Comprehensive Review on Application Progress of Marine Collagen Cross-Linking Modification in Bone Repairs
Abstract
:1. Introduction
2. Introduction of Marine Collagen
2.1. Characteristics and Physicochemical Properties of Marine Collagen
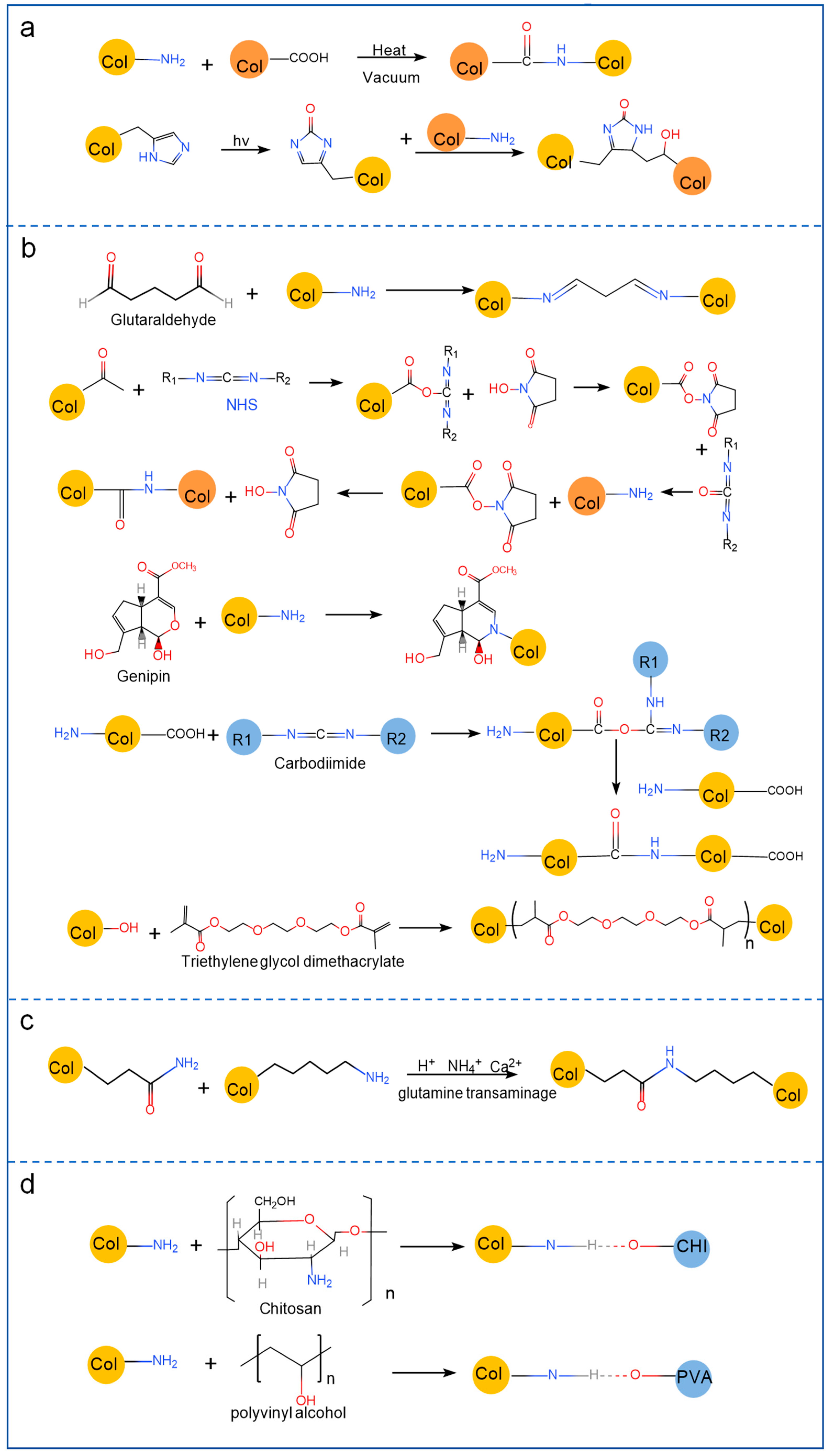
2.2. Modification of Marine Collagen
2.2.1. Physical Modification
2.2.2. Chemical Modification
2.2.3. Enzymatic Modification
2.2.4. Polymer Material Blending Modification
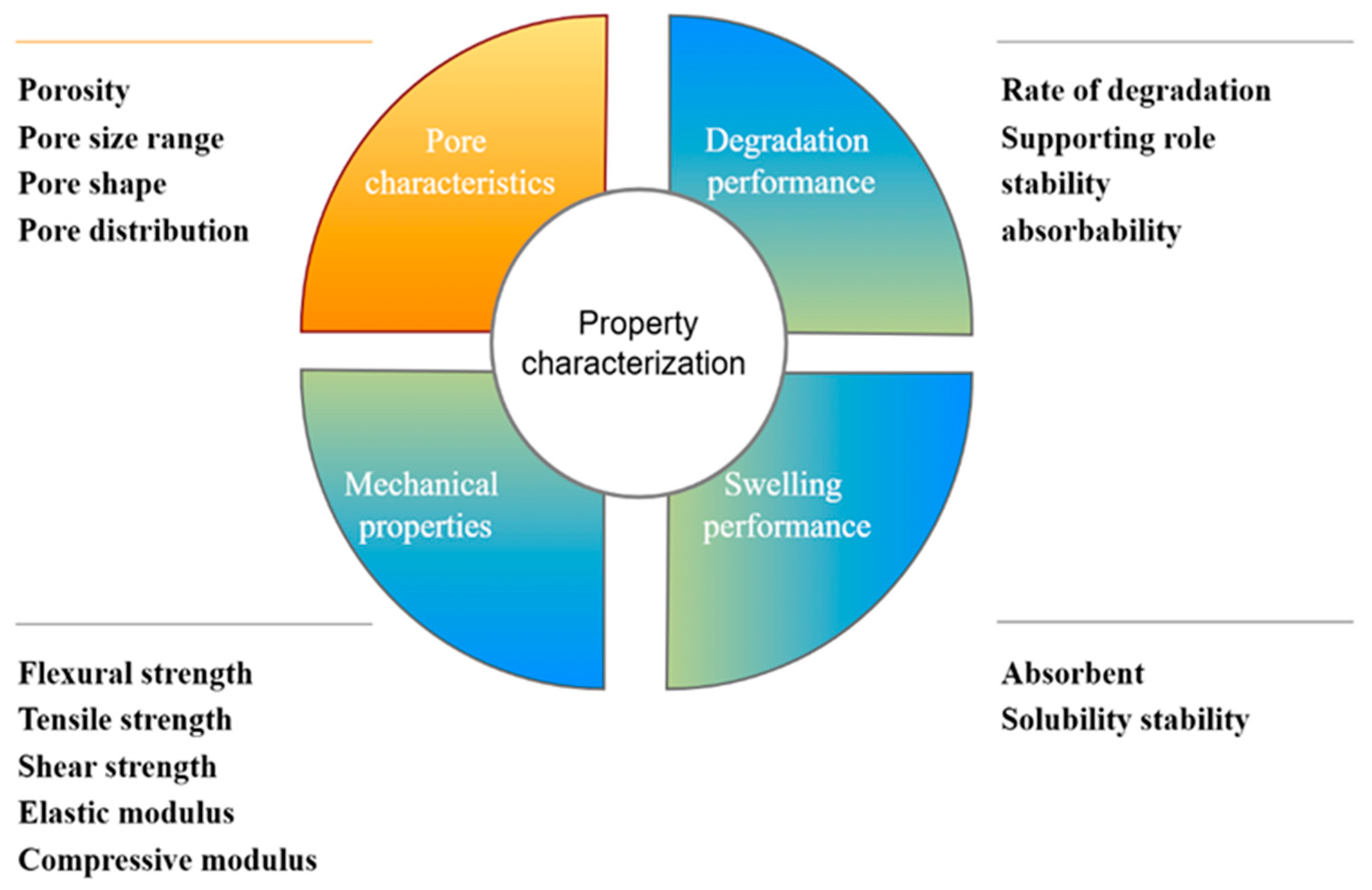
3. Property Characterization of Collagen Application in Bone Repair
3.1. Pore Characteristics
3.2. Degradation Performance
3.3. Mechanical Properties
3.3.1. Modulus
3.3.2. Strength
3.4. Swelling Performance
4. Application of Marine Collagen to Restore Injured Bone Tissue
4.1. Collagen Hydrogels
4.1.1. Collagen Hydrogels Bound with Organic Materials
4.1.2. Collagen Hydrogels Bound with Inorganic Materials
4.2. Composite Scaffolds
4.2.1. Marine Collagen Composite Scaffolds Bound by Organic Materials
4.2.2. Marine Collagen Composite Scaffolds Bound by Inorganic Materials
4.3. Collagen Membranes
4.4. Collagen Sponges
5. Future and Challenge
5.1. Safety
5.2. Usability
5.3. Standardization
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hu, Y.; Jing, Y.; Geng, Z.; Su, J. Bone Repair Biomaterials: A Perspective from Immunomodulation. Adv. Funct. Mater. 2022, 32, 2208639. [Google Scholar] [CrossRef]
- Liu, C. Application of marine collagen for stem-cell-based therapy and tissue regeneration (Review). Med. Int. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Vijayalekha, A.; Anandasadagopan, S.K.; Pandurangan, A.K. An Overview of Collagen-Based Composite Scaffold for Bone Tissue Engineering. Appl. Biochem. Biotechnol. 2023, 195, 4617–4636. [Google Scholar] [CrossRef]
- Cadar, E.; Pesterau, A.-M.; Prasacu, I.; Ionescu, A.-M.; Pascale, C.; Dragan, A.-M.L.; Sirbu, R.; Tomescu, C.L. Marine Antioxidants from Marine Collagen and Collagen Peptides with Nutraceuticals Applications: A Review. Antioxidants 2024, 13, 919. [Google Scholar] [CrossRef]
- Shaik, M.I.; Rahman, S.H.A.; Yusri, A.S.; Ismail-Fitry, M.R.; Kumar, N.S.S.; Sarbon, N.M. A review on the processing technique, physicochemical, and bioactive properties of marine collagen. J. Food Sci. 2024, 89, 5205–5229. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2021, 74, 92–103. [Google Scholar] [CrossRef]
- Nadine, S.; Fernandes, I.J.; Correia, C.R.; Mano, J.F. Close-to-native bone repair via tissue-engineered endochondral ossification approaches. iScience 2022, 25, 105370. [Google Scholar] [CrossRef]
- Rahman, M.A. Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar]
- Kim, S.-K. Marine Nutraceuticals: Prospects and Perspectives; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Fassini, D.; Wilkie, I.C.; Pozzolini, M.; Ferrario, C.; Sugni, M.; Rocha, M.S.; Giovine, M.; Bonasoro, F.; Silva, T.H.; Reis, R.L. Diverse and Productive Source of Biopolymer Inspiration: Marine Collagens. Biomacromolecules 2021, 22, 1815–1834. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gonçalves, C.; Sousa, R.O.; Reis, R.L.; Oliveira, J.M.; Silva, T.H. Extraction and Purification of Biopolymers from Marine Origin Sources Envisaging Their Use for Biotechnological Applications. Mar. Biotechnol. 2024, 26, 1079–1119. [Google Scholar] [CrossRef]
- Wosicka-Frąckowiak, H.; Poniedziałek, K.; Woźny, S.; Kuprianowicz, M.; Nyga, M.; Jadach, B.; Milanowski, B. Collagen and Its Derivatives Serving Biomedical Purposes: A Review. Polymers 2024, 16, 2668. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef]
