Insights into the Mechanism Underpinning Composite Molecular Docking During the Self-Assembly of Fucoidan Biopolymers with Peptide Nanofibrils
Abstract
1. Introduction
2. Results and Discussion
2.1. Co-Assembly of Fucoidan and Fmoc-FRGDF to Form Composite Hydrogels
2.2. Particle Size, Zeta Potential, and Turbidity
2.3. Water-Holding Capacity
2.4. Water State Analysis
2.5. Thermal Stability
2.6. Thermodynamic Properties
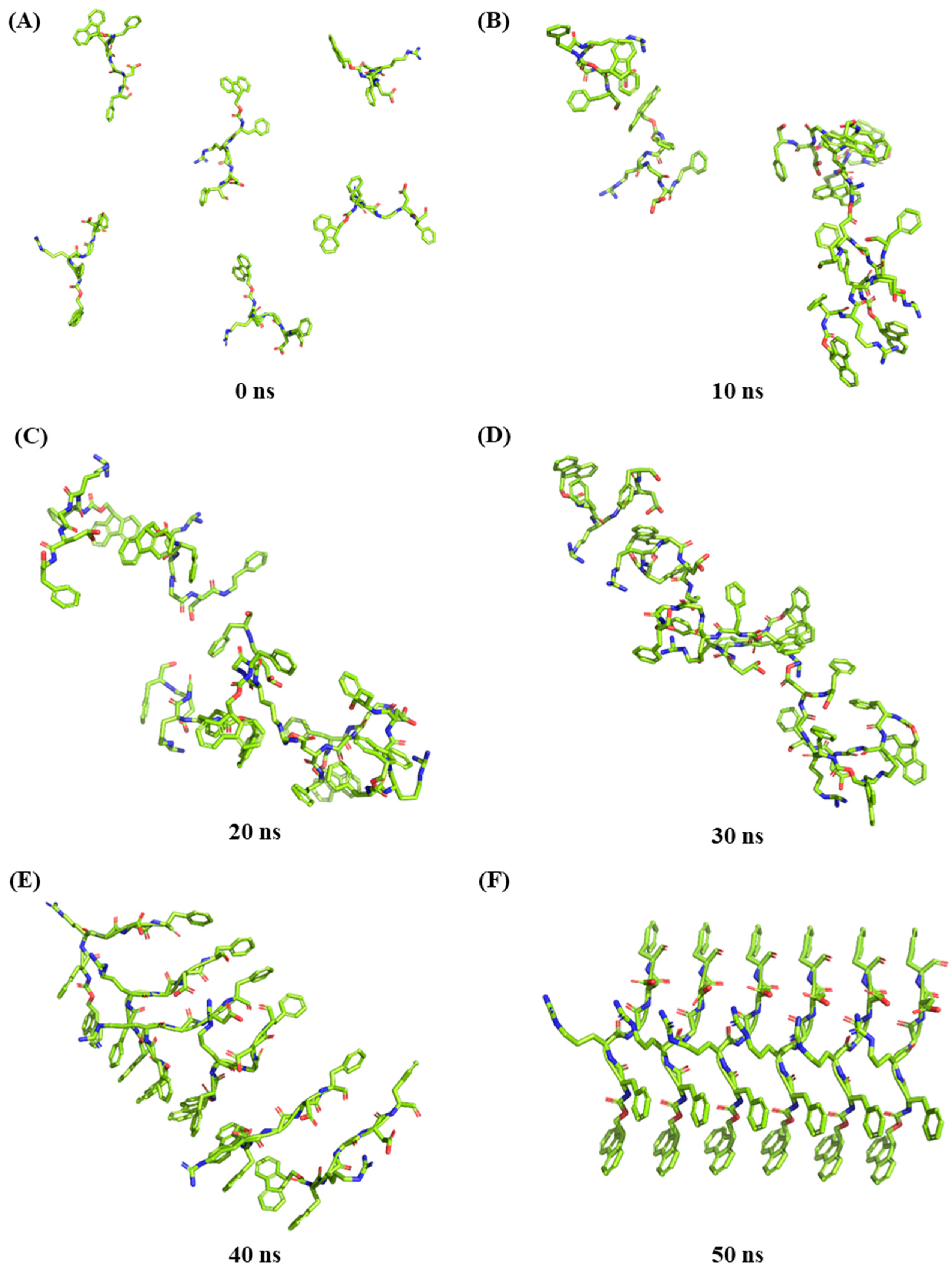
2.7. Molecular Dynamic Simulation of Peptide Self-Assembly
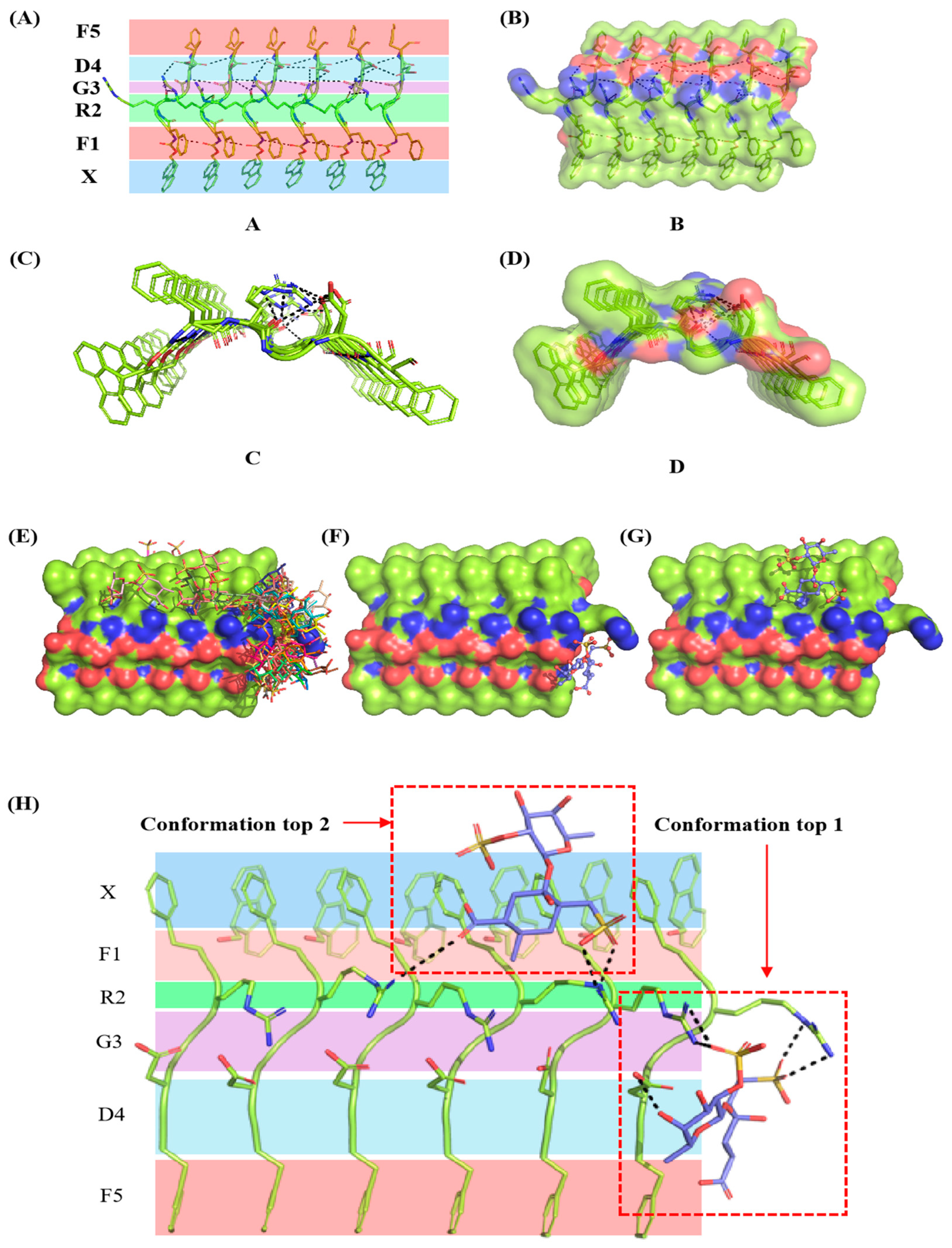
2.8. Binding Mode Analysis of Fucoidan
3. Materials and Methods
3.1. Material
3.2. Preparation of Fucoidan/Fmoc-FRGDF Composite Hydrogels
3.3. Particle Size and Zeta Potential
3.4. Turbidity
3.5. Determination of Water-Holding Capacity
3.6. Synchronous Thermal Analyzer Analysis
3.7. Low-Frequency Nuclear Magnetic Resonance Analysis
3.8. Isothermal Titration Calorimetry
3.9. Molecular Simulation
3.10. Molecular Docking
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, D. Recent Advances in Hydrogels; ACS Publications: Washington, DC, USA, 2022; Volume 34, pp. 1987–1989. [Google Scholar]
- Li, R.; Pavuluri, S.; Bruggeman, K.; Long, B.M.; Parnell, A.J.; Martel, A.; Parnell, S.R.; Pfeffer, F.M.; Dennison, A.J.; Nicholas, K.R. Coassembled nanostructured bioscaffold reduces the expression of proinflammatory cytokines to induce apoptosis in epithelial cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, B. Short peptide-based smart thixotropic hydrogels. Gels 2022, 8, 569. [Google Scholar] [CrossRef]
- Li, R.; Horgan, C.C.; Long, B.; Rodriguez, A.L.; Mather, L.; Barrow, C.J.; Nisbet, D.R.; Williams, R.J. Tuning the mechanical and morphological properties of self-assembled peptide hydrogels via control over the gelation mechanism through regulation of ionic strength and the rate of pH change. RSC Adv. 2015, 5, 301–307. [Google Scholar] [CrossRef]
- Wang, L.; Shen, G.; Yan, X. Bio-inspired short peptide self-assembly: From particles to functional materials. Particuology 2022, 64, 14–34. [Google Scholar] [CrossRef]
- Li, R.; Tai, M.-R.; Wu, Y.-R.; Zhou, Q.-L.; Xia, Q.-Y.; Zhong, S.-Y.; Qi, Y.; Barrow, C.J.; Williams, R.J. Controlling the supramolecular ordering of fish gelatin via simultaneous assembly with fucoidan resulting in nanostructural modification and enhanced rheological performance. LWT 2023, 184, 115078. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Q.-L.; Chen, S.-T.; Tai, M.-R.; Cai, H.-Y.; Ding, R.; Liu, X.-F.; Chen, J.-P.; Luo, L.-X.; Zhong, S.-Y. Chemical Characterization and Immunomodulatory Activity of Fucoidan from Sargassum hemiphyllum. Mar. Drugs 2022, 21, 18. [Google Scholar] [CrossRef]
