Abstract
A new cyclic diamine, 1,5-diazacyclohenicosane (1), was isolated from samples of the marine sponge Mycale sp. collected at Lamu Island (Kenya). Its structure was determined by a combination of spectroscopic techniques, including (+)-HRESIMS and 1D and 2D NMR spectroscopy. The compound displayed cytotoxicity at the μM level against three human tumor cell lines.
1. Introduction
Sponges continue to be a rich source of metabolites with interesting biological properties [1,2]. Among them, specimens belonging to the genus Mycale have been the subject of extensive research leading to the isolation and identification of a wide variety of novel structures. Some of these Mycale metabolites display interesting biological properties such as the antiviral and antitumour activities detected in the mycalamides [3,4], the potent cytotoxicity exhibited by the mycalolides [5–7], pateamine [8], and peloruside A [9] or the histone deacetylase inhibitory properties displayed by the cyclic tetrapeptides azumamides A–E [10].
As part of our continuing program to search for new anticancer agents, the chemical composition of samples of the marine sponge Mycale sp. collected at Lamu Island (Kenya) was investigated due to the cytotoxicity displayed by their organic extracts. Herein we report the isolation, structural characterization and cytotoxic activity of a new cyclic diamine, 1,5-diazacyclohenicosane (1), obtained by bioassay-guided fractionation of extracts of this sponge.
2. Results and Discussion
Freshly collected samples of Mycale sp. were immediately frozen and transported stored in dry ice to PharmaMar. The frozen sponge was triturated and extracted with a 1:1 mixture of MeOH:CH2Cl2, and the organic extract subjected to reversed-phase VLC on Polygoprep C18 silica gel to yield a bioactive fraction containing compound 1 (Figure 1).
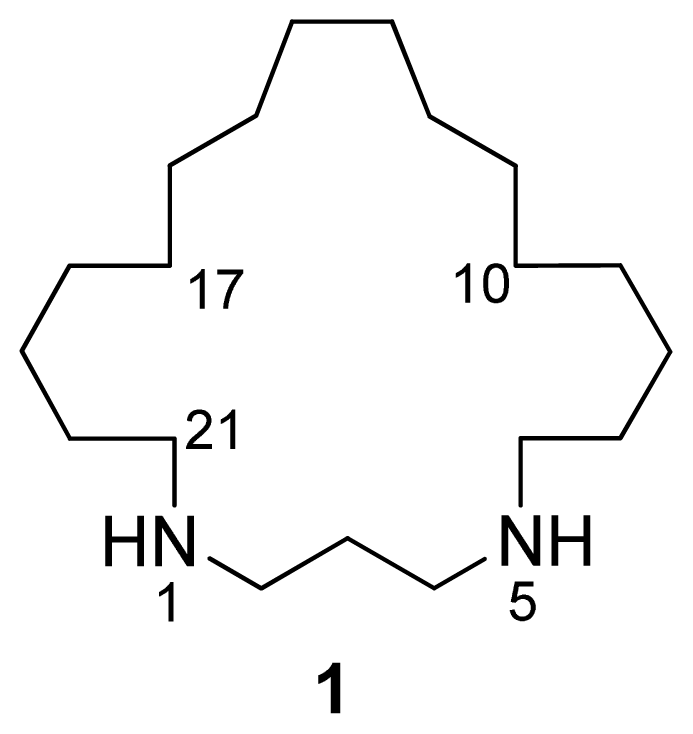
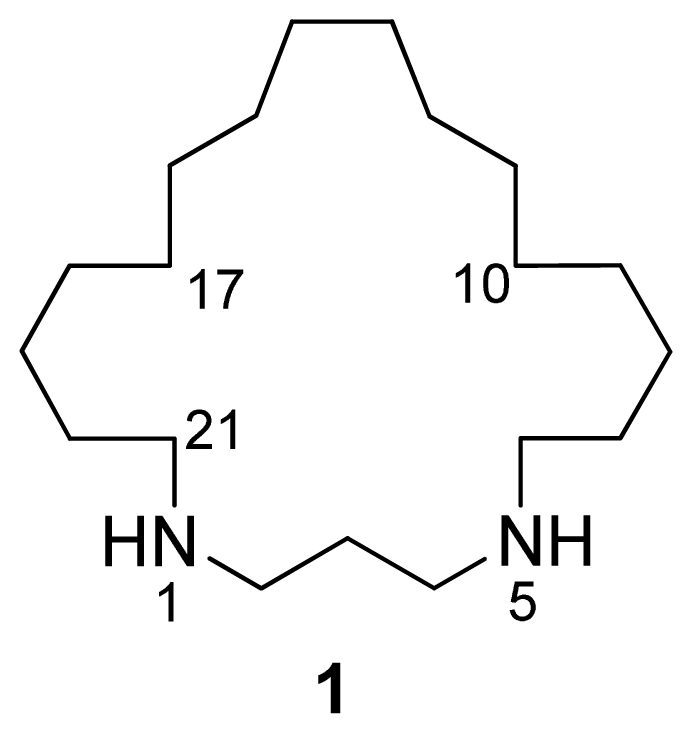
Figure 1.
