Exploring the Antimicrobial Potential of Hallachrome, a Defensive Anthraquinone from the Marine Worm Halla parthenopeia (Polychaeta)
Abstract
1. Introduction
2. Results
2.1. Antibacterial Susceptibility Testing and MIC Determination
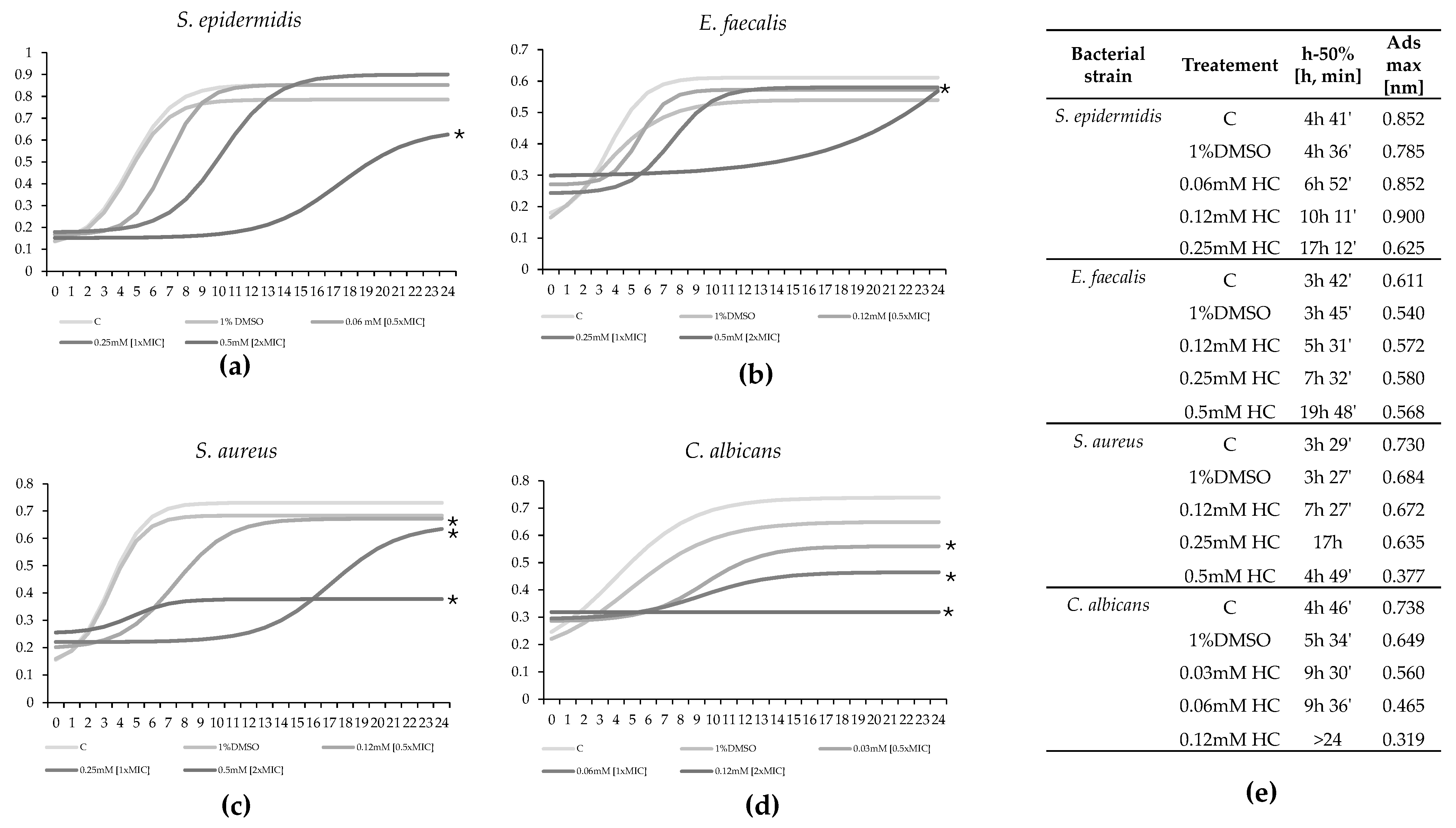
2.2. Time Kill Studies
2.3. Determination of Membrane Permeability Alteration (Cristal Violet Assay)
2.4. HC Activity on Hyphae Formation, Biofilm Formation, and Mature Biofilm
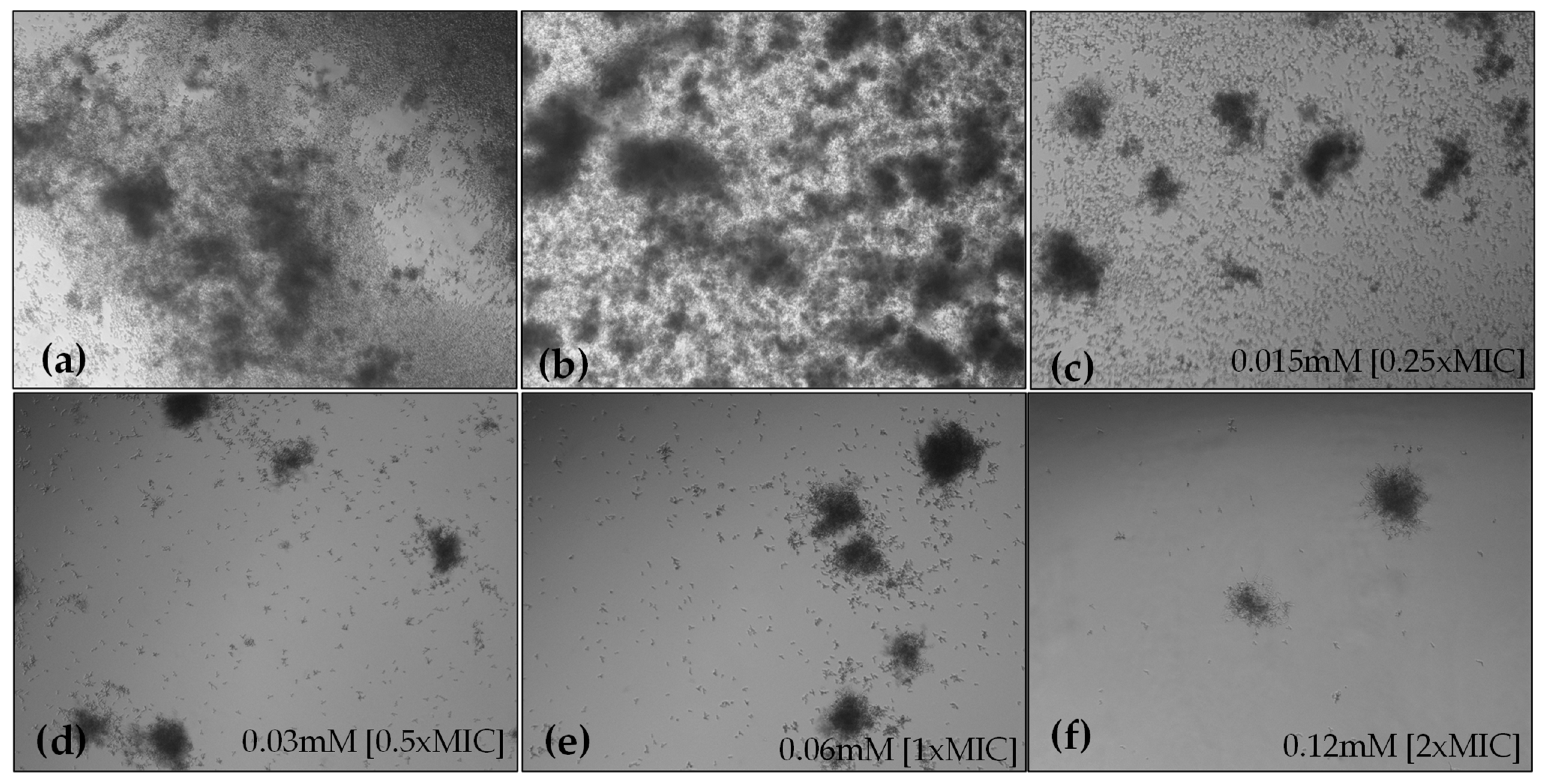
2.4.1. Hyphae Formation
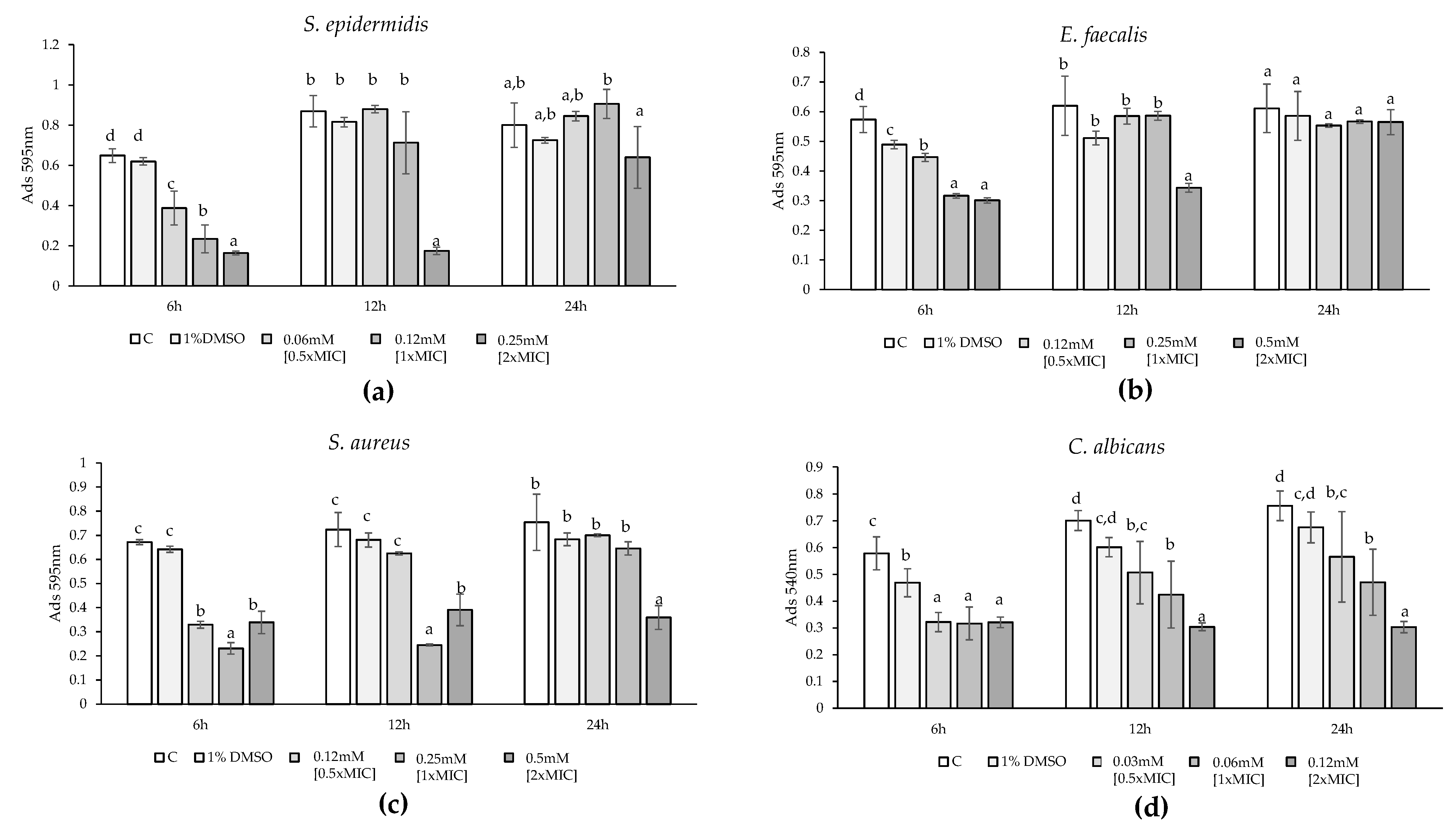
2.4.2. Biofilm Formation
2.4.3. Mature Biofilms
3. Discussion
4. Materials and Methods
4.1. H. parthenopeia Maintenance and Hallachrome Collection
4.2. Microbial Strains, Cultivation, and Chemicals Used in Testing
4.3. Antibacterial Susceptibility Testing and MIC Determination
4.4. Time-Kill Studies
4.5. Determination of Membrane Permeability Alteration via a Cristal Violet Assay
4.6. Determination of HC Activity on Biofilm
4.7. Determination of HC Activity on Biofilm: Microscopic Study
4.8. Determination of HC Activity on Mature Biofilm by Fluorescence Microscopy Assay
4.9. C. albicans Hyphae Formation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- WHO World Antimicrobial Awareness Week-WAAW from 18 to 24 November 2020. Available online: https://www.who.int/campaigns/world-antimicrobial-awareness-week/2020 (accessed on 25 June 2024).
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J., Jr. Trends in Antimicrobial Drug Development: Implications for the Future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- López, Y.; Cepas, V.; Soto, S.M. The Marine Ecosystem as a Source of Antibiotics. In Grand Challenges in Marine Biotechnology; Rampelotto, P., Trincone, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–18. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.; Vásquez, V.; Cabrera, A.; Martínez, M.; Carpio, J. In Silico and In Vitro Identification of 1,8-Dihydroxy-4,5-dinitroanthraquinone as a New Antibacterial Agent against Staphylococcus aureus and Enterococcus faecalis. Molecules 2024, 29, 203. [Google Scholar] [CrossRef]
