Purpuramine R, a New Bromotyrosine Isolated from Pseudoceratina cf. verrucosa Collected in the Kingdom of Tonga
Abstract
1. Introduction
2. Results
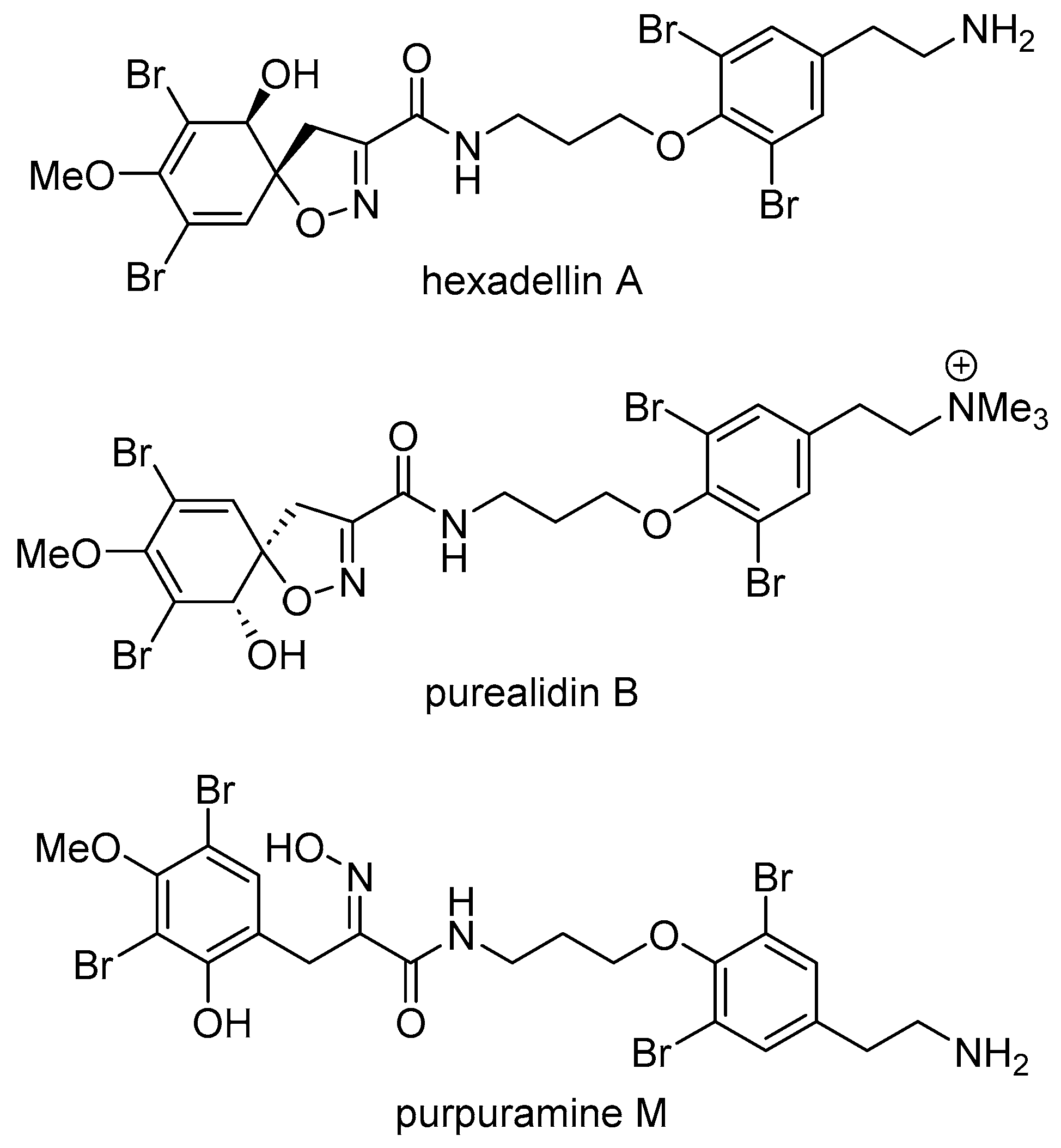
2.1. Structure Elucidation of Purpuramine R (1)
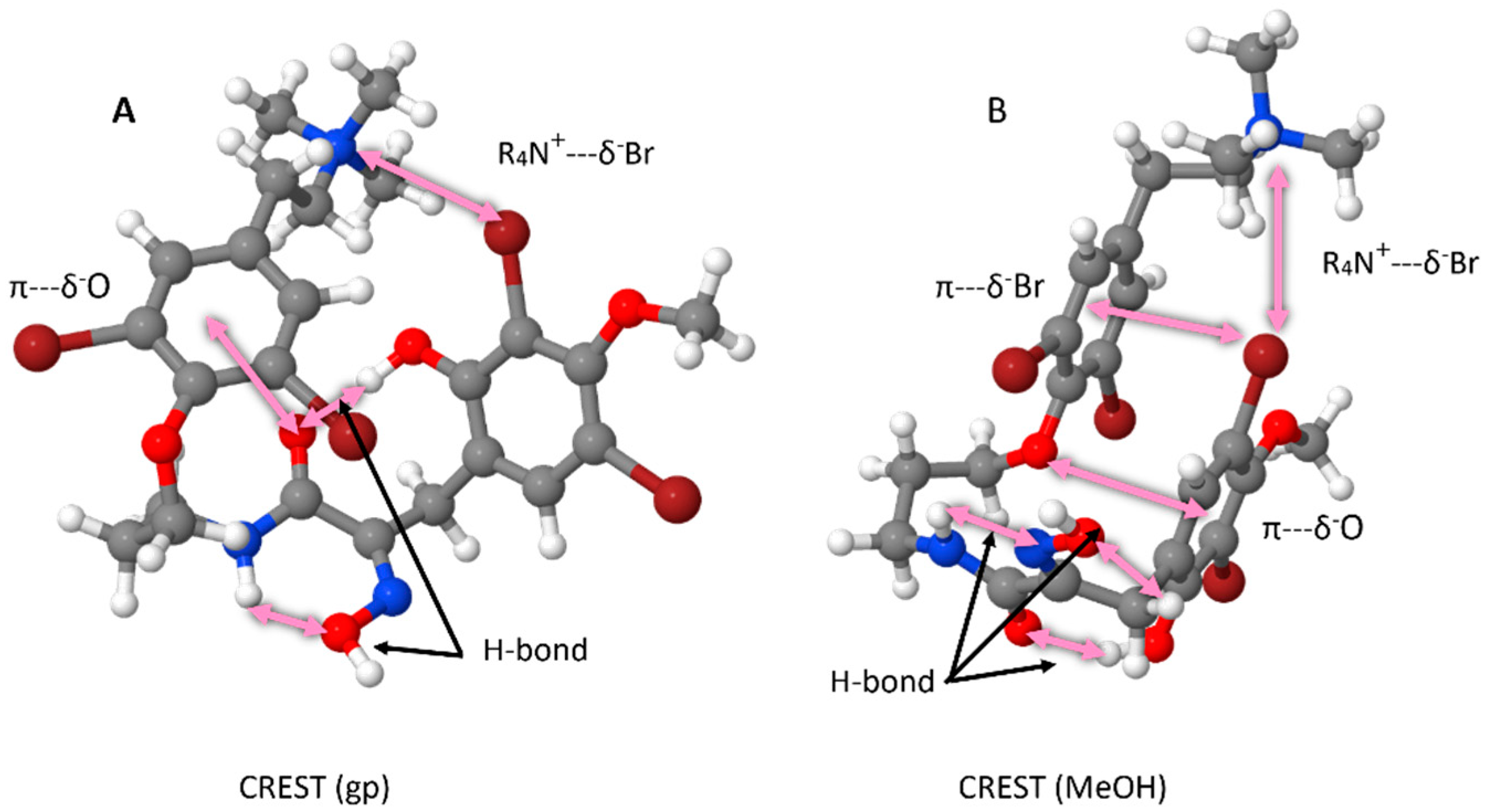
2.2. Conformational Analysis of Purpuramine R (1)
2.3. Bioactivity of Purpuramine R (1)
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Sponge Material
3.3. Isolation of Purpuramine R (1)
3.4. Computational Chemistry
3.5. Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Davis, R.A.; Feng, Y.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines A-C, antibacterial bromotyrosine-derived metabolites from the marine sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 75, 1001–1005. [Google Scholar] [CrossRef]
- Calcul, L.; Inman, W.D.; Morris, A.A.; Tenney, K.; Ratnam, J.; McKerrow, J.H.; Valeriote, F.A.; Crews, P. Additional insights on the bastadins: Isolation of analogues from the sponge Ianthella cf. reticulata and exploration of the oxime configurations. J. Nat. Prod. 2010, 73, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Quiñoá, E.; Crews, P. Phenolic constituents of Psammaplysilla. Tetrahedron Lett. 1987, 28, 3229–3232. [Google Scholar] [CrossRef]
- Hooper, J.N.A.; Soest, R.W.M.; Willenz, P. Systema Porifera A Guide to the Classification of Sponges; Springer: Berlin, Germany, 2002; Volume 1. [Google Scholar]
