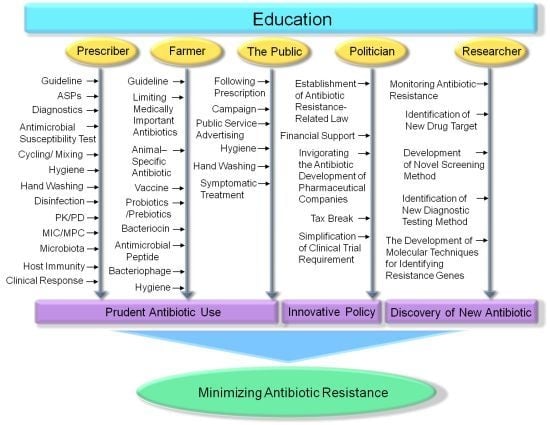
Strategies to Minimize Antibiotic Resistance
Abstract
:1. Introduction
2. Appropriate Antibiotic Prescribing


3. Antimicrobial Stewardship Programs
4. Education
5. Hygiene and Disinfection
6. Veterinary Medicine
7. The Development of Novel Antibiotics

8. Conclusions

Acknowledgments
Conflicts of Interest
References
- Palmer, A.C.; Kishony, R. Understanding, predicting and manipulating the genotypic evolution of antibiotic resistance. Nat. Rev. Genet. 2013, 14, 243–248. [Google Scholar] [CrossRef]
- Meyer, E.; Schwab, F.; Schroeren-Boersch, B.; Gastmeier, P. Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: Secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Crit. Care 2010, 14. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Mantengoli, E.; Docquier, J.D.; Musmanno, R.A.; Coratza, G. Epidemiology of infections caused by multiresistant Gram-negatives: ESBLs, MBLs, panresistant strains. New Microbiol. 2007, 30, 332–339. [Google Scholar]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr.; Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the infectious diseases society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Cantas, L.; Shah, S.Q.; Cavaco, L.M.; Manaia, C.M.; Walsh, F.; Popowska, M.; Garelick, H.; Burgmann, H.; Sorum, H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Otter, J.A.; French, G.L. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 2010, 10, 227–239. [Google Scholar] [CrossRef]
- Brown, S.; Bantar, C.; Young, H.K.; Amyes, S.G. Limitation of Acinetobacter baumannii treatment by plasmid-mediated carbapenemase ARI-2. Lancet 1998, 351, 186–187. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef]
- Strahilevitz, J.; Jacoby, G.A.; Hooper, D.C.; Robicsek, A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin. Microbiol. Rev. 2009, 22, 664–689. [Google Scholar] [CrossRef]
- Fischer, J.; Rodriguez, I.; Schmoger, S.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Salmonella enterica subsp. enterica producing VIM-1 carbapenemase isolated from livestock farms. J. Antimicrob. Chemother. 2013, 68, 478–480. [Google Scholar] [CrossRef]
- Fischer, J.; Rodriguez, I.; Schmoger, S.; Friese, A.; Roesler, U.; Helmuth, R.; Guerra, B. Escherichia coli producing VIM-1 carbapenemase isolated on a pig farm. J. Antimicrob. Chemother. 2012, 67, 1793–1795. [Google Scholar] [CrossRef]
- Almeida Da Silva, P.E.; Palomino, J.C. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: Classical and new drugs. J. Antimicrob. Chemother. 2011, 66, 1417–1430. [Google Scholar] [CrossRef]
- Dye, C. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat. Rev. Microbiol. 2009, 7, 81–87. [Google Scholar] [CrossRef]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O.; Group, E.-E.W. Critical shortage of new antibiotics in development against multidrug-resistant bacteria-time to react is now. Drug Resist. Updat. 2011, 14, 118–124. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, S.H.; Cha, S.S.; Lee, S.H. New disturbing trend in antimicrobial resistance of Gram-negative pathogens. PLoS Pathog. 2009, 5, e1000221. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, I.K.; Lee, S.H. New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med. Res. Rev. 2012, 32, 216–232. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, S.H.; Cha, S.-S.; Lee, S.H. A lack of drugs for antibiotic-resistant Gram-negative bacteria. Nat. Rev. Drug Discov. 2007, 6. [Google Scholar] [CrossRef]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E., Jr. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef]
- Livermore, D.M. Minimising antibiotic resistance. Lancet Infect. Dis. 2005, 5, 450–459. [Google Scholar] [CrossRef]
- Barber, M.; Rozwadowska-Dowzenko, M. Infection by penicillin-resistant Staphylococci. Lancet 1948, 2, 641–644. [Google Scholar]
- Levy, S.B. The challenge of antibiotic resistance. Sci. Am. 1998, 278, 46–53. [Google Scholar] [CrossRef]
