Low-Molecular-Weight Fucoidan Inhibits Thromboinflammation and Ameliorates Deep Vein Thrombosis via Targeting S100A8/A9
Abstract
:1. Introduction
2. Results
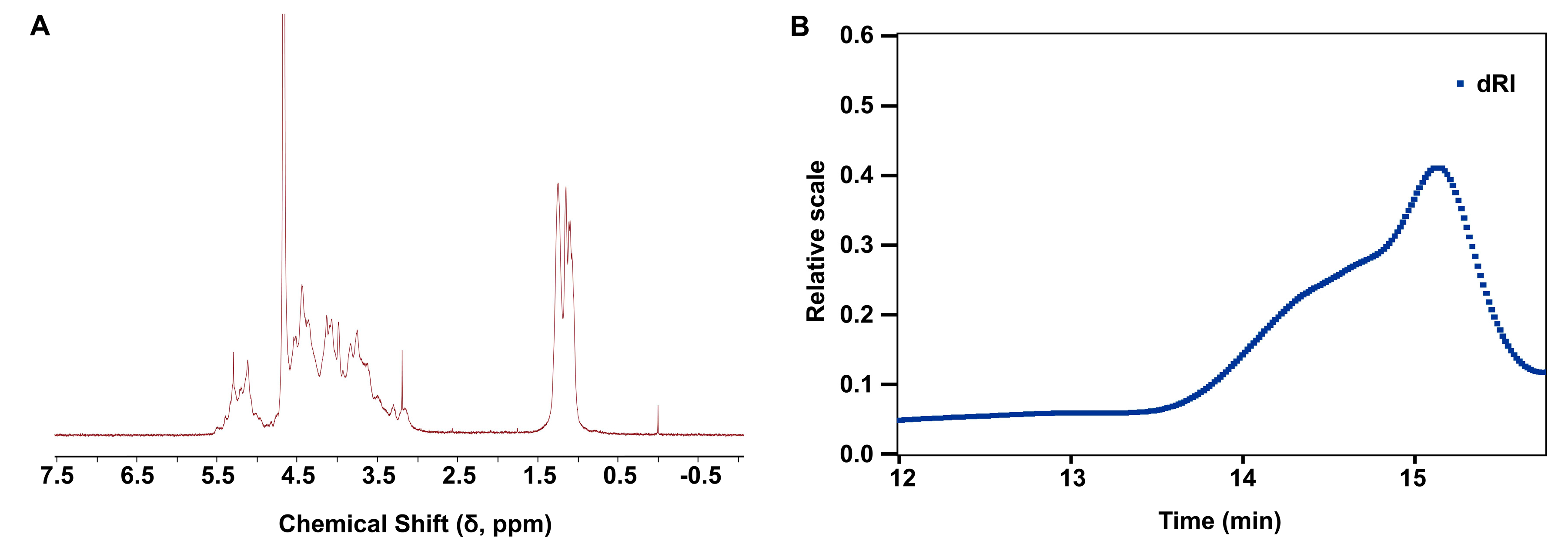
2.1. Structural Characterization and Physicochemical Properties of LMF
2.2. LMF Inhibited S100A8/A9-Augmented Platelet Aggregation
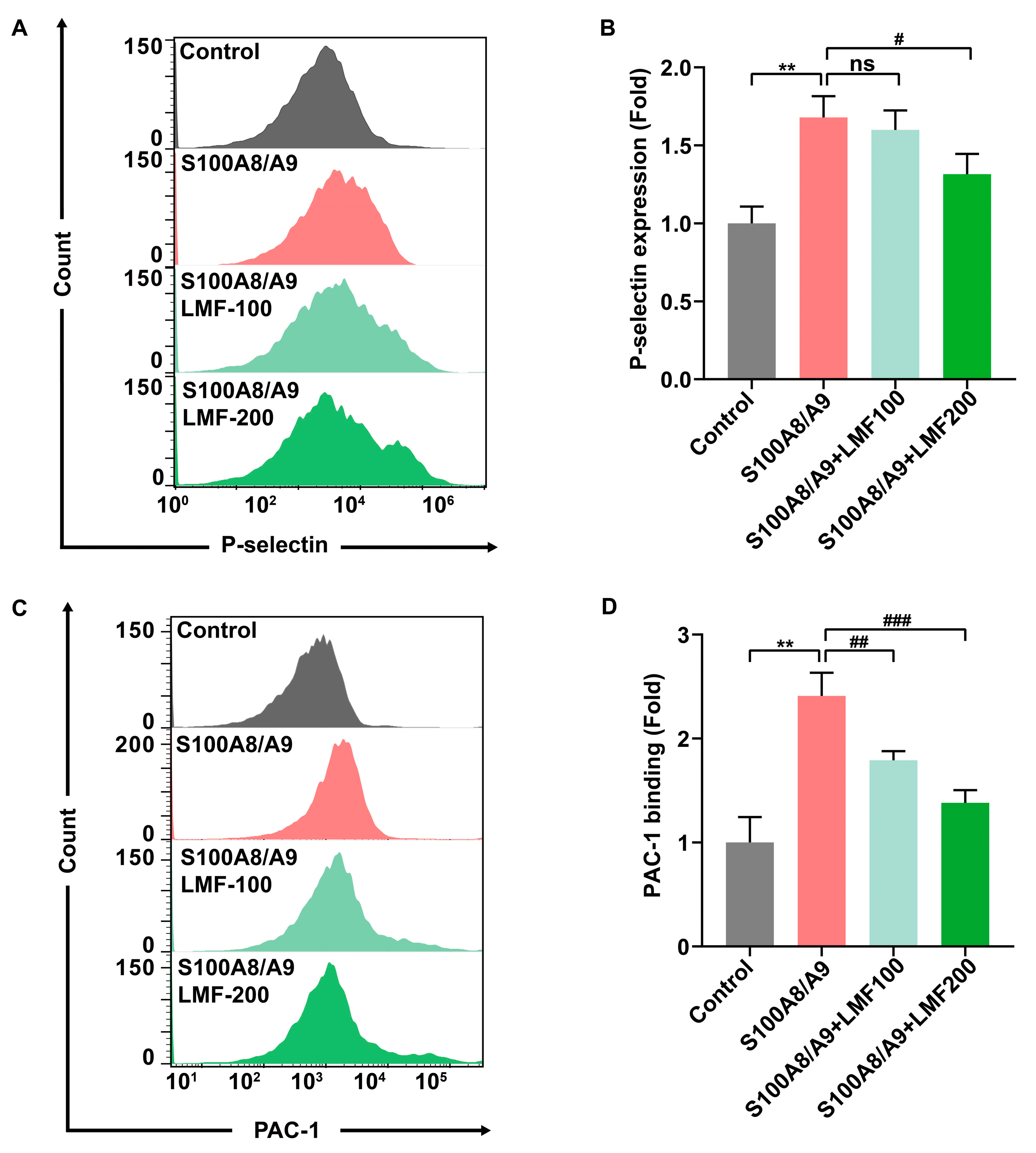
2.3. LMF Inhibited S100A8/A9-Induced Platelet Activation and Secretion
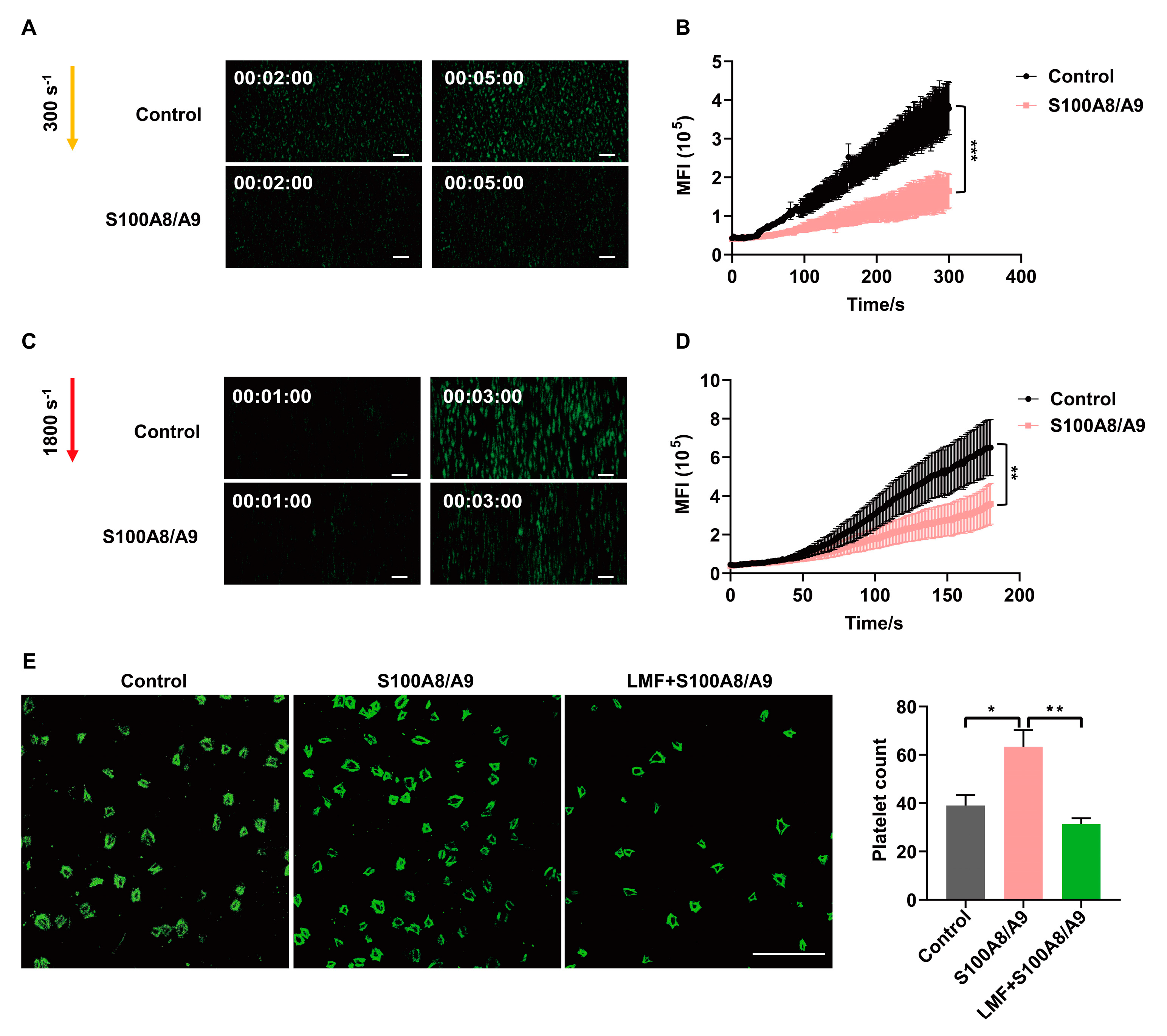
2.4. LMF Inhibited S100A8/A9-Promoted Platelet Adhesion on Immobilized Fibrinogen
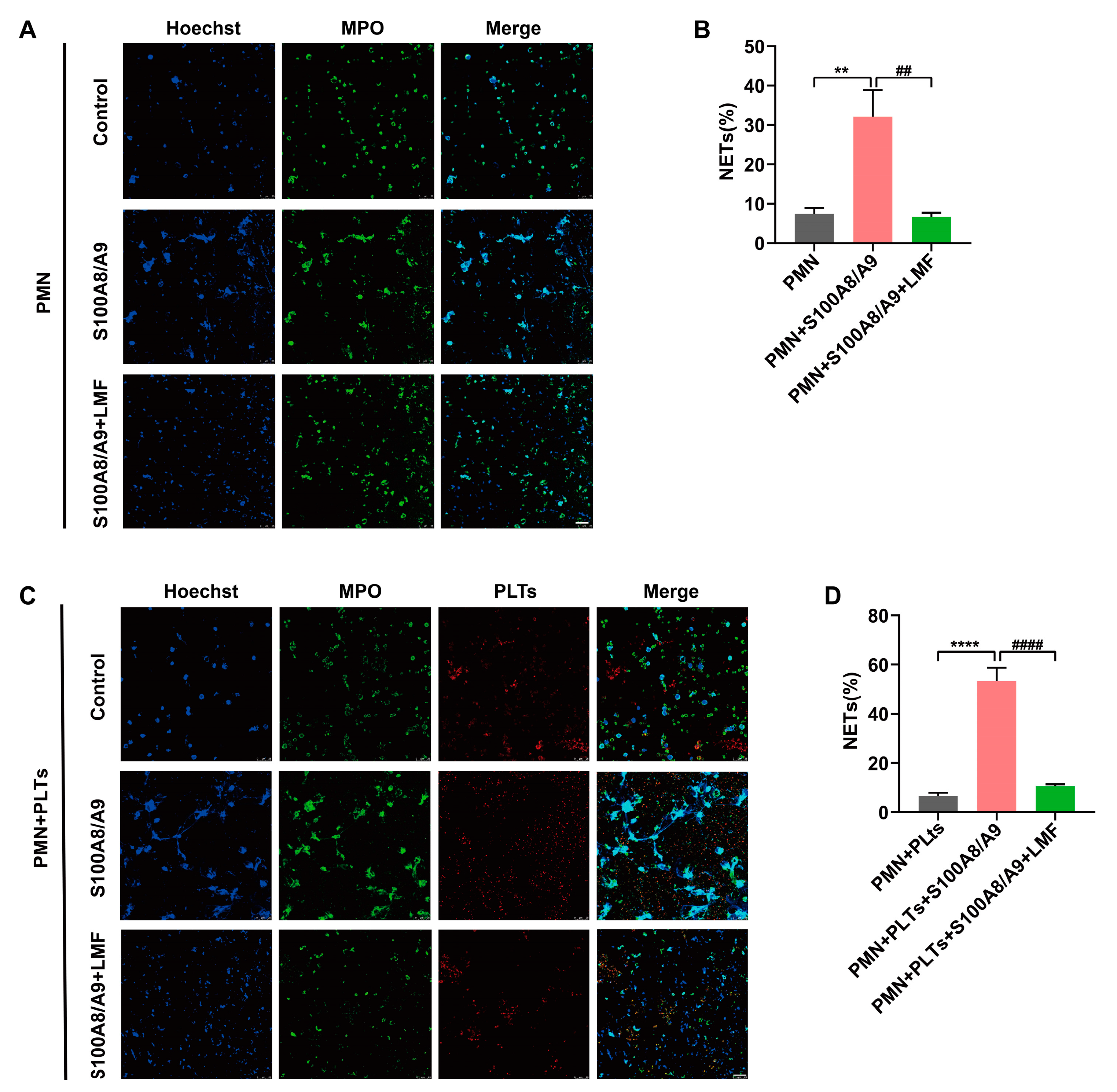
2.5. LMF Inhibited S100A8/A9-Induced NET Formation
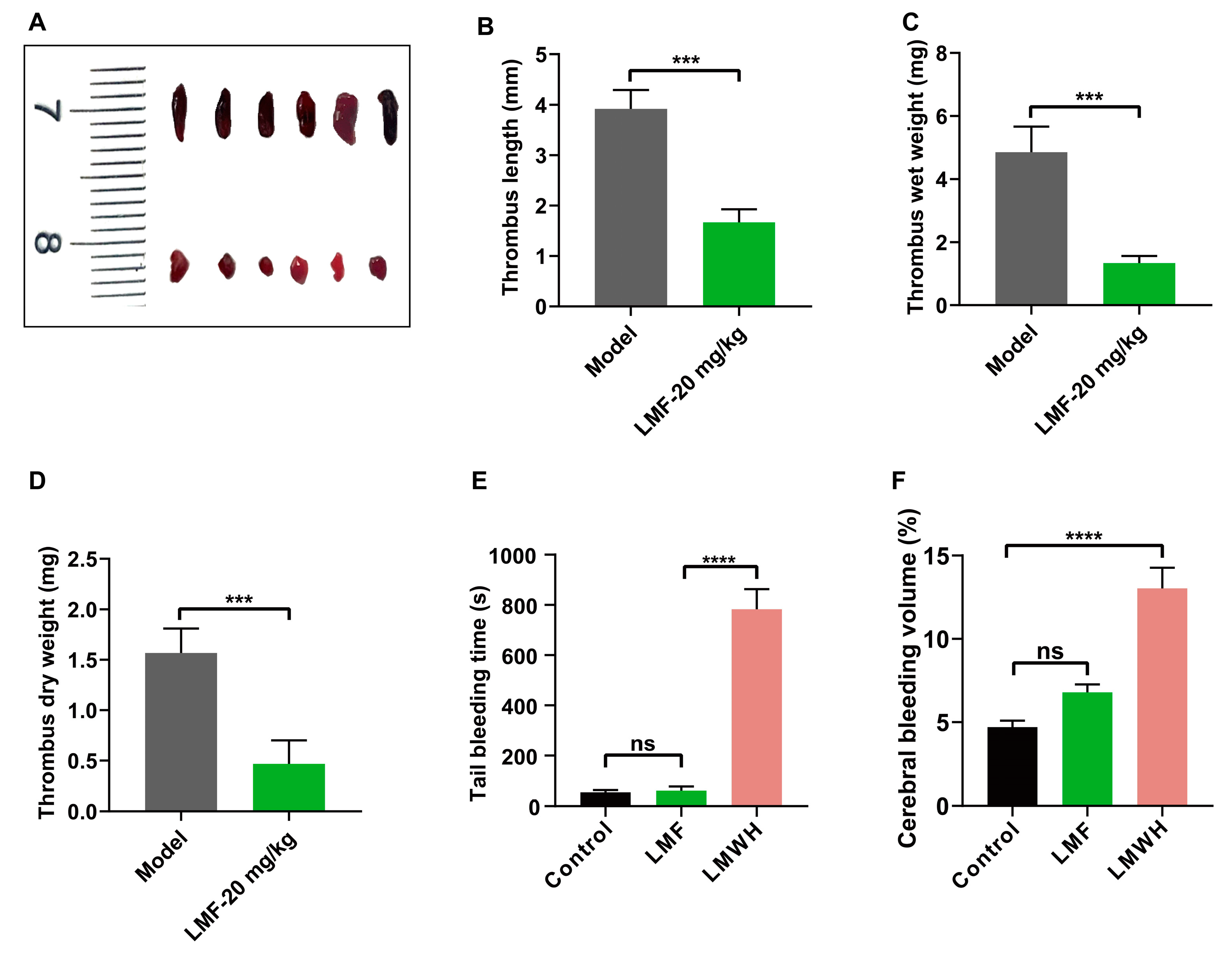
2.6. LMF Ameliorated DVT Development Without Perturbing Hemostasis
3. Discussion
4. Materials and Methods
4.1. Analysis of NMR and Physicochemical Properties
4.2. Animals
4.3. Platelet Isolation and Aggregation Assay
4.4. Flow Cytometry
4.5. Surface Plasmon Resonance (SPR) Analysis
4.6. Ex Vivo Perfusion Chamber
4.7. Platelet Adhesion and Confocal Microscopy
4.8. Isolation of Neutrophils and NET Formation Assay (In Vitro)
4.9. Murine Model of Deep Vein Thrombosis
4.10. Tail Bleeding
4.11. Collagenase-Induced Cerebral Bleeding
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, F.; Tritschler, T.; Kahn, S.R.; Rodger, M.A. Venous thromboembolism. Lancet 2021, 398, 64–77. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Potere, N.; Abbate, A.; Kanthi, Y.; Carrier, M.; Toldo, S.; Porreca, E.; Di Nisio, M. Inflammasome Signaling, Thromboinflammation, and Venous Thromboembolism. JACC. Basic Transl. Sci. 2023, 8, 1245–1261. [Google Scholar] [CrossRef]
