Investigating the Mechanism of Action of Ipomoea pes-caprae in the Treatment of Rheumatoid Arthritis Based on Serum Metabolomics and Network Pharmacology
Abstract
1. Introduction
2. Results
2.1. Effects of IPE on AI Score and Left Hind Toe Swelling in RA Rats
2.2. Pathological Section of Ankle Synovial Tissue and Micro-CT of the Ankle Joint of Rats
2.3. Effect of IPE on Inflammatory Factors in Serum
2.4. Characterization of the Chemical Constituents of IPE
2.5. Identification and Relative Contents of the Absorbed Components in Plasma of the Con + IPE, WCM + IPE, and WHM + IPE Groups
2.6. Pharmacokinetic Analysis of the Absorbed Components of IPE in the Plasma
2.7. Serum Metabolomics Analysis of IPE in RA Rats
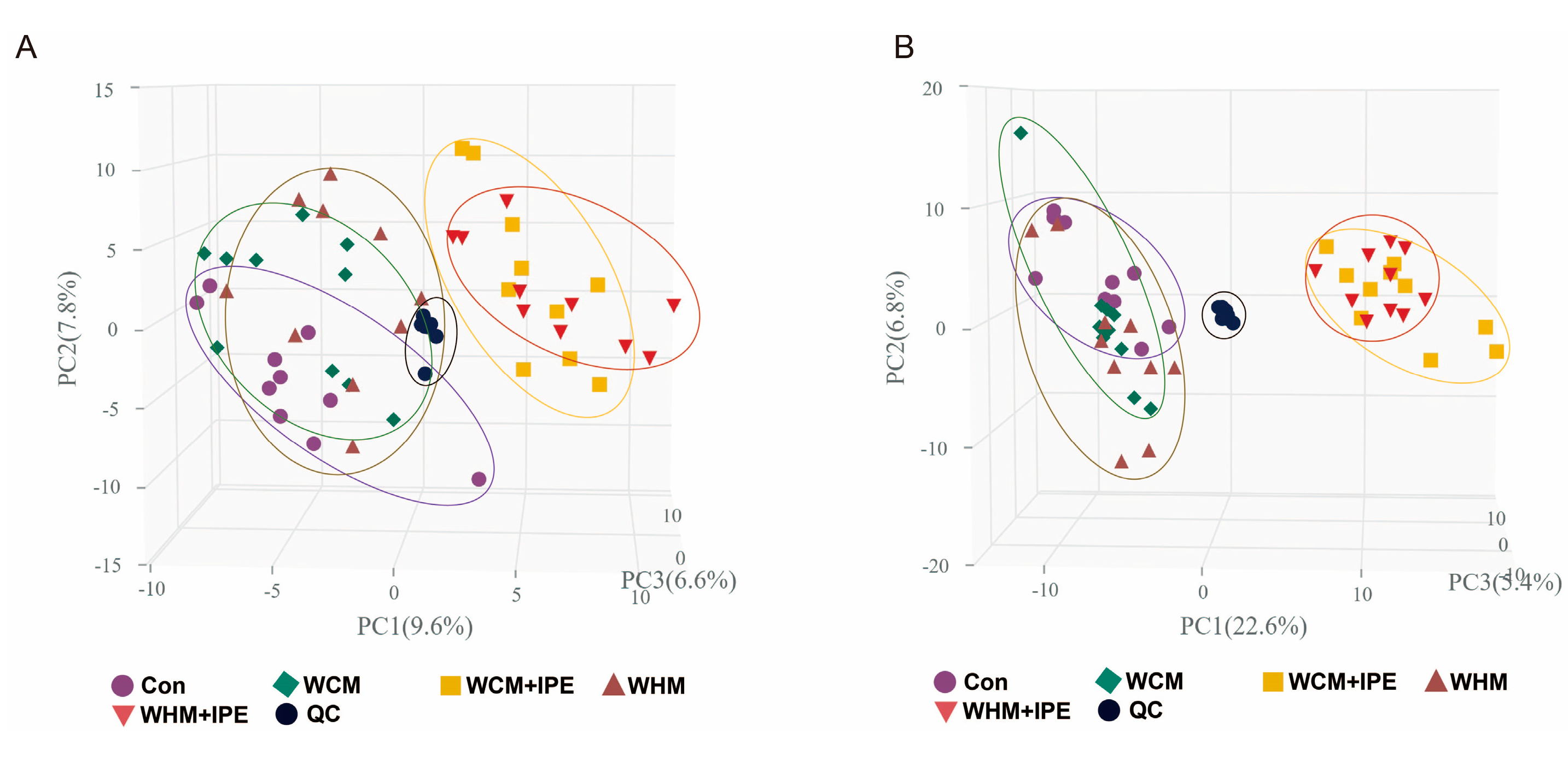
2.7.1. PCA of Serum Samples
2.7.2. OPLS-DA Analysis of Serum Samples
2.7.3. Identification of Endogenous Biomarkers
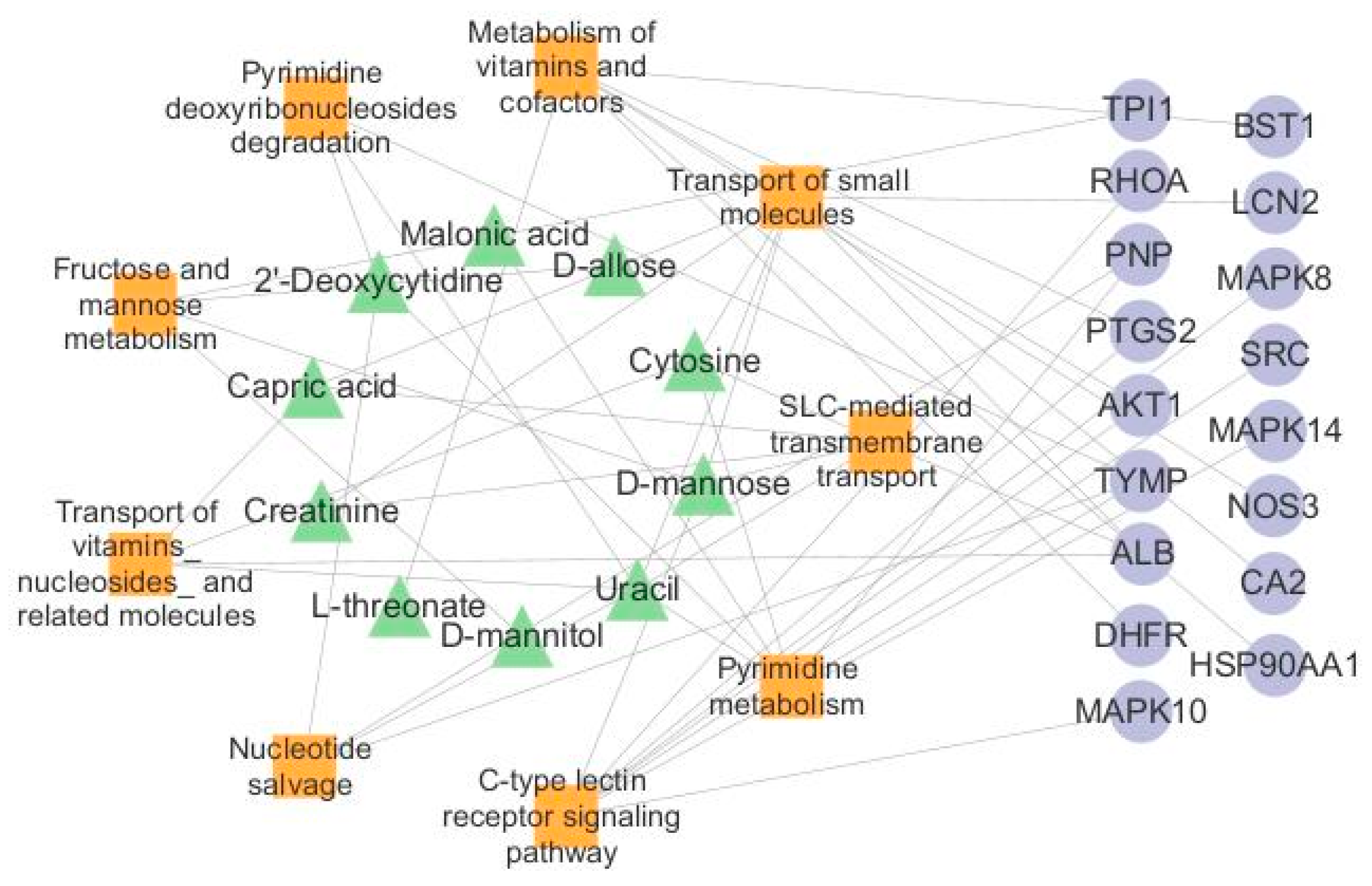
2.7.4. Analysis of the Major Metabolic Pathways in IPE
2.8. Network Pharmacological Analysis of IPE Against RA
2.9. Molecular Docking of Absorbed Components in the Plasma to Key Targets
2.10. Network Analysis of Endogenous Biomarkers and Targets
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents and Standards
4.2. Preparation of Plant Extracts
4.2.1. Preparation of IPE Paste
4.2.2. Preparation of IPE Samples
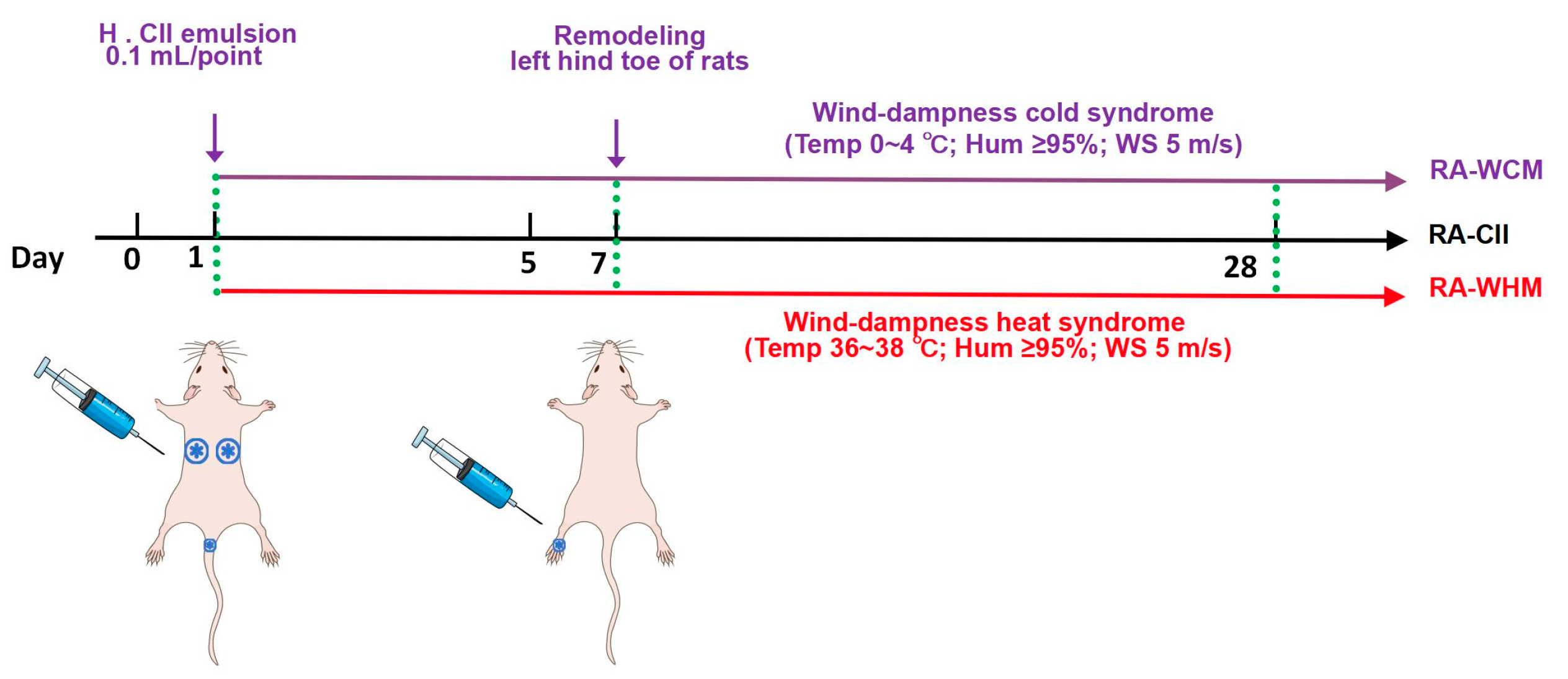
4.3. Animal Grouping and Drug Administration
4.4. Animal Model Construction
4.5. Joint Inflammation AI Score Evaluation and Left Hind Toe Swelling Analysis
4.6. Serum Sample Collection
4.7. Histopathology and Micro-CT Analysis
4.8. Chromatographic and Mass Spectrometry Conditions for UPLC-MS
4.9. Pharmacokinetic Analysis
4.10. Serum Metabolomics Analysis
4.11. Multivariate Data Analysis
4.12. Network Pharmacology Analysis
4.13. Molecular Docking
4.14. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | Rheumatoid arthritis |
| NSAIDS | Nonsteroidal anti-inflammatory drugs |
| CORT | Corticosteroids |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| TNF-α | Tumor Necrosis Factor-α |
| IL-6 | Interleukin- 6 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| FLS | Fibroblast-like synoviocytes |
| CRP | C-reactive protein |
| RF | Rheumatoid factor |
| TNF-α | Tumor necrosis factor-α |
| TGF-β | Transforming growth factor-β |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| Akt/mTOR | Akt/mammalian target of rapamycin |
| LTB4 | Leukotriene B4 |
| PI3K/Akt | Phosphatidylinositol 3-kinases/Akt |
| MAPK | Mitogen-activated protein kinase |
| JNK | C-Jun N-terminal kinase |
| ERK | Extracellular regulated protein kinases |
| C/ebp | CCAAT/enhancer-binding protein |
References
- Li, Z. Arthromyodynia Theory; Guangdong Science & Technology Press, Ltd.: Guangzhou, China, 1987. [Google Scholar]
- Jiang, Q. Practical Chinese Medicine Clinical Medicine Series Practical Chinese Medicine Rheumatology Immunology; China Press of Traditional Chinese Medicine: Beijing, China, 2022. [Google Scholar]
- Xie, C.Q.; Jia, P.; Hu, L. Research Progress on Pathogenesis and Drug Treatment of Rheumatoid Arthritis. China Mod. Doctor. 2024, 62, 145–148. [Google Scholar]
- Jia, W.; Yu, S.; Liu, X.; Le, Q.; He, X.; Yu, L.; He, J.; Yang, L.; Gao, H. Ethanol Extract of Limonium Bicolor Improves Dextran Sulfate Sodium-Induced Ulcerative Colitis by Alleviating Inflammation and Restoring Gut Microbiota Dysbiosis in Mice. Mar. Drugs 2024, 22, 175. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma Longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, Y.L.; Yan, K.; Li, H.; Zhang, X.; Essola, J.M.; Ding, C.; Chang, K.; Qing, G.; Zhang, F.; et al. Traditional Chinese Medicine Formulae Qy305 Reducing Cutaneous Adverse Reaction and Diarrhea by Its Nanostructure. Adv. Sci. 2024, 11, e2306140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, H.L.; Gong, X.; Luo, C.G. Guidelines of Diagnosis and Treatment of Rheumatoid Arthritis Disease and Syndrome Combination. J. Tradit. Chin. Med. 2018, 59, 1794–1800. [Google Scholar]
