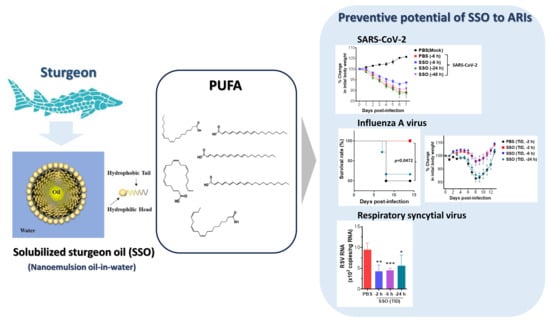
Exploring the Preventive Potential of Solubilized Sturgeon Oil on Acute Infection with Respiratory Viruses
Abstract
:1. Introduction
2. Results
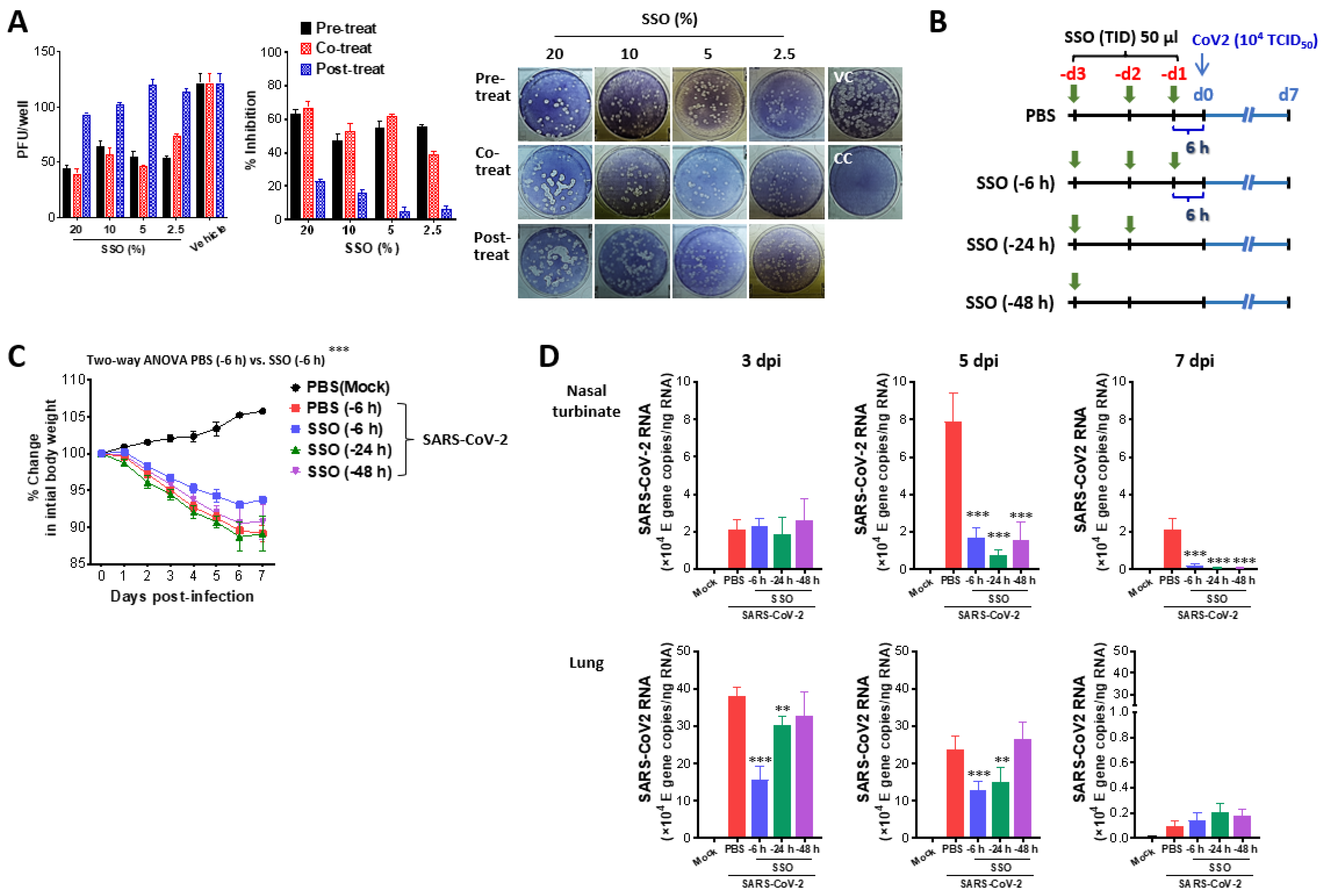
2.1. SSO Pre-Treatment Mitigates Lung Inflammation with Reduced Viral Burden After SARS-CoV-2 Infection
2.1.1. SSO Pre-Treatment Inhibits SARS-CoV-2 Replication and Ameliorates Morbidity in Hamster Infection Model
