Nannochloropsis Lipids and Polyunsaturated Fatty Acids: Potential Applications and Strain Improvement
Abstract
1. Introduction
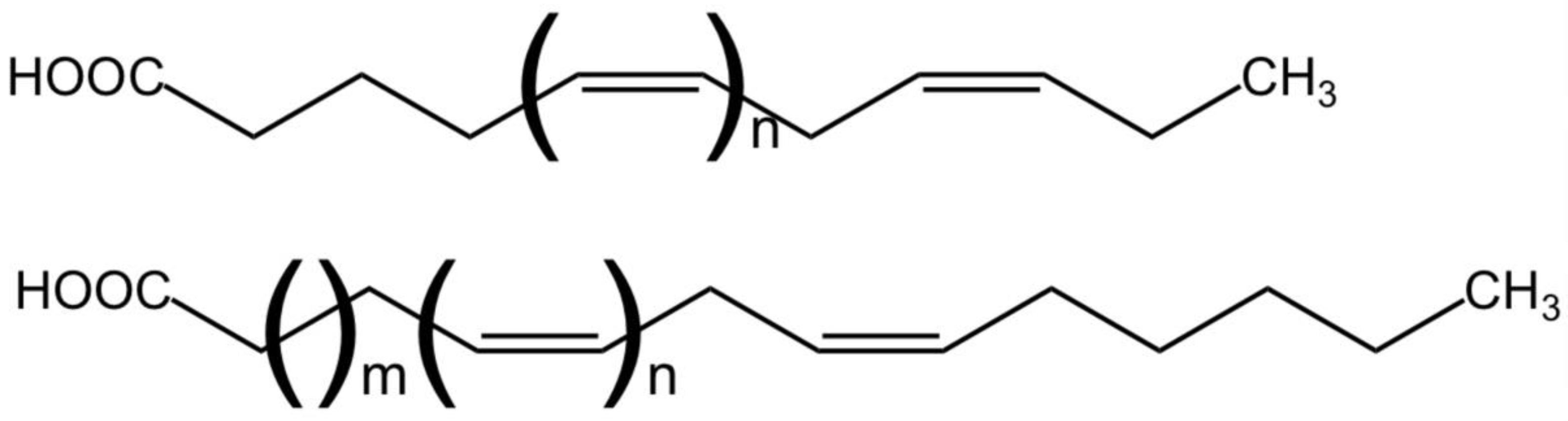
2. Lipid Classes and Polyunsaturated Fatty Acids
2.1. PUFA Synthesis Pathways
2.2. Occurrence of PUFAs in Nature
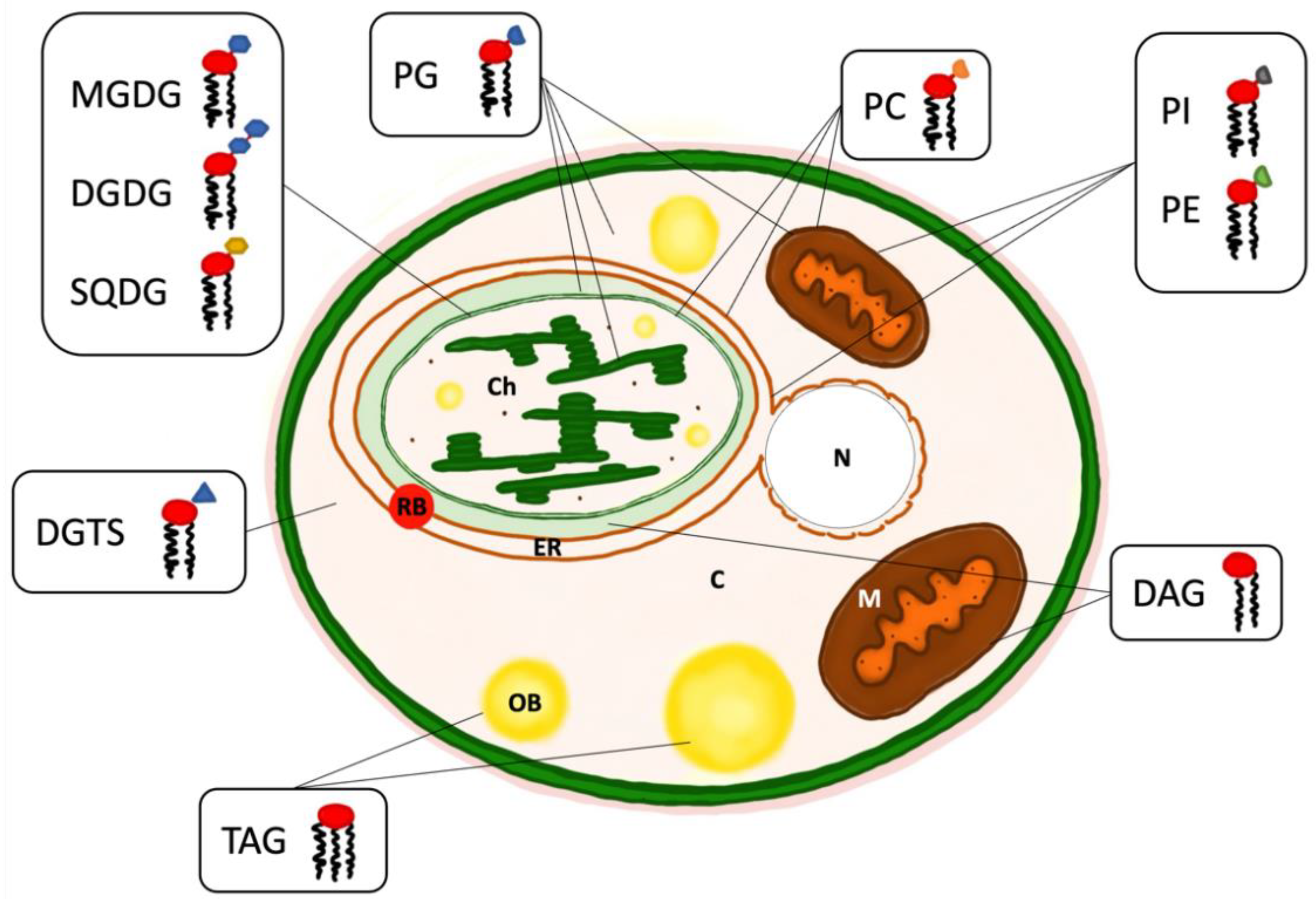
3. Intracellular Organisation of Lipids
4. PUFA Applications
4.1. Nutrition: Food and Feed
4.1.1. Food
Meat Quality
Dairy
4.1.2. Feed in Aquaculture
| Animal Species | Microalgae Species | Feed Incorporation (%) | Effect | Reference |
|---|---|---|---|---|
| Juvenile turbot (Scophthalmus maximus L.) | Nannochloropsis sp. | 2.5, 5, 7.5 and 10 | Improved weight gain, enhanced antioxidant capacity, increased digestive enzyme activity at 5% incorporation. | Qiao et al. [105] |
| Pacific white shrimp | Nannochloropsis spp. | 0.5, 1, 2 | At 1% incorporation, a trend towards lower mortality and increased ROS production was observed. At 2% incorporation, there was a significant increase in resistance to thermal shock and lower mortality and highest production of ROS. | Guimarães et al. [106] |
| Senegalese sole (Solea senegalensis) | Nannochloropsis gaditana | 3 | Increased dry weight; improved growth performance; slight decrease in glutathione levels. | Peixoto et al. [107] |
| Nile tilapia (Oreochromis niloticus) | Nannochloropsis oculata | 5, 10 | At 5%, improved growth performance, increased crude protein, enhanced lipid profile (higher high-density lipoproteins, lower low-density lipoproteins). At 10% further improvement in growth performance, significant increase in ω-3 PUFA, better antioxidant response, higher EPA and DHA content. | Zahran et al. [108] |
| Kuruma Shrimp (Marsupenaeus japonicus) | Nannochloropsis sp. | 1, 4, 7 | A 1% improved survival rate and increased body weight compared to control diet; 4%, alonside increased body weight, also improved feed efficiency and body lipid content; 7% incorporation increased stress tolerance and improved fatty acid profile with higher EPA and DHA. | Adissin et al. [109] |