- Wang, Y.; Chen, S.; Du, K.; Liang, C.; Wang, S.; Owusu Boadi, E.; Li, J.; Pang, X.; He, J.; Chang, Y.X. Traditional Herbal Medicine: Therapeutic Potential in Rheumatoid Arthritis. J. Ethnopharmacol. 2021, 279, 114368. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, P.C.; Feng, J.; Chen, Z.L.; Wang, Q.H.; Kuang, H.X. Application of Metabonomics in Study on Traditional Chinese Medicine. Chin. Tradit. Herb. Drugs. 2015, 46, 756–765. [Google Scholar]
- Li, Z.; Yuan, J.; Dai, Y.; Xia, Y. Integration of Serum Pharmacochemistry and Metabolomics to Reveal the Underlying Mechanism of Shaoyao-Gancao-Fuzi Decoction to Ameliorate Rheumatoid Arthritis. J. Ethnopharmacol. 2024, 326, 117910. [Google Scholar] [CrossRef]
- Peng, W.; Yang, Y.; Lu, H.; Shi, H.; Jiang, L.; Liao, X.; Zhao, H.; Wang, W.; Liu, J. Network Pharmacology Combines Machine Learning, Molecular Simulation Dynamics and Experimental Validation to Explore the Mechanism of Acetylbinankadsurin a in the Treatment of Liver Fibrosis. J. Ethnopharmacol. 2024, 323, 117682. [Google Scholar] [CrossRef]
- Zhang, D.F.; Hu, C.J.; Hu, K.F. Application and Prospect of Network Pharmacology in the Field of Traditional Chinese Medicine. J. Med. Inf. 2024, 45, 30–36+56. [Google Scholar]
- Guan, H.S.; Wang, S.G. Chinese Marine Herb; Shanghai Scientific & Technical Publishers: Shanghai, China, 2009. [Google Scholar]
- Ge, Y.C.; Luo, J.Q.; Wu, Y.H.; Li, Y.X.; Huo, W.Z.; Yu, B.W. Chemical Constituents from Ipomoea pes-caprae. J. Chin. Med. Mater. 2016, 39, 2251–2255. [Google Scholar]
- Deng, J.G.; Hao, E.W.; Hou, X.T. Marine Chinese Medicine; Guangxi Science and Technology Publishing House Co., Ltd.: Nanning, China, 2018. [Google Scholar]
- Bilal, M.; Qindeel, M.; Nunes, L.V.; Duarte, M.T.S.; Ferreira, L.F.R.; Soriano, R.N.; Iqbal, H.M.N. Marine-Derived Biologically Active Compounds for the Potential Treatment of Rheumatoid Arthritis. Mar. Drugs 2020, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.H.; Deng, J.G.; Qin, J.F.; Hao, E.W.; Wei, W.; Xie, J.L.; Hou, X.T. Research Progress on Chemical Constituents of Marine Medicine Ipomoea pes-caprae and Their Pharmacological Activities. Chin. Tradit. Herb. Drugs. 2018, 49, 955–964. [Google Scholar]
- Wei, Y.T.; Hao, E.W.; Xia, Z.S.; Jiang, Q.Y.; Du, Z.C.; Deng, J.G.; Hou, X.T. Study on the Therapeutic Effect of Ipomoea pes-caprae Extract on Collagen-Induced Arthritis Model Rats. Guangxi Sci. 2021, 28, 626–633. [Google Scholar]
- Huang, C.T.; Yang, X.; Hou, X.T.; Du, Z.C.; Yu, C.L.; Mo, Q.; Zhao, F.C.; Wei, Y.T.; Hao, E.W.; Deng, J.G. Effect of Aqueous Extract of Chinese Marine Medicine Houteng on Pedal Swelling in Rheumatoid Arthritis Rats with Fengshihanbi or Fengshirebi Syndrome. Pharmacol. Clin. Chin. Mater. Med. 2021, 37, 109–113. [Google Scholar]
- Ma, X.W.; Zhang, H.; Deng, L.L.; Yan, R.; Fang, S.M. Research Progress in Roles of Mitogen Activated Protein Kinase Signalling Pathway in Pathogenesis of Rheumatoid Arthritis. Chin. J. Pharmacol. Toxicol. 2022, 36, 297–302. [Google Scholar]
- Choy, E.H.S.; Calabrese, L.H. Neuroendocrine and Neurophysiological Effects of Interleukin 6 in Rheumatoid Arthritis. Rheumatology 2018, 57, 1885–1895. [Google Scholar] [CrossRef]
- Cheng, X.; Cui, N.; Hou, X.; Pi, Z.; Deng, J.; Liu, S. Uncovering the Effective Substances and Mechanism of Ipomoea pes-caprae against Rheumatoid Arthritis Using Liquid Chromatography-Mass Spectrometry and Targeted Network Pharmacology. J. Sep. Sci. 2023, 46, e2200856. [Google Scholar] [CrossRef]
- Miyoshi, M.; Liu, S. Collagen-Induced Arthritis Models. Methods Mol. Biol. 2018, 1868, 3–7. [Google Scholar]