- El-Demerdash, A.; Atanasov, A.G.; Horbanczuk, O.K.; Tammam, M.A.; Abdel-Mogib, M.; Hooper, J.N.A.; Sekeroglu, N.; Al-Mourabit, A.; Kijjoa, A. Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review. Mar. Drugs 2019, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Han, X.; Mi, Y.; Cui, Y.; Fu, A.; Liu, K.; Li, X.; Tang, X.; Li, G. Anti-inflammatory and cytotoxicity nitrogenous merosesquiterpenoids from the sponge Pseudoceratina purpurea. Phytochemistry 2024, 226, 114220. [Google Scholar] [CrossRef]
- Yu, X.; Han, X.; Cui, Y.; Fu, A.; Liu, K.; Zhang, W.; Tang, X.; Li, G. Pseudoceranoids A-J, Sesquiterpene-Based Meroterpenoids with Cytotoxicity from the Sponge Pseudoceratina purpurea. J. Nat. Prod. 2023, 86, 2710–2717. [Google Scholar] [CrossRef]
- Gotsbacher, M.P.; Karuso, P. New antimicrobial bromotyrosine analogues from the sponge Pseudoceratina purpurea and its predator Tylodina corticalis. Mar. Drugs 2015, 13, 1389–1409. [Google Scholar] [CrossRef]
- Jang, J.H.; van Soest, R.W.; Fusetani, N.; Matsunaga, S. Pseudoceratins A and B, antifungal bicyclic bromotyrosine-derived metabolites from the marine sponge Pseudoceratina purpurea. J. Org. Chem. 2007, 72, 1211–1217. [Google Scholar] [CrossRef]
- Taufa, T.; Subramani, R.; Northcote, P.T.; Keyzers, R.A. Natural Products from Tongan Marine Organisms. Molecules 2021, 26, 4534. [Google Scholar] [CrossRef]
- Taufa, T.; Singh, A.J.; Harland, C.R.; Patel, V.; Jones, B.; Halafihi, T.; Miller, J.H.; Keyzers, R.A.; Northcote, P.T. Zampanolides B-E from the Marine Sponge Cacospongia mycofijiensis: Potent Cytotoxic Macrolides with Microtubule-Stabilizing Activity. J. Nat. Prod. 2018, 81, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Bracegirdle, J.; Gordon, D.P.; Harvey, J.E.; Keyzers, R.A. Kinase-Inhibitory Nucleoside Derivatives from the Pacific Bryozoan Nelliella nelliiformis. J. Nat. Prod. 2020, 83, 547–551. [Google Scholar] [CrossRef]
