Update on Methodologies Available for Ciguatoxin Determination: Perspectives to Confront the Onset of Ciguatera Fish Poisoning in Europe †
Abstract
:1. Introduction
2. General Considerations about CFP
2.1. Origin of CFP and toxic fish
2.2. Epidemiology and symptomatology
2.3. Organisms producing CTXs
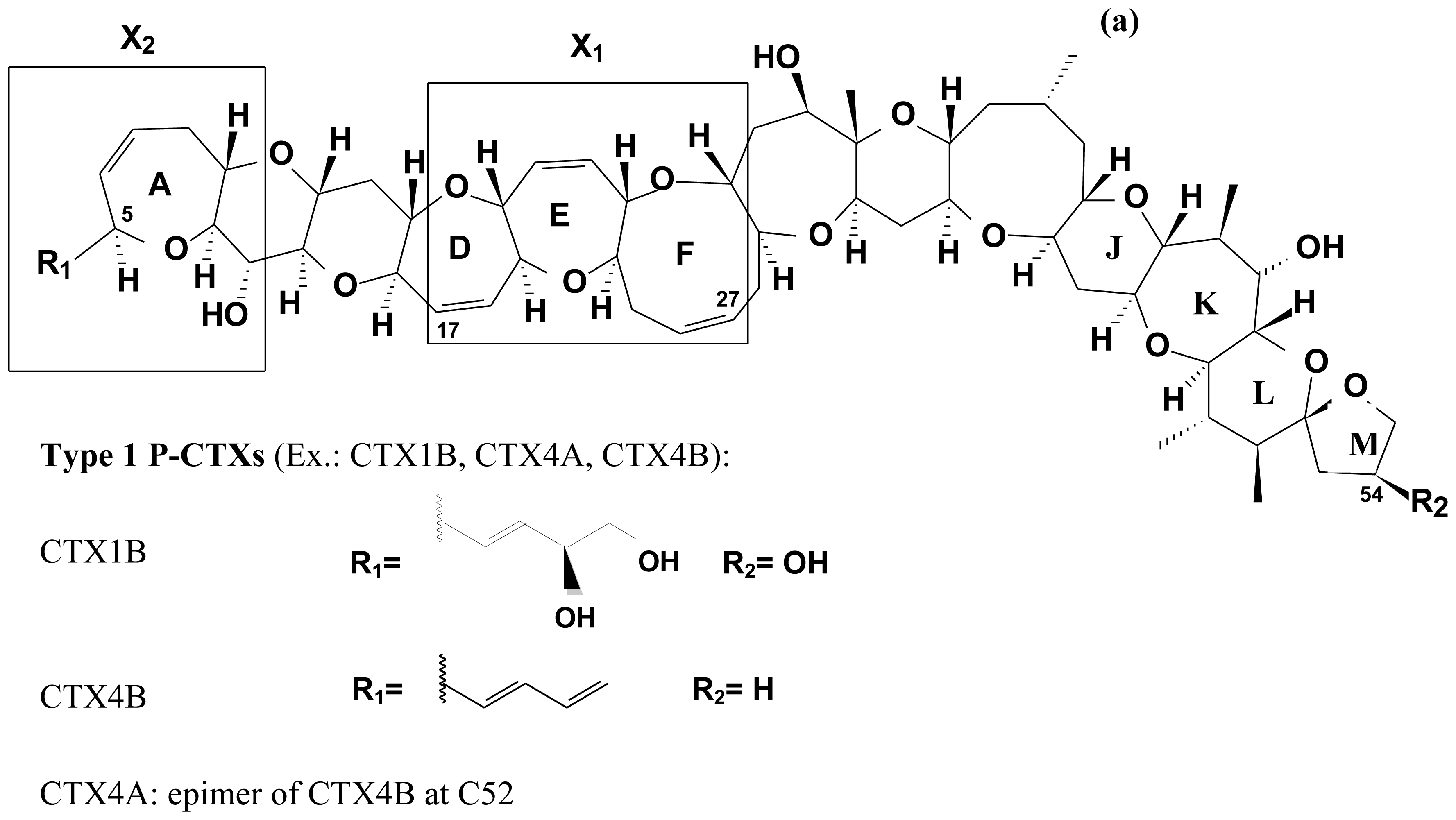
2.4. Chemical structure of CTXs and geographical variability
2.5. Toxicity and mechanisms of action
3. Methodologies for CTXs Determination
3.1. Importance of CTX determination
3.2. Sample preparation for CTXs determination
3.2.1. Fish samples
3.2.2. Microalgal samples
3.3. Methods for CTXs determination
3.3.1. Bioassays with animals: The MBA for CTXs
3.3.2. Bioassay with tissues
3.3.3. In vitro bioassays: CBA
3.3.4. Immunoassays
3.3.5. Pharmacological assay: Receptor Binding Assay
3.3.6. Physico-chemical analysis: High performance liquid chromatography coupled with spectroscopic (UV, FLD) or spectrometric (MS/MS) methods
3.3.6.1. High-performance liquid chromatography with UV detection
3.3.6.2. High-performance liquid chromatography with fluorescence detection
3.3.6.3. High-performance liquid chromatography with mass spectrometry detection
4. Perspectives to Confront the Onset of Ciguatera in Europe
4.1. Ciguatera in Europe
4.2. Gambierdiscus species in the Mediterranean Sea and Macaronesian waters (Canary Islands)
4.3. EU regulation for CTXs
4.4. A risk analysis approach to confront CFP
4.4.1. CFP risk assessment
4.4.2. CFP risk management
4.4.3. CFP risk communication
4.5. Application of risk analysis for CFP in Europe
- - Favor that the competent authority will lead actions related to risk analysis for CFP, in coordination with the different agencies implicated.
- - Identify research agencies implicated in CFP to improve risk analysis.
- - Provide the correct framework within the EU to have competent laboratories for the analysis of CTXs in food.
- - Systematically record possible cases of CFP with strict identification of symptoms and nature and origin of suspicious food.
- - Structural and toxicological characterization of CTXs and other toxins present in food and microalgae associated with CFP. Pursue recognition of CTX presence in Seriola spp. in the NE-Atlantic and consider extending analysis to other fish located in areas with presence of Gambierdiscus spp.
- - Follow benthic microalgal distribution of hazardous species with special focus on Gambierdiscus spp.
- - Evaluate exposure to CTXs.
- - Revise current legislation on CFP and foresee the set-up of expert analysis groups to identify deficiencies and future needs.
- - Establish preliminary monitoring programs in areas where CFP is present.
- - Centralize records of CFP cases in Europe for epidemiology surveillance.
- - Establish CFP treatment protocol(s).
- - Work in association with food import companies, local fisheries agencies in ciguatera areas and tourism agencies to establish action and communication protocols.
- - Define a protocol for a widespread communication strategy to the general public to be used in case of a ciguatera episode.
- - Define and implement selective communication targeted at agencies and specific bodies.
5. Conclusions
Acknowledgements
References and Notes
- In the whole text, Europe is not understood as the political entity which includes overseas territories but as a continent (geographical definition).
- Yasumoto, T. Chemistry, etiology, and food chain dynamics of marine toxins. Proc. Jpn. Acad., Ser. B 2005, 81, 43–51. [Google Scholar]
- Lewis, RJ. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar]
- Bottein Dechraoui, M-Y; Wang, Z; Ramsdell, JS. Optimization of ciguatoxin extraction method from blood for Pacific ciguatoxin (P-CTX-1). Toxicon 2007, 49, 100–105. [Google Scholar]
- Moulignier, A; Binet, D; Frottier, J. Ciguatera fish poisoning: also in Europe. Br. Med. J 1995, 59, 192. [Google Scholar]
- Bavastrelli, M; Bertucci, P; Midulla, M; Giardini, O; Sanguigni, S. Ciguatera fish poisoning: an emerging syndrome in Italian travelers. J. Travel Med 2001, 8, 139–142. [Google Scholar]
- de Fouw, J; Egmond, H; Speijers, G. Ciguatera fish poisoning: a review RIVM report 388802021. 2001.
- de Haro, L; Pommier, P; Valli, M. Emergence of imported ciguatera in Europe: report of 18 cases at the poison control centre of Marseille. Clin. Toxicol 2003, 41, 927–930. [Google Scholar]
- Pérez-Arellano, J; Luzardo, O; Brito, A; Cabrera, M; Zumbado, M; Carranza, C; Angel-Moreno, A; Dickey, R; Boada, L. Ciguatera Fish Poisoning, Canary Islands. Emerg. Infect. Dis 2005, 11, 1981–1982. [Google Scholar]
- Gouveia, N; Delgado, J; Gouveia, N; Vale, P. Primeiro registo da ocorrência de episódios do tipo ciguatérico no arquipélago da Madeira. Abstract book of the X Reuniao Oberica, Fitoplancton Toxico e Biotoxinas, Lisbon, Portugal, May 2009.
- Aligizaki, K. Spread of Potentially Toxic Benthic Dinoflagellates in the Mediterranean Sea: A Response to Climate Change? Abstract book of the CIESM Workshop n°40: “Phytoplankton response to Mediterranean environmental change”, Tunis, Tunisia, 2009.
- Aligizaki, K; Katikou, P; Nikolaidis, G. Toxic benthic Dinoflagellates Spreading and Potential Risk in the Mediterranean Sea. Abstract book of the 7th International Conference in Molluscan Shellfish Safety, Nantes, France, 14–19 June 2009.
- Aligizaki, K; Nikolaidis, G. Morphological identification of two tropical dinoflagellates of the genera Gambierdiscus and Sinophysis in the Mediterranean Sea. J. Biol. Res 2008, 9, 75–82. [Google Scholar]
- Aligizaki, K; Nikolaidis, G; Fraga, S. Is Gambierdiscus expanding to new areas? Harmful Algae 2008, 36, 6–7. [Google Scholar]
- Fraga, S; Riobó, P; Diogène, J; Paz, B; Franco, JM. Toxic and Potentially Toxic Benthic Dinoflagellates Observed in Macaronesia (NE Atlantic Archipelago). Abstract book of the 11th International Conference on Harmful Algae, Capetown, South Africa, 2004; p. 115.
- Yasumoto, T; Murata, M. Marine toxins. Chem. Rev 1993, 93, 1897–1909. [Google Scholar]
- Yasumoto, T; Fukui, M; Sasaki, K; Sugiyama, K. Determinations of marine toxins in foods. J AOAC Int 1995, 78, 574–582. [Google Scholar]
- Lehane, L. Ciguatera update. Med. J. Aust 2000, 172, 176–179. [Google Scholar]
- Lehane, L; Lewis, RJ. Ciguatera: recent advances but the risk remains. Int. J. Food Microbiol 2000, 61, 91–125. [Google Scholar]
- Lewis, RJ. Ciguatera: Australian perspectives on a global problem. Toxicon 2006, 48, 799–809. [Google Scholar]
- Li, C; Zhou, Y; Zhang, L; Shen, Q. A review: studies on ciguatoxin. J. Shanghai Ocean Univ 2009, 18, 365–371. [Google Scholar]
- Yasumoto, T. Chemical Structures of Implicated Toxins. Abstract book of the Ciguatera and related biotoxins worshop, Noumea, New Caledonia, 2008.