4.1.3. Cosmeceuticals
4.1.4. Pharmaceutical Applications of PUFAs Obtained from Nannochloropsis sp.
5. Detection and Analysis of Lipids and PUFAs
5.1. Offline
5.1.1. Titration Methods
5.1.2. Colorimetric Methods
5.1.3. Fluorometric Methods
5.1.4. Gas Chromatography
5.1.5. Mass Spectrometry
5.1.6. Vibrational Spectroscopy
5.2. Online
5.2.1. Autofluorescence and Fluorescence from Dyes
5.2.2. Fluorescence Activated Cell Sorting
5.2.3. Raman Spectroscopy
6. Strategies to Modulate PUFA Content in Polar and Neutral Lipids in Nannochloropsis sp.
6.1. Culture Conditions
6.1.1. Temperature
6.1.2. Light
6.1.3. Nutrient Availability
Nitrogen
Phosphorus
6.1.4. Two-Step Cultivation
6.2. Microalgae Strain Improvement
6.2.1. Random Mutagenesis
Mutagenic Agent
Selection with Pathway Inhibitors
| Growth Inhibitor | Concentration | Improvement | Study |
|---|---|---|---|
| Cerulenin | 25 μM | 29% EPA increase | Chaturvedi et al. [200] |
| Cerulenin and Galvestine-1 | 50/60 μM and 10 μM | Increased membrane lipids and EPA 1.4-fold | Razali et al. [201] |
| DCMU 1 | 2 μM | EPA increase | Zhang et al. [202] |
| Erythromycin | 50 μg/mL | 12% EPA increase | Chaturvi et al. [200] |
| Quizalofop | 50 and 70 μM | PUFA, EPA, and TFA increase | Chaturvi et al. [203] |
6.2.2. Adaptive Laboratory Evolution
| Microalgae | Strain Improvement Method | Mutagenic Agent/Selective Pressure | Improvement | Study |
|---|---|---|---|---|
| Nannochloropsis gaditana | RM | EMS | Increased productivity | Perin et al. [212] |
| Nannochloropsis gaditana | RM | EMS | Increased photosynthetic activity and productivity; decreased chl content | Perin et al. [213] |
| Nannochloropsis gaditana | RM | EMS | Increased lipid productivity | Cecchin et al. [214] |
| Nannochloropsis gaditana | RM | EMS | Increased lipids and ketocarotenoid productivity | Cecchin et al. [215] |
| Nannochloropsis oceanica | RM | Heavy ion irradiation | Increased growth rate, chl-a content, and lipid productivity | Ma et al. [70] |
| Nannochloropsis oceanica | RM | EMS and NTG | Increased lipid productivity | Wang et al. [216] |
| Nannochloropsis oceanica | RM | Nuclear radiation | Increased biomass productivity and higher oxygen evolution rate | Lu et al. [217] |
| Nannochloropsis oculata | RM | DCMU | Increased EPA | Jimin et al. [204] |
| Nannochloropsis oculata | RM | MNU | Increased PUFA, EPA, and TFA | Chaturvedi et al. [205] |
| Nannochloropsis oculata | RM | UV 320–400 nm | Higher lipids: chl | Srinivas et al. [218] |
| Nannochloropsis oculata | RM | UV 345 nm | Higher lipid content; increased ω-3 and ω-6 | Moha-Léon et al. [219] |
| Nannochloropsis oculata | RM and ALE | EMS and temperature stress | Increased temperature tolerance by 10 °C and lipid productivity and content | Arora et al. [220] |
| Nannochloropsis oculata | RM | EMS | Increased PUFA, carbohydrate, and pigment productivity | Arora et al. [221] |
| Nannochloropsis oculata | RM | EMS | Increased membrane lipids and EPA content | Razali et al. [201] |
| Nannochloropsis oculate ST-6 | RM | EMS | EPA increase | Chaturvedi et al. [200] |
| Nannochloropsis sp. | RM | EMS | Increased lipid productivity | Anandarajah et al. [222] |
| Nannochloropsis sp. | RM | EMS | TFA increase; PUFA decrease | Doan et al. [162] |
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results; UN DESA/POP/2022/TR/NO. 3; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food Commodities from Microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The Role of Microalgae in the Bioeconomy. N. Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Li, P.; Li, R.; Zhong, J. In Situ Biodiesel Production from Fast-Growing and High Oil Content Chlorella Pyrenoidosa in Rice Straw Hydrolysate. J. Biomed. Biotechnol. 2011, 2011, 141207. [Google Scholar] [CrossRef]
- Yan, D.; Lu, Y.; Chen, Y.F.; Wu, Q. Waste Molasses Alone Displaces Glucose-Based Medium for Microalgal Fermentation towards Cost-Saving Biodiesel Production. Bioresour. Technol. 2011, 102, 6487–6493. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic Cultivation of Auxenochlorella Protothecoides Using Forest Biomass as a Feedstock for Sustainable Biodiesel Production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, Hopes, and the Way Forward for Microalgal Biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef]
- Ferrer-Ledo, N.; Stegemüller, L.; Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Growth and Fatty Acid Distribution over Lipid Classes in Nannochloropsis oceanica Acclimated to Different Temperatures. Front. Plant Sci. 2023, 14, 1078998. [Google Scholar] [CrossRef]
- Van Vooren, G.; Le Grand, F.; Legrand, J.; Cuiné, S.; Peltier, G.; Pruvost, J. Investigation of Fatty Acids Accumulation in Nannochloropsis oculata for Biodiesel Application. Bioresour. Technol. 2012, 124, 421–432. [Google Scholar] [CrossRef]
- Taleb, A.; Pruvost, J.; Legrand, J.; Marec, H.; Le-Gouic, B.; Mirabella, B.; Legeret, B.; Bouvet, S.; Peltier, G.; Li-Beisson, Y.; et al. Development and Validation of a Screening Procedure of Microalgae for Biodiesel Production: Application to the Genus of Marine Microalgae Nannochloropsis. Bioresour. Technol. 2015, 177, 224–232. [Google Scholar] [CrossRef]
- Buhani; Wijayanti, T.A.; Suharso; Sumadi; Ansori, M. Application of Modified Green Algae Nannochloropsis sp. as Adsorbent in the Simultaneous Adsorption of Methylene Blue and Cu(II) Cations in Solution. Sustain. Environ. Res. 2021, 31, 17. [Google Scholar] [CrossRef]
- Parsy, A.; Sambusiti, C.; Baldoni-Andrey, P.; Elan, T.; Périé, F. Cultivation of Nannochloropsis oculata in Saline Oil & Gas Wastewater Supplemented with Anaerobic Digestion Effluent as Nutrient Source. Algal Res. 2020, 50, 101966. [Google Scholar] [CrossRef]
- Mohseni, A.; Kube, M.; Fan, L.; Roddick, F.A. Potential of Chlorella Vulgaris and Nannochloropsis Salina for Nutrient and Organic Matter Removal from Municipal Wastewater Reverse Osmosis Concentrate. Environ. Sci. Pollut. Res. 2020, 27, 26905–26914. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kwon, Y.M.; Kim, K.W.; Kim, J.Y.H. Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications. Mar. Drugs 2021, 19, 690. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; Pagnussatt, F.A.; Lemos, A.C.; Nicolli, C.P.; Del Ponte, E.M.; Badiale-Furlong, E. Nannochloropsis sp. and Spirulina sp. as a Source of Antifungal Compounds to Mitigate Contamination by Fusarium graminearum Species Complex. Curr. Microbiol. 2019, 76, 930–938. [Google Scholar] [CrossRef]
- Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef]
- Hussein, H.A.; Mohamad, H.; Mohd Ghazaly, M.; Laith, A.A.; Abdullah, M.A. Anticancer and Antioxidant Activities of Nannochloropsis oculata and Chlorella sp. Extracts in Co-Application with Silver Nanoparticle. J. King Saud. Univ. Sci. 2020, 32, 3486–3494. [Google Scholar] [CrossRef]
- Plankton24—Nannochloropsis | Plankton24 Plankton & Fish Food | Food & Feed Machines. Available online: https://reef-aquarium-store.com/plankton24-nannochloropsis (accessed on 21 May 2024).
- Nannochloropsis Freeze-Dried Phytoplankton Feed Artemia Shrimp Coral Algova (50 g): Amazon.de: Pet Supplies. Available online: https://www.amazon.de/-/en/Nannochloropsis-Freeze-Dried-Phytoplankton-Artemia/dp/B00TVARE3G/ref=sr_1_3?dchild=1&keywords=nannochloropsis&qid=1619543022&sr=8-3&th=1 (accessed on 21 May 2024).
