Abstract
In screening endophytic fungi from Costa Rica for bioactivity, fungal culture CR200, isolated from a buttonwood tree, was found to contain compounds that initiate DNA damage in a test strain of E. coli (Biochemical Induction Assay, BIA) and inhibit growth of Gram-positive bacteria, including antibiotic-resistant strains. Two new bisanthraquinones (cytoskyrins A and B) and five new related octaketides (cytosporones A-E) were isolated from fermentation broths of this fungus. Cytoskyrin A exhibited potent in-vitro antibacterial (MICs against Gram-positive bacteria, 0.03 – 0.25 μg/mL) and DNA-damaging activities (10 ng/spot), whereas cytoskyrin B was inactive in these assays. Among the cytosporones, only D and E exhibited Gram-positive activity, but they were inactive in the BIA. Mechanistically, cytoskyrin A specifically inhibited DNA synthesis in E. coli imp at its MIC; however, it also moderately inhibited protein synthesis at 2x its MIC. Cytoskyrin A exhibited poor cytotoxicity against tumor cell lines (IC50 > 5 μg/mL) compared to known antitumor agents. The nuclear ribosomal internal transcribed spacer region of CR200 was found to share highest similarity (94–96%) with Cytospora spp. Micro- and macroscopic morphological observations of the conidia and conidiomata, respectively, also suggested this fungus to be a Cytospora sp.
1. Introduction
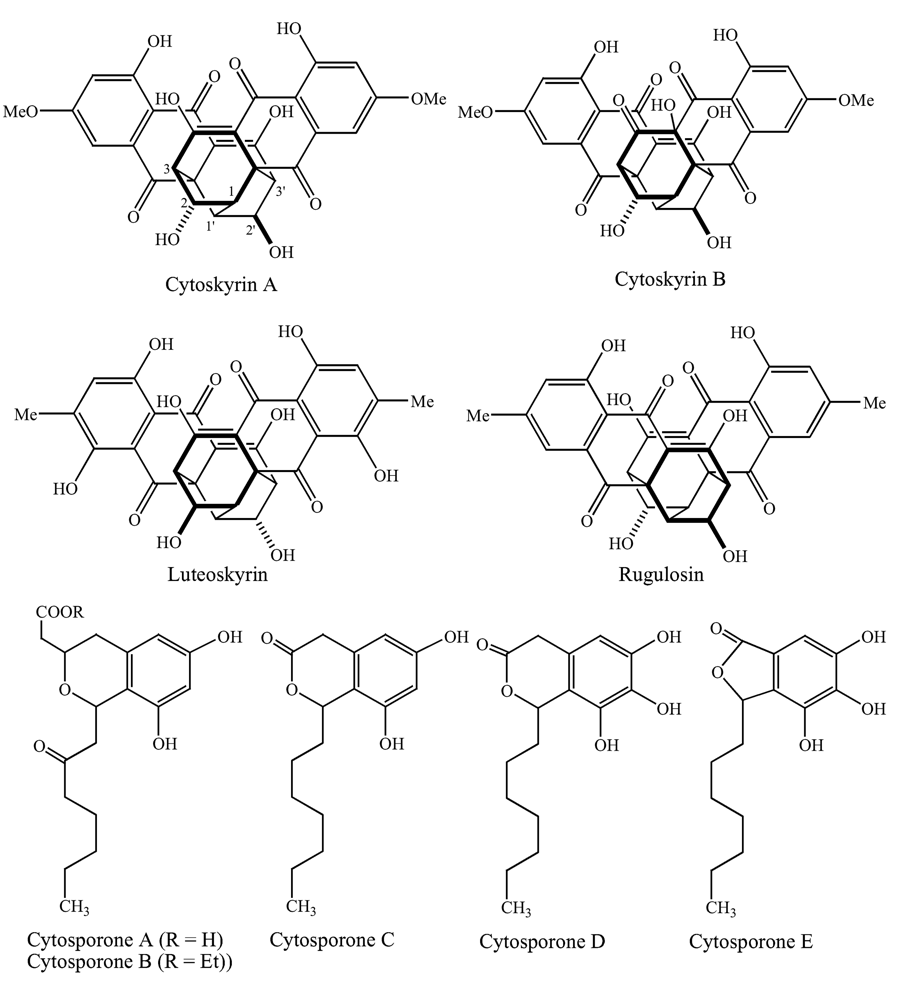
Fungi, one of the most diverse groups of organisms, are known to produce a wide variety of medically important metabolites. Of the 1.5 million species estimated to be in existence only a small fraction (ca. 1%) have been studied [1]. In our continued efforts to discover novel compounds from filamentous fungi, culture CR200, obtained from a Costa Rican buttonwood tree, was found to be active in the Biochemical Induction Assay (BIA), which detects compounds that initiate DNA damage [2] and against a panel of antibiotic-resistant bacteria. As previously reported, two new bisanthraquinones (cytoskyrins A and B, Figure 1) and five new related octaketides (cytosporones A-E) were isolated from fermentation broths of this culture [3, 4]. Since then similar bisanthraquinones have been isolated from a number of different fungi [5, 6] and even synthesized [7–10]. In this paper, we report the biological activities of the isolated compounds as well as the taxonomy and fermentation of CR200. Some of these findings were previously presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapeutic Agents and the Society for Industrial Microbiology meetings [11, 12].