- Ma, J.F.; Hou, X.J.; Liu, X.P.; Wang, Y.; Ding, M.G.; Zheng, W.; Zhu, Y.L. Establishment and Evaluation of Cold Syndrome Animal Model with Rheumatoid Arthritis. China J. Tradit. Chin. Med. Pharm. 2016, 31, 1967–1970. [Google Scholar]
- Yang, D.Y.; Zhao, Z.T.; Chen, J.L.; Zhu, T.T. Replication and Evaluation of Rat Model of Rheumatoid Arthritis with Heat Arthralgia Syndrome. Lishizhen Med. Mater. Med. Res. 2021, 32, 2042–2045. [Google Scholar]
- Chen, Q.; Zhou, W.; Huang, Y.; Tian, Y.; Wong, S.Y.; Lam, W.K.; Ying, K.Y.; Zhang, J.; Chen, H. Umbelliferone and Scopoletin Target Tyrosine Kinases on Fibroblast-Like Synoviocytes to Block Nf-Κb Signaling to Combat Rheumatoid Arthritis. Front. Pharmacol. 2022, 13, 946210. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, W.; Jin, L. Caffeic Acid Alleviates Inflammatory Response in Rheumatoid Arthritis Fibroblast-Like Synoviocytes by Inhibiting Phosphorylation of Iκb Kinase A/Β and Iκbα. Int. Immunopharmacol. 2017, 48, 61–66. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Z.; Xia, N.; Zhang, W.; Wei, Y.; Huang, J.; Ren, Z.; Meng, F.; Yang, L. Anti-Arthritic Activity of Ferulic Acid in Complete Freund’s Adjuvant (Cfa)-Induced Arthritis in Rats: Jak2 Inhibition. Inflammopharmacology 2020, 28, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Zhao, Q.; Ding, Y.; Zhang, B.; Kong, L. Protocatechuic Acid Inhibits Proliferation, Migration and Inflammatory Response in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 969–976. [Google Scholar] [CrossRef]
- Rzodkiewicz, P.; Gąsińska, E.; Gajewski, M.; Bujalska-Zadrożny, M.; Szukiewicz, D.; Maśliński, S. Esculetin Reduces Leukotriene B4 Level in Plasma of Rats with Adjuvant-Induced Arthritis. Reumatologia 2016, 54, 161–164. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the Il-23/Il-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Afifi, N.; Medhat, B.M.; Abdel Ghani, A.M.; Mohamed Ali Hassan, H.G.E.; Behiry, M.E. Value of Albumin-Fibrinogen Ratio and Crp-Albumin Ratio as Predictor Marker of Disease Activity in Egyptian Ra Patients, Correlated with Musculoskeletal Sonography. Res. Rev. 2020, 12, 241–248. [Google Scholar] [CrossRef]
- Ishizuka, M.; Nagata, H.; Takagi, K.; Iwasaki, Y.; Shibuya, N.; Kubota, K. Clinical Significance of the C-Reactive Protein to Albumin Ratio for Survival after Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 900–907. [Google Scholar] [CrossRef]
- Tominaga, T.; Nonaka, T.; Sumida, Y.; Hidaka, S.; Sawai, T.; Nagayasu, T. The C-Reactive Protein to Albumin Ratio as a Predictor of Severe Side Effects of Adjuvant Chemotherapy in Stage Iii Colorectal Cancer Patients. PLoS ONE 2016, 11, e0167967. [Google Scholar] [CrossRef]
- Sahebari, M.; Ayati, R.; Mirzaei, H.; Sahebkar, A.; Hejazi, S.; Saghafi, M.; Saadati, N.; Ferns, G.A.; Ghayour-Mobarhan, M. Serum Trace Element Concentrations in Rheumatoid Arthritis. Biol. Trace Elem. Res. 2016, 171, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, J.; Tan, B.; Zhu, F.; Fang, L. Hypercoagulable State Is Associated with Nf-Kappa B Activation and Increased Inflammatory Factors in Patients with Rheumatoid Arthritis. J. Cell Mol. Immunol. 2016, 32, 364–368. [Google Scholar]
- Liu, Q.; Wang, J.; Ding, C.; Chu, Y.; Jiang, F.; Hu, Y.; Li, H.; Wang, Q. Sinomenine Alleviates Rheumatoid Arthritis by Suppressing the Pi3k-Akt Signaling Pathway, as Demonstrated through Network Pharmacology, Molecular Docking; Experimental Validation. Drug Des. Devel. Ther. 2024, 18, 3523–3545. [Google Scholar] [CrossRef]
- Aihaiti, Y.; Tuerhong, X.; Zheng, H.; Cai, Y.; Yang, M.; Xu, P. Peroxiredoxin 4 Regulates Tumor-Cell-Like Characteristics of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis through Pi3k/Akt Signaling Pathway. Clin. Immunol. 2022, 237, 108964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.H.; Xu, J.J.; Zhu, D.C.; Li, J.F.; Zhang, C.X.; Lin, N.; Gao, W.Y. Glycyrrhizin Inhibits Osteoarthritis Development through Suppressing the Pi3k/Akt/Nf-Κb Signaling Pathway in Vivo and in Vitro. Food Funct. 2020, 11, 2126–2136. [Google Scholar] [CrossRef]