- Yao, W.; Qiu, H.-M.; Cheong, K.-L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef]
- Tai, M.-R.; Ji, H.-W.; Chen, J.-P.; Liu, X.-F.; Song, B.-B.; Zhong, S.-Y.; Rifai, A.; Nisbet, D.R.; Barrow, C.J.; Williams, R.J. Biomimetic triumvirate nanogel complexes via peptide-polysaccharide-polyphenol self-assembly. Int. J. Biol. Macromol. 2023, 251, 126232. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Shangguan, X.; Wang, H.; Zhang, L.; Bansal, N. Characteristics of fish gelatin-anionic polysaccharide complexes and their applications in yoghurt: Rheology and tribology. Food Chem. 2021, 343, 128413. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, H.; Fang, Y.; Lu, J.; Yang, W.; Qiao, Z.; Lou, Q.; Xu, D.; Zhang, J. Comparison of gelling properties and flow behaviors of microbial transglutaminase (MTGase) and pectin modified fish gelatin. J. Texture Stud. 2019, 50, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, F.; Li, D.; Wu, G.; Zhang, H. Preparation, structure and stability of protein-pterostilbene nanocomplexes coated by soybean polysaccharide and maltodextrin. Food Biosci. 2022, 49, 101899. [Google Scholar] [CrossRef]
- Riquelme, N.; Zúñiga, R.; Arancibia, C. Physical stability of nanoemulsions with emulsifier mixtures: Replacement of tween 80 with quillaja saponin. LWT 2019, 111, 760–766. [Google Scholar] [CrossRef]
- Su, J.; Wang, X.; Li, W.; Chen, L.; Zeng, X.; Huang, Q.; Hu, B. Enhancing the viability of Lactobacillus plantarum as probiotics through encapsulation with high internal phase emulsions stabilized with whey protein isolate microgels. J. Agric. Food Chem. 2018, 66, 12335–12343. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Boyd-Moss, M.; Long, B.; Martel, A.; Parnell, A.; Dennison, A.J.; Barrow, C.J.; Nisbet, D.R.; Williams, R.J. Facile control over the supramolecular ordering of self-assembled peptide scaffolds by simultaneous assembly with a polysacharride. Sci. Rep. 2017, 7, 4797. [Google Scholar] [CrossRef]
