Two C23-Steroids and a New Isocoumarin Metabolite from Mangrove Sediment-Derived Fungus Penicillium sp. SCSIO 41429
Abstract
1. Introduction
2. Results and Discussion
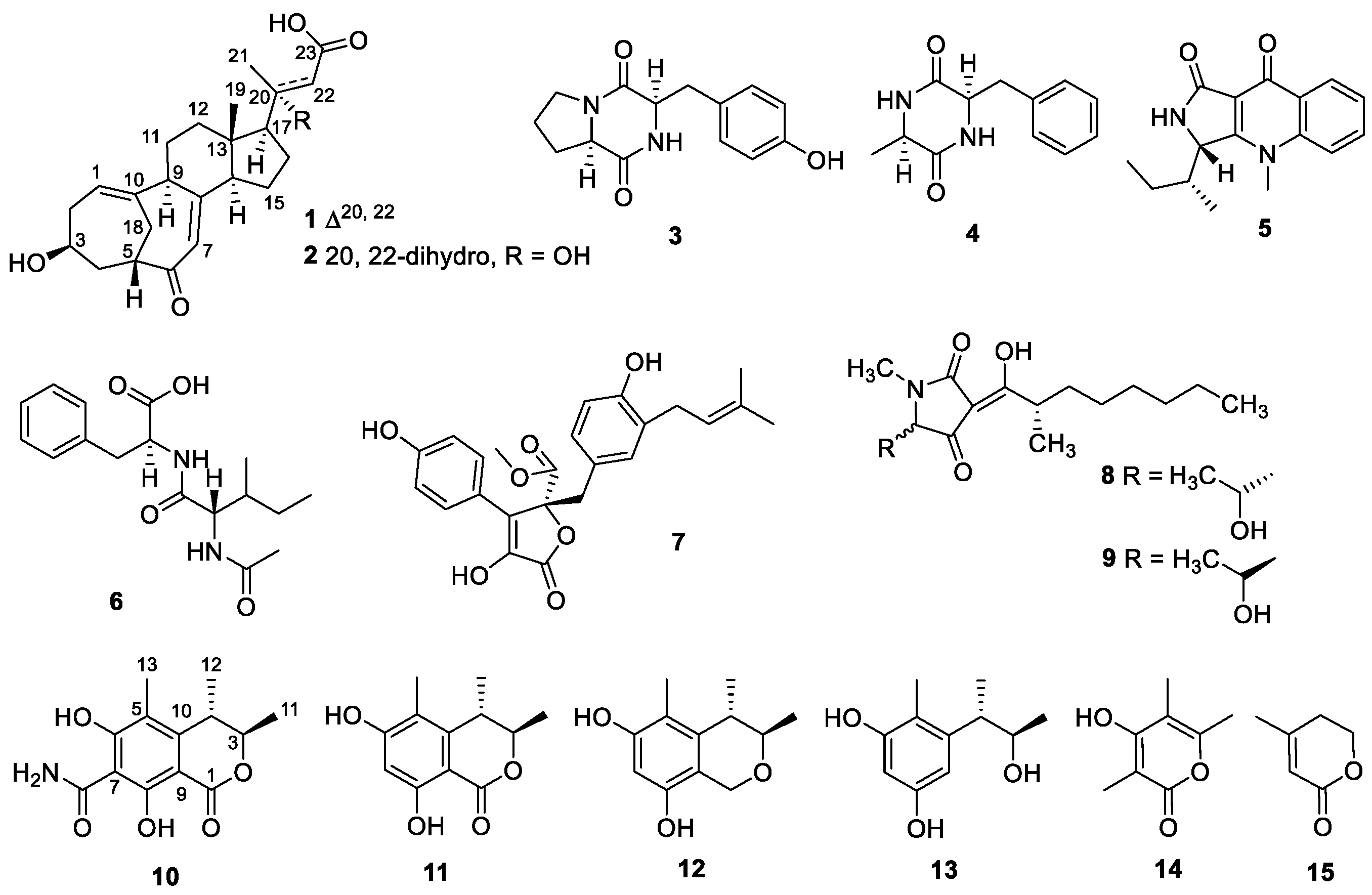
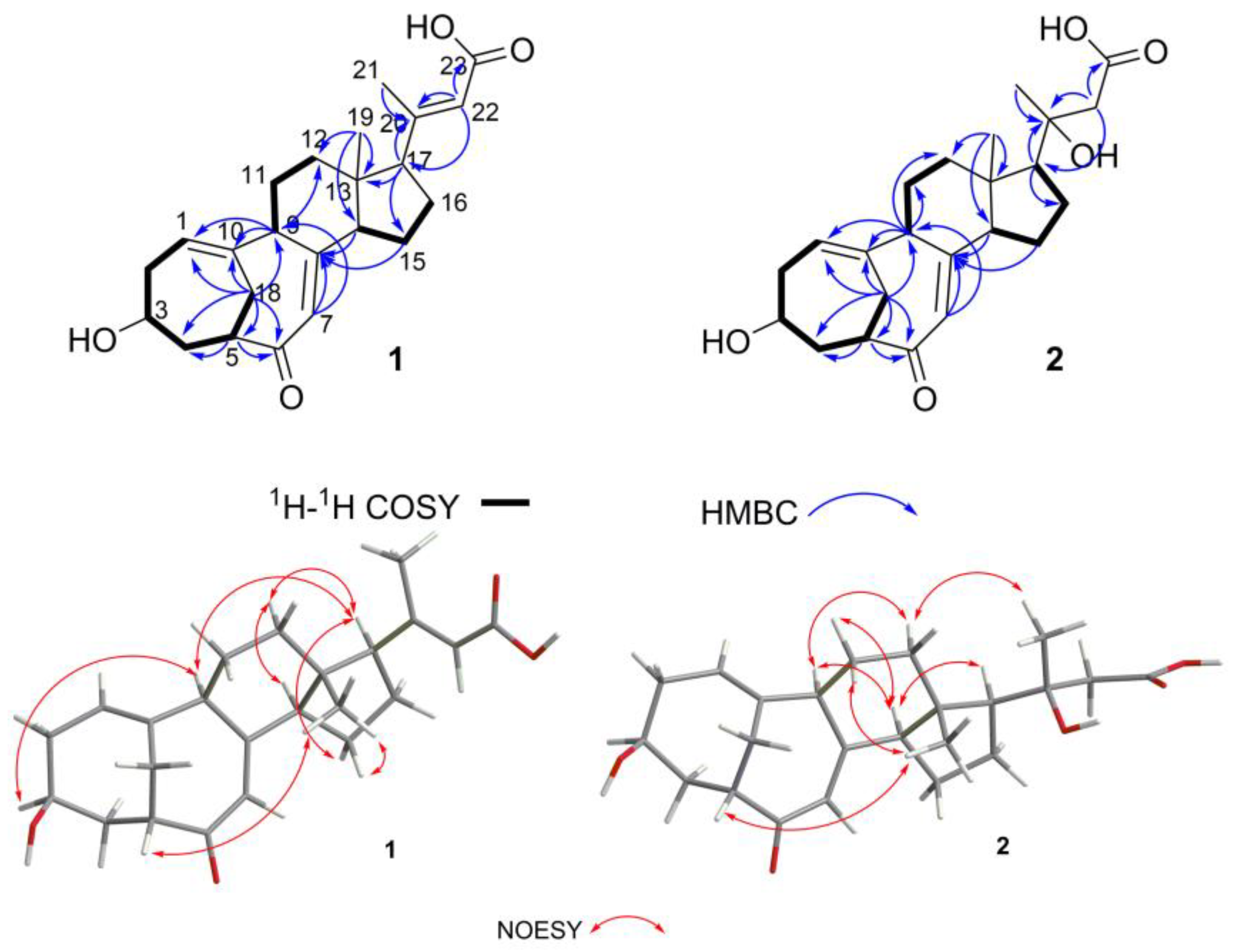
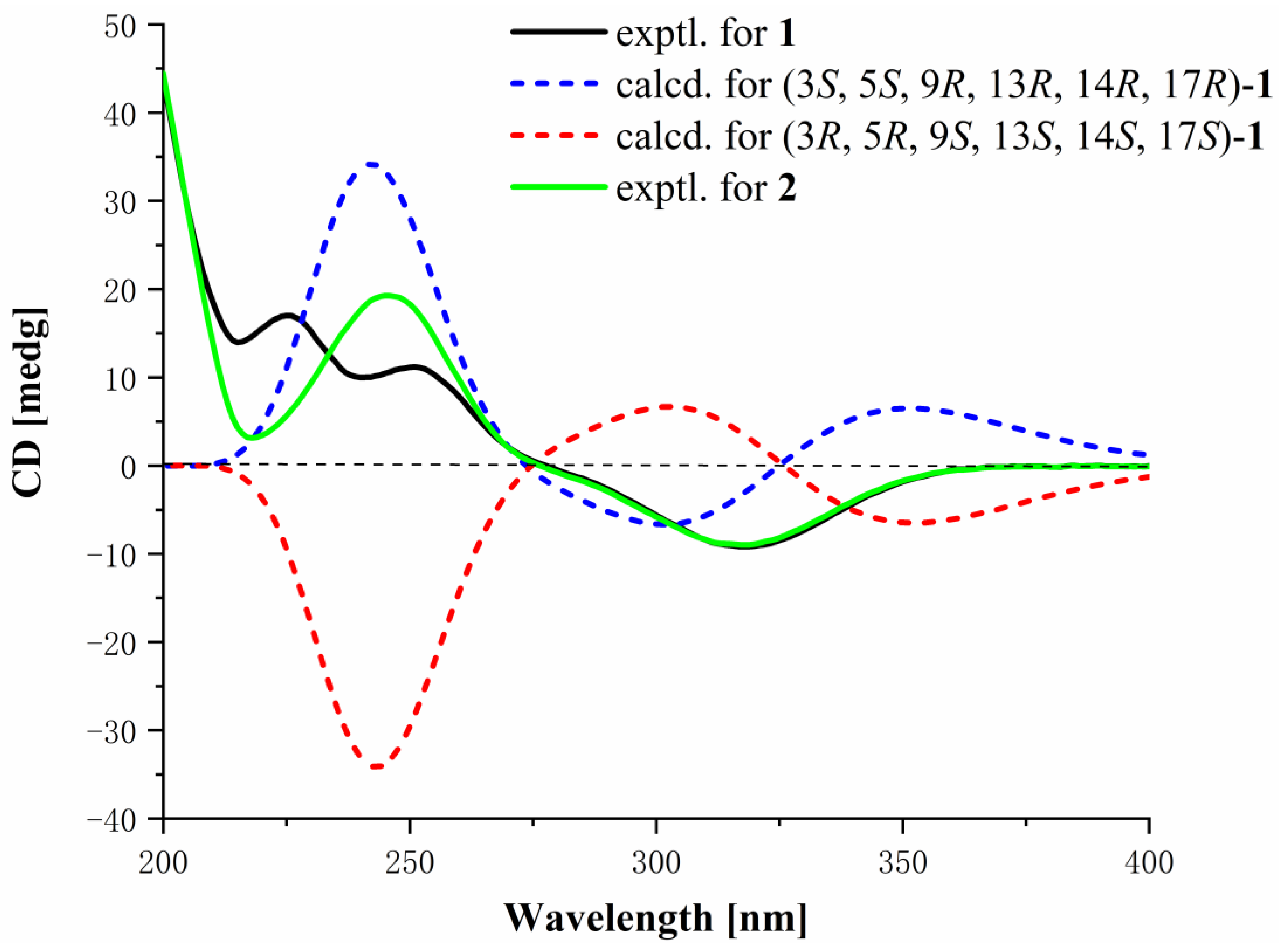
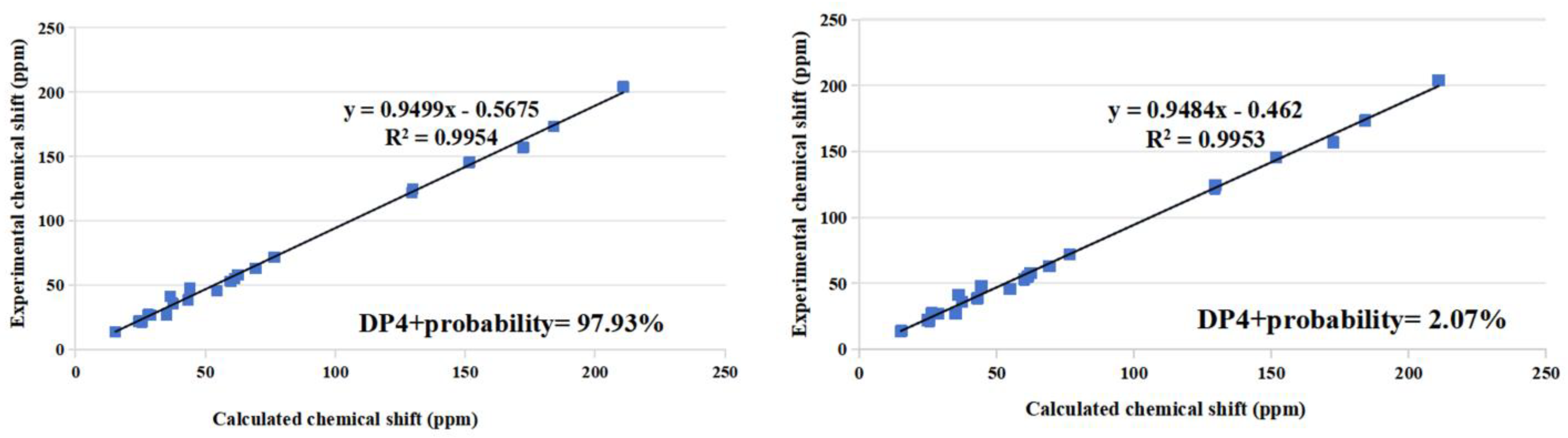
Structural Identification of New Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
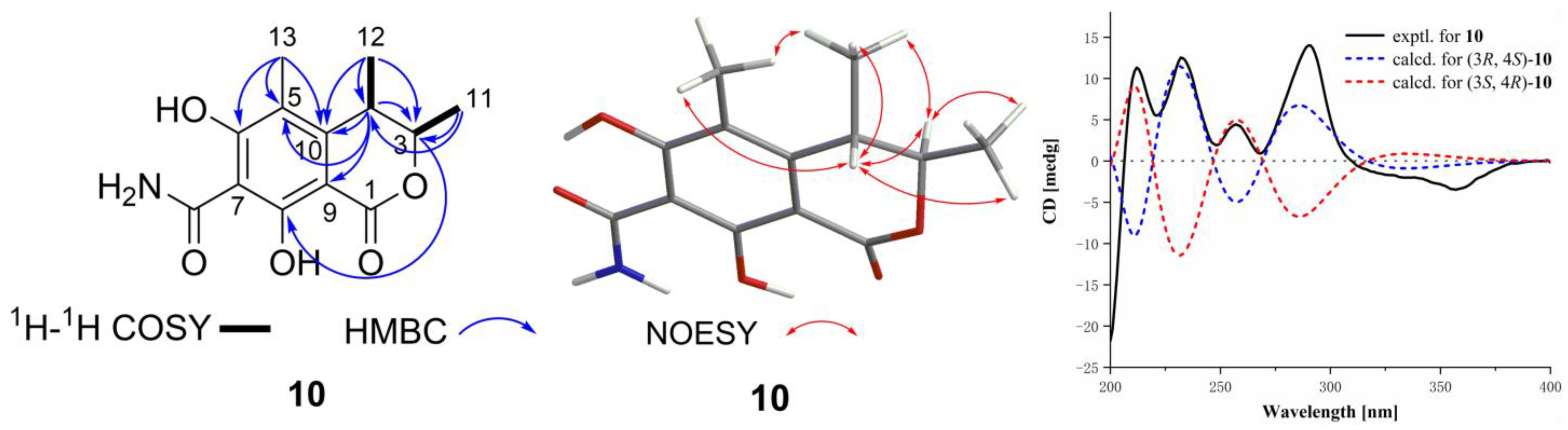
3.4. Structural Elucidation of the New Compounds 1, 2, and 10
3.5. Pancreatic Lipase Inhibition Activity In Vitro Assay
3.6. DPPH Free Radical Scavenging Assay
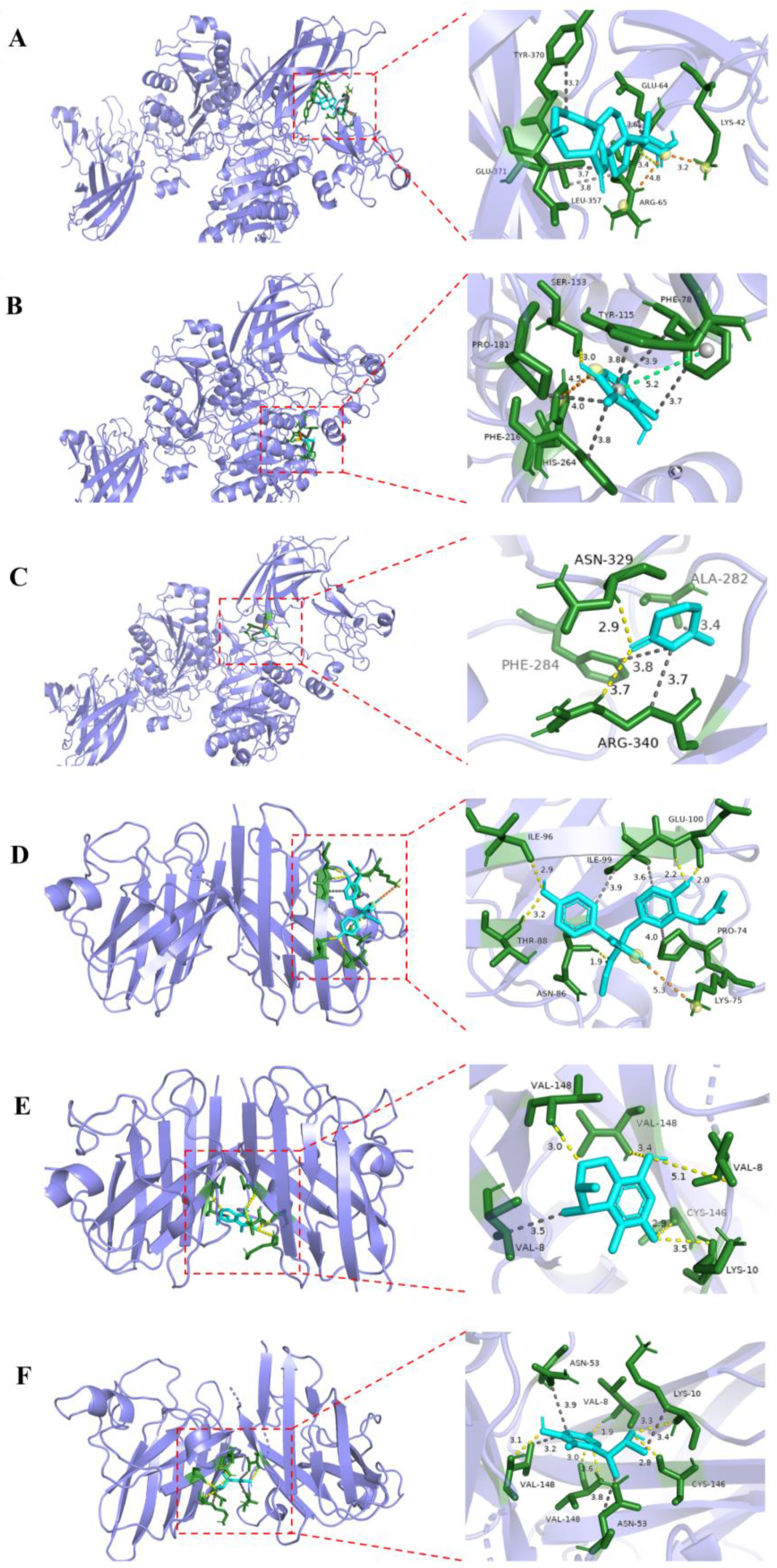
3.7. Molecular Docking Analysis
3.8. ECD Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilding, J. The future of obesity treatment. EXS 2000, 89, 181–191. [Google Scholar] [PubMed]
- Rahul, B.B.; Kamlesh, B.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar]
- Drent, M.L.; Veen, E.A.d.v. First clinical studies with orlistat: A short review. Obes. Res. 1995, 4, 623S–625S. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Gulcin, A. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Fisher, F.J.; Shahriar, M. β-Lactams from the ocean. Mar. Drugs 2023, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Deepika, N.P.; Rahman, M.H.; Duraiswamy, B. The emerging role of marine natural products for the treatment of parkinson’s disease. CNS Neurol. Disord. 2023, 22, 801–816. [Google Scholar] [CrossRef]
- Manoharan, S.; Perumal, E. Potential role of marine bioactive compounds in cancer signaling pathways: A review. Eur. J. Pharmacol. 2022, 936, 175330. [Google Scholar] [CrossRef]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef]
- Zeng, W.; Huang, G.; Wang, B.; Cai, J.; Zheng, C. Secondary metabolites and biological activities of Penicillium spp. fungi of mangrove origin (2007–2020). Chin. J. Org. Chem. 2021, 41, 4255–4278. [Google Scholar] [CrossRef]
- Chen, C.; Chen, W.; Pang, X.; Liao, S.; Wang, J.; Lin, X.; Yang, B.; Zhou, X.; Luo, X.; Liu, Y. Pyrrolyl 4-quinolone alkaloids from the mangrove endophytic fungus Penicillium steckii SCSIO 41025: Chiral resolution, configurational assignment, and enzyme inhibitory activities. Phytochemisty 2021, 186, 112730. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, M.; Chen, C.; Yang, B.; Gao, C.; Liu, Y.; Luo, X.; Tan, Y.; Zhou, X. Peniditerpenoids A and B: Oxidized indole diterpenoids with osteoclast differentiation inhibitory activity from a