- Lob, S.H.; Badal, R.E.; Bouchillon, S.K.; Hawser, S.P.; Hackel, M.A.; Hoban, D.J. Epidemiology and susceptibility of Gram-negative appendicitis pathogens: SMART 2008–2010. Surg. Infect. 2013, 14, 203–208. [Google Scholar] [CrossRef]
- Rossi, F.; Baquero, F.; Hsueh, P.R.; Paterson, D.L.; Bochicchio, G.V.; Snyder, T.A.; Satishchandran, V.; McCarroll, K.; DiNubile, M.J.; Chow, J.W. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative Bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). J. Antimicrob. Chemother. 2006, 58, 205–210. [Google Scholar] [CrossRef]
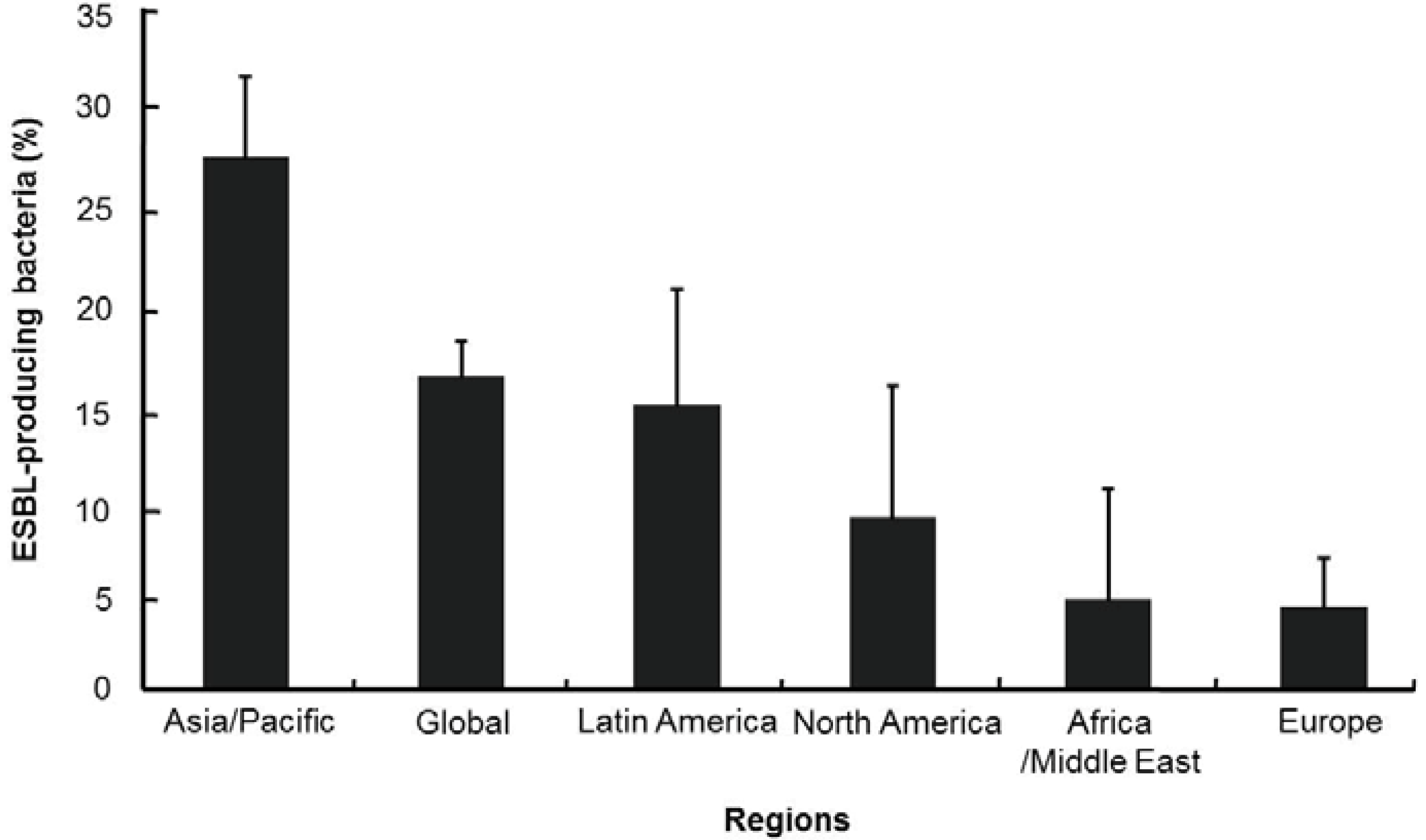
- Chen, Y.H.; Hsueh, P.R.; Badal, R.E.; Hawser, S.P.; Hoban, D.J.; Bouchillon, S.K.; Ni, Y.; Paterson, D.L. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative Bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J. Infect. 2011, 62, 280–291. [Google Scholar] [CrossRef]
- Hawser, S.P.; Bouchillon, S.K.; Hoban, D.J.; Badal, R.E.; Hsueh, P.R.; Paterson, D.L. Emergence of high levels of extended-spectrum-β-lactamase-producing Gram-negative Bacilli in the Asia-Pacific region: Data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob. Agents Chemother. 2009, 53, 3280–3284. [Google Scholar] [CrossRef]
- Hsueh, P.R. Study for Monitoring Antimicrobial Resistance Trends (SMART) in the Asia-Pacific region, 2002–2010. Int. J. Antimicrob. Agents 2012, 40, S1–S3. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Badal, R.E.; Hawser, S.P.; Hoban, D.J.; Bouchillon, S.K.; Ni, Y.; Paterson, D.L. Epidemiology and antimicrobial susceptibility profiles of aerobic and facultative Gram-negative Bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region: 2008 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). Int. J. Antimicrob. Agents 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Ko, W.C.; Hsueh, P.R. Increasing extended-spectrum β-lactamase production and quinolone resistance among Gram-negative Bacilli causing intra-abdominal infections in the Asia/Pacific region: Data from the Smart Study 2002–2006. J. Infect. 2009, 59, 95–103. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, Y.S.; Toh, H.S.; Lee, Y.L.; Liu, Y.M.; Ho, C.M.; Lu, P.L.; Liu, C.E.; Chen, Y.H.; Wang, J.H.; et al. Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia-Pacific region: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002–2010. Int. J. Antimicrob. Agents 2012, 40, S4–S10. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Hoban, D.J.; Carmeli, Y.; Chen, S.Y.; Desikan, S.; Alejandria, M.; Ko, W.C.; Binh, T.Q. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J. Infect. 2011, 63, 114–123. [Google Scholar] [CrossRef]
- Lu, P.L.; Liu, Y.C.; Toh, H.S.; Lee, Y.L.; Liu, Y.M.; Ho, C.M.; Huang, C.C.; Liu, C.E.; Ko, W.C.; Wang, J.H.; et al. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents. 2012, 40, S37–S43. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Carmeli, Y. Mortality and delay in effective therapy associated with extended-spectrum β-lactamase production in Enterobacteriaceae bacteraemia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2007, 60, 913–920. [Google Scholar] [CrossRef]
- Paterson, D.L.; Ko, W.C.; von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: Implications of production of extended-spectrum β-lactamases. Clin. Infect. Dis. 2004, 39, 31–37. [Google Scholar] [CrossRef]
- Lee, N.Y.; Huang, W.H.; Tsui, K.C.; Hsueh, P.R.; Ko, W.C. Carbapenem therapy for bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2011, 70, 150–153. [Google Scholar] [CrossRef]
- Bryan, J. Developments in antimicrobial resistance and treatment. Future Microbiol. 2011, 6, 715–720. [Google Scholar] [CrossRef]
- General Medical Council. Tomorrow’s Doctors: Out-Comes and Standards for Undergraduate Medical Education; General Medical Council: London, UK, 2009. Available online: http://www.gmc-uk.org/TomorrowsDoctors_2009.pdf_39260971.pdf (accessed on 4 September 2013).