- Sardi, J.C.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J. Candida Species: Current Epidemiology, Pathogenicity, Biofilm Formation, Natural Antifungal Products and New Therapeutic Options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans Natural Products, Sources of New Antifungal Drugs: A Review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The Effects of Aloe Emodin-Mediated Antimicrobial Photodynamic Therapy on Drug-Sensitive and Resistant Candida albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Long, Y.; Mao, Y.; Jiang, F.; Lu, Y. 2-Alkyl-anthraquinones Inhibit Candida albicans Biofilm via Inhibiting the Formation of Matrix and Hyphae. Res. Microbiol. 2022, 173, 103955. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Eom, Y.B. Zerumbone Inhibits Candida albicans Biofilm Formation and Hyphal Growth. Can. J. Microbiol. 2019, 65, 713–721. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs Over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.C.; Fenical, W. Antibacterials from the Sea. Chemistry 2010, 16, 12512–12525. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.M.; Swaileh, Z.; Ammar, M.; Jaghama, W.; Yousef, M.; Karaman, R.; Bufo, S.A.; Scrano, L. Antifungal and Antibacterial Activities of Isolated Marine Compounds. Toxins 2023, 15, 93. [Google Scholar] [CrossRef]
- Struck, T.; Paul, C.; Hill, N.; Hartmann, S.; Hosel, C.; Kube, M.; Lieb, B.; Meyer, A.; Tiedmannm, R.; Purschke, G.; et al. Phylogenomic Analyses Unravel Annelid Evolution. Nature 2011, 471, 95–98. [Google Scholar] [CrossRef]
- Rodrigo, A.P.; Costa, P.M. The Hidden Biotechnological Potential of Marine Invertebrates: The Polychaeta Case Study. Environ. Res. 2019, 173, 270–280. [Google Scholar] [CrossRef]