2.1.2. Attenuated Lung Inflammation in SARS-CoV-2-Infected Hamster After SSO Pre-Treatment
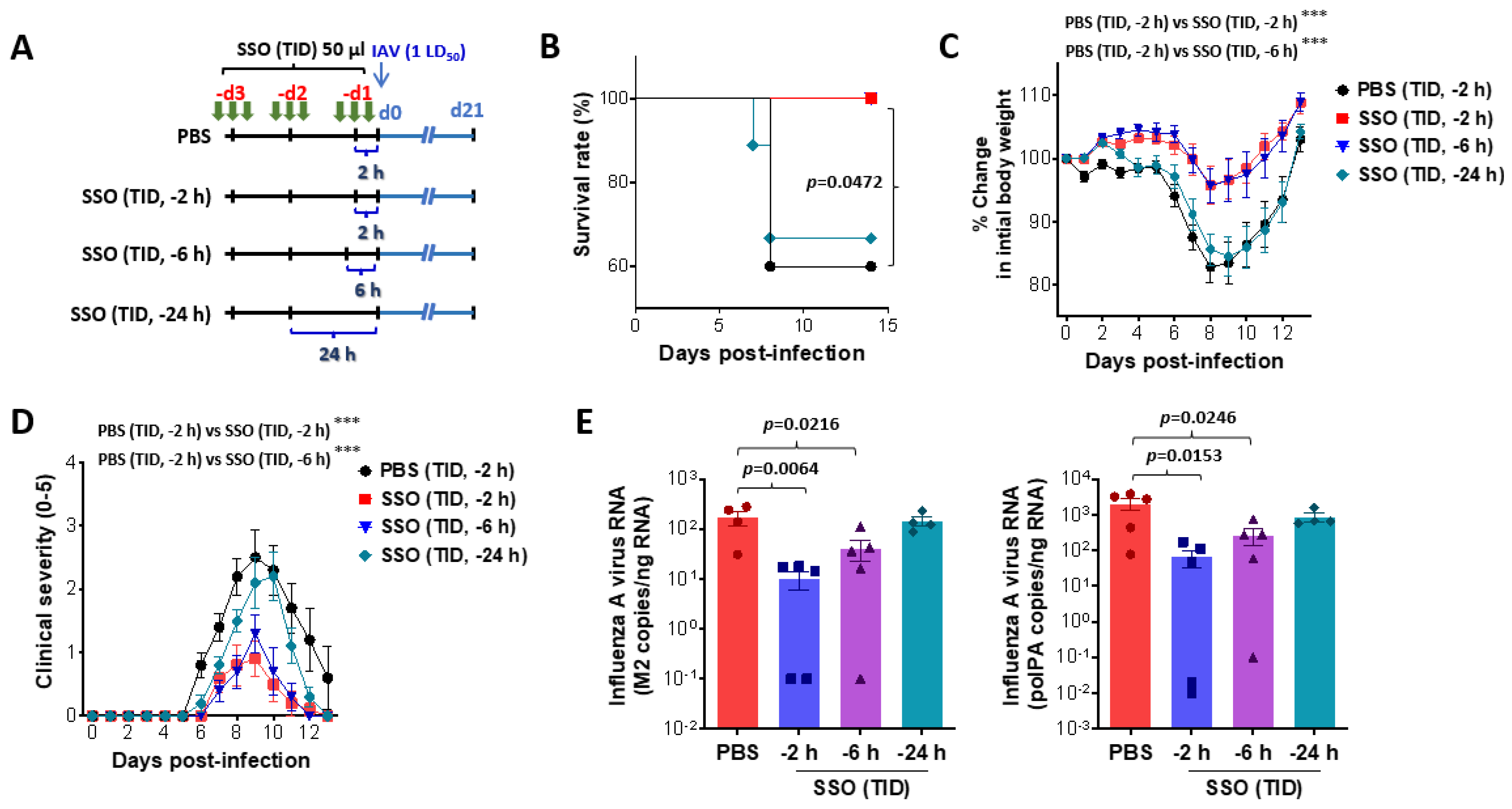
2.2. Pre-Treatment of SSO Attenuates Mortality and Morbidity Caused by Infection with IAV
2.2.1. Attenuation of Mortality and Morbidity by Pre-Treatment of SSO at Least 6 H Prior to IAV Infection
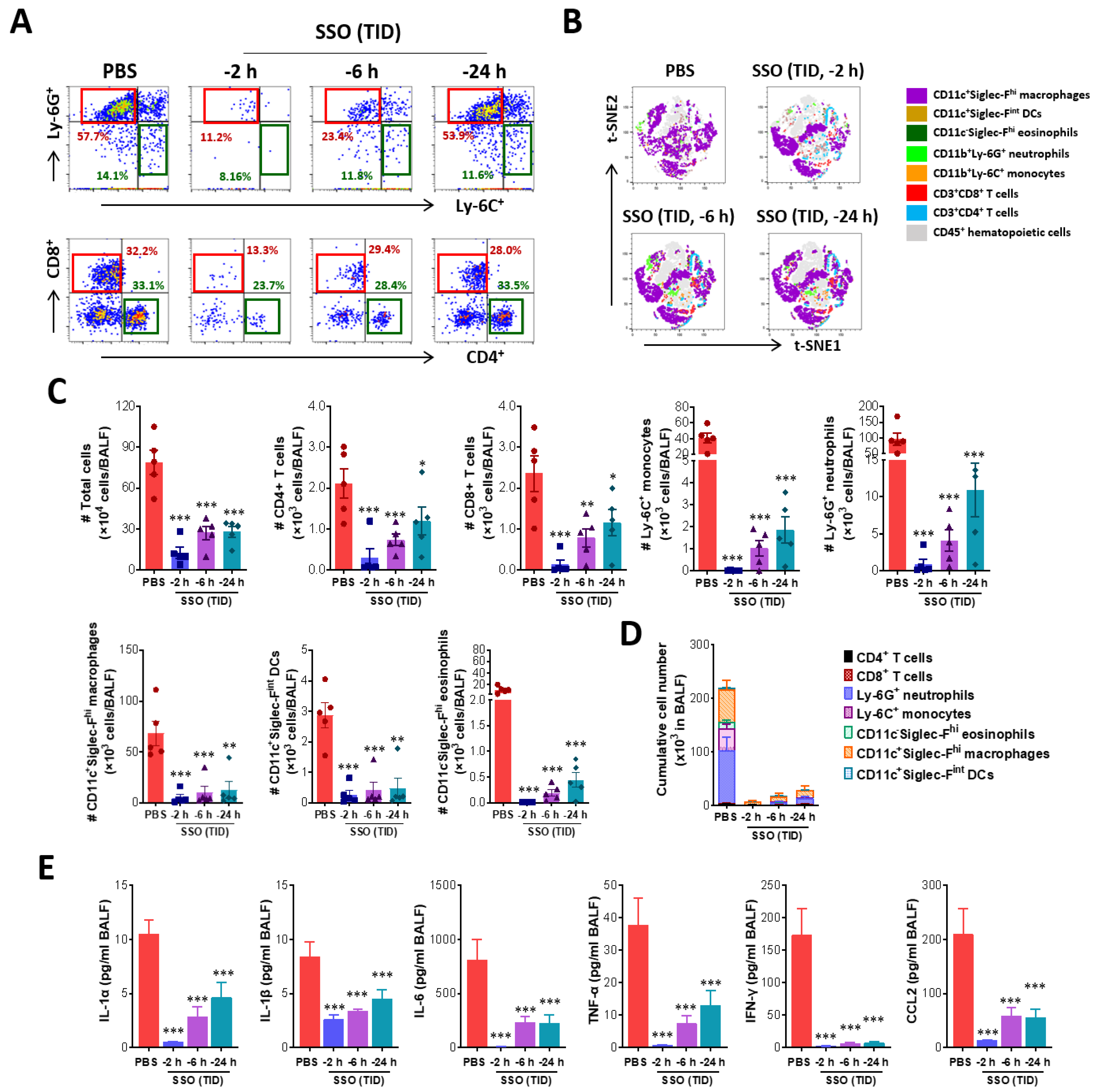