- Bracegirdle, J.; Robertson, L.P.; Hume, P.A.; Page, M.J.; Sharrock, A.V.; Ackerley, D.F.; Carroll, A.R.; Keyzers, R.A. Lamellarin Sulfates from the Pacific Tunicate Didemnum ternerratum. J. Nat. Prod. 2019, 82, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Woolner, V.H.; Gordon, R.M.A.; Miller, J.H.; Lein, M.; Northcote, P.T.; Keyzers, R.A. Halogenated Meroditerpenoids from a South Pacific Collection of the Red Alga Callophycus serratus. J. Nat. Prod. 2018, 81, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, P.R. Dictyoceratida, Dendroceratida and Verongida from the New Caledonia Lagoon (Porifera: Demospongiae). Mem. Qld. Mus. 1995, 38, 1–51. [Google Scholar]
- Morris, S.A.; Andersen, R.J. Nitrogenous Metabolites from the Deep Water Sponge Hexadella sp. Can. J. Chem. 1989, 67, 677–681. [Google Scholar] [CrossRef]
- Dai, J.; Parrish, S.M.; Yoshida, W.Y.; Yip, M.L.R.; Turkson, J.; Kelly, M.; Williams, P. Bromotyrosine-derived metabolites from an Indonesian marine sponge in the family Aplysinellidae (Order Verongiida). Bioorg. Med. Chem. Lett. 2016, 26, 499–504. [Google Scholar] [CrossRef]
- Arabshahi, L.; Schmitz, F.J. Brominated tyrosine metabolites from an unidentified sponge. J. Org. Chem. 1987, 52, 3584–3586. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-Binding Quantum Chemistry Methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Moriou, C.; Lacroix, D.; Petek, S.; El-Demerdash, A.; Trepos, R.; Leu, T.M.; Florean, C.; Diederich, M.; Hellio, C.; Debitus, C.; et al. Bioactive Bromotyrosine Derivatives from the Pacific Marine Sponge Suberea clavata (Pulitzer-Finali, 1982). Mar. Drugs 2021, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, J.; Hamann, M.T. The marine bromotyrosine derivatives. Alkaloids Chem. Biol. 2005, 61, 59–262. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Shaala, L.A. Psammaplysins: Insights from Natural Sources, Structural Variations, and Pharmacological Properties. Mar. Drugs 2022, 20, 663. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Marmann, A.; Lin, W.; Proksch, P. Sponge derived bromotyrosines: Structural diversity through natural combinatorial chemistry. Nat. Prod. Commun. 2015, 10, 219–231. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M.; Agemi, K.; Shigemori, H.; Ishibashi, M.; Sasaki, T.; Mikami, Y. Purealidins B and C, New Bromotyrosine Alkaloids from the Okinawan Marine Sponge Psammaplysilla pupurea. Tetrahedron 1991, 47, 6617–6622. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView; Version 6.1.1; Semichem Inc.: Shawnee Mission, KS, USA, 2019. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Version C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- CHEmical SHIft REpository with Coupling Constants Added Too. Available online: http://cheshirenmr.info/index.htm (accessed on 1 August 2023).
- Expanding the Limits of Computational Chemistry. Available online: https://gaussian.com/uvvisplot/ (accessed on 1 July 2023).