- Coutinho, M.C.L.; Teixeira, V.L.; Santos, C.S.G. A Review of “Polychaeta” Chemicals and Their Possible Ecological Role. J. Chem. Ecol. 2018, 44, 72–94. [Google Scholar] [CrossRef]
- Simonini, R.; Iori, D.; Forti, L.; Righi, S.; Prevedelli, D. Ecotoxicity of Hallachrome, an Unusual 1-2 Anthraquinone Excreted by the Infaunal Polychaete Halla parthenopeia: Evidence for a Chemical Defence? Invertebr. Surviv. J. 2019, 16, 84–91. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; Cunha, N.B.; Carneiro, J.A.; Costa, R.A.; Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Cuvillier-Hot, V.; Boidin-Wichlacz, C.; Tasiemski, A. Polychaetes as Annelid Models to Study Ecoimmunology of Marine Organisms. J. Mar. Sci. Technol. 2014, 22, 9–14. [Google Scholar]
- Rajanbabu, V.; Chen, J.Y.; Wu, J.L. Antimicrobial Peptides from Marine Organisms. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 747–758. [Google Scholar] [CrossRef]
- Chain, B.M.; Anderson, R.S. Antibacterial of the Coelomic Fluid from the Polychaeta, Glycera dibranchiata. Partial Purification and Biochemical Characterization of the Active Factor. Biol. Bull. 1983, 164, 41–49. [Google Scholar] [CrossRef]
- Higa, T.; Scheuer, P.J. Thelepin, a New Metabolite from the Marine Annelid Thelepus setosus. J. Am. Chem. Soc. 1974, 96, 2246–2248. [Google Scholar] [CrossRef]
- Stabili, L.; Licciano, M.; Giangrande, A.; Gerardi, C.; De Pascali, S.A.; Fanizzi, F.P. First Insight on the Mucus of the Annelid Myxicola infundibulum (Polychaeta, Sabellidae) as a Potential Prospect for Drug Discovery. Mar. Drugs 2019, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Prota, G.; D’Agostino, M.; Misuraca, G. The Structure of Hallachrome: 7-Hydroxy-8-methoxy-6-methyl-1, 2-Anthraquinone. J. Chem. Soc. Perkin Trans. 1972, 13, 1614–1616. [Google Scholar] [CrossRef]