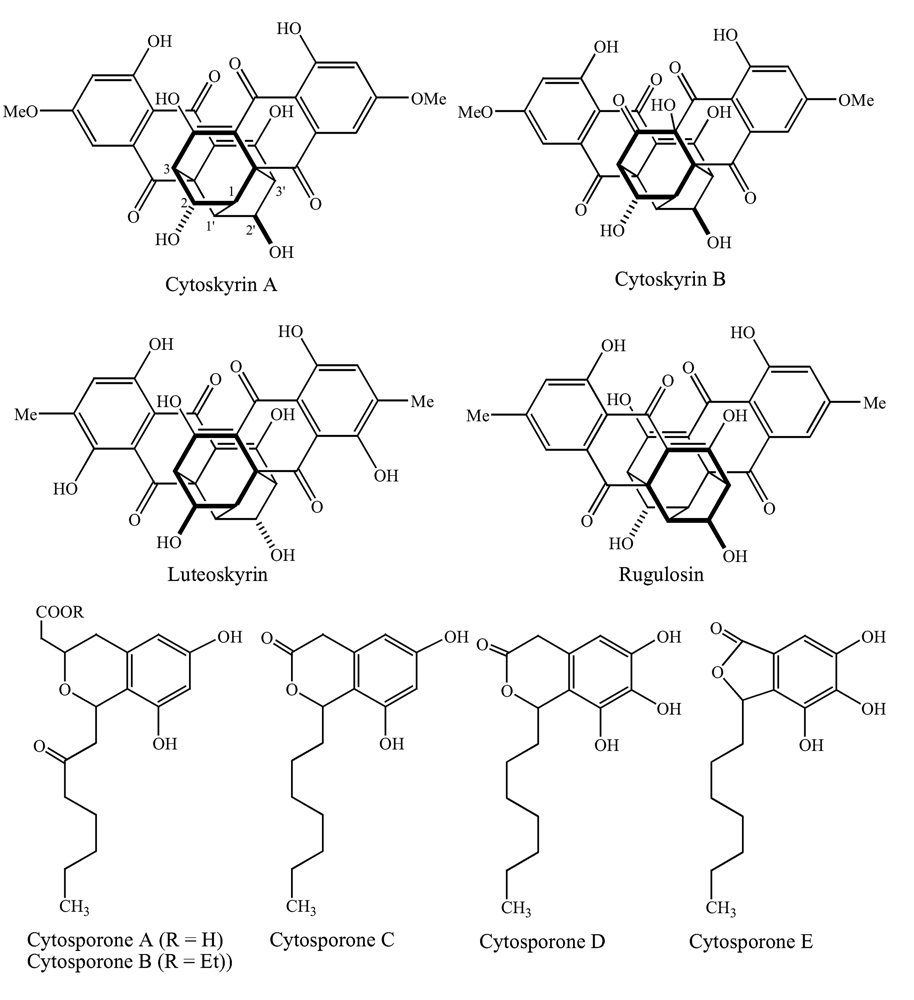
Figure 1.
Chemical structures of cytoskyrins and related bisanthraquinones, and cytosporones.
2. Results and Discussion
Culture Morphology
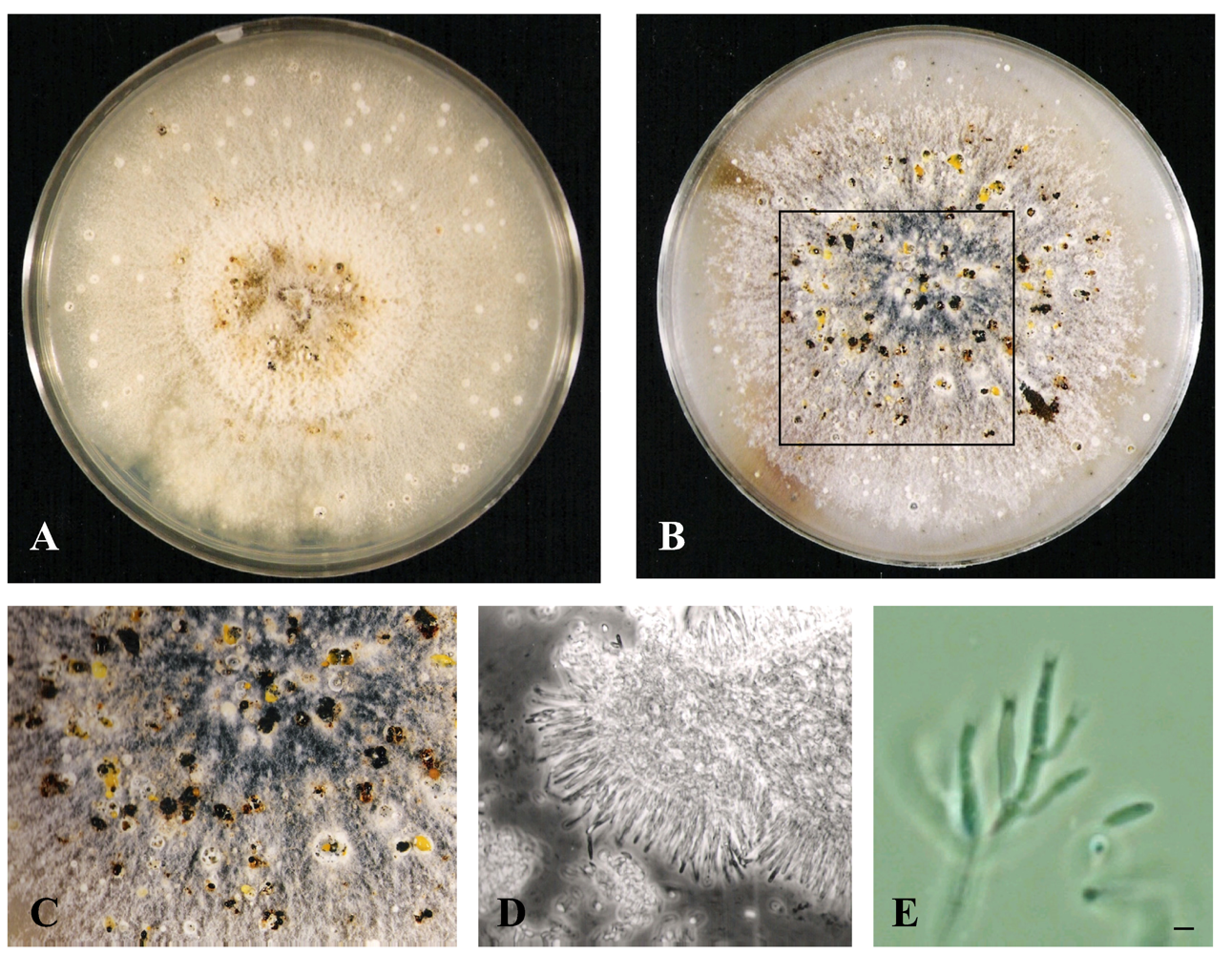
Growth of CR200 on potato dextrose agar (PDA), cornmeal agar (CMA) and Leonian’s agar (LA) were examined. The organism grew rather well on all three media, but the most differentiation and texture occurred on PDA (Table 1). CR200 grew robustly on PDA at 22°C reaching a diameter of 74 mm after only 7 days of growth and a maximum of 81 mm by 14 days (Figure 2A). Surface morphology was generally floccose to felty throughout the incubation period. Colony color progressed