- Friedman, M; Fleming, L; Fernandez, M; Bienfang, P; Schrank, K; Dickey, R; Bottein, M; Backer, L; Ayyar, R; Weisman, R. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–479. [Google Scholar]
- Dickey, RW; Plakas, SM. Ciguatera: A Public Health Perspective. Toxicon 2009. [Google Scholar] [CrossRef]
- Mills, AR. Poisonous Fish in the South Pacific. J. Trop. Med. Hyg 1956, 59, 99–103. [Google Scholar]
- Randall, J. A review of ciguatera, tropical fish poisoning, with a tentative explanation of its cause. Bull. Mar. Sci 1958, 8, 236–267. [Google Scholar]
- Yasumoto, T; Nakajima, I; Bagnis, R; Adachi, R. Finding of a dinoflagellate as a likely culprit of ciguatera. Bull. Jpn. Soc. Sci. Fish 1977, 43, 1021–1026. [Google Scholar]
- Adachi, R; Fukuyo, Y. The thecal structure of a marine toxic dinoflagellate Gambierdiscus toxicus gen. and sp. nov. collected in a ciguatera-endemic area. Bull. Jpn. Soc. Sci. Fish 1979, 45, 67–71. [Google Scholar]
- Satake, M; Ishibashi, Y; Legrand, A; Yasumoto, T. Isolation and structure of ciguatoxin-4 A, a new ciguatoxin precursor, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem 1996, 60, 2103–2105. [Google Scholar]
- Yasumoto, T; Inoue, A; Bagnis, R; Garçon, M. Ecological survey on a dinoflagellate possibly responsible for the induction of ciguatera. Bull. Jpn. Soc. Sci. Fish 1979, 45, 395–399. [Google Scholar]
- Halstead, B. Poisonous and Venomous Marine Animals of the World; Darwin Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Oshiro, N; Yogi, K; Asato, S; Sasaki, T; Tamanaha, K; Hirama, M; Yasumoto, T; Inafuku, Y. Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon 2009. [Google Scholar] [CrossRef]
- Darius, HT; Ponton, D; Revel, T; Cruchet, P; Ung, A; Fouc, MT; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar]
- Chinain, M; Darius, H; Ung, A; Fouc, M; Revel, T; Cruchet, P; Pauillac, S; Laurent, D. Ciguatera risk management in French Polynesia: The case study of Raivavae Island (Australes Archipelago). Toxicon 2009. [Google Scholar] [CrossRef]
- Vernoux, J; Lahlou, N; Abbad, A; Riyeche, N; Magras, L. A study of the distribution of ciguatoxin in individual Caribbean fish. Acta Tropica 1985, 42, 225–233. [Google Scholar]
- Pearn, J. Neurology of ciguatera. Br. Med. J 2001, 70, 4–8. [Google Scholar]
- Chevaldonne, P. Ciguatera and the saupe, Sarpa salpa (L.), in the Mediterranean: A possible misinterpretation. J. Fish Biol 1990, 37, 503–504. [Google Scholar]
- Ting, J; Brown, A; Pearn, J. Ciguatera poisoning: an example of a public health challenge. Aust. N. Z. J. Publ. Health 1998, 22, 140–142. [Google Scholar]
- Bagnis, R; Kuberski, T; Laugier, S. Clinical Observations on 3,009 Cases of Ciguatera (Fish Poisoning) in the South Pacific. Am. J. Trop. Med. Hyg 1979, 28, 1067–1073. [Google Scholar]
- Glaziou, P; Legrand, A. The epidemiology of ciguatera fish poisoning. Toxicon 1994, 32, 863–873. [Google Scholar]
- Ruff, T; Lewis, R. Clinical aspects of ciguatera: an overview. Mere. Qld Museum, Brisbane 1994, 34, 609–619. [Google Scholar]
- Chateau-Degat, M; Dewailly, E; Cerf, N; Nguyen, N; Huin-Blondey, M; Hubert, B; Laudon, F; Chansin, R. Temporal trends and epidemiological aspects of ciguatera in French Polynesia: a 10-year analysis. Trop. Med. Int. Health 2007, 12, 485–492. [Google Scholar]
- Darius, HT; Revel, T; Ung, A; Cruchet, P; Tchou Fouc, M; Chinain, M. The Situation of Ciguatera Fish Poisoning in French Polynesia from 2000 to 2004. Abstract book of the 12th International Conférence on Harmful Algae, Copenhage, Denmark, 2007.
- Quod, JP; Turquet, J. Ciguatera in Réunion Island (SW Indian Ocean): Epidemiology and clinical patterns. Toxicon 1996, 34, 779–785. [Google Scholar]
- Lewis, RJ; Sellin, M; Poli, MA; Norton, RS; MacLeod, JK; Sheil, MM. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar]
- Lewis, R; Sellin, M. Multiple ciguatoxins in the flesh of fish. Toxicon 1992, 30, 915–919. [Google Scholar]
- Fleming, LE; Baden, DG; Bean, JA; Weisman, R; Blythe, DG. Reguera, B, Blanco, J, Fernandez, ML, Wyatt, T, Eds.; Seafood toxin diseases: issues in epidemiology and community outreach. In Harmful Algae; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Galicia, Spain, 1998; pp. 245–248. [Google Scholar]
- Lewis, RJ. Ciguatera management. SPC Live Reef Fish Inf. Bull 2000, 7, 11–13. [Google Scholar]
- Laurent, D; Joannot, P; Amade, P; Maesse, P; Colmet-Daage, B. Knowledge on ciguatera in Noumea (New-Caledonia). Fourth International Conference on Ciguatera Fish Poisoning, Tahiti, French Polynesia, May 1992; 85, p. 520.
- Baumann, F; Bourrat, M-B; Pauillac, S. Prevalence, Symptoms and Chronicity of Ciguatera in New Caledonia: Results from an adult population survey conducted in Noumea during 2005. Toxicon 2009. [Google Scholar] [CrossRef]
- Laboute, P; Grandperrin, R. Poissons de Nouvelle-Calédonie; IRD Edition Catherine Ledru: Nouméa, Nouvelle-Calédonie, 2000; p. 520. [Google Scholar]
- Hokama, Y. Ciguatera fish poisoning. J. Clin. Lab. Anal 1988, 2, 44–50. [Google Scholar]
- Chateau-Degat, M-L; Huin-Blondey, M-O; Chinain, M; Darius, T; Legrand, A-M; Nguyen, NL; Laudon, F; Chansin, R; Dewailly, E. Prevalence of Chronic Symptoms of Ciguatera Disease in French Polynesian Adults. Am. J. Trop. Med. Hyg 2007, 77, 842–846. [Google Scholar]
- Glaziou, P; Martin, P. Study of factors that influence the clinical response to ciguatera fish poisoning. Toxicon 1993, 31, 1151. [Google Scholar]
- Palafox, N; Jain, L; Pinano, A; Gulick, T; Williams, R; Schatz, I. Successful treatment of ciguatera fish poisoning with intravenous mannitol. JAMA 1988, 259, 2740–2742. [Google Scholar]
- Blythe, D; Fleming, L; Ram Ayyar, D; De Sylva, D; Baden, D; Schrank, K. Mannitol Therapy for acute and chronic ciguatera fish poisoning. Mere. Qld Museum, Brisbane 1994, 34, 465–470. [Google Scholar]
- Bagnis, R; Spiegel, A; Boutin, J; Burucoa, C; Nguyen, L; Cartel, J; Capdevielle, P; Imbert, P; Prigent, D; Gras, C. Evaluation of the efficacy of mannitol in the treatment of ciguatera in French Polynesia. Médecine tropicale: revue du Corps de santé colonial 1992, 52, 67–73. [Google Scholar]
- Perez, C; Vasquez, P; Perret, C. Treatment of ciguatera poisoning with gabapentin. N. Engl. J. Med 2001, 344, 692. [Google Scholar]
- Lange, W; Kreider, S; Hattwick, M; Hobbs, J. Potential benefit of tocainide in the treatment of ciguatera: report of three cases. Am. J. Med 1988, 84, 1087–1088. [Google Scholar]
- Bourdy, G; Cabalionb, P; Amadea, P; Laurenta, D. Traditional remedies used in the Western Pacific for the treatment of ciguatera poisoning. J. Etlinopharniacol 1992, 36, 163–174. [Google Scholar]
- Boydron-Le Garrec, R; Benoit, E; Sauviat, M-P; Lewis, RJ; Molgó, J; Laurent, D. Ability of some plant extracts, traditionally used to treat ciguatera fish poisoning, to prevent the in vitro neurotoxicity produced by sodium channel activators. Toxicon 2005, 46, 625–634. [Google Scholar]
- Kumar-Roiné, S. La ciguatéra: nouvelles perspectives thérapeutiques; Université de Nouvelle-Calédonie: Nouméa, New Caledonia, 2009. [Google Scholar]
- Mattei, C; Wen, P; Nguyen-Huu, T; Alvarez, M; Benoit, E. Brevenal Inhibits Pacific Ciguatoxin-1B-Induced Neurosecretion from Bovine. PLoS ONE 2008, 3, 1–9. [Google Scholar]
- Nguyen-Huu, TD; Mattei, C; Wen, PJ; Bourdelais, AJ; Lewis, RJ; Benoit, E; Baden, DG; Molgó, J; Meunier, FA. Ciguatoxin-induced catecholamine secretion in bovine chromaffin cells: Mechanism of action and reversible inhibition by brevenal. Toxicon 2009. [Google Scholar] [CrossRef] [Green Version]
- Villareal, T; Hanson, S; Qualia, S; Jester, E; Granade, H; Dickey, R. Petroleum production platforms as sites for the expansion of ciguatera in the northwestern Gulf of Mexico. Harmful Algae 2007, 6, 253–259. [Google Scholar]
- Faust, M. Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species. J. Phycol 1995, 31, 996–1003. [Google Scholar]
- Holmes, M. Gambierdiscus yasumotoi sp. nov.(Dinophyceae), a toxic benthic dinoflagellate from Southeastern Asia. J. Phycol 1998, 34, 661–668. [Google Scholar]
- Chinain, M; Faust, M; Pauillac, S. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol 1999, 35, 1282–1296. [Google Scholar]
- Chinain, M; Darius, H; Ung, A; Cruchet, P; Wang, Z; Ponton, D; Laurent, D; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2009. [Google Scholar] [CrossRef]
- Rhodes, LL; Smith, KF; Munday, R; Selwood, AI; McNabb, PS; Holland, PT; Bottein, M-Y. Toxic dinoflagellates (Dinophyceae) from Rarotonga, Cook Islands. Toxicon 2009. [Google Scholar] [CrossRef]
- Tester, P; Faust, M; Vandersea, M; Kibler, S; Chinain, M; Holmes, M; Holland, C; Litaker, R. Gambierdiscus toxicus: taxonomic uncertainties concerning Gambierdiscus toxicus: proposed epitype. Proceeding of the 12th International Conference on Harmful Algae, Copenhagen Denmark, 2008; pp. 269–271.