2.2.2. SSO Pre-Treatment Ameliorates Lung Inflammation with Reduced Production of Cytokine Secretion After IAV Infection
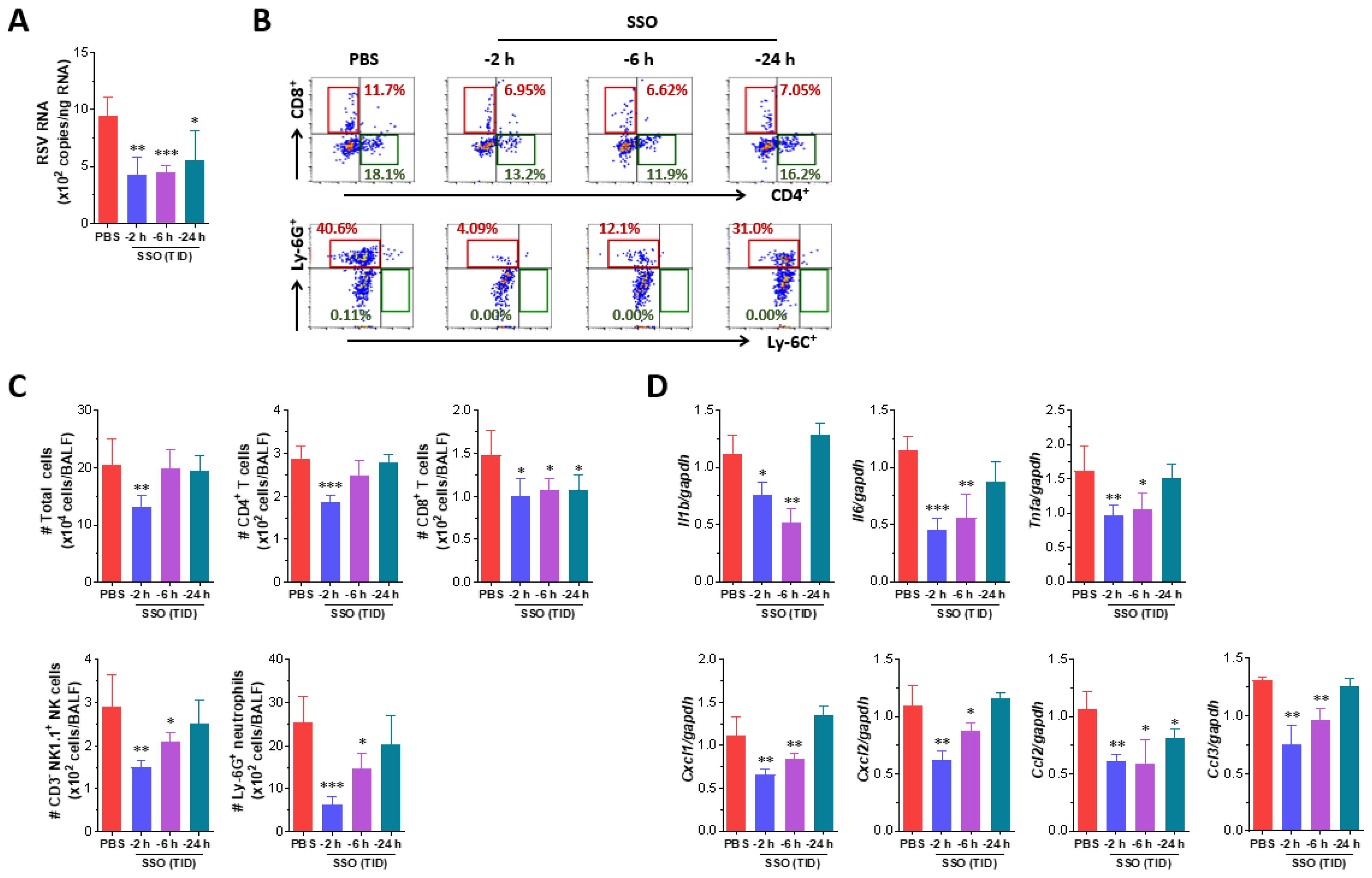
2.3. Attenuated Infection of RSV by Pre-Treatment of SSO
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals, Cells, and Viruses
4.3. Antibodies and Reagents
4.4. Preparation and Analysis of SSO
4.5. Acute Infection Models of Respiratory Viruses
4.6. Collection and Analysis of Bronchoalveolar Lavage Fluid (BALF)
4.7. Quantitative Real-Time RT-PCR for Determination of Viral Burden and Cytokine Expression
4.8. Determination of Secreted Cytokine Proteins
4.9. Flow Cytometric Analysis
4.10. Histopathological Examinations
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates 2021: Deaths by Cause, Age, Sex, by Country and by Region. Available online: https://www.who.int/data/global-health-estimates (accessed on 19 February 2025).
- GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Wang, W.; Bhushan, G.L.; Paz, S.; Stauft, C.B.; Selvaraj, P.; Goguet, E.; Bishop-Lilly, K.A.; Subramanian, R.; Vassell, R.; Lusvarghi, S.; et al. Antigenic cartography using hamster sera identifies SARS-CoV-2 JN.1 evasion seen in human XBB.1.5 booster sera. bioRxiv 2024. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, H.Y.; Ren, L.L.; Lu, Q.B.; Ren, X.; Zhang, C.H.; Wang, Y.F.; Lin, S.H.; Zhang, X.A.; Li, J.; et al. Chinese Centers for Disease Control and Prevention (CDC) Etiology of Respiratory Infection Surveillance Study Team Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 2021, 12, 5026. [Google Scholar] [CrossRef]
- Schüz, M.L.; Dallmeyer, L.; Fragkou, P.C.; Omony, J.; Krumbein, H.; Hünerbein, B.L.; Skevaki, C. Global prevalence of respiratory virus infections in adults and adolescents during the COVID-19 pandemic: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 137, 16–24. [Google Scholar] [CrossRef]
- Metz, C.; Schmid, A.; Veldhoen, S. Increase in complicated upper respiratory tract infection in children during the 2022/2023 winter season-a post coronavirus disease 2019 effect? Pediatr. Radiol. 2024, 54, 49–57. [Google Scholar] [CrossRef]
- Goto, T.; Kawai, N.; Bando, T.; Takasaki, Y.; Shindo, S.; Sato, T.; Tani, N.; Chong, Y.; Ikematsu, H. Virological and Clinical Outcomes of Influenza Outpatients Treated With Baloxavir, Oseltamivir, or Laninamivir in the 2023–2024 Season. Influenza Other Respir. Viruses 2024, 18, e70042. [Google Scholar] [CrossRef]
- Park, S.; Je, N.K.; Kim, D.W.; Park, M.; Heo, J. Current status and clinical outcomes of pharmacotherapies according to SARS-CoV-2 mutations in patients with mild-to-moderate COVID-19: A retrospective single center study. BMC Infect. Dis. 2024, 24, 871. [Google Scholar] [CrossRef]
- Chan, C.C.; Guo, Q.; Chan, J.F.; Tang, K.; Cai, J.P.; Chik, K.K.; Huang, Y.; Dai, M.; Qin, B.; Ong, C.P.; et al. Identification of novel small-molecule inhibitors of SARS-CoV-2 by chemical genetics. Acta Pharm. Sin. B 2024, 14, 4028–4044. [Google Scholar] [CrossRef]
- Suzaki, I.; Kobayashi, H. Coronavirus Disease 2019 and Nasal Conditions: A Review of Current Evidence. Vivo 2021, 35, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Seo, M.Y. SARS-CoV-2 Infection (COVID-19) and Rhinologic Manifestation: Narrative Review. J. Pers. Med. 2022, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Winther, B.; Gwaltney, J.M., Jr.; Mygind, N.; Hendley, J.O. Viral-induced rhinitis. Am. J. Rhinol. 1998, 12, 17–20. [Google Scholar] [CrossRef]
- Hadjichrysanthou, C.; Beukenhorst, A.L.; Koch, C.M.; Alter, G.; Goudsmit, J.; Anderson, R.M.; de Wolf, F. Exploring the Role of Antiviral Nasal Sprays in the Control of Emerging Respiratory Infections in the Community. Infect. Dis. Ther. 2022, 11, 2287–2296. [Google Scholar] [CrossRef]
- Unger-Manhart, N.; Morokutti-Kurz, M.; Zieglmayer, P.; Russo, A.; Siegl, C.; König-Schuster, M.; Koller, C.; Graf, P.; Graf, C.; Lemell, P.; et al. Decongestant Effect of “Coldamaris Akut”, a Carrageenan- and Sorbitol-Containing Nasal Spray in Seasonal Allergic Rhinitis. Int. J. Gen. Med. 2024, 17, 5105–5121. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Moakes, R.J.A.; Grover, L.M. Low Acyl Gellan as an Excipient to Improve the Sprayability and Mucoadhesion of Iota Carrageenan in a Nasal Spray to Prevent Infection With SARS-CoV-2. Front. Med. Technol. 2021, 16, 687681. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/Xanthan Gum Based Hydrogels as Potential Carrier for an Antiviral Drug: Fabrication, Characterization, and Safety Evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef]
- Ben, C. Drug-Free Nasal Spray Prevents Infection by Trapping Viruses in the Nose. Available online: https://newatlas.com/disease/pcans-nasal-spray-traps-viruses (accessed on 23 December 2024).