- Huang, F.; Jiang, M.; Wen, H.; Wu, F.; Liu, W.; Tian, J.; Shao, H. Dietary vitamin C requirement of genetically improved farmed Tilapia, Oreochromis niloticus. Aquac. Res. 2014, 47, 689–697. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.-L.; Li, H.-R.; Liu, J.-X.; Cheng, J.-S.; Qi, X.-Y.; Ye, S.-J.; Gong, H.-L.; Zhao, X.-H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.-X.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Zhang, R.; Brooker, C.; Whitehouse, L.L.E.; Thomson, N.H.; Wood, D.; Tronci, G. Mechanical and suture-holding properties of a UV-cured atelocollagen membrane with varied crosslinked architecture. Biomed. Mater. 2024, 19, 065036. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Impact of UV- and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials. Acta Biomater. 2019, 100, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Kishimoto, M.; Tanaka, T.; Yamane, H. Effect of the Application of a Dehydrothermal Treatment on the Structure and the Mechanical Properties of Collagen Film. Materials 2020, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [PubMed]
- Bazrafshan, Z.; Stylios, G.K. A novel approach to enhance the spinnability of collagen fibers by graft polymerization. Mater. Sci. Eng. C 2019, 94, 108–116. [Google Scholar] [CrossRef]
- Delgado, L.M.; Fuller, K.; Zeugolis, D.I. Collagen cross-linking: Biophysical, biochemical, and biological response analysis. Tissue Eng. Part A 2017, 23, 1064–1077. [Google Scholar]
- Zhang, T.; Yu, Z.; Ma, Y.; Chiou, B.-S.; Liu, F.; Zhong, F. Modulating physicochemical properties of collagen films by cross-linking with glutaraldehyde at varied pH values. Food Hydrocoll. 2022, 124, 107270. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, S.; Liu, Y.; Guo, F.; Miao, Q.; Huang, H. A composite hydrogel scaffold based on collagen and carboxymethyl chitosan for cartilage regeneration through one-step chemical crosslinking. Int. J. Biol. Macromol. 2022, 226, 706–715. [Google Scholar] [CrossRef]
- Ludmila, S.; Victoria, C.; Angelina, S.; Diana, F.; Andrey, K.; Natalia, V.; Olga, S.; Evgeny, S. New Composite Materials with Cross-Linked Structures Based on Grafted Copolymers of Acrylates on Cod Collagen. Appl. Sci. 2023, 13, 5455. [Google Scholar] [CrossRef]
- Ellingson, A.; Pancheri, N.; Schiele, N. Regulators of collagen crosslinking in developing and adult tendons. Eur. Cells Mater. 2022, 43, 130–152. [Google Scholar] [CrossRef]
- Sommer, A.; Dederko-Kantowicz, P.; Staroszczyk, H.; Sommer, S.; Michalec, M. Enzymatic and Chemical Cross-Linking of Bacterial Cellulose/Fish Collagen Composites—A Comparative Study. Int. J. Mol. Sci. 2021, 22, 3346. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Xiao, S.; Zhang, Y.; Chen, D.; Liu, X.; Wu, Y. Efficacy of enzyme-induced collagen crosslinking on porcine cornea. Exp. Ther. Med. 2024, 27, 87. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, R.; Ebara, M.; Uto, K. Tunable enzymatically degradable hydrogels for controlled cargo release with dynamic mechanical properties. Soft Matter 2023, 19, 6224–6233. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Y.; Zheng, X.; Yu, G.; Dan, N.; Dan, W.; Li, Z.; Chen, Y.; Liu, X. Origin of critical nature and stability enhancement in collagen matrix based biomaterials: Comprehensive modification technologies. Int. J. Biol. Macromol. 2022, 216, 741–756. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Rao, W.; Cao, L.; Zhang, D. Molecular interaction analysis between collagen and chitosan blend film based on infrared spectroscopy. Trans. Chin. Soc. Agric. Eng. 2018, 34, 285–291. [Google Scholar]
- Xiang, Z.-X.; Gong, J.-S.; Li, H.; Shi, W.-T.; Jiang, M.; Xu, Z.-H.; Shi, J.-S. Heterologous expression, fermentation strategies and molecular modification of collagen for versatile applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 5268–5289. [Google Scholar] [CrossRef]
- Rui, L.; Yang, M.; Zhu, D.; Han, W.; Dang, X. High-modulus nanocomposite scaffold based on waterborne polyurethane grafted collagen polypeptide/hydroxyapatite for potential bone healing. Mater. Today Commun. 2021, 27, 102222. [Google Scholar] [CrossRef]