Structure of compound 1.
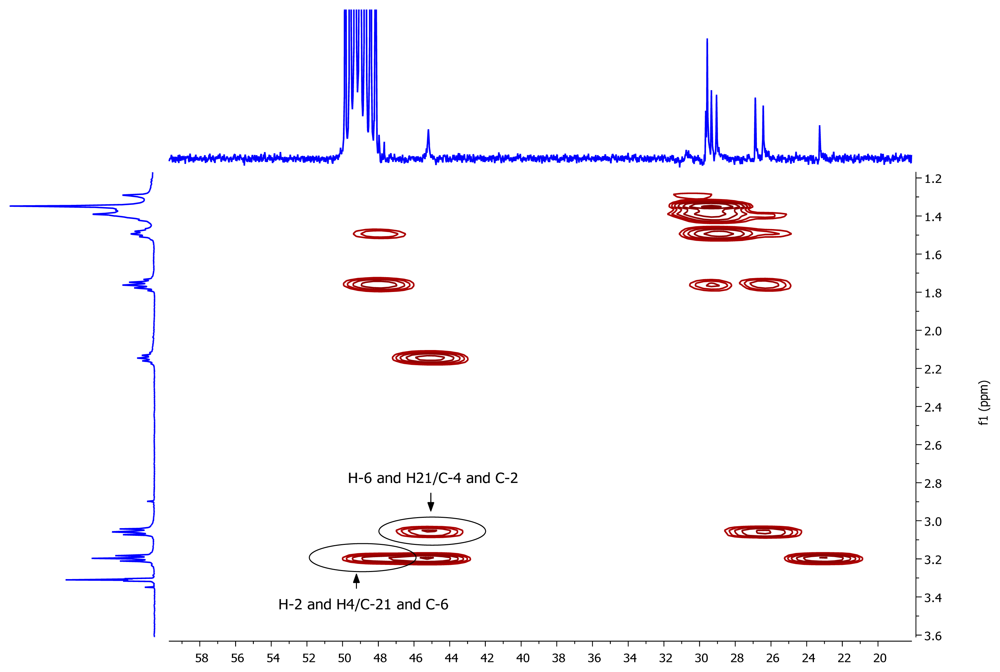
A pseudomolecular ion at m/z 297.3266 in the (+)-HRESIMS of 1 accounted for a molecular formula of C19H40N2 (calcd. for C19H41N2 297.3264), requiring one degree of unsaturation. The existence of an element of symmetry in the structure of the compound was evidenced by the presence of only nine resonance signals, one of them with double intensity, in its 13C-NMR spectrum (Table 1). Only methylene groups were present in the structure of 1, according to the edited HSQC spectrum. The chemical shifts of two of the 13C signals (48.2 and 45.2 ppm), accounting for four carbon atoms, indicated that these atoms were attached to nitrogen. COSY correlations measured in CD3OD (Table 1) established the presence of two spin systems in the molecule: from C-2 to C-4 and from C-6 to C-21. Finally, key HMBC cross-peaks observed between H-2/H-4 and C-21/C-6, and between H-6/H-21 and C-4/C-2 (Figure 2) connected both spin systems and established the identity of compound 1 as 1,5-diazacyclohenicosane.

Table 1.
NMR data of compound 1 (CD3OD, 500/75 MHz).