- Yuan, F.L.; Li, X.; Lu, W.G.; Sun, J.M.; Jiang, D.L.; Xu, R.S. Epidermal Growth Factor Receptor (Egfr) as a Therapeutic Target in Rheumatoid Arthritis. Clin. Rheumatol. 2013, 32, 289–292. [Google Scholar] [CrossRef]
- Gul, B.; Anwar, R.; Saleem, M.; Ahmad, M.; Ullah, M.I.; Kamran, S. Attenuation of Cfa-Induced Arthritis through Regulation of Inflammatory Cytokines and Antioxidant Mechanisms by Solanum Nigrum L. Leaves Extracts. Inflammopharmacology 2023, 31, 3281–3301. [Google Scholar] [CrossRef]
- Hughes, C.D.; Ryan, S.E.; Steel, K.J.A.; van den Beukel, M.D.; Trouw, L.A.; van Schie, K.A.J.; Toes, R.E.M.; Menon, B.; Kirkham, B.W.; Taams, L.S. Type 17-Specific Immune Pathways Are Active in Early Spondyloarthritis. RMD Open. 2023, 9, e003328. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Zhou, J.; Cai, Y.; Li, Z.; Sun, J.; Xie, Z.; Hao, G. Metabolic Effects of Quercetin on Inflammatory and Autoimmune Responses in Rheumatoid Arthritis Are Mediated through the Inhibition of Jak1/Stat3/Hif-1α Signaling. Mol. Med. 2024, 30, 170. [Google Scholar] [CrossRef]
- Wang, M.; Tian, T.; Yu, S.; He, N.; Ma, D. Th17 and Treg Cells in Bone Related Diseases. Clin. Dev. Immunol. 2013, 2013, 203705. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.T.; Zhao, Y.K.; Chen, J.L.; Zhu, T.T.; Yan, X.K.; Zhang, Y.F. Effect of Different Suspension Moxibustion Methods on Syndrome Characteristics of Rats with Rheumatoid Arthritis of Heat Bi Syndrome Based on “Moxibustion Can Be Used for Heat Syndrome”. Chin. Acupunct. Moxibustion 2023, 43, 1062–1069. [Google Scholar]
- Wang, Y.G.; Fu, J.X.; Zhang, C.; Hu, S.Q.; Zhao, H.B. Flower Scent Component Changes During the Flowering Process in Symplocos Sumuntia. J. Zhejiang A. F. Univ. 2016, 33, 516–523. [Google Scholar]
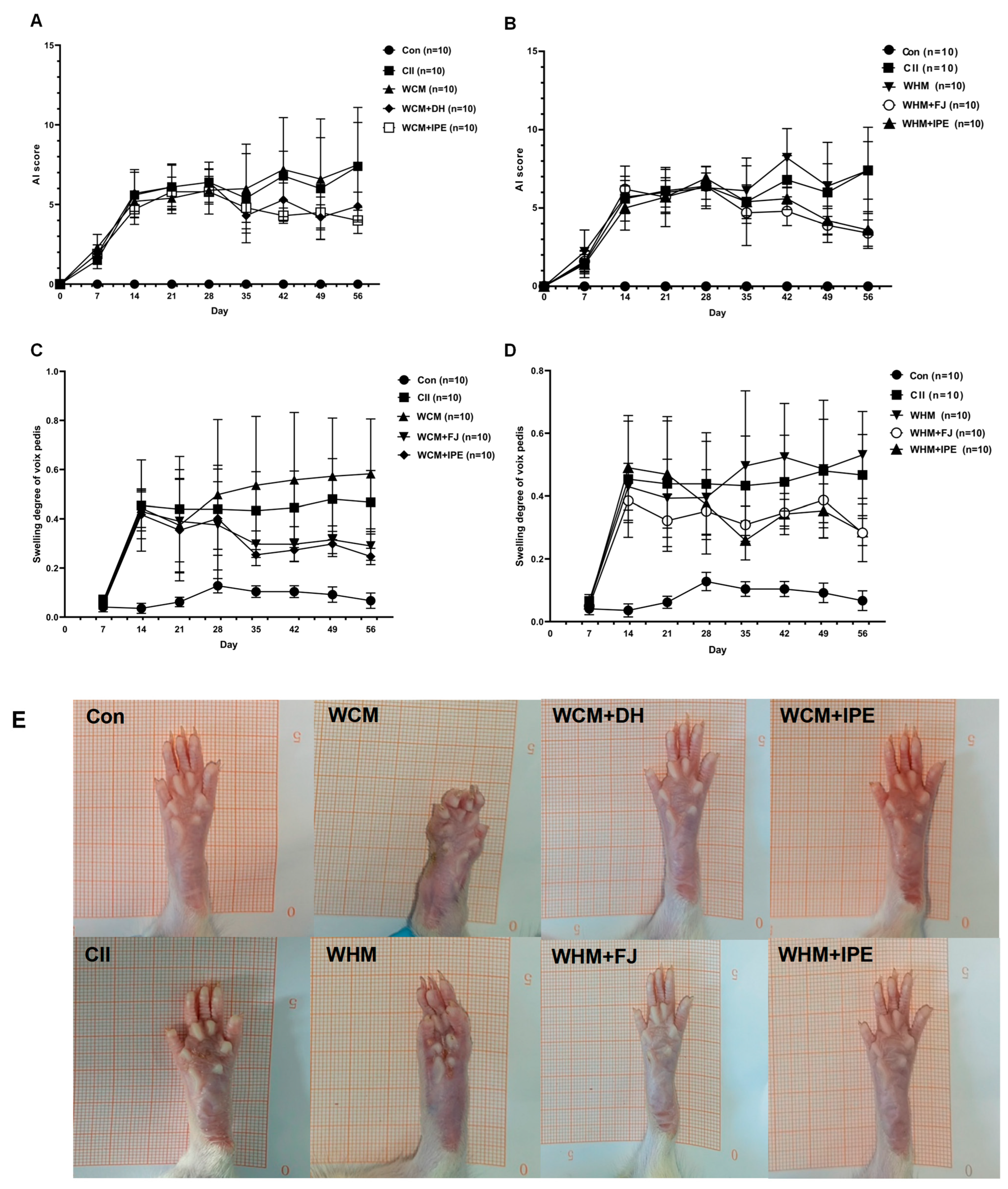








| Group | Dose (g/kg) | AI Score | |
|---|---|---|---|
| Day 28 (Prior to First Dose) | Day 56 (Post Last Dose) | ||
| Con | - | 0 ± 0 | 0 ± 0 |
| CII | - | 6.4 ± 0.8 ** | 7.4 ± 2.8 ** |
| WCM | - | 5.9 ± 0.9 ** | 7.5 ± 3.6 ** |
| WHM | - | 6.3 ± 1.3 ** | 7.4 ± 1.8 ** |
| WCM + DH | 9 | 6.4 ± 1.3 ** | 4.9 ± 0.9 Δ |
| WHM + FJ | 9 | 6.9 ± 0.7 ** | 3.6 ± 1.2 # |
| WCM + IPE | 13.5 | 5.8 ± 1.4 ** | 4.0 ± 0.8 Δ |
| WHM + IPE | 13.5 | 6.4 ± 1.3 ** | 3.4 ± 0.8 # |
| Group | Dose (g/kg) | left Hind Toes Swelling | |
|---|---|---|---|
| Day 28 (Prior to First Dose) | Day 56 (Post Last Dose) | ||
| Con | - | 0.13 ± 0.03 | 0.07 ± 0.03 |
| CII | - | 0.44 ± 0.16 ** | 0.47 ± 0.13 ** |
| WCM | - | 0.50 ± 0.31 ** | 0.58 ± 0.22 ** |
| WHM | - | 0.40 ± 0.18 ** | 0.53 ± 0.14 ** |
| WCM + DH | 9 | 0.38 ± 0.24 ** | 0.29 ± 0.06 Δ |
| WHM + FJ | 9 | 0.35 ± 0.07 ** | 0.28 ± 0.09 # |
| WCM + IPE | 13.5 | 0.40 ± 0.15 ** | 0.25 ± 0.03 Δ |
| WHM + IPE | 13.5 | 0.37 ± 0.11 ** | 0.29 ± 0.04 # |
| Peak No. | Formula | Retention Time (min) | Fragment Ion | Relative Molecular Mass (m/z) | Error (ppm) ×106 | Fragment Ions in the Positive/Negative ion Mode (m/z) | Identification | Relative Content (%) | Identification Type | |
|---|---|---|---|---|---|---|---|---|---|---|
| Precursor Mass | Found at Mass | |||||||||
| 1 | C7H12O6 | 0.53 | [M−H]− | 191.0561 | 191.0562 | 0.4 | 173.0459, 127.0402, 109.0297 | Quinic acid | 1.918 | Organic acid |
| 2 | C4H4O4 | 0.59 | [M−H]− | 115.0036 | 115.0037 | 0.3 | 97.9325, 71.0130 | Fumaric acid | 0.129 | Organic acid |
| 3 | C4H6O5 | 0.6 | [M−H]− | 133.0143 | 133.0144 | 1.1 | 71.0139, 72.9931, 59.0138, 115.0039 | Malic acid | 0.626 | Organic acid |
| 4 | C6H8O7 | 0.72 | [M−H]− | 191.0197 | 191.0200 | 1.4 | 129.0198, 111.0091 | Citric acid | 1.839 | Organic acid |
| 5 | C10H13N5O4 | 0.76 | [M+H]+ | 268.1040 | 268.1039 | −0.5 | 136.0615, 119.0351 | Adenosine | 0.441 | Nucleoside |