- Benayahu, D.; Benayahu, Y. A Unique Marine-Derived Collagen: Its Characterization towards Biocompatibility Applications for Tissue Regeneration. Mar. Drugs 2021, 19, 419. [Google Scholar] [CrossRef]
- Jeyachandran, D.; Cerruti, M. Glass, Ceramic, Polymeric, and Composite Scaffolds with Multiscale Porosity for Bone Tissue Engineering. Adv. Eng. Mater. 2023, 25, 2201743. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Neto, A.S.; Ferreira, J.M.F. Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering. Materials 2018, 11, 1702. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-Chemical Characterization and Biological Tests of Collagen/Silk Fibroin/Chitosan Scaffolds Cross-Linked by Dialdehyde Starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Aslam, M.A.; Bin Abdullah, M.F.; Stojanović, G.M. Current Perspectives of Protein in Bone Tissue Engineering: Bone Structure, Ideal Scaffolds, Fabrication Techniques, Applications, Scopes, and Future Advances. ACS Appl. Bio Mater. 2024, 7, 5082–5106. [Google Scholar] [CrossRef]
- Shi, Q.; Shui, H.; Chen, Q.; Li, Z.-Y. How does mechanical stimulus affect the coupling process of the scaffold degradation and bone formation: An in silico approach. Comput. Biol. Med. 2020, 117, 103588. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Gallentine, S.C.; Powell, H.M. Collagen-Based Electrospun Materials for Tissue Engineering: A Systematic Review. Bioengineering 2021, 8, 39. [Google Scholar] [CrossRef]
- Pripatnanont, P.; Chankum, C.; Meesane, J.; Thonglam, J. Physical and biological performances of a semi-resorbable barrier membrane based on silk fibroin-glycerol-fish collagen material for guided bone regeneration. J. Biomater. Appl. 2021, 36, 930–942. [Google Scholar] [CrossRef]
- Jin, S.; Sun, F.; Zou, Q.; Huang, J.; Zuo, Y.; Li, Y.; Wang, S.; Cheng, L.; Man, Y.; Yang, F.; et al. Fish Collagen and Hydroxyapatite Reinforced Poly(lactide-co-glycolide) Fibrous Membrane for Guided Bone Regeneration. Biomacromolecules 2019, 20, 2058–2067. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Janorkar, A.V. Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr. Opin. Biomed. Eng. 2020, 17, 100253. [Google Scholar] [CrossRef]
- Turner, C.H. Yield Behavior of Bovine Cancellous Bone. J. Biomech. Eng. 1989, 111, 256–260. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Romero, A.; Guerrero, A. Influence of collagen concentration and glutaraldehyde on collagen-based scaffold properties. J. Biomed. Mater. Res. Part A 2016, 104, 1462–1468. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and Mechanical Properties Significantly Influence Cell Attachment, Proliferation, and Migration Within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Nikogeorgos, N.; Ajalloueian, A.; Fossum, M.; Lee, S.; Chronakis, I.S. Compressed collagen constructs with optimized mechanical properties and cell interactions for tissue engineering applications. Int. J. Biol. Macromol. 2018, 108, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Woods, B.; Marques, A.L.P.; Moreira-Silva, J.; Silva, T.H.; Cryan, S.-A.; Reis, R.L.; O’Brien, F.J. Multifunctional Biomaterials from the Sea: Assessing the Effects of Chitosan Incorporation into Collagen Scaffolds on Mechanical and Biological Functionality. Acta Biomater. 2016, 43, 160–169. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Crosslinking of hybrid scaffolds produced from collagen and chitosan. Int. J. Biol. Macromol. 2019, 139, 262–269. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89A, 363–369. [Google Scholar] [CrossRef]
- Hidalgo-Vicelis, J.L.; Alvarez-Perez, M.A.; Miranda-Castro, S.P.; Piña-Barba, M.C. Type I Collagen-chitosan Membranes Crosslinked Chemically with N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide Hydrochloride for Guided Bone Regeneration: A Comparative Study. Fibers Polym. 2020, 21, 262–272. [Google Scholar] [CrossRef]