from white to taupe and buff with some yellow-brown at the colony center. Conidiomata (pycnidia) were visible by day 14 as white to light brown dots partially submerged into the agar (Figure 2A). By the 21st to 28th days, some conidiomata appeared dark brown (Figures 2B). About five to ten percent of them oozed a spore mass as yellow cirrhi in globules (Figures 2C) while others leaked a clear to brownish exudate. The conidiomata displayed elongated necks/beaks, some of them with extensive branching after an extended incubation of six weeks to several months.

Table 1.
Morphological characteristics in different fungal media.
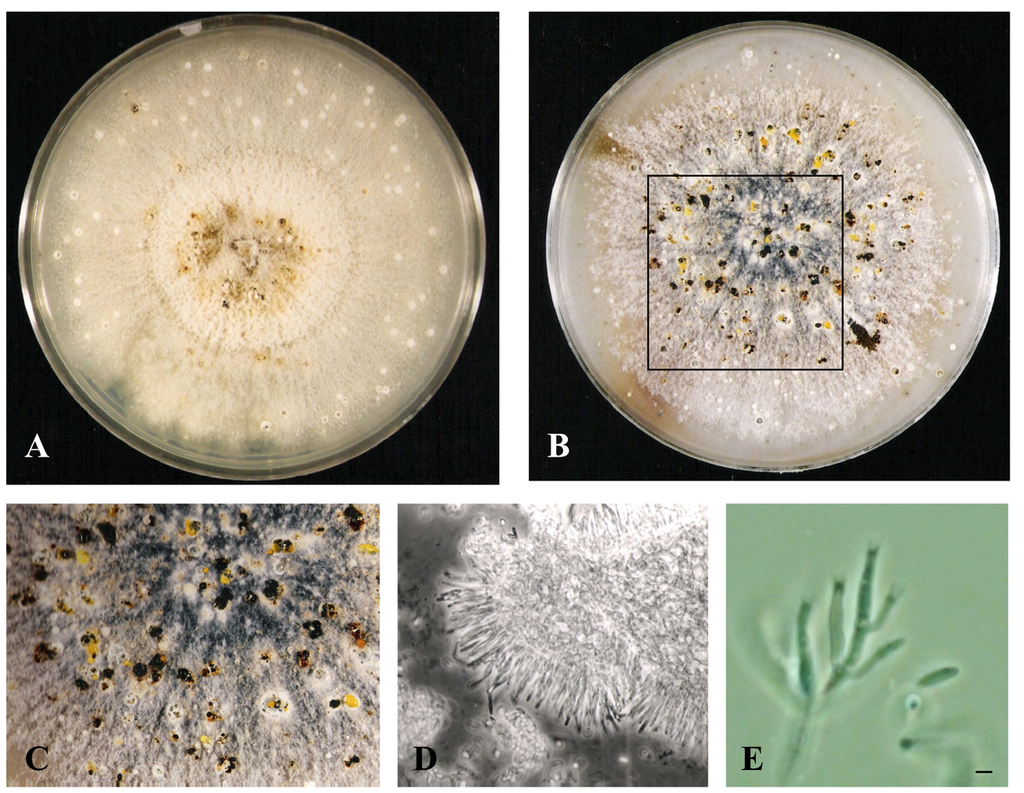
Figure 2.