- Richlen, ML; Morton, SL; Barber, PH; Lobel, PS. Phylogeography, morphological variation and taxonomy of the toxic dinoflagellate Gambierdiscus toxicus (Dinophyceae). Harmful Algae 2008, 7, 614–629. [Google Scholar]
- Litaker, R; Vandersea, M; Faust, M; Kibler, S; Chinain, M; Holmes, M; Holland, W; Tester, P. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar]
- Holmes, M; Lewis, R; Poli, M; Gillespie, N. Strain dependent production of ciguatoxin precursors (gambiertoxins) by Gambierdiscus toxicus (Dinophyceae) in culture. Toxicon 1991, 29, 761–775. [Google Scholar]
- Chinain, M; Germain, M; Deparis, X; Pauillac, S; Legrand, A. Seasonal abundance and toxicity of the dinoflagellate Gambierdiscus spp.(Dinophyceae), the causative agent of ciguatera in Tahiti, French Polynesia. Mar. Biol 1999, 135, 259–267. [Google Scholar]
- Sakami, T; Nakahara, H; Chinain, M; Ishida, Y. Effects of epiphytic bacteria on the growth of the toxic dinoflagellate Gambierdiscus toxicus (Dinophyceae). J. Exp. Mar. Biol. Ecol 1999, 233, 231–246. [Google Scholar]
- Lartigue, J; Jester, E; Dickey, R; Villareal, T. Nitrogen source effects on the growth and toxicity of two strains of the ciguatera-causing dinoflagellate Gambierdiscus toxicus. Harmful Algae 2009, 8, 781–791. [Google Scholar]
- Llewellyn, LE. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the South Pacific: Reassessing the link between ciguatera and climate change. Toxicon 2009. [Google Scholar] [CrossRef]
- Doucette, GJ; Kodama, M; Franca, S; Gallacher, S. Anderson, DM, Cembella, AD, Hallegraeff, GM, Eds.; Bacterial interactions with harmful algal bloom species: bloom ecology, toxinogenesis, and cytology. In Physiological Ecology of Harmful Algal Blooms; Springer-Verlag: Berlin, Heidelberg, Germany, 1998; Volume G41, pp. 619–649. [Google Scholar]
- Laurent, D; Kerbrat, AS; Darius, HT; Girard, E; Golubic, S; Benoit, E; Sauviat, MP; Chinain, M; Molgo, J; Pauillac, S. Are cyanobacteria involved in Ciguatera Fish Poisoning-like outbreaks in New Caledonia? Harmful Algae 2008, 7, 827–838. [Google Scholar]
- Méjean, A; Peyraud-Thomas, C; Kerbrat, AS; Golubic, S; Pauillac, S; Chinain, M; Laurent, D. First identification of the neurotoxin homoanatoxin-a from mats of Hydrocoleum lyngbyaceum (marine cyanobacterium) possibly linked to giant clam poisoning in New Caledonia. Toxicon 2009. [Google Scholar] [CrossRef]
- Golubic, S; Abed, R; Pali ska, K; Pauillac, S; Chinain, M; Laurent, D. Marine toxic cyanobacteria: Diversity, environmental responses and hazards. Toxicon 2009. [Google Scholar] [CrossRef]
- Holmes, M; Lewis, R; Gillespie, N. Toxicity of Australian and French polynesian strains of Gambierdiscus toxicus (Dinophyceae) grown in culture: characterization of a new type of maitotoxin. Toxicon 1990, 28, 1159–1172. [Google Scholar]
- Satake, M; Murata, M; Yasumoto, T. Gambierol: a new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc 1993, 115, 361–362. [Google Scholar]
- Nagai, H; Murata, M; Torigoe, K; Satake, M; Yasumoto, T. Gambieric acids, new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gambierdiscus toxicus. J. Org. Chem 1992, 57, 5448–5453. [Google Scholar]
- Nagai, H; Mikami, Y; Yazawa, K; Gonoi, T; Yasumoto, T. Biological activities of novel polyether antifungals, gambieric acids A and B from a marine dinoflagellate Gambierdiscus toxicus. J. Antibiot 1993, 46, 520–522. [Google Scholar]
- Ohizumi, Y; Yasumoto, T. Contractile response of the rabbit aorta to maitotoxin, the most potent marine toxin. J. Physiol 1983, 337, 711–721. [Google Scholar]
- Yasumoto, T; Bagnis, R; Vernoux, J. Toxicity of the surgeonfishes II. Properties of the principal water soluble toxin. Bull. Jpn. Soc. Sci. Fish 1976, 42, 359–365. [Google Scholar]
- Cuypers, E; Abdel-Mottaleb, Y; Kopljar, I; Rainier, J; Raes, A; Snyders, D; Tytgat, J. Gambierol, a toxin produced by the dinoflagellate Gambierdiscus toxicus, is a potent blocker of voltage-gated potassium channels. Toxicon 2008, 51, 974–983. [Google Scholar]
- Yasumoto, T. The chemistry and biological function of natural marine toxins. Chem. Rec 2001, 1, 228–242. [Google Scholar]
- Ito, E; Suzuki-Toyota, F; Toshimori, K; Fuwa, H; Tachibana, K; Satake, M; Sasaki, M. Pathological effects on mice by gambierol, possibly one of the ciguatera toxins. Toxicon 2003, 42, 733–740. [Google Scholar]
- Legrand, A; Fukui, M; Cruchet, P; Ishibashi, Y; Yasumoto, T. Characterization of ciguatoxins from different fish species and wild Gambierdiscus toxicus. Proceeding of the 3rd International Conference on Ciguatera, Puerto Rico, 1992; Tosteson, TR, Ed.; Polysciences publications: Quebec, Canada, 1992; pp. 25–32. [Google Scholar]
- Murata, M; Naoki, H; Iwashita, T; Matsunaga, S; Sasaki, M; Yokoyama, A; Yasumoto, T. Structure of maitotoxin. J. Am. Chem. Soc 1993, 115, 2060–2062. [Google Scholar]
- Vernoux, J; Lewis, R. Isolation and characterization of Caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon 1997, 35, 889–900. [Google Scholar]
- Hamilton, B; Hurbungs, M; Jones, A; Lewis, RJ. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar]
- Hamilton, B; Hurbungs, M; Vernoux, J-P; Jones, A; Lewis, RJ. Isolation and characterization of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar]
- Pottier, I; Vernoux, J; Jones, A; Lewis, R. Characterization of multiple Caribbean ciguatoxins and congeners in individual specimens of horse-eye jack (Caranx latus) by high-performance liquid chromatography/mass spectrometry. Toxicon 2002, 40, 929–939. [Google Scholar]
- Pottier, I; Vernoux, J; Jones, A; Lewis, R. Analysis of toxin profiles in three different fish species causing ciguatera fish poisoning in Guadeloupe, French West Indies. Food Addit. Contam 2002, 19, 1034–1042. [Google Scholar]
- Satake, M; Murata, M; Yasumoto, T. The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron Lett 1993, 34, 1975–1978. [Google Scholar]
- Murata, M; Legrand, A; Ishibashi, Y; Fukui, M; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc 1990, 112, 4380–4386. [Google Scholar]
- Legrand, A-M; Teai, T; Cruchet, P; Satake, M; Murata, K; Yasumoto, T. Two structural types of ciguatoxin involved in ciguatera fish poisoning in French Polynesia. VIII International Conference on Harmful Algae; Reguera, B, Blanco, J, Fernandez, ML, Wyatt, T, Eds.; Xunta de Galicia and International Oceanographic Commission of UNESCO: Paris, France, 1998; pp. 473–475. [Google Scholar]
- Lewis, R; Vernoux, J; Breretons, I. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc 1998, 120, 5914–5920. [Google Scholar]
- Molgó, J; Comella, J; Legrand, A. Ciguatoxin enhances quantal transmitter release from frog motor nerve terminals. Br. J. Pharmacol 1990, 99, 695. [Google Scholar]
- Benoit, E; Juzans, P; Legrand, A; Molgo, J. Nodal swelling produced by ciguatoxin-induced selective activation of sodium channels in myelinated nerve fibers. Neuroscience 1996, 71, 1121–1131. [Google Scholar]
- Mattei, C; Dechraoui, M; Molgó, J; Meunier, F; Legrand, A; Benoit, E. Neurotoxins targetting receptor site 5 of voltage-dependent sodium channels increase the nodal volume of myelinated axons. J. Neurosci. Res 1999, 55, 666–673. [Google Scholar]
- Molgó, J; Shimahara, T; Legrand, A. Ciguatoxin, extracted from poisonous morays eels, causes sodium-dependent calcium mobilization in NG108-15 neuroblastoma x glioma hybrid cells. Neurosci. Lett 1993, 158, 147–150. [Google Scholar]
- Hidalgo, J; Liberona, J; Molgó, J; Jaimovich, E. Pacific ciguatoxin-1b effect over Na+ and K+ currents, inositol 1,4,5-triphosphate content and intracellular Ca2+ signals in cultured rat myotubes. Br. J. Pharmacol 2002, 137, 1055–1062. [Google Scholar]
- Bidard, J; Vijverberg, H; Frelin, C; Chungue, E; Legrand, A; Bagnis, R; Lazdunski, M. Ciguatoxin is a novel type of Na+ channel toxin. J. Biol. Chem 1984, 259, 8353. [Google Scholar]
- Lombet, A; Bidard, J; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett 1987, 219, 355–359. [Google Scholar]
- Lewis, R. Immunological, biochemical and chemical features of ciguatoxins- implications for the detection of ciguateric fish. Mere. Qld Museum, Brisbane 1994, 34, 541–548. [Google Scholar]
- Lewis, RJ; Molgó, J; Adams, DJ. Botana, LM, Ed.; Ciguatera toxins: Pharmacology of toxins involved in ciguatera and related fish poisonings. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Marcel Dekker: New York, NY, USA, 2000; pp. 419–447. [Google Scholar]
- Kumar-Roine, S; Matsui, M; Chinain, M; Laurent, D; Pauillac, S. Modulation of inducible nitric oxide synthase gene expression in RAW 264.7 murine macrophages by Pacific ciguatoxin. Nitric Oxide-Biol. Chem 2008, 19, 21–28. [Google Scholar]
- Racciatti, D; Vecchiet, J; Ceccomancini, A; Ricci, F; Pizzigallo, E. Chronic fatigue syndrome following a toxic exposure. Sci. Total Environ 2001, 270, 27–31. [Google Scholar]
- Pall, M. Common etiology of posttraumatic stress disorder, fibromyalgia, chronic fatigue syndrome and multiple chemical sensitivity via elevated nitric oxide/peroxynitrite. Med. Hypotheses 2001, 57, 139–145. [Google Scholar]
- Teixeira, C; de Oliveira, J; Baracat, J; Priviero, F; Okuyama, C; Rodrigues Netto, N; Fregonesi, A; Antunes, E; De Nucci, G. Nitric oxide release from human corpus cavernosum induced by a purified scorpion toxin. Urology 2004, 63, 184–189. [Google Scholar]
- Sauviat, M; Boydron-Le Garrec, R; Masson, J; Lewis, R; Vernoux, J; Molgó, J; Laurent, D; Benoit, E. Mechanisms involved in the swelling of erythrocytes caused by Pacific and Caribbean ciguatoxins. Blood Cell. Mol. Dis 2006, 36, 1–9. [Google Scholar]
- Desesso, J; Scialli, A; Goeringer, G. D-mannitol, a specific hydroxyl free radical scavenger, reduces the developmental toxicity of hydroxyurea in rabbits. Birth Defects Res. Part A: Clin. Mol. Teratol 1994, 49, 248–259. [Google Scholar]
- Leiro, J; Álvarez, E; Arranz, J; Siso, I; Orallo, F. In vitro effects of mangiferin on superoxide concentrations and expression of the inducible nitric oxide synthase, tumour necrosis factor- and transforming growth factor-genes. Biochem. Pharmacol 2003, 65, 1361–1371. [Google Scholar]
- Dickey, RW. Botana, LM, Ed.; Ciguatera Toxins: Chemistry, Toxicology and Detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 2nd ed; CRC Press-Taylor & Francis: Boca Raton, FL, USA, 2008; pp. 479–500. [Google Scholar]
- Dickey, RW; Granade, HR; Jester, ELE; Abraham, A; El Said, KR; Plakas, SM. A tiered method for determination of Caribbean and Pacific ciguatoxins in fish and formulation of regulatory advisory levels. Abstract book of the Ciguatera and Related Biotoxins Workshop, Noumea, New Caledonia, 2008.
