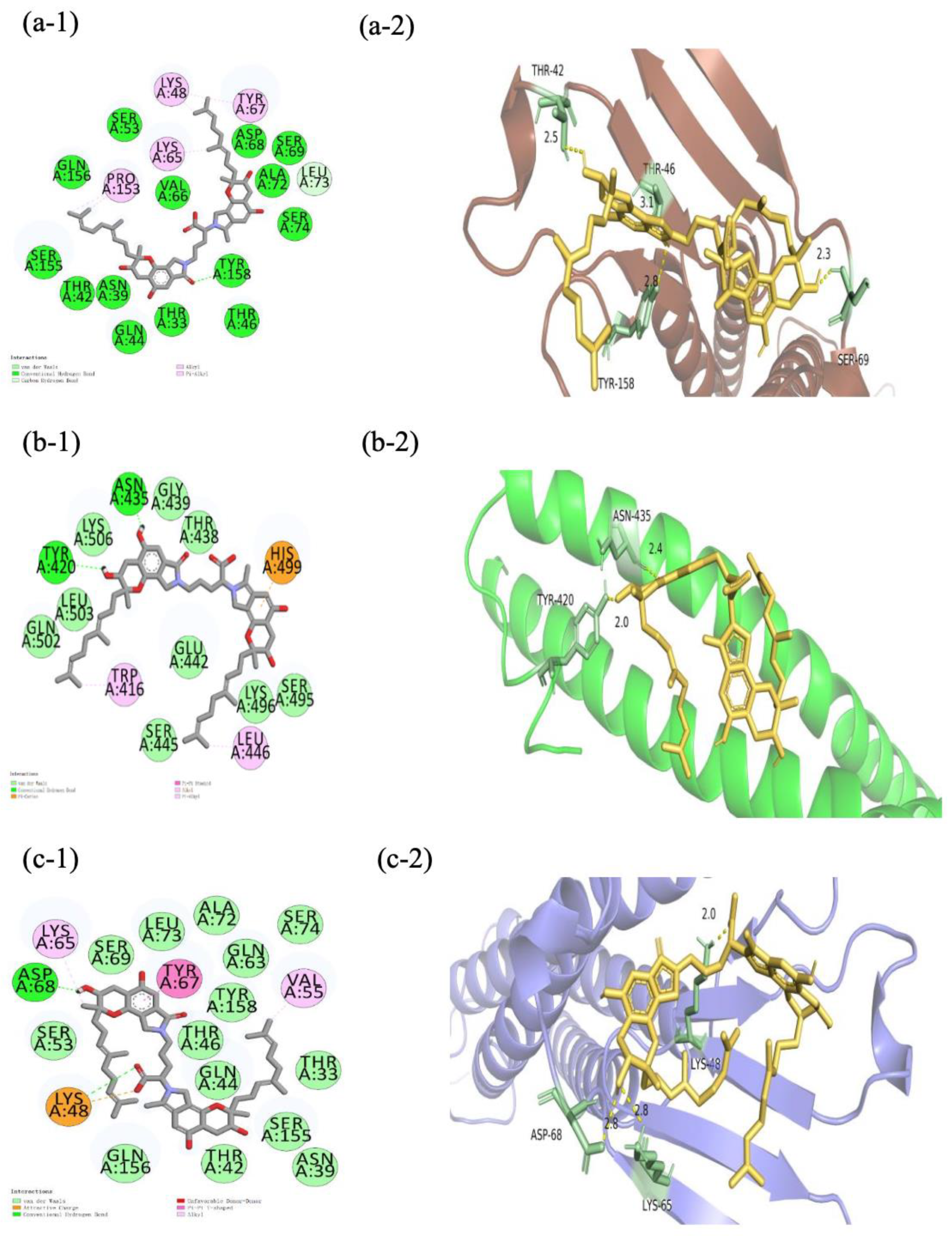
2.6. Molecular Docking of FGFC1 to TJC Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. The Preparation of the FGFC1 Solutions
4.3. Cell Cultures
4.4. CCK-8 Assay
4.5. Molecular Docking
4.6. TEER Assay
4.7. Immunofluorescent Staining Microscopy
4.8. RNA Extraction from hCMEC/D3 Cells and Quantitative RT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Rihani, S.B.; Batarseh, Y.S.; Kaddoumi, A. The Blood-Brain Barrier in Health and Disease. Int. J. Mol. Sci. 2023, 24, 9261. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914–931. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Han, H.; Li, B.; Guo, Z.; Fu, J.; Wu, W. The Rare Marine Bioactive Compounds in Neurological Disorders and Diseases: Is the Blood-Brain Barrier an Obstacle or a Target? Mar. Drugs 2023, 21, 406. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef] [PubMed]
- Romanitan, M.O.; Popescu, B.O.; Winblad, B.; Bajenaru, O.; Bogdanovic, N. Occludin is overexpressed in Alzheimer’s disease and vascular dementia. J. Cell. Mol. Med. 2007, 11, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Di, G.; Gao, C.C.; He, G.; Wang, X.; Han, Y.L.; Sun, L.A.; Zhou, M.L.; Jiang, X. FTY720 Reduces Endothelial Cell Apoptosis and Remodels Neurovascular Unit after Experimental Traumatic Brain Injury. Int. J. Med. Sci. 2021, 18, 304–313. [Google Scholar] [CrossRef]
- Pomilio, C.; Presa, J.; Oses, C.; Vinuesa, A.; Bentivegna, M.; Gregosa, A.; Riudavets, M.; Sevlever, G.; Galvan, V.; Levi, V.; et al. Loss of Direct Vascular Contact to Astrocytes in the Hippocampus as an Initial Event in Alzheimer’s Disease. Evidence from Patients, In Vivo and In Vitro Experimental Models. Mol. Neurobiol. 2024, 61, 5142–5160. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Qiao, S.; Yang, D.; Li, Z.; Xu, J.; Li, W.; Su, L.; Liu, W. Occludin degradation makes brain microvascular endothelial cells more vulnerable to reperfusion injury in vitro. J. Neurochem. 2021, 156, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Denes, L.; de-Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Lonikar, N.; Choudhari, P.; Bhusnuare, O. Insilico analysis of marine indole alkaloids for design of adenosine A2A receptor antagonist. J. Biomol. Struct. Dyn. 2021, 39, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Saito, A.; Fujimura, M.; Shimizu, H.; Mekawy, M.; Hasumi, K.; Tominaga, T. Stachybotrys microspora triprenyl phenol-7, a novel fibrinolytic agent, suppresses superoxide production, matrix metalloproteinase-9 expression, and thereby attenuates ischemia/reperfusion injury in rat brain. Neurosci. Lett. 2011, 503, 110–114. [Google Scholar] [CrossRef]
- Hang, S.; Chen, H.; Wu, W.; Wang, S.; Fang, Y.; Sheng, R.; Tu, Q.; Guo, R. Progress in Isoindolone Alkaloid Derivatives from Marine Microorganism: Pharmacology, Preparation, and Mechanism. Mar. Drugs 2022, 20, 405. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, S.; Zhang, B.; Duan, N.; Zhou, R.; Yan, S.; Elango, J.; Liu, N.; Wu, W. FGFC1 Exhibits Anti-Cancer Activity via Inhibiting NF-κB Signaling Pathway in EGFR-Mutant NSCLC Cells. Mar. Drugs 2022, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Hashimoto, T.; Nobe, K.; Hasumi, K.; Honda, K. A novel finding of a low-molecular-weight compound, SMTP-7, having thrombolytic and anti-inflammatory effects in cerebral infarction of mice. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 382, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wu, W.; Su, T.; Chen, J.; Zhu, Q.; Zhang, C.; Wang, X.; Bao, B. Effects of a novel marine natural product: Pyrano indolone alkaloid fibrinolytic compound on thrombolysis and hemorrhagic activities in vitro and in vivo. Arch. Pharm. Res. 2015, 38, 1530–1540. [Google Scholar] [CrossRef]
- Su, T.; Bao, B.; Yan, T.; Zhang, C.; Bu, Y.; Wu, W. Response surface methodology to optimize marine microbe culture for producing fungi fibrinolytic compound. Sheng Wu Gong Cheng Xue Bao 2013, 29, 857–861. [Google Scholar]
- Wang, Y.; Chen, H.; Sheng, R.; Fu, Z.; Fan, J.; Wu, W.; Tu, Q.; Guo, R. Synthesis and Bioactivities of Marine Pyran-Isoindolone Derivatives as Potential Antithrombotic Agents. Mar. Drugs 2021, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Anasooya Shaji, C.; Robinson, B.D.; Yeager, A.; Beeram, M.R.; Davis, M.L.; Isbell, C.L.; Huang, J.H.; Tharakan, B. The Tri-phasic Role of Hydrogen Peroxide in Blood-Brain Barrier Endothelial cells. Sci. Rep. 2019, 9, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Yamada, K.; Shibata, Y.; Imada, C. Metabolites Produced by a New Lactiplantibacillus plantarum Strain BF1-13 Isolated from Deep Seawater of Izu-Akazawa Protect the Intestinal Epithelial Barrier from the Dysfunction Induced by Hydrogen Peroxide. Mar. Drugs 2022, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Lye, M.F.; Anderson, J.M.; Lavie, A. Domain swapping within PDZ2 is responsible for dimerization of ZO proteins. J. Biol. Chem. 2007, 282, 37710–37716. [Google Scholar] [CrossRef] [PubMed]




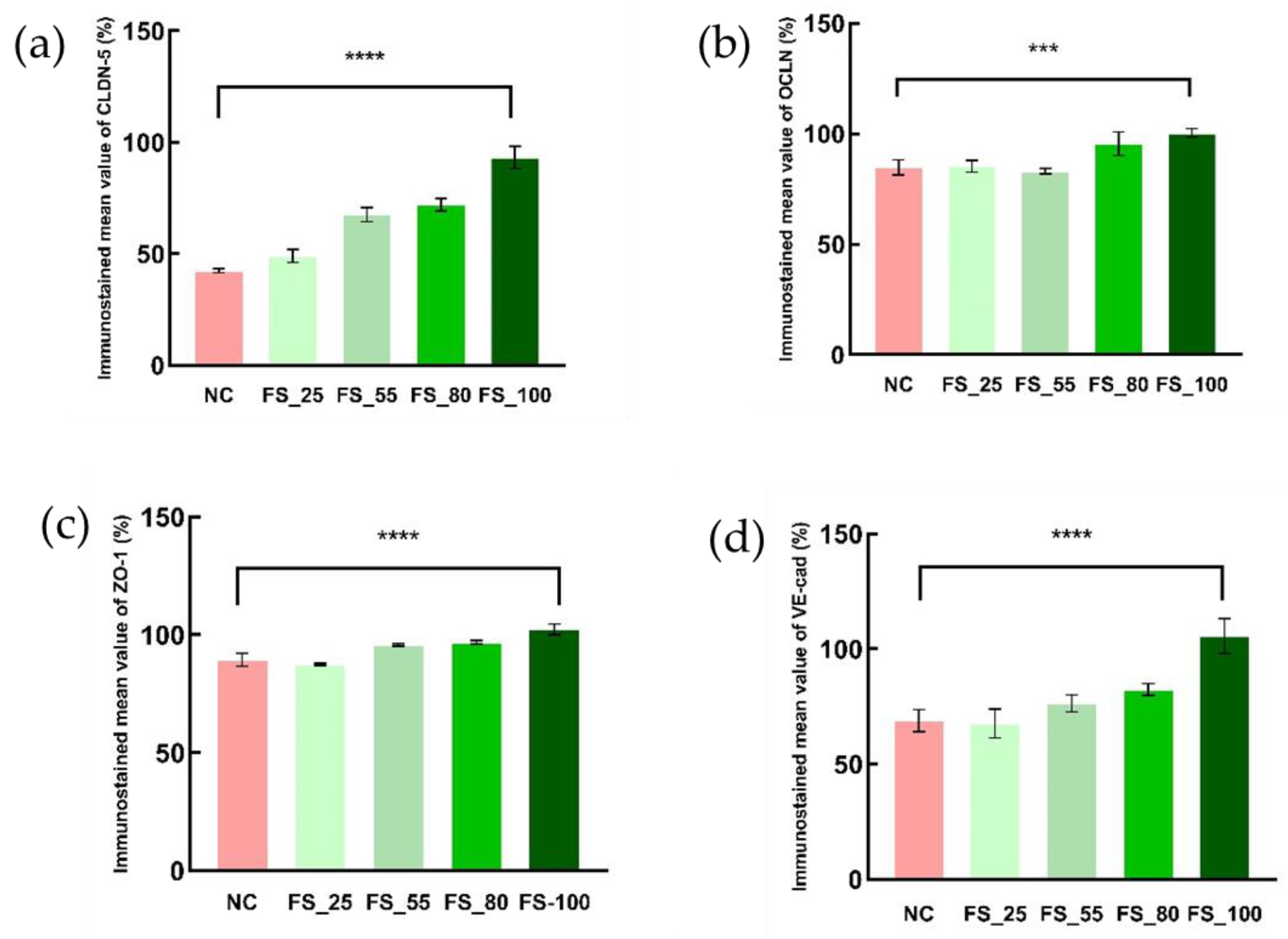



| Mean Integral Optical Density (Relative to the Blank) | NC | FS | |||
|---|---|---|---|---|---|
| 25 µM | 55 µM | 80 µM | 100 µM | ||