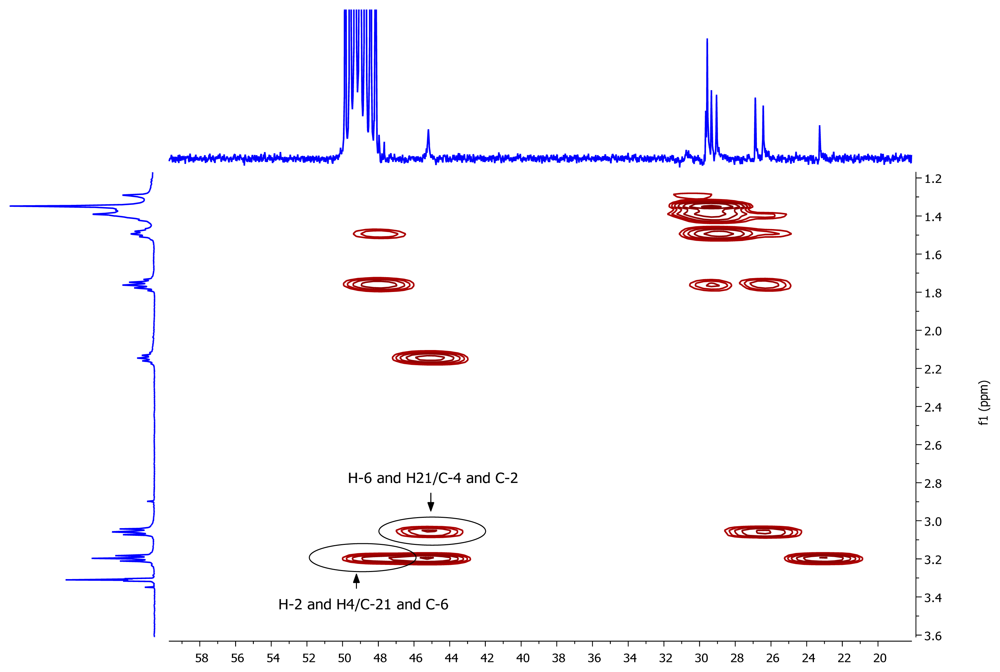
Figure 2.
Key HMBC correlations for compound 1.
The cytotoxic activity of 1 was tested against three human tumor cell lines, including lung (A549), colon (HT29) and breast (MDA-MB-231) tissues. The compound exhibited moderate activity with GI50 values in the micromolar range and no selectivity against the cell lines tested: 5.41 μM (A549), 5.07 μM (HT29) and 5.74 μM (MDA-MB-231). Doxorubicin displayed values of 0.32 μM (A549), 0.36 μM (HT29) and 0.26 μM (MDA-MB-231) when tested as a positive control under the same conditions.
In summary, a new cytotoxic cyclic diamine, 1,5-diazacyclohenicosane (1), has been isolated from samples of the Kenyan sponge Mycale sp. Its structure resembles that of other cyclic amines previously characterized from sponge samples such as the motuporamines isolated from the Papua New Guinea sponge Xestospongia exigua [11–12] or halichlorensin obtained from South African specimens of Halichlona tulearensis [13–14]. This structural similarity together with the interesting anti-invasive and anti-angiogenic properties displayed by the motuporamine family suggest that further studies to assess the potential of our compound in this area of research are merited.
3. Experimental Section
3.1. General
NMR spectra were recorded on Varian “Unity 500” (500 MHz, 1H) or Varian “Unity 300” (75 MHz, 13C) spectrometers. Chemical shifts were reported in ppm using residual CD3OD (δ 3.31 for 1H and 49.0 for 13C) as internal reference. HMBC experiments were optimized for a 3JCH of 8 Hz. (+)-HRESIMS was performed on a QSTAR Applied Biosystems spectrometer. (+)-ESIMS were recorded using an Agilent 1100 Series LC/MSD spectrometer.
3.2. Animal material
Mycale sp. was collected in August 2005 by SCUBA diving at depths between 8 and 24 m at Lamu Island (Kenya) (3º 37′ 07″ S, 39º 53′ 43″ E). The material was identified by Dr. José Luis Carballo from the Universidad Nacional Autónoma (México). A voucher specimen is deposited at PharmaMar (ORMA037245).
3.3. Extraction and isolation
The frozen organism (14 g) was triturated and extracted with a 1:1 mixture of CH2Cl2:MeOH (3 × 200 mL). The extract was concentrated to yield a crude of 1.20 g. This material was subjected to VLC on Polygoprep RP-18 with a stepped gradient from H2O:MeOH 3:1 to MeOH. The bioactive fraction eluted with H2O:MeOH 1:3 contained pure compound 1 (12.9 mg).
1,5-Diazacyclohenicosane (1). Pale yellow amorphous solid; 1H- (500 MHz) and 13C-NMR (75 MHz) see Table 1; (+)-ESIMS m/z 297 [M+H]+; (+)-HRESIMS m/z 297.3266 [M+H]+ (calcd. for C19H41N2, 297.3264).
3.4. Biological activity
A549 (ATCC CCL-185), lung carcinoma; HT29 (ATCC HTB-38), colorectal carcinoma and MDA-MB-231 (ATCC HTB-26), breast adenocarcinoma cell lines were obtained from the ATCC. Cell lines were maintained in RPMI medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine and 100 U/mL penicillin and streptomycin, at 37 ºC and 5% CO2. Triplicate cultures were incubated for 72 h in the presence or absence of test compounds (at ten concentrations ranging from 10 to 0.0026 μg/mL). For quantitative estimation of cytotoxicity, the colorimetric sulforhodamine B (SRB) method was used [15]. Briefly, cells were washed twice with PBS, fixed for 15 min in 1% glutaraldehyde solution, rinsed twice in PBS, and stained in 0.4% SRB solution for 30 min at room temperature. Cells were then rinsed several times with 1% acetic acid solution and air-dried. Sulforhodamine B was then extracted in 10 mM trizma base solution and the absorbance measured at 490 nm. Results are expressed as GI50, the concentration that causes 50% inhibition in cell growth after correction for cell count at the start of the experiment (NCI algorithm). Doxorubicin and DMSO (solvent) were used as the positive and negative controls in this assay. Prism 3.03 from GraphPad was used for the statistical analysis of the cell growth inhibition results.