- Ferri, A.; Righi, S.; Prevedelli, D.; Simonini, R. Optimal Growth and Feeding Behaviour of the Valuable Bait Halla parthenopeia (Polychaeta: Oenonidae) in Small-Scale Aquaculture. Aquac. Res. 2024, 50, 1627–1634. [Google Scholar] [CrossRef]
- Ferri, A.; Costa, P.M.; Simonini, R. Unveiling the Microanatomy of Secretory Organs in Halla parthenopeia (Oenonidae): Implications for the Feeding Strategies and Defence Mechanisms of a Carnivorous Burrowing Polychaete. J. Anat. 2024; submitted. [Google Scholar]
- Diaz-Munoz, G.; Miranda, I.L.; Sartori, S.K.; De Rezende, D.C.; Diaz, M.A. Anthraquinones: An Overview. Stud. Nat. Prod. Chem. 2018, 58, 313–338. [Google Scholar] [CrossRef]
- Malmir, M.; Serrano, R.; Silva, O. Anthraquinones as Potential Antimicrobial Agents—A Review. In Antimicrobial research: Novel bioknowledge and educational programs, 1st ed.; Antonio, M.V., Ed.; Formatex Research Center S.L.: Badajoz, Spain, 2017; Volume 1, pp. 55–61. [Google Scholar]
- Friedman, M.; Xu, A.; Lee, R.; Nguyen, D.N.; Phan, T.A.; Hamada, S.M.; Panchel, R.; Tam, C.C.; Kim, J.H.; Cheng, L.W.; et al. The Inhibitory Activity of Anthraquinones against Pathogenic Protozoa, Bacteria, and Fungi and the Relationship to Structure. Molecules 2020, 25, 3101. [Google Scholar] [CrossRef]
- Qun, T.; Zhou, T.; Hao, J.; Wang, C.; Zhang, K.; Xu, J.; Zhou, W. Antibacterial Activities of Anthraquinones: Structure–Activity Relationships and Action Mechanisms. RSC Med. Chem. 2023, 14, 1446–1471. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Folliero, V.; Palma, F.; Crudele, V.; Finamore, E.; Sanna, G.; Manzin, A.; De Filippis, A.; Galdiero, M.; Franci, G. Anthraquinone Rhein Exhibits Antibacterial Activity against Staphylococcus aureus. Appl. Sci. 2022, 12, 8691. [Google Scholar] [CrossRef]