- Zheng, H.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Effect of κ-carrageenan on gelation and gel characteristics of Antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019, 278, 644–652. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Pan, N.; Kong, B.; Xia, X. Effect of ice structuring protein on the quality of quick-frozen patties subjected to multiple freeze-thaw cycles. Meat Sci. 2021, 172, 108335. [Google Scholar] [CrossRef]
- Chen, H.; Wu, D.; Ma, W.; Wu, C.; Tian, Y.; Wang, S.; Du, M. Strong fish gelatin hydrogels enhanced by carrageenan and potassium sulfate. Food Hydrocoll. 2021, 119, 106841. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, T.; Yu, F.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of curdlan on the texture and structure of Alaska pollock surimi gels treated at 120 C. Int. J. Food Prop. 2018, 21, 1778–1788. [Google Scholar] [CrossRef]
- Li, L.; Ren, L.; Wang, L.; Liu, S.; Zhang, Y.; Tang, L.; Wang, Y. Effect of water state and polymer chain motion on the mechanical properties of a bacterial cellulose and polyvinyl alcohol (BC/PVA) hydrogel. Rsc Adv. 2015, 5, 25525–25531. [Google Scholar] [CrossRef]
- Yin, W.; Su, R.; Qi, W.; He, Z. A casein-polysaccharide hybrid hydrogel cross-linked by transglutaminase for drug delivery. J. Mater. Sci. 2012, 47, 2045–2055. [Google Scholar] [CrossRef]
- Wee, M.; Yusoff, R.; Lin, L.; Xu, Y. Effect of polysaccharide concentration and charge density on acid-induced soy protein isolate-polysaccharide gels using HCl. Food Struct. 2017, 13, 45–55. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; Gao, Y. Interaction and formation mechanism of binary complex between zein and propylene glycol alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Mousavi, M.; Askari, G.; Kiani, H.; Adt, I.; Gharsallaoui, A. Thermodynamic and physiochemical insights into chickpea protein-Persian gum interactions and environmental effects. Int. J. Biol. Macromol. 2018, 119, 1052–1058. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; Zujovic, Z.; Akbarinejad, A.; Nieuwoudt, M.; Seal, C.K.; McGillivray, D.J. The interactions between the two negatively charged polysaccharides: Gum Arabic and alginate. Food Hydrocoll. 2021, 112, 106343. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Naqvi, A.A.; Mohammad, T.; Hasan, G.M.; Hassan, M.I. Advancements in docking and molecular dynamics simulations towards ligand-receptor interactions and structure-function relationships. Curr. Top. Med. Chem. 2018, 18, 1755–1768. [Google Scholar] [CrossRef]