- Taravel, M.; Domard, A. Collagen and its interaction with chitosan. Biomaterials 1995, 16, 865–871. [Google Scholar] [CrossRef]
- Yang, X.; Yang, D.; Lin, X.; Li, D.; Shi, W.; Xiang, Z.; Mu, C.; Ge, L.; Li, D.; Xu, Z. Effect of Dehydrothermal Treatment on the Structure and Properties of a Collagen-Based Heterogeneous Bilayer Membrane. ACS Appl. Polym. Mater. 2023, 5, 3427–3438. [Google Scholar] [CrossRef]
- Valipour, F.; Rahimabadi, E.Z.; Rostamzad, H. Preparation and characterization of wound healing hydrogel based on fish skin collagen and chitosan cross-linked by dialdehyde starch. Int. J. Biol. Macromol. 2023, 253, 126704. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef]
- Mredha, T.I.; Kitamura, N.; Nonoyama, T.; Wada, S.; Goto, K.; Zhang, X.; Nakajima, T.; Kurokawa, T.; Takagi, Y.; Yasuda, K.; et al. Anisotropic tough double network hydrogel from fish collagen and its spontaneous in vivo bonding to bone. Biomaterials 2017, 132, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Zhang, Y.; Wu, L.; Zhong, Q.; Li, X.; Shen, S.; Xu, X.; Cao, X.; Zhou, Z.; Wong, H.M.; et al. Biomimetic mineralization of collagen from fish scale to construct a functionally gradient lamellar bone-like structure for guided bone regeneration. Int. J. Biol. Macromol. 2024, 281, 136454. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Y.; Pan, H.; Chang, C.; Ao, N.; Xu, H.; Zhang, Z.; Hu, P.; Li, R.; Duan, S.; et al. Preparation and evaluation of stingray skin collagen/oyster osteoinductive composite scaffolds. J. Biomater. Sci. Polym. Ed. 2023, 34, 1360–1381. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.N.; Gonçalves, C.; Oliveira, J.M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Innovative methodology for marine collagen–chitosan–fucoidan hydrogels production, tailoring rheological properties towards biomedical application. Green Chem. 2021, 23, 7016–7029. [Google Scholar] [CrossRef]
- Lu, X.-L.; Miao, H.-L.; Liu, F.; Gao, M.-Q. Analysis of the biocompatibility of a new PLGA/fish skin collagen conjugated electrostatic spinning film. Shanghai Kou Qiang Yi Xue = Shanghai J. Stomatol. 2018, 27, 244–247. [Google Scholar]
- Martins, E.; Diogo, G.S.; Pires, R.; Reis, R.L.; Silva, T.H. 3D Biocomposites Comprising Marine Collagen and Silica-Based Materials Inspired on the Composition of Marine Sponge Skeletons Envisaging Bone Tissue Regeneration. Mar. Drugs 2022, 20, 718. [Google Scholar] [CrossRef]
- Yunoki, S.; Nagai, N.; Suzuki, T.; Munekata, M. Novel biomaterial from reinforced salmon collagen gel prepared by fibril formation and cross-linking. J. Biosci. Bioeng. 2004, 98, 40–47. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.-M.; Moon, S.-Y.; Jeong, Y.-I.; Kim, C.S.; Lee, S.-Y. Biomedical Membrane of Fish Collagen/Gellan Gum Containing Bone Graft Materials. Materials 2022, 15, 2954. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Kogami, H.; Nakayama, H.; Nagata, M.; Yoshie, H. Osteogenic activity of human periosteal sheets cultured on salmon collagen-coated ePTFE meshes. J. Mater. Sci. Mater. Med. 2009, 21, 731–739. [Google Scholar] [CrossRef]
- Ben Azaza, Y.; van der Lee, A.; Li, S.; Nasri, M.; Nasri, R. Chitosan/collagen-based hydrogels for sustainable development: Phycocyanin controlled release. Sustain. Chem. Pharm. 2022, 31, 100905. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Salamone, M.; Barbarino, E.; Barbarino, M.; Nicosia, A.; Ghersi, G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 5798. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; Glattauer, V.; Onofrillo, C.; Duchi, S.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Suitability of Marine- and Porcine-Derived Collagen Type I Hydrogels for Bioprinting and Tissue Engineering Scaffolds. Mar. Drugs 2022, 20, 366. [Google Scholar] [CrossRef]