Morphological characteristics of culture CR200 grown on PDA for 14 days (A), PDA for 28 days (B), enlarged view of the boxed area on B (C), CR200 phase contrast image of conidioma excised from a 28 day culture (D), and phase contrast image of conidiogenous cells with collerettes and a conidium (bar = 2.0 μm) (E).
CR200 grew most rapidly on LA, but remained flat or submerged in the agar. Mycelia were colorless or translucent becoming light olive-gray by day 14. Conidiomata also formed on LA by day 14, but were completely submerged, olive-gray and larger than those that formed on PDA.
CR200 grew more slowly on all three media at 33°C. The growth stress at the higher temperature was indicated by altered morphologies. On PDA, a red-brown to brown soluble pigment was produced, while on LA both the surface and reverse displayed red-brown to brown pigmentation. Conidiomata did not form on any media at 33°C. Conidiophores were hyaline, unbranched to highly branched at the base and/or mid-height and formed a continuous layer (Figure 2D, E). Conidiogenous cells were enteroblastic, phialidic, tapering to the apices with collerettes up to 2 μm long (Figure 2E). Conidia were hyaline, allantoid and measured about 5 to 6 μm long and 1.5 μm wide (Figure 2E).
Phylogenetics
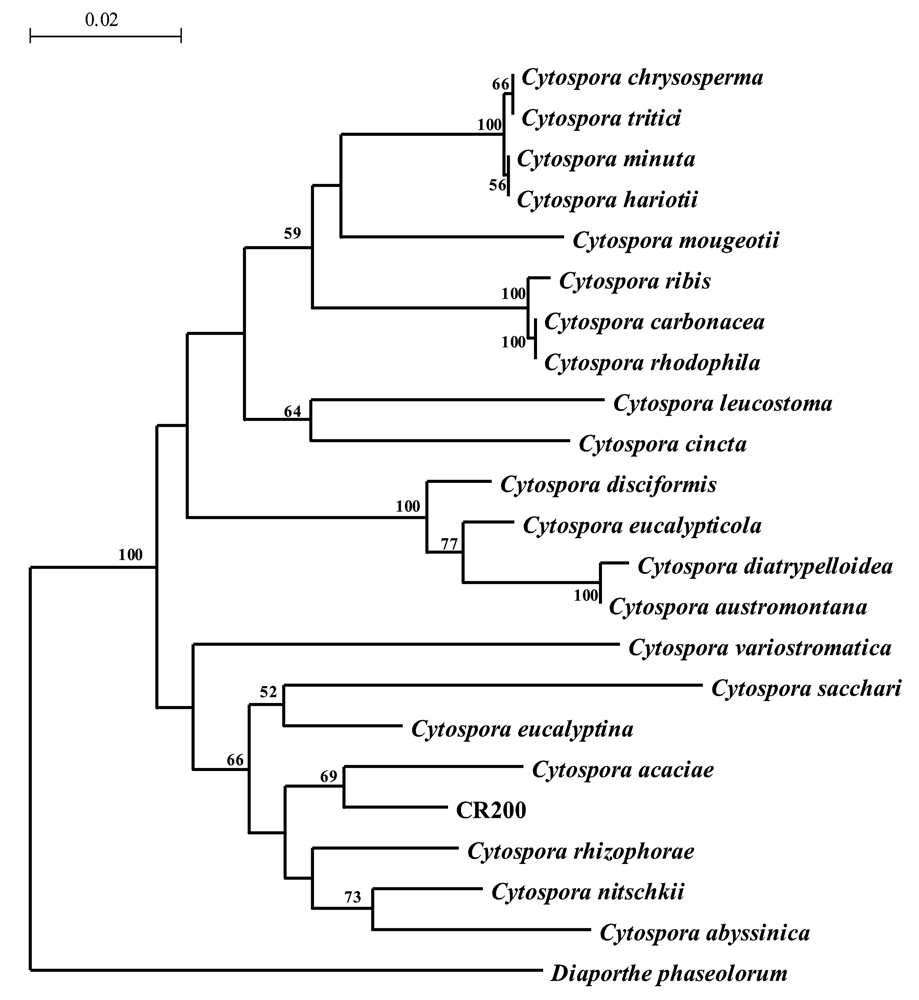
The ITS of CR200 was found to be 517 bp in length. A BLAST search of the ITS of CR200 revealed that it is most similar to C. acaciae (96%). A phylogenetic tree (Figure 2) of the ITS of 22 Cytospora spp. placed CR200 in a clade with C. acaciae, C. rhizophorae, C. nitschkii, and C. abyssinica. However, CR200 branched deeply from C. acaciae (supported by 69 bootstrap replicates), indicating that it is a distinct species.