- Cay, N.; Ipek, A.; Gumus, M.; Birkan, Z.; Ozmen, E. Platelet activity indices in patients with deep vein thrombosis. Clin. Appl. Thromb./Hemost. Off. J. Int. Acad. Clin. Appl. Thromb./Hemost. 2012, 18, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ma, J.; Wu, D.; Fang, C.; Wang, Z.; Guo, T.; Mo, J. Neutrophil extracellular traps mediate deep vein thrombosis: From mechanism to therapy. Front. Immunol. 2023, 14, 1198952. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, S.; Solar, C.; Tapia, R.; Pereira, J.; Fuentes, E.; Palomo, I. Pathophysiology of deep vein thrombosis. Clin. Exp. Med. 2023, 23, 645–654. [Google Scholar] [CrossRef]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Colicchia, M.; Schrottmaier, W.C.; Perrella, G.; Reyat, J.S.; Begum, J.; Slater, A.; Price, J.; Clark, J.C.; Zhi, Z.; Simpson, M.J.; et al. S100A8/A9 drives the formation of procoagulant platelets through GPIbα. Blood 2022, 140, 2626–2643. [Google Scholar] [CrossRef]
- Sprenkeler, E.G.G.; Zandstra, J.; van Kleef, N.D.; Goetschalckx, I.; Verstegen, B.; Aarts, C.E.M.; Janssen, H.; Tool, A.T.J.; van Mierlo, G.; van Bruggen, R.; et al. S100A8/A9 Is a Marker for the Release of Neutrophil Extracellular Traps and Induces Neutrophil Activation. Cells 2022, 11, 236. [Google Scholar] [CrossRef]
- Bai, B.; Xu, Y.; Chen, H. Pathogenic roles of neutrophil-derived alarmins (S100A8/A9) in heart failure: From molecular mechanisms to therapeutic insights. Br. J. Pharmacol. 2023, 180, 573–588. [Google Scholar] [CrossRef]