mangrove-sediment-derived Penicillium sp. J. Nat. Prod. 2024, 87, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Cai, J.; Chen, Y.; Zhu, Y.; Chen, C.; Yang, B.; Zhou, X.; Tao, H. Two new sesquiterpenoids and a new shikimic acid metabolite from mangrove sediment-derived fungus Roussoella sp. SCSIO 41427. Mar. Drugs 2024, 22, 103. [Google Scholar] [CrossRef]
- Chen, C.; Chen, W.; Tao, H.; Yang, B.; Zhou, X.; Luo, X.; Liu, Y. Diversified polyketides and nitrogenous compounds from the mangrove endophytic fungus Penicillium steckii SCSIO 41025. Chin. J. Chem. 2021, 39, 2132–2140. [Google Scholar] [CrossRef]
- Du, L.; Zhu, T.; Fang, Y.; Gu, Q.; Zhu, W. Unusual C25 steroid isomers with bicyclo [4.4.1]A/B rings from a volcano ash-derived fungus Penicillium citrinum. J. Nat. Prod. 2008, 71, 1343–1351. [Google Scholar] [CrossRef]
- Jayatilake, S.G.; Thornton, P.M.; Leonard, C.A.; Grimwade, E.J.; Baker, J.B. Metabolites from an antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef]
- Yang, H.; Wei, N.; Jiang, Q.; Chen, G.; Wen, L. Otoprotective compounds from the metabolites derived from mangrove symbiotic actinomycete Streptomyces sp. 1624105. Chem. Nat. Compd. 2021, 57, 792–794. [Google Scholar] [CrossRef]