- Farooq, U.; Khan, S.; Naz, S.; Khan, A.; Khan, A.; Ahmed, A.; Khan, A.R. Three New Anthraquinone Derivatives Isolated from Symplocos racemosa and Their Antibiofilm Activity. Chin. J. Nat. Med. 2017, 15, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; AL-Dhabi, N.A.; Balachandran, C.; Raj, M.K.; Arasu, M.V.; Ignacimuthu, S. Novel 1,5,7-Trihydroxy-3-Hydroxy Methyl Anthraquinone Isolated from Terrestrial Streptomyces sp. (eri-26) with Antimicrobial and Molecular Docking Studies. Appl. Biochem. Biotechnol. 2014, 174, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Ryu, Y.; Lee, J. Calcium-Chelating Alizarin and Other Anthraquinones Inhibit Biofilm Formation and the Hemolytic Activity of Staphylococcus aureus. Sci. Rep. 2016, 6, 19267. [Google Scholar] [CrossRef]
- Chen, H.; Du, K.; Sun, Y.J.; Hao, Z.Y.; Zhang, Y.L.; Bai, J.; Wang, Q.H.; Hu, H.Y.; Feng, W.S. Solanrubiellin A, a Hydroanthraquinone Dimer with Antibacterial and Cytotoxic Activity from Solanum lyratum. Nat. Prod. Res. 2020, 34, 3176–3181. [Google Scholar] [CrossRef]
- Zhang, C.; Ondeyka, J.G.; Zink, D.L.; Basilio, A.; Vicente, F.; Collado, J.; Singh, S.B. Isolation, Structure and Antibacterial Activity of Pleosporone from a Pleosporalean Ascomycete Discovered by Using Antisense Strategy. Bioorg. Med. Chem. 2009, 17, 2162–2166. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, M.; Masłyk, M.; Kubiński, K.; Golczyk, H. Emodin, a Natural Inhibitor of Protein Kinase CK2, Suppresses Growth, Hyphal Development, and Biofilm Formation of Candida albicans. Yeast 2017, 34, 253–265. [Google Scholar] [CrossRef]
- Kang, K.; Fong, W.P.; Tsang, P.W. Novel Antifungal Activity of Purpurin against Candida Species in vitro. Med. Mycol. 2010, 48, 904–911. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Coombes, P.H.; Mulholland, D.A.; Van Staden, J. Emodin, an Antibacterial Anthraquinone from the Roots of Cassia occidentalis. S. Afr. J. Bot. 2006, 72, 295–297. [Google Scholar] [CrossRef]