- Su, M.; Chen, C.; Li, S.; Li, M.; Zeng, Z.; Zhang, Y.; Xia, L.; Li, X.; Zheng, D.; Lin, Q.; et al. Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis. Nat. Cardiovasc. Res. 2022, 1, 732–747. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Fan, Y.; Ma, R.; An, Y.; Chen, G.; Xie, Y. Discovery of protein biomarkers for venous thromboembolism in non-small cell lung cancer patients through data-independent acquisition mass spectrometry. Front. Oncol. 2023, 13, 1079719. [Google Scholar] [CrossRef] [PubMed]
- Fitton, H.J.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Yao, Y.; Yim, E.K.F. Fucoidan for cardiovascular application and the factors mediating its activities. Carbohydr. Polym. 2021, 270, 118347. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Mackeigan, D.T.; Shoara, A.A.; Xu, R.; Bhoria, P.; Karakas, D.; Ma, W.; Cerenzia, E.; Chen, Z.; Hoard, B.; et al. Dual roles of fucoidan-GPIbα interaction in thrombosis and hemostasis: Implications for drug development targeting GPIbα. J. Thromb. Haemost. JTH 2023, 21, 1274–1288. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Kessinger, C.W.; Schmaier, A.; Jaffer, F.A.; Simon, D.I. Myeloid-related protein-14 regulates deep vein thrombosis. JCI Insight 2017, 2, e91356. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Furniss, J.A.; Vautrinot, J.; Williams, C.M.; Walsh, T.G.; Brill, A.; Amulic, B.; Poole, A.W. Critical role for platelet Ral GTPases in regulating venous thrombosis in mice. J. Thromb. Haemost. JTH 2024, 23, 254–261. [Google Scholar] [CrossRef]
- Goudswaard, L.J.; Williams, C.M.; Khalil, J.; Burley, K.L.; Hamilton, F.; Arnold, D.; Milne, A.; Lewis, P.A.; Heesom, K.J.; Mundell, S.J.; et al. Alterations in platelet proteome signature and impaired platelet integrin α(IIb)β(3) activation in patients with COVID-19. J. Thromb. Haemost. JTH 2023, 21, 1307–1321. [Google Scholar] [CrossRef]