Fermentation
Production of cytoskyrin A as measured by BIA activity was quite heterogeneous in the 100-flask fermentation. Approximately 87% of the flask fermentations were active in the BIA, with 42% displaying very good activity and 45% with fair to poor activity. The remaining 13% of the flask fermentations did not exhibit activity in the BIA, suggesting no production of cytoskyrin A. Separation of the cells from the broth and extraction of both with ethyl acetate revealed that the majority of cytoskyrin A was associated with the cells, but is also present in the fermentation broths. The higher shaking speed of 200 rpm produced activity detected by the BIA sooner than the lower shaking speed of 100 rpm, whether incubated at 22°C or 28°C. Interestingly, BIA activity was not detected from the whole broths of CR200 fermented at 22°C and 100 rpm, even after 21 days of incubation.
Biological activities
DNA-damaging activities of the CR200 fermentation broth and pure compounds were tested by the biochemical induction assay (BIA). Cytoskyrin A demonstrated a minimum inducing concentration of 12 ng/spot in this assay, suggesting this compound as a potent DNA-damaging agent in bacteria (Table 2). Related bisanthraquinones such as rugulosin and luteoskyrin, known DNA intercalating agents, were toxic but did not induce the positive BIA response under the used assay conditions. These agents exhibit induction in the BIA only after a prolonged preincubation period. Cytoskyrin B, also produced by CR200, was inactive in the BIA. Cytoskyrin A appears to have the structural requirements to exhibit potent DNA-damaging activity in bacteria. Cytoskyrin A also exhibited excellent activity against the Gram-positive bacteria and the E. coli imp. However, it did not have activity against other Gram-negative bacteria, indicating that it may have difficulty crossing the outer membrane barrier (Table 3). Cytoskyrin B was inactive against the bacterial isolates tested (Tables 2 and 3). Minor differences in the chemical structures appear to affect the bioactivity of this class of compound (Figure 1). Among the five novel cytosporones A-E isolated from the CR200 fermentations, only D and E were active against Gram-positive bacteria and yeast (Table 3).

Table 2.
BIA activity of Cytoskyrins and known anticancer drugs.

Table 3.
Antimicrobial Activity of Cytoskyrins and Cytosporones.
Inhibition of DNA, RNA, and protein synthesis was determined by measuring the incorporation of 3H-Tdr, 3H-Udr, and 3H-AA, respectively, into TCA-precipitable material of a logarithmic-phase culture of E. coli imp. Control drugs affected the anticipated macromolecular processes (Table 4). Cytoskyrin A specifically inhibited the incorporation of 3H-thymidine into DNA (Table 4). Incorporation of amino acids into protein was also affected at higher concentrations. Based on the BIA activity and the specific inhibition of DNA synthesis in E. coli imp, cytoskyrin A appears to be a potent DNA-damaging agent. Since DNA-damaging agents also constitute an important class of anticancer drugs, cytoskyrin A was tested for antiproliferative activity against four human tumor cell lines and it was found to have moderate cytoxicity with IC50 values in the range of 4 to 24 μg/mL (Table 5).

Table 4.
Effects on the incorporation of radiolabeled precursors into macromolecules in E. coli imp.

Table 5.
Cytotoxicity (IC50 in μg/mL) of Cytoskyrin A and known antitumor agents.
3. Conclusions
The cytoskyrins and cytosporones isolated from Cytospora sp. CR200 were first disclosed as novel compounds in 2000 by Brady et al [3,4]. Since then other researchers have reported the production of similar compounds by several different fungi [5, 6]. Unlike the related compound luteoskyrin, cytoskyrin A exhibited potent activity in the bacterial BIA and is therefore capable of directly or indirectly initiating DNA damage. Selective inhibition of DNA synthesis (measured by the incorporation of radiolabeled thymidine) in E. coli further confirmed its mode of action. Unfortunately, cytoskyrin A was less toxic to human cell lines than luteoskyrin and could not be used as an anticancer agent. Although luteoskyrin and cytoskyrins are chemically related, the mechanisms-of-action of these compounds appear to be different in prokaryotic and eukaryotic cells.
CR200 was isolated as an endophyte from a tissue sample of a branch of Conocarpus erecta. Although the fruiting structures of this fungus were never observed on its host, morphology of CR200 in culture and phylogenetic analysis of the ITS identified CR200 as a Cytospora sp. Further classification of CR200 to the species level would be difficult because no comprehensive keys are available for distinguishing Cytospora spp. in culture. Adams et al. [21] have shown that sequencing and comparative analysis of the ITS can be important to the identification of Cytospora spp., yet ITS data exists for less than 30 of the more than 300 species of Cytospora that have been described. Considering the importance of these fungi in the production of useful natural products such as antibiotics [22], angiotensin inhibitors [23] and HIV integrase inhibitors [24] efforts to sequence the ITS of validly described species would greatly aid in the future classification of Cytospora spp.
4. Experimental
Morphological Taxonomy
Fungal culture CR200 was isolated from a branch of Conocarpus erecta (Buttonwood tree) in the Guanacaste National Park, Costa Rica (collection permit number 246.94). CR200 was streaked on Difco potato dextrose agar (PDA) and grown for 7–14 days at 22 ºC. Mycelia and conidia were washed with potato dextrose broth (PDB) containing 25 % glycerol, and 1 mL aliquots were transferred into cryovials for long-term storage at -140ºC at Wyeth Research, Pearl River, NY. An additional copy is kept at the repository of the National Institute of Biodiversity, INBio Code 12652.