- Song, T.; Tang, M.; Ge, H.; Chen, M.; Lian, X.; Zhang, Z. Novel bioactive penicipyrroether A and pyrrospirone J from the marine-derived Penicillium sp. ZZ380. Mar. Drugs 2019, 17, 292. [Google Scholar] [CrossRef]
- Tang, X.; Liu, S.; Yan, X.; Tang, B.; Fang, M.; Wang, X.; Wu, Z.; Qiu, Y. Two new cytotoxic compounds from a deep-sea Penicillum citreonigrum XT20-134. Mar. Drugs 2019, 17, 509. [Google Scholar] [CrossRef]
- Triana, R.D.; Sanro, T.; Sofa, F.; Muhammad, H. α-Glucosidase inhibitor compounds from Aspergillus terreus RCC1 and their antioxidant activity. Med. Chem. Res. 2014, 24, 737–743. [Google Scholar]
- Li, S.; Mou, Q.; Xu, X.; Qi, S.; Leung, P.H.M. Synergistic antibacterial activity between penicillenols and antibiotics against methicillin-resistant Staphylococcus aureus. R. Soc. Open Sci. 2018, 5, 172466. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Tian, L.; Zhu, T.; Wang, W.; Du, L.; Fang, Y.; Gu, Q.; Zhu, W. Isocoumarin derivatives from the sea squirt-derived fungus Penicillium stoloniferum QY2-10 and the halotolerant fungus Penicillium notatum B-52. Arch. Pharm. Res. 2007, 30, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, X.; Ge, Y.; Yin, Q.; Wu, X.; Tang, J.; Zhang, Z.; Wu, B. Citrinin monomer, trimer, and tetracyclic alkaloid derivatives from the hydrothermal vent-associated fungus Penicillium citrinum TW132-59. J. Org. Chem. 2022, 87, 13270–13279. [Google Scholar] [CrossRef]