- Abdelkarim, O.H.; Wijffels, R.H.; Barbosa, M.J. Microalgal Lipid Production: A Comparative Analysis of Nannochloropsis and Microchloropsis Strains. J. Appl. Phycol. 2024, 37, 15–34. [Google Scholar] [CrossRef]
- Chua, E.T.; Schenk, P.M. A Biorefinery for Nannochloropsis: Induction, Harvesting, and Extraction of EPA-Rich Oil and High-Value Protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef]
- Janssen, J.H.; Wijffels, R.H.; Barbosa, M.J. Lipid Production in Nannochloropsis gaditana during Nitrogen Starvation. Biology 2019, 8, 5. [Google Scholar] [CrossRef]
- Banerjee, A.; Maiti, S.K.; Guria, C.; Banerjee, C. Metabolic Pathways for Lipid Synthesis under Nitrogen Stress in Chlamydomonas and Nannochloropsis. Biotechnol. Lett. 2017, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Poliner, E.; Farré, E.M.; Benning, C. Advanced Genetic Tools Enable Synthetic Biology in the Oleaginous Microalgae Nannochloropsis sp. Plant Cell Rep. 2018, 37, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Canini, D.; Ceschi, E.; Perozeni, F. Toward the Exploitation of Sustainable Green Factory: Biotechnology Use of Nannochloropsis spp. Biology 2024, 13, 292. [Google Scholar] [CrossRef]
- Ajjawi, I.; Verruto, J.; Aqui, M.; Soriaga, L.B.; Coppersmith, J.; Kwok, K.; Peach, L.; Orchard, E.; Kalb, R.; Xu, W.; et al. Lipid Production in Nannochloropsis Gaditana Is Doubled by Decreasing Expression of a Single Transcriptional Regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar] [CrossRef]
- Pereira, H.; Schulze, P.S.C.; Schüler, L.M.; Santos, T.; Barreira, L.; Varela, J. Fluorescence Activated Cell-Sorting Principles and Applications in Microalgal Biotechnology. Algal Res. 2018, 30, 113–120. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A Comprehensive Classification System for Lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; Van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS Comprehensive Classification System for Lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis—Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; Woodhead Publishing Limited: Cambridge, UK, 2010. [Google Scholar]
- Willye, J.M.; Sherwood, L.M.; Woolverton, C.J. Prescott, Harley, and Klein’s Microbiology, 7th ed.; McGraw-Hill: New York, NY, USA, 2008; Volume 356, ISBN 978-0-07-299291-5. [Google Scholar]
- Muñoz, C.F.; Südfeld, C.; Naduthodi, M.I.S.; Weusthuis, R.A.; Barbosa, M.J.; Wijffels, R.H.; D’Adamo, S. Genetic Engineering of Microalgae for Enhanced Lipid Production. Biotechnol. Adv. 2021, 52, 107836. [Google Scholar] [CrossRef]
- Sousa, S.C.; Freitas, A.C.; Gomes, A.M.; Carvalho, A.P. Extraction of Nannochloropsis Fatty Acids Using Different Green Technologies: The Current Path. Mar. Drugs 2023, 21, 365. [Google Scholar] [CrossRef]
- Le, H.D.; Meisel, J.A.; de Meijer, V.E.; Gura, K.M.; Puder, M. The Essentiality of Arachidonic Acid and Docosahexaenoic Acid. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 165–170. [Google Scholar] [CrossRef]
- Wen, Y.T.; Dai, J.H.; Gao, Q. Effects of Omega-3 Fatty Acid on Major Cardiovascular Events and Mortality in Patients with Coronary Heart Disease: A Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; Kita, T.; et al. Effects of Eicosapentaenoic Acid on Major Coronary Events in Hypercholesterolaemic Patients (JELIS): A Randomised Open-Label, Blinded Endpoint Analysis. Lancet 2007, 369, 1090–1098. [Google Scholar]
- Marchioli, R. Dietary Supplementation with N-3 Polyunsaturated Fatty Acids and Vitamin E after Myocardial Infarction: Results of the GISSI-Prevenzione Trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Cardiovasc. Dis. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L. Polyunsaturated Fatty Acids: Conversion to Lipid Mediators, Roles in Inflammatory Diseases and Dietary Sources. Int. J. Mol. Sci. 2023, 24, 8838. [Google Scholar] [CrossRef]
- De Santis, A.; Varela, Y.; Sot, J.; D’Errico, G.; Goñi, F.M.; Alonso, A. Omega-3 Polyunsaturated Fatty Acids Do Not Fluidify Bilayers in the Liquid-Crystalline State. Sci. Rep. 2018, 8, 16240. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of Polyunsaturated Fatty Acids, from Mediators to Membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Jouhet, J. Importance of the Hexagonal Lipid Phase in Biological Membrane Organization. Front. Plant Sci. 2013, 4, 494. [Google Scholar] [CrossRef]
- Kobayashi, K. Role of Membrane Glycerolipids in Photosynthesis, Thylakoid Biogenesis and Chloroplast Development. J. Plant Res. 2016, 129, 565–580. [Google Scholar] [CrossRef]
- Latowski, D.; Åkerlund, H.E.; Strzałka, K. Violaxanthin De-Epoxidase, the Xanthophyll Cycle Enzyme, Requires Lipid Inverted Hexagonal Structures for Its Activity. Biochemistry 2004, 43, 4417–4420. [Google Scholar] [CrossRef]
- Dolch, L.J.; Rak, C.; Perin, G.; Tourcier, G.; Broughton, R.; Leterrier, M.; Morosinotto, T.; Tellier, F.; Faure, J.D.; Falconet, D.; et al. A Palmitic Acid Elongase Affects Eicosapentaenoic Acid and Plastidial Monogalactosyldiacylglycerol Levels in Nannochloropsis. Plant Physiol. 2017, 173, 742–759. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Biochemistry and Biotechnology of Lipid Accumulation in the Microalga Nannochloropsis oceanica. J. Agric. Food Chem. 2022, 70, 11500–11509. [Google Scholar] [CrossRef] [PubMed]
- Vieler, A.; Wu, G.; Tsai, C.H.; Bullard, B.; Cornish, A.J.; Harvey, C.; Reca, I.B.; Thornburg, C.; Achawanantakun, R.; Buehl, C.J.; et al. Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga Nannochloropsis oceanica CCMP1779. PLoS Genet. 2012, 8, e1003064. [Google Scholar] [CrossRef]
- Han, D.; Jia, J.; Li, J.; Sommerfeld, M.; Xu, J.; Hu, Q. Metabolic Remodeling of Membrane Glycerolipids in the Microalga Nannochloropsis oceanica under Nitrogen Deprivation. Front. Mar. Sci. 2017, 4, 242. [Google Scholar] [CrossRef]
- Schneider, J.C.; Roessler, P. Radiolabeling Studies of Lipids and Fatty Acids in Nannochloropsis (Eustigmatophyceae), an Oleaginous Marine Alga. J. Phycol. 1994, 30, 594–598. [Google Scholar] [CrossRef]
- Arao, T.; Sakaki, T.; Yamada, M. Biosynthesis of Polyunsaturated Lipids in the Diatom, Phaeodactylum tricornutum. Phytochemistry 1994, 36, 629–635. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Schneider, J.C.; Livne, A.; Sukenik, A.; Roessler, P.G. A Mutant of Nannochloropsis Deficient in Eicosapentaenoic Acid Production. Phytochemistry 1995, 40, 807–814. [Google Scholar] [CrossRef]
- Guo, F.; Bunn, S.E.; Brett, M.T.; Kainz, M.J. Polyunsaturated Fatty Acids in Stream Food Webs—High Dissimilarity among Producers and Consumers. Freshw. Biol. 2017, 62, 1325–1334. [Google Scholar] [CrossRef]
- Brett, M.T.; Mueller-Navarra, D.C. The Role of Highly Unsaturated Fatty Acids in Aquatic Foodweb Processes. Freshw. Biol. 1997, 38, 483–499. [Google Scholar] [CrossRef]
- Monroig, Ó.; Tocher, D.R.; Navarro, J.C. Biosynthesis of Polyunsaturated Fatty Acids in Marine Invertebrates: Recent Advances in Molecular Mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, H.; Kang, S.B.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Twining, C.W.; Brenna, J.T.; Hairston, N.G.; Flecker, A.S. Highly Unsaturated Fatty Acids in Nature: What We Know and What We Need to Learn. Oikos 2016, 125, 749–760. [Google Scholar] [CrossRef]
- Kaye, Y.; Grundman, O.; Leu, S.; Zarka, A.; Zorin, B.; Didi-Cohen, S.; Khozin-Goldberg, I.; Boussiba, S. Metabolic Engineering toward Enhanced LC-PUFA Biosynthesis in Nannochloropsis oceanica: Overexpression of Endogenous Δ12 Desaturase Driven by Stress-Inducible Promoter Leads to Enhanced Deposition of Polyunsaturated Fatty Acids in TAG. Algal Res. 2015, 11, 387–398. [Google Scholar] [CrossRef]
- Abida, H.; Dolch, L.J.; Meï, C.; Villanova, V.; Conte, M.; Block, M.A.; Finazzi, G.; Bastien, O.; Tirichine, L.; Bowler, C.; et al. Membrane Glycerolipid Remodeling Triggered by Nitrogen and Phosphorus Starvation in Phaeodactylum tricornutum. Plant Physiol. 2015, 167, 118–136. [Google Scholar] [CrossRef]