- Joshi, A.; Schmidt, L.E.; Burnap, S.A.; Lu, R.; Chan, M.V.; Armstrong, P.C.; Baig, F.; Gutmann, C.; Willeit, P.; Santer, P.; et al. Neutrophil-Derived Protein S100A8/A9 Alters the Platelet Proteome in Acute Myocardial Infarction and Is Associated with Changes in Platelet Reactivity. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 49–62. [Google Scholar] [CrossRef]
- Colicchia, M.; Perrella, G.; Gant, P.; Rayes, J. Novel mechanisms of thrombo-inflammation during infection: Spotlight on neutrophil extracellular trap-mediated platelet activation. Res. Pract. Thromb. Haemost. 2023, 7, 100116. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Bercu, A.; Wang, Y.A.; Woollard, K.J.; Mangin, P.; Vanhoorelbeke, K.; Crawley, J.T.B.; Salles, C. II, The GPIbα intracellular tail-role in transducing VWF- and collagen/GPVI-mediated signaling. Haematologica 2022, 107, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Laridan, E.; Martinod, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [Google Scholar] [CrossRef]
- Aggarwal, A.; Fullam, L.; Brownstein, A.P.; Maynard, G.A.; Ansell, J.; Varga, E.A.; Friedman, R.J.; Rickles, F.R. Deep vein thrombosis (DVT) and pulmonary embolism (PE): Awareness and prophylaxis practices reported by patients with cancer. Cancer Investig. 2015, 33, 405–410. [Google Scholar] [CrossRef] [PubMed]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; Lavalle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Wagner, B.; Nagler, M.; Koeck, T.; Ten Cate, V.; Prochaska, J.H.; Heitmeier, S.; Meyer, I.; Gerdes, C.; Laux, V.; et al. Comprehensive platelet phenotyping supports the role of platelets in the pathogenesis of acute venous thromboembolism-results from clinical observation studies. EBioMedicine 2020, 60, 102978. [Google Scholar] [CrossRef]