- Song, T.; Chen, M.; Ge, Z.; Chai, W.; Li, X.; Zhang, Z.; Lian, X. Bioactive penicipyrrodiether A, an adduct of GKK1032 analogue and phenol A derivative, from a marine-sourced fungus Penicillium sp. ZZ380. J. Org. Chem. 2018, 83, 13395–13401. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, L.; Zhou, H.; Hu, H.; Hou, G. Chemical composition and protective effect of guava (Psidium guajava L.) leaf extract on piglet intestines. J. Sci. Food Agric. 2021, 101, 2767–2778. [Google Scholar] [CrossRef]
- Shintani, R.; Hayashi, T. Rhodium-catalyzed asymmetric 1, 4-addition of sodium tetraarylborates to β, β-disubstituted α, β-unsaturated esters. Org. Lett. 2011, 13, 350–352. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, S.B.; Ahn, J.H.; Turk, A.; Kwon, E.B.; Kim, M.O.; Hwang, B.Y.; Lee, M.K. Xanthones from the stems of Cudrania tricuspidata and their inhibitory effects on pancreatic lipase and fat accumulation. Bioorg. Chem. 2019, 92, 103234. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Cai, J.; Long, J.; Yang, B.; Lin, X.; Wang, J.; Xiao, J.; Liu, Y.; Zhou, X. New azaphthalide and phthalide derivatives from the marine coral-derived fungus Aspergillus sp. SCSIO 41405. Phytochem. Lett. 2021, 43, 94–97. [Google Scholar] [CrossRef]