- Endo, K.; Mizusawa, N.; Shen, J.R.; Yamada, M.; Tomo, T.; Komatsu, H.; Kobayashi, M.; Kobayashi, K.; Wada, H. Site-Directed Mutagenesis of Amino Acid Residues of D1 Protein Interacting with Phosphatidylglycerol Affects the Function of Plastoquinone QB in Photosystem II. Photosynth. Res. 2015, 126, 385–397. [Google Scholar] [CrossRef]
- Han, F.; Pei, H.; Hu, W.; Han, L.; Zhang, S.; Ma, G. Effect of High-Temperature Stress on Microalgae at the End of the Logarithmic Phase for the Efficient Production of Lipid. Environ. Technol. 2016, 37, 2649–2657. [Google Scholar] [CrossRef]
- Da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef]
- Canãvate, J.P.; Armada, I.; Riós, J.L.; Hachero-Cruzado, I. Exploring Occurrence and Molecular Diversity of Betaine Lipids across Taxonomy of Marine Microalgae. Phytochemistry 2016, 124, 68–78. [Google Scholar] [CrossRef]
- Mühlroth, A.; Winge, P.; El Assimi, A.; Jouhet, J.; Maréchal, E.; Hohmann-Marriott, M.F.; Vadstein, O.; Bonesa, A.M. Mechanisms of Phosphorus Acquisition and Lipid Class Remodeling under P Limitation in a Marine Microalga. Plant Physiol. 2017, 175, 1543–1559. [Google Scholar] [CrossRef]
- Murakami, H.; Nobusawa, T.; Hori, K.; Shimojima, M.; Ohta, H. Betaine Lipid Is Crucial for Adapting to Low Temperature and Phosphate Deficiency in Nannochloropsis. Plant Physiol. 2018, 177, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Breuer, G.; Lamers, P.P.; Janssen, M.; Wijffels, R.H.; Martens, D.E. Opportunities to Improve the Areal Oil Productivity of Microalgae. Bioresour. Technol. 2015, 186, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal Triacylglycerols as Feedstocks for Biofuel Production: Perspectives and Advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Solovchenko, A.E. Physiological Role of Neutral Lipid Accumulation in Eukaryotic Microalgae under Stresses. Russ. J. Plant Physiol. 2012, 59, 167–176. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Zhu, M.; Yu, C.; Cao, Y.; Zhang, D.; Zhou, G. Increased Lipid Productivity and TAG Content in Nannochloropsis by Heavy-Ion Irradiation Mutagenesis. Bioresour. Technol. 2013, 136, 360–367. [Google Scholar] [CrossRef]
- Maeda, Y.; Nojima, D.; Yoshino, T.; Tanaka, T. Structure and Properties of Oil Bodies in Diatoms. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160408. [Google Scholar] [CrossRef]
- Goold, H.; Beisson, F.; Peltier, G.; Li-Beisson, Y. Microalgal Lipid Droplets: Composition, Diversity, Biogenesis and Functions. Plant Cell Rep. 2015, 34, 545–555. [Google Scholar] [CrossRef]
- Gee, C.W.; Andersen-Ranberg, J.; Boynton, E.; Rosen, R.Z.; Jorgens, D.; Grob, P.; Holman, H.Y.N.; Niyogi, K.K. Implicating the Red Body of Nannochloropsis in Forming the Recalcitrant Cell Wall Polymer Algaenan. Nat. Commun. 2024, 15, 5456. [Google Scholar] [CrossRef]
- Murakami, R.; Hashimoto, H. Unusual Nuclear Division in Nannochloropsis oculata (Eustigmatophyceae, Heterokonta) Which May Ensure Faithful Transmission of Secondary Plastids. Protist 2009, 160, 41–49. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and Composition of the Nannochloropsis Gaditana Cell Wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef]
- Al-Hoqani, U.; Young, R.; Purton, S. The Biotechnological Potential of Nannochloropsis. Perspect. Phycol. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Flori, S.; Jouneau, P.H.; Finazzi, G.; Maréchal, E.; Falconet, D. Ultrastructure of the Periplastidial Compartment of the Diatom Phaeodactylum tricornutum. Protist 2016, 167, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Domoto, N.; Koenen, M.E.; Havenaar, R.; Mikajiri, A.; Chu, B. The Bioaccessibility of Eicosapentaenoic Acid Was Higher from Phospholipid Food Products than from Mono- and Triacylglycerol Food Products in a Dynamic Gastrointestinal Model. Food Sci. Nutr. 2013, 1, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.; Schneider, I.; Meyer, H.; Neubronner, J.; Von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into Plasma Phospholipids in Response to Different Omega-3 Fatty Acid Formulations—A Comparative Bioavailability Study of Fish Oil vs. Krill Oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- Ramesh Kumar, B.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as Rich Source of Polyunsaturated Fatty Acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Berquin, I.M.; Edwards, I.J.; Chen, Y.Q. Multi-Targeted Therapy of Cancer by Omega-3 Fatty Acids. Cancer Lett. 2008, 269, 363–377. [Google Scholar] [CrossRef]
- Adkins, Y.; Kelley, D.S. Mechanisms Underlying the Cardioprotective Effects of Omega-3 Polyunsaturated Fatty Acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary Omega 3 Fatty Acids and the Developing Brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Dinicolantonio, J.J. The Importance of a Balanced ω-6 to ω-3 Ratio in the Prevention and Management of Obesity. Open Heart 2016, 3, 385. [Google Scholar] [CrossRef] [PubMed]
- Dinicolantonio, J.J.; O’keefe, J.; O’keefe, J.H. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo. Med. 2021, 118, 5–453. [Google Scholar]
- Saini, R.K.; Keum, Y.S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as Sources of Omega-3 Polyunsaturated Fatty Acids: Biotechnological Aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae N-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Jensen, I.J.; Eilertsen, K.E.; Otnæs, C.H.A.; Mæhre, H.K.; Elvevoll, E.O. An Update on the Content of Fatty Acids, Dioxins, PCBs and Heavy Metals in Farmed, Escaped and Wild Atlantic Salmon (Salmo salar L.) in Norway. Foods 2020, 9, 1901. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors Influencing Fatty Acids in Meat and the Role of Antioxidants in Improving Meat Quality. Br. J. Nutr. 1997, 78, S49–S60. [Google Scholar] [CrossRef]
- Rey, A.I.; Daza, A.; López-Carrasco, C.; López-Bote, C.J. Feeding Iberian Pigs with Acorns and Grass in Either Free-Range or Confinement Affects the Carcass Characteristics and Fatty Acids and Tocopherols Accumulation in Longissimus Dorsi Muscle and Backfat. Meat Sci. 2006, 73, 66–74. [Google Scholar] [CrossRef]
- Najafi, M.H.; Zeinoaldini, S.; Ganjkhanlou, M.; Mohammadi, H.; Hopkins, D.L.; Ponnampalam, E.N. Performance, Carcass Traits, Muscle Fatty Acid Composition and Meat Sensory Properties of Male Mahabadi Goat Kids Fed Palm Oil, Soybean Oil or Fish Oil. Meat Sci. 2012, 92, 848–854. [Google Scholar] [CrossRef]
- Dang, D.X.; Kim, I.H. Coated Refined Fish Oil Supplementation Improves Growth Performance and Meat Quality in Finishing Pigs. Livest. Sci. 2022, 265, 105099. [Google Scholar] [CrossRef]
- Díaz, M.T.; Pérez, C.; Sánchez, C.I.; Lauzurica, S.; Cañeque, V.; González, C.; De La Fuente, J. Feeding Microalgae Increases Omega 3 Fatty Acids of Fat Deposits and Muscles in Light Lambs. J. Food Compos. Anal. 2017, 56, 115–123. [Google Scholar] [CrossRef]
- Bruneel, C.; Lemahieu, C.; Fraeye, I.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of Microalgal Feed Supplementation on Omega-3 Fatty Acid Enrichment of Hen Eggs. J. Funct. Foods 2013, 5, 897–904. [Google Scholar] [CrossRef]
- Wu, B.; Xie, Y.; Xu, S.; Lv, X.; Yin, H.; Xiang, J.; Chen, H.; Wei, F. Comprehensive Lipidomics Analysis Reveals the Effects of Different Omega-3 Polyunsaturated Fatty Acid-Rich Diets on Egg Yolk Lipids. J. Agric. Food Chem. 2020, 68, 15048–15060. [Google Scholar] [CrossRef] [PubMed]
- Boeckaert, C.; Vlaeminck, B.; Dijkstra, J.; Issa-Zacharia, A.; Van Nespen, T.; Van Straalen, W.; Fievez, V. Effect of Dietary Starch or Micro Algae Supplementation on Rumen Fermentation and Milk Fatty Acid Composition of Dairy Cows. J. Dairy Sci. 2008, 91, 4714–4727. [Google Scholar] [CrossRef]
- Lamminen, M.; Halmemies-Beauchet-Filleau, A.; Kokkonen, T.; Jaakkola, S.; Vanhatalo, A. Different Microalgae Species as a Substitutive Protein Feed for Soya Bean Meal in Grass Silage Based Dairy Cow Diets. Anim. Feed. Sci. Technol. 2019, 247, 112–126. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Jennings, S.; Holmes, R.; Harle, J.; Merino, G.; Allen, J.I.; Holt, J.; Dulvy, N.K.; Barange, M. Potential Consequences of Climate Change for Primary Production and Fish Production in Large Marine Ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2979–2989. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Matulich, K.L.; Hooper, D.U.; Byrnes, J.E.; Duffy, E.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.I.; Gonzalez, A. The Functional Role of Producer Diversity in Ecosystems. Am. J. Bot. 2011, 98, 572–592. [Google Scholar] [CrossRef]