- Bagnis, R; Bennett, J; Prieur, C; Legrand, A. The Dynamics of Three Toxic Benthic Dinoflagellates and the Toxicity of Ciguateric Surgeonfish in French Polynesia; Elsevier: New York, NY, USA, 1985; pp. 177–182. [Google Scholar]
- Roeder, K; Erler, K; Kibler, S; Tester, P. Characteristic profiles of Ciguatera toxins in different strains of Gambierdiscus spp. Toxicon 2009. [Google Scholar] [CrossRef]
- Morton, S; Norris, D; Bomber, J. Effect of temperature, salinity and light intensity on the growth and seasonality of toxic dinoflagellates associated with ciguatera. J. Exp. Mar. Biol. Ecol 1992, 157, 79–90. [Google Scholar]
- Lewis, RJ; Yang, AJ; Jones, A. Rapid extraction combined with LC-tandem mass spectrometry (CREM-LC/MS/MS) for the determination of ciguatoxins in ciguateric fish flesh. Toxicon 2009, 54, 62–66. [Google Scholar]
- Stewart, I; Eaglesham, G; Poole, S; Graham, G; Paulo, C; Wickramasinghe, W; Sadler, R; Shaw, G. Establishing a public health analytical service based upon chemical methods for detecting and quantifying Pacific ciguatoxin in fish samples. Toxicon 2009. [Google Scholar] [CrossRef]
- Scheuer, P; Takahaski, W; Tsutsumi, J; Yoshida, T. Ciguatoxin: isolation and chemical structure. Science 1967, 155, 1267–1268. [Google Scholar]
- Hashimoto, Y; Yasumoto, T; Kamiya, H; Yoshida, T. Occurrence of ciguatoxin and ciguaterin in ciguatoxic fishes in the Ryukyu and Amami Islands. Bull. Jpn. Soc. Sci. Fish 1969, 35, 327–332. [Google Scholar]
- Wong, C-K; Hung, P; Lee, KLH; Kam, K-M. Study of an outbreak of ciguatera fish poisoning in Hong Kong. Toxicon 2005, 46, 563–571. [Google Scholar]
- Lewis, RJ. Hallegraeff, G, Anderson, D, Cembella, A, Eds.; Detection of Ciguatoxins and related Benthic Dinoflagellate Toxins: in vivo and in vitro Methods. In Manual on Harmful Marine Microalgae; IOC Manuals and Guides No.33; UNESCO: Paris, France, 1995; Volume 33, pp. 135–161. [Google Scholar]
- Lewis, RJ. Hallegraeff, GM, Anderson, DM, Cembella, AD, Eds.; Detection of toxins associated with ciguatera fish poisoning. In Manual on Harmful Marine Microalgae; UNESCO: Paris France, 2003; pp. 267–277. [Google Scholar]
- Wong, C; Hung, P; Lee, K; Kam, K. Solid-phase extraction clean-up of ciguatoxin-contaminated coral fish extracts for use in the mouse bioassay. Food Addit. Contam 2009, 26, 236–247. [Google Scholar]
- Darius, T. Institut Louis Malardé: Papeete (French Polinesia), personal communication; 2010.
- Satake, M; Ishimaru, T; Legrand, A; Yasumoto, T. Smayda, TJ, Shimizu, Y, Eds.; Isolation of a ciguatoxin analog from cultures of Gambierdiscus toxicus. In Toxic Phytoplankton Blooms in the Sea; Elsevier: New York, NY, USA, 1993; pp. 575–579. [Google Scholar]
- Satake, M; Ishibashi, Y; Legrand, A; Yasumoto, T. Short Communication: Isolation and structure of ciguatoxin-4A, a new ciguatoxin precursor, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem 1997, 60, 2103–2105. [Google Scholar]
- Legrand, A; Litaudon, M; Genthon, J; Bagnis, R; Yasumoto, T. Isolation and some properties of ciguatoxin. J. Appl. Phycol 1989, 1, 183–188. [Google Scholar]
- Caillaud, A; Cañete, E; de la Iglesia, P; Giménez, G; Diogène, J. Cell-based assay coupled with chromatographic fractioning: A strategy for marine toxins detection in natural samples. Toxicol. in Vitro 2009, 23, 1591–1596. [Google Scholar]
- Caillaud, A; Yasumoto, T; Diogène, J. Detection and quantification of maitotoxin-like compounds using a Neuroblastoma (Neuro-2a) cell based assay. Application to the screening of maitotoxin-like compounds in Gambierdiscus spp. Toxicon 2010, 56, 36–44. [Google Scholar]
- Banner, A; Helfrich, P; Scheuer, P; Yoshida, T. Research on ciguatera in the tropical Pacific. In Proceedings of the 16th ann. Session Gulf; Caribbean Fisheries Institute, 1963; pp. 84–98. [Google Scholar]
- Hirama, M; Oishi, T; Uehara, H; Inoue, M; Maruyama, M; Oguri, H; Satake, M. Total synthesis of ciguatoxin CTX3C. Science 2001, 294, 1904–1907. [Google Scholar]
- Inoue, M; Miyazaki, K; Uehara, H; Maruyama, M; Hirama, M. First-and second-generation total synthesis of ciguatoxin CTX3C. Proc. Nat. Acad. Sci. USA 2004, 101, 12013. [Google Scholar]
- Inoue, M; Uehara, H; Maruyama, M; Hirama, M. Practical total synthesis of ciguatoxin CTX3C by improved protective group strategy. Org. Lett 2002, 4, 4551–4554. [Google Scholar]
- Inoue, M; Miyazaki, K; Ishihara, Y; Tatami, A; Ohnuma, Y; Kawada, Y; Komano, K; Yamashita, S; Lee, N; Hirama, M. Synthesis of Ciguatoxin (CTX1B). Synfacts 2006, 12, 1193–1193. [Google Scholar]
- Inoue, M; Miyazaki, K; Ishihara, Y; Tatami, A; Ohnuma, Y; Kawada, Y; Komano, K; Yamashita, S; Lee, N; Hirama, M. Synthesis of Ciguatoxin (CTX1B). J. Am. Chem. Soc 2006, 128, 9352–9354. [Google Scholar]
- Akinari, H; Isobe, M. Total Synthesis of Ciguatoxin. Angew. Chem 2009, 48, 2941–2945. [Google Scholar]
- Fuwa, H; Kainuma, N; Satake, M; Sasaki, M. Synthesis and biological evaluation of gambierol analogues. Bioorg. Med. Chem. Lett 2003, 13, 2519–2522. [Google Scholar]
- Dechraoui, M-Y; Naar, J; Pauillac, S; Legrand, A-M. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon 1999, 37, 125–143. [Google Scholar]
- Pottier, I; Hamilton, B; Jones, A; Lewis, R; Vernoux, J. Identification of slow and fast-acting toxins in a highly ciguatoxic barracuda (Sphyraena barracuda) by HPLC/MS and radiolabeled ligand binding. Toxicon 2003, 42, 663–672. [Google Scholar]
- Dechraoui, MYB; Tiedeken, JA; Persad, R; Wang, Z; Granade, HR; Dickey, RW; Ramsdell, JS. Use of two detection methods to discriminate ciguatoxins from brevetoxins: Application to great barracuda from Florida Keys. Toxicon 2005, 46, 261–270. [Google Scholar]
- Campora, C; Hokama, Y; Tamaru, C; Anderson, B; Vincent, D. Evaluating the Risk of Ciguatera Fish Poisoning from Reef Fish Grown at Marine Aquaculture Facilities in Hawai’i. J. World Aquac. Soc 2010, 41, 61–70. [Google Scholar]
- Campora, CE; Dierking, J; Tamaru, CS; Hokama, Y; Vincent, D. Detection of ciguatoxin in fish tissue using sandwich ELISA and neuroblastoma cell bioassay. J. Clin. Lab. Anal 2008, 22, 246–253. [Google Scholar]
- Bienfang, P; Oben, B; DeFelice, S; Moeller, P; Huncik, K; Oben, P; Toonen, R; Daly-Engel, T; Bowen, B. Ciguatera: the detection of neurotoxins in carnivorous reef fish from the coast of Cameroon, West Africa. Afr. J. Mar. Sci 2008, 30, 533–540. [Google Scholar]
- Banner, A. Jones, AO, Endean, R, Eds.; Ciguatera: a disease from coral reef fish. In Biology and Geology of Coral Reefs; Academic Press: London, UK, 1976; Volume 3. [Google Scholar]
- Banner, A; Scheuer, P; Sasaki, S; Helfrich, P; Alender, C. Observations on ciguatera-type toxin in fish. Ann. N.Y. Acad. Sci 1960, 90, 770–787. [Google Scholar]
- Bagnis, R; Chanteau, S; Chungue, E; Drollet, J; Lechat, I; Legrand, A; Pompon, A; Prieur, C; Roux, J; Tetaria, C. Comparison of the cat bioassay. The mouse bioassay and the mosquito bioassay to detect ciguatoxicity in fish. Proceedings of the fifth international coral reef congress, Tahiti, French Polynesia, 1985; pp. 491–496.
- Kosaki, T; Stephens, B; Anderson, H. Marine toxins from the pacific: III. Comparative bio-assay of ciguatoxin(s) in the mouse and chicken. Proceeding of the West Pharmacol Soc 1968, 126–128. [Google Scholar]
- Granade, H; Cheng, P; Doorenbos, N. Ciguatera I: brine shrimp (Artemia salina L) larval assay for ciguatera toxins. J. Pharm. Sci 1976, 65, 1414–1415. [Google Scholar]
- Labrousse, H; Matile, L. Toxicological biotest on Diptera larvae to detect ciguatoxins and various other toxic substances. Toxicon 1996, 34, 881–891. [Google Scholar]
- Park, D. Evolution of methods for assessing ciguatera toxins in fish. Rev. Environ. Contam. Toxicol 1994, 136, 1. [Google Scholar]
- Botana, LM. Botana, L, Ed.; The Mouse Bioassay as a universal Detector. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 2nd ed; CRC Pres-Taylor & Francis: Boca Raton, FL, USA, 2008; pp. 597–628. [Google Scholar]
- European Union Commission Regulation EC 86/609/EEC. Off. J. Eur. Union 1986, L358, 1–28.