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All spd-Block Elements (Z = 1-86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Montenegro, P.; Pham, G.; Abdoul-Latif, F.; Taffin-de-Givenchy, E.; Mehiri, M. Marine Bromotyrosine Derivatives in Spotlight: Bringing Discoveries and Biological Significance. Mar. Drugs 2024, 22, 132. [Google Scholar] [CrossRef]


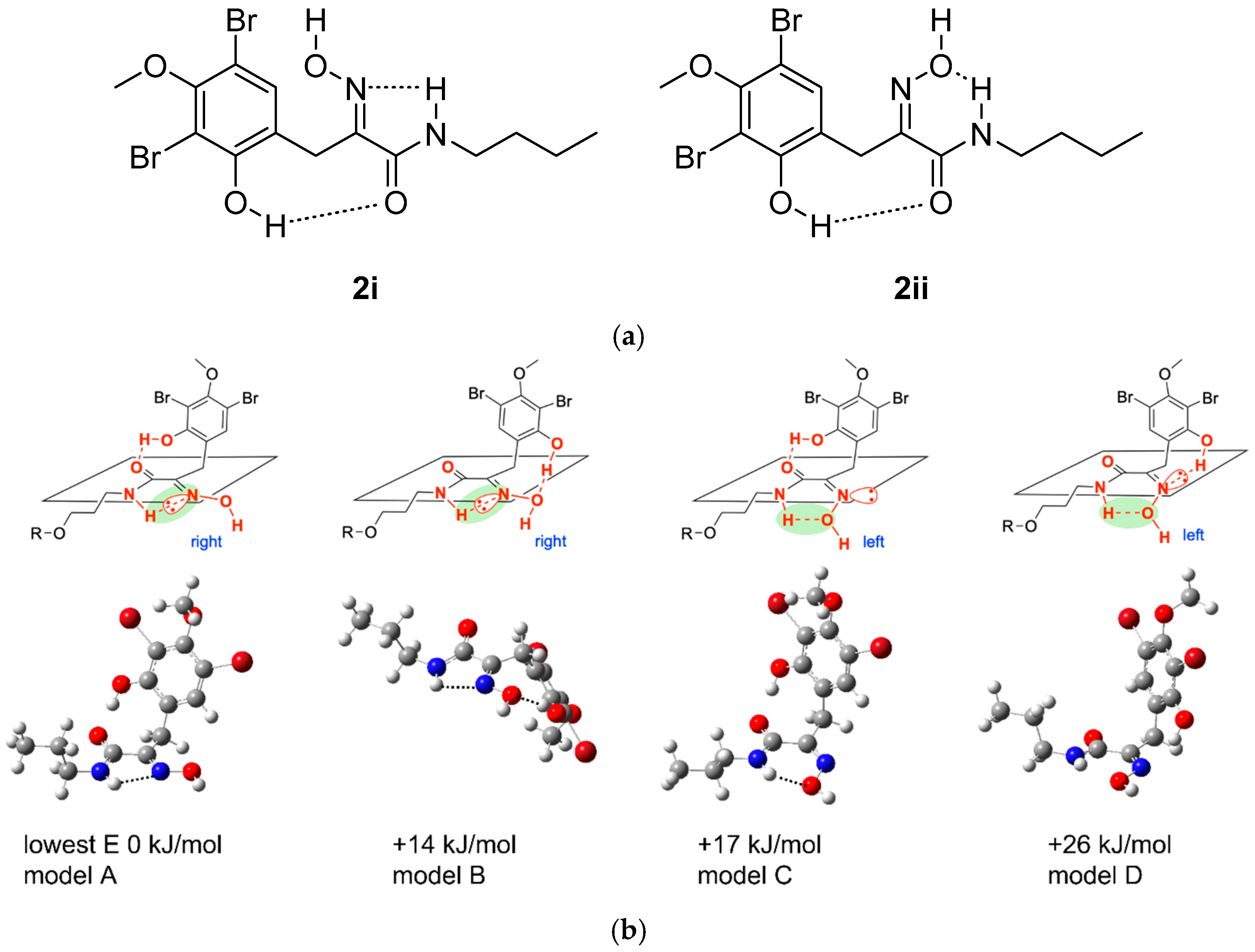
| Oxime Configuration | C-7 | C-8 | C-9 | C-10 | MAE a | |
|---|---|---|---|---|---|---|
| Experimental | 25.6 | 154.9 | 166.8 | 37.9 | 0.00 | |
| Model A | E | 25.8 | 153.6 | 164.0 | 42.7 | 2.33 |
| Model B | E | 25.5 | 157.8 | 160.3 | 42.3 | 4.13 |
| Model C | Z | 34.4 | 153.0 | 159.8 | 44.2 | 5.89 |
| Model D | Z | 34.3 | 154.4 | 157.3 | 42.7 | 6.36 |
| Position | 13C | 1H | ||||
|---|---|---|---|---|---|---|
| δ (ppm) | Type | 1JC,H (Hz) | δ (ppm) | Mult. | J (Hz) | |
| 1 | 123.0 | C | - | - | ||
| 2 | 151.7 a | C | - | - | ||
| 3 | 108.8 a | C | - | - | ||
| 4 | 154.7 a | C | - | - | ||
| 5 | 106.8 a | C | - | - | ||
| 6 | 134.5 | CH | 162 | 7.42 | s | |
| 7 | 25.6 | CH2 | 137 | 3.81 | s, 2H | |
| 8 | 154.9 a | C | - | - | ||
| 9 | 166.8 a | C | - | - | ||
| 10 | 37.9 | CH2 | 134 | 3.58 | t, 2H | 6.7 |
| 11 | 30.5 | CH2 | 126 | 2.10 | quin, 2H | 6.4 |
| 12 | 72.2 | CH2 | 148 | 4.02 | t, 2H | 5.9 |
| 13 | 153.5 a | C | - | - | ||
| 14/18 | 119.5 | C | - | - | ||
| 15/17 | 134.6 | CH | 170 | 7.58 | s, 2H | |
| 16 | 136.0 a | C | - | - | ||
| 19 | 28.8 | CH2 | 128 | 3.09 | m, 2H | |
| 20 | 67.7 | CH2 | 148 | 3.54 | m, 2H | |
| 21 | 53.6–53.7 | N+(CH3)3 | 148 | 3.20 | s, 9H | |