| CLDN-5 | 42.51% | 49.10% | 67.64% | 72.11% | 93.29% |
| OCLN | 84.76% | 85.29% | 83.09% | 95.52% | 100.37% |
| ZO-1 | 89.38% | 87.48% | 95.75% | 96.83% | 102.33% |
| VE-cad | 68.91% | 67.67% | 76.50% | 82.42% | 105.67% |
| FGFC1-CLDN-5 | FGFC1-OCLN | FGFC1-ZO-1 | FGFC1-VE-cad | |||
|---|---|---|---|---|---|---|
| (PDZ1) | (PDZ2) | (PDZ3) | ||||
| Hydrogen bonding | TYR158 | TYR420, ASN435 | SER:57, SER:39, SER:57, ARG:74 | LYS87, SER6 | ASP86 | |
| Electrostatic | HIS499 | LYS B:8 | LYS48 | |||
| Hydrophobic | LEU73, PRO158, LYS65, LYS48, TYR68 | TRP416, LEU446 | LYS109, ARG108, HIS46, VAL55 | GLU250, TYR198, LEU202, ILE249 | LYS A:8, LYS A:87, GLY45 | VAL55, TYR67, LYS48 |
| Binding energy | −6.9 kcal/mol | −6.1 kcal/mol | −7.7 kcal/mol | −5.4 kcal/mol | −5.0 kcal/mol | −7.2 kcal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, X.; Han, H.; Sun, H.; Zhang, H.; Wu, W. Protection of Tight Junctional Complexes between hCMEC/D3 Cells by Deep-Sea Fibrinolytic Compound FGFC1. Mar. Drugs 2024, 22, 341. https://doi.org/10.3390/md22080341
Diao X, Han H, Sun H, Zhang H, Wu W. Protection of Tight Junctional Complexes between hCMEC/D3 Cells by Deep-Sea Fibrinolytic Compound FGFC1. Marine Drugs. 2024; 22(8):341. https://doi.org/10.3390/md22080341
Chicago/Turabian StyleDiao, Xiaozhen, Hui Han, Haoyu Sun, Haixing Zhang, and Wenhui Wu. 2024. "Protection of Tight Junctional Complexes between hCMEC/D3 Cells by Deep-Sea Fibrinolytic Compound FGFC1" Marine Drugs 22, no. 8: 341. https://doi.org/10.3390/md22080341
APA StyleDiao, X., Han, H., Sun, H., Zhang, H., & Wu, W. (2024). Protection of Tight Junctional Complexes between hCMEC/D3 Cells by Deep-Sea Fibrinolytic Compound FGFC1. Marine Drugs, 22(8), 341. https://doi.org/10.3390/md22080341