- Aleva, R.M.; Boersma, W.G.; Dutch Thoracic, S. Guideline “diagnosis and treatment of community-acquired pneumonia” from the Dutch thoracic society. Ned. Tijdschr. Geneeskd. 2005, 149, 2501–2507. [Google Scholar]
- American Thoracic, S.; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef]
- Hayashi, Y.; Paterson, D.L. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin. Infect. Dis. 2011, 52, 1232–1240. [Google Scholar] [CrossRef]
- Hoffken, G.; Lorenz, J.; Kern, W.; Welte, T.; Bauer, T.; Dalhoff, K.; Dietrich, E.; Ewig, S.; Gastmeier, P.; Grabein, B.; et al. S3-guideline on ambulant acquired pneumonia and deep airway infections. Pneumologie 2005, 59, 612–664. [Google Scholar]
- McNulty, C.A.; Lecky, D.M.; Farrell, D.; Kostkova, P.; Adriaenssens, N.; Koprivova Herotova, T.; Holt, J.; Touboul, P.; Merakou, K.; Koncan, R.; et al. Overview of e-Bug: An antibiotic and hygiene educational resource for schools. J. Antimicrob. Chemother. 2011, 66, v3–v12. [Google Scholar] [CrossRef]
- Muto, C.A.; Jernigan, J.A.; Ostrowsky, B.E.; Richet, H.M.; Jarvis, W.R.; Boyce, J.M.; Farr, B.M.; SHEA. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect. Control Hosp. Epidemiol. 2003, 24, 362–386. [Google Scholar]
- Kett, D.H.; Cano, E.; Quartin, A.A.; Mangino, J.E.; Zervos, M.J.; Peyrani, P.; Cely, C.M.; Ford, K.D.; Scerpella, E.G.; et al. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: An observational, multicentre cohort study. Lancet Infect. Dis. 2011, 11, 181–189. [Google Scholar] [CrossRef]
- Nicasio, A.M.; Eagye, K.J.; Kuti, E.L.; Nicolau, D.P.; Kuti, J.L. Length of stay and hospital costs associated with a pharmacodynamic-based clinical pathway for empiric antibiotic choice for ventilator-associated pneumonia. Pharmacotherapy 2010, 30, 453–462. [Google Scholar] [CrossRef]
- Nicasio, A.M.; Eagye, K.J.; Nicolau, D.P.; Shore, E.; Palter, M.; Pepe, J.; Kuti, J.L. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J. Crit. Care 2010, 25, 69–77. [Google Scholar] [CrossRef]
- Kumar, A.; Zarychanski, R.; Light, B.; Parrillo, J.; Maki, D.; Simon, D.; Laporta, D.; Lapinsky, S.; Ellis, P.; Mirzanejad, Y.; et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: A propensity-matched analysis. Crit. Care Med. 2010, 38, 1773–1785. [Google Scholar] [CrossRef]
- Park, H.K.; Song, J.U.; Um, S.W.; Koh, W.J.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Jeon, K. Clinical characteristics of health care-associated pneumonia in a Korean teaching hospital. Respir. Med. 2010, 104, 1729–1735. [Google Scholar] [CrossRef]
- Shindo, Y.; Sato, S.; Maruyama, E.; Ohashi, T.; Ogawa, M.; Hashimoto, N.; Imaizumi, K.; Sato, T.; Hasegawa, Y. Health-care-associated pneumonia among hospitalized patients in a Japanese community hospital. Chest 2009, 135, 633–640. [Google Scholar] [CrossRef]
- Brito, V.; Niederman, M.S. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr. Opin. Infect. Dis. 2009, 22, 316–325. [Google Scholar] [CrossRef]
- Owens, R.C., Jr. Antimicrobial stewardship: Concepts and strategies in the 21st century. Diagn. Microbiol. Infect. Dis. 2008, 61, 110–128. [Google Scholar] [CrossRef]
- Drew, R.H. Antimicrobial stewardship programs: How to start and steer a successful program. J. Manag. Care Pharm. 2009, 15, S18–S23. [Google Scholar]
- Madaras-Kelly, K.J.; Remington, R.E.; Lewis, P.G.; Stevens, D.L. Evaluation of an intervention designed to decrease the rate of nosocomial methicillin-resistant Staphylococcus aureus infection by encouraging decreased fluoroquinolone use. Infect. Control Hosp. Epidemiol. 2006, 27, 155–169. [Google Scholar] [CrossRef]
- Harbarth, S.; Cosgrove, S.; Carmeli, Y. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 2002, 46, 1619–1628. [Google Scholar] [CrossRef]
- Calil, R.; Marba, S.T.; von Nowakonski, A.; Tresoldi, A.T. Reduction in colonization and nosocomial infection by multiresistant bacteria in a neonatal unit after institution of educational measures and restriction in the use of cephalosporins. Am. J. Infect. Control 2001, 29, 133–138. [Google Scholar] [CrossRef]
- Go, E.S.; Urban, C.; Burns, J.; Kreiswirth, B.; Eisner, W.; Mariano, N.; Mosinka-Snipas, K.; Rahal, J.J. Clinical and molecular epidemiology of acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet 1994, 344, 1329–1332. [Google Scholar] [CrossRef]
- Rahal, J.J.; Urban, C.; Segal-Maurer, S. Nosocomial antibiotic resistance in multiple gram-negative species: Experience at one hospital with squeezing the resistance balloon at multiple sites. Clin. Infect. Dis. 2002, 34, 499–503. [Google Scholar] [CrossRef]
- Cook, P.P.; Rizzo, S.; Gooch, M.; Jordan, M.; Fang, X.; Hudson, S. Sustained reduction in antimicrobial use and decrease in methicillin-resistant Staphylococcus aureus and Clostridium difficile infections following implementation of an electronic medical record at a tertiary-care teaching hospital. J. Antimicrob. Chemother. 2011, 66, 205–209. [Google Scholar] [CrossRef]
- Moehring, R.W.; Anderson, D.J. Antimicrobial stewardship as part of the infection prevention effort. Curr. Infect. Dis. Rep. 2012, 14, 592–600. [Google Scholar] [CrossRef]
- Paterson, D.L. The role of antimicrobial management programs in optimizing antibiotic prescribing within hospitals. Clin. Infect. Dis. 2006, 42, S90–S95. [Google Scholar] [CrossRef]
- Beardsley, J.R.; Williamson, J.C.; Johnson, J.W.; Luther, V.P.; Wrenn, R.H.; Ohl, C.C. Show me the money: Long-term financial impact of an antimicrobial stewardship program. Infect. Control Hosp. Epidemiol. 2012, 33, 398–400. [Google Scholar] [CrossRef]
- Carling, P.; Fung, T.; Killion, A.; Terrin, N.; Barza, M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect. Control Hosp. Epidemiol. 2003, 24, 699–706. [Google Scholar]
- Standiford, H.C.; Chan, S.; Tripoli, M.; Weekes, E.; Forrest, G.N. Antimicrobial stewardship at a large tertiary care academic medical center: Cost analysis before, during, and after a 7-year program. Infect. Control Hosp. Epidemiol. 2012, 33, 338–345. [Google Scholar] [CrossRef]