- Aili, R.; Nakata, H.; Miyasaka, M.; Kuroda, S.; Tamura, Y.; Yokoi, T.; Kawashita, M.; Shimada, Y.; Kasugai, S.; Marukawa, E. Evaluation of a hydroxyapatite-crosslinked fish gelatin membranes. J. Dent. Sci. 2024, 19, 900–908. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Qiu, M.; Zheng, Y.; Shi, X.; Yang, J. Biomimetic injectable and bilayered hydrogel scaffold based on collagen and chondroitin sulfate for the repair of osteochondral defects. Int. J. Biol. Macromol. 2023, 257, 128593. [Google Scholar] [CrossRef]
- Kim, T.-H.; Oh, G.-W.; Heo, S.-Y.; Heo, S.-J.; Kim, Y.-M.; Lee, D.-S.; Kang, H.W.; Kim, H.-W.; Lee, B.; Choi, I.-W.; et al. 3D-printed polycaprolactone/collagen/alginate scaffold incorporating phlorotannin for bone tissue regeneration: Assessment of sub-chronic toxicity. Int. J. Biol. Macromol. 2024, 282 Pt 6, 137480. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Williams, D.S.; Sotelo, C.G.; Pérez-Martín, R.I.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Marine origin biomaterials using a compressive and absorption methodology as cell-laden hydrogel envisaging cartilage tissue engineering. Mater. Sci. Eng. C 2022, 137, 212843. [Google Scholar] [CrossRef]
- Diogo, G.S.; Permuy, M.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Serra, J.; González, P.; Munõz, F.; Pirraco, R.P.; Reis, R.L.; et al. In vivo assessment of marine vs bovine origin collagen-based composite scaffolds promoting bone regeneration in a New Zealand rabbit model. Mater. Sci. Eng. C 2024, 159, 213813. [Google Scholar] [CrossRef]
- Bernhardt, A.; Paul, B.; Gelinsky, M. Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects. Mar. Drugs 2018, 16, 91. [Google Scholar] [CrossRef]
- Oh, G.-W.; Nguyen, V.-T.; Heo, S.-Y.; Ko, S.-C.; Kim, C.S.; Park, W.S.; Choi, I.-W.; Jung, W.-K. 3D PCL/fish collagen composite scaffolds incorporating osteogenic abalone protein hydrolysates for bone regeneration application: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2020, 32, 355–371. [Google Scholar] [CrossRef]
- Diogo, G.S.; López-Senra, E.L.; Pirraco, R.P.; Canadas, R.F.; Fernandes, E.M.; Serra, J.; Pérez-Martín, R.I.; Sotelo, C.G.; Marques, A.P.; González, P.; et al. Marine Collagen/Apatite Composite Scaffolds Envisaging Hard Tissue Applications. Mar. Drugs 2018, 16, 269. [Google Scholar] [CrossRef]
- Muthukumar, T.; Aravinthan, A.; Sharmila, J.; Kim, N.S.; Kim, J.-H. Collagen/chitosan porous bone tissue engineering composite scaffold incorporated with Ginseng compound K. Carbohydr. Polym. 2016, 152, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Venkatesan, J.; Janapala, V.R.; Kim, S.-K. Biophysicochemical evaluation of chitosan-hydroxyapatite-marine sponge collagen composite for bone tissue engineering. J. Biomed. Mater. Res. Part A 2012, 100, 486–495. [Google Scholar] [CrossRef]
- Kim, S.-C.; Heo, S.-Y.; Oh, G.-W.; Yi, M.; Jung, W.-K. A 3D-Printed Polycaprolactone/Marine Collagen Scaffold Reinforced with Carbonated Hydroxyapatite from Fish Bones for Bone Regeneration. Mar. Drugs 2022, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Choi, C.H.; Mun, F.; Lee, J.; Kim, H.; Jung, W.-K.; Jang, C.H.; Kim, G. A polycaprolactone/fish collagen/alginate biocomposite supplemented with phlorotannin for hard tissue regeneration. RSC Adv. 2017, 7, 2009–2018. [Google Scholar] [CrossRef]
- Ahmed, Z.; Powell, L.C.; Matin, N.; Mearns-Spragg, A.; Thornton, C.A.; Khan, I.M.; Francis, L.W. Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity. Mar. Drugs 2021, 19, 405. [Google Scholar] [CrossRef]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish collagen and alginate: Combined marine materials for superior chondrogenesis of hMSC. Mater. Sci. Eng. C 2016, 64, 190–198. [Google Scholar] [CrossRef]