- Paull, J.R.A.; Luscombe, C.A.; Seta, A.; Heery, G.P.; Bobardt, M.D.; Gallay, P.A.; Constant, S.; Castellarnau, A. Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium. Sci. Rep. 2024, 14, 21259. [Google Scholar] [CrossRef]
- Rennie, P.; Bowtell, P.; Hull, D.; Charbonneau, D.; Lambkin-Williams, R.; Oxford, J. Low pH gel intranasal sprays inactivate influenza viruses in vitro and protect ferrets against influenza infection. Respir. Res. 2007, 8, 38. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef]
- Arts, M.T.; Kohler, C.C. Health and condition in fish: The influence of lipids on membrane competency and immune response. In Lipids in Aquatic Ecosystems, 1st ed.; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer Nature Link: New York, NY, USA, 2009; pp. 237–256. [Google Scholar]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enteral. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Jackman, J.A.; Arabyan, E.; Zakaryan, H.; Elrod, C.C. Glycerol Monolaurate Inhibits Wild-Type African Swine Fever Virus Infection in Porcine Macrophages. Pathogens 2023, 12, 1193. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Truong, A.D.; Ly, D.V.; Hoang, T.V.; Chu, N.T.; Nguyen, H.T.; Dang, A.T.K.; De Vos, M.; Lannoo, K.; Bruggeman, G.; et al. The potential anti-African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using epidemic ASFV strain circulating in Vietnam. Open Vet. J. 2021, 11, 346–355. [Google Scholar] [CrossRef]
- Sagna, A.; Nair, R.V.R.; Hulyalkar, N.; Rajasekharan, S.; Nair, V.T.G.; Sivakumar, K.C.; Suja, S.R.; Baby, S.; Sreekumar, E. Ethyl palmitate, an anti-chikungunya virus principle from Sauropus androgynus, a medicinal plant used to alleviate fever in ethnomedicine. J. Ethnopharmacol. 2023, 309, 116366. [Google Scholar] [CrossRef] [PubMed]
- Suo, X.; Wang, J.; Wang, D.; Fan, G.; Zhu, M.; Fan, B.; Yang, X.; Li, B. DHA and EPA inhibit porcine coronavirus replication by alleviating ER stress. J. Virol. 2023, 97, e0120923. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, C.; Huang, X.; Jansen, C.A.; Savelkoul, H.F.J.; Liu, G. Linoleic acid stimulation results in TGF-beta1 production and inhibition of PEDV infection in vitro. Virology 2023, 581, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sardana, K.; Sachdeva, S. Role of nutritional supplements in selected dermatological disorders: A review. J. Cosmet. Dermatol. 2022, 21, 85–98. [Google Scholar] [CrossRef]
- Nasrollahi, S.A.; Ayatollahi, A.; Yazdanparast, T.; Samadi, A.; Hosseini, H.; Shamsipour, M.; Akhlaghi, A.A.; Yadangi, S.; Abels, C.; Firooz, A. Comparison of linoleic acid-containing water-in-oil emulsion with urea-containing water-in-oil emulsion in the treatment of atopic dermatitis: A randomized clinical trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 21–28. [Google Scholar] [CrossRef]
- Fiocchi, A.; Sala, M.; Signoroni, P.; Banderali, G.; Agostoni, C.; Riva, E. The efficacy and safety of gamma-linolenic acid in the treatment of infantile atopic dermatitis. J. Int. Med. Res. 1997, 22, 24–32. [Google Scholar] [CrossRef]
- Simon, D.; Eng, P.A.; Borelli, S.; Kägi, R.; Zimmermann, C.; Zahner, C.; Drewe, J.; Hess, L.; Ferrari, G.; Lautenschlager, S.; et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv. Ther. 2014, 31, 180–188. [Google Scholar] [CrossRef]