- Yasumoto, T; Raj, U; Bagnis, R. Seafood Poisonings in Tropical Regions; Laboratory of Food Hygiene, Faculty of Agriculture: Tohoku University: Japan, 1984; p. 74. [Google Scholar]
- Lewis, RJ; Sellin, M. Recovery of ciguatoxin from fish flesh. Toxicon 1993, 31, 1333–1336. [Google Scholar]
- Dickey, RW; Granade, HR; McClure, FD. Evaluation of a solid-phase immunobed assay for detection of ciguatera-related biotoxins in caribbean finfish. Memoir. Queensl. Mus 1994, 34, 481–488. [Google Scholar]
- Hoffman, P; Granade, H; McMillan, J. The mouse ciguatoxin bioassay: a dose-response curve and symptomatology analysis. Toxicon 1983, 21, 363–369. [Google Scholar]
- Miyahara, J; Akau, C; Yasumoto, T. Effects of ciguatoxin and maitotoxin on the isolated guinea pig atria. Res. Comm. Chem. Pathol. Pharmacol 1979, 25, 177. [Google Scholar]
- Kimura, LH; Hokama, Y; Abad, MA; Oyama, M; Miyahara, JT. Comparison of three different assays for the assessment of ciguatoxin in fish tissues: radioimmunoassay, mouse bioassay and in vitro guinea pig atrium assay. Toxicon 1982, 20, 907–912. [Google Scholar]
- Eisenbrand, G; Pool-Zobel, B; Baker, V; Balls, M; Blaauboer, BJ; Boobis, A; Carere, A; Kevekordes, S; Lhuguenot, JC; Pieters, R; Kleiner, J. Methods of in vitro toxicology. Food Chem. Toxicol 2002, 40, 193–236. [Google Scholar]
- Rossini, G. Functional assays in marine biotoxin detection. Toxicology 2005, 207, 451–462. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth 1983, 65, 55–63. [Google Scholar]
- Tubaro, A; Florio, C; Luxich, E; Vertua, R; Della Loggia, R; Yasumoto, T. Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon 1996, 34, 965–974. [Google Scholar]
- Manger, R; Leja, L; Lee, S; Hungerford, J; Hokama, Y; Dickey, R; Granade, H; Lewis, R; Yasumoto, T; Wekell, M. Detection of sodium channel toxins: directed cytotoxicity assays of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. J. AOAC Int 1995, 78, 521–527. [Google Scholar]
- Manger, R; Leja, L; Lee, S; Hungerford, J; Wekell, M. Tetrazolium-based cell bioassay for neurotoxins active on voltage-sensitive sodium channels: semiautomated assay for saxitoxins, brevetoxins, and ciguatoxins. Anal. Biochem 1993, 214, 190–194. [Google Scholar]
- Ledreux, A; Krys, S; Bernard, C. Suitability of the Neuro-2a cell line for the detection of palytoxin and analogues (neurotoxic phycotoxins). Toxicon 2009, 53, 300–308. [Google Scholar]
- Cañete, E; Diogène, J. Comparative study of the use of neuroblastoma cells (Neuro-2a) and neuroblastoma × glioma hybrid cells (NG108-15) for the toxic effect quantification of marine toxins. Toxicon 2008, 52, 541–550. [Google Scholar]
- Kogure, K; Tamplin, M; Simidu, U; Colwell, R. A tissue culture assay for tetrodotoxin, saxitoxin and related toxins. Toxicon 1988, 26, 191–197. [Google Scholar]
- Catterall, W. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol 1980, 20, 15–43. [Google Scholar]
- Catterall, W; Nirenberg, M. Sodium Uptake Associated with Activation of Action Potential Ionophores of Cultured Neuroblastoma and Muscle Cells. Proc. Nat. Acad. Sci. USA 1973, 70, 3759–3763. [Google Scholar]
- Catterall, W. Molecular properties of voltage-sensitive sodium channels. Annu. Rev. Biochem 1986, 55, 953–985. [Google Scholar]
- Jellett, J; Marks, L; Stewart, J; Dorey, M; Watson-Wright, W; Lawrence, J. Paralytic shellfish poison (saxitoxin family) bioassays: automated endpoint determination and standardization of the in vitro tissue culture bioassay, and comparison with the standard mouse bioassay. Toxicon 1992, 30, 1143–1156. [Google Scholar]
- Gallacher, S; Birkbeck, T. A tissue culture assay for direct detection of sodium channel blocking toxins in bacterial culture supernates. FEMS Microbiol. Lett 1992, 92, 101–108. [Google Scholar]
- Shimojo, R; Iwaoka, W. A rapid hemolysis assay for the detection of sodium channel-specific marine toxins. Toxicology 2000, 154, 1–7. [Google Scholar]
- Jellett, J; Stewart, J; Laycock, M. Toxicological evaluation of saxitoxin, neosaxitoxin, gonyautoxin II, gonyautoxin II plus III and decarbamoylsaxitoxin with the mouse neuroblastoma cell bioassay. Toxicol. in Vitro 1995, 9, 57–65. [Google Scholar]
- Catterall, W. Anderson, DM, White, AW, Baden, DG, Eds.; The voltage-sensitive sodium channel: a receptor for multiple toxins. In Toxic Dinoflagellates; Elsevier: New York, NY, USA, 1985; pp. 329–344. [Google Scholar]
- Dickey, R; Jester, E; Granade, R; Mowdy, D; Moncreiff, C; Rebarchik, D; Robl, M; Musser, S; Poli, M. Monitoring brevetoxins during a Gymnodinium breve red tide: comparison of sodium channel specific cytotoxicity assay and mouse bioassay for determination of neurotoxic shellfish toxins in shellfish extracts. Nat. Toxins 1999, 7, 157–165. [Google Scholar]
- Jellett, J; Belland, E; Doucette, L; Windust, A; Gallacher, S; Quilliam, M. Validation of the Maritime in Vitro Shellfish Test (MIST™) kits for marine biotoxins. Canadian technical report of fisheries and aquatic sciences/Rapport technique canadien des sciences halieutiques et aquatiques 1999, 2261, 120. [Google Scholar]
- Birinyi-Strachan, L; Gunning, S; Lewis, R; Nicholson, G. Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol. Appl. Pharmacol 2005, 204, 175–186. [Google Scholar]
- Ghiaroni, V; Fuwa, H; Inoue, M; Sasaki, M; Miyazaki, K; Hirama, M; Yasumoto, T; Rossini, G; Scalera, G; Bigiani, A. Effect of ciguatoxin 3C on voltage-gated Na+ and K+ currents in mouse taste cells. Chem. Senses 2006, 31, 673–680. [Google Scholar]
- Bottein Dechraoui, M-Y; Ramsdell, JS. Type B brevetoxins show tissue selectivity for voltage-gated sodium channels: comparison of brain, skeletal muscle and cardiac sodium channels. Toxicon 2003, 41, 919–927. [Google Scholar]
- Fairey, E; Bottein Dechraoui, M; Sheets, M; Ramsdell, J. Modification of the cell based assay for brevetoxins using human cardiac voltage dependent sodium channels expressed in HEK-293 cells. Biosens. Bioelectron 2001, 16, 579–586. [Google Scholar]
- Cañete, E; Diogène, J. Improvements in the use of neuroblastomaxglioma hybrid cells (NG108–15) for the toxic effect quantification of marine toxins. Toxicon 2010, 55, 381–389. [Google Scholar]
- Bottein Dechraoui, M; Wacksman, J; Ramsdell, J. Species selective resistance of cardiac muscle voltage gated sodium channels: characterization of brevetoxin and ciguatoxin binding sites in rats and fish. Toxicon 2006, 48, 702–712. [Google Scholar]
- Caillaud, A; Cañete, E; Fraga, S; Mallat, E; Diogène, J. Toxicidad de la dinoflagelaqda Gambierdiscus sp. aislada de las Islas Canarias. Proceedings of the IXe Reunión Ibérica, Avances y Tendencias en Fitoplancton Tóxico y Biotoxinas, Cartagena, Spain, 2008; pp. 303–309.
- Yasumoto, T. Search for ciguatoxin producing clones of Gambierdiscus toxicus on Okinawan coastal reefs. Abstract book of the Ciguatera and Related Biotoxins Workshop, Noumea, NC, USA, 2008.
- Bottein, M-Y; Wang, Z; Ramsdell, JS. Toxicokinetics of Pacific ciguatoxin-1 (P-CTX-1) in rats. Abstract book of the Ciguatera and related Biotoxins Workshop, Noumea, NC, USA, 2008.
- Caillaud, A. IRTA: Sant Carles de la Rapita (Tarragona, Spain), unpublished data; 2010.
- Liebsch, M; Spielmann, H. Currently available in vitro methods used in the regulatory toxicology. Toxicol. Lett 2002, 127, 127–134. [Google Scholar]
- Satake, M; Fukui, M; Legrand, A; Cruchet, P; Yasumoto, T. Isolation and structures of new ciguatoxin analogs, 2, 3-dihydroxyCTX3C and 51-hydroxyCTX3C, accumulated in tropical reef fish. Tetrahedron Lett 1998, 39, 1197–1198. [Google Scholar]
- Hokama, Y; Banner, A; Boylan, D. A radioimmunoassay for the detection of ciguatoxin. Toxicon 1977, 15, 317–325. [Google Scholar]
- Hokama, Y; Honda, S; Kobayashi, M; Nakagawa, L; Asahina, A; Miyahara, J. Natori, S, Hashimoto, K, Ueno, Y, Eds.; Monoclonal antibody (MAb) in detection of ciguatoxin (CTX) and related polyethers by the stick-enzyme immunoassay (S-EIA) in fish tissues associated with ciguatera poisoning. In Mycotoxins and Phycotoxins ‘88; Elsevier: Amsterdam, The Netherland, 1988; pp. 303–310. [Google Scholar]
- Hokama, Y; Osugi, A; Honda, S; Matsuo, M. Monoclonal antibodies in the detection of ciguatoxin and other toxic polyethers in fish tissues by a rapid poke stick test. Fifth International Coral Reef Congress, Tahiti, French Polynesia; Gabrie, C, Salvat, B, Eds.; 1985; pp. 449–456. [Google Scholar]
- Kimura, L; Abad, M; Hokama, Y. Evaluation of the radioimmunoassay (RIA) for detection of ciguatoxin (CTX) in fish tissues. J. Fish Biol 1982, 21, 671–680. [Google Scholar]
- Hokama, Y; Abad, M; Kimura, L. A rapid enzyme-immunoassay for the detection of ciguatoxin in contaminated fish tissues. Toxicon 1983, 21, 817–824. [Google Scholar]
- Hokama, Y; Asahina, A; Hong, T; Shang, E; Miyahara, J. Evaluation of the stick enzyme immunoassay in Caranx sp. and Seriola dumerili associated with ciguatera. J. Clin. Lab. Anal 1990, 4, 363–366. [Google Scholar]
- Hokama, Y; Hong, T; Isobe, M; Ichikawa, Y; Yasumoto, T. Cross-reactivity of highly purified okadaic acid (OA), synthetic, spiroketal east sphere of OA and ciguatoxin. J. Clin. Lab. Anal 1992, 6, 54–58. [Google Scholar]
- Ganal, C; Asahina, A; Hokama, Y; Miyahara, J. Characterization of marine toxin (s) in Myripristis sp. by immunological, mouse toxicity, and guinea pig assays. J. Clin. Lab. Anal 1993, 7, 41–45. [Google Scholar]
- Hokama, Y; Asahina, A; Shang, E; Hong, T; Shirai, J. Evaluation of the Hawaiian reef fishes with the solid phase immunobead assay. J. Clin. Lab. Anal 1993, 7, 26–30. [Google Scholar]
- Inoue, M; Lee, N; Tsumuraya, T; Fujii, I; Hirama, M. Use of monoclonal antibodies as an effective strategy for treatment of ciguatera poisoning. Toxicon 2009, 53, 802–805. [Google Scholar]
- Nagumo, Y; Oguri, H; Shindo, Y; Sasaki, S; Oishi, T; Hirama, M; Tomioka, Y; Mizugaki, M; Tsumuraya, T. Concise synthesis of ciguatoxin ABC-ring fragments and surface plasmon resonance study of the interaction of their BSA conjugates with monoclonal antibodies. Bioorg. Med. Chem. Lett 2001, 11, 2037–2040. [Google Scholar]