| 6 | C4H6O4 | 0.79 | [M−H]− | 117.0193 | 117.0193 | 0 | 117.0193, 99.9260, 73.0294 | Succinic acid | 0.034 | Organic acid |
| 7 | C10H13N5O5 | 0.8 | [M−H]− | 282.0844 | 282.0846 | 0.8 | 150.0422, 133.0156 | Guanosine | 0.104 | Adenosine |
| 8 | C7H6O5 | 1.03 | [M−H]− | 169.0143 | 169.0141 | −0.7 | 79.0185, 125.0250, 124.0251, 69.0338, 51.0242 | Gallic acid | 0.015 | Organic acid |
| 9 | C7H6O4 | 1.75 | [M−H]− | 153.0193 | 153.0195 | 1.3 | 108.0221, 109.0290 | Protocatechuic acid | 0.296 | Organic acid |
| 10 | C16H18O9 | 2.03 | [M−H]− | 353.0878 | 353.0880 | 0.6 | 191.0579, 179.0347, 135.0453, 161.0245 | Neochlorogenic acid | 11.241 | Organic acid |
| 11 | C7H6O3 | 2.36 | [M−H]− | 137.0244 | 137.0245 | 0.1 | 137.0245, 119.0140, 109.0296, 108.0219, 93.0343 | Protocatechuic aldehyde | 1.34 | Organic acid |
| 12 | C7H6O3 | 2.36 | [M−H]− | 137.0244 | 137.0245 | 0.1 | 137.0245, 93.0343, 65.0033 | Salicylic acid | 1.34 | Organic acid |
| 13 | C15H16O9 | 2.38 | [M−H]− | 339.0722 | 339.0721 | −0.2 | 177.0192, 133.0295 | Esculin | 0.041 | Coumarins |
| 14 | C8H8O3 | 2.97 | [M−H]− | 151.0401 | 151.0400 | −0.5 | 136.0168, 108.0216 | Methyl 4-hydroxybenzoate | 0.043 | Organic acid |
| 15 | C16H18O9 | 3.03 | [M−H]− | 353.0878 | 353.0879 | 0.2 | 191.0584, 173.0455, 135.0450, 179.0350, 161.0246 | Chlorogenic acid | 15.739 | Organic acid |
| 16 | C16H18O9 | 3.03 | [M−H]− | 353.0878 | 353.0879 | 0.2 | 1910561, 179.0349, 135.0451, 161.0241 | Cryptochlorogenic acid | 2.335 | Organic acid |
| 17 | C10H8O4 | 3.08 | [M+H]+ | 193.0495 | 193.0494 | −0.7 | 178.0262, 150,0313, 133.0285, 122.0363 | Scopoletin | 0.575 | Coumarins |
| 18 | C9H10O2 | 3.11 | [M−H]− | 149.0608 | 149.0606 | −1.1 | 149.0581, 121.0688 | 4-Hydroxy-3-methoxystyrene | 0.021 | Organic acid |
| 19 | C10H10O4 | 3.13 | [M−H]− | 193.0506 | 195.0504 | −1 | 134.0360, 133.0270 | Ferulic acid | 0.14 | Organic acid |
| 20 | C9H6O4 | 3.18 | [M+H]+ | 179.0339 | 179.0337 | −1 | 151.0398, 123.0443, | Esculetin | 0.053 | Coumarins |
| 21 | C9H8O4 | 3.25 | [M−H]− | 179.0350 | 179.0349 | −0.3 | 135.0453, 134.0375, 107.0504, 89.0398, 79.0554 | Caffeic acid | 3.633 | Organic acid |
| 22 | C17H20N4O6 | 3.89 | [M+H]− | 377.1456 | 377.1455 | −0.1 | 377.1427, 243.0877, 172.0873, 319.1363 | Vitamine B2 | 0.201 | Vitamins |
| 23 | C9H6O2 | 4.51 | [M+H]+ | 147.0441 | 147.0441 | 0 | 119.0487 | Coumarin | 0.097 | Coumarins |
| 24 | C27H30O17 | 4.73 | [M−H]− | 625.1410 | 625.1410 | 0 | 625.1413, 463.1073, 301.0361, 271.0254 | Quercetin-3-O-sophoroside | 0.095 | Flavonoids |
| 25 | C10H10O3 | 4.91 | [M+H]− | 179.0703 | 179.0609 | −1.9 | 133.1009, 105.0700 | Mellein | 0.029 | Organic acid |
| 26 | C10H8O4 | 4.98 | [M+H]− | 193.0495 | 193.0492 | −1.8 | 178.0259, 150.0310, 133.0284, 122.0362 | Scopoletin | 0.787 | Coumarins |
| 27 | C10H10O4 | 5.11 | [M−H]− | 193.0506 | 193.0503 | −1.4 | 133.0285, 134.0361 | Methyl caffeate acid | 0.042 | Organic acid |
| 28 | C27H30O16 | 5.39 | [M−H]− | 609.1461 | 609.1458 | −0.5 | 609.1455, 301.0349, 300.0273, 271.0245 | Rutin | 0.155 | Flavonoids |
| 29 | C21H20O12 | 5.59 | [M−H]− | 463.0882 | 463.0883 | 0.2 | 301.0354, 300.0276, 271.0249, 255.0300 | Isoquercitrin | 0.762 | Flavonoids |
| 30 | C21H20O12 | 5.71 | [M−H]− | 463.0882 | 463.0881 | −0.1 | 301.0353, 300.0275, 271.0249, 255.0299 | Quercetin-7-O-β-D-glucopyranoside | 2.043 | Flavonoids |
| 31 | C7H6O3 | 5.75 | [M−H]− | 137.0244 | 137.0242 | −1.8 | 93.0348, 65.0396 | p-Hydroxybenzoic acid | 0.074 | Organic acid |
| 32 | C27H30O15 | 5.88 | [M−H]− | 593.1512 | 593.1511 | −0.3 | 284.0331 | Kaempferol-3-O-rutinoside | 0.026 | Flavonoids |
| 33 | C25H24O12 | 6.09 | [M−H]− | 515.1195 | 515.1194 | −0.2 | 353.0875, 191.0557, 179.5898, 173.0475 | Isochlorogenic acid B | 19.91 | Organic acid |
| 34 | C11H12O4 | 6.138 | [M+H]+ | 209.0808 | 209.0808 | 0 | 103.0544 | Ethyl Caffeate | 0.074 | Organic acid |