- Stamey, T.A.; Bragonje, J. Resistance to nalidixic acid. A misconception due to underdosage. JAMA 1976, 236, 1857–1860. [Google Scholar] [CrossRef]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilback, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Caron, W.P.; Mousa, S.A. Prevention strategies for antimicrobial resistance: A systematic review of the literature. Infect. Drug Resist. 2010, 3, 25–33. [Google Scholar]
- Canton, R.; Morosini, M.I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Restricting the selection of antibiotic-resistant mutants: A general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 2001, 33, S147–S156. [Google Scholar] [CrossRef]
- Pulido, M.R.; Garcia-Quintanilla, M.; Martin-Pena, R.; Cisneros, J.M.; McConnell, M.J. Progress on the development of rapid methods for antimicrobial susceptibility testing. J. Antimicrob. Chemother. 2013, in press. [Google Scholar]
- Van Belkum, A.; Dunne, W.M., Jr. Next-generation antimicrobial susceptibility testing. J. Clin. Microbiol. 2013, 51, 2018–2024. [Google Scholar] [CrossRef]
- Goff, D.A.; Jankowski, C.; Tenover, F.C. Using rapid diagnostic tests to optimize antimicrobial selection in antimicrobial stewardship programs. Pharmacotherapy 2012, 32, 677–687. [Google Scholar] [CrossRef]
- Khoruts, A.; Dicksved, J.; Jansson, J.K.; Sadowsky, M.J. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol. 2010, 44, 354–360. [Google Scholar]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; Gonzalez, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Robinson, C.J.; Young, V.B. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes 2010, 1, 279–284. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjolund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 2010, 5, e9836. [Google Scholar] [CrossRef]
- Antonopoulos, D.A.; Huse, S.M.; Morrison, H.G.; Schmidt, T.M.; Sogin, M.L.; Young, V.B. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect. Immun. 2009, 77, 2367–2375. [Google Scholar] [CrossRef]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef]
- Dessein, R.; Gironella, M.; Vignal, C.; Peyrin-Biroulet, L.; Sokol, H.; Secher, T.; Lacas-Gervais, S.; Gratadoux, J.J.; Lafont, F.; Dagorn, J.C.; et al. Toll-like receptor 2 is critical for induction of Reg3β expression and intestinal clearance of Yersinia pseudotuberculosis. Gut 2009, 58, 771–776. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Frutos Rde, L.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Hill, D.A.; Hoffmann, C.; Abt, M.C.; Du, Y.; Kobuley, D.; Kirn, T.J.; Bushman, F.D.; Artis, D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal. Immunol. 2010, 3, 148–158. [Google Scholar] [CrossRef]
- Umenai, T.; Hirai, H.; Shime, N.; Nakaya, T.; Asahara, T.; Nomoto, K.; Kita, M.; Tanaka, Y.; Imanishi, J. Eradication of the commensal intestinal microflora by oral antimicrobials interferes with the host response to lipopolysaccharide. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 633–641. [Google Scholar] [CrossRef]
- Pelissier, M.A.; Vasquez, N.; Balamurugan, R.; Pereira, E.; Dossou-Yovo, F.; Suau, A.; Pochart, P.; Magne, F. Metronidazole effects on microbiota and mucus layer thickness in the rat gut. FEMS Microbiol. Ecol. 2010, 73, 601–610. [Google Scholar]
- Faure, S.; Perrin-Guyomard, A.; Delmas, J.M.; Chatre, P.; Laurentie, M. Transfer of plasmid-mediated CTX-M-9 from Salmonella enterica serotype Virchow to Enterobacteriaceae in human flora-associated rats treated with cefixime. Antimicrob. Agents Chemother. 2010, 54, 164–169. [Google Scholar] [CrossRef]
- Lester, C.H.; Frimodt-Moller, N.; Sorensen, T.L.; Monnet, D.L.; Hammerum, A.M. In vivo transfer of the vanA resistance gene from an Enterococcus faecium isolate of animal origin to an E. faecium isolate of human origin in the intestines of human volunteers. Antimicrob. Agents Chemother. 2006, 50, 596–599. [Google Scholar] [CrossRef]
- Lofmark, S.; Jernberg, C.; Billstrom, H.; Andersson, D.I.; Edlund, C. Restored fitness leads to long-term persistence of resistant Bacteroides strains in the human intestine. Anaerobe 2008, 14, 157–160. [Google Scholar] [CrossRef]
- Sjolund, M.; Wreiber, K.; Andersson, D.I.; Blaser, M.J.; Engstrand, L. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann. Intern. Med. 2003, 139, 483–487. [Google Scholar] [CrossRef]
- Kale-Pradhan, P.B.; Jassal, H.K.; Wilhelm, S.M. Role of Lactobacillus in the prevention of antibiotic-associated diarrhea: A meta-analysis. Pharmacotherapy 2010, 30, 119–126. [Google Scholar] [CrossRef]
- Kinnebrew, M.A.; Ubeda, C.; Zenewicz, L.A.; Smith, N.; Flavell, R.A.; Pamer, E.G. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J. Infect. Dis. 2010, 201, 534–543. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Patel, A.; Song, X.; Miller, R.E.; Speck, K.; Banowetz, A.; Hadler, R.; Sinkowitz-Cochran, R.L.; Cardo, D.M.; Srinivasan, A. Impact of different methods of feedback to clinicians after postprescription antimicrobial review based on the Centers For Disease Control and Prevention’s 12 steps to prevent antimicrobial resistance among hospitalized adults. Infect. Control Hosp. Epidemiol. 2007, 28, 641–646. [Google Scholar] [CrossRef]
- Camins, B.C.; King, M.D.; Wells, J.B.; Googe, H.L.; Patel, M.; Kourbatova, E.V.; Blumberg, H.M. Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital: A randomized controlled trial. Infect. Control Hosp. Epidemiol. 2009, 30, 931–938. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Seo, S.K.; Bolon, M.K.; Sepkowitz, K.A.; Climo, M.W.; Diekema, D.J.; Speck, K.; Gunaseelan, V.; Noskin, G.A.; Herwaldt, L.A.; et al. Evaluation of postprescription review and feedback as a method of promoting rational antimicrobial use: A multicenter intervention. Infect. Control Hosp. Epidemiol. 2012, 33, 374–380. [Google Scholar] [CrossRef]
- Muller, B.; Becker, K.L.; Schachinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; Ritz, R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef]
- Jones, A.E.; Fiechtl, J.F.; Brown, M.D.; Ballew, J.J.; Kline, J.A. Procalcitonin test in the diagnosis of bacteremia: A meta-analysis. Ann. Emerg. Med. 2007, 50, 34–41. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Craig, J.C.; McLean, A.S. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 210–217. [Google Scholar] [CrossRef]
- Bal, A.M.; Kumar, A.; Gould, I.M. Antibiotic heterogeneity: From concept to practice. Ann. N. Y. Acad. Sci. 2010, 1213, 81–91. [Google Scholar] [CrossRef]