- Brill, A.; Fuchs, T.A.; Chauhan, A.K.; Yang, J.J.; De Meyer, S.F.; Köllnberger, M.; Wakefield, T.W.; Lämmle, B.; Massberg, S.; Wagner, D.D. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 2011, 117, 1400–1407. [Google Scholar] [CrossRef]
- Reinhardt, C.; von Brühl, M.L.; Manukyan, D.; Grahl, L.; Lorenz, M.; Altmann, B.; Dlugai, S.; Hess, S.; Konrad, I.; Orschiedt, L.; et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J. Clin. Investig. 2008, 118, 1110–1122. [Google Scholar] [CrossRef]
- Lan, H.; Tong, Z.; Jiao, Y.; Han, H.; Ma, Y.; Li, Y.; Jia, X.; Hu, B.; Zhang, W.; Zhong, M.; et al. Deep Vein Thrombosis Is Facilitated by Endothelial-Derived Extracellular Vesicles via the PDI-GRP94-GPIIb/IIIa Pathway in Mice. J. Clin. Med. 2023, 12, 4265. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Monroe, D.M.; Roberts, H.R.; Hoffman, M.R. Tissue factor de-encryption: Ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 1999, 10, 201–210. [Google Scholar] [CrossRef]
- Mereweather, L.J.; Constantinescu-Bercu, A.; Crawley, J.T.B.; Salles, C. II, Platelet-Neutrophil Crosstalk in Thrombosis. Int. J. Mol. Sci. 2023, 24, 1266. [Google Scholar] [CrossRef]
- Schönichen, C.; Montague, S.J.; Brouns, S.L.N.; Burston, J.J.; Cosemans, J.; Jurk, K.; Kehrel, B.E.; Koenen, R.R.; Ní Áinle, F.; O’Donnell, V.B.; et al. Antagonistic Roles of Human Platelet Integrin αIIbβ3 and Chemokines in Regulating Neutrophil Activation and Fate on Arterial Thrombi Under Flow. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, C.; Gao, H.; Bilodeau, M.L.; Zhang, Z.; Croce, K.; Liu, S.; Morooka, T.; Sakuma, M.; Nakajima, K.; et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J. Clin. Investig. 