| 35 | C15H10O6 | 6.15 | [M+H]+ | 287.0550 | 287.0540 | −3.5 | 153.0185, 121.0291 | Kaempferol | 0.147 | Flavonoids |
| 36 | C21H20O11 | 6.15 | [M−H]− | 447.0933 | 447.0929 | −1 | 284.0327, 255.0295, 227.0346 | Astragalin | 0.123 | Flavonoids |
| 37 | C26H32O11 | 6.16 | [M−H]− | 519.1872 | 519.1871 | −0.4 | 357.1344, 151.0402, 136.0165 | (+)-Pinoresinol-β-D-glucoside | 0.108 | Flavonoids |
| 38 | C25H24O12 | 6.31 | [M−H]− | 515.1195 | 515.1192 | −0.6 | 353.0875, 191.6309, 179.0347, 173.0457 | Isochlorogenic acid A | 12.564 | Organic acid |
| 39 | C22H26O8 | 6.41 | [M−H]− | 417.1555 | 417.1550 | −1.2 | 387.1084, 181.0503, 166.0269, 137.0242 | Syringaresinol | 0.167 | Organic acid |
| 40 | C25H24O12 | 6.75 | [M−H]− | 515.1195 | 515.1191 | −0.8 | 353.0873, 191.0555, 179.0357, 173.0480 | Isochlorogenic acid C | 19.627 | Organic acid |
| 41 | C26H26O12 | 8.01 | [M−H]− | 529.1352 | 529.1348 | −0.7 | 367.1033, 353,0870, 179.0350, 173.0454 | 3,5-Di-caffeoylquinic acid or 4,5-Di -caffeoylquinicacidmethyl Ester or 3,4-Di- caffeoylquinic acid methyl ester | 0.302 | Organic acid |
| 42 | C34H30O14 | 8.26 | [M−H]− | 661.1563 | 661.1561 | −0.3 | 515.1193, 499.1245, 353.0880, 191.0558, 179.0349 | 3,5-Di-O-caffeoyl-4-O-coumaroylquinic acid | 0.27 | Organic acid |
| 43 | C18H16O6 | 9.53 | [M+H]+ | 329.1020 | 329.1021 | 0.5 | 314.0787, 299.0552 | 4′-Hydroxy-3′,5,7, -trimethoxyflavone | 0.242 | Flavonoids |
| 44 | C36H58O10 | 10.27 | [M+FA−H]− | 695.4012 | 695.4005 | −1.0 | 487.3421 | Pedunculoside | 0.102 | Terpene |
| 45 | C43H36O16 | 10.93 | [M−H]− | 807.1931 | 807.1925 | −0.8 | 645.1607, 499.1238, 179.0354 | 4,5-Di-O-caffeoyl-1,3-Di-O-couma-roylquinicacid | 0.021 | Organic acid |
| 46 | C42H62O16 | 11.84 | [M−H]− | 821.3965 | 821.3968 | 0.3 | 821.3948, 351.0563 | Glycyrrhizic Acid | 0.009 | Organic acid |
| 47 | C13H14O2 | 17.76 | [M+H]+ | 203.1067 | 203.1066 | −0.4 | 185.0957, 161.0960, 121.0653 | 2-Hydroxy-4,4,7-trimethyl-1(4H)-naphthalenone | 0.086 | Organic acid |
| 48 | C61H106O24 | 20.97 | [M+FA−H]− | 1267.7056 | 1267.7057 | 0.2 | 965.4911, 947.4778, 545.3325, 417.2858, 146.9649 | Pescapreins XXX | 0.001 | Resin glycosides |
| 49 | C18H34O2 | 24.36 | [M−H]− | 281.2481 | 281.2479 | −2.5 | 281.7474 | Oleic acid | 0.032 | Organic acid |
| Group | No. | Retention Time (min) | Formula | Fragment Ion | Relative Molecular Mass (m/z) | Error (ppm) ×106 | Fragment Ions in the Positive/Negative Ion Mode (m/z) | Identification | Relative Content (%) | >Identification Type | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Precursor Mass | Found at Mass | ||||||||||
| Con + IPE | 1 | 1.98 | C7H6O4 | [M−H]− | 153.0193 | 153.0197 | 2.5 | 109.0305, 108.0225 | Protocatechuic acid | 1.04 | Prototypical or metabolic components |
| 2 | 3.26 | C10H8O4 | [M+H]+ | 193.0495 | 193.0495 | 0 | 178.0269, 150.0323, 133.0297, 122.0378 | Scopoletin | 5.83 | Prototype components | |
| 3 | 3.34 | C9H8O4 | [M−H]− | 179.035 | 179.0353 | 1.6 | 135.0453, 117.0340, 89.0391 | Caffeic acid | 33.04 | Prototypical or metabolic components | |
| 4 | 3.42 | C9H6O4 | [M+H]+ | 179.0339 | 179.0343 | 2.0 | 123.0455 | Esculetine | 0.39 | Prototype components | |
| 5 | 3.6 | C10H10O4 | [M−H]− | 193.0506 | 193.0507 | 0.3 | 134.0380, 133.0296 | Ferulic acid | 17.23 | Prototypical or metabolic components | |
| 6 | 5.22 | C10H8O4 | [M+H]+ | 193.0495 | 193.0493 | −1.0 | 178.0270, 150.0317, 133.0299, 122.0378 | Isoscopoletin | 2.7 | Prototype components | |
| 7 | 5.39 | C10H10O4 | [M−H]− | 193.0506 | 193.0511 | 2.6 | 134.0373, 133.0289 | Methyl caffeate acid | 1.57 | Prototypical or metabolic components | |
| 8 | 6.05 | C7H6O3 | [M−H]− | 137.0244 | 137.0242 | −1.2 | 93.0334, 65.0391 | p-Hydroxybenzoic acid | 38.2 | Prototypical or metabolic components | |
| WCM + IPE | 9 | 1.75 | C7H6O4 | [M−H]− | 153.0193 | 153.0196 | 2 | 109.0291, 108.0207 | Protocatechuic acid | 1.14 | Prototypical or metabolic components |