- Houston, S.J.S.; Karalazos, V.; Tinsley, J.; Tocher, D.R.; Glencross, B.D.; Monroig, Ó. A Comparison of Regression Models for Defining EPA + DHA Requirements Using the Gilthead Seabream (Sparus aurata) as a Model Species. Aquaculture 2022, 556, 738308. [Google Scholar] [CrossRef]
- Qiao, H.; Hu, D.; Ma, J.; Wang, X.; Wu, H.; Wang, J. Feeding Effects of the Microalga Nannochloropsis sp. on Juvenile Turbot (Scophthalmus maximus L.). Algal Res. 2019, 41, 101540. [Google Scholar] [CrossRef]
- Guimarães, A.M.; Guertler, C.; Pereira, G.D.V.; Coelho, J.d.R.; Rezende, P.C.; Nóbrega, R.O.; Vieira, F.D.N. Nannochloropsis spp. As Feed Additive for the Pacific White Shrimp: Effect on Midgut Microbiology, Thermal Shock Resistance and Immunology. Animals 2021, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.; Pinto, W.; Gonçalves, A.T.; Machado, M.; Reis, B.; Silva, J.; Navalho, J.; Dias, J.; Conceição, L. Benjamín Costas, & Microalgal Biomasses Have Potential as Ingredients in Microdiets for Senegalese Sole (Solea senegalensis) Post-Larvae. J. Appl. Phycol. 2021, 33, 2241–2250. [Google Scholar] [CrossRef]
- Zahran, E.; Elbahnaswy, S.; Ahmed, F.; Ibrahim, I.; Khaled, A.A.; Eldessouki, E.A. Nutritional and Immunological Evaluation of Nannochloropsis oculata as a Potential Nile Tilapia-Aquafeed Supplement. BMC Vet. Res. 2023, 19, 1–18. [Google Scholar] [CrossRef]
- Adissin, T.O.O.; Manabu, I.; Shunsuke, K.; Saichiro, Y.; Moss, A.S.; Dossou, S. Effects of Dietary Nannochloropsis sp. Powder and Lipids on the Growth Performance and Fatty Acid Composition of Larval and Postlarval Kuruma Shrimp, Marsupenaeus japonicus. Aquac. Nutr. 2020, 26, 186–200. [Google Scholar] [CrossRef]
- Meguro, S.; Arai, Y.; Masukawa, Y.; Uie, K.; Tokimitsu, I. Relationship between Covalently Bound Ceramides and Transepidermal Water Loss (TEWL). Arch. Dermatol. Res. 2000, 292, 463–468. [Google Scholar] [CrossRef]
- Ekanayake-Mudiyanselage, S.; Aschauer, H.; Schmook, F.P.; Jensen, J.-M.; Meingassner, J.G.; Proksch, E. Expression of Epidermal Keratins and the Cornified Envelope Protein Involucrin Is Influenced by Permeability Barrier Disruption. J. Investig. Dermatol. 1998, 111, 517–523. [Google Scholar] [CrossRef]
- Orengo, F.; Black, H.S.; Wolf, J.E. Influence of Fish Oil Supplementation on the Minimal Erythema Dose in Humans. Arch. Dermatol. Res. 1992, 284, 219–221. [Google Scholar] [CrossRef]
- Rhodes, L.E.; Durham, B.H.; Fraser, W.D.; Friedmatm, P. Dietary Fish Oil Reduces Basal and Ultraviolet B-Generated PGE 2 Levels in Skin and Increases the Threshold to Provocation of Polymorphic Light Eruption. J. Investig. Dermatol. 1995, 105, 532–535. [Google Scholar] [CrossRef]
- Shahbakhti, H.; Watson, R.E.B.; Azurdia, R.M.; Ferreira, C.Z.; Garmyn, M.; Rhodes, L.E. Influence of Eicosapentaenoic Acid, an Omega-3 Fatty Acid, on Ultraviolet-B Generation of Prostaglandin-E2 and Proinflammatory Cytokines Interleukin-1β, Tumor Necrosis Factor-α, Interleukin-6 and Interleukin-8 in Human Skin In Vivo. Photochem. Photobiol. 2007, 80, 231–235. [Google Scholar] [CrossRef]
- Biernacki, M.; Conde, T.; Stasiewicz, A.; Surażyński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation. Int. J. Mol. Sci. 2023, 24, 14323. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Mastroeni, S.; Melchi, F.; Pilla, M.A.; Antonelli, G.; Camaioni, D.; Alotto, M.; Pasquini, P. A Protective Effect of the Mediterranean Diet for Cutaneous Melanoma. Int. J. Epidemiol. 2008, 37, 1018–1029. [Google Scholar] [CrossRef]
- Hakim, I.A.; Harris, R.B.; Ritenbaugh, C. Fat Intake and Risk of Squamous Cell Carcinoma of the Skin. Nutr. Cancer 2000, 36, 155–162. [Google Scholar] [CrossRef]
- Baum, C.L.; Arpey, C.J. Normal Cutaneous Wound Healing: Clinical Correlation with Cellular and Molecular Events. Am. Soc. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 Fatty Acids Effect on Wound Healing. Wound Repair. Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef]
- da Silva Ferreira, V.; Sant’Anna, C. Impact of Culture Conditions on the Chlorophyll Content of Microalgae for Biotechnological Applications. World J. Microbiol. Biotechnol. 2017, 33, 1–8. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New Diacylglyceryltrimethylhomoserines from the Marine Microalga Nannochloropsis Granulata and Their Nitric Oxide Inhibitory Activity. J. Appl. Phycol. 2013, 25, 1513–1521. [Google Scholar] [CrossRef]
- Conde, T.; Neves, B.; Couto, D.; Melo, T.; Lopes, D.; Pais, R.; Batista, J.; Cardoso, H.; Silva, J.L.; Domingues, P.; et al. Polar Lipids of Marine Microalgae Nannochloropsis oceanica and Chlorococcum Amblystomatis Mitigate the LPS-Induced Pro-Inflammatory Response in Macrophages. Mar. Drugs 2023, 21, 629. [Google Scholar] [CrossRef]
- Khatib, S.; Artoul, F.; Paluy, I.; Boluchevsky, L.; Kvitnitsky, E.; Vaya, J. Nannochloropsis sp. Ethanol Extract Prevents Macrophage and LDL Oxidation and Enhances PON1 Activity through the Principal Active Compound Lyso-Diacylglyceryltrimethylhomoserine (Lyso-DGTS). J. Appl. Phycol. 2018, 30, 1679–1689. [Google Scholar] [CrossRef]
- Rao, A.; Briskey, D.; Nalley, J.O.; Ganuza, E. Omega-3 Eicosapentaenoic Acid (Epa) Rich Extract from the Microalga Nannochloropsis Decreases Cholesterol in Healthy Individuals: A Double-Blind, Randomized, Placebo-Controlled, Three-Month Supplementation Study. Nutrients 2020, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, G.G.; Aricetti, J.A.; Tubino, M. A Simple, Fast, and Green Titrimetric Method for the Determination of the Iodine Value of Vegetable Oils Without Wijs Solution (ICl). Food Anal. Methods 2016, 9, 2479–2483. [Google Scholar] [CrossRef]
- Kyriakidis, N.B.; Katsiloulis, T. Calculation of Iodine Value from Measurements of Fatty Acid Methyl Esters of Some Oils: Comparison with the Relevant American Oil Chemists Society Method. J. Am. Oil Chem. Soc. 2000, 77, 1235–1238. [Google Scholar] [CrossRef]
- Baptista, P.; Felizardo, P.; Menezes, J.C.; Neiva Correia, M.J. Multivariate near Infrared Spectroscopy Models for Predicting the Iodine Value, CFPP, Kinematic Viscosity at 40 °C and Density at 15 °C of Biodiesel. Talanta 2008, 77, 144–151. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; de Souza, C.O.; Vich, D.V.; de Carvalho, G.C.; Nascimento, M.A. Screening Microalgae Strains for Biodiesel Production: Lipid Productivity and Estimation of Fuel Quality Based on Fatty Acids Profiles as Selective Criteria. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Selvarajan, R.; Felföldi, T.; Tauber, T.; Sanniyasi, E.; Sibanda, T.; Tekere, M. Screening and Evaluation of Some Green Algal Strains (Chlorophyceae) Isolated from Freshwater and Soda Lakes for Biofuel Production. Energies 2015, 8, 7502–7521. [Google Scholar] [CrossRef]
- Zhivich, A.B.; Koldobskii, G.I.; Ostrovskii, V.A. Methods of Preparing Tetrazolium Salts. Chem. Heterocycl Compd. (N. Y.) 1990, 26, 1319–1328. [Google Scholar] [CrossRef]
- Ryan, J.; Farr, H.; Visnovsky, S.; Vyssotski, M.; Visnovsky, G. A Rapid Method for the Isolation of Eicosapentaenoic Acid-Producing Marine Bacteria. J. Microbiol. Methods 2010, 82, 49–53. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, L.J.; Liu, Z.; Xu, H.B. Isolating Mortierella Alpina Strains of High Yield of Arachidonic Acid. Lett. Appl. Microbiol. 2004, 39, 332–335. [Google Scholar] [CrossRef]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. A Quick Colorimetric Method for Total Lipid Quantification in Microalgae. J. Microbiol. Methods 2016, 125, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid Quantification of Microalgal Lipids in Aqueous Medium by a Simple Colorimetric Method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Balduyck, L.; Veryser, C.; Goiris, K.; Bruneel, C.; Muylaert, K.; Foubert, I. Optimization of a Nile Red Method for Rapid Lipid Determination in Autotrophic, Marine Microalgae Is Species Dependent. J. Microbiol. Methods 2015, 118, 152–158. [Google Scholar] [CrossRef]