Acknowledgements
The authors thank the assistance of Dr. Susana González in recording the NMR experiments, Dr. José Luis Carballo for the taxonomic identification of the sample, Dr. Luis F. García-Fernández for performing the cytotoxicity assays, Santiago Bueno and Carlos de Eguilior for collection of the sponge, and Dr. Simon Munt for the revision of the text. Thanks are also due to the Ministry of Livestock and Fisheries Development (Fisheries Department), Republic of Kenya, for the permission to collect the samples. This article is dedicated to Prof. Alejandro F. Barrero on the occasion of his 60th birthday.
- Samples Availability: Samples of compound 1 are available from the authors.
References and Notes
- Blunt, JW; Copp, BR; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2009, 26, 170–244, and previous papers in this series. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2002, 19, 1–49, and previous papers in this series. [Google Scholar]
- Perry, NB; Blunt, JW; Munro, MHG; Pannel, LK. Mycalamide A, an antiviral compound from a New Zealand sponge of the genus Mycale. J Am Chem Soc 1988, 110, 4850–4851. [Google Scholar]
- Perry, NB; Blunt, JW; Munro, MHG; Thompson, AM. Antiviral and antitumor agents from a New Zealand sponge, Mycale sp. 2. Structures and solution conformations of mycalamides A and B. J Org Chem 1990, 55, 223–227. [Google Scholar]
- Fusetani, N; Yasumuro, K; Matsunaga, S; Hashimoto, K. Mycalolides A-C, hybrid macrolides of ulapualides and halichondramide, from a sponge of the genus Mycale. Tetrahedron Lett 1989, 30, 2809–2812. [Google Scholar]
- Matsunaga, S; Sugawara, T; Fusetani, N. New mycalolides from the marine sponge Mycale magellanica and their interconversion. J Nat Prod 1998, 61, 1164–1167. [Google Scholar]
- Phuwapraisirisan, P; Matsunaga, S; van Soest, RWM; Fusetani, N. Isolation of a new mycalolide from the marine sponge Mycale izuensis. J Nat Prod 2002, 65, 942–943. [Google Scholar]
- Northcote, PT; Blunt, JW; Munro, MHG. Pateamine: A potent cytotoxin from the New Zealand marine sponge, Mycale sp. Tetrahedron Lett 1991, 32, 6411–6414. [Google Scholar]
- West, LM; Northcote, PT; Battershill, CN. Peloruside A: A potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J Org Chem 2000, 65, 445–449. [Google Scholar]
- Nakao, Y; Yoshida, S; Matsunaga, S; Shindoh, N; Terada, Y; Nagai, K; Yamashita, JK; Ganesan, A; van Soest, RWM; Fusetani, N. Azumamides A-E: Histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew Chem Int Ed 2006, 45, 7553–7557. [Google Scholar]
- Williams, DE; Lassota, P; Andersen, RJ. Motuporamines A-C, cytotoxic alkaloids isolated from the marine sponge Xestospongia exigua (Kirkpatrick). J Org Chem 1998, 63, 4838–4841. [Google Scholar]
- Williams, DE; Craig, KS; Patrick, B; McHardy, LM; van Soest, R; Roberge, M; Andersen, RJ. Motuporamines, anti-invasion and anti-angiogenic alkaloids from the marine sponge Xestospongia exigua (Kirkpatrick): Isolation, structure elucidation, analogue synthesis, and conformational analysis. J Org Chem 2002, 67, 245–258. [Google Scholar]
- Koren-Goldshlager, G; Kashman, Y; Schleyer, M. Haliclorensin, a novel diamino alkaloid from the marine sponge Haliclona tulearensis. J Nat Prod 1998, 61, 282–284. [Google Scholar]
- Heinrich, MR; Kashman, Y; Spiteller, P; Steglich, W. Revision of the structure of haliclorensin to (S)-7-methyl-1,5-diazacyclotetradecane and confirmation of the new structure by synthesis. Tetrahedron 2001, 57, 9973–9978. [Google Scholar]
- Skehan, P; Storeng, R; Scudiero, D; Monks, A; McMahon, J; Vistica, D; Warren, JT; Bokesch, H; Kenney, S; Boyd, MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990, 82, 1107–1112. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).