| 10 | 2.15 | C7H6O3 | [M−H]− | 137.0244 | 137.0246 | 1.6 | 108.0226, 93.0345 | Protocatechuic aldehyde | 1.68 | Prototype components | |
| 11 | 3.16 | C10H8O4 | [M+H]+ | 193.0495 | 193.0494 | −0.1 | 178.0271, 150.0322, 133.0289, 122.0369 | Scopoletin | 4.71 | Prototype components | |
| 12 | 3.19 | C9H8O4 | [M−H]− | 179.0350 | 179.0352 | 1.2 | 135.0454, 117.0346, 89.0400 | Caffeic acid | 30.2 | Prototypical or metabolic components | |
| 13 | 3.31 | C9H6O4 | [M+H]+ | 179.0339 | 179.0340 | 0.8 | 151.0398, 123.0442 | Esculetine | 0.5 | Prototype components | |
| 14 | 3.48 | C10H10O4 | [M−H]− | 193.0506 | 193.0506 | 0 | 134.0363, 133.0291 | Ferulic acid | 20.09 | Prototypical or metabolic components | |
| 15 | 4.69 | C9H6O2 | [M+H]+ | 147.0441 | 147.0440 | −0.7 | 119.0500 | Coumarin | 1.77 | Prototype components | |
| 16 | 5.18 | C10H8O4 | [M+H]+ | 193.0495 | 193.0486 | −5 | 178.0270, 150.0321, 133.0291, 122.0366 | Isoscopoletin | 7.8 | Prototype components | |
| 17 | 5.32 | C10H10O4 | [M−H]− | 193.0506 | 193.0505 | 0.6 | 133.0285, 134.0361 | Methyl caffeate acid | 2.45 | Prototypical or metabolic components | |
| 18 | 5.96 | C7H6O3 | [M−H]− | 137.0244 | 137.0242 | −1.3 | 93.0334, 65.0390 | p-Hydroxybenzoic acid | 29.65 | Prototypical or metabolic components | |
| WHM + IPE | 19 | 1.79 | C7H6O4 | [M−H]− | 153.0193 | 153.0192 | −0.9 | 109.0308, 108.0219 | Protocatechuic acid | 0.84 | Prototypical or metabolic components |
| 20 | 3.23 | C10H8O4 | [M+H]+ | 193.0495 | 193.0497 | 1 | 178.0280, 150.0334, 133.0299, 122.0369 | Scopoletin | 5.56 | Prototype components | |
| 21 | 3.32 | C9H8O4 | [M−H]− | 179.0350 | 179.0349 | −0.7 | 135.0454, 107.0509, 89.0391 | Caffeic acid | 34.24 | Prototypical or metabolic components | |
| 22 | 3.41 | C9H6O4 | [M+H]+ | 179.0339 | 179.0342 | 1.7 | 151.0141, 123.0439 | Esculetine | 0.58 | Prototype components | |
| 23 | 3.58 | C10H10O4 | [M−H]− | 193.0506 | 193.0505 | −0.6 | 134.0369, 133.0294 | Ferulic acid | 17.71 | Prototypical or metabolic components | |
| 24 | 5.21 | C10H8O4 | [M+H]+ | 193.0495 | 193.0489 | −3.2 | 178.0259, 150.0310, 133.0284, 122.0368 | Isoscopoletin | 5 | Prototype components | |
| 25 | 5.38 | C10H10O4 | [M−H]− | 193.0506 | 193.0508 | 1 | 134.0510, 133.0292 | Methyl caffeate acid | 2.02 | Prototypical or metabolic components | |
| 26 | 6.05 | C7H6O3 | [M−H]− | 137.0244 | 137.0241 | −2.1 | 93.0333, 65.0391 | p-Hydroxybenzoic acid | 34.04 | Prototypical or metabolic components | |
| Parameters | AUC0~t (μg·h·L−1) | MRT0~t (h) | Tmax (h) | Cmax (μg·L−1) | t1/2 (h) | |
|---|---|---|---|---|---|---|
| Group Component | ||||||
| Con + IPE | Caffeic acid | 2078.79 ± 349.44 | 4.18 ± 1.82 | 0.85 ± 0.22 | 702.53 ± 189.91 | 4.51 ± 3.03 |
| Ferulic acid | 60.56 ± 27.27 | 1.30 ± 0.28 | 0.45 ± 0.10 | 35.76 ± 10.90 | 1.51 ± 0.51 | |
| Protocatechuic acid | 504.08 ± 177.40 | 1.90 ± 0.42 | 0.66 ± 0.25 | 268.86 ± 90.17 | 1.93 ± 1.01 | |
| WCM + IPE | Caffeic acid | 1130.16 ± 162.90 | 3.32 ± 0.35 | 0.62 ± 0.14 | 341.80 ± 123.30 | 2.82 ± 1.07 |
| Ferulic acid | 34.92 ± 22.61 | 1.05 ± 0.35 | 0.33 ± 0.12 | 26.15 ± 11.58 | 1.02 ± 0.46 | |
| Protocatechuic acid | 193.59 ± 99.99 | 1.59 ± 0.38 | 0.41 ± 0.12 | 121.05 ± 77.40 | 1.46 ± 0.32 | |
| WHM + IPE | Caffeic acid | 2301.61 ± 409.95 | 4.53 ± 1.34 | 0.93 ± 0.12 | 709.49 ± 194.27 | 3.82 ± 2.55 |
| Ferulic acid | 103.90 ± 41.20 | 2.27 ± 0.42 | 0.7 ± 0.27 | 38.75 ± 16.20 | 1.31 ± 0.32 | |
| Protocatechuic acid | 306.73 ± 98.26 | 1.83 ± 0.24 | 0.62 ± 0.14 | 145.46 ± 53.24 | 1.25 ± 0.36 |
| Group | No. | Metabolite | Formula | HMDB ID | WHM vs. Con | WHM + IPE vs. WHM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIP | Difference Multiple | p Value | Trend | VIP | Difference Multiple | p Value | Trend | |||||
| WHM + IPE | 1 | Urocanic acid | C6H6N2O2 | 0000301 | 1.008 | 0.5471 | 0.001 | ↓ | 14.11 | 309.5 | 0.000 | ↑ |