- Erezuma, I.; Lukin, I.; Desimone, M.; Zhang, Y.S.; Dolatshahi-Pirouz, A.; Orive, G. Progress in self-healing hydrogels and their applications in bone tissue engineering. Mater. Sci. Eng. C 2022, 146, 213274. [Google Scholar] [CrossRef]
- Ochi, M.; Uchio, Y.; Kawasaki, K.; Wakitani, S.; Iwasa, J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. Bone Jt. J. 2002, 84, 571–578. [Google Scholar]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Benhnia, M.R.-E.; Perez-Puyana, V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers 2022, 14, 272. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Zheng, F.; Ju, M.; Lü, Y.; Hua, Y.; Yao, W.; Wu, H.; Zhao, M.; Han, S.; Wei, Y.; Liu, R. Carp scales derived double cross-linking hydrogels achieve collagen peptides sustained-released for bone regeneration. Int. J. Biol. Macromol. 2023, 255, 128276. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; An, X.; Duan, S.; Jiang, Z.; Liu, X.; Zhao, X.; Li, Y. A comparative study on cross-linking of fibrillar gel prepared by tilapia collagen and hyaluronic acid with EDC/NHS and genipin. Int. J. Biol. Macromol. 2022, 213, 639–650. [Google Scholar] [CrossRef]
- Jang, C.H.; Ahn, S.H.; Yang, G.-H.; Kim, G.H. A MSCs-laden polycaprolactone/collagen scaffold for bone tissue regeneration. RSC Adv. 2016, 6, 6259–6265. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Q.; Xu, Y.; Wang, Y.; Han, X.; Wang, Z.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. The effect of LyPRP/collagen composite hydrogel on osteogenic differentiation of rBMSCs. Regen. Biomater. 2021, 8, rbaa053. [Google Scholar] [CrossRef]
- Chang, M.C.; Kim, B.-G.; Whang, J.-H. Compressive strength enhancement of artificial bone using hydroxyapatite/fish-collagen nanocomposite. J. Korean Ceram. Soc. 2020, 57, 321–330. [Google Scholar] [CrossRef]
- Jang, C.H.; Kim, W.; Kim, G. Effects of fibrous collagen/CDHA/hUCS biocomposites on bone tissue regeneration. Int. J. Biol. Macromol. 2021, 176, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wenhui, W.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, P.; Shen, T.; Zhang, Y.; Ye, J.; Gao, C. Influence of pore architectures of silk fibroin/collagen composite scaffolds on the regeneration of osteochondral defects in vivo. J. Mater. Chem. B 2019, 8, 391–405. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Abtahi, S.; Chen, X.; Shahabi, S.; Nasiri, N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater. Au 2023, 3, 394–417. [Google Scholar] [CrossRef]
- Bahrizadeh, F.; Lisar, H.A.; Naderi, N.J. A survey on osteogenic effect of collagen-membrane derived from Rutilus kutum swim bladder in rat calvaria. Dent. Res. J. 2021, 18, 55. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Stepwise Proliferation and Chondrogenic Differentiation of Mesenchymal Stem Cells in Collagen Sponges under Different Microenvironments. Int. J. Mol. Sci. 2022, 23, 6406. [Google Scholar] [CrossRef]
- Ghadimi, T.; Latifi, N.; Hivechi, A.; Sarmadi, V.H.; Shahbazi, S.B.; Amini, N.; Milan, P.B.; Abbaszadeh, A.; Larijani, G.; Fathalian, H.; et al. Sargassum glaucescens Extract/Marine-Derived Collagen Blend Sponge and Their Properties for Wound Healing. Mar. Biotechnol. 2025, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Katrilaka, C.; Karipidou, N.; Petrou, N.; Manglaris, C.; Katrilakas, G.; Tzavellas, A.N.; Pitou, M.; Tsiridis, E.E.; Choli-Papadopoulou, T.; Aggeli, A. Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering. Materials 2023, 16, 4425. [Google Scholar] [CrossRef] [PubMed]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.J.; Han, D.K.; Choi, S.H.; Chai, J.K.; Cho, K.S.; Kim, C.K.; Kim, C.S. Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J. Periodontol. 2005, 76, 1667–1674. [Google Scholar]
