Sphaerococcenol A Derivatives: Design, Synthesis, and Cytotoxicity
Abstract
:1. Introduction
2. Results
2.1. Hemi-Synthesis of New Sphaerococcenol A Analogues
2.2. Cytotoxicity on Malignant Cell Lines
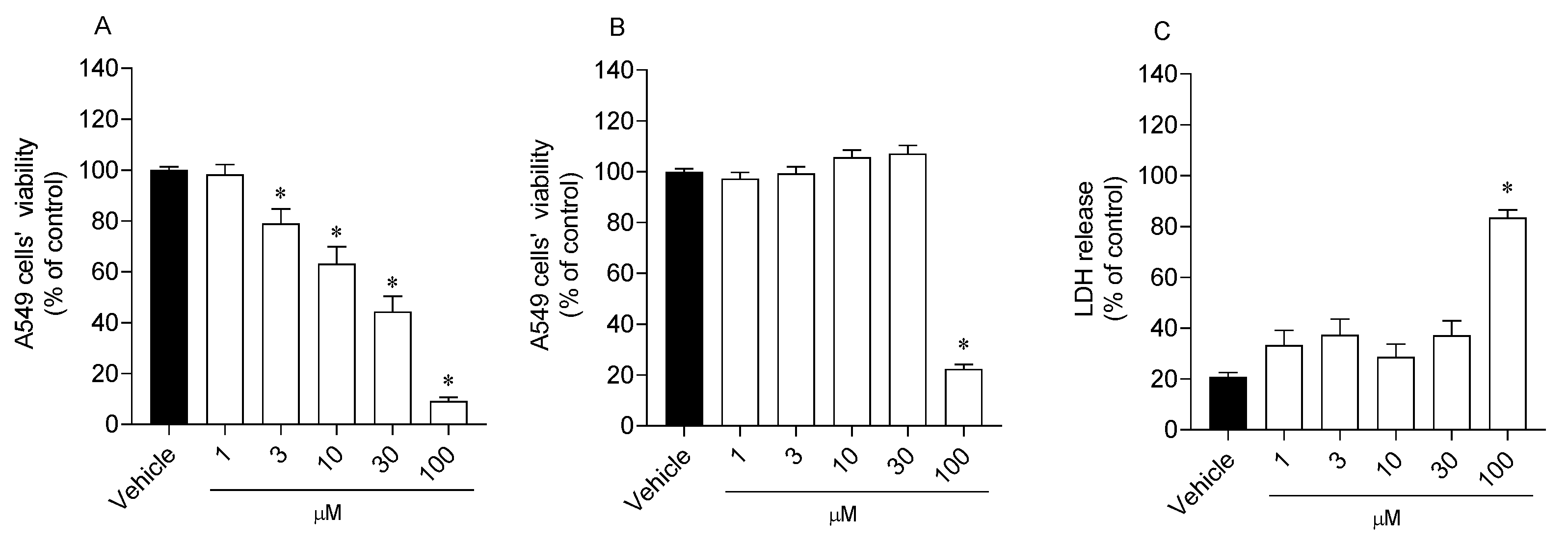
2.3. Effects of Analogue 1 on Cell Viability and Mitochondrial Function
2.4. Reactive Oxygen Species Levels
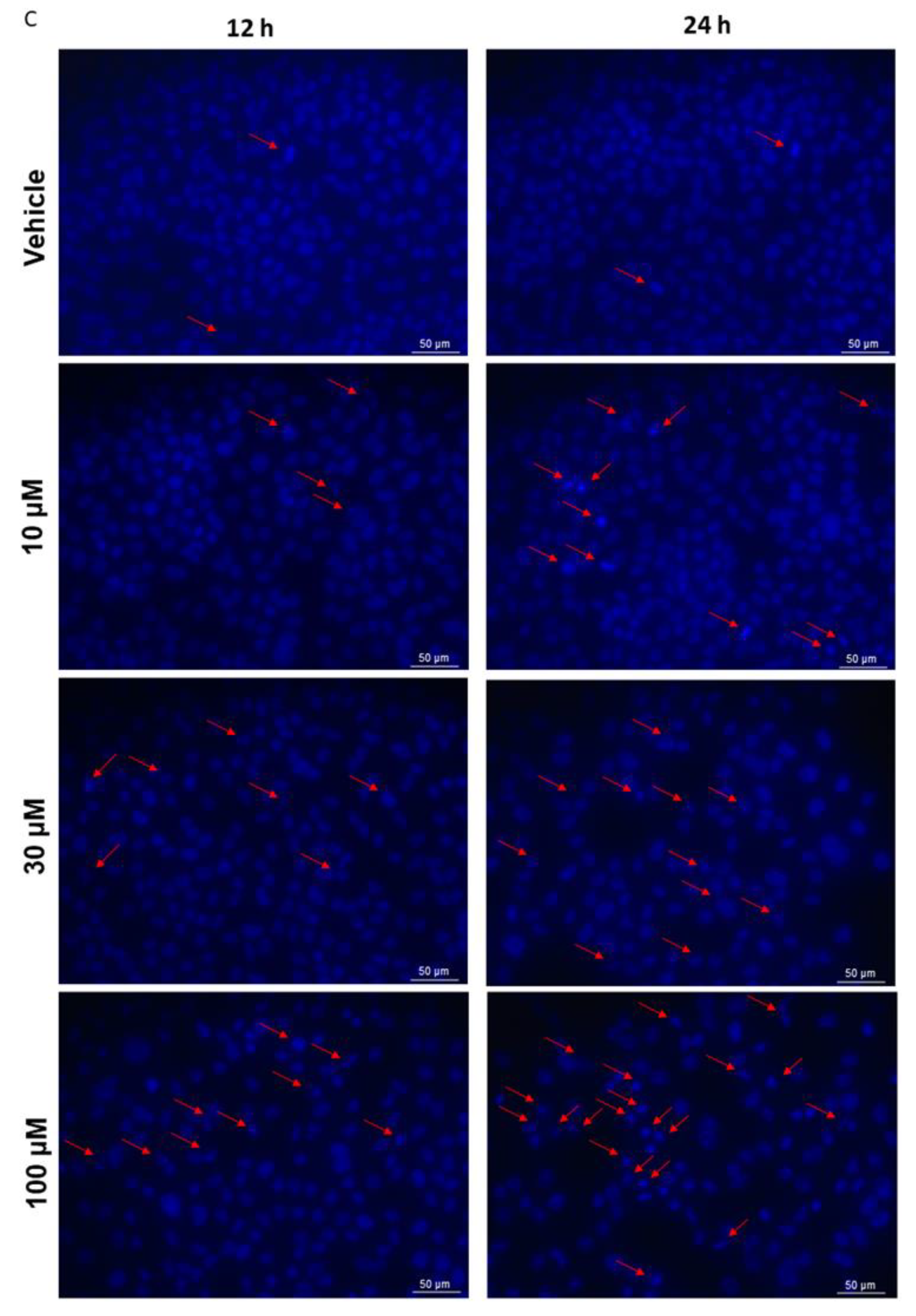
2.5. Mitochondrial Dysfunction and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Hemi-Synthesis of Analogues
4.1.1. Reagents
4.1.2. Extraction and Isolation of Sphaerococcenol A
4.1.3. General Procedure for Thio-Michael Addition to Sphaerococcenol A (GP1)
(4S,4aS,4bS,8S,8aS,10aS)-8a-(Bromomethyl)-4-hydroxy-8-isopropyl-4,10a-dimethyl-1-(phenylthio)-1,4,4a,4b,7,8,8a,9,10,10a-decahydrophenanthren-3(2H)-one (1)
(4S,4aS,4bS,8S,8aS,10aS)-8a-(Bromomethyl)-4-hydroxy-8-isopropyl-4,10a-dimethyl-1-(propylthio)-1,4,4a,4b,7,8,8a,9,10,10a-decahydrophenanthren-3(2H)-one (2 and 3)
(4S,4aS,4bS,8S,8aS,10aS)-1-(Benzylthio)-8a-(bromomethyl)-4-hydroxy-8-isopropyl-4,10a-dimethyl-1,4,4a,4b,7,8,8a,9,10,10a-decahydrophenanthren-3(2H)-one (4)
4.1.4. Reduction of Sphaerococcenol A
4.1.5. Studies on Thiol Attack: Thiol-Michael Addition versus SN2
Sphaerococcenol A with Nucleophile in Excess (20.0 equiv)
1-Bromo-2,2-d’imethylpropane as Surrogate Molecule
4.2. Cell Culture Conditions
4.2.1. Cytotoxicity of Compounds
4.2.2. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT)
4.2.3. Lactate Dehydrogenase (LDH) Activity
4.2.4. Calcein-AM Assay
4.3. Levels of Reactive Oxygen Species
4.4. Mitochondrial Membrane Potential
4.5. Biomarkers Related to Apoptosis
4.5.1. Caspase-3 Activity
4.5.2. DAPI Staining
4.6. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef]
- Tripathi, D.; Hajra, K.; Maity, D. Recent Advancement of Bio-Inspired Nanoparticles in Cancer Theragnostic. J. Nanotheranostics 2023, 4, 299–322. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Singh, V.; Khurana, A.; Navik, U.; Allawadhi, P.; Bharani, K.K.; Weiskirchen, R. Apoptosis and Pharmacological Therapies for Targeting Thereof for Cancer Therapeutics. Sci 2022, 4, 15. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about clinically approved and Preclinically investigated marine natural products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide—Identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Vagias, C.; Roussis, V. Sphaeroane and neodolabellane diterpenes from the red alga Sphaerococcus coronopifolius. Mar. Drugs 2009, 7, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Etahiri, S.; Bultel-Poncé, V.; Caux, C.; Guyot, M. New Bromoditerpenes from the Red Alga Sphaerococcus coronopifolius. J. Nat. Prod. 2001, 64, 1024–1027. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Afonso, M.B.; Guedes, R.A.; Guedes, R.C.; Alvariño, R.; Pinteus, S.; Gaspar, H.; Goettert Márcia, I.; Alfonso, A.; et al. Disclosing the antitumour potential of the marine bromo diterpene sphaerococcenol A on distinct cancer cellular models. Biomed. Pharmacother. 2022, 149, 112886. [Google Scholar] [CrossRef]
- Alves, C.; Serrano, E.; Silva, J.; Rodrigues, C.; Pinteus, S.; Gaspar, H.; Botana, L.M.; Alpoim, M.C.; Pedrosa, R. Sphaerococcus coronopifolius bromoterpenes as potential cancer stem cell-targeting agents. Biomed. Pharmacother. 2020, 128, 110275. [Google Scholar] [CrossRef]
- Rodrigues, D.; Alves, C.; Horta, A.; Pinteus, S.; Silva, J.; Culioli, G.; Thomas, O.P.; Pedrosa, R. Antitumor and Antimicrobial Potential of Bromoditerpenes Isolated from the Red Alga, Sphaerococcus coronopifolius. Mar. Drugs 2015, 13, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Vagias, C.; Bruyère, C.; Lamoral-Theys, D.; Kiss, R.; Roussis, V. Structure and in vitro antitumor activity evaluation of brominated diterpenes from the red alga Sphaerococcus coronopifolius. Bioorganic Med. Chem. 2010, 18, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Cafieri, F.; Napoli, L.D.; Fattorusso, E. Base-induced rearrangement of Sphaerococcenol A. Tetrahedron 1978, 34, 1225–1226. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Sorkin, B.C.; Kuszak, A.J.; Bloss, G.; Fukagawa, N.K.; Hoffman, F.A.; Jafari, M.; Barrett, B.; Brown, P.N.; Bushman, F.D.; Casper, S.J.; et al. Improving natural product research translation: From source to clinical trial. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 41–65. [Google Scholar] [CrossRef]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Prousis, K.C.; Kikionis, S.; Ioannou, E.; Morgana, S.; Faimali, M.; Piazza, V.; Calogeropoulou, T.; Roussis, V. Synthesis and Antifouling Activity Evaluation of Analogs of Bromosphaerol, a Brominated Diterpene Isolated from the Red Alga Sphaerococcus coronopifolius. Mar. Drugs 2022, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Quesada, A.; Vagias, C.; Moreau, D.; Roussakis, C.; Roussis, V. Cytotoxic bromoditerpenes from the red alga Sphaerococcus coronopifolius. Tetrahedron 2008, 64, 5184–5190. [Google Scholar] [CrossRef]
- Riss, T.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Riss, T.; Niles, A.; Moravec, R.; Karassina, N.; Vidugiriene, J. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2019. [Google Scholar]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-acetyoxymethyl cytotoxicity assay: Standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.R.; Anadón, A.; Wang, X.; et al. Cancer Metabolism: The Role of ROS in DNA Damage and Induction of Apoptosis in Cancer Cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef]
- Pelicano, H.; Feng, L.; Zhou, Y.; Carew, J.S.; Hileman, E.O.; Plunkett, W.; Keating, M.J.; Huang, P. Inhibition of Mitochondrial Respiration: A Novel Strategy to Enhance Drug-Induced Apoptosis in Human Leukemia Cells by a Reactive Oxygen Species-Mediated Mechanism. J. Biol. Chem. 2003, 278, 37832–37839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Chen, G.-L.; Liu, Y.; Zhuang, X.-C.; Guo, M.-Q. Stimulation of ROS Generation by Extract of Warburgia ugandensis Leading to G0/G1 Cell Cycle Arrest and Antiproliferation in A549 Cells. Antioxidants 2021, 10, 1559. [Google Scholar] [CrossRef]
- Hasanzadeh, D.; Mahdavi, M.; Dehghan, G.; Charoudeh, H.N. Farnesiferol C induces cell cycle arrest and apoptosis mediated by oxidative stress in MCF-7 cell line. Toxicol. Rep. 2017, 4, 420–426. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Rosa, S.D.; Stefano, S.D.; Scarpelli, P.; Zavodnik, N. Terpenes from the Red Alga Spaherococcus coronopifolius of the North Adriatic Sea. Phytochemistry 1988, 27, 1875–1878. [Google Scholar] [CrossRef]
- Chipoline, C.; Fonseca, A.C.C.d.; Costa, G.R.M.; de Souza, M.P.; Rabelo, V.W.-H.; de Queiroz, L.N.; de Souza, T.L.F.; de Almeida, E.C.P.; Abreu, P.A.; Pontes, B.; et al. Molecular mechanism of action of new 1,4-naphthoquinones tethered to 1,2,3-1H-triazoles with cytotoxic and selective effect against oral squamous cell carcinoma. Bioorg. Chem. 2020, 101, 103984. [Google Scholar]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]