- Alves, D.S.; Pérez-Fons, L.; Estepa, A.; Micol, V. Membrane-Related Effects Underlying the Biological Activity of the Anthraquinones Emodin and Barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef]
- Raghuveer, D.; Pai, V.V.; Murali, T.S.; Nayak, R. Exploring Anthraquinones as Antibacterial and Antifungal Agents. ChemistrySelect 2023, 8, e202204537. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Yu, J.; Feng, Q.; Zhao, L.; Song, H.; Wang, W. Antibacterial Mode of Action of 1,8-Dihydroxy-Anthraquinone from Porphyra haitanensis against Staphylococcus aureus. Nat. Prod. Res. 2014, 29, 976–979. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [CrossRef]
- Xiang, H.; Cao, F.; Ming, D.; Zheng, Y.; Dong, X.; Zhong, X.; Mu, D.; Li, B.; Zhong, L.; Cao, J.; et al. Aloe-Emodin Inhibits Staphylococcus aureus Biofilms and Extracellular Protein Production at the Initial Adhesion Stage of Biofilm Development. Appl. Microbiol. Biotechnol. 2017, 101, 6671–6681. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm Formation by the Fungal Pathogen Candida albicans: Development, Architecture, and Drug Resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Park, S.J.; Han, K.H.; Park, J.Y.; Choi, S.J.; Lee, K.H. Influence of Bacterial Presence on Biofilm Formation of Candida albicans. Yonsei Med. J. 2014, 55, 449–458. [Google Scholar] [CrossRef]
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus Infection; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Manoharan, R.K.; Lee, J.H.; Kim, Y.G.; Lee, J. Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Gauwerky, K.; Borelli, C.; Korting, H.C. Targeting Virulence: A New Paradigm for Antifungals. Drug Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef]
- Iori, D.; Forti, L.; Massamba-N’Siala, G.; Prevedelli, D.; Simonini, R. Toxicity of the Purple Mucus of the Polychaete Halla parthenopeia (Oenonidae) Revealed by a Battery of Ecotoxicological Bioassays. Sci. Mar. 2014, 78, 589–595. [Google Scholar] [CrossRef]