- Nagumo, Y; Oguri, H; Tsumoto, K; Shindo, Y; Hirama, M; Tsumuraya, T; Fujii, I; Tomioka, Y; Mizugaki, M; Kumagai, I. Phage-display selection of antibodies to the left end of CTX3C using synthetic fragments. J. Immunol. Meth 2004, 289, 137–146. [Google Scholar]
- Oguri, H; Hirama, M; Tsumuraya, T; Fujii, I; Maruyama, M; Uehara, H; Nagumo, Y. Synthesis-based approach toward direct sandwich immunoassay for ciguatoxin CTX3C. J. Am. Chem. Soc 2003, 125, 7608–7612. [Google Scholar]
- Tsumoto, K; Yokota, A; Tanaka, Y; Ui, M; Tsumuraya, T; Fujii, I; Kumagai, I; Nagumo, Y; Oguri, H; Inoue, M. Critical contribution of aromatic rings to specific recognition of polyether rings: The case of ciguatoxin CTX3C-ABC and its specific antibody 1C49. J. Biol. Chem 2008, 283, 12259–12266. [Google Scholar]
- Tsumuraya, T; Fujii, I; Hirama, M. Production of monoclonal antibodies for sandwich immunoassay detection of Pacific ciguatoxins. Toxicon 2010. [Google Scholar] [CrossRef]
- Tsumuraya, T; Fujii, I; Inoue, M; Tatami, A; Miyazaki, K; Hirama, M. Production of monoclonal antibodies for sandwich immunoassay detection of ciguatoxin 51-hydroxyCTX3C. Toxicon 2006, 48, 287–294. [Google Scholar]
- Ui, M; Tanaka, Y; Tsumuraya, T; Fujii, I; Inoue, M; Hirama, M; Tsumoto, K. How protein recognizes ladder-like polycyclic ethers - Interactions between ciguatoxin (CTX3C) fragments and its specific antibody 10C9. J. Biol. Chem 2008, 283, 19440–19447. [Google Scholar]
- Harlow, E; Lane, D. Antibodies: A Laboratory Manual; Cold Spring Harbour Laboratory Press: New York, NY, USA, 1988; p. 695. [Google Scholar]
- Murata, M; Legrand, A; Ishibashi, Y; Yasumoto, T. Structures of ciguatoxin and its congener. J. Am. Chem. Soc 1989, 111, 8929–8931. [Google Scholar]
- Baden, D; Mende, T; Szmant, A; Trainer, V; Edwards, R; Roszell, L. Brevetoxin binding: molecular pharmacology versus immunoassay. Toxicon 1988, 26, 97–103. [Google Scholar]
- Baden, D; Mende, T; Walling, J; Schultz, D. Specific antibodies directed against toxins of Ptychodiscus brevis (Florida’s red tide dinoflagellate). Toxicon 1984, 22, 783–789. [Google Scholar]
- Melinek, R; Rein, K; Schultz, D; Baden, D. Brevetoxin PbTx-2 immunology: differential epitope recognition by antibodies from two goats. Toxicon 1994, 32, 883–890. [Google Scholar]
- Naar, J; Branaa, P; Bottein-Dechraoui, M; Chinain, M; Pauillac, S. Polyclonal and monoclonal antibodies to PbTx-2-type brevetoxins using minute amount of hapten protein conjugates obtained in a reversed micellar medium. Toxicon 2001, 39, 869–878. [Google Scholar]
- Poli, M; Rein, K; Baden, D. Radioimmunoassay for PbTx-2-type brevetoxins: epitope specificity of two anti-PbTx sera. J. AOAC Int 1995, 78, 538–542. [Google Scholar]
- Pauillac, S; Halmos, T; Labrousse, H; Antonakis, K; Avrameas, S. Production of highly specific monoclonal antibodies to monensin and development of a microELISA to detect this antibiotic. J. Immunol. Meth 1993, 164, 165–173. [Google Scholar]
- Naar, J; Branaa, P; Chinain, M; Pauillac, S. An Improved Method for the Microscale Preparation and Characterization of Hapten- Protein Conjugates: The Use of Cholesterol as a Model for Nonchromophore Hydroxylated Haptens. Bioconjugate Chem 1999, 10, 1143–1149. [Google Scholar]
- Habeeb, A. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem 1966, 14, 328–336. [Google Scholar]
- Pauillac, S; Naar, J; Mouratou, B; Guesdon, J. Application of a modified version of Habeeb’s trinitrophenylation method for the characterization of hapten protein conjugates in a reversed micellar medium. J. Immunol. Meth 2002, 263, 75–83. [Google Scholar]
- Friguet, B; Chaffotte, A; Djavadi-Ohaniance, L; Goldberg, M. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Meth 1985, 77, 305–319. [Google Scholar]
- Yasumoto, T; Igarashi, T; Legrand, A; Cruchet, P; Chinain, M; Fujita, T; Naokis, H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectroscopy. J. Am. Chem. Soc 2000, 122, 4988–4989. [Google Scholar]
- Sasaki, M; Inoue, M; Tachibana, K. Synthetic studies toward ciguatoxin. Stereocontrolled construction of the KLM ring fragment. J. Org. Chem 1994, 59, 715–717. [Google Scholar]
- Pauillac, S; Sasaki, M; Inoue, M; Naar, J; Branaa, P; Chinain, M; Tachibana, K; Legrand, A. Characterization of mice antisera elicited with a ciguatoxin tetracyclic synthetic ring fragment (JKLM) conjugated to carrier proteins. Toxicon 2000, 38, 669–685. [Google Scholar]
- Campora, CE; Hokama, Y; Ebesu, JSM. Comparative analysis of purified Pacific and Caribbean ciguatoxin congeners and related marine toxins using a modified elisa technique. J. Clin. Lab. Anal 2006, 20, 121–125. [Google Scholar]
- Campora, CE; Hokama, Y; Yabusaki, K; Isobe, M. Development of an enzyme-linked immunosorbent assay for the detection of ciguatoxin in fish tissue using chicken immunoglobulin Y. J. Clin. Lab. Anal 2008, 22, 239–245. [Google Scholar]
- Catterall, WA; Risk, M. Toxin T46 from Ptychodiscus brevis (formely Gymnodinium breve) enhances activation of voltage-sensitive sodium channels by veratridine. Mol. Pharmacol 1981, 19, 345–348. [Google Scholar]
- Barchi, R; Weigele, J. Characteristics of saxitoxin binding to the sodium channel of sarcolemma isolated from rat skeletal muscle. J. Physiol 1979, 295, 383–396. [Google Scholar]
- Al-Sabi, A; McArthur, J; Ostroumov, V; French, R. Marine toxins that target voltage-gated sodium channels. Mar. Drugs 2006, 4, 157–192. [Google Scholar]
- Arias, H. Marine toxins targeting ion channels. Mar. Drugs 2006, 4, 37–69. [Google Scholar]
- Llewellyn, L; Doyle, J. Microtitre plate assay for paralytic shellfish toxins using saxiphilin: gauging the effects of shellfish extract matrices, salts and pH upon assay performance. Toxicon 2001, 39, 217–224. [Google Scholar]
- Vieytes, M; Cabado, A; Alfonso, A; Louzao, M; Botana, A; Botana, L. Solid-phase radioreceptor assay for paralytic shellfish toxins. Anal. Biochem 1993, 211, 87–93. [Google Scholar]
- Doucette, G; Logan, M; Ramsdell, J; Van Dolah, F. Development and preliminary validation of a microtiter plate-based receptor binding assay for paralytic shellfish poisoning toxins. Toxicon 1997, 35, 625–636. [Google Scholar]
- Llewellyn, L; Doyle, J; Negri, A. A high-throughput, microtiter plate assay for paralytic shellfish poisons using the saxitoxin-specific receptor, saxiphilin. Anal. Biochem 1998, 261, 51–56. [Google Scholar]
- Llewellyn, L; Negri, A; Doyle, J; Baker, P; Beltran, E; Neilans, B. Radioreceptor assays for sensitive detection and quantitation of saxitoxin and its analogues from strains of the freshwater cyanobacterium, Anabaena circinalis. Environ. Sci. Tech 2001, 35, 1445–1451. [Google Scholar]
- Ruberu, S; Liu, Y; Wong, C; Perera, S; Langlois, G; Doucette, G; Powell, C. Receptor binding assay for paralytic shellfish poisoning toxins: optimization and interlaboratory comparison. J. AOAC Int 2003, 86, 737–745. [Google Scholar]
- Usup, G; Leaw, C; Cheah, M; Ahmad, A; Ng, B. Analysis of paralytic shellfish poisoning toxin congeners by a sodium channel receptor binding assay. Toxicon 2004, 44, 37–43. [Google Scholar]
- Wang, D. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar]
- Sharkey, R; Jover, E; Couraud, F; Baden, D; Catterall, W. Allosteric modulation of neurotoxin binding to voltage-sensitive sodium channels by Ptychodiscus brevis toxin 2. Mol. Pharmacol 1987, 31, 273–278. [Google Scholar]
- Poli, M; Mende, T; Baden, D. Anderson, DM, White, AW, Baden, DG, Eds.; Characterization of the Ptychodiscus brevis polyether binding component in excitable membranes. In Toxic Dinoflagellates; Elsevier: New York, NY, USA, 1985; pp. 357–362. [Google Scholar]
- Poli, M; Mende, T; Baden, D. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol 1986, 30, 129–135. [Google Scholar]
- Nicholson, G; Lewis, R. Ciguatoxins: Cyclic polyether modulators of voltage-gated ion channel function. Mar. Drugs 2006, 4, 82–118. [Google Scholar]
- Poli, M; Lewis, R; Dickey, R; Musser, S; Buckner, C; Carpenter, L. Identification of Caribbean ciguatoxins as the cause of an outbreak of fish poisoning among US soldiers in Haiti. Toxicon 1997, 35, 733–741. [Google Scholar]
- Poli, M; Musser, S; Dickey, R; Eilers, P; Hall, S. Neurotoxic shellfish poisoning and brevetoxin metabolites: a case study from Florida. Toxicon 2000, 38, 981–993. [Google Scholar]
- Whitney, P; Delgado, J; Baden, D. Complex behavior of marine animal tissue extracts in the competitive binding assay of brevetoxins with rat brain synaptosomes. Nat. Toxins 1997, 5, 193–200. [Google Scholar]
- Trainer, V; Baden, D; Catterall, W. Detection of marine toxins using reconstituted sodium channels. J. AOAC Int 1995, 78, 570–573. [Google Scholar]
- Woofter, R; Dechraoui, M; Garthwaite, I; Towers, N; Gordon, C; Córdova, J; Ramsdell, J. Measurement of brevetoxin levels by radioimmunoassay of blood collection cards after acute, long-term, and low-dose exposure in mice. Environ. Health Perspect 2003, 111, 1595. [Google Scholar]
- Van Dolah, FM; Finley, EL; Haynes, BL; Doucette, GJ; Moeller, PD; Ramsdell, JS. Development of rapid and sensitive high throughput pharmacologic assays for marine phycotoxins. Nat. Toxins 1994, 2, 189–196. [Google Scholar]
- Dodd, P; Hardy, J; Oakley, A; Edwardson, J; Perry, E; Delaunoy, J. A rapid method for preparing synaptosomes: comparison, with alternative procedures. Brain Res 1981, 226, 107–118. [Google Scholar]
- Pottier, I; Vernoux, J; Lewis, R. Ciguatera fish poisoning in the Caribbean islands and Western Atlantic. Rev. Environ. Contam. Toxicol 2001, 168, 99–141. [Google Scholar]
- Tosteson, T; Ballantine, D; Durst, H. Seasonal frequency of ciguatoxic barracuda in southwest Puerto Rico. Toxicon 1988, 26, 795–801. [Google Scholar]
- Darius, T. Institut Louis Malardé: Papeete, French Polinesia, unpublished work; 2010.