- Kollef, M.H. Is antibiotic cycling the answer to preventing the emergence of bacterial resistance in the intensive care unit? Clin. Infect. Dis. 2006, 43, S82–S88. [Google Scholar] [CrossRef]
- Masterton, R.G. Antibiotic heterogeneity. Int. J. Antimicrob. Agents 2010, 36, S15–S18. [Google Scholar] [CrossRef]
- Piper, G.L.; Kaplan, L.J. Antibiotic heterogeneity optimizes antimicrobial prescription and enables resistant pathogen control in the intensive care unit. Surg. Infect. 2012, 13, 194–202. [Google Scholar] [CrossRef]
- Bruno-Murtha, L.A.; Brusch, J.; Bor, D.; Li, W.; Zucker, D. A pilot study of antibiotic cycling in the community hospital setting. Infect. Control Hosp. Epidemiol. 2005, 26, 81–87. [Google Scholar]
- Pakyz, A.L.; Farr, B.M. Rates of Stenotrophomonas maltophilia colonization and infection in relation to antibiotic cycling protocols. Epidemiol. Infect. 2009, 137, 1679–1683. [Google Scholar] [CrossRef]
- Van Loon, H.J.; Vriens, M.R.; Fluit, A.C.; Troelstra, A.; van der Werken, C.; Verhoef, J.; Bonten, M.J. Antibiotic rotation and development of Gram-negative antibiotic resistance. Am. J. Respir. Crit. Care Med. 2005, 171, 480–487. [Google Scholar] [CrossRef]
- Warren, D.K.; Hill, H.A.; Merz, L.R.; Kollef, M.H.; Hayden, M.K.; Fraser, V.J.; Fridkin, S.K. Cycling empirical antimicrobial agents to prevent emergence of antimicrobial-resistant Gram-negative bacteria among intensive care unit patients. Crit. Care Med. 2004, 32, 2450–2456. [Google Scholar] [CrossRef]
- Cumpston, A.; Craig, M.; Hamadani, M.; Abraham, J.; Hobbs, G.R.; Sarwari, A.R. Extended follow-up of an antibiotic cycling program for the management of febrile neutropenia in a hematologic malignancy and hematopoietic cell transplantation unit. Transpl. Infect. Dis. 2013, 15, 142–149. [Google Scholar] [CrossRef]
- Bergstrom, C.T.; Lo, M.; Lipsitch, M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc. Natl. Acad. Sci. USA 2004, 101, 13285–13290. [Google Scholar] [CrossRef]
- Beardmore, R.E.; Pena-Miller, R. Antibiotic cycling versus mixing: The difficulty of using mathematical models to definitively quantify their relative merits. Math. Biosci. Eng. 2010, 7, 923–933. [Google Scholar] [CrossRef]
- Beardmore, R.E.; Pena-Miller, R. Rotating antibiotics selects optimally against antibiotic resistance, in theory. Math. Biosci. Eng. 2010, 7, 527–552. [Google Scholar] [CrossRef]
- Bennett, K.M.; Scarborough, J.E.; Sharpe, M.; Dodds-Ashley, E.; Kaye, K.S.; Hayward, T.Z., III; Vaslef, S.N. Implementation of antibiotic rotation protocol improves antibiotic susceptibility profile in a surgical intensive care unit. J. Trauma 2007, 63, 307–311. [Google Scholar] [CrossRef]
- Chong, Y.; Shimoda, S.; Yakushiji, H.; Ito, Y.; Miyamoto, T.; Kamimura, T.; Shimono, N.; Akashi, K. Antibiotic rotation for febrile neutropenic patients with hematological malignancies: Clinical significance of antibiotic heterogeneity. PLoS One 2013, 8, e54190. [Google Scholar] [CrossRef]
- Goulart, C.P.; Mahmudi, M.; Crona, K.A.; Jacobs, S.D.; Kallmann, M.; Hall, B.G.; Greene, D.C.; Barlow, M. Designing antibiotic cycling strategies by determining and understanding local adaptive landscapes. PLoS One 2013, 8, e56040. [Google Scholar] [CrossRef]
- Hashino, S.; Morita, L.; Kanamori, H.; Takahata, M.; Onozawa, M.; Nakagawa, M.; Kawamura, T.; Fujisawa, F.; Kahata, K.; Izumiyama, K.; et al. Clinical impact of cycling the administration of antibiotics for febrile neutropenia in Japanese patients with hematological malignancy. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 173–178. [Google Scholar] [CrossRef]
- Sarraf-Yazdi, S.; Sharpe, M.; Bennett, K.M.; Dotson, T.L.; Anderson, D.J.; Vaslef, S.N. A 9-Year retrospective review of antibiotic cycling in a surgical intensive care unit. J. Surg. Res. 2012, 176, e73–e78. [Google Scholar] [CrossRef]
- Takesue, Y.; Nakajima, K.; Ichiki, K.; Ishihara, M.; Wada, Y.; Takahashi, Y.; Tsuchida, T.; Ikeuchi, H. Impact of a hospital-wide programme of heterogeneous antibiotic use on the development of antibiotic-resistant Gram-negative bacteria. J. Hosp. Infect. 2010, 75, 28–32. [Google Scholar] [CrossRef]
- Martinez, J.A.; Nicolas, J.M.; Marco, F.; Horcajada, J.P.; Garcia-Segarra, G.; Trilla, A.; Codina, C.; Torres, A.; Mensa, J. Comparison of antimicrobial cycling and mixing strategies in two medical intensive care units. Crit. Care Med. 2006, 34, 329–336. [Google Scholar] [CrossRef]
- Sandiumenge, A.; Diaz, E.; Rodriguez, A.; Vidaur, L.; Canadell, L.; Olona, M.; Rue, M.; Rello, J. Impact of diversity of antibiotic use on the development of antimicrobial resistance. J. Antimicrob. Chemother. 2006, 57, 1197–1204. [Google Scholar] [CrossRef]
- Sandiumenge, A.; Lisboa, T.; Gomez, F.; Hernandez, P.; Canadell, L.; Rello, J. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE Organisms. Chest 2011, 140, 643–651. [Google Scholar] [CrossRef]
- Canton, R.; Bryan, J. Global antimicrobial resistance: From surveillance to stewardship. Part 2: Stewardship initiatives. Expert Rev. Anti. Infect. Ther. 2012, 10, 1375–1377. [Google Scholar] [CrossRef]
- Pulcini, C.; Cua, E.; Lieutier, F.; Landraud, L.; Dellamonica, P.; Roger, P.M. Antibiotic misuse: A prospective clinical audit in a French university hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 277–280. [Google Scholar] [CrossRef]
- Harnden, A.; Perera, R.; Brueggemann, A.B.; Mayon-White, R.; Crook, D.W.; Thomson, A.; Mant, D. Respiratory infections for which general practitioners consider prescribing an antibiotic: A prospective study. Arch. Dis. Child. 2007, 92, 594–597. [Google Scholar]
- Wise, R. The relentless rise of resistance? J. Antimicrob. Chemother. 2004, 54, 306–310. [Google Scholar] [CrossRef]
- Denes, E.; Prouzergue, J.; Ducroix-Roubertou, S.; Aupetit, C.; Weinbreck, P. Analysis of antibiotic prescriptions done by general practitions for urinary tract infections. Clin. Microbiol. Infect. 2012, 31, 3079–3083. [Google Scholar] [CrossRef]
- Katsarolis, I.; Antoniadou, A.; Poulakou, G. Antibiotic prescribing habits in primary care adult respiratory tract infections. Clin. Microbiol. Infect. 2002, 156, 1114–1119. [Google Scholar]