2014, 124, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Goette, N.P.; Lev, P.R.; Baroni Pietto, M.C.; Marin Oyarzún, C.P.; Castro Ríos, M.A.; Moiraghi, B.; Sackmann, F.; Kamiya, L.J.; Verri, V.; et al. Elevated levels of damage-associated molecular patterns HMGB1 and S100A8/A9 coupled with toll-like receptor-triggered monocyte activation are associated with inflammation in patients with myelofibrosis. Front. Immunol. 2024, 15, 1365015. [Google Scholar]
- Du, F.; Ding, Z.; Rönnow, C.F.; Rahman, M.; Schiopu, A.; Thorlacius, H. S100A9 induces reactive oxygen species-dependent formation of neutrophil extracellular traps in abdominal sepsis. Exp. Cell Res. 2022, 421, 113405. [Google Scholar] [CrossRef]
- Sreejit, G.; Abdel-Latif, A.; Athmanathan, B.; Annabathula, R.; Dhyani, A.; Noothi, S.K.; Quaife-Ryan, G.A.; Al-Sharea, A.; Pernes, G.; Dragoljevic, D.; et al. Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction. Circulation 2020, 141, 1080–1094. [Google Scholar] [CrossRef]
- Andrews, R.K.; Arthur, J.F.; Gardiner, E.E. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb. Haemost. 2014, 112, 659–665. [Google Scholar] [CrossRef]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-Neutrophil Interplay: Insights into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef]
- Liu, T.; Scallan, C.D.; Broze, G.J., Jr.; Patarroyo-White, S.; Pierce, G.F.; Johnson, K.W. Improved coagulation in bleeding disorders by Non-Anticoagulant Sulfated Polysaccharides (NASP). Thromb. Haemost. 2006, 95, 68–76. [Google Scholar] [CrossRef]