- CLSI M100-Ed29; CLSI Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; Volume 39.
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (An Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between Natural Products and Antibiotics against Infectious Diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-Related Gene Expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed]




| Bacterial Strain | MIC (mM) | Inhibition Rate |
|---|---|---|
| Candida albicans ATCC 10231 | 0.06 | 97% |
| Enterococcus faecalis ATCC 29212 | 0.25 | 88% |
| Staphylococcus aureus ATCC 6538 | 0.25 | 92% |
| Staphylococcus epidermidis ATCC 12228 | 0.125 | 98% |
| Pseudomonas aeruginosa ATCC9027 | >2 | 0% |
| Escherichia coli ATCC25922 | >2 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferri, A.; Simonini, R.; Sabia, C.; Iseppi, R. Exploring the Antimicrobial Potential of Hallachrome, a Defensive Anthraquinone from the Marine Worm Halla parthenopeia (Polychaeta). Mar. Drugs 2024, 22, 380. https://doi.org/10.3390/md22090380
Ferri A, Simonini R, Sabia C, Iseppi R. Exploring the Antimicrobial Potential of Hallachrome, a Defensive Anthraquinone from the Marine Worm Halla parthenopeia (Polychaeta). Marine Drugs. 2024; 22(9):380. https://doi.org/10.3390/md22090380
Chicago/Turabian StyleFerri, Anita, Roberto Simonini, Carla Sabia, and Ramona Iseppi. 2024. "Exploring the Antimicrobial Potential of Hallachrome, a Defensive Anthraquinone from the Marine Worm Halla parthenopeia (Polychaeta)" Marine Drugs 22, no. 9: 380. https://doi.org/10.3390/md22090380
APA StyleFerri, A., Simonini, R., Sabia, C., & Iseppi, R. (2024). Exploring the Antimicrobial Potential of Hallachrome, a Defensive Anthraquinone from the Marine Worm Halla parthenopeia (Polychaeta). Marine Drugs, 22(9), 380. https://doi.org/10.3390/md22090380