- Pulcini, C.; Gyssens, I.C. How to educate prescribers in antimicrobial stewardship practices. Virulence 2013, 4, 192–202. [Google Scholar] [CrossRef]
- Davey, P.; Garner, S.; Professional Education Subgroup of SACAR. Professional education on antimicrobial prescribing: A report from the Specialist Advisory Committee on Antimicrobial Resistance (SACAR) professional education subgroup. J. Antimicrob. Chemother. 2007, 60, i27–i32. [Google Scholar] [CrossRef]
- Dryden, M.S.; Cooke, J.; Davey, P. Antibiotic stewardship—More education and regulation not more availability? J. Antimicrob. Chemother. 2009, 64, 885–888. [Google Scholar] [CrossRef]
- Finch, R.G.; Metlay, J.P.; Davey, P.G.; Baker, L.J.; International Forum on Antibiotic Resistance Colloquium. Educational interventions to improve antibiotic use in the community: Report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002. Lancet Infect. Dis. 2004, 4, 44–53. [Google Scholar] [CrossRef]
- Lecky, D.M.; McNulty, C.A.; Touboul, P.; Herotova, T.K.; Benes, J.; Dellamonica, P.; Verlander, N.Q.; Kostkova, P.; Weinberg, J. Evaluation of e-Bug, an educational pack, teaching about prudent antibiotic use and hygiene, in the Czech Republic, France and England. J. Antimicrob. Chemother. 2010, 65, 2674–2684. [Google Scholar] [CrossRef]
- McNulty, C.A.; Cookson, B.D.; Lewis, M.A. Education of healthcare professionals and the public. J. Antimicrob. Chemother. 2012, 67, i11–i18. [Google Scholar] [CrossRef]
- McNulty, C.A.; Boyle, P.; Nichols, T.; Clappison, P.; Davey, P. The public’s attitudes to and compliance with antibiotics. J. Antimicrob. Chemother. 2007, 60, i63–i68. [Google Scholar] [CrossRef]
- Huttner, B.; Goossens, H.; Verheij, T.; Harbarth, S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect. Dis. 2010, 10, 17–31. [Google Scholar] [CrossRef]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar]
- Weber, D.J.; Rutala, W.A.; Miller, M.B.; Huslage, K.; Sickbert-Bennett, E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control 2010, 38, S25–S33. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control 2007, 35, S165–S193. [Google Scholar] [CrossRef]
- Allegranzi, B.; Pittet, D. Role of hand hygiene in healthcare-associated infection prevention. J. Hosp. Infect. 2009, 73, 305–315. [Google Scholar] [CrossRef]
- Sax, H.; Allegranzi, B.; Chraiti, M.N.; Boyce, J.; Larson, E.; Pittet, D. The World Health Organization hand hygiene observation method. Am. J. Infect. Control. 2009, 37, 827–834. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC. Infect. Dis. 2006, 6. [Google Scholar] [CrossRef]
- Peacock, J.E., Jr.; Marsik, F.J.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: Introduction and spread within a hospital. Ann. Intern. Med. 1980, 93, 526–532. [Google Scholar] [CrossRef]
- Pittet, D.; Hugonnet, S.; Harbarth, S.; Mourouga, P.; Sauvan, V.; Touveneau, S.; Perneger, T.V. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet 2000, 356, 1307–1312. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Boyce, J.; World Health Organization World Alliance for Patient Safety First Global Patient Safety Challenge Core Group of Experts. The World Health Organization Guidelines on Hand Hygiene in Health Care and their consensus recommendations. Infect. Control Hosp. Epidemiol. 2009, 30, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Conly, J.M.; Hill, S.; Ross, J.; Lertzman, J.; Louie, T.J. Handwashing practices in an intensive care unit: The effects of an educational program and its relationship to infection rates. Am. J. Infect. Control 1989, 17, 330–339. [Google Scholar] [CrossRef]
- Pessoa-Silva, C.L.; Hugonnet, S.; Pfister, R.; Touveneau, S.; Dharan, S.; Posfay-Barbe, K.; Pittet, D. Reduction of health care associated infection risk in neonates by successful hand hygiene promotion. Pediatrics 2007, 120, e382–e390. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Guzman, S.; Safdar, N. Reduction in nosocomial infection with improved hand hygiene in intensive care units of a tertiary care hospital in Argentina. Am. J. Infect. Control 2005, 33, 392–397. [Google Scholar] [CrossRef]
- Salama, M.F.; Jamal, W.Y.; Mousa, H.A.; Al-Abdulghani, K.A.; Rotimi, V.O. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J. Infect. Public Health 2013, 6, 27–34. [Google Scholar] [CrossRef]
- Erasmus, V.; Brouwer, W.; van Beeck, E.F.; Oenema, A.; Daha, T.J.; Richardus, J.H.; Vos, M.C.; Brug, J. A qualitative exploration of reasons for poor hand hygiene among hospital workers: Lack of positive role models and of convincing evidence that hand hygiene prevents cross-infection. Infect. Control Hosp. Epidemiol. 2009, 30, 415–419. [Google Scholar] [CrossRef]
- Sax, H.; Uckay, I.; Richet, H.; Allegranzi, B.; Pittet, D. Determinants of good adherence to hand hygiene among healthcare workers who have extensive exposure to hand hygiene campaigns. Infect. Control Hosp. Epidemiol. 2007, 28, 1267–1274. [Google Scholar] [CrossRef]
- Jumaa, P.A. Hand hygiene: Simple and complex. Int. J. Infect. Dis. 2005, 9, 3–14. [Google Scholar] [CrossRef]
- Schneider, J.; Moromisato, D.; Zemetra, B.; Rizzi-Wagner, L.; Rivero, N.; Mason, W.; Imperial-Perez, F.; Ross, L. Hand hygiene adherence is influenced by the behavior of role models. Pediatr. Crit. Care Med. 2009, 10, 360–363. [Google Scholar] [CrossRef]
- Hartstein, A.I.; Denny, M.A.; Morthland, V.H.; LeMonte, A.M.; Pfaller, M.A. Control of methicillin-resistant Staphylococcus aureus in a hospital and an intensive care unit. Infect. Control Hosp. Epidemiol. 1995, 16, 405–411. [Google Scholar]
- Patterson, J.E.; Vecchio, J.; Pantelick, E.L.; Farrel, P.; Mazon, D.; Zervos, M.J.; Hierholzer, W.J., Jr. Association of contaminated gloves with transmission of Acinetobacter calcoaceticus var. anitratus in an intensive care unit. Am. J. Med. 1991, 91, 479–483. [Google Scholar] [CrossRef]
- Tenorio, A.R.; Badri, S.M.; Sahgal, N.B.; Hota, B.; Matushek, M.; Hayden, M.K.; Trenholme, G.M.; Weinstein, R.A. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant Enterococcus species by health care workers after patient care. Clin. Infect. Dis. 2001, 32, 826–829. [Google Scholar] [CrossRef]