- Puhari, S.S.M.; Yuvaraj, S.; Vasudevan, V.; Ramprasath, T.; Rajkumar, P.; Arunkumar, K.; Amutha, C.; Selvam, G.S. Isolation and characterization of fucoidan from four brown algae and study of the cardioprotective effect of fucoidan from Sargassum wightii against high glucose-induced oxidative stress in H9c2 cardiomyoblast cells. J. Food Biochem. 2022, 46, e14412. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Liu, M.; Xu, R.; Wang, G.; Li, J.; Chen, P.; Ma, W.; Mwangi, J.; Lu, Q.; Duan, Z.; et al. The 14-3-3ζ-c-Src-integrin-β3 complex is vital for platelet activation. Blood 2020, 136, 974–988. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Neves, M.A.D.; Zhu, G.; Carrim, N.; Yu, R.; Gupta, S.; Marshall, J.; Rotstein, O.; Peng, J.; et al. GPIbα is required for platelet-mediated hepatic thrombopoietin generation. Blood 2018, 132, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Mackeigan, D.T.; Shoara, A.A.; Bhoria, P.; Zhu, G.; Karakas, D.; Ma, W.; Chen, Z.Y.; Xu, R.; Slavkovic, S.; et al. Novel GPIb-independent platelet aggregation induced by botrocetin: Implications for diagnosis and antithrombotic therapy. J. Thromb. Haemost. JTH 2024, 22, 3249–3265. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, G.; Xu, R.; Shen, C.; Ni, H.; Lai, R. Soy Isoflavones Inhibit Both GPIb-IX Signaling and αIIbβ3 Outside-In Signaling via 14-3-3ζ in Platelet. Molecules 2021, 26, 4911. [Google Scholar] [CrossRef]
- Shen, C.; Liu, M.; Tian, H.; Li, J.; Xu, R.; Mwangi, J.; Lu, Q.; Hao, X.; Lai, R. Conformation-Specific Blockade of αIIbβ3 by a Non-RGD Peptide to Inhibit Platelet Activation without Causing Significant Bleeding and Thrombocytopenia. Thromb. Haemost. 2020, 120, 1432–1441. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Shen, C.; Hao, X.; Sun, P.; Li, P.; Xu, T.; Hu, C.; Rose, O.; Zhou, H.J.N.i. Salivary factor LTRIN from Aedes aegypti facilitates the transmission of Zika virus by interfering with the lymphotoxin-β receptor. Nat. Immunol. 