| 2 | D-allose | C6H12O6 | 0001151 | 7.123 | 0.6662 | 0.024 | ↓ | 2.675 | 1.436 | 0.021 | ↑ | |
| 3 | D-mannitol | C6H14O6 | 0000765 | 1.555 | 0.6053 | 0.000 | ↓ | 2.681 | 5.095 | 0.000 | ↑ | |
| 4 | Hippuric acid | C9H9NO3 | 0000714 | 5.063 | 0.4753 | 0.000 | ↓ | 10.65 | 10.78 | 0.000 | ↑ | |
| 5 | L-threonate | C4H8O5 | 0000943 | 1.954 | 0.7518 | 0.001 | ↓ | 3.403 | 3.074 | 0.000 | ↑ | |
| 6 | Pentachlorophenol | C₆HCl₅O | 0041974 | 1.228 | 0.6585 | 0.004 | ↓ | 1.730 | 3.064 | 0.000 | ↑ | |
| 7 | Pyrocatechol | C₆H₆O₂ | 0000957 | 1.071 | 0.4134 | 0.013 | ↓ | 2.782 | 18.23 | 0.000 | ↑ | |
| 8 | 1-Methyl-l-histidine | C₇H11N₃O₂ | 0000001 | 1.469 | 1.294 | 0.041 | ↑ | 3.027 | 0.060 | 0.000 | ↓ | |
| 9 | Creatinine | C4H7N3O | 0000562 | 8.835 | 0.8639 | 0.012 | ↓ | 10.49 | 1.213 | 0.000 | ↑ | |
| 10 | DL-normetanephrine | C₈H11NO₃ | 0000819 | 2.890 | 0.6666 | 0.036 | ↓ | 6.720 | 3.023 | 0.000 | ↑ | |
| 11 | Malonic acid | C3H4O4 | 0000691 | 20.16 | 0.6487 | 0.004 | ↓ | 22.96 | 1.609 | 0.000 | ↑ | |
| WCM + IPE | 12 | Urocanic acid | C6H6N2O2 | 0000301 | 1.043 | 0.7180 | 0.037 | ↓ | 15.12 | 268.1 | 0.000 | ↑ |
| 13 | Capric acid | C10H20O2 | 0000511 | 1.791 | 1.312 | 0.010 | ↑ | 2.166 | 0.4037 | 0.000 | ↓ | |
| 14 | O-succinyl-L-homoserine | C8H13NO6 | 0255868 | 1.068 | 1.262 | 0.040 | ↑ | 1.001 | 0.6845 | 0.016 | ↓ | |
| 15 | Octanoic acid | C8H16O2 | 0000482 | 2.684 | 1.501 | 0.008 | ↑ | 2.251 | 0.4895 | 0.000 | ↓ | |
| 16 | p-Coumaryl alcohol | C9H10O2 | 0003654 | 1.418 | 0.6516 | 0.041 | ↓ | 2.614 | 5.756 | 0.000 | ↑ | |
| 17 | Uracil | C₄H₄N₂O₂ | 0000300 | 10.96 | 1.440 | 0.000 | ↑ | 5.442 | 0.7988 | 0.035 | ↓ | |
| 18 | γ-Aminobutyric acid | C4H9NO2 | 0000112 | 2.308 | 1.230 | 0.015 | ↑ | 2.497 | 0.7888 | 0.008 | ↓ | |
| 19 | 2′-Deoxycytidine | C9H13N3O4 | 0000014 | 3.011 | 1.124 | 0.011 | ↑ | 3.165 | 0.8917 | 0.008 | ↓ | |
| 20 | Cytosine | C4H5N3O | 0000630 | 4.876 | 1.114 | 0.016 | ↑ | 4.607 | 0.9116 | 0.049 | ↓ | |
| 21 | D-mannose | C6H12O6 | 0000169 | 2.547 | 0.5833 | 0.000 | ↓ | 1.072 | 1.337 | 0.025 | ↑ | |
| Gene | Name | Degree |
|---|---|---|
| ALB | Albumin | 44 |
| AKT1 | AKT serine/threonine kinase 1 Gene | 41 |
| EGFR | Epidermal growth factor receptor | 37 |
| CASP3 | Caspase-3 | 36 |
| Identification | Ion Pair (m/z) | Collecting Model | Voltage (V) | Collision Energy (V) |
|---|---|---|---|---|
| Caffeic acid | 179.0350→135.0428 | [M−H]− | −80 | −35 |
| Ferulic acid | 193.0506→134.0411 | [M−H]− | −80 | −35 |
| Protocatechuic acid | 153.0193→109.0294 | [M−H]− | −80 | −35 |
| Syringic acid | 197.0556→123.0091 | [M−H]− | −80 | −20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, F.; Li, S.; Pan, X.; Wen, J.; Xie, J.; Du, Z.; Hao, E.; Deng, J.; Hou, X. Investigating the Mechanism of Action of Ipomoea pes-caprae in the Treatment of Rheumatoid Arthritis Based on Serum Metabolomics and Network Pharmacology. Mar. Drugs 2025, 23, 114. https://doi.org/10.3390/md23030114
Zhong F, Li S, Pan X, Wen J, Xie J, Du Z, Hao E, Deng J, Hou X. Investigating the Mechanism of Action of Ipomoea pes-caprae in the Treatment of Rheumatoid Arthritis Based on Serum Metabolomics and Network Pharmacology. Marine Drugs. 2025; 23(3):114. https://doi.org/10.3390/md23030114
Chicago/Turabian StyleZhong, Fangfei, Siwei Li, Xianglong Pan, Juan Wen, Jinling Xie, Zhengcai Du, Erwei Hao, Jiagang Deng, and Xiaotao Hou. 2025. "Investigating the Mechanism of Action of Ipomoea pes-caprae in the Treatment of Rheumatoid Arthritis Based on Serum Metabolomics and Network Pharmacology" Marine Drugs 23, no. 3: 114. https://doi.org/10.3390/md23030114
APA StyleZhong, F., Li, S., Pan, X., Wen, J., Xie, J., Du, Z., Hao, E., Deng, J., & Hou, X. (2025). Investigating the Mechanism of Action of Ipomoea pes-caprae in the Treatment of Rheumatoid Arthritis Based on Serum Metabolomics and Network Pharmacology. Marine Drugs, 23(3), 114. https://doi.org/10.3390/md23030114