For macroscopic morphology, an agar plug of CR200 was placed in duplicate onto PDA, cornmeal agar (CMA, Oxoid) and Leonian’s agar (LA, 1.2 g KH2PO4, 0.6 g MgSO4, 6.25 g maltose, 6.25 g Difco malt extract, 15.0 g Bacto agar/L of distilled water) and the culture plates were incubated at 22 °C and 33 °C. Colony morphology and size (in mm) were noted on the 7th, 14th, and 21st days of incubation. For microscopic analysis, CR200 was grown on PDA for 21 days. The conidiomata were observed at 1x to 6.3x magnification under an Olympus brand stereoscope. A smash prep was prepared by aseptic excision of several conidiomata, which were placed on a microscope slide with water (10 μL) and gently flattened with a coverslip. Microscopic observations were performed with an Olympus B071 phase-contrast microscope at 50x and 1,250x magnification.
Molecular Taxonomy
Isolation of the genomic DNA, PCR amplification and direct sequencing of the nuclear ribosomal ITS1-5.8S-ITS2 region have been described previously [13]. Briefly, mycelia (50–100 mg) were lysed in lysis buffer (0.5 mL, 2% Triton X-100, 1% SDS, 100 mM NaCl, 10 mM Tris pH 8.0, 1 mM EDTA). Proteins were precipitated by the addition of 25:24:1 phenol/chloroform/isoamyl alcohol (0.5 mL, Sigma). The aqueous and organic layers were separated by centrifugation at 14,000 rpm for 5 minutes. The aqueous layer was pipetted into a new microfuge tube and the DNA was precipitated by addition of 3 M sodium acetate (0.1 mL) and isopropanol (0.8 mL). The nuclear ribosomal ITS1-5.8S-ITS2 region was amplified with primers ITS1 and ITS4 [14]. Amplification of the ITS was confirmed by gel electrophoresis. The PCR product was cleaned and sequenced directly with an ABI 3700 sequencer with the ABI Prism DNA sequencing kit and Big Dye terminators version 3.0 (Applied Biosystems).
For this study, the ITS were sequenced for Cytospora sp. CR200, Cytospora cincta ATCC 32673, Cytospora chrysosperma ATCC 56609, Cytospora leucostoma ATCC 42549 (deposited as Leucostoma persoonii), Cytospora rhizophorae ATCC 38475, and Cytospora sacchari ATCC 32322 and assigned the following GenBank accessions: DQ996039-DQ996044.
To determine the most closely related fungi, the ITS of CR200 was compared to other sequences in the GenBank database by BLASTN 2.2.2 analysis. The ITS of CR200 and several Cytospora spp. (Table 6) were aligned in Clustal X 1.81 with the multiple alignment parameters set at default values. A phylogenetic tree (Figure 2) was generated in TREECON 1.3b [15] with the distances calculated according to the Jukes and Cantor [16] method and insertions and deletions not taken into account. Bootstrap values were calculated using 1,000 bootstrap samples, and neighbor-joining was used to infer tree topologies. The phylogenetic tree was rooted with Diaporthe phaseolorum.

Table 6.
Cytospora spp. used in the phylogenetic analysis.
Fermentation
Fermentation of CR200 for the production of the cytoskyrins was performed in a 6-liter batch. For seed fermentation, culture mycelia grown for seven days on Bennetts’ agar (pH 7.0, containing per liter: dextrose 10 g, beef extract 0.77 g, yeast extract 1 g, NZ amine 2 g, agar 15 g) were scraped into thirty 25x150 mm Pyrex culture tubes each containing PDB (11 mL). Seed tubes were incubated for four days at 22 °C and 160 rpm. On the fourth day the contents of the seed tubes were combined into a sterile vessel and gently macerated to obtain a homogeneous inoculum. One hundred Erlenmeyer flasks (250 mL), each containing sterile PDB (60 mL) were inoculated with the seed culture (5% v/v), and fermented for 21 days at 24 °C and 200 rpm. Additional flasks (five flasks for each condition) were inoculated to study the effects of temperature (22 °C versus 28 °C) and aeration (shaking at 100 rpm versus 200 rpm) on the production of cytoskyrin A, which was measured by testing whole broths in the BIA. Fermentation of CR200 for the production of the cytosporones was performed in a 10-liter batch. First-stage seed conditions were the same as described above except that only three seed tubes were inoculated. For the second stage, the contents of the seed tubes were added to PDB (500 mL in a 2.8 L Fernbach flask) and incubated at 22°C, 200 rpm for three days. For production fermentation, 10 L of PDB in a 10 L bioreactor (Bioflo 3000, New Brunswick Scientific) were inoculated at 5% v/v with second-stage and incubated at 24 °C, 350 rpm for 15 days.
Bioactivities
In vitro Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations (MICs) were determined by the microbroth dilution method [17, 18]. Briefly, inocula were adjusted to a density of 1x107 to 5x107 CFU/mL and 5 μL were added to minimal medium or Mueller-Hinton broth (MHB) (100 μL, containing the drug at concentrations ranging from 0.03 to 128 μg/mL) in the wells of a microtiter plate. The MIC was defined as the lowest concentration of the antibiotic that prevented visual turbidity after 18 h of incubation at 37 °C.