- Yen, G.; Chen, H. Antionxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Cai, J.; Chen, Y.; Zhu, Y.; Yang, B.; Zhou, X.; Liu, Y.; Tao, H. Discovery of enzyme inhibitors from mangrove sediment derived fungus Trichoderma harzianum SCSIO 41051. Chem. Biodivers. 2024, 21, e202400070. [Google Scholar] [CrossRef]
| Pos. | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 122.2, CH | 5.56 (dd, 8.5, 6.0) | 122.0, CH | 5.53 (dd, 8.5, 6.0) |
| 2α | 35.9, CH2 | 2.04~2.08 (overlapped) | 35.9, CH2 | 2.04~2.13 (overlapped) |
| 2β | 2.28~2.37 (overlapped) | 2.34 (m) | ||
| 3 | 63.0, CH | 3.12 (m) | 63.0, CH | 3.12 (m) |
| 4α | 41.4, CH2 | 1.50~1.62 (overlapped) | 41.3, CH2 | 1.41~1.53 (overlapped) |
| 4β | 2.64 (m) | 2.63 (m) | ||
| 5 | 48.1, CH | 2.69 (m) | 48.1, CH | 2.68 (m) |
| 6 | 204.1, C | 204.1, C | ||
| 7 | 124.5, CH | 5.43 (s) | 124.6, CH | 5.39 (s) |
| 8 | 156.5, C | 156.9, C | ||
| 9 | 53.1, CH | 2.86 (dd, 12.0, 5.5) | 53.1, CH | 2.81 (dd, 12.0, 5.5) |
| 10 | 145.4, C | 145.6, C | ||
| 11α | 27.4, CH2 | 1.50~1.62 (overlapped) | 27.2, CH2 | 1.41~1.53 (overlapped) |