| 22 | 60.8 | CH3 | 145 | 3.80 | s, 3H | |
| S. aureus ATCC 25923 (µg/mL) | E. coli W3110 (µg/mL) | E. coli W3110 ΔtolC (µg/mL) | K. pneumoniae KPLN49 (µg/mL) | |
|---|---|---|---|---|
| Purpuramine R (1) | 16 (21.1) | >32 (42.2) | 16 (21.1) | >32 (42.2) |
| Gentamicin (positive control) | 0.25 (0.52) | 0.5 (1.05) | 0.5 (1.05) | >32 (42.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Garcia, J.L.; Lee-Harwood, H.; Ackerley, D.; Kelly, M.; Matoto, S.V.; Hunt, P.; Singh, A.J.; Keyzers, R.A. Purpuramine R, a New Bromotyrosine Isolated from Pseudoceratina cf. verrucosa Collected in the Kingdom of Tonga. Mar. Drugs 2025, 23, 186. https://doi.org/10.3390/md23050186
Ramirez-Garcia JL, Lee-Harwood H, Ackerley D, Kelly M, Matoto SV, Hunt P, Singh AJ, Keyzers RA. Purpuramine R, a New Bromotyrosine Isolated from Pseudoceratina cf. verrucosa Collected in the Kingdom of Tonga. Marine Drugs. 2025; 23(5):186. https://doi.org/10.3390/md23050186
Chicago/Turabian StyleRamirez-Garcia, Jennie L., Hannah Lee-Harwood, David Ackerley, Michelle Kelly, S. Vailala Matoto, Patricia Hunt, A. Jonathan Singh, and Robert A. Keyzers. 2025. "Purpuramine R, a New Bromotyrosine Isolated from Pseudoceratina cf. verrucosa Collected in the Kingdom of Tonga" Marine Drugs 23, no. 5: 186. https://doi.org/10.3390/md23050186
APA StyleRamirez-Garcia, J. L., Lee-Harwood, H., Ackerley, D., Kelly, M., Matoto, S. V., Hunt, P., Singh, A. J., & Keyzers, R. A. (2025). Purpuramine R, a New Bromotyrosine Isolated from Pseudoceratina cf. verrucosa Collected in the Kingdom of Tonga. Marine Drugs, 23(5), 186. https://doi.org/10.3390/md23050186