- Tao, X.; Huang, Y.; Wang, C.; Chen, F.; Yang, L.; Ling, L.; Che, Z.; Chen, X. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Technol. 2020, 55, 33–45. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, L.; Zhou, B.; Zhang, S.; Ma, X.; Zhou, H.; Tuo, X. Elucidation on the interaction between transferrin and ascorbic acid: A study based on spectroscopic analysis, molecular docking technology, and antioxidant evaluation. J. Mol. Liq. 2022, 360, 119413. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Ahmad, I.; Pati, S.; Ghosh, A.; Sarkar, T.; Rabha, B.; Patel, H.; Baishya, D.; Edinur, H.A.; Abdul Kari, Z. Potent bioactive compounds from seaweed waste to combat cancer through bioinformatics investigation. Front. Nutr. 2022, 9, 889276. [Google Scholar] [CrossRef] [PubMed]
- Mysinger, M.M.; Shoichet, B.K. Rapid context-dependent ligand desolvation in molecular docking. J. Chem. Inf. Model. 2010, 50, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Zhou, F.; Xiang, T.; Wen, L.; Wu, Z.; Cao, J. Mechanism of Gusuibu (Drynariae rhizoma)-Buguzhi (Psoraleae fructus) drug pair on treatment of bone metastasis cancer pain based on network pharmacology and molecular docking. J. Hunan Univ. Chin. Med. 2021, 41, 1372–1380. [Google Scholar]
- Chu, L.; Yang, L.; Li, J.; Lin, L.; Zheng, G. Effect of Smilax china L. starch on the gel properties and interactions of calcium sulfate-induced soy protein isolate gel. Int. J. Biol. Macromol. 2019, 135, 127–132. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Obadi, M.; Chen, Z.; Xu, B. Effects of saccharides on the rheological and gelling properties and water mobility of egg white protein. Food Hydrocoll. 2020, 108, 106038. [Google Scholar] [CrossRef]


| Sample | Particle Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| A0 | 748 ± 82 ab | −31 ± 5 ab | 0.81 ± 0.20 ab |
| A2 | 896 ± 89 a | −39 ± 1 c | 0.96 ± 0.04 a |
| A4 | 851 ± 170 a | −37 ± 3 c | 0.80 ± 0.17 ab |
| A6 | 609 ± 104 b | −36 ± 2 c | 0.65 ± 0.11 bc |
| A8 | 884 ± 171 a | −34 ± 1 bc | 0.77 ± 0.08 abc |
| Fucoidan solution | 522 ± 24 b | −26 ± 1 a | 0.54 ± 0.07 c |
| Sample | Distribution of Relaxation Time T2/ms | Proportion of Relaxation Time Peak Area P2/% | ||||||
|---|---|---|---|---|---|---|---|---|
| T2b1 | T2b2 | T22 | T23 | P2b1 | P2b2 | P22 | P23 | |
| A0 | 0.91 ± 0.02 a | 8.4 1± 0.06 a | 36.22 ± 0.17 b | 1320 ± 13 a | 3.69 ± 0.59 c | 1.84 ± 0.57 ab | 5.76 ± 0.21 b | 88.63 ± 0.65 a |