- Südfeld, C.; Hubáček, M.; D’Adamo, S.; Wijffels, R.H.; Barbosa, M.J. Optimization of High-Throughput Lipid Screening of the Microalga Nannochloropsis oceanica Using BODIPY 505/515. Algal Res. 2021, 53, 102138. [Google Scholar] [CrossRef]
- Guzmán, H.M.; de la Jara Valido, A.; Duarte, L.C.; Presmanes, K.F. Estimate by Means of Flow Cytometry of Variation in Composition of Fatty Acids from Tetraselmis Suecica in Response to Culture Conditions. Aquac. Int. 2010, 18, 189–199. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Strasser, B.C.; Scott, J.A.; Ross, G.M. Optimization of Staining Conditions for Microalgae with Three Lipophilic Dyes to Reduce Precipitation and Fluorescence Variability. Cytom. Part A 2012, 81, 618–626. [Google Scholar] [CrossRef]
- Gao, F.; Teles (Cabanelas, ITD), I.; Wijffels, R.H.; Barbosa, M.J. Process Optimization of Fucoxanthin Production with Tisochrysis lutea. Bioresour. Technol. 2020, 315, 123894. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Alonzo, F.; Mayzaud, P.; Francé’, F. Spectrofluorometric Quantification of Neutral and Polar Lipids in Zooplankton Using Nile Red. Mar. Chem. 1999, 67, 289–301. [Google Scholar] [CrossRef]
- Niko, Y.; Klymchenko, A.S. Emerging Solvatochromic Push-Pull Dyes for Monitoring the Lipid Order of Biomembranes in Live Cells. J. Biochem. 2021, 170, 163–174. [Google Scholar] [CrossRef]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Jouhet, J.; Alves, E.; Boutté, Y.; Darnet, S.; Domergue, F.; Durand, T.; Fischer, P.; Fouillen, L.; Grube, M.; Joubès, J.; et al. Plant and Algal Lipidomes: Analysis, Composition, and Their Societal Significance. Prog. Lipid Res. 2024, 96, 101290. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.; Figueiredo, A.R.P.; da Costa, E.; Couto, D.; Silva, J.; Domingues, M.R.; Domingues, P. Ethanol Extraction of Polar Lipids from Nannochloropsis oceanica for Food, Feed, and Biotechnology Applications Evaluated Using Lipidomic Approaches. Mar. Drugs 2021, 19, 593. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Melo, T.; Lopes, D.; Couto, D.; Marques, F.; Domingues, M.R. Applications of Lipidomics in Marine Organisms: Progress, Challenges and Future Perspectives. Mol. Omics 2022, 18, 357–386. [Google Scholar] [CrossRef]
- Jouhet, J.; Lupette, J.; Clerc, O.; Magneschi, L.; Bedhomme, M.; Collin, S.; Roy, S.; Maréchal, E.; Rébeillé, F. LC-MS/MS versus TLC plus GC Methods: Consistency of Glycerolipid and Fatty Acid Profiles in Microalgae and Higher Plant Cells and Effect of a Nitrogen Starvation. PLoS ONE 2017, 12, e0182423. [Google Scholar] [CrossRef]
- Mossoba, M.M.; Milosevic, V.; Milosevic, M.; Kramer, J.K.G.; Azizian, H. Determination of Total Trans Fats and Oils by Infrared Spectroscopy for Regulatory Compliance. Anal. Bioanal. Chem. 2007, 389, 87–92. [Google Scholar] [CrossRef]
- Chmielarz, M.; Sampels, S.; Blomqvist, J.; Brandenburg, J.; Wende, F.; Sandgren, M.; Passoth, V. FT-NIR: A Tool for Rapid Intracellular Lipid Quantification in Oleaginous Yeasts. Biotechnol. Biofuels 2019, 12, 169. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Heraud, P.; Gupta, A.; Puri, M.; McNaughton, D.; Barrow, C.J. FTIR Microspectroscopy for Rapid Screening and Monitoring of Polyunsaturated Fatty Acid Production in Commercially Valuable Marine Yeasts and Protists. Analyst 2013, 138, 6016–6031. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Chen, T.; Yang, B.; Jiang, Y.; Wei, D.; Chen, F. Rapid Characterization of Fatty Acids in Oleaginous Microalgae by Near-Infrared Spectroscopy. Int. J. Mol. Sci. 2015, 16, 7045–7056. [Google Scholar] [CrossRef]
- Sandnes, J.M.; Ringstad, T.; Wenner, D.; Heyerdahl, P.H.; Källqvist, T.; Gislerød, H.R. Real-Time Monitoring and Automatic Density Control of Large-Scale Microalgal Cultures Using near Infrared (NIR) Optical Density Sensors. J. Biotechnol. 2006, 122, 209–215. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Yan, C.; Cong, W. Determination of Intracellular Lipid and Main Fatty Acids of Nannochloropsis oceanica by ATR-FTIR Spectroscopy. J. Appl. Phycol. 2022, 34, 343–352. [Google Scholar] [CrossRef]
- Gao, F.; Sá, M.; Teles, I.; Wijffels, R.H.; Barbosa, M.J. Production and Monitoring of Biomass and Fucoxanthin with Brown Microalgae under Outdoor Conditions. Biotechnol. Bioeng. 2021, 118, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.; Ferrer-Ledo, N.; Wijffels, R.; Crespo, J.G.; Barbosa, M.; Galinha, C.F. Monitoring of Eicosapentaenoic Acid (EPA) Production in the Microalgae Nannochloropsis oceanica. Algal Res. 2020, 45, 101766. [Google Scholar] [CrossRef]
- Sá, M.; Bertinetto, C.G.; Ferrer-Ledo, N.; Jansen, J.J.; Wijffels, R.; Crespo, J.G.; Barbosa, M.; Galinha, C.F. Fluorescence Spectroscopy and Chemometrics for Simultaneous Monitoring of Cell Concentration, Chlorophyll and Fatty Acids in Nannochloropsis oceanica. Sci. Rep. 2020, 10, 7688. [Google Scholar] [CrossRef]
- Sá, M.; Ferrer-Ledo, N.; Gao, F.; Bertinetto, C.G.; Jansen, J.; Crespo, J.G.; Wijffels, R.H.; Barbosa, M.; Galinha, C.F. Perspectives of Fluorescence Spectroscopy for Online Monitoring in Microalgae Industry. Microb. Biotechnol. 2022, 15, 1824–1838. [Google Scholar] [CrossRef]
- Sharma, S.K.; Nelson, D.R.; Abdrabu, R.; Khraiwesh, B.; Jijakli, K.; Arnoux, M.; O’Connor, M.J.; Bahmani, T.; Cai, H.; Khapli, S.; et al. An Integrative Raman Microscopy-Based Workflow for Rapid In Situ Analysis of Microalgal Lipid Bodies. Biotechnol. Biofuels 2015, 8, 164. [Google Scholar] [CrossRef]
- Thai, T.; Doan, Y.; Obbard, J.P. Enhanced Intracellular Lipid in Nannochloropsis sp. via Random Mutagenesis and Flow Cytometric Cell Sorting. Algal Res. 2012, 1, 17–21. [Google Scholar] [CrossRef]
- Wu, H.; Volponi, J.V.; Oliver, A.E.; Parikh, A.N.; Simmons, B.A.; Singh, S. In Vivo Lipidomics Using Single-Cell Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 3809–3814. [Google Scholar] [CrossRef]
- Ling Jiang, F.; Ikeda, I.; Ogawa, Y.; Endo, Y. Terahertz Absorption Spectra of Fatty Acids and Their Analogues. J. Oleo Sci. 2011, 60, 339–343. [Google Scholar] [CrossRef]
- Cantarero, S.I.; Flores, E.; Allbrook, H.; Aguayo, P.; Vargas, C.A.; Tamanaha, J.E.; Scholz, J.B.C.; Bach, L.T.; Löscher, C.R.; Riebesell, U.; et al. Lipid Remodeling in Phytoplankton Exposed to Multi-Environmental Drivers in a Mesocosm Experiment. Biogeosciences 2024, 21, 3927–3958. [Google Scholar] [CrossRef]
- Chini Zittelli, G.; Rodolfi, L.; Tredici, M.R. Mass Cultivation of Nannochloropsis sp. in Annular Reactors. J. Appl. Phycol. 2003, 15, 107–114. [Google Scholar] [CrossRef]
- Sukenik, A.; Zmora, O.; Carmeli, Y. Biochemical Quality of Marine Unicellular Algae with Special Emphasis on Lipid Composition. II. Nannochloropsis sp. Aquaculture 1993, 117, 313–326. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of Light and Temperature on Fatty Acid Production in Nannochloropsis Salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Solovchenko, A.; Lukyanov, A.; Solovchenko, O.; Didi-Cohen, S.; Boussiba, S.; Khozin-Goldberg, I. Interactive Effects of Salinity, High Light, and Nitrogen Starvation on Fatty Acid and Carotenoid Profiles in Nannochloropsis oceanica CCALA 804. Eur. J. Lipid Sci. Technol. 2014, 116, 635–644. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane Fluidity and Its Roles in the Perception of Environmental Signals. Biochim. Biophys. Acta Biomembr. 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Renaud, S.M.; Van Thinh, L.; Lambrinidis, G.; Parry, D.L. Effect of Temperature on Growth, Chemical Composition and Fatty Acid Composition of Tropical Australian Microalgae Grown in Batch Cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Teoh, M.L.; Phang, S.M.; Chu, W.L. Response of Antarctic, Temperate, and Tropical Microalgae to Temperature Stress. J. Appl. Phycol. 2013, 25, 285–297. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Physiol. Plant 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Sukenik, A. Ecophysiological Considerations in the Optimization of Eicosapentaenoic Acid Production by Nannochloropsis sp. (Eustigmatophyceae). Bioresour. Technol. 1991, 35, 263–269. [Google Scholar] [CrossRef]