| 11β | 1.71~1.80 (overlapped) | 1.64~1.80 (overlapped) | ||
| 12α | 36.9, CH2 | 1.71~1.80 (overlapped) | 38.8, CH2 | 2.04~2.13 (overlapped) |
| 12β | 1.50~1.62 (overlapped) | 1.41~1.53 (overlapped) | ||
| 13 | 47.1, C | 45.7, C | ||
| 14 | 54.5, CH | 2.28~2.37 (overlapped) | 55.3, CH | 2.04~2.13 (overlapped) |
| 15α | 23.9, CH2 | 1.71~1.80 (overlapped) | 22.1, CH2 | 1.41~1.53 (overlapped) |
| 15β | 1.85 (m) | 1.41~1.53 (overlapped) | ||
| 16α | 22.29, CH2 | 1.50~1.62 (overlapped) | 21.6, CH2 | 1.64~1.80 (overlapped) |
| 16β | 1.50~1.62 (overlapped) | 1.87 (m) | ||
| 17 | 59.9, CH | 2.42 (t, 9.5) | 58.0, CH | 1.64~1.80 (overlapped) |
| 18 | 27.2, CH2 | 2.48 (overlapped) | 27.1, CH2 | 2.47 (overlapped) |
| 19 | 13.3, CH3 | 0.53 (s) | 13.8, CH3 | 0.76 (s) |
| 20 | 156.5, C | 72.0, C | ||
| 21 | 19.8, CH3 | 2.10 (s) | 27.4, CH3 | 1.30 (s) |
| 22 | 117.4, CH | 5.66 (s) | 47.9, CH2 | 2.24 (d, 5.0) |
| 23 | 167.6, C | 173.5, C | ||
| Pos. | δC, Type | δH (J in Hz) |
|---|---|---|
| 1 | 170.2, C | |
| 3 | 80.2, CH | 4.86 (q, 6.6) |
| 4 | 33.9, CH | 3.18 (q, 7.1) |
| 5 | 111.9, C | |
| 6 | 168.8, C | |
| 7 | 100.4, C | |
| 8 | 166.0, C | |
| 9 | 94.2, C | |
| 10 | 144.8, C | |
| 11 | 18.6, CH3 | 1.22 (d, 6.6) |
| 12 | 18.6, CH3 | 1.17 (d, 7.1) |