| A2 | 1.01 ± 0.03 a | 3.71 ± 0.58 c | 30.85 ± 1.58 b | 1154 ± 7 b | 6.09 ± 0.27 a | 0.36 ± 0.13 c | 5.66 ± 0.34 b | 88.11 ± 0.08 a |
| A4 | 0.37 ± 0.01 b | 7 ± 1 b | 45 ± 6 a | 505 ± 4 c | 4.66 ± 0.68 b | 1.62 ± 0.40 b | 6.94 ± 0.34 a | 86.59 ± 1.08 b |
| A6 | 0.48 ± 0.09 b | 1.90 ± 0.62 d | 30 ± 1 b | 471 ± 9 d | 4.65 ± 0.39 b | 0.65 ± 0.26 c | 7.47 ± 0.48 a | 86.22 ± 0.28 b |
| A8 | 0.38 ± 0.20 b | 3.81 ± 0.14 c | 47 ± 5 a | 433 ± 6 e | 3.65 ± 0.42 c | 2.51 ± 0.45 a | 7.41 ± 0.39 a | 86.95 ± 0.18 b |
| Binding Ratio (n) | Kd (µM) | ΔH (kJ/mol) | ΔS (kJ/mol) | TΔS (kJ/mol) | ΔG (kJ/mol) |
|---|---|---|---|---|---|
| 1.68 | 6.66 | −45.80 | −0.050 | −16.22 | −29.57 |
| Items | Conformation Top 1 | Conformation Top 2 |
|---|---|---|
| ΔGvdw | −8.08 kJ/mol | −7.53 kJ/mol |
| ΔGH-bond | −10.13 kJ/mol | −8.62 kJ/mol |
| ΔGdesol | −4.90 kJ/mol | −7.70 kJ/mol |
| ΔGele | −25.70 kJ/mol | −23.86 kJ/mol |
| ΔGint | −48.81 kJ/mol | −47.72 kJ/mol |
| ΔGtor | 16.24 kJ/mol | 16.24 kJ/mol |
| ΔGbind | −32.57 kJ/mol | −31.48 kJ/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Tai, M.-R.; Su, X.-N.; Ji, H.-W.; Chen, J.-P.; Liu, X.-F.; Song, B.-B.; Zhong, S.-Y.; Nisbet, D.R.; Barrow, C.J.; et al. Insights into the Mechanism Underpinning Composite Molecular Docking During the Self-Assembly of Fucoidan Biopolymers with Peptide Nanofibrils. Mar. Drugs 2025, 23, 169. https://doi.org/10.3390/md23040169
Li R, Tai M-R, Su X-N, Ji H-W, Chen J-P, Liu X-F, Song B-B, Zhong S-Y, Nisbet DR, Barrow CJ, et al. Insights into the Mechanism Underpinning Composite Molecular Docking During the Self-Assembly of Fucoidan Biopolymers with Peptide Nanofibrils. Marine Drugs. 2025; 23(4):169. https://doi.org/10.3390/md23040169
Chicago/Turabian StyleLi, Rui, Min-Rui Tai, Xian-Ni Su, Hong-Wu Ji, Jian-Ping Chen, Xiao-Fei Liu, Bing-Bing Song, Sai-Yi Zhong, David. R. Nisbet, Colin J. Barrow, and et al. 2025. "Insights into the Mechanism Underpinning Composite Molecular Docking During the Self-Assembly of Fucoidan Biopolymers with Peptide Nanofibrils" Marine Drugs 23, no. 4: 169. https://doi.org/10.3390/md23040169
APA StyleLi, R., Tai, M.-R., Su, X.-N., Ji, H.-W., Chen, J.-P., Liu, X.-F., Song, B.-B., Zhong, S.-Y., Nisbet, D. R., Barrow, C. J., & Williams, R. J. (2025). Insights into the Mechanism Underpinning Composite Molecular Docking During the Self-Assembly of Fucoidan Biopolymers with Peptide Nanofibrils. Marine Drugs, 23(4), 169. https://doi.org/10.3390/md23040169