- Nieminen, P.; Westenius, E.; Halonen, T.; Mustonen, A. Fatty acid composition in tissues of the farmed Siberian sturgeon (Acipenser baerii). Food Chem. 2014, 159, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, Z.; Wang, J.; Jin, W.; Abdu, H.I.; Pei, J.; Wang, Q.; Abd El-Aty, A.M. A review of the nutritional value and biological activities of sturgeon processed byproducts. Front. Nutr. 2022, 9, 1024309. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, Y.K.; Park, J.H.; Kim, S.H.; Park, C.S.; Kim, H.J.; Lee, C.K. Therapeutic efficacy and mechanism of solubilized sturgeon oil in a mouse model of house dust mite-induced atopic dermatitis 115. J. Funct. Foods 2024, 115, 106093. [Google Scholar] [CrossRef]
- Kim, J.; An, J.; Lee, Y.K.; Ha, G.; Ban, H.; Kong, H.; Lee, H.; Song, Y.; Lee, C.K.; Kim, S.B.; et al. Hair Growth Promoting Effects of Solubilized Sturgeon Oil and Its Correlation with the Gut Microbiome. Pharmaceuticals 2024, 17, 1112. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K. Method for Manufacturing Composition for Treating Atopic Dermatitis Containing Sturgeon Water-Soluble Oil. Korea Patent 10-2555616, 11 July 2023. [Google Scholar]
- Pandey, K.; Acharya, A.; Mohan, M.; Ng, C.L.; Reid, S.P.; Byrareddy, S.N. Animal models for SARS-CoV-2 research: A comprehensive literature review. Transbound. Emerg. Dis. 2021, 68, 1868–1885. [Google Scholar] [CrossRef]
- Wang, R.Y.; Wang, Y.; Wang, X.X.; Wu, B.Y.; Wu, H.P.; Hu, H.Z. Analysis of infection indicators and risk factors for influenza A after the COVID-19 pandemic. New Microbiol. 2024, 47, 269–274. [Google Scholar]
- O’Connor, T.; Borsig, L.; Heikenwalder, M. CCL2-CCR2 Signaling in Disease Pathogenesis. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 105–118. [Google Scholar] [CrossRef]
- Nakagome, K.; Nagata, M. Innate Immune Responses by Respiratory Viruses, Including Rhinovirus, During Asthma Exacerbation. Front. Immunol. 2022, 13, 865973. [Google Scholar] [CrossRef]
- Mazur, N.I.; Caballero, M.T.; Nunes, M.C. Severe respiratory syncytial virus infection in children: Burden, management, and emerging therapies. Lancet 2024, 404, 1143–1156. [Google Scholar] [CrossRef]
- Zar, H.J.; Cacho, F.; Kootbodien, T.; Mejias, A.; Ortiz, J.R.; Stein, R.T.; Hartert, T.V. Early-life respiratory syncytial virus disease and long-term respiratory health. Lancet Respir. Med. 2024, 12, 810–821. [Google Scholar] [CrossRef]
- Montes, J.B.T.; Montes, D.; Miele, A.; Baik-Han, W.; Gulati, G.; Lew, L.Q. The Impact of COVID-19 Pandemic on Respiratory Syncytial Virus Infection in Children. Pulm. Med. 2024, 2024, 2131098. [Google Scholar] [CrossRef] [PubMed]
- Zaderer, V.; Diem, G.; Posch, W.; Jakschitz, T.; Bonn, G.K.; Bellmann-Weiler, R.; Huber, L.A.; Wilflingseder, D. P80 natural essence spray and lozenges provide respiratory protection against Influenza A, B, and SARS-CoV-2. Respir. Res. 2024, 25, 102. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Patel, A.B.; Vora, L.K.; Singla, R.K.; Shah, P.; Uversky, V.N.; Apostolopoulos, V. Nitric Oxide and its Derivatives Containing Nasal Spray and Inhalation Therapy for the Treatment of COVID-19. Curr. Pharm. Des. 2022, 28, 3658–3670. [Google Scholar] [CrossRef] [PubMed]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. Polyunsaturated omega-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci. Rep. 2021, 11, 5207. [Google Scholar] [CrossRef]