- Nakamura, T.; Shirakata, Y.; Shinohara, Y.; Miron, R.J.; Furue, K.; Noguchi, K. Osteogenic potential of recombinant human bone morphogenetic protein-9/absorbable collagen sponge (rhBMP-9/ACS) in rat critical size calvarial defects. Clin. Oral Investig. 2016, 21, 1659–1665. [Google Scholar] [CrossRef]
- Lo, K.W.-H.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Q.; Deng, B.; Morita, Y.; Ju, Y.; Song, G. Construction of tendon replacement tissue based on collagen sponge and mesenchymal stem cells by coupled mechano-chemical induction and evaluation of its tendon repair abilities. Acta Biomater. 2018, 74, 247–259. [Google Scholar] [CrossRef]
- Yamamoto, K.; Igawa, K.; Sugimoto, K.; Yoshizawa, Y.; Yanagiguchi, K.; Ikeda, T.; Yamada, S.; Hayashi, Y. Biological Safety of Fish (Tilapia) Collagen. BioMed Res. Int. 2014, 2014, 630757. [Google Scholar] [CrossRef]
- Alves, A.; Costa-Gouveia, J.; de Castro, J.V.; Sotelo, C.; Vázquez, J.; Pérez-Martín, R.; Torrado, E.; Neves, N.; Reis, R.; Castro, A.; et al. Study of the immunologic response of marine-derived collagen and gelatin extracts for tissue engineering applications. Acta Biomater. 2022, 141, 123–131. [Google Scholar] [CrossRef]
- Elkhenany, H.; Soliman, M.W.; Atta, D.; El-Badri, N. Innovative Marine-Sourced Hydroxyapatite, Chitosan, Collagen, and Gelatin for Eco-Friendly Bone and Cartilage Regeneration. J. Biomed. Mater. Res. Part A 2025, 113, e37833. [Google Scholar]
- Guo, X.; Ma, Y.; Wang, H.; Yin, H.; Shi, X.; Chen, Y.; Gao, G.; Sun, L.; Wang, J.; Wang, Y.; et al. Status and developmental trends in recombinant collagen preparation technology. Regen. Biomater. 2024, 11, rbad106. [Google Scholar] [CrossRef]
- Diogo, G.S.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. From Its Nature to Its Function: Marine-Collagen-Based-Biomaterials for Hard Tissue Applications. Tissue Eng. Part B Rev. 2024, 30, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Ding, C.; Xu, M.; Hu, M.; Zhang, R. Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels 2024, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z. Recent Developments in Bio-Ink Formulations Using Marine-Derived Biomaterials for Three-Dimensional (3D) Bioprinting. Mar. Drugs 2024, 22, 134. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Wang, Y.; Wang, X.; Qian, D.; Yan, J.; Sun, Z.; Cui, P.; Yu, L.; Wu, J.; et al. Marine biomaterials in biomedical nano/micro-systems. J. Nanobiotechnol. 2023, 21, 408. [Google Scholar] [CrossRef]



| Collagen Source | Binding Materials | Cross-Linking Agent | Mechanical Behavior | Forms of Action | Site of Action | Action Cells | Example |
|---|---|---|---|---|---|---|---|
| Swim bladder Bester sturgeon fish | HAP | GA, genipin, and N,N′-methylenebis (acrylamide) | Increased modulus, similar to articular cartilage | Hydrogel | Cartilage of the knee bones | [62] | |
| Scale | Epigallocatechin gallate | Increased modulus of elasticity and reduced degradation rate | GBR membrane | Skull | Sprague Dawley rat bone marrow mesenchymal stem cells | [63] | |
| Stingray skin | Oyster shell powder | Genipin | Improved mechanical properties | Composite bracket | MC3T3-E1 cells | [64] | |
| Jellyfish | Fucose (derived from brown algae) and chitosan (from the squid barrier) | Natural cross-linking (polyelectrolyte interaction) | Increased viscosity and adhesion values | Hydrogel | [65] | ||
| Medical-grade fish skin | PLA-glycolic acid | Conjugated electrospinning technique | Nanofiber membrane | Mouse fibroblasts, L929 | [66] | ||
| Cod skin | Silica-based materials | EDC | Increased compression modulus and improved swelling properties | 3D composite stent | L929 fibroblasts | [67] | |
| Salmon skin | Femoral condyles | EDC | Improved mechanical properties | Fibril gel | [68] | ||