- Higerd, TB; Babinchak, JA; Scheuer, PJ; Jollow, DJ. Resolution of Ciguatera-Associated Toxins Using High-Performance Liquid Chromatography (HPLC). Mar. Fish. Rev 1986, 48, 23–28. [Google Scholar]
- Legrand, AM; Cruchet, P; Bagnis, R; Murata, M; Ishibashi, Y; Yasumoto, T. Graneli, E, Sundstrom, B, Edler, L, Anderson, DM, Eds.; Chromatographic and spectral evidence for the presence of multiple ciguatera toxins. In Toxic Marine Phytoplankton; Elsevier: New York, NY, USA, 1990; pp. 374–378. [Google Scholar]
- Legrand, A; Teai, T; Crochet, P; Satake, M; Murata, K; Yasumoto, T. Two structural types of ciguatoxins involved on ciguatera fish poisoning in French Polynesia. Proceeding of the VIII International conference on Harmful algae, Vigo, Spain, 1997.
- Goto, J; Goto, N; Shamsa, F; Saito, M; Komatsu, S; Suzaki, K; Nambara, T. New sensitive derivatization of hydroxysteroids for high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 1983, 147, 397–400. [Google Scholar]
- Legrand, AM; Fukui, M; Cruchet, P; Ishibashi, Y; Yasumoto, T. Characterization of ciguatoxins from different fish species and wild G. toxicus. Proceeding of the 3rd International Conference on Ciguatera, Puerto Rico, 1990.
- Pauillac, S; Bléhaut, J; Cruchet, P; Lotte, C; Legrand, A. Lassus, P, Erad, E, Gentien, P, Marcaillou, C, Eds.; Recent advances in detection of ciguatoxins in French Polynesia. In Harmful Algal Blooms; Lavoisier: Intercept Paris, France, 1995; pp. 801–808. [Google Scholar]
- Lee, JS; Murata, M; Yasumoto, T. Analytical methods for determination of diarrhetic shellfish toxins. In Mycotoxins and Phycotoxins ‘88; Elsevier: Amsterdam, The Netherland, 1989; pp. 327–334. [Google Scholar]
- Yasumoto, T; Satake, M; Fukui, M; Nagai, H; Murata, K; Legrand, AM. Smayda, TJ, Shimizu, Y, Eds.; A turning point in ciguatera study. In Toxic Phytoplankton Blooms in the Sea; Elsevier Science Publishers: New York, NY, USA, 1993; pp. 455–460. [Google Scholar]
- Dickey, R; Bencsath, F; Granade, H; Lewis, R. Liquid chromatographic mass spectrometric methods for the determination of marine polyether toxins. Bulletin de la Société de pathologie exotique 1992, 85, 514–515. [Google Scholar]
- Sick, L; Hansen, D; Babinchak, J; Higerd, B. An HPLC-Fluorescence Method for Identifying a Toxic Fraction Extracted from the Marine Dinoflagellate Gambierdiscus toxicus. Mar. Fish. Rev 1986, 48, 29–34. [Google Scholar]
- Oshima, Y; Machida, M; Sasaki, K; Tamaoki, Y; Yasumoto, T. Liquid chromatographic-fluorometric analysis of paralytic shellfish toxins. Agric. Biol. Chem 1984, 48, 1707–1711. [Google Scholar]
- Franco, J; Fernández-Vila, P. Separation of paralytic shellfish toxins by reversed phase high performance liquid chromatography, with postcolumn reaction and fluorimetric detection. Chromatographia 1993, 35, 613–620. [Google Scholar]
- Lewis, R; Holmes, M; Alewood, P; Jones, A. Ionspray mass spectrometry of ciguatoxin-1, maitotoxin-2 and-3, and related marine polyether toxins. Nat. Toxins 1994, 2, 56–63. [Google Scholar]
- Lewis, R; Jones, A. Characterization of ciguatoxins and ciguatoxin congeners present in ciguateric fish by gradient reverse-phase high-performance liquid chromatography/mass spectrometry. Toxicon 1997, 35, 159–168. [Google Scholar]
- Lewis, R; Jones, A; Vernouxs, J. HPLC/tandem electrospray mass spectrometry for the determination of Sub-ppb levels of Pacific and Caribbean ciguatoxins in crude extracts of fish. Anal. Chem 1999, 71, 247–250. [Google Scholar]
- Hamilton, B; Whittle, N; Shaw, G; Eaglesham, G; Moore, M; Lewis, R. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2009. [Google Scholar] [CrossRef]
- Lewis, R; Norton, R; Brereton, I; Eccles, C. Ciguatoxin-2 is a diastereomer of ciguatoxin-3. Toxicon 1993, 31, 637. [Google Scholar]
- Nicholson, G; Lewis, R. Ciguatoxins: Cyclic polyether modulators of voltage-gated Iion channel function. Mar. Drugs 2006, 4, 82–118. [Google Scholar]
- Lewis, RJ; Molgó, J; Adams, DJ. Botana, LM, Ed.; Ciguatoxins: Cyclic Polyether Modulators of Voltage-gated Iion Channel Function. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection. Food Science and Technology Series; Marcel Dekker Inc: New York, NY, USA, 2000; Volume 103, pp. 419–447. [Google Scholar]
- Herzberg, A. Toxicity of Siganus luridus (Rüppell) on the Mediterranean coast of Israel. Aquaculture 1973, 2, 89–91. [Google Scholar]
- Raikhlin-Eisenkraft, B; Bentur, Y. Rabbitfish (“Aras”): An unusual source of ciguatera poisoning. Isr. Med. Assoc. J 2002, 4, 28–30. [Google Scholar]
- Spanier, E; Finkelstein, Y; Raikhlin-Eisenkraft, B. Toxicity of the saupe, Sarpa salpa (Linnaeus, 1758), on the Mediterranean coast of Israel. J. Fish Biol 1989, 34, 635–636. [Google Scholar]
- Bentur, Y; Spanier, E. Evaluation of Ciguatoxin on the Eastern Mediterranean Coast. J. Toxicol. Clin. Toxicol 2004, 42, 754. [Google Scholar]
- Bentur, Y; Spanier, E. Ciguatoxin-like substances in edible fish on the eastern Mediterranean. Clin. Toxicol 2007, 45, 695–700. [Google Scholar]
- de Haro, L; Pommier, P. Hallucinatory fish poisoning (ichthyoallyeinotoxism): two case reports from the western Mediterranean and literature review. Clin. Toxicol 2006, 44, 185–188. [Google Scholar]
- Silva, ES. Contribution à l’étude du micro-plancton, de Dakar et des régions maritimes voisines. Bulletin de l’ Institut Français d’Afrique Noire. Ser A 1956, 335–371. [Google Scholar]
- Caillaud, A; Fraga, S; Aligizaki, K; Mohammad-Noor, N; Nikolaidis, G; Moestrup, O; Diogène, J. Desarrollo de un ensayo celular para la detección de maitotoxinas en Gambierdiscus spp. Estudio comparativo entre cepas de distinctas procedencias. Abstract book of the X Reuniao Oberica, Fitoplancton Toxico e Biotoxinas, Lisbon, Portugal, 2009.
- Fraga, S. Instituto Español de Oceanografía: Vigo, Spain, unpublished data; 2010.
- Fraga, S. Global Climate Change and Harmful Algal Blooms (HABs). Abstract book of the 4th European Phycological Congress, Oviedo, Spain, 23–27 July 2007; p. 41.
- Bianchi, C. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar]
- Salat, J; Pascual, J. The oceanographic and meteorological station at L’Estartit (NW Mediterranean). Tracking long-term hydrological change in the Mediterranean Sea. CIESM Workshop Ser 2002, 16, 29–32. [Google Scholar]
- Mangialajo, L; Bertolotto, R; Cattaneo-Vietti, R; Chiantore, M; Grillo, C; Lemée, R; Melchiorre, N; Moretto, P; Povero, P; Ruggieri, N. The toxic benthic dinoflagellate Ostreopsis ovata: Quantification of proliferation along the coastline of Genoa, Italy. Mar. Pollut. Bull 2008, 56, 1209–1214. [Google Scholar]
- Aligizaki, K; Nikolaidis, G. The presence of the potentially toxic genera Ostreopsis and Coolia (Dinophyceae) in the North Aegean sea, Greece. Harmful Algae 2006, 5, 717–730. [Google Scholar]
- Vila, M; Garcés, E; Masó, M. Potentially toxic epiphytic dinoflagellate assemblages on macroalgae in the NW Mediterranean. Aquat. Microb. Ecol 2001, 26, 51–60. [Google Scholar]
- Turki, S. Distribution of toxic dinoflagellates along the leaves of seagrass Posidonia oceanica and Cymodocea nodosa from the Gulf of Tunis. Cahiers De Biologie Marine 2005, 46, 29–34. [Google Scholar]
- Penna, A; Vila, M; Fraga, S; Giacobbe, MG; Andreoni, F; Riobó, P; Vernesi, C. Characterization of Ostreopsis and Coolia (Dinophyceae) isolates in the western Mediterranean Sea based upon morphology, toxicity and internal transcribed spacer 5.8s rDNA sequences. J. Phycol 2005, 41, 212–225. [Google Scholar]
- Penna, A; Fraga, S; Battocchi, C; Casabianca, S; Giacobbe, MG; Riobó, P; Vernesi, C. A phylogeographical study of the toxic benthic dinoflagellate genus Ostreopsis Schmidt. J. Biogeogr 2010, 1–12. [Google Scholar]
- Bomber, J; Morton, S; Babinchak, J; Norris, D; Morton, J. Epiphytic Dinoflagellates of Drift AlgaeAnother Toxigenic Community in the Ciguatera Food Chain. Bull. Mar. Sci 1988, 43, 204–214. [Google Scholar]
- Hales, S; Weinstein, P; Woodward, A. Ciguatera (Fish Poisoning), El Nino, and Pacific Sea Surface Temperatures. Ecosyst. Health 1999, 5, 20–25. [Google Scholar]
- Moore, S; Trainer, V; Mantua, N; Parker, M; Laws, E; Backer, L; Fleming, L. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4. [Google Scholar]
- Shears, NT; Ross, PM. Blooms of benthic dinoflagellates of the genus Ostreopsis; an increasing and ecologically important phenomenon on temperate reefs in New Zealand and worldwide. Harmful Algae 2009, 8, 916–925. [Google Scholar]
- Matsumoto, T; Nagashima, Y; Kusuhara, H; Sugiyama, Y; Ishizaki, S; Shimakura, K; Shiomi, K. Involvement of carrier-mediated transport system in uptake of tetrodotoxin into liver tissue slices of puffer fish Takifugu rubripes. Toxicon 2007, 50, 173–179. [Google Scholar]
- Hop, H; Borga, K; Gabrielsen, G; Kleivane, L; Skaare, J. Food web magnificaton of persistent organic pollutants in poikilotherms and homeotherms. Environ. Sci. Technol 2002, 36, 2589–2597. [Google Scholar]
- Perry, A; Low, P; Ellis, J; Reynolds, J. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar]
- Stebbing, A; Turk, S; Wheeler, A; Clarke, K. Immigration of southern fish species to southwest England linked to warming of the North Atlantic (1960–2001). J. Mar. Biol. Assoc. UK 2002, 82, 177–180. [Google Scholar]
- European Union Commission Regulation EC 1021/2008. Off. J. Eur. Union 2008, L277, 15–17.
- European Union Commission Regulation EC 2074/2005. Off. J. Eur. Union 2005, L338, 27–59.
- European Union Commission Regulation EC 1664/2006. Off. J. Eur. Union 2006, L320, 13–45.