| 13 | 9.1, CH3 | 2.01 (s) |
| 7-CONH2 | 175.1, C | 14.86 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Chen, C.; Cai, J.; Chen, Y.; Zhu, Y.; Yang, B.; Zhou, X.; Liu, Y.; Tao, H. Two C23-Steroids and a New Isocoumarin Metabolite from Mangrove Sediment-Derived Fungus Penicillium sp. SCSIO 41429. Mar. Drugs 2024, 22, 393. https://doi.org/10.3390/md22090393
Huang L, Chen C, Cai J, Chen Y, Zhu Y, Yang B, Zhou X, Liu Y, Tao H. Two C23-Steroids and a New Isocoumarin Metabolite from Mangrove Sediment-Derived Fungus Penicillium sp. SCSIO 41429. Marine Drugs. 2024; 22(9):393. https://doi.org/10.3390/md22090393
Chicago/Turabian StyleHuang, Lishan, Chunmei Chen, Jian Cai, Yixin Chen, Yongyan Zhu, Bin Yang, Xuefeng Zhou, Yonghong Liu, and Huaming Tao. 2024. "Two C23-Steroids and a New Isocoumarin Metabolite from Mangrove Sediment-Derived Fungus Penicillium sp. SCSIO 41429" Marine Drugs 22, no. 9: 393. https://doi.org/10.3390/md22090393
APA StyleHuang, L., Chen, C., Cai, J., Chen, Y., Zhu, Y., Yang, B., Zhou, X., Liu, Y., & Tao, H. (2024). Two C23-Steroids and a New Isocoumarin Metabolite from Mangrove Sediment-Derived Fungus Penicillium sp. SCSIO 41429. Marine Drugs, 22(9), 393. https://doi.org/10.3390/md22090393