2018, 19, 342–353. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Li, M.; Tang, X.; Liao, Z.; Shen, C.; Cheng, R.; Fang, M.; Wang, G.; Li, Y.; Tang, S.; Xie, L.; et al. Hypoxia and low temperature upregulate transferrin to induce hypercoagulability at high altitude. Blood 2022, 140, 2063–2075. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zhao, W.; Fang, S.; Zhao, J.; Wang, W.; Zhou, S.; Wang, T.; Fang, X.; Chen, X.; Awais, M.; et al. Low-Molecular-Weight Fucoidan Inhibits Thromboinflammation and Ameliorates Deep Vein Thrombosis via Targeting S100A8/A9. Mar. Drugs 2025, 23, 180. https://doi.org/10.3390/md23050180
Feng Y, Zhao W, Fang S, Zhao J, Wang W, Zhou S, Wang T, Fang X, Chen X, Awais M, et al. Low-Molecular-Weight Fucoidan Inhibits Thromboinflammation and Ameliorates Deep Vein Thrombosis via Targeting S100A8/A9. Marine Drugs. 2025; 23(5):180. https://doi.org/10.3390/md23050180
Chicago/Turabian StyleFeng, Yiting, Weiqing Zhao, Siwen Fang, Jingwen Zhao, Wanshuai Wang, Shaoyun Zhou, Tianyu Wang, Xinke Fang, Xue Chen, Muhammad Awais, and et al. 2025. "Low-Molecular-Weight Fucoidan Inhibits Thromboinflammation and Ameliorates Deep Vein Thrombosis via Targeting S100A8/A9" Marine Drugs 23, no. 5: 180. https://doi.org/10.3390/md23050180
APA StyleFeng, Y., Zhao, W., Fang, S., Zhao, J., Wang, W., Zhou, S., Wang, T., Fang, X., Chen, X., Awais, M., Cai, C., Shen, C., & Liu, M. (2025). Low-Molecular-Weight Fucoidan Inhibits Thromboinflammation and Ameliorates Deep Vein Thrombosis via Targeting S100A8/A9. Marine Drugs, 23(5), 180. https://doi.org/10.3390/md23050180