- Hagihara, M.; Yamashita, M.; Ariyoshi, T.; Eguchi, S.; Minemura, A.; Miura, D.; Higashi, S.; Oka, K.; Nonogaki, T.; Mori, T.; et al. Clostridium butyricum-induced omega-3 fatty acid 18-HEPE elicits anti-influenza virus pneumonia effects through interferon-lambda upregulation. Cell Rep. 2022, 41, 111755. [Google Scholar] [CrossRef]
- Hoyer, A.; Rehbinder, E.M.; Färdig, M.; Asad, S.; Lødrup Carlsen, K.C.; Endre, K.M.A.; Granum, B.; Haugen, G.; Hedlin, G.; Monceyron Jonassen, C.; et al. Filaggrin mutations in relation to skin barrier and atopic dermatitis in early infancy. Br. J. Dermatol. 2022, 186, 544–552. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the Roles of Filaggrin in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequence (5′–3′) | Gene Bank ID |
|---|---|---|
| IL-1β | F: AAG TGA TAT TCT CCA TGA GCT TTG T R: TTC TTC TTT GGG TAT TGC TTG G | NM_008361.4 |
| IL-6 | F: AAC GAT GAT GCA CTT GCA GA R: GAG CAT TGG AAA TTG GGG TA | NM_0.31168.2 |
| TNF-a | F: CGT CGT AGC AAA CCA CCA AG R: TTG AAG AGA ACC TGG GAG TA | NM_013693.3 |
| Cxcl1 | F: CGC TGC TGC TGC TGG CCA CCA R: GGC TAT GAC TTC GGT TTG GGT GCA | NM_008176.3 |
| Cxcl2 | F: ATC CAGAGC TTG AGT GTGACG R: AAG GCA AAC TTT TTG ACC GCC | NM_009140.2 |
| Ccl2 | F: AAA AAC CTG GAT CGG AAC CAA R: CGG GTC AAC TTC ACA TTC AAA G | NM_011333.3 |
| Ccl3 | F: CCA AGT CTT CTC AGC GCC AT R: GAA TCT TCC GGC TGT AGG AG | NM_011337.2 |
| GAPDG | F: AAC GAC CCC TTC ATT GAC R: TCC ACG ACA TAC TCA GCA C | NM_001289726.1 |
| SARS-CoV-2 | F: ACAGGTACGTTAATAGTTAATAGCGT | OX489524.1 |
| R: ATATTGCAGCAGTACGCACACA | ||
| Probe: [FAM]ACACTAGCCATCCTTAC[BHQ1] | ||
| IAV | a F: CGGTCCAAATTCCTGCTGA | CY121306.1 |
| R: CATTGGGTTCCTTCCATCCA | ||
| Probe: [HEX]CCAAGTCATGAAGGAGA[BHQ1] | ||
| IAV | b F: CTTCTAACCGAGGTCGAAACGTA | OK022520.1 |
| R: GGTGACAGGATTGGTCTTGTCTTTA | ||
| Probe: [FAM]TCAGGCCCCCTCAAAGC[BHQ1] | ||
| RSV-A | F: TTGGATCTGCAATCGCCA | M22643.1 |
| R: CTTTTGATCTTGTTCACTTCTCCTTCT | ||
| Probe: [HEX]TGGCACTGCTGTATCTA[BHQ1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.O.; Uyangaa, E.; Lee, Y.-K.; Yun, S.-H.; Yu, M.; Kim, H.J.; Cho, H.W.; Byeon, H.W.; Lee, C.-K.; Eo, S.K. Exploring the Preventive Potential of Solubilized Sturgeon Oil on Acute Infection with Respiratory Viruses. Mar. Drugs 2025, 23, 112. https://doi.org/10.3390/md23030112
Park SO, Uyangaa E, Lee Y-K, Yun S-H, Yu M, Kim HJ, Cho HW, Byeon HW, Lee C-K, Eo SK. Exploring the Preventive Potential of Solubilized Sturgeon Oil on Acute Infection with Respiratory Viruses. Marine Drugs. 2025; 23(3):112. https://doi.org/10.3390/md23030112
Chicago/Turabian StylePark, Seong Ok, Erdenebileg Uyangaa, Yong-Kwang Lee, Suk-Hyun Yun, Minyeong Yu, Hyo Jin Kim, Hye Won Cho, Hee Won Byeon, Chong-Kil Lee, and Seong Kug Eo. 2025. "Exploring the Preventive Potential of Solubilized Sturgeon Oil on Acute Infection with Respiratory Viruses" Marine Drugs 23, no. 3: 112. https://doi.org/10.3390/md23030112
APA StylePark, S. O., Uyangaa, E., Lee, Y.-K., Yun, S.-H., Yu, M., Kim, H. J., Cho, H. W., Byeon, H. W., Lee, C.-K., & Eo, S. K. (2025). Exploring the Preventive Potential of Solubilized Sturgeon Oil on Acute Infection with Respiratory Viruses. Marine Drugs, 23(3), 112. https://doi.org/10.3390/md23030112