Biochemical Induction Assay (BIA)
A slightly modified version of the previously described agar plate BIA method was used [2]. Briefly, an overnight culture of E. coli BR513 grown in LBE broth (100 mL/500 mL Erlenmeyer flask) at 37 °C and 200 rpm was inoculated into molten soft agar (40 mL, 1 %) at 50 °C to give a final A600 of 0.15 (1–3 x 107 cfu/mL). The inoculated soft agar was immediately poured onto a LBE agar base layer previously poured into a 9″x9″ Nunc plate. After 15 min, five μL of each of the two-fold serially diluted solutions of the test compounds were spotted on the agar surface. The plate was incubated at 37 °C for 3.5 h and then overlayed with soft-agar (40 mL), containing fast blue RR (87 mg) and 6-bromo-2-naphthyl-β-D-galactopyranoside (BNG, 13 mg). A red-violet color produced around the sample spot was read as a positive response.
Incorporation of Radiolabeled Precursors
Macromolecular synthesis in E. coli imp, an outer membrane permeability mutant [19], was studied by measuring the incorporation of appropriate radiolabeled precursors into trichloroacetic acid (TCA)-precipitable material [18]. E. coli imp was grown at 37 °C, 200 rpm in modified minimal medium (50 mL medium/250 mL Erlenmeyer flask) to an A450 of 0.20. Aliquots (100 μL) were dispensed into microtiter wells containing antibacterial agents and the plates were incubated for 10 min at 37 °C with vigorous agitation. Cells were pulse-labeled for 5 min by adding the following radiolabeled precursors at the indicated final concentrations: 3H-thymidine (Tdr), 2 μCi/ml with 0.05 μg unlabeled thymidine/mL; 3H-Uridine (Udr), 2 μCi with 2.5 μg unlabeled uridine/mL; or 3H-amino acid mixture (AA), 2.5 μCi/mL. To determine specific incorporation into DNA, RNA, and protein, chilled (4 °C) TCA (100 μL, 10%) supplemented with unlabeled precursors (0.5 mg/mL) was added to each well, and the plate was immediately refrigerated for 1 h. The precipitate was collected on a glass fiber filter (Wallac filtermat B, Wallac 1205–404) using a Skatron 96-well cell harvester (Model 11050) programmed for a 3 sec prewet with chilled DI water, a 12 sec wash with 5% chilled TCA and a 5 sec drying cycle. Filter mats were dried for 7 min at high power in a microwave oven (Quasar, 700 Watts), solid scintillant (MeltilexB, Pharmacia 1205–402) was applied, and the isotope that was retained on the filter was quantitated in an LKB Betaplate scintillation counter (Wallac 1205). The levels of incorporation of 3H-Tdr, 3H-Udr, and 3H-AA were expressed as the percent of the untreated control.
Antiproliferative Activity
Four human carcinoma cell lines, A431 (epidermoid carcinoma), SKBR3 (breast carcinoma), MDA-MB-435 (breast carcinoma), and SW620 (colon carcinoma) were used for cell proliferation assay. All cell lines were obtained from the American Type Culture Collection (ATCC). Cells were maintained in RPMI-1640 medium supplemented with 5% fetal bovine serum. Cells were plated in 96-well plates at the densities of 5.0x 104/mL. On the next day, compounds were dosed at 0.5, 5, 50, 500, and 5000 ng/mL range and cultured for 2 days. At the end of incubation, cell survival was determined by the sulforhodamine B assay as previously described [20]. The IC50 values were obtained from the growth curves.
Acknowledgements
We thank Drs. M. Greenstein, W. M. Maiese and G. Carter at Wyeth Research, Pearl River, NY and Dr. J. Clardy, Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA for their guidance and support. We thank for Dr. Y. Wang, Wyeth Research, for providing the antiproliferative data. We also thank Dr. Gerard Adams, Department of Plant Pathology, Michigan State University, East Lansing, MI for the sequencing and depositing of many ITS sequences of Cytospora spp into GenBank. The work at Harvard Medical School was funded by NIH CA067786 (JC) and NIH CA24487 (JC).