- Clabots, C.R.; Gerding, S.J.; Olson, M.M.; Peterson, L.R.; Gerding, D.N. Detection of asymptomatic Clostridium difficile carriage by an alcohol shock procedure. J. Clin. Microbiol. 1989, 27, 2386–2387. [Google Scholar]
- Wullt, M.; Odenholt, I.; Walder, M. Activity of three disinfectants and acidified nitrite against Clostridium difficile spores. Infect. Control Hosp. Epidemiol. 2003, 24, 765–768. [Google Scholar]
- Boyce, J.M.; Ligi, C.; Kohan, C.; Dumigan, D.; Havill, N.L. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect. Control Hosp. Epidemiol. 2006, 27, 479–483. [Google Scholar] [CrossRef]
- Vernaz, N.; Sax, H.; Pittet, D.; Bonnabry, P.; Schrenzel, J.; Harbarth, S. Temporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficile. J. Antimicrob. Chemother. 2008, 62, 601–607. [Google Scholar] [CrossRef]
- Mathur, P. Hand hygiene: Back to the basics of infection control. Indian J. Med. Res. 2011, 134, 611–620. [Google Scholar] [CrossRef]
- Grabsch, E.A.; Burrell, L.J.; Padiglione, A.; O’Keeffe, J.M.; Ballard, S.; Grayson, M.L. Risk of environmental and healthcare worker contamination with vancomycin-resistant Enterococci during outpatient procedures and hemodialysis. Infect. Control Hosp. Epidemiol. 2006, 27, 287–293. [Google Scholar] [CrossRef]
- Perry, C.; Marshall, R.; Jones, E. Bacterial contamination of uniforms. J. Hosp. Infect. 2001, 48, 238–241. [Google Scholar] [CrossRef]
- Zachary, K.C.; Bayne, P.S.; Morrison, V.J.; Ford, D.S.; Silver, L.C.; Hooper, D.C. Contamination of gowns, gloves, and stethoscopes with vancomycin-resistant Enterococci. Infect. Control Hosp. Epidemiol. 2001, 22, 560–564. [Google Scholar]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef]
- Hardy, K.J.; Oppenheim, B.A.; Gossain, S.; Gao, F.; Hawkey, P.M. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect. Control Hosp. Epidemiol. 2006, 27, 127–132. [Google Scholar] [CrossRef]
- Dancer, S.J.; White, L.F.; Lamb, J.; Girvan, E.K.; Robertson, C. Measuring the effect of enhanced cleaning in a UK hospital: A prospective cross-over study. BMC Med. 2009, 7. [Google Scholar] [CrossRef] [Green Version]
- Goodman, E.R.; Platt, R.; Bass, R.; Onderdonk, A.B.; Yokoe, D.S.; Huang, S.S. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci on surfaces in intensive care unit rooms. Infect. Control Hosp. Epidemiol. 2008, 29, 593–599. [Google Scholar] [CrossRef]
- Hayden, M.K.; Bonten, M.J.; Blom, D.W.; Lyle, E.A.; van de Vijver, D.A.; Weinstein, R.A. Reduction in acquisition of vancomycin-resistant Enterococcus after enforcement of routine environmental cleaning measures. Clin. Infect. Dis. 2006, 42, 1552–1560. [Google Scholar]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarella, L. 2007 Guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. Am. J. Infect. Control 2007, 35, S65–S164. [Google Scholar] [CrossRef]
- Catalano, M.; Quelle, L.S.; Jeric, P.E.; Di Martino, A.; Maimone, S.M. Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J. Hosp. Infect. 1999, 42, 27–35. [Google Scholar] [CrossRef]
- Jawad, A.; Seifert, H.; Snelling, A.M.; Heritage, J.; Hawkey, P.M. Survival of Acinetobacter baumannii on dry surfaces: Comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 1998, 36, 1938–1941. [Google Scholar]
- Kim, K.H.; Fekety, R.; Batts, D.H.; Brown, D.; Cudmore, M.; Silva, J., Jr.; Waters, D. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J. Infect. Dis. 1981, 143, 42–50. [Google Scholar] [CrossRef]
- Dubberke, E.R.; Gerding, D.N.; Classen, D.; Arias, K.M.; Podgorny, K.; Anderson, D.J.; Burstin, H.; Calfee, D.P.; Coffin, S.E.; Fraser, V.; et al. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect. Control Hosp. Epidemiol. 2008, 29, S81–S92. [Google Scholar] [CrossRef]
- Dancer, S.J.; White, L.; Robertson, C. Monitoring environmental cleanliness on two surgical wards. Int. J. Environ. Health Res. 2008, 18, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Oelberg, D.G.; Joyner, S.E.; Jiang, X.; Laborde, D.; Islam, M.P.; Pickering, L.K. Detection of pathogen transmission in neonatal nurseries using DNA markers as surrogate indicators. Pediatrics 2000, 105, 311–315. [Google Scholar] [CrossRef]
- White, L.F.; Dancer, S.J.; Robertson, C.; McDonald, J. Are hygiene standards useful in assessing infection risk? Am. J. Infect. Control 2008, 36, 381–384. [Google Scholar] [CrossRef] [Green Version]
- Carling, P.C.; Briggs, J.L.; Perkins, J.; Highlander, D. Improved cleaning of patient rooms using a new targeting method. Clin. Infect. Dis. 2006, 42, 385–388. [Google Scholar] [CrossRef]
- McEwen, S.A. Antibiotic use in animal agriculture: What have we learned and where are we going? Anim. Biotechnol. 2006, 17, 239–250. [Google Scholar] [CrossRef]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef]
- Roura, E.; Homedes, J.; Klasing, K.C. Prevention of immunologic stress contributes to the growth-permitting ability of dietary antibiotics in chicks. J. Nutr. 1992, 122, 2383–2390. [Google Scholar]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Anderson, A.D.; Nelson, J.M.; Rossiter, S.; Angulo, F.J. Public health consequences of use of antimicrobial agents in food animals in the United States. Microb. Drug Resist. 2003, 9, 373–379. [Google Scholar] [CrossRef]
- Anthony, F.; Acar, J.; Franklin, A.; Gupta, R.; Nicholls, T.; Tamura, Y.; Thompson, S.; Threlfall, E.J.; Vose, D.; van Vuuren, M.; et al. Antimicrobial resistance: Responsible and prudent use of antimicrobial agents in veterinary medicine. Rev. Sci. Tech. 2001, 20, 829–839. [Google Scholar]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Roe, M.T.; Pillai, S.D. Monitoring and identifying antibiotic resistance mechanisms in bacteria. Poult. Sci. 2003, 82, 622–626. [Google Scholar]
- Alexander, T.W.; Yanke, J.L.; Reuter, T.; Topp, E.; Read, R.R.; Selinger, B.L.; McAllister, T.A. Longitudinal characterization of antimicrobial resistance genes in feces shed from cattle fed different subtherapeutic antibiotics. BMC Microbiol. 2011, 11. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Bager, F.; Madsen, M.; Christensen, J.; Aarestrup, F.M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 1997, 31, 95–112. [Google Scholar] [CrossRef]
- Coque, T.M.; Tomayko, J.F.; Ricke, S.C.; Okhyusen, P.C.; Murray, B.E. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob. Agents Chemother. 1996, 40, 2605–2609. [Google Scholar]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef]