| Fish | Gellan gum | D-PBS | Increased tensile strength | GBR membrane | Skull | L292 cells | [69] |
| Salmon | Expanded polytetrafluoroethylene | Periosteal sheet | Alveolar bone | [70] | |||
| Bluefin tuna | Blue crab chitosan | Enhanced stability, strength, and viscoelasticity | Hydrogel | [71] | |||
| Jellyfish | Hydroxyphenylpropionic acid | Horseradish peroxidase, hydrogen-peroxide-catalyzed oxidative coupling (enzymatic cross-linking) | Stiffness improvement | Hydrogel | Nasal septal cartilage | Chondrocytes | [72] |
| Macruronus novazealandii skin | Methacrylates | Ultraviolet (physical cross-linking) | Proper viscosity and shear thinning | 3D-printed bioink hydrogel | L929 fibroblasts | [73] | |
| Nile tilapia skin | HAP | Heat cross-linking | Superior strength, higher flexibility, elasticity, and heat resistance | GBR membrane | MC3T3-E1 cells | [74] | |
| Scale | Oxidized chondroitin sulfate, polyethylene glycol acrylate (PEGDA) | Ammonium persulfate, TEMED | Increased compressive strength | Double-layer hydrogel stent | Articular cartilage | ADMSCs | [75] |
| Paralichthys olivaceus skin | Polycaprolactone, alginate | EDC | Higher water absorption capacity | 3D scaffold | Femur area | [76] | |
| Jellyfish and blue shark skin | Chitosan, fucoidan | Natural cross-linking | Increased elastic–solid properties and mechanical stability | Hydrogel | Articular cartilage tissue | ATDC5 | [77] |
| Blue shark skin | BAp | EDC | Higher compressive modulus | Composite scaffolds | Distal lateral part of femoral condyle | [78] | |
| Jellyfish | Mineralized salmon collagen that mimics biology | EDC | Duplex brackets | Osteochondral tissue | Human mesenchymal stromal cells | [79] | |
| Osteogenic abalone | Poly(ε-caprolactone). | EDC, NHS | Decreased stress rate and increased strain rate | 3D bracket | Shin | Mouse mesenchymal stem cells | [80] |
| Shark skin | Apatite, a marine mineral derived from shark teeth | EDC/NHS or HMDI | Increased ductility | 3D bracket | Hard tissue | Osteoblast-like cell lines | [81] |
| Fish scales | Chitosan, HAP, and β-tricalcium phosphate | GA | Reduced degradation rate and improved swelling properties | Composite stents | Osteoporosis | NIH/3T3, MG-63 | [82] |
| Sponge | Chitosan, HAP | Natural cross-linking | Decreased water absorption | Composite stents | Osteoblast-like MG-63 cells | [83] | |
| Skin of alfalfa | HAP carbonate, polycaprolactone | Increased modulus of elasticity | 3D printed scaffold | Skull | MC3T3-E1 | [84] | |
| Fish | Alginate, polycaprolactone, mesenchymal tannins solution | EDC | Composite stents | Thigh bone | MG63 cells | [85] | |
| Jellyfish | EDC | Higher thermal stability | Bracket | Cartilage tissue of bovine joints | Bovine cartilage protein cells | [86] | |
| Jellyfish | Alginate | EDC | High elasticity | Bracket | Cartilage | hMSC | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, X.; Geng, X.; Li, W.; Cui, H.; Wang, Y.; Qin, S. Comprehensive Review on Application Progress of Marine Collagen Cross-Linking Modification in Bone Repairs. Mar. Drugs 2025, 23, 151. https://doi.org/10.3390/md23040151
Zhai X, Geng X, Li W, Cui H, Wang Y, Qin S. Comprehensive Review on Application Progress of Marine Collagen Cross-Linking Modification in Bone Repairs. Marine Drugs. 2025; 23(4):151. https://doi.org/10.3390/md23040151
Chicago/Turabian StyleZhai, Xiaofei, Xinrong Geng, Wenjun Li, Hongli Cui, Yunqing Wang, and Song Qin. 2025. "Comprehensive Review on Application Progress of Marine Collagen Cross-Linking Modification in Bone Repairs" Marine Drugs 23, no. 4: 151. https://doi.org/10.3390/md23040151
APA StyleZhai, X., Geng, X., Li, W., Cui, H., Wang, Y., & Qin, S. (2025). Comprehensive Review on Application Progress of Marine Collagen Cross-Linking Modification in Bone Repairs. Marine Drugs, 23(4), 151. https://doi.org/10.3390/md23040151