- Alboresi, A.; Perin, G.; Vitulo, N.; Diretto, G.; Block, M.; Jouhet, J.; Meneghesso, A.; Valle, G.; Giuliano, G.; Maréchal, E.; et al. Light Remodels Lipid Biosynthesis in Nannochloropsis Gaditana by Modulating Carbon Partitioning between Organelles. Plant Physiol. 2016, 171, 2468–2482. [Google Scholar] [CrossRef]
- Ferrer-Ledo, N.; van Oossanen, S.; Wijffels, R.H.; Evers, W.A.C.; Südfeld, C.; Janssen, M.; Teles Dominguez Cabanelas, I.; Barbosa, M.J. Effect of Incident Light and Light Gradients on Eicosapentaenoic Acid Distribution between Lipid Classes in Nannochloropsis oceanica. J. Appl. Phycol. 2024, 37, 163–179. [Google Scholar] [CrossRef]
- Paliwal, C.; Mitra, M.; Bhayani, K.; Bharadwaj, S.V.V.; Ghosh, T.; Dubey, S.; Mishra, S. Abiotic Stresses as Tools for Metabolites in Microalgae. Bioresour. Technol. 2017, 244, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.; Minnaard, J.; Dubinsky, Z. Photoacclimation in the Marine Alga Nannochloropsis sp. (Eustigmatophyte): A Kinetic Study. J. Plankton Res. 1996, 18, 1797–1818. [Google Scholar] [CrossRef]
- Turpin, D.H. Effects of Inorganic N Availability on Algal Photosynthesis and Carbon Metabolism. J. Phycol. 1991, 27, 14–20. [Google Scholar] [CrossRef]
- Adams, C.; Godfrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding Precision Nitrogen Stress to Optimize the Growth and Lipid Content Tradeoff in Oleaginous Green Microalgae. Bioresour. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae Lipid and Biomass for Biofuel Production: A Comprehensive Review on Lipid Enhancement Strategies and Their Effects on Fatty Acid Composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Simionato, D.; Block, M.A.; La Rocca, N.; Jouhet, J.; Maréchal, E.; Finazzi, G.; Morosinotto, T. The Response of Nannochloropsis Gaditana to Nitrogen Starvation Includes de Novo Biosynthesis of Triacylglycerols, a Decrease of Chloroplast Galactolipids, and Reorganization of the Photosynthetic Apparatus. Eukaryot. Cell 2013, 12, 665–676. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, X.; Yang, M.; Liu, J.; Yao, C.; Xue, S. Glycerolipid Remodeling Triggered by Phosphorous Starvation and Recovery in Nannochloropsis oceanica. Algal Res. 2019, 39, 101451. [Google Scholar] [CrossRef]
- Matsui, H.; Shiozaki, K.; Okumura, Y.; Ishikawa, M.; Waqalevu, V.; Hayasaka, O.; Honda, A.; Kotani, T. Effects of Phosphorous Deficiency of a Microalga Nannochloropsis oculata on Its Fatty Acid Profiles and Intracellular Structure and the Effectiveness in Rotifer Nutrition. Algal Res. 2020, 49, 101905. [Google Scholar] [CrossRef]
- Su, C.H.; Chien, L.J.; Gomes, J.; Lin, Y.S.; Yu, Y.K.; Liou, J.S.; Syu, R.J. Factors Affecting Lipid Accumulation by Nannochloropsis oculata in a Two-Stage Cultivation Process. J. Appl. Phycol. 2011, 23, 903–908. [Google Scholar] [CrossRef]
- Mitra, M.; Patidar, S.K.; Mishra, S. Integrated Process of Two Stage Cultivation of Nannochloropsis sp. for Nutraceutically Valuable Eicosapentaenoic Acid along with Biodiesel. Bioresour. Technol. 2015, 193, 363–369. [Google Scholar] [CrossRef]
- Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Mar. Drugs 2022, 20, 440. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, S.; Sojin, K.; Santhanam, P.; Dhanalakshmi, B.; Latha, S.; Park, M.S.; Kim, M.K. Triggering of Fatty Acids on Tetraselmis sp. by Ethyl Methanesulfonate Mutagenic Treatment. Bioresour. Technol. Rep. 2018, 2, 21–28. [Google Scholar] [CrossRef]
- Barten, R.; Peeters, T.; Navalho, S.; Fontowicz, L.; Wijffels, R.H.; Barbosa, M. Expanding the Upper-Temperature Boundary for the Microalga Picochlorum sp. (BPE23) by Adaptive Laboratory Evolution. Biotechnol. J. 2022, 17, 2100659. [Google Scholar] [CrossRef]
- Gregory, T.R. Understanding Natural Selection: Essential Concepts and Common Misconceptions. Evol. Educ. Outreach 2009, 2, 156–175. [Google Scholar] [CrossRef]
- Barrett, R.D.H.; Schluter, D. Adaptation from Standing Genetic Variation. Trends Ecol. Evol. 2008, 23, 38–44. [Google Scholar] [CrossRef]
- Morgan, A.D.; Ness, R.W.; Keightley, P.D.; Colegrave, N. Spontaneous Mutation Accumulation in Multiple Strains of the Green Alga, Chlamydomonas Reinhardtii. Evolution 2014, 68, 2589–2602. [Google Scholar] [CrossRef]
- Kodym, A.; Afza, R. Physical and Chemical Mutagenesis. Methods Mol. Biol. 2003, 236, 189–204. [Google Scholar] [CrossRef]
- Kumawat, S.; Rana, N.; Bansal, R.; Vishwakarma, G.; Mehetre, S.T.; Das, B.K.; Kumar, M.; Kumar Yadav, S.; Sonah, H.; Raj Sharma, T.; et al. Expanding Avenue of Fast Neutron Mediated Mutagenesis for Crop Improvement. Plants 2019, 8, 164. [Google Scholar] [CrossRef]
- Liu, S.; Xu, J.; Chen, W.; Fu, H.; Ma, L.Y.; Xu, H.; Xinnian, L.; Wu, M.; Ma, F. Enhancement of Lipid Productivity in Green Microalgae Chlorella sp. via Fast Neutron Irradiation. Biomass Bioenergy 2016, 91, 196–203. [Google Scholar] [CrossRef]
- Najafi, M.B.H.; Pezeshki, P. Bacterial Mutation; Types, Mechanisms and Mutant Detection Methods: A Review. Eur. Sci. J. 2013, 4. [Google Scholar]
- Wang, W.; Wei, T.; Fan, J.; Yi, J.; Li, Y.; Wan, M.; Wang, J.; Bai, W. Repeated Mutagenic Effects of 60Co-γ Irradiation Coupled with High-Throughput Screening Improves Lipid Accumulation in Mutant Strains of the Microalgae Chlorella Pyrenoidosa as a Feedstock for Bioenergy. Algal Res. 2018, 33, 71–77. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Haliloğlu, K.; Tosun, M.; Bujak, H.; Eren, B.; Demirel, F.; Szulc, P.; Karagöz, H.; Selwet, M.; Özkan, G.; et al. Ethyl Methanesulfonate (EMS) Mutagen Toxicity-Induced DNA Damage, Cytosine Methylation Alteration, and IPBS-Retrotransposon Polymorphisms in Wheat (Triticum aestivum L.). Agronomy 2023, 13, 1767. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The Lipid Biochemistry of Eukaryotic Algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Fujita, Y. Isolation of Enhanced Eicosapentaenoic Acid Producing Mutants of Nannochloropsis oculata ST-6 Using Ethyl Methane Sulfonate Induced Mutagenesis Techniques and Their Characterization at MRNA Transcript Level. Phycol. Res. 2006, 54, 208–219. [Google Scholar] [CrossRef]
- Wan Razali, W.A.; Evans, C.A.; Pandhal, J. Comparative Proteomics Reveals Evidence of Enhanced EPA Trafficking in a Mutant Strain of Nannochloropsis oculata. Front. Bioeng. Biotechnol. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Sun, X.; Yang, G.; Zhang, X.; Gao, Z. Fatty Acid Composition Analyses of the DCMU Resistant Mutants of Nannochloropsis oculata (Eustigmatophyceae). J. Ocean. Univ. Qingdao 2003, 2, 65–68. [Google Scholar]
- Chaturvedi, R.; Uppalapati, S.R.; Alamsjah, M.A.; Fujita, Y. Isolation of Quizalofop-Resistant Mutants of Nannochloropsis oculata (Eustigmatophyceae) with High Eicosapentaenoic Acid Following N-Methyl-N-Nitrosourea-Induced Random Mutagenesis. J. Appl. Phycol. 2004, 16, 135–144. [Google Scholar] [CrossRef]
- Pansook, S.; Incharoensakdi, A.; Phunpruch, S. Effects of the Photosystem II Inhibitors CCCP and DCMU on Hydrogen Production by the Unicellular Halotolerant Cyanobacterium Aphanothece Halophytica. Sci. World J. 2019, 2019, 1030236. [Google Scholar] [CrossRef]
- Botté, C.Y.; Deligny, M.; Roccia, A.; Bonneau, A.L.; Saïdani, N.; Hardré, H.; Aci, S.; Yamaryo-Botté, Y.; Jouhet, J.; Dubots, E.; et al. Chemical Inhibitors of Monogalactosyldiacylglycerol Synthases in Arabidopsis thaliana. Nat. Chem. Biol. 2011, 7, 834–842. [Google Scholar] [CrossRef]