- Cuny, C.; Friedrich, A.; Kozytska, S.; Layer, F.; Nubel, U.; Ohlsen, K.; Strommenger, B.; Walther, B.; Wieler, L.; Witte, W. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. Int. J. Med. Microbiol. 2010, 300, 109–117. [Google Scholar] [CrossRef]
- Horton, R.A.; Randall, L.P.; Snary, E.L.; Cockrem, H.; Lotz, S.; Wearing, H.; Duncan, D.; Rabie, A.; McLaren, I.; Watson, E.; et al. Fecal carriage and shedding density of CTX-M extended-spectrum β-lactamase-producing Escherichia coli in cattle, chickens, and pigs: Implications for environmental contamination and food production. Appl. Environ. Microbiol. 2011, 77, 3715–3719. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L.; Toleman, M.A.; Walsh, T.R. Does broad-spectrum β-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J. Antimicrob. Chemother. 2011, 66, 689–692. [Google Scholar] [CrossRef]
- Colquhoun, D.J.; Aarflot, L.; Melvold, C.F. gyrA and parC Mutations and associated quinolone resistance in Vibrio anguillarum serotype O2b strains isolated from farmed Atlantic cod (Gadus morhua) in Norway. Antimicrob. Agents Chemother. 2007, 51, 2597–2599. [Google Scholar] [CrossRef]
- Shah, S.Q.; Colquhoun, D.J.; Nikuli, H.L.; Sorum, H. Prevalence of antibiotic resistance genes in the bacterial flora of integrated fish farming environments of Pakistan and Tanzania. Environ. Sci. Technol. 2012, 46, 8672–8679. [Google Scholar]
- Rhodes, G.; Huys, G.; Swings, J.; McGann, P.; Hiney, M.; Smith, P.; Pickup, R.W. Distribution of oxytetracycline resistance plasmids between aeromonads in hospital and aquaculture environments: Implication of Tn1721 in dissemination of the tetracycline resistance determinant tetA. Appl. Environ. Microbiol. 2000, 66, 3883–3890. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Potter, A.; Gerdts, V.; Littel-van den Hurk, S. Veterinary vaccines: Alternatives to antibiotics? Anim. Health Res. Rev. 2008, 9, 187–199. [Google Scholar] [CrossRef]
- Boklund, A.; Alban, L.; Mortensen, S.; Houe, H. Biosecurity in 116 Danish fattening swineherds: Descriptive results and factor analysis. Prev. Vet. Med. 2004, 66, 49–62. [Google Scholar] [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Anderson, R.C.; Harvey, R.B.; Genovese, K.J.; Kennedy, C.N.; Venn, D.W.; Nisbet, D.J. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim. Health Res. Rev. 2008, 9, 217–225. [Google Scholar] [CrossRef]
- Castanon, J.I. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Atterbury, R.J. Bacteriophage biocontrol in animals and meat products. Microb. Biotechnol. 2009, 2, 601–612. [Google Scholar] [CrossRef]
- Joerger, R.D. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003, 82, 640–647. [Google Scholar]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Butler, M.S.; Cooper, M.A. Screening strategies to identify new antibiotics. Curr. Drug Targets. 2012, 13, 373–387. [Google Scholar] [CrossRef]
- Giamarellou, H.; Poulakou, G. Multidrug-resistant Gram-negative infections: What are the treatment options? Drugs 2009, 69, 1879–1901. [Google Scholar]
- Silver, L.L. Are natural products still the best source for antibacterial discovery? The bacterial entry factor. Expert Opin. Drug Discov. 2008, 3, 487–500. [Google Scholar] [CrossRef]
- O’Shea, R.; Moser, H.E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef]
- Projan, S.J. Why is big Pharma getting out of antibacterial drug discovery? Curr. Opin. Microbiol. 2003, 6, 427–430. [Google Scholar] [CrossRef]
- Eidorial Office. Regulatory watch: Non-inferiority-trial discussions impact new drug applications. Nat. Rev. Drug Discov. 2009, 8. [CrossRef]
- Hogberg, L.D.; Heddini, A.; Cars, O. The global need for effective antibiotics: Challenges and recent advances. Trends Pharmacol. Sci. 2010, 31, 509–515. [Google Scholar] [CrossRef]
- Projan, S.J.; Shlaes, D.M. Antibacterial drug discovery: Is it all downhill from here? Clin. Microbiol. Infect. 2004, 10, 18–22. [Google Scholar] [CrossRef]
- Infectious Diseases Society of America. The 10 × 20 initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 2010, 50, 1081–1083. [CrossRef]
- Morel, C.M.; Mossialos, E. Stoking the antibiotic pipeline. BMJ 2010, 340. [Google Scholar] [CrossRef]
- Council of the European Union. Council Conclusions on Innovative Incentives for Effective Antibiotics. Available online: http://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/lsa/111608.pdf (accessed on 4 September 2013).
- Giannakaki, V.; Miyakis, S. Novel antimicrobial agents against multi-drug-resistant Gram-positive bacteria: An overview. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 182–188. [Google Scholar] [CrossRef]
- Karras, G.; Giannakaki, V.; Kotsis, V.; Miyakis, S. Novel antimicrobial agents against multi-drug-resistant Gram-negative bacteria: An overview. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 175–181. [Google Scholar]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Lloyd, D.H. Alternatives to conventional antimicrobial drugs: A review of future prospects. Vet. Dermatol. 2012, 23, 299–304. [Google Scholar] [CrossRef]
- Jawetz, E. Antimicrobial chemotherapy. Annu. Rev. Microbiol. 1956, 10, 85–114. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, C.-R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to Minimize Antibiotic Resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274-4305. https://doi.org/10.3390/ijerph10094274
Lee C-R, Cho IH, Jeong BC, Lee SH. Strategies to Minimize Antibiotic Resistance. International Journal of Environmental Research and Public Health. 2013; 10(9):4274-4305. https://doi.org/10.3390/ijerph10094274
Chicago/Turabian StyleLee, Chang-Ro, Ill Hwan Cho, Byeong Chul Jeong, and Sang Hee Lee. 2013. "Strategies to Minimize Antibiotic Resistance" International Journal of Environmental Research and Public Health 10, no. 9: 4274-4305. https://doi.org/10.3390/ijerph10094274
APA StyleLee, C.-R., Cho, I. H., Jeong, B. C., & Lee, S. H. (2013). Strategies to Minimize Antibiotic Resistance. International Journal of Environmental Research and Public Health, 10(9), 4274-4305. https://doi.org/10.3390/ijerph10094274