| IC50 (µM) | |||||||
|---|---|---|---|---|---|---|---|
| A549 | SI | DU-145 | SI | MCF-7 | SI | FL83B | |
| Sphaerococcenol A | 14.99 * (12.30–18.19) | 2.5 | 3.75 c,e,* (3.17–4.44) | 10.0 | 6.53 c,* (4.43–9.57) | 5.7 | 37.40 (31.49–44.92) |
| Compound 1 | 18.70 (12.82–26.76) | 1.8 | 15.82 c,e (13.33–18.92) | 2.2 | 16.44 c (11.46–22.40) | 2.1 | 34.56 (17.89–64.68) |
| Compound 2 | 33.78 (27.63–41.19) | 1.4 | 25.80 e (17.97–36.39) | 1.9 | 70.26 * (49.57–101.7) | 0.7 | 48.02 a (36.77–64.07) |
| Compound 3 | 22.53 (18.36–27.56) | 2.5 | 20.13 e (16.43–24.52) | 2.8 | 14.31 c,* (10.01–21.61) | 4.0 | 56.69 (35.40–98.96) |
| Compound 4 | 35.45 a (29.72–42.43) | 1.1 | 70.11 a (39.63–145.8) | 0.6 | 30.40 c (23.46–39.22) | 1.3 | 40.32 (32.10–50.92) |
| Compound 5 | 22.68 * (18.17–27.88) | 3.7 | 18.53 e,* (16.25–21.68) | 4.4 | 31.95 c,* (23.36–45.45) | 2.6 | 82.12 a,b,c,d,e (67.32–n.d.) |
| Compound 6 | 33.54 * (29.48–38.55) | 1.9 | 20.46 e,* (16.73–24.92) | 3.1 | 22.19 c,* (14.49–31.98) | 2.9 | 63.29 b,* (53.21–75.49) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, D.; Fortunato, M.A.G.; Silva, J.; Pingo, M.; Martins, A.; Afonso, C.A.M.; Pedrosa, R.; Siopa, F.; Alves, C. Sphaerococcenol A Derivatives: Design, Synthesis, and Cytotoxicity. Mar. Drugs 2024, 22, 408. https://doi.org/10.3390/md22090408
Sousa D, Fortunato MAG, Silva J, Pingo M, Martins A, Afonso CAM, Pedrosa R, Siopa F, Alves C. Sphaerococcenol A Derivatives: Design, Synthesis, and Cytotoxicity. Marine Drugs. 2024; 22(9):408. https://doi.org/10.3390/md22090408
Chicago/Turabian StyleSousa, Dídia, Milene A. G. Fortunato, Joana Silva, Mónica Pingo, Alice Martins, Carlos A. M. Afonso, Rui Pedrosa, Filipa Siopa, and Celso Alves. 2024. "Sphaerococcenol A Derivatives: Design, Synthesis, and Cytotoxicity" Marine Drugs 22, no. 9: 408. https://doi.org/10.3390/md22090408
APA StyleSousa, D., Fortunato, M. A. G., Silva, J., Pingo, M., Martins, A., Afonso, C. A. M., Pedrosa, R., Siopa, F., & Alves, C. (2024). Sphaerococcenol A Derivatives: Design, Synthesis, and Cytotoxicity. Marine Drugs, 22(9), 408. https://doi.org/10.3390/md22090408