References and Notes
- Hawksworth, DL. The fungal dimension of biodiversity: magnitude, significance and conservation. Mycol Res 1991, 95, 641–655. [Google Scholar]
- Greenstein, M; Wildey, MJ; Maiese, WM. Borders, DB, Doyle, TW, Eds.; The biochemical induction assay and its application in the detection of the calicheamicins. In Enediyne Antibiotics as Antitumor Agents; Marcel Dekker, Inc: New York NY, 1993; pp. 17–27. [Google Scholar]
- Brady, SF; Singh, MP; Janso, JE; Clardy, J. Cytoskyrin A and B, new BIA active bisanthraquinones isolated from an endophytic fungus. Org Lett 2000, 2, 4047–4049. [Google Scholar]
- Brady, SF; Wagenaar, MM; Singh, MP; Janso, JE; Clardy, J. The cytosporones, new octaketide cytotoxins isolated from an endophytic fungus. Org Lett 2000, 2, 4043–4046. [Google Scholar]
- Jadulco, R; Brauers, G; Edrada, RA; Ebel, R; Wray, V; Sudarsono; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J Nat Prod 2002, 65, 730–733. [Google Scholar]
- Agusta, A; Ohashi, K; Shibuya, H. Bisanthraquinone metabolites produced by the endophytic fungus Diaporthe sp. Chem Pharm Bull 2006, 54, 579–582. [Google Scholar]
- Nicolaou, KC; Papageorgiou, CD; Piper, JL; Chadha, RK. Total synthesis of (+)-rugulosin and (+)-2,2′-epi-cytoskyrin A through cascade reactions. Angew Chem Int Ed 2005, 44, 5846–5851. [Google Scholar]
- Nicolaou, KC; Lim, YH; Papageorgiou, CD; Piper, JL. Total synthesis of (+)-rugulosin and (+)-2,2′-epi-cytoskyrin A through cascade reactions. Angew Chem Int Ed 2005, 44, 7917–7921. [Google Scholar]
- Snider, BB; Gao, X. Efficient syntheses of rugulosin analogues. J Org Chem 2005, 70, 6863–6869. [Google Scholar]
- Nicolaou, KC; Lim, YH; Piper, JL; Papageorgiou, CD. Total syntheses of 2,2′-epi-cytoskyrin A, rugulosin, and the alleged structure of rugulin. J Am Chem Soc 2007, 129, 4001–4013. [Google Scholar]
- Singh, MP; Janso, JE; Luckman, SW; Brady, SF; Clardy, J; Maiese, WM; Greenstein, M. Biological and mechanistic activities of cytoskyrins and cytosporones produced by the endophytic Cytospora sp. CR200. Program and Abstracts of Papers of 40th Intersci. Conf. Antimicrob. Agents Chemother, Toronto, Canada; 2000. [Google Scholar]
- Janso, J; Luckman, SW; Hucul, J; Brady, SF; Clardy, J; Maiese, WM; Greenstein, M; Singh, MP. Cytoskyrin A and Cytosporone D: two novel compounds produced by the endophytic fungus CR200: Taxonomy, Fermentation and Bioactivity. Program and Abstracts of Papers of Soc. Indust. Microbiol. Meeting, San Diego, CA; 2000. [Google Scholar]
- Janso, J; Bernan, VS; Greenstein, M; Bugni, T; Ireland, CM. Penicillium dravuni:a new marine-derived species from an alga in Fiji. Mycologia 2005, 2, 444–453. [Google Scholar]
- White, TJ; Brun, TD; Lee, S; Taylor, J. Innis, MA, Gelfand, DH, Sninsky, JJ, White, TJ, Eds.; Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications; Academic Press: New York NY, 1990; pp. 315–322. [Google Scholar]
- Van De Peer, Y; DeWachter, R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comp Appl Biosci 1994, 10, 569–570. [Google Scholar]
- Jukes, TH; Cantor, CR. Munro, HH, Ed.; Mammalian protein metabolism; Academic Press: New York NY, 1969; pp. 21–132. [Google Scholar]
- National Committee for Clinical Laboratory Standards, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A; National Committee for Clinical Laboratory Standards: Villanova PA, 1991.
- Singh, MP; Petersen, PJ; Weiss, WJ; Kong, F; Greenstein, M. Saccharomicins, novel heptadecaglycoside antibiotics produced by S. espanaensis: Antibacterial and mechanistic activities. Antimicrob Agents Chemother 2000, 44, 2154–2159. [Google Scholar]
- Sampson, BA; Misra, R; Benson, SA. Identification and characterization of a new gene of Escherichia coli K-12 involved in outer membrane permeability. Genetics 1989, 122, 491–501. [Google Scholar]
- Kubota, T; Takahara, T; Nagata, N; Furukawa, T; Kase, S; Tanino, S; Ishibiki, K; Kitajima, M. Colorimetric chemosensitivity testing using sulforhodamine B. J Surg Oncol 1993, 52, 83–88. [Google Scholar]
- Adams, GC; Wingfield, MJ; Common, R; Roux, J. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol 2005, 52, 1–144. [Google Scholar]
- He, H; Janso, JE; Williamson, RT; Yang, HY; Carter, GT. Cytosporacin, a highly unsaturated polyketide: Application of the ACCORD-ADEQUATE experiment to the structural determination of natural products. J Org Chem 2003, 68, 6079–6082. [Google Scholar]
- Steven-Miles, S; Goetz, MA; Bills, GF; Giacobbe, RA; Tkacz, JS; Chang, RSL; Mojena, M; Martin, I; Diez, MT; Pelaez, F; Hensens, OD; Jones, T; Burg, RW; Kong, YL; Huang, L. Discovery of an angiotensin II binding inhibitor from a Cytospora sp. using semiautomated screening procedures. J Antibiot 1995, 49, 119–123. [Google Scholar]
- Singh, SB; Jayasuriya, H; Guan, Z; Silverman, KC; Lingham, RB; Dombrowski, AW; Hazuda, DJ; Polishook, JD. (Merck). HIV Integrase Inhibitors. US Pat 6,541,515, 2003. [Google Scholar]