- European Union Commission Regulation EC 1244/2007. Off. J. Eur. Union 2007, L281, 12–18.
- European Union Commission Regulation EC 854/2004. Off. J. Eur. Union 2004, L226, 83–127.
- Guzmán-Pérez, SE; Douglas, LP. Botana, LM, Ed.; Ciguatera toxins: Chemistry and Detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Ledoux, M; Fremy, J. Phytoplankton, phycotoxins and seafood poisoning. Recueil de Medecine Veterinaire 1994, 170, 129–139. [Google Scholar]
- Bagnis, R. Socioeconomic impact of ciguatera in French Polynesia. Proceeding of the International Conference on Ciguatera Fish Poisoning, Papeete, French Polynesia, 1992.
- FAO, Food and nutrition paper 80, Risk assessment for ciguatoxins. In Marine Biotoxins Food and Agricultural Organization of the United Nations; FAO: Roma, Italy, 2004; p. 222.
- Bruslé, J. Ciguatera Fish Poisoning-A Review. Sanitary and Economic Aspects; Les éditions INSERM: Paris, France, 1997; p. 150. [Google Scholar]
- Kumar-Roiné, S; Matsui, M; Reybier, K; Darius, HT; Chinain, M; Pauillac, S; Laurent, D. Ability of certain plant extracts traditionally used to treat ciguatera fish poisoning to inhibit nitric oxide production in RAW 264.7 macrophages. J. Ethnopharmacol 2009, 123, 369–377. [Google Scholar]








| Origin | Number of rings | Number of carbons | Examples of CTX | Molecular Weigh | Source | |
|---|---|---|---|---|---|---|
| Pacific (P-) | Type I | 13 | 60 | CTX (CTX1B, CTX-1) | 1110.6 | carnivorous fish |
| CTX2A2 (CTX-2, 52-epi-54-deoxyCTX) | 1094.5 | carnivorous fish | ||||
| CTX2B2 (CTX-3, 54-deoxyCTX) | 1094.5 | carnivorous fish | ||||
| CTX4A | 1060.8 | G. toxicus, G. polynesiensis | ||||
| CTX4B (GTX-4B, Gt 4b) | 1060.8 | G. toxicus, G. polynesiensis, herbivorous fish | ||||
| Type II | 13 | 57 | CTX3C | 1022.8 | G. toxicus, G. polynesiensis, herbivorous fish | |
| CTX2A1 (2,3-dihydroxyCTX3C) | 1056.0 | carnivorous fish | ||||
| Caribbean (C-) | 14 | 62 | CTX-1 | 1140.7 | carnivorous fish | |
| CTX-2 | 1140.7 | carnivorous fish | ||||
| Indian (I-) | nd | nd | CTX-1 | 11406 | carnivorous fish | |
| CTX-2 | 1140.6 | carnivorous fish | ||||
| CTX-3 | 1157.6 | carnivorous fish | ||||
| CTX-4 | 1157.6 | carnivorous fish | ||||
| Name | Alternative name | Molecular ion [M + H]+ | Source | References |
|---|---|---|---|---|
| Pacific ciguatoxins | ||||
| P-CTX-1 | CTX1B | 1111.6 | Carnivorous fish | [45,271] |
| P-CTX-2 | CTX2A2; 52-epi-54-deoxyCTX | 1095.5 | Carnivorous fish | [45,271,274] |
| P-CTX-3 | CTX2B2; 54-deoxyCTX | 1095.5 | Carnivorous fish | [45,271] |
| 49-epi-CTX-3C | CTX-3B | 1023.6 | G. toxicus | [69,99] |
| M-seco-CTX-3C | 1041.6 | G. toxicus | [69,99] | |
| CTX-3C | 1023.6 | G. toxicus | [99,122] | |
| 2,3-dihydroxyCTX-3C | CTX-2A1 | 1057.6 | Carnivorous fish/G. toxicus | [197,122] |
| 51-hydroxyCTX-3C | CTX-2C1 | 1039.5 | Carnivorous fish | [197] |
| CTX-4B | GT-4B | 1061.6 | G. toxicus Herbivorous fish | [29,122,227] |
| 52-epi-ciguatoxin-4B | CTX-4A; GT-4A | 1061.6 | G. toxicus | [100,122,227] |
| Caribbean ciguatoxins | ||||
| C-CTX-1 | 1141.6 | Carnivorous fish | [94,97,98,102,147,272] | |
| C-CTX-2 | 56-epi-C-CTX-1 | 1141.6 | Carnivorous fish | [94,97,98,102,147] |
| C-CTX-1127 | 1127.6 | Carnivorous fish | [97,98,147] | |
| C-CTX-1143 | 1143.6 | Carnivorous fish | [97,98,147] | |
| C-CTX-1157 | 1157.6 | Carnivorous fish | [97,98,147] | |
| C-CTX-1159 | 1159.6 | Carnivorous fish | [97,98,147] | |
| Indian ciguatoxins | ||||
| I-CTX-1 | 1141.6 | Carnivorous fish | [96] | |
| I-CTX-2 | 1141.6 | Carnivorous fish | [95,96] | |
| I-CTX-3 | 1157.6 | Carnivorous fish | [95,96] | |
| I-CTX-4 | 1157.6 | Carnivorous fish | [95,96] | |
| Level of information | Factors to be addressed | Impact and application (examples) within and beyond risk assessment |
|---|---|---|
| Food intoxication and social aspects | ||
| Food | Toxin content, unequivocal species identification, origin of food, traceability | Species based risk association, hazard characterization, food retrievals, elaboration of safe lists, identification of irregular practices, fraud. |
| Symptoms | General, gastrointestinal, cardiovascular, neurological, time lapses, duration, intensity | CFP diagnosis, regional characterization |
| Epidemiology | Clinical records, restaurant records, intoxication surveys, unreported cases, treatment | Risk characterization, populations at risk, therapy assessment, alternative food sources, epidemiological survey, databases |
| Food habits, market | Consumer surveys, fish trade and sales, fishing practices | Eating habits, exposure assessment, identify areas at risk |
| Ethnology | Avoidances, remedies | Historical learning, local palliative measures, identify population exposed |
| Environment | ||
| Environmental data | Latitude, temperature, salinity, turbulence, turbidity, currents, stratification | Microalgal distribution, ecophysiology, toxin production, fish distribution, fish migration, prediction of potential expansion |
| Habitat | Benthic structure, benthic communities, coral status (bleaching), anthropogenic activities | Surface availability for Gambierdiscus growth, population succession |
| Toxins | Chemical structure, toxicological potency, toxicological factor for each CTX derivative | Toxin characterization, hazard prediction according to toxin analysis, regional differences according to toxin profiles |
| Microalgae | Gambierdiscus spp., other benthic dinoflagellates and microalgal species producing toxins, species distribution and population dynamics, toxin content of natural populations and of clone cultures | Identification of causative toxin producer species, comparative approach among sites, identification of hot spots, temporal variations, intra-specific variation, definition of monitoring strategies |
| Fish | Taxonomy, toxin content, transmission, bioaccumulation and metabolization. Toxins: intra-specific variation/organs/age-size. Trophic level: herbivorous, carnivorous. Behavior: Migration | Species identification, risk associated to species, age, size Species distribution Toxic potency, identification of indicative species |
| Level of action | Factors to be addressed | Impact and application (examples) |
|---|---|---|
| Coordination between implicated agencies | Define agency to coordinate actions on CFP at regional level Define a common strategy for the management of CFP Centralization of the information and multi-lateral communication Define competent authorities Identify toxins addressed, including derivatives | Optimizes reaction and preventive actions Reduces the impact and cost of CFP |
| Legislation | Define acceptable levels of toxins in food Official methods for toxin recognition CFP ranking of species or groups of fish to be avoided or controlled Responsibilities of fishermen and retailers Import/export policies | Provides a framework for strategy and decision making and defines responsibilities |
| Laboratory | CTX standards Reference material Methodology set up and standardization | CTX identification and quantification |
| Monitoring | Toxin content in fish with validated methods Fish identification Gambierdiscus spp. population dynamics Toxin content in Gambierdiscus spp. with validated methods Environmental data (e.g., seawater temperature) | Optimizes the strategy for toxin recognition in food and the identification of fish and areas at risk Reduces CFP records Relation between onset of ciguatera and climate change |
| CFP records | Symptom diagnosis Confirmation of CTX in blood/plasma of patients Regional-based epidemiological data | Provides morbidity statistics Characterizes specific regional symptoms Reduce misidentification of other types of non ciguatera ichtyosarcotoxisms |
| CFP treatment | Medical board awareness on CFP Definition of treatment protocols Availabiliity of treatments | Reduces personal CFP symptoms Reduces hospitalization and economic impact |
| Industry/Market/Consumer | Define alternative food sources and avoidable bad practices Define own strategies to overcome CFP according to regional recommendations Report CFP intoxications Traceability of processed food | Reduce CFP records Reduce CFP impact on health and industry (fisheries and tourism) |
| Level of communication | Factors to be addressed | Impact and application (examples) |
|---|---|---|
| Widespread | Listing of species and areas at risk Listing of CFP symptoms Define consumers actions to take in case of CFP Report CFP episodes Report ciguatera warnings and specific instructions during episodes Evaluate public perception and knowledge on ciguatera | Avoidance of fishing and marketing selected species of fish Avoidance of viscera consumption Favor CFP reporting by consumers Improve risk communication strategies |
| Selective target | Facilitate communication and training of administration staff, retailers, fishermen, travel agencies and consumers Listing of CFP symptoms to favor CFP reporting by medical staff Disseminate protocols of communication among agencies Facilitate transfer of information to research agencies involved in the study of ciguatera Evaluate professional perception and knowledge on ciguatera | Reduce CFP impact Improve CFP epidemiological records Understand efficiency of CFP therapies Improve scientific comprehension of ciguatera Improve risk communication strategies |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Caillaud, A.; De la Iglesia, P.; Darius, H.T.; Pauillac, S.; Aligizaki, K.; Fraga, S.; Chinain, M.; Diogène, J. Update on Methodologies Available for Ciguatoxin Determination: Perspectives to Confront the Onset of Ciguatera Fish Poisoning in Europe. Mar. Drugs 2010, 8, 1838-1907. https://doi.org/10.3390/md8061838
Caillaud A, De la Iglesia P, Darius HT, Pauillac S, Aligizaki K, Fraga S, Chinain M, Diogène J. Update on Methodologies Available for Ciguatoxin Determination: Perspectives to Confront the Onset of Ciguatera Fish Poisoning in Europe. Marine Drugs. 2010; 8(6):1838-1907. https://doi.org/10.3390/md8061838
Chicago/Turabian StyleCaillaud, Amandine, Pablo De la Iglesia, H. Taiana Darius, Serge Pauillac, Katerina Aligizaki, Santiago Fraga, Mireille Chinain, and Jorge Diogène. 2010. "Update on Methodologies Available for Ciguatoxin Determination: Perspectives to Confront the Onset of Ciguatera Fish Poisoning in Europe" Marine Drugs 8, no. 6: 1838-1907. https://doi.org/10.3390/md8061838
APA StyleCaillaud, A., De la Iglesia, P., Darius, H. T., Pauillac, S., Aligizaki, K., Fraga, S., Chinain, M., & Diogène, J. (2010). Update on Methodologies Available for Ciguatoxin Determination: Perspectives to Confront the Onset of Ciguatera Fish Poisoning in Europe. Marine Drugs, 8(6), 1838-1907. https://doi.org/10.3390/md8061838