- Gabashvili, I.S.; Gregory, S.T.; Valle, M.; Grassucci, R.; Worbs, M.; Wahl, M.C.; Dahlberg, A.E.; Frank, J. The Polypeptide Tunnel System in the Ribosome and Its Gating in Erythromycin Resistance Mutants of L4 and L22. Mol. Cell 2001, 8, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Moreno-Garrido, I.; Blasco, J.; Araújo, C.V.M. Effect of Erythromycin and Modulating Effect of CeO2 NPs on the Toxicity Exerted by the Antibiotic on the Microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Environ. Pollut. 2018, 242, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Barten, R.; van Workum, D.J.M.; de Bakker, E.; Risse, J.; Kleisman, M.; Navalho, S.; Smit, S.; Wijffels, R.H.; Nijveen, H.; Barbosa, M.J. Genetic Mechanisms Underlying Increased Microalgal Thermotolerance, Maximal Growth Rate, and Yield on Light Following Adaptive Laboratory Evolution. BMC Biol. 2022, 20, 1–14. [Google Scholar] [CrossRef]
- Darwin, C. The Origin of the Species: 150th Anniversary Edition; Signet Classics: New York, NY, USA, 2009. [Google Scholar]
- Dettman, J.R.; Rodrigue, N.; Melnyk, A.H.; Wong, A.; Bailey, S.F.; Kassen, R. Evolutionary Insight from Whole-Genome Sequencing of Experimentally Evolved Microbes. Mol. Ecol. 2012, 21, 2058–2077. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, T.J.; Lenski, R.E.; Ebert, D.; Hollis, B.; Olivieri, I.; Whitlock, M.C. Experimental Evolution. Trends Ecol. Evol. 2012, 27, 547–560. [Google Scholar] [CrossRef]
- Perin, G.; Segalla, A.; Basso, S.; Simionato, D.; Meneghesso, A.; Sforza, E.; Bertucco, A.; Morosinotto, T. Biotechnological Optimization of Light Use Efficiency in Nannochloropsis Cultures for Biodiesel Production. Chem. Eng. Trans. 2014, 37, 763–768. [Google Scholar] [CrossRef]
- Perin, G.; Bellan, A.; Segalla, A.; Meneghesso, A.; Alboresi, A.; Morosinotto, T. Generation of Random Mutants to Improve Light-Use Efficiency of Nannochloropsis Gaditana Cultures for Biofuel Production. Biotechnol. Biofuels 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Cecchin, M.; Berteotti, S.; Paltrinieri, S.; Vigliante, I.; Iadarola, B.; Giovannone, B.; Maffei, M.E.; Delledonne, M.; Ballottari, M. Improved Lipid Productivity in Nannochloropsis Gaditana in Nitrogen-Replete Conditions by Selection of Pale Green Mutants. Biotechnol. Biofuels 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Cecchin, M.; Cazzaniga, S.; Martini, F.; Paltrinieri, S.; Bossi, S.; Maffei, M.E.; Ballottari, M. Astaxanthin and Eicosapentaenoic Acid Production by S4, a New Mutant Strain of Nannochloropsis gaditana. Microb. Cell Fact. 2022, 21, 1–15. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Yang, G.; Han, J.; Thomsen, L. Kehou Pan Breeding 3 Elite Strains of Nannochloropsis oceanica by Nitrosoguanidine Mutagenesis and Robust Screening. Algal Res. 2016, 19, 104–108. [Google Scholar] [CrossRef]
- Lu, H.; Cheng, J.; Wang, Z.; Zhang, X.; Chen, S.; Zhou, J. Enhancing Photosynthetic Characterization and Biomass Productivity of Nannochloropsis oceanica by Nuclear Radiation. Front. Energy Res. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Srinivas, R.; Ochs, C. Effect of UV-A Irradiance on Lipid Accumulation in Nannochloropsis oculata. Photochem. Photobiol. 2012, 88, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Moha-León, J.D.; Pérez-Legaspi, I.A.; Ortega-Clemente, L.A.; Rubio-Franchini, I.; Ríos-Leal, E. Improving the Lipid Content of Nannochloropsis oculata by a Mutation-Selection Program Using UV Radiation and Quizalofop. J. Appl. Phycol. 2019, 31, 191–199. [Google Scholar] [CrossRef]
- Arora, N.; Lo, E.; Philippidis, G.P. A Two-Prong Mutagenesis and Adaptive Evolution Strategy to Enhance the Temperature Tolerance and Productivity of Nannochloropsis oculata. Bioresour. Technol. 2022, 364, 128101. [Google Scholar] [CrossRef]
- Arora, N.; Yen, H.W.; Philippidis, G.P. Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability 2020, 12, 5125. [Google Scholar] [CrossRef]
- Anandarajah, K.; Mahendraperumal, G.; Sommerfeld, M.; Hu, Q. Characterization of Microalga Nannochloropsis sp. Mutants for Improved Production of Biofuels. Appl. Energy 2012, 96, 371–377. [Google Scholar] [CrossRef]

| Food | ω-3 | ω-6 | ||
|---|---|---|---|---|
| EPA | LA | ARA | ||
| Dairy | Butter 1 | 0.024 | 2.25 | 0.104 |
| Cheddar | 0.01 | 0.939 | 0.051 | |
| Eggs | * | 1.46 | 0.02 | |
| Greek Yogurt 2 | - | 0.01 | - | |
| Parmesan | 0.008 | 0.999 | 0.027 | |
| Whole milk | 0.001 | 0.097 | 0.004 | |
| Fish | Bluefin tuna 3 | 0.252 | 0.06 | - |
| Haddock 4 | 0.042 | 0.014 | 0.009 | |
| Pollock 4 | 0.049 | 0.005 | 0.005 | |
| Salmon 3,4 [92] | 0.4 | 0.1 | - | |
| Tuna 5 | 0.025 | 0.013 | 0.022 | |
| Fish oils | Cod liver | 6.9 | 0.935 | 0.935 |
| Salmon | 13 | 1.54 | 0.675 | |
| Sardine | 10.1 | 2.01 | 1.76 | |
| Legume products | Hummus | - | 6.81 | 0.005 |
| Peanut butter | * | 9.73 | * | |
| Soy milk | - | 0.988 | - | |
| Meat | Beef loin 6 | 0.002 | 0.362 | 0.064 |
| Chicken breast 7 | 0.004 | 0.599 | 0.086 | |
| Cured bacon 6 | 0.003 | 5.27 | 0.16 | |
| Ham | - | 0.446 | 0.053 | |
| Oils | Coconut | - | 1.68 | - |
| Olive | 0.001 | 9.74 | 0.044 | |
| Sunflower | - | 13 | 0.02 | |
| Vegetables | Broccoli 8 | * | 0.017 | - |
| Nannochloropsis | Whole biomass (dry) | 2.24 | 0.36 | 0.69 |
| Nannochloropsis extract | Fish feed ingredient (NannoStarGOLD, AlgaSpring) | 13 | * | 1.8 |
| Nannochloropsis-enriched oil | Food supplement (OMEGA-3, Iwi) | 25 | * | * |
| Food supplement (Almega PL®, Qualitas Health Lda) | 2.3 | 50.8 | * | |
| Methods | Processing Time | Principle | Lipid Class Target | Accuracy | Scale | Application |
|---|---|---|---|---|---|---|
| Spectrofluorometry | Off- and online | Fluorescence | NL; PL; EPA | Medium/High | Laboratory | LQM |
| Flow cytometry | Off- and online | Fluorescence | NL; PL | High | Laboratory | HTS and LQM |
| Fatty Acid Methyl Ester (FAME) analysis | Offline | Chromatography | NL; PL; TFA | High | Lab- to commercial | SA |
| Iodine value (IV) | Offline | Titration/Spectroscopy/Chromatography | NL; PL; TFA | Medium/High | Lab- to commercial | SA |
| Mass spectrometry (GC-MS; DI-MS; LC-MS) | Offline | Electron ionisation | NL; PL; PUFA | Medium/High | Laboratory | SA |
| NIR-FTIR | Off- and online | Spectroscopy | PUFA | High | Laboratory | LQM |
| Raman spectroscopy | Off- and online | Spectroscopy | PUFA | High | Laboratory | HTS and SA |
| Solvatochromism | Off- and online | Absorbance/Fluorescence | NL, PL | Medium/High | Laboratory | HTS |
| Tetrazolium (TTC) assay | Offline | Colorimetry | PUFA | Low | Laboratory | HTS |
| Sulpho-phospho-vanillin (SPV) assay | Offline | Colorimetry | NL; PUFA | Low/Medium | Laboratory | HTS |
| TerHz | Offline | Spectroscopy | TFA | High | Laboratory | SA |
| Lipid Class | Condition | Low Light | High Light | ||
|---|---|---|---|---|---|
| Method | LC-MS/MS [49] | TLC-GC/FID [8] | LC-MS/MS [49] | TLC-GC/FID [8] | |
| Diacylglycerol–trimethyl–homoserine (DGTS) | 0.8 | 0.7 | 0.4 | 0.6 | |
| Digalactosyldiacylglycerol (DGDG) | 1 | 1.4 | 0.9 | 0.9 | |
| Monogalactosyldiacyglycerol (MGDG) | 3.2 | 2.6 | 1.5 | 0.5 | |
| Phosphatidylcholine (PC) | 1.9 | 1.4 | 0.6 | 1.2 | |
| Phosphatidylethanolamine (PE) | 0.4 | 0.2 | 0.4 | 0.3 | |
| Phosphatidylglycerol (PG) | 3.2 | 0.8 | 1.5 | 0.5 | |
| Phosphatidylinositol (PI) | 0.5 | 0.4 | 0.2 | 0.3 | |
| Sulphoquinovosyldiacylglycerol (SQDG) | 1.8 | 1 | 2.4 | 0.6 | |
| Triacylglycerols (TAG) | 0.2 | 2.3 | 11.4 | 10.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navalho, S.; Ferrer-Ledo, N.; Barbosa, M.J.; Varela, J. Nannochloropsis Lipids and Polyunsaturated Fatty Acids: Potential Applications and Strain Improvement. Mar. Drugs 2025, 23, 128. https://doi.org/10.3390/md23030128
Navalho S, Ferrer-Ledo N, Barbosa MJ, Varela J. Nannochloropsis Lipids and Polyunsaturated Fatty Acids: Potential Applications and Strain Improvement. Marine Drugs. 2025; 23(3):128. https://doi.org/10.3390/md23030128
Chicago/Turabian StyleNavalho, Sofia, Narcis Ferrer-Ledo, Maria J. Barbosa, and João Varela. 2025. "Nannochloropsis Lipids and Polyunsaturated Fatty Acids: Potential Applications and Strain Improvement" Marine Drugs 23, no. 3: 128. https://doi.org/10.3390/md23030128
APA StyleNavalho, S., Ferrer-Ledo, N., Barbosa, M. J., & Varela, J. (2025). Nannochloropsis Lipids and Polyunsaturated Fatty Acids: Potential Applications and Strain Improvement. Marine Drugs, 23(3), 128. https://doi.org/10